KOH-Based Hydrothermal Synthesis of Iron-Rich Titanate Nanosheets Assembled into 3D Hierarchical Architectures from Natural Ilmenite Mineral Sands
Abstract
:1. Introduction
2. Materials and Methods
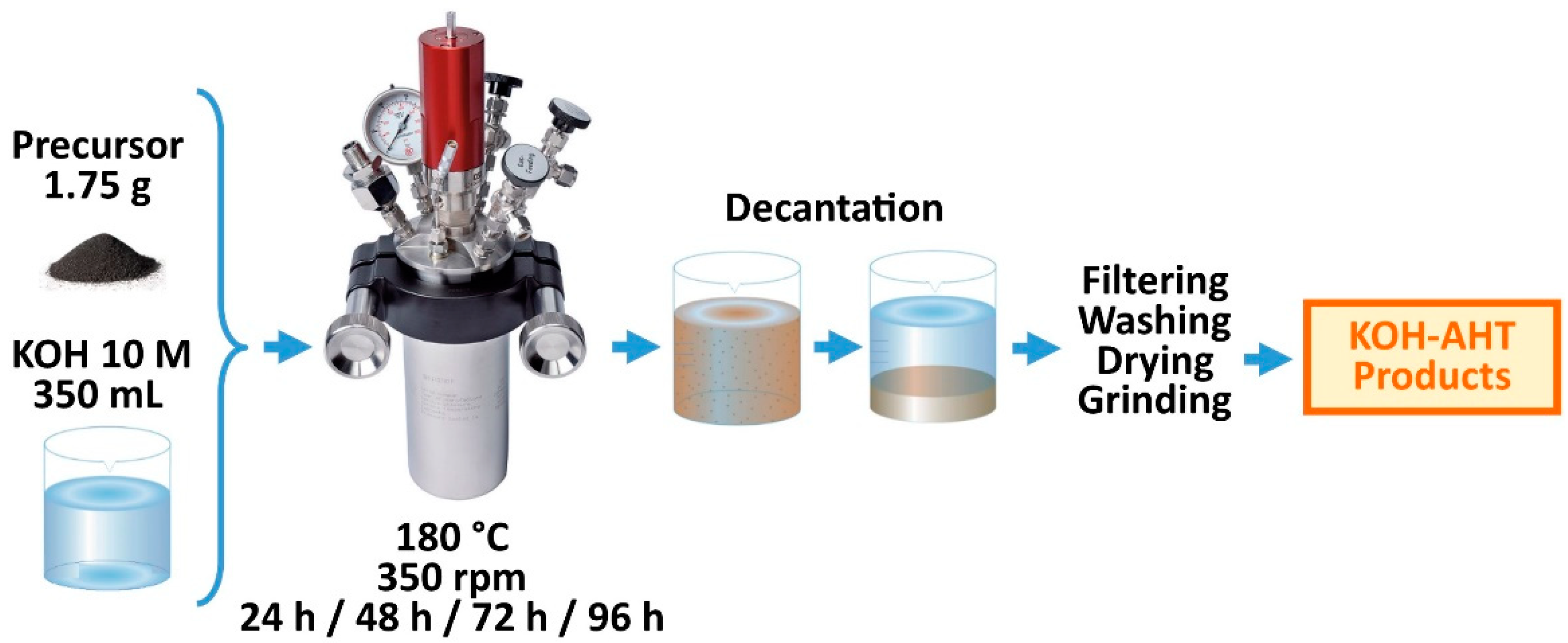
2.1. KOH-AHT Procedure
2.2. Characterization of KOH-AHT Products
3. Results
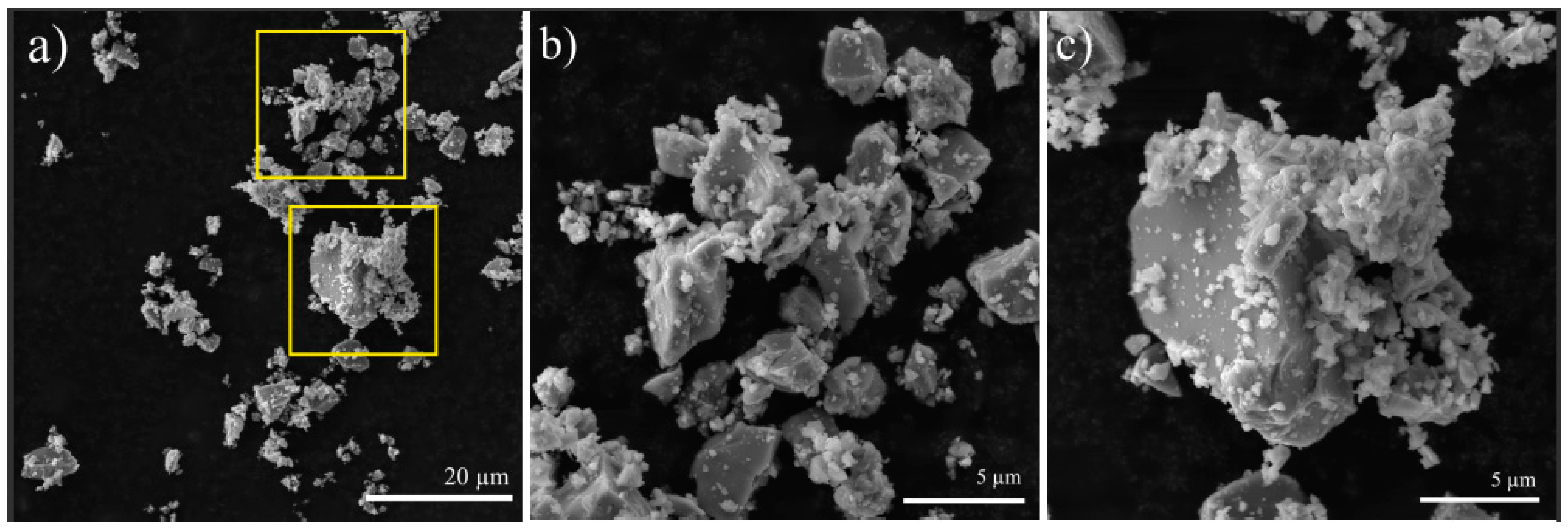
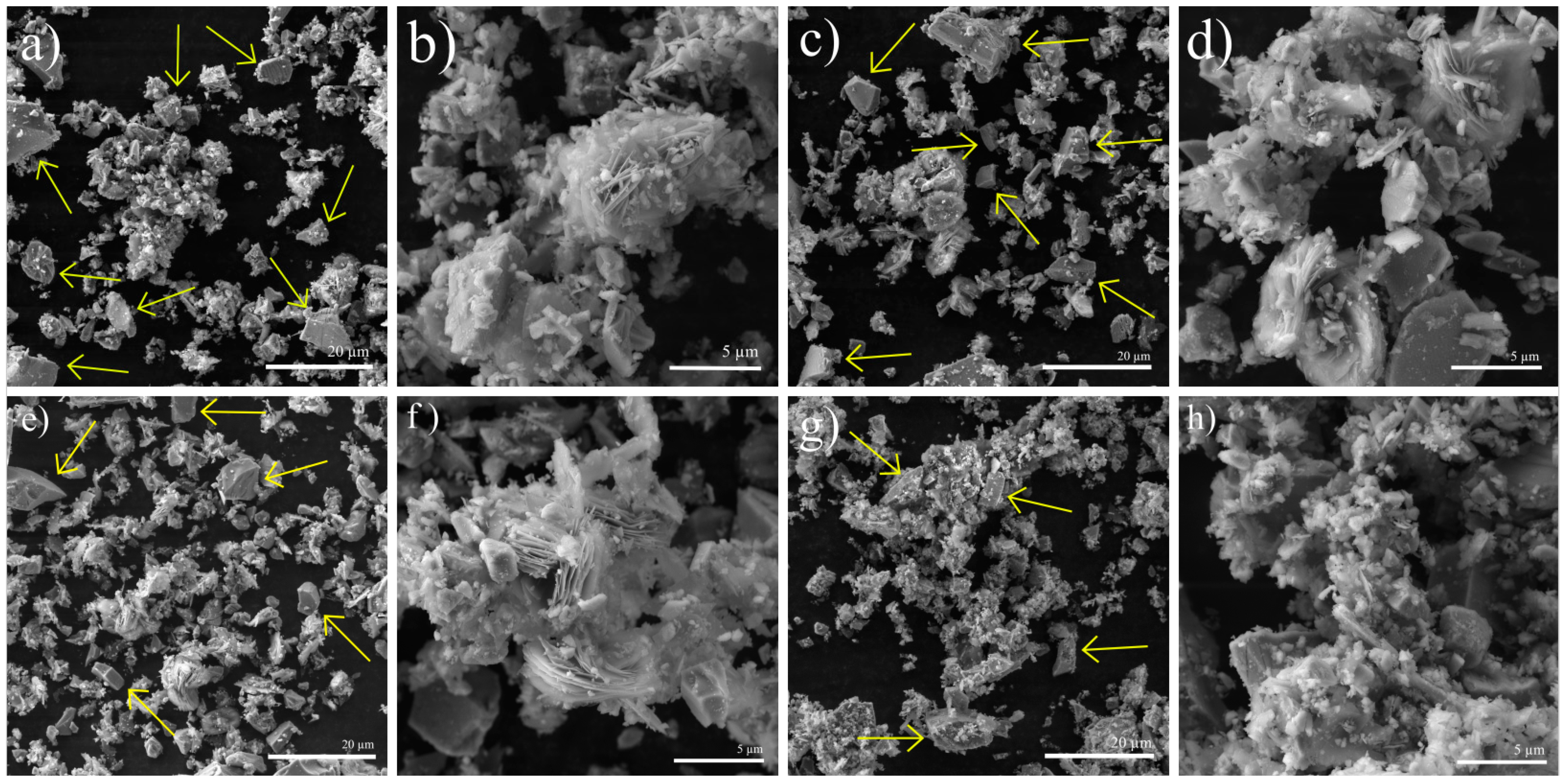
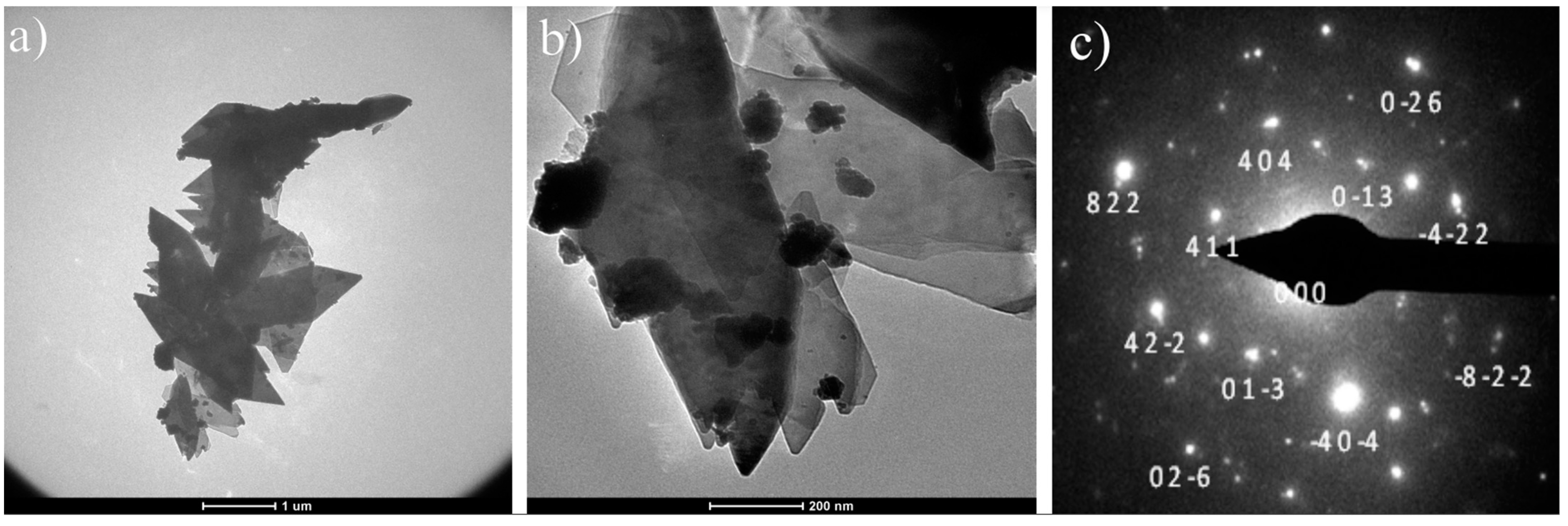
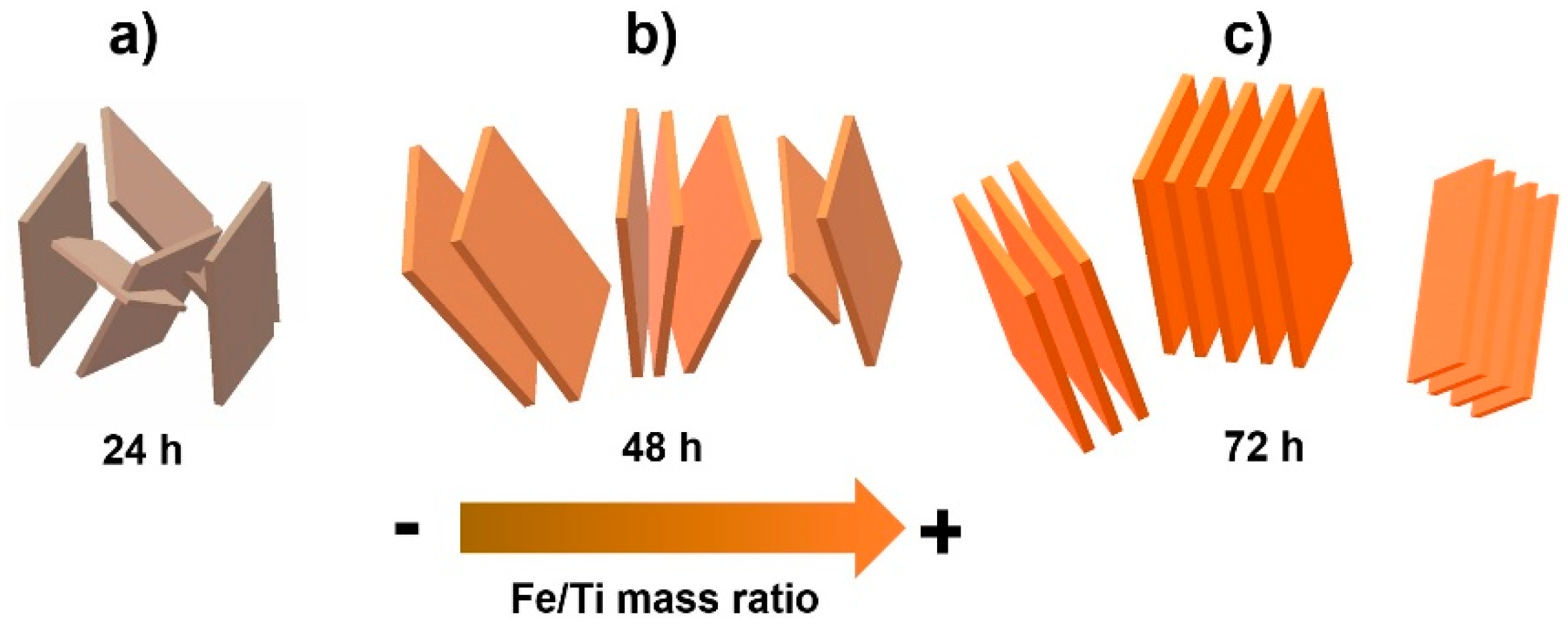
3.1. SEM and TEM Analyses
3.2. XRPD Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.; Du, G.H.; Peng, L.-M. Trititanate Nanotubes Made via a Single Alkali Treatment. Adv. Mater. 2002, 14, 1208–1211. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Parmon, V.N.; Lapkin, A.A.; Walsh, F.C. The effect of hydrothermal conditions on the mesoporous structure of TiO2 nanotubes. J. Mater. Chem. 2004, 14, 3370–3377. [Google Scholar] [CrossRef]
- Morgado, E.; De Abreu, M.A.S.; Pravia, O.R.C.; Marinkovic, B.A.; Jardim, P.M.; Rizzo, F.C.; Araújo, A.S. A study on the structure and thermal stability of titanate nanotubes as a function of sodium content. Solid State Sci. 2006, 8, 888–900. [Google Scholar] [CrossRef]
- Morgan, D.L.; Triani, G.; Blackford, M.G.; Raftery, N.A.; Frost, R.L.; Waclawik, E.R. Alkaline hydrothermal kinetics in titanate nanostructure formation. J. Mater. Sci. 2011, 46, 548–557. [Google Scholar] [CrossRef]
- Kochkar, H.; Lakhdhar, N.; Berhault, G.; Bausach, M.; Abdelhamid, G. Optimization of the alkaline hydrothermal route to titanate nanotubes by a doehlert matrix experience design. J. Phys. Chem. C 2009, 113, 1672–1679. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, J.; Li, L.; Zheng, J.; Zhang, L.; Gong, L.; Wang, Z.; Zhu, Z. A systematic study on evolution mechanism of titanate nanostructures in the hydrothermal process. Chem. Phys. Lett. 2011, 508, 258–264. [Google Scholar] [CrossRef]
