Adsorption Difference of Octadecylamine on (002) and (131) Crystal Planes of Fine Muscovite and Its Guidance on Fine Muscovite Flotation
Abstract
1. Introduction
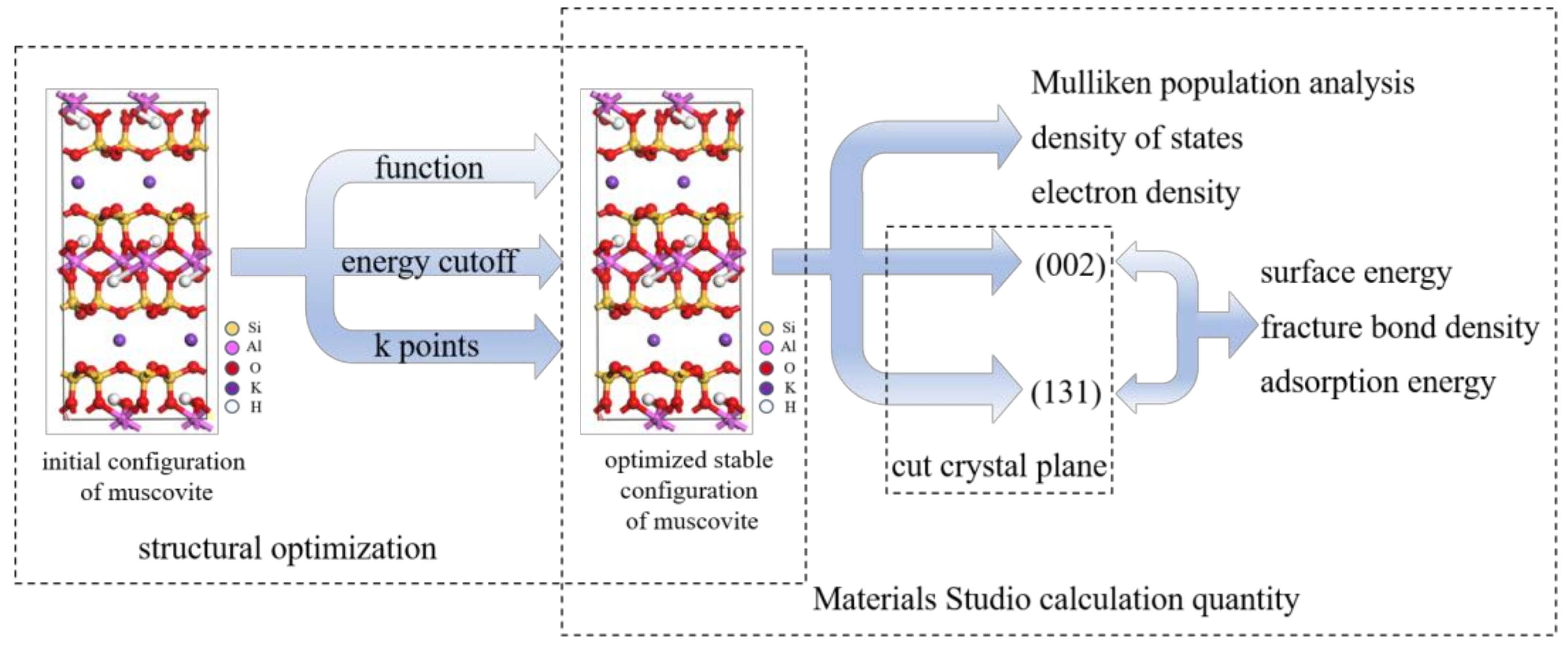
2. Theoretical and Experimental Sections
2.1. Models
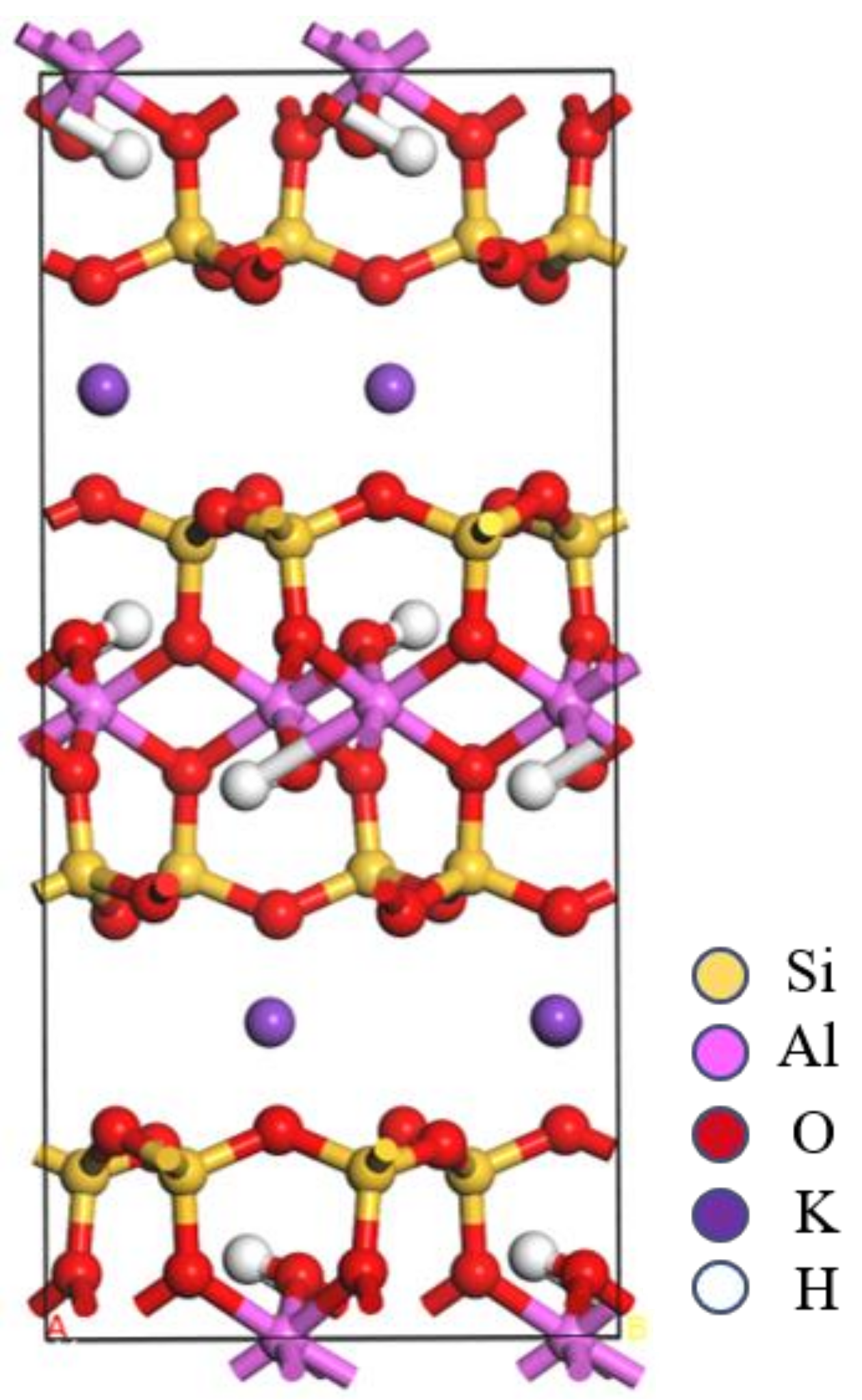
2.2. Crystal Structure Optimization
2.3. Calculation Quantity
