Viability of Morisca Powder Tailings for Ceramic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
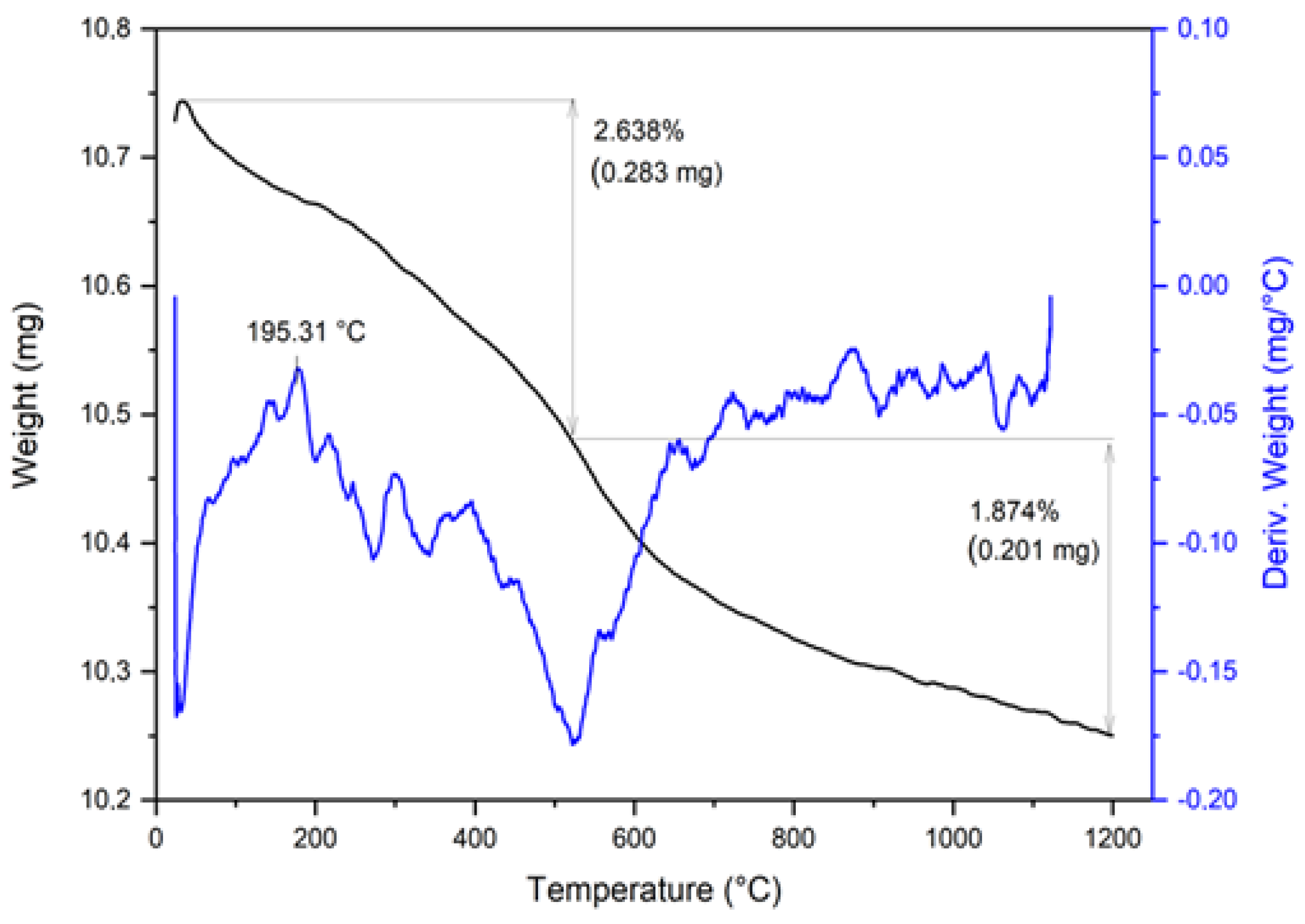
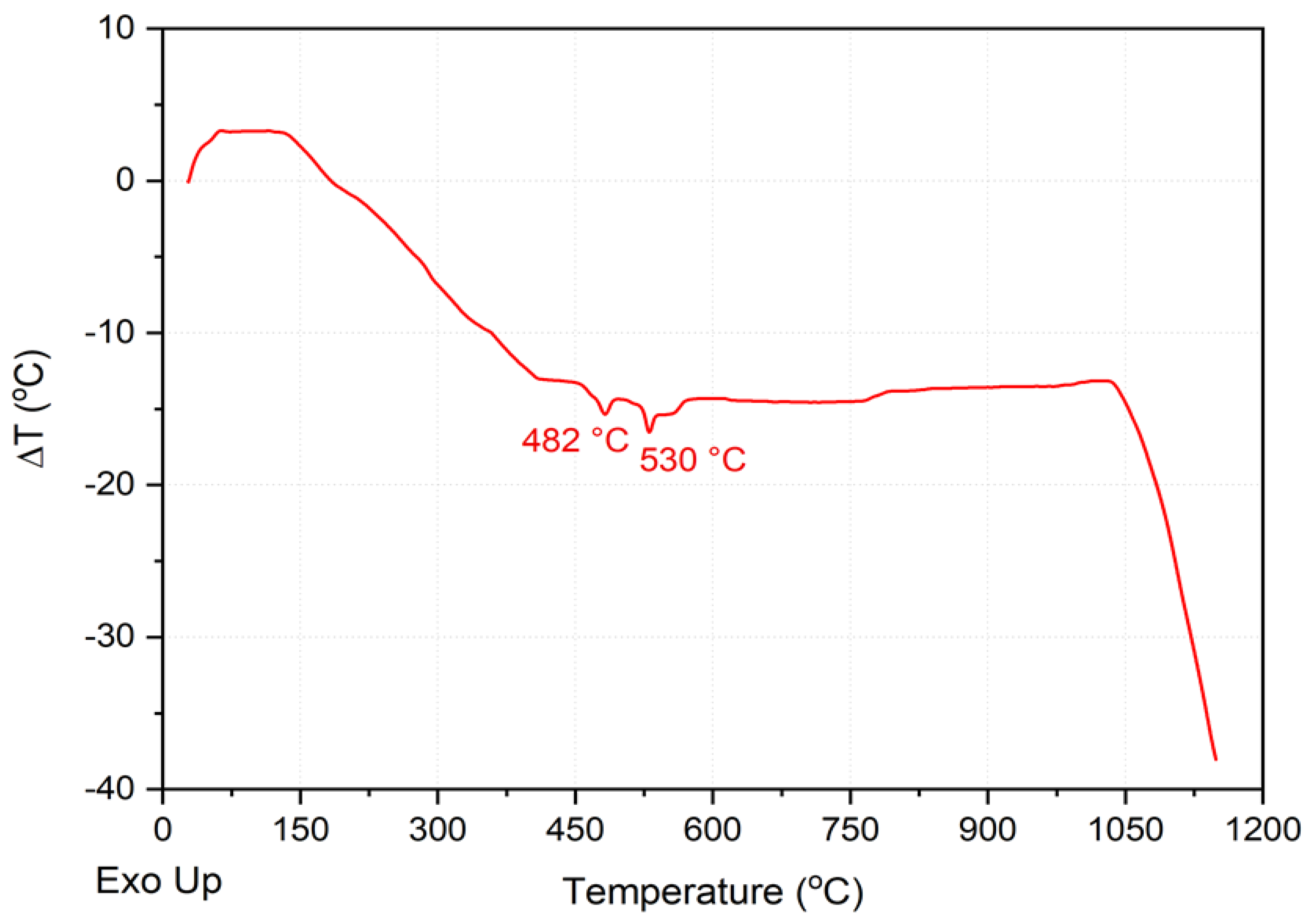
