Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Elemental Analysis
2.3. Samples
2.4. Distribution Coefficients (Kd) Experiments
2.5. Speciation of U and Hg-Computational Modelling
2.6. Ion Exchange Study of U and Hg
2.6.1. Kinetics Experiments
2.6.2. Continuous Mode Experiments
2.6.3. Effect of Extraction Cycles
3. Results
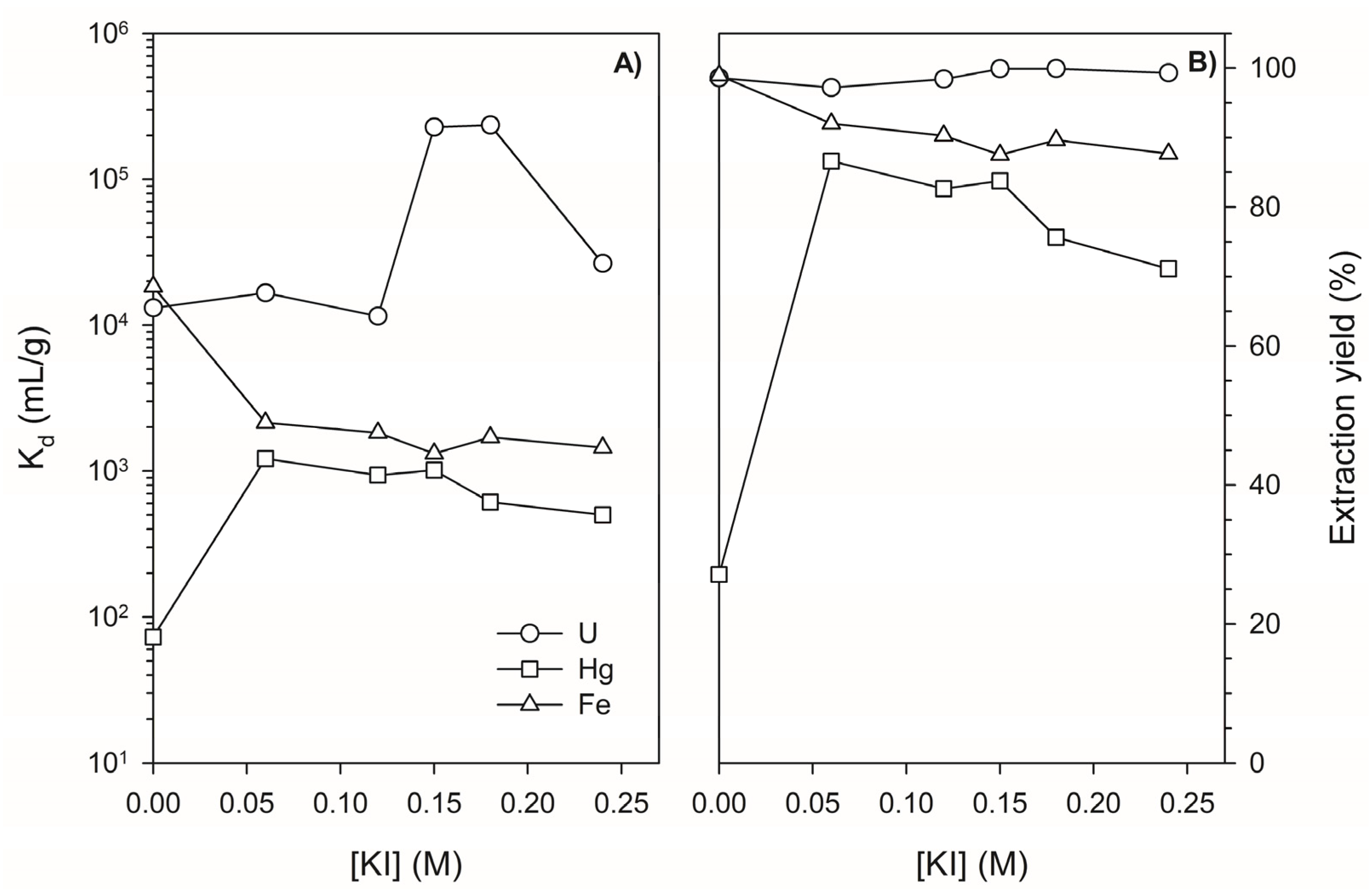
3.1. Determination of Distribution Coefficients for Major Elements, Hg, and U on Lewatit TP260 in the Absence and Presence of KI
3.2. Predicted Complexes of U and Hg at Different Concentration of KI in SCRW Leaching Solution
3.3. Retention of U and Hg from SCRW Leaching Solution by Lewatit TP260—Batch Mode Experimentation
3.4. Retention of U and Hg from SCRW Leaching Solution by Lewatit TP260—Continuous Mode Experimentation
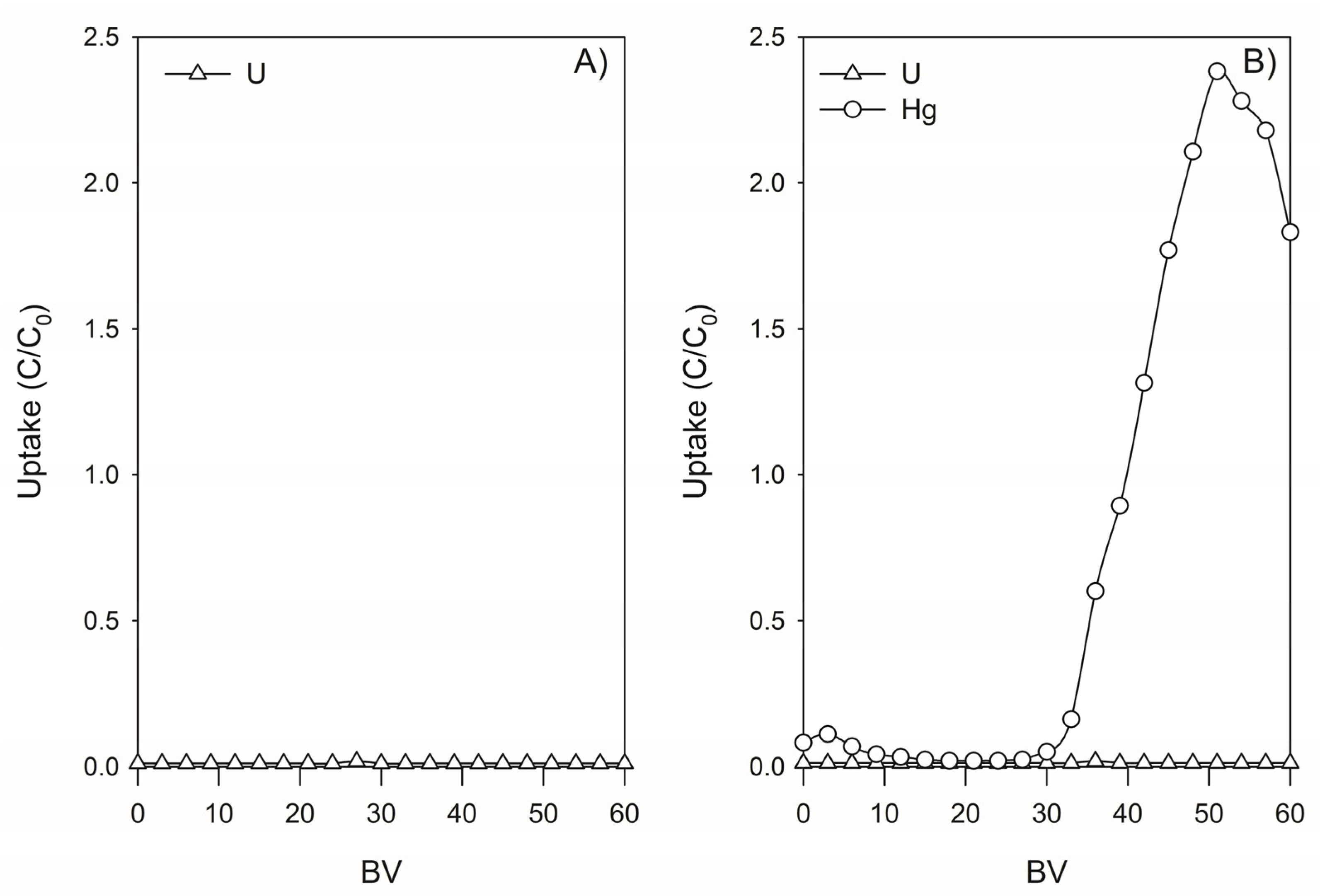
3.4.1. Assessment Using SCRW’ Leaching Solution
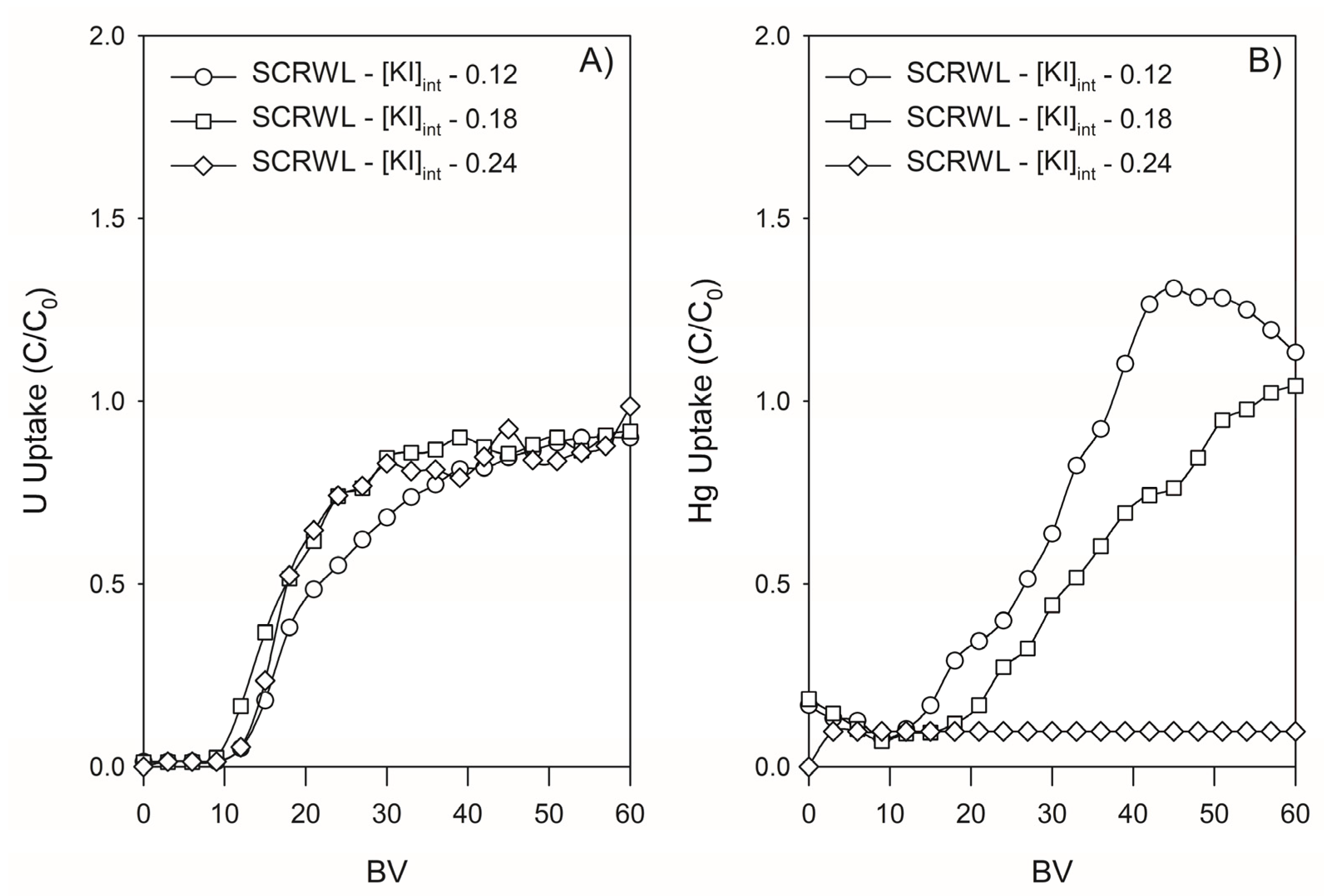
3.4.2. Retention of U and Hg by Lewatit TP260 in SCRW Leaching Solution
3.4.3. Effect of the Flow Rate and the Geometry
3.5. Retention of U from SCRW Leaching Solution by Lewatit TP260—Extraction Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council. Molybdenum-99/Technetium-99m Supply Reliability. Medical Isotope Production without Highly Enriched Uranium; National Academies Press: Washington, DC, USA, 2009; pp. 55–65. [Google Scholar]
- Sameh, A.H. Production Cycle for Large Scale Fission Mo-99 Separation by the Processing of Irradiated LEU Uranium Silicide Fuel Element Targets. Sci. Technol. Nucl. 2013, 14, 704846. [Google Scholar] [CrossRef] [Green Version]