2.3.1. Population Analysis
2.3.2. Surface Energy and Fracture Bond Density
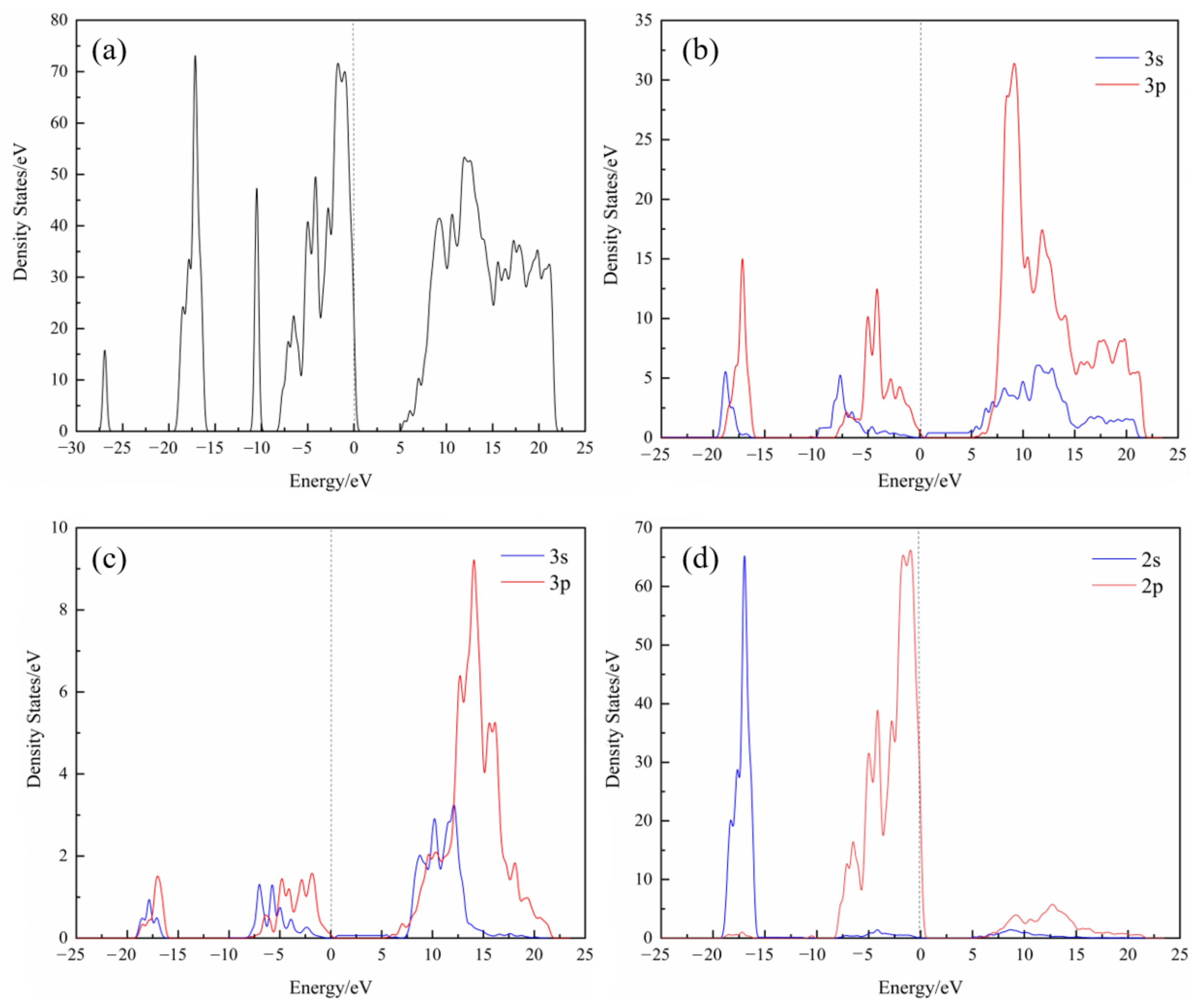
2.3.3. State Density
2.3.4. Electron Density