2.2. Chemical, Mineralogical, and Thermal Analysis Tests
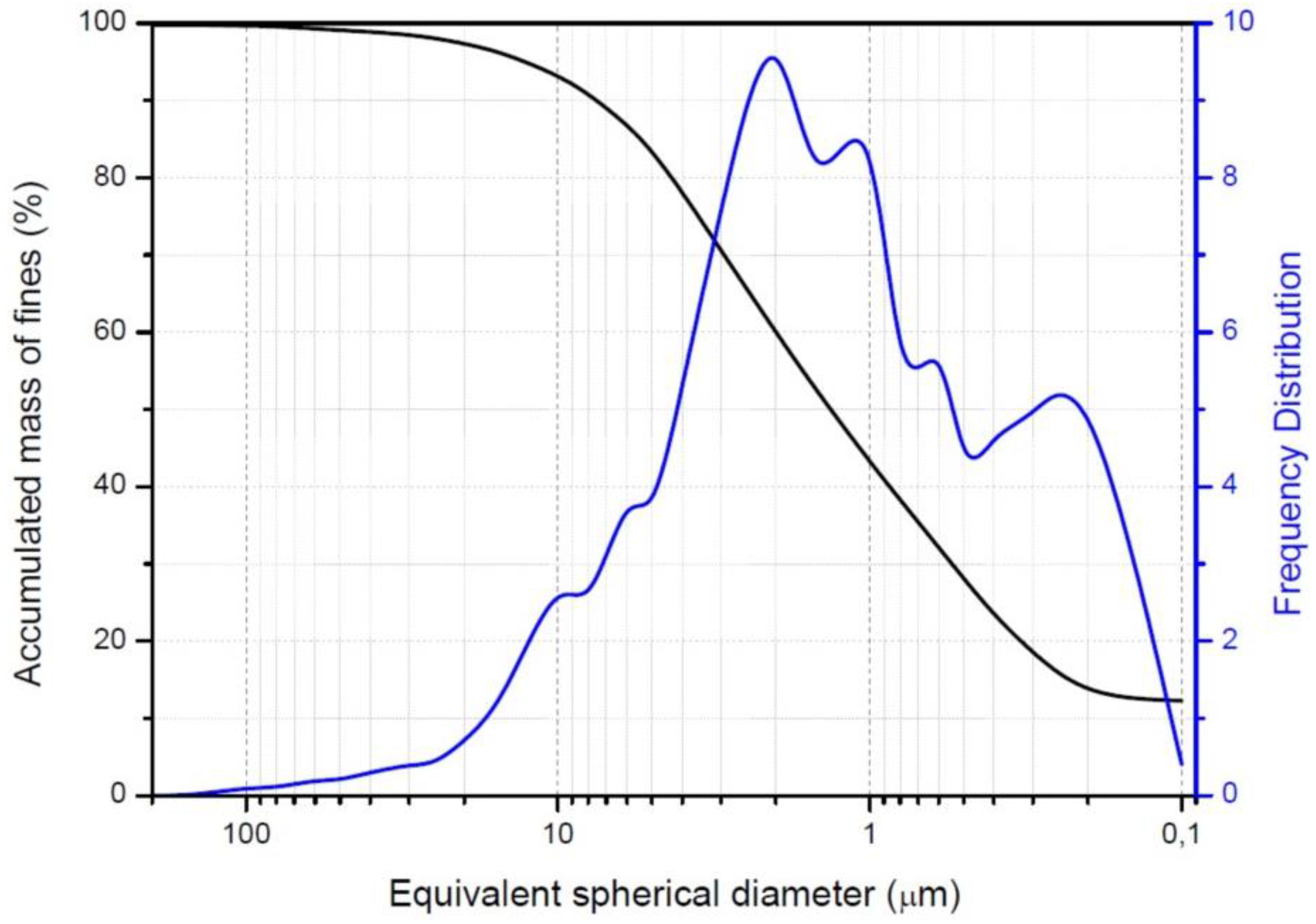
2.3. Particle Analysis
2.4. Consistency Limits