- Wymer, R.G.; Blanco, R.E. Uranium-aluminum alloy dissolution. Ind. Eng. Chem. 1957, 49, 59–61. [Google Scholar] [CrossRef]
- Ethier, A.; Whynot, J.; O’Connor, N.; Briden, N. Evaluation of Potential Mercury Releases from Medical Isotope Waste. CNL Nucl. Rev. 2014, 2, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Bond, M.J.; Silke, R.; Stuart, M.; Carr, J.; Rowan, D.J. A weight-of-evidence approach to the assessment of ecological risk from historical contamination of Ottawa river sediments near Chalk River Laboratories. CNL Nucl. Rev. 2015, 4, 155–170. [Google Scholar] [CrossRef]
- Reynier, N.; Lastra, R.; Laviolette, C.; Bouzoubaâ, N.; Chapman, M. Optimization and validation of a chemical process for uranium, mercury and cesium leaching from cemented radioactive wastes. CNL Nucl. Rev. 2015, 4, 131–139. [Google Scholar] [CrossRef]
- Abowslama, E.; Ebraheem, E.; Sam, A.K. Precipitation and purification of uranium from rock phosphate. J. Radioanal. Nucl. Chem. 2014, 299, 815–818. [Google Scholar] [CrossRef]
- Gupta, R.; Pandey, V.M.; Pranesh, S.R.; Chakravarty, A.B. Study of an improved technique for precipitation of uranium from eluted solution. Hydrometallurgy 2004, 71, 429–434. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.N.; Manchanda, V.K.; Husain, M.; Prasad, A.K.; Parmar, V.S. N,N,N′,N′-Tetraoctyl Diglycolamide (TODGA): A Promising Extractant for Actinide-Partitioning from High-Level Waste (HLW). Solvent Extr. Ion Exch. 2005, 23, 463–479. [Google Scholar] [CrossRef]
- Patil, A.B.; Pathak, P.N.; Shinde, V.S.; Alyapyshev, M.Y.; Babain, V.A.; Mohapatra, P.K. A novel solvent system containing a dipicolinamide in room temperature ionic liquids for actinide ion extraction. J. Radioanal. Nucl. Chem. 2015, 305, 521–528. [Google Scholar] [CrossRef]