- Doong, R.; Kao, I. Fabrication and Characterization of Nanostructured Titanate Materials by the Hydrothermal Treatment Method. Recent Pat. Nanotechnol. 2008, 2, 84–102. [Google Scholar] [CrossRef]
- Simpraditpan, A.; Wirunmongkol, T.; Pavasupree, S.; Pecharapa, W. Simple hydrothermal preparation of nanofibers from a natural ilmenite mineral. Ceram. Int. 2013, 39, 2497–2502. [Google Scholar] [CrossRef]
- Suzuki, Y.; Pavasupree, S.; Yoshikawa, S.; Kawahata, R. Natural rutile-derived titanate nanofibers prepared by direct hydrothermal processing. J. Mater. Res. 2005, 20, 1063–1070. [Google Scholar] [CrossRef]
- Jardim, P.M.; Mancic, L.; Marinkovic, B.A.; Milosevic, O.; Rizzo, F. Nax-yHyTi2-xFexO4•nH2O nanosheets with lepidocrocite-like layered structure synthesized by hydrothermal treatment of ilmenite sand. Cent. Eur. J. Chem. 2011, 9, 415–421. [Google Scholar] [CrossRef]
- Costa, A.M.L.M.; Marinkovic, B.A.; Suguihiro, N.M.; Smith, D.J.; Da Costa, M.E.H.M.; Paciornik, S. Fe-doped nanostructured titanates synthesized in a single step route. Mater. Charact. 2015, 99, 150–159. [Google Scholar] [CrossRef]
- Mancic, L.T.; Marinkovic, B.A.; Jardim, P.M.; Milosevic, O.B.; Rizzo, F. Precursor Particle Size as the Key Parameter for Isothermal Tuning of Morphology from Nanofibers to Nanotubes in the Na2-xHxTinO2n+1 System through Hydrothermal Alkali Treatment of Rutile Mineral Sand. Cryst. Growth Des. 2009, 9, 2152–2158. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Pontón, P.I.; Resende, J.M.; Letichevsky, S.; Habran, M.; Viol, J.B.; Pandoli, O.; Mancic, L. Lepidocrocite-like ferrititanate nanosheets and their full exfoliation with quaternary ammonium compounds. Mater. Des. 2015, 85, 197–204. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Mancic, L.; Jardim, P.M.; Milosevic, O.; Rizzo, F. Hydrothermal synthesis of NaxFexTi2-xO4 from natural ilmenite sand: A CaFe2O4 structure type compound. Solid State Commun. 2008, 145, 346–350. [Google Scholar] [CrossRef]