2.5. Specimens and Technological Tests
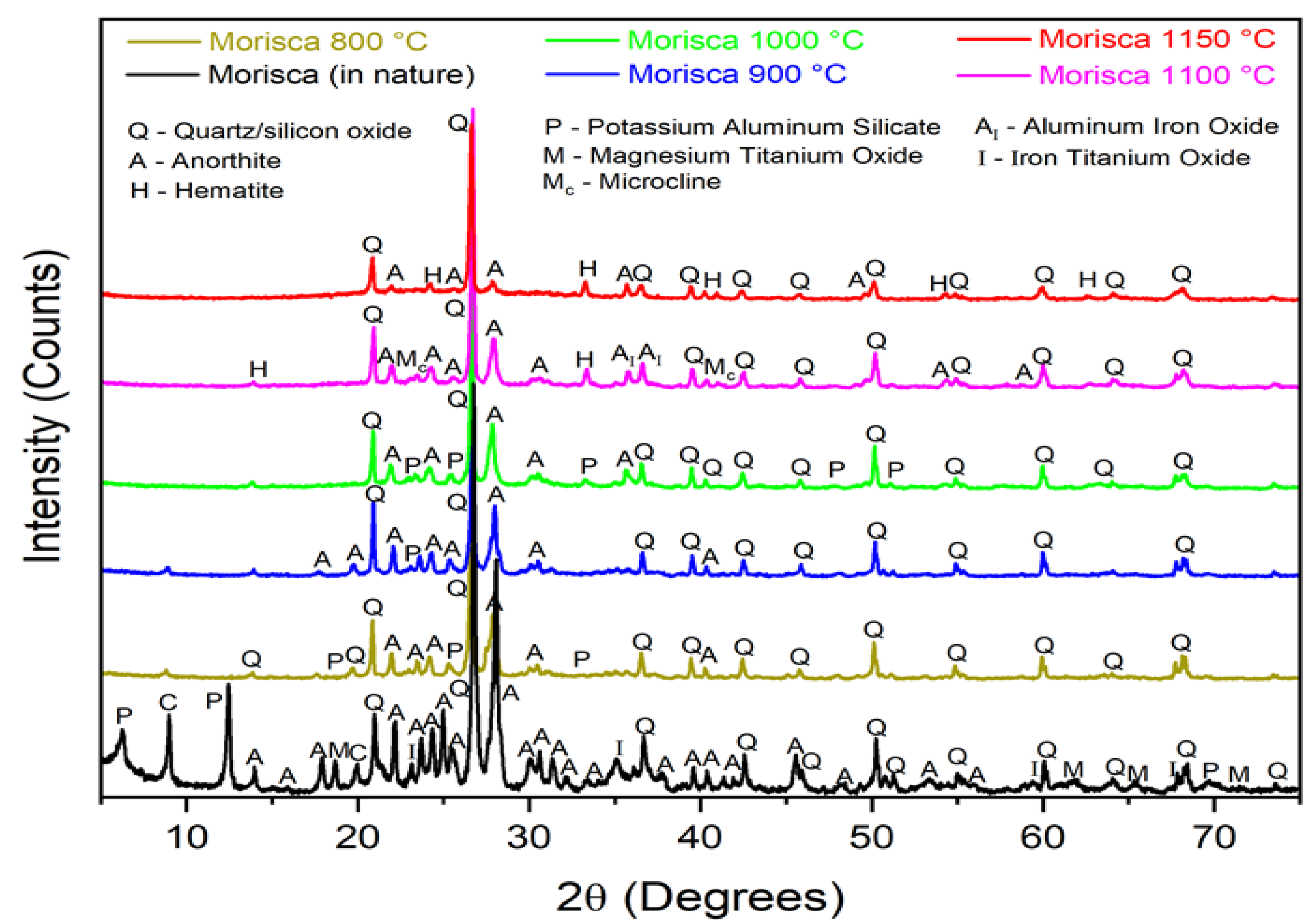
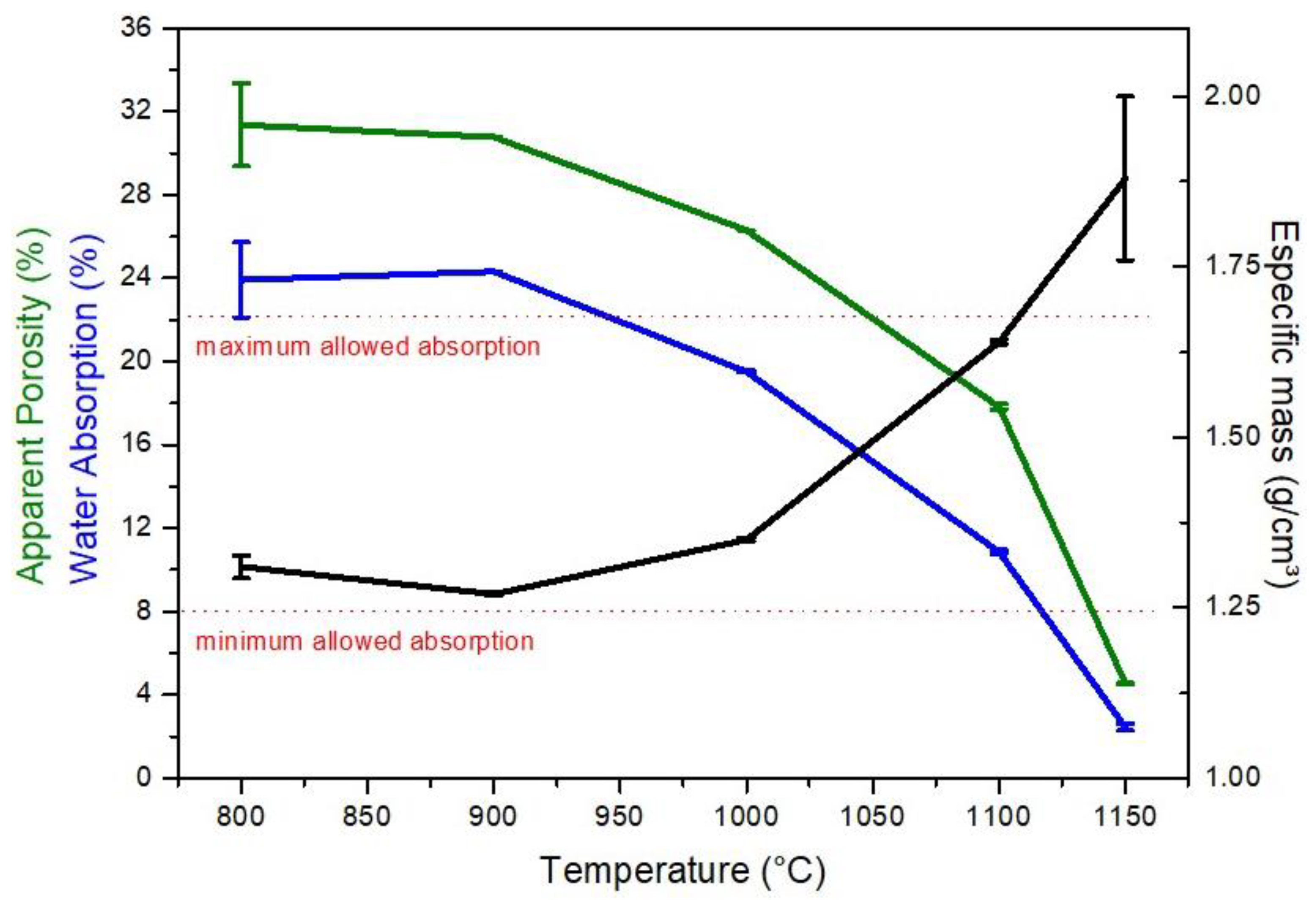
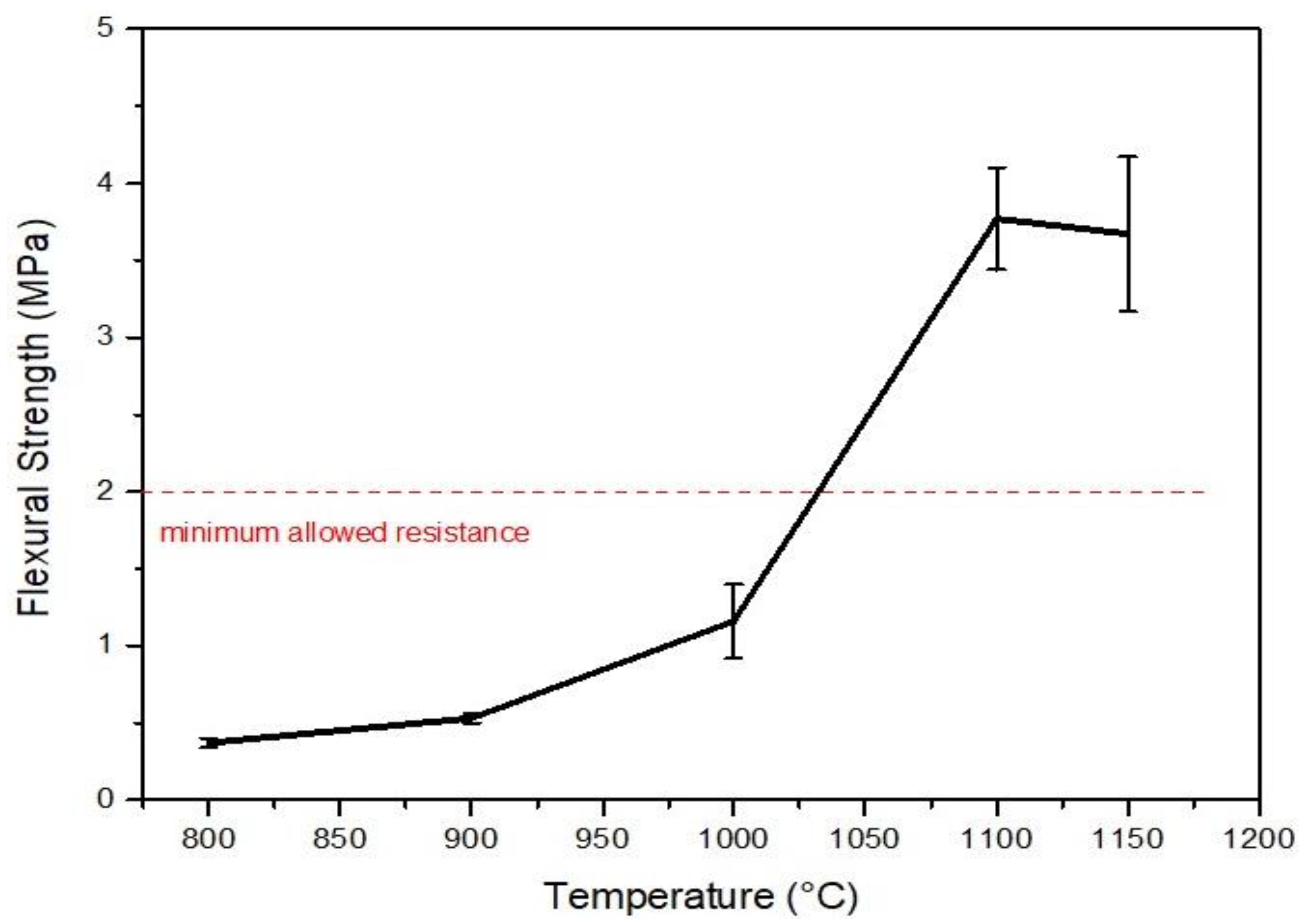
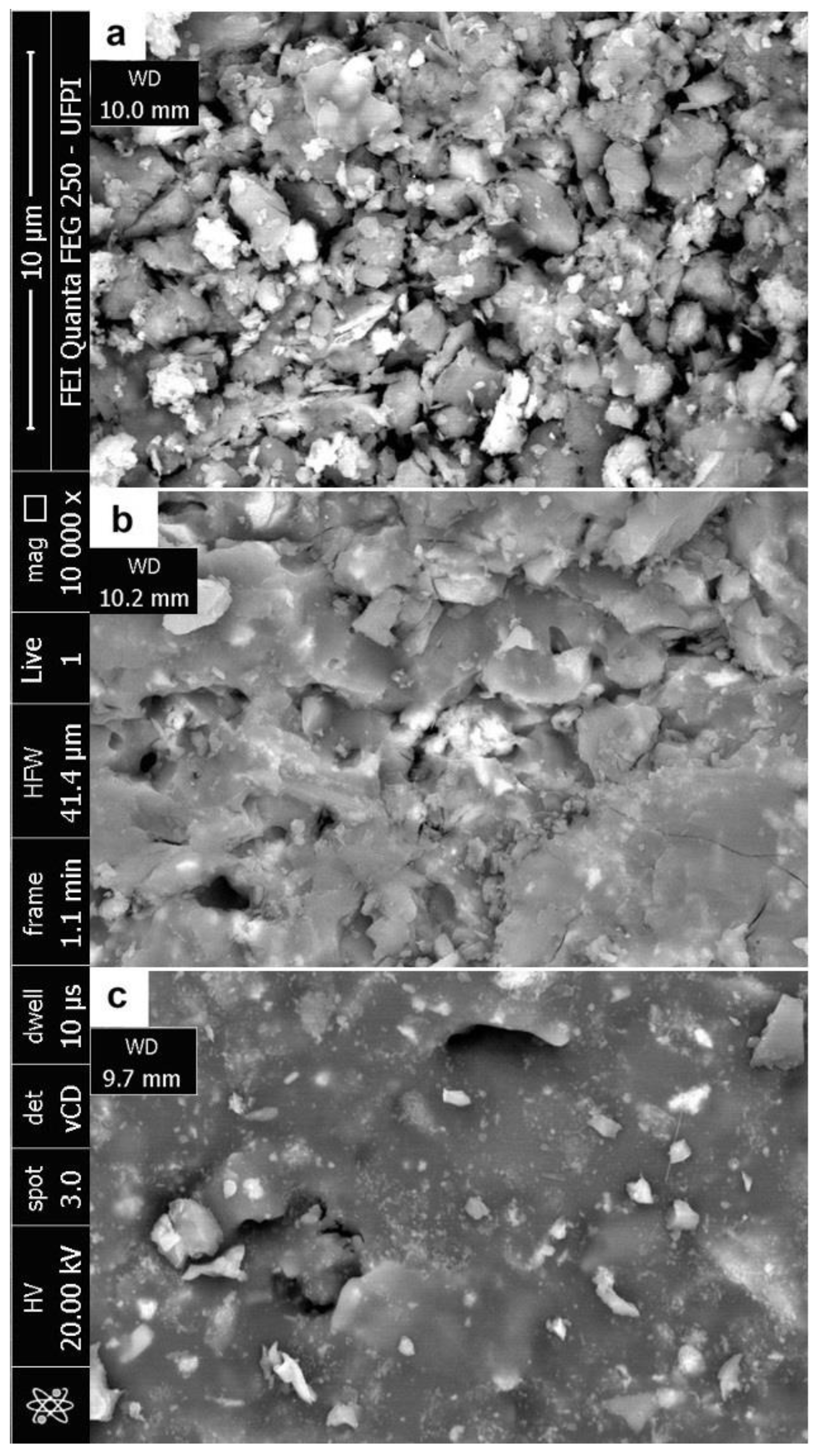