- Jensen, M.P.; Chiarizia, R.; Ulicki, J.S.; Spindler, B.D.; Murphy, D.J.; Hossain, M.M.; Roca-Sabio, A.; de Blas, A.; Rodríguez-Blas, T. Solvent Extraction Separation of Trivalent Americium from Curium and the Lanthanides. Solvent Extr. Ion Exch. 2015, 33, 329–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Huang, X.; Wang, C.; Zhu, Z.; Zhang, G. Synergistic extraction of rare earths by mixture of HDEHP and HEH/EHP in sulfuric acid medium. J. Rare Earth 2008, 26, 688–692. [Google Scholar] [CrossRef]
- Sood, D.D.; Patil, S.K. Chemistry of nuclear fuel reprocessing: Current status. J. Radioanal. Nucl. Chem. 1996, 203, 547–573. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ferella, F.; De Michelis, I.; Vegliò, F. Treatment of fluid catalytic cracking spent catalysts to recover lanthanum and cerium: Comparison between selective precipitation and solvent extraction. J. Ind. Eng. Chem. 2015, 24, 92–97. [Google Scholar] [CrossRef]
- Chan, G.Y.; Drew, M.G.; Hudson, M.J.; Iveson, P.B.; Liljenzin, J.O.; Skålberg, M.; Spjuth, L.; Madic, C. Solvent extraction of metal ions from nitric acid solution using N,N′-substituted malonamides. Experimental and crystallographic evidence for two mechanisms of extraction, metal complexation and ion-pair formation. J. Chem. Soc. Dalton 1997, 4, 649–660. [Google Scholar] [CrossRef]
- El-Nadi, Y.A.; El-Hefny, N.E.; Aly, H.F. Solvent extraction and recovery of Y(III) and Yb(III) from fluorspar mineral. Int. J. Miner. Met. Mater. 2013, 20, 713–719. [Google Scholar] [CrossRef]