- Lagos, K.J.; Marinkovic, B.A.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. Towards iron-titanium oxide nanostructures from ecuadorian black mineral sands. Minerals 2021, 11, 122. [Google Scholar] [CrossRef]
- Wang, A.; Si, Y.; Yin, H.; Chen, J.; Huo, J. Synthesis of Na-, Fe-, and Mg-containing titanate nanocomposites starting from ilmenite and NaOH and adsorption kinetics, isotherms, and thermodynamics of Cu(II), Cd(II), and Pb(II) cations. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 249, 114411. [Google Scholar] [CrossRef]
- Piera, E.; Tejedor-Tejedor, M.I.; Zorn, M.E.; Anderson, M.A. Relationship concerning the nature and concentration of Fe(III) species on the surface of TiO2 particles and photocatalytic activity of the catalyst. Appl. Catal. B Environ. 2003, 46, 671–685. [Google Scholar] [CrossRef]
- Enhessari, M.; Razi, M.K.; Etemad, L.; Parviz, A.; Sakhaei, M. Structural, optical and magnetic properties of the Fe2TiO5 nanopowders. J. Exp. Nanosci. 2014, 9, 167–176. [Google Scholar] [CrossRef]
- Preda, S.; Umek, P.; Zaharescu, M.; Anastasescu, C.; Petrescu, S.V.; Gîfu, C.; Eftemie, D.I.; State, R.; Papa, F.; Balint, I. Iron-Modified Titanate Nanorods for Oxidation of Aqueous Light Irradiation. Catalysts 2022, 12, 666. [Google Scholar] [CrossRef]
- Chezhina, N.V.; Piir, I.V.; Krasnov, A.G.; Koroleva, M.S.; Kellerman, D.G.; Semenov, V.G.; Shalaeva, E.V.; Leonidov, I.I.; Shein, I.R. Structure and Magnetic Properties of a Nanosized Iron-Doped Bismuth Titanate Pyrochlore. Inorg. Chem. 2022, 61, 13369–13378. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, A.; Singh, L.; Mishra, S.K.; Babu, P.D.; Asokan, K.; Kumar, S.; Chen, C.L.; Yang, K.S.; Wei, D.H.; et al. Structural, magnetic and electronic properties of iron doped barium strontium titanate. RSC Adv. 2016, 6, 112363–112369. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sobolev, A.N.; Yaroslavtsev, S.A.; Rusakov, V.S.; Kulova, T.L.; Skundin, A.M.; Yaroslavtsev, A.B. Influence of iron doping on structure and electrochemical properties of Li4Ti5O12. Electrochim. Acta 2016, 219, 524–530. [Google Scholar] [CrossRef]