3. Results and Discussion
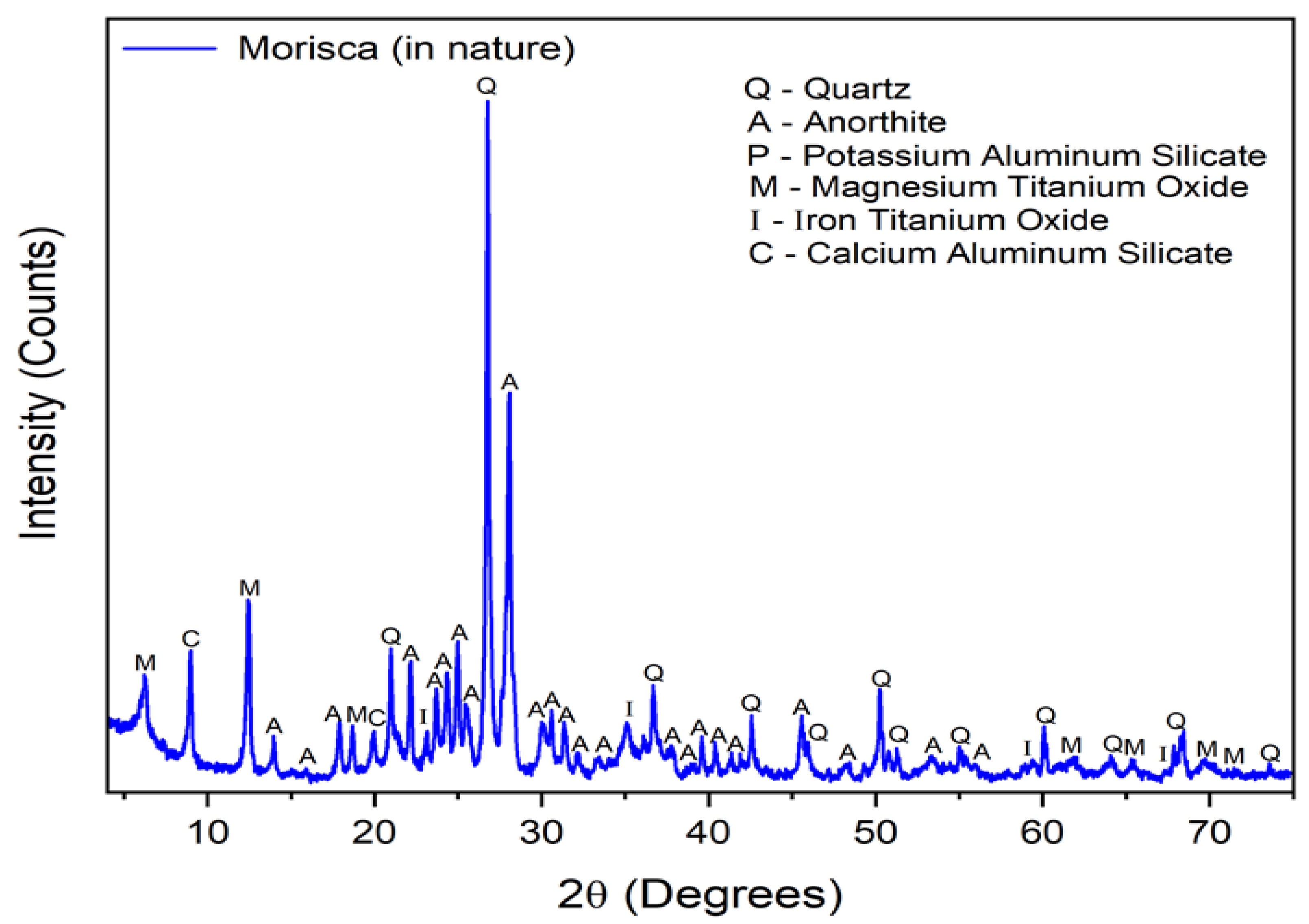
3.1. Raw Material Characterisation (before Firing)
3.2. Characterisation after Burning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abirochas. Brazilian Association of the Ornamental Rock Industry. Balance of Brazilian Exports and Imports of Ornamental Stones in 2019. Report 01/2020. Available online: https://abirochas.com.br/wp-content/uploads/2022/01/Informe-01_2020-Balanco-2019-1.pdf (accessed on 23 March 2021).