- Preston, J.S.; du Preez, A.C. Solvent extraction of platinum-group metals from hydrochloric acid solutions by dialkyl sulphoxides. Solvent Extr. Ion Exch. 2002, 20, 359–374. [Google Scholar] [CrossRef]
- Shimojo, K.; Nakai, A.; Okamura, H.; Saito, T.; Ohashi, A.; Naganawa, H. Comprehensive extraction study using N, N-dioctyldiglycolamic acid. Anal. Sci. 2014, 30, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Liu, Y.; Li, S.; Ding, S.; Jin, Y.; Wang, Z.; Hu, X.; Zhang, L. Selective Extraction of Americium(III) over Europium(III) Ions with Pyridylpyrazole Ligands: Structure–Property Relationships. Eur. J. Inorg. Chem. 2017, 3, 651–658. [Google Scholar] [CrossRef]
- Yuezhoua, W.; Ruiqina, L.; Yana, W.; Jianhuaa, Z.; Xinpenga, W.; Zia, C. Chromatographic Separation of Actinides and Fission Products. Energy Procedia 2013, 39, 110–119. [Google Scholar]
- Sharma, B.K.; Rajamani, P.; Mathur, P.K. Use of type-II strong base anion exchange resins for ion exchange chromatographic separation of isotopes of boron. Indian J. Chem. Technol. 1997, 4, 308–316. [Google Scholar]
- McGarvey, F.X.; Ungar, J. The Influence of Resin Functional Group on the Ion- Exchange Recovery of Uranium. J. S. Afr. I Min. Metall. 1981, 81, 93–100. [Google Scholar]
- Strelow, F.W.E.; Bothma, C.J.C. Anion Exchange and a Selectivity Scale for Elements in Sulfuric Acid Media with a Strongly Basic Resin Anal. Chem. 1967, 39, 595–599. [Google Scholar]