- Akieh, M.N.; Lahtinen, M.; Väisänen, A.; Sillanpää, M. Preparation and characterization of sodium iron titanate ion exchanger and its application in heavy metal removal from waste waters. J. Hazard. Mater. 2008, 152, 640–647. [Google Scholar] [CrossRef]
- Fàbrega, C.; Andreu, T.; Cabot, A.; Morante, J.R. Location and catalytic role of iron species in TiO2: Fe photocatalysts: An EPR study. J. Photochem. Photobiol. A Chem. 2010, 211, 170–175. [Google Scholar] [CrossRef]
- Dosen, A.; Pontón, P.I.; Marinkovic, B.A. Thermally induced phase transformations of lepidocrocite-like ferrititanate nanosheets synthesized from a low cost precursor by hydrothermal method. Mater. Chem. Phys. 2017, 197, 138–144. [Google Scholar] [CrossRef]
- Harito, C.; Bavykin, D.V.; Light, M.E.; Walsh, F.C. Titanate nanotubes and nanosheets as a mechanical reinforcement of water-soluble polyamic acid: Experimental and theoretical studies. Compos. Part B Eng. 2017, 124, 54–63. [Google Scholar] [CrossRef]
- Tamayo-Aguilar, A.; Guaman, M.V.; Guerrero, V.H.; Lagos, K.J.; Costa, C.A.; Nascimento, C.R.; Marinkovic, B.A.; Ponton, P.I. Mechanical properties of amine-cured epoxy composites reinforced with pristine protonated titanate nanotubes. J. Mater. Res. Technol. 2020, 9, 15771–15778. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Ipsale, S.; Rizzo, G.; Baratto, C.; Faglia, G.; Sberveglieri, G. Pd- and Ca-doped iron oxide for ethanol vapor sensing. Mater. Sci. Eng. B 2007, 139, 41–47. [Google Scholar] [CrossRef]
- Cao, W.; Tan, O.K.; Pan, J.S.; Zhu, W.; Gopal Reddy, C. V XPS characterization of xα-Fe2O3–(1−x)ZrO2 for oxygen gas sensing application. Mater. Chem. Phys. 2002, 75, 67–70. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 Nanotubes in Gas Sensor and Lithium-Ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhao, Y.; Zhu, B.; Kong, F.; Wang, D.; Wu, S.; Huang, W.; Zhang, S. H2S sensing characteristics of Pt-doped α-Fe2O3 thick film sensors. Sensors Actuators B Chem. 2007, 125, 79–84. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Patil, S.A.; Shinde, D.V.; Waghmare, S.D.; Zate, M.K.; Mane, R.S.; Han, S.H. Hematite nanostructures: Morphology-mediated liquefied petroleum gas sensors. Sens. Actuators B Chem. 2013, 188, 669–674. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Cressey, B.A.; Light, M.E.; Walsh, F.C. An aqueous, alkaline route to titanate nanotubes under atmospheric pressure conditions. Nanotechnology 2008, 19, 275604. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Cressey, B.A.; Walsh, F.C. Low-temperature synthesis of titanate nanotubes in aqueous KOH. Aust. J. Chem. 2007, 60, 95–98. [Google Scholar] [CrossRef]
- An, Y.; Yin, D.; Song, L.; Chen, Y.; Gao, K. Hydrothermal synthesis and characterisation of titanate nanomaterial thin films in mixed aqueous NaOH/KOH. Micro Nano Lett. 2020, 15, 313–316. [Google Scholar] [CrossRef]
- Lagos, K.J.; Marinkovic, B.A.; Dosen, A.; Guamán, M.V.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. Data on phase and chemical compositions of black sands from “El Ostional” beach situated in Mompiche, Ecuador. Data Brief 2020, 32, 106214. [Google Scholar] [CrossRef]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Blesa, M.A.; Morando, P.J.; Regazzoni, A.E. Chemical Dissolution of Metal Oxides; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315891415. [Google Scholar]
- Meng, X.; Wang, Q.; Duan, L.; Chung, J.S. Catalytic combustion of diesel soot over perovskite-type catalyst: Potassium titanates. Kinet. Catal. 2012, 53, 560–564. [Google Scholar] [CrossRef]
- Ide, Y.; Shirae, W.; Takei, T.; Mani, D.; Henzie, J. Merging Cation Exchange and Photocatalytic Charge Separation Efficiency in an Anatase/K2Ti4O9 Nanobelt Heterostructure for Metal Ions Fixation. Inorg. Chem. 2018, 57, 6045–6050. [Google Scholar] [CrossRef]