- Silva, E. Governor Visits Mine in Juazeiro do Piauí. 2004. Available online: http://www.piaui.pi.gov.br/materia.php?id=6462 (accessed on 23 March 2021).
- Wicander, R.; Monroe, J.S. Fundamentals of Geology; Cengage Learning: São Paulo, Brazil, 2014. [Google Scholar]
- Chioli Filho, C.; Rodrigues, E.P. Applications Guide for Cladding Rocks: Bula Project; ABIROCHAS: São Paulo, Brazil, 2009; 119p. [Google Scholar]
- Araújo, A.M.M.; Santos, L.C.M.L.; Barbosa, D.; Sousa, A.A.P.; Silva, J.P., Jr.; Barbosa, F.S. Analysis of Quartzite Mining Activity in the Quilombola Sumidouro Community, Queimada Nova—Piauí. CPMTC. Geonomos 2017, 25, 50–56. [Google Scholar]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Leonelli, C.; Manfredini, T. Recycling of industrial wastes in ceramic manufacturing: State of art and glass case studies. Ceram. Int. 2016, 42, 13333–13338. [Google Scholar] [CrossRef]
- Silva, M.C.A.; Leão, V.A.; Reis, E.L. Incorporation of quartzite fines in the production of red ceramics. J. Clean. Prod. 2021, 288, 125098. [Google Scholar] [CrossRef]
- Carreiro, M.E.A.; Santo, R.C.; Silva, V.J.; Lira, H.L.; Neves, G.A.; Menezes, R.R.; Santana, L.N.L. Residue of quartzite—alternative raw material for use in structural ceramics. Ceramica 2016, 62, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Suvorova, O.V.; Selivanova, E.A.; Mikhailova, J.A.; Masloboev, V.A.; Makarov, D.V. Ceramic products from mining and metallurgical waste. Appl. Sci. 2020, 10, 3515. [Google Scholar] [CrossRef]
- Suvorova, O.; Kumarova, V.; Nekipelov, D.; Selivanova, E.; Makarov, D.; Masloboev, V. Construction ceramics from ore dressing waste in Murmansk region, Russia. Constr. Build. Mater. 2017, 153, 783–789. [Google Scholar] [CrossRef]
- Siqueira, A.A.; Alexandre, J.; Azevedo, A.R.G.; Botelho, L.C.G.; Paes, A.L.C.; Pinheiro, V.D.; Monteiro, S.N. Evaluation of the incorporation of waste from the paper industry into red ceramics. In Proceedings of the 74th ABM Annual Congress International ABM Week 2019, São Paulo, Brazil, 1–3 October 2019. [Google Scholar]
- Cipriano, P.B.; Rezende, R.T.O.; Ferraz, A.D.V. Production of red ceramic using clay from gypsum mining and gypsum residue. Acta Bras. 2019, 3, 25–29. [Google Scholar] [CrossRef]
- Gurgel, J.F.S.; Xavier, S.C.R.; Amaral, I.B.C.; Araújo, A.D.; Reis, A.B. Coprocessing of tailings of the garimpo areinha (Diamantina/MG) for red ceramics production. Rev. Matéria 2020, 25, e12814. [Google Scholar]
- Belmonte, L.J.; Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E.; Vestbø, A.P. Screening of heavy metal containing waste types for use as raw material in Arctic clay-based bricks. Environ. Sci. Pollut. Res. 2018, 25, 32831–32843. [Google Scholar] [CrossRef] [Green Version]
- Saboya, F.; Xavier, G.C.; Alexandre, J. The use of the powder marble by-product to enhance the properties of brick ceramic. Constr. Build. Mater. 2007, 21, 1950–1960. [Google Scholar] [CrossRef]
- Acchar, W.; Vieira, F.A.; Segadães, A.M. Using ornamental stone cutting rejects as raw materials for red clay ceramic products: Properties and microstructure development. Mater. Sci. Eng. A 2006, 435–436, 606–610. [Google Scholar] [CrossRef]
- Segadães, A.M.; Carvalho, M.A.; Acchar, W. Using marble and granite rejects to enhance the processing of clay products. Appl. Clay Sci. 2005, 30, 42–52. [Google Scholar] [CrossRef]
- Souza, H.N.; Reis, E.L.; Lima, R.M.F.; Cipriano, R.A.S. Using soapstone waste with diesel oil adsorbed as raw material for red ceramic products. Ceram. Int. 2016, 42, 16205–16211. [Google Scholar] [CrossRef]
- Pinheiro, B.C.A.; Holanda, J.N.F. Processing of red ceramics incorporated with encapsulated petroleum waste. J. Mater. Process Technol. 2009, 209, 5606–5610. [Google Scholar] [CrossRef]
- NBR 6457; Soil Samples—Preparation for Compaction Tests and Characterisation Tests. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2016.
- ABNT NBR 6459; Soil—Liquid Limit Determination. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2017.
- NBR 7180; Soil—Plasticity Limit Determination. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2016.
- NBR 15270-3; Ceramic Components Part 3: Structural and Non-Structural Ceramic Blocks—Test Methods. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2017.
- NBR ISO 10545-3; Ceramic Tiles Part 3: Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and Bulk Density. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2020.