- Hashikin, N.A.A.; Yeong, C.H.; Abdullah, B.J.J.; Ng, K.H.; Chung, L.Y.; Dahalan, R.; Perkins, A.C. Neutron Activated Samarium-153 Microparticles for Transarterial Radioembolization of Liver Tumour with Post-Procedure Imaging Capabilities. PLoS ONE 2015, 10, e0138106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, Y.; Nelson, F. Anion-exchange Studies. XXV. The Rare Earth in Nitrate Solutions. J. Phys. Chem. 1959, 63, 77–79. [Google Scholar] [CrossRef]
- Kadous, A.; Didi, M.A.; Villemin, D. Removal of uranium(VI) from acetate medium using Lewatit TP 260 resin. J. Radioanal. Nucl. Chem. 2011, 288, 553–561. [Google Scholar] [CrossRef]
- Esma, B.; Omar, A.; Amine, D.M. Comparative study on lanthanum(III) sorption onto Lewatit TP 207 and Lewatit TP 260. J. Radioanal. Nucl. Chem. 2014, 299, 439–446. [Google Scholar] [CrossRef]
- Yuchi, A.; Sato, T.; Morimoto, Y.; Mizuno, H.; Wada, H. Adsorption Mechanism of Trivalent Metal Ions on Chelating Resins Containing Iminodiacetic Acid Groups with Reference to Selectivity. Anal. Chem. 1997, 69, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rösch, F. Processing of Generator-Produced 68Ga for Medical Application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Torralvo, F.A.; Fernández-Pereira, C. Recovery of germanium from real fly ash leachates by ion-exchange extraction. Miner. Eng. 2011, 24, 35–41. [Google Scholar] [CrossRef]
- Reynier, N.; Lastra, R.; Laviolette, C.; Fiset, J.F.; Bouzoubaâ, N.; Chapman, M. Comparison of Uranium Recovery by Ion Exchange from Sulfuric Acid Leaching solution in Iodide and Chloride Media. Solvent Extr. Ion Exch. 2016, 34, 188–200. [Google Scholar] [CrossRef]
- Lewatit MonoPlus TP260. Product Information. Edition: 2021-08-31. Available online: https://lanxess.com/en/Products-and-Solutions/Products/l/LEWATIT-MonoPlus-TP-260 (accessed on 6 March 2023).