2.3.5. Adsorption Energy
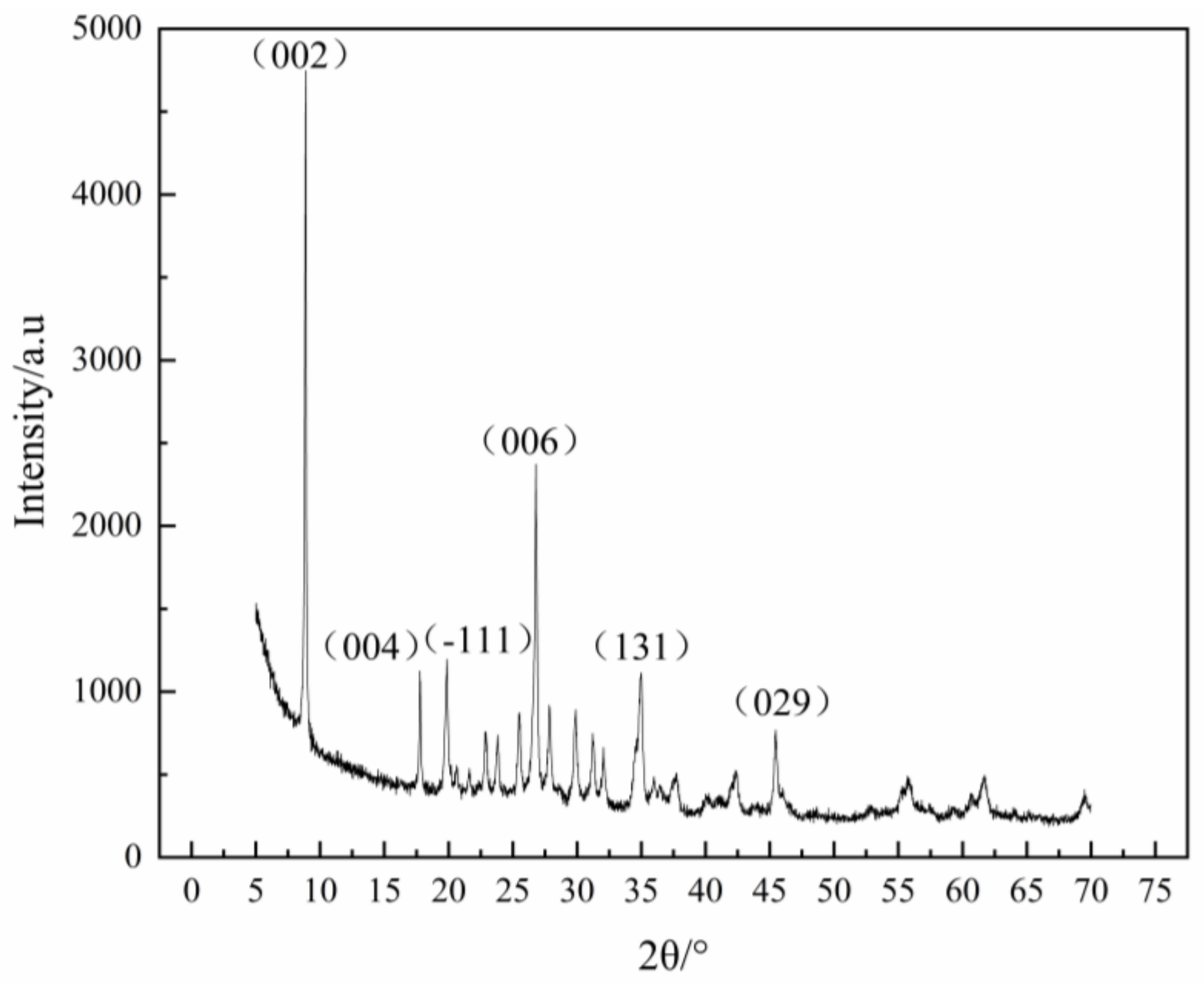
2.4. XRD
2.5. Flotation Test
3. Results and Discussion
3.1. Population Analysis and Electron Density of Muscovite