- Saothayanun, T.K.; Sirinakorn, T.T.; Ogawa, M. Ion Exchange of Layered Alkali Titanates (Na2Ti3O7, K2Ti4O9, and Cs2Ti5O11) with Alkali Halides by the Solid-State Reactions at Room Temperature. Inorg. Chem. 2020, 59, 4024–4029. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Byeon, S.H. Structural and morphological behavior of TiO2 rutile obtained by hydrolysis reaction of Na2Ti3O7. Bull. Korean Chem. Soc. 2004, 25, 1051–1054. [Google Scholar] [CrossRef]
- Zainullina, V.M.; Zhukov, V.P.; Denisova, T.A.; Maksimova, L.G. Electronic structure and chemical bonding in monoclinic and cubic Li2-xHxTiO3 (0 ≤ x ≤ 2). J. Struct. Chem. 2003, 44, 180–186. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Lu, X.; Liu, F.; Dang, Y.; Li, M.; Ruan, M.; Wu, M.; Zhu, C.; Mani, T.; Suib, S.L.; Gao, P.X. Transition-metal doped titanate nanowire photocatalysts boosted by selective ion-exchange induced defect engineering. Appl. Surf. Sci. 2022, 591, 153116. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. A critical review on the recent progress of synthesizing techniques and fabrication of TiO2-based nanotubes photocatalysts. Appl. Catal. A Gen. 2014, 481, 127–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Luo, W.; Taleb, A. Large-scale synthesis route of TiO2 nanomaterials with controlled morphologies using hydrothermal method and TiO2 aggregates as precursor. Nanomaterials 2021, 11, 365. [Google Scholar] [CrossRef]
- Yang, W.; Chen, H.; Lu, J. Assembly of stacked In2O3 nanosheets for detecting trace NO2 with ultrahigh selectivity and promoted recovery. Appl. Surf. Sci. 2021, 539, 148217. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Walsh, F.C. Titanate and Titania Nanotubes; Nanoscience & Nanotechnology Series; Royal Society of Chemistry: Cambridge, UK, 2009; ISBN 978-1-84755-910-4. [Google Scholar]
- Wu, D.; Liu, J.; Zhao, X.; Li, A.; Chen, Y.; Ming, N. Sequence of events for the formation of titanate nanotubes, nanofibers, nanowires, and nanobelts. Chem. Mater. 2006, 18, 547–553. [Google Scholar] [CrossRef]