- Pappas, R.S. Sample Preparation Problem Solving for Inductively Coupled Plasma-Mass Spectrometry with Liquid Introduction Systems I. Solubility, Chelation, and Memory Effects. Spectroscopy 2012, 27, 20–31. [Google Scholar] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; U.S. Geological Survey: Denver, CO, USA, 2013; Volume 6, pp. 1–497. [Google Scholar]
- Subramonian, S.; Clifford, D.; Vijjeswarapu, W. Evaluating Ion Exchange for Removing Radium From Groundwater. J. Am. Water Works Ass. 1990, 82, 61–70. [Google Scholar] [CrossRef]
- Trambouze, P. Countercurrent two-phase flow fixed bed catalytic reactors. Chem. Eng. Sci. 1990, 45, 2269–2275. [Google Scholar] [CrossRef]
- Alguacil, F.J.A. Kinetic study of Cadmium(II) Adsorption on Lewatit TP260 Resin. J. Chem. Res. 2003, 3, 144–146. [Google Scholar] [CrossRef]
- Cerpa, A.; Alguacil, F.J.; Lado, I.; Lopez, A.; Lopez, F.A. Removal of Ni (II) and Co (II) ions from acidic solutions by Lewatit TP-260 resin. Desalin. Water Treat. 2017, 70, 169–174. [Google Scholar] [CrossRef]
- Clever, H.L.; Johnson, S.A.; Derrick, M.E. The solubility of mercury and some sparingly soluble mercury salts in water and aqueous electrolyte solutions. J. Phys. Chem. Ref. Data 1985, 14, 631–680. [Google Scholar] [CrossRef]
- Sawicki, M.; Lecerclé, D.; Grillon, G.; Le Gall, B.; Sérandour, A.L.; Poncy, J.L.; Taran, F. Bisphosphonate sequestering agents. Synthesis and preliminary evaluation for in vitro and in vivo uranium(VI) chelation. Eur. J. Med. Chem. 2008, 43, 2768–2777. [Google Scholar] [CrossRef]
- Vukovic, S.; Hay, B.P.; Bryantsev, V.S. Predicting stability constants for uranyl complexes using density functional theory. Inorg. Chem. 2015, 54, 3995–4001. [Google Scholar] [CrossRef]
- Fan, L.; Huang, G.; Yang, S.; Xie, Y.; Liu, W.; Shi, J. Preparation of phosphate-functionalized biopolymer/graphene oxide gels for enhanced selective adsorption of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2021, 329, 555–564. [Google Scholar] [CrossRef]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 1995. [Google Scholar]
- Lapidus, L.; Amundson, N.R. Mathematics of adsorption in beds. The effect of longitudinal diffusion in ion exchange and chromatographic columns. J. Phys. Chem 1952, 56, 984–988. [Google Scholar] [CrossRef]
- Nesterenko, P.N.; Shaw, M.J.; Hill, S.J.; Jones, P. Aminophosphonate-functionalized silica: A versatile chromatographic stationary phase for high-performance chelation ion chromatography. Microchem. J. 1999, 62, 58–69. [Google Scholar] [CrossRef]



| Instrumental Parameters | ICP-OES | ICP-MS |
|---|---|---|
| RF Power (W) | 1250 | 1500 |
| Plasma gas flow (L/min) | 15 | 14 |
| Auxiliary gas flow (L/min) | 1.5 | 0.8 |
| Nebulizer gas flow (L/min) | 0.75 | 0.78 |
| Replicates | 3 | 3 |
| Replicate Time (s) | 10 | 30 |
| Stabilization Time (s) | 30 | 10 |
| Sample flow rate (rpm) | 14 | 15 |