- Netto, R.C.B. Ceramic Tests; SENAI-SP Editora: São Paulo, Brazil, 2019; 448p. [Google Scholar]
- Zanelli, C.; Marrocchino, E.; Guarini, G.; Toffano, A.; Vaccaro, C.; Dondi, M. Recycling Construction and Demolition Residues in Clay Bricks. Appl. Sci. 2021, 11, 8918. [Google Scholar] [CrossRef]
- Santos, G.R. Characterization of Clays in the Municipalities of Sidrolândia and Rio Verde in Mato Grosso/MS. Master’s Thesis, Federal University of Mato Grosso do Sul, Campo Grande, Brazil, 2007. [Google Scholar]
- Grun, E. Characterisation of Clays from Canelinha/SC and Study of Ceramic Mass Formulations. Master’s Thesis, State University of Santa Catarina, Joinville, Brazil, 2007. [Google Scholar]
- Setz, L.F.G.; Silva, A.C. Ceramic Processing without Mystery; Blucher Ltd.: São Paulo, Brazil, 2019; p. 255. [Google Scholar]
- Tognotti, R.; Gracher, H. Firing Defects Caused by Quartz in Monoporozas. Cerâmica Ind. 2001, 6, 40–45. [Google Scholar]
- Barnes, G. Soil Mechanics: Principles and Practice, 4th ed.; Palgrave Macmillan: London, UK, 2016. [Google Scholar]
- Almeida, K.S.; Soares, R.A.L.; Matos, J.M.E. Characterization of clay deposit in the central region of piauí for use in the ceramic industry. Rev. Matéria 2020, 25, 1–13. [Google Scholar] [CrossRef]
- Caputo, H.P. Soil Mechanics and It’s Fundamental Applications, 6th ed.; LTC: Rio de Janeiro, Brazil, 1996. [Google Scholar]
- Hasan, M.; Sayed, A.; Hossain, M.A.; Hossain, M.; Sohel, M.H. Determination of Consistency Limits of Different Agricultural Soils. Intern. Inv. J. Agric. Soil Scie. 2017, 5, 1–7. [Google Scholar]
- Vigneron, T.Q.G.; Vieira, C.M.F.; Delaqua, G.C.G.; Vernilli, F., Jr.; Neto, Â.C. Incorporation of waste in red mold flux ceramic. J. Mater. Res. Technol. 2019, 8, 5707–5715. [Google Scholar] [CrossRef]
- Sesma, N.M. Study of the Properties of a Red Ceramic Sintered at Different Temperatures. Master’s Thesis, School of Engineering of Lorena-University of São Paulo, São Paulo, Brazil, 2014. [Google Scholar]
- NBR 15270-1; Ceramic Components—Part 1: Ceramic Blocks for Sealing Masonry—Terminology and Requirements. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2016.
- NBR 15310; Ceramic Components—Tiles—Terminology, Requirements and Test Methods. Brazilian Association of Standards and Techniques (ABNT): São Paulo, Brazil, 2005.
- Dantas, A.P.A.; Acchar, W.; Leite, J.Y.P.L.; Araújo, F.S.D. Utilisation of Residue of Ornamental Rocks in the Porcelained Stoneware. Holos 2010, 26, 92–108. [Google Scholar] [CrossRef]
- Murmu, A.L.; Patel, A. Towards sustainable bricks production: An overview. Constr. Build. Mater. 2018, 165, 112–125. [Google Scholar] [CrossRef]
- Zhang, L. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]




| Variables | Equations | Description | |
| Water Absorption () | Equation (1) | = dry weight = wet weight = apparent volume = initial length = final length | |
| Apparent Porosity () | Equation (2) | ||
| Apparent Specific Mass () | Equation (3) | ||
| Drying and Firing Linear Shrinkage () | Equation (4) | ||
| Total Retraction | Equation (5) | ||
| Oxides | SiO₂ | Al₂O₃ | Fe₂O₃ | K₂O | TiO₂ | MgO | CaO | P₂O5 |
|---|---|---|---|---|---|---|---|---|
| (%) | 70.55 | 14.24 | 9.10 | 2.49 | 1.70 | 1.40 | 0.33 | 0.33 |
| Sample | Fine Quarry Materials (%) | Silt (%) | Sand (%) | Average Diameter |
|---|---|---|---|---|
| v% ≤ 2 μm | 2 μm < v% ≤ 20 μm | v% > 20 μm | μm | |
| Morisca | 60 | 37.4 | 2.6 | 1.34 |
| Sample | Liquid Limit (LL) | Plastic Limit (PL) | Plasticity Index (PI) |
|---|---|---|---|
| % | % | % | |
| Morisca | 38.24 | 24.61 | 13.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho Jr., A.A.d.; Leite, K.d.S.; Elias de Matos, J.M. Viability of Morisca Powder Tailings for Ceramic Applications. Minerals 2023, 13, 459. https://doi.org/10.3390/min13040459
Carvalho Jr. AAd, Leite KdS, Elias de Matos JM. Viability of Morisca Powder Tailings for Ceramic Applications. Minerals. 2023; 13(4):459. https://doi.org/10.3390/min13040459
Chicago/Turabian StyleCarvalho Jr., Antônio Alves de, Kelson de Sousa Leite, and José Milton Elias de Matos. 2023. "Viability of Morisca Powder Tailings for Ceramic Applications" Minerals 13, no. 4: 459. https://doi.org/10.3390/min13040459