- Xu, L.; Fang, H.; Li, S.; Zhu, J.; Pan, C.; Pan, Y.; Feng, Q. Tailored Hydrothermal Synthesis of Specific Facet-Dominated TiO2 Nanocrystals from Lepidocrocite-Type Layered Titanate Nanosheets: Systematical Investigation and Enhanced Photocatalytic Performance. Langmuir 2020, 36, 4477–4495. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cao, Y.; Deng, Z.; Tong, H. Formation of titanate nanostructures under different NaOH concentration and their application in wastewater treatment. J. Solid State Chem. 2011, 184, 712–719. [Google Scholar] [CrossRef]
- Chen, X.; Cen, C.; Tang, Z.; Zeng, W.; Chen, D.; Fang, P.; Chen, Z. The key role of ph value in the synthesis of titanate nanotubes-loaded manganese oxides as a superior catalyst for the selective catalytic reduction of NO with NH3. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef]
- Kim, T.W.; Ha, H.W.; Paek, M.J.; Hyun, S.H.; Baek, I.H.; Choy, J.H.; Hwang, S.J. Mesoporous iron oxide-layered titanate nanohybrids: Soft-chemical synthesis, characterization, and photocatalyst application. J. Phys. Chem. C 2008, 112, 14853–14862. [Google Scholar] [CrossRef]
- Munoz, M.; Domínguez, P.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Naturally-occurring iron minerals as inexpensive catalysts for CWPO. Appl. Catal. B Environ. 2017, 203, 166–173. [Google Scholar] [CrossRef]
- Muzenda, C.; Nkwachukwu, O.V.; Arotiba, O.A. Synthetic Ilmenite (FeTiO3) Nanoparticles as a Heterogeneous Electro-Fenton Catalyst for the Degradation of Tetracycline in Wastewater. Ind. Eng. Chem. Res. 2022, 61, 11417–11428. [Google Scholar] [CrossRef]
- El-Sayed Abdo, A.; El-Sarraf, M.A.; Gaber, F.A. Utilization of ilmenite/epoxy composite for neutrons and gamma rays attenuation. Ann. Nucl. Energy 2003, 30, 175–187. [Google Scholar] [CrossRef]
- Kara, H.; Karabul, Y.; Kılıç, M.; İçelli, O.; Güven Özdemir, Z. Volcanic Rock Reinforced Epoxy Composites for Gamma Ray Shielding. Eur. J. Sci. Technol. 2019, 15, 552–560. [Google Scholar] [CrossRef]