| Wavelength (nm)/Isotope | Mg(279.553), Al(237.312), Ca(422.673), Fe(238.204), In(190.794), Hg(184.887), U(385.957), Tl(190.794), In(230.606), Ar(737.212) | 24Mg, 27Al, 44Ca, 56Fe, 200Hg, 202Hg 232Th, 238U 103Rh, 115In |
| SCRW Leaching Solution | KI a (M) | KI a (g/L) | U (mg/L) | Hg (mg/L) | ||
|---|---|---|---|---|---|---|
| Kinetics | Column | Kinetics | Column | |||
| SCRWL-[KI]int—0.12 | 0.12 | 20 | 137 | 164 | 72 | 74 |
| SCRWL-[KI]int—0.15 | 0.15 | 25 | - | 152 | - | 87 |
| SCRWL-[KI]int—0.18 | 0.18 | 30 | 134 | 186 | 118 | 82 |
| SCRWL-[KI]int—0.24 | 0.24 | 40 | 107 | 168 | 104 | 62 |
| Parameter | Concentration (mol/L) | Concentration (mg/L) |
|---|---|---|
| U(VI) | 0.0004 | 95.2 |
| Hg2+ | 0.0005 | 100 |
| Fe3+ | 0.013 | 726 |
| Al3+ | 0.056 | 1511 |
| Mg2+ | 0.0061 | 148 |
| K+ | 0.015; 0.15; 0.30 | 587; 5865; 11,730 |
| I− | 0.015; 0.15; 0.30 | 1904; 19,036; 38,071 |
| N(V) | 0.072 | 1008.5 |
| S(VI) | 1 | 32,660 |
| Ca2+ | 0.5 * | 20,039 |
| pH | 1.82 | 1.82 |
| Sample Identification | Concentration | |||
|---|---|---|---|---|
| U (mg/L) | Hg (mg/L) | Ca (mg/L) | KI (M) | |
| SCRWL’-0.15-U | 115 | - | 351 | 0.15 |
| SCRWL’-0.15-U.Hg | 100 | 97 | 317 | 0.15 |
| U Extraction (%) | Hg Extraction (%) | |||
|---|---|---|---|---|
| Bed Volume | Mean Uptake (C/C0) | Standard Deviation | Mean Uptake (C/C0) | Standard Deviation |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5 | 0.37 | 0.09 | 10.32 | 1.39 |
| 10 | 0.75 | 0.18 | 41.69 | 5.87 |
| 15 | 6.74 | 4.06 | 90.51 | 2.23 |
| 20 | 16.51 | 1.97 | 105.02 | 3.20 |
| 25 | 29.84 | 3.59 | 107.62 | 1.28 |
| 30 | 42.49 | 4.47 | 104.97 | 5.88 |
| 35 | 55.31 | 3.34 | 102.76 | 4.45 |
| 40 | 60.54 | 5.52 | 107.42 | 5.64 |
| 45 | 69.72 | 3.36 | 101.76 | 2.83 |
| 50 | 73.91 | 3.09 | 100.86 | 4.10 |
| Cycles | Steps | U (% ± STD) | Hg (% ± STD) | Fe (% ± STD) |
|---|---|---|---|---|
| 1 | Adsorption | 87 ± 1% | 64 ± 1% | 18 ± 2% |
| Desorption | 82 ± 5% | 5 ± 1% | 0% | |
| 2 | Adsorption | 75 ± 2% | 56 ± 4% | 54 ± 4% |
| Desorption | 80 ± 13% | 5 ± 1% | 0% | |
| 3 | Adsorption | 62 ± 2% | 29 ± 9% | 56 ± 1% |
| Desorption | 82 ± 14% | 6 ± 2% | 0% | |
| 4 | Adsorption | 58 ± 1% | 26 ± 1% | 44 ± 6% |
| Desorption | 84 ± 3% | 6 ± 1% | 0% | |
| 5 | Adsorption | 54 ± 1% | 22 ± 1% | 50 ± 1% |
| Desorption | 84 ± 7% | 5 ± 1% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courchesne, M.; Couture, R.-M.; Basque, J.; Reynier, N.; Larivière, D. Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media. Minerals 2023, 13, 405. https://doi.org/10.3390/min13030405
Courchesne M, Couture R-M, Basque J, Reynier N, Larivière D. Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media. Minerals. 2023; 13(3):405. https://doi.org/10.3390/min13030405
Chicago/Turabian StyleCourchesne, Maxime, Raoul-Marie Couture, Justine Basque, Nicolas Reynier, and Dominic Larivière. 2023. "Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media" Minerals 13, no. 3: 405. https://doi.org/10.3390/min13030405