- Eren Belgin, E.; Aycik, G.A.; Kalemtas, A.; Pelit, A.; Dilek, D.A.; Kavak, M.T. Preparation and characterization of a novel ionizing electromagnetic radiation shielding material: Hematite filled polyester based composites. Radiat. Phys. Chem. 2015, 115, 43–48. [Google Scholar] [CrossRef]


| Sample | Morphology | Fe Content (wt. %) | Ti Content (wt. %) | K Content (wt. %) | Fe/Ti Mass Ratio |
|---|---|---|---|---|---|
| Precursor | Irregular particles | 75.40 ± 1.51 | 24.60 ± 0.55 | - | ~3.1 |
| 24 h | Nanosheets | 73.51 ± 1.29 | 23.21 ± 1.24 | 3.28 ± 0.24 | ~3.2 |
| 48 h | Nanosheets | 81.54 ± 0.63 | 17.01 ± 0.43 | 1.44 ± 0.10 | ~4.8 |
| 72 h | Nanosheets | 87.06 ± 3.69 | 12.18 ± 0.49 | 0.75 ± 0.04 | ~7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagos, K.J.; Marinkovic, B.A.; Dosen, A.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. KOH-Based Hydrothermal Synthesis of Iron-Rich Titanate Nanosheets Assembled into 3D Hierarchical Architectures from Natural Ilmenite Mineral Sands. Minerals 2023, 13, 406. https://doi.org/10.3390/min13030406
Lagos KJ, Marinkovic BA, Dosen A, Debut A, Vizuete K, Guerrero VH, Pardo E, Pontón PI. KOH-Based Hydrothermal Synthesis of Iron-Rich Titanate Nanosheets Assembled into 3D Hierarchical Architectures from Natural Ilmenite Mineral Sands. Minerals. 2023; 13(3):406. https://doi.org/10.3390/min13030406
Chicago/Turabian StyleLagos, Karina J., Bojan A. Marinkovic, Anja Dosen, Alexis Debut, Karla Vizuete, Victor H. Guerrero, Emilio Pardo, and Patricia I. Pontón. 2023. "KOH-Based Hydrothermal Synthesis of Iron-Rich Titanate Nanosheets Assembled into 3D Hierarchical Architectures from Natural Ilmenite Mineral Sands" Minerals 13, no. 3: 406. https://doi.org/10.3390/min13030406
APA StyleLagos, K. J., Marinkovic, B. A., Dosen, A., Debut, A., Vizuete, K., Guerrero, V. H., Pardo, E., & Pontón, P. I. (2023). KOH-Based Hydrothermal Synthesis of Iron-Rich Titanate Nanosheets Assembled into 3D Hierarchical Architectures from Natural Ilmenite Mineral Sands. Minerals, 13(3), 406. https://doi.org/10.3390/min13030406