3.2. Anisotropy of Fracture Bond Density and Surface Energy at (002) and (131) Planes of Muscovite
3.3. State Density of Muscovite
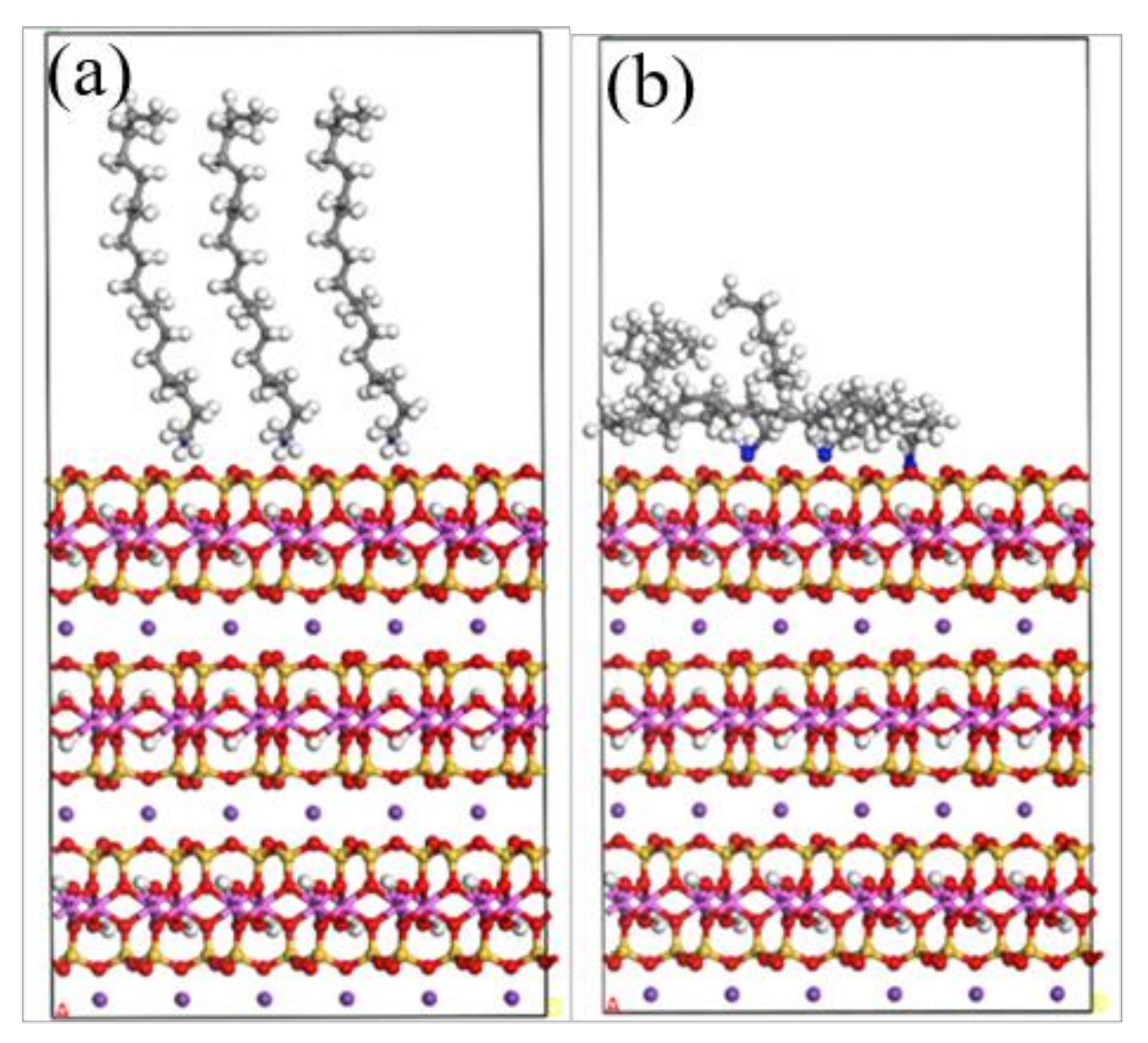
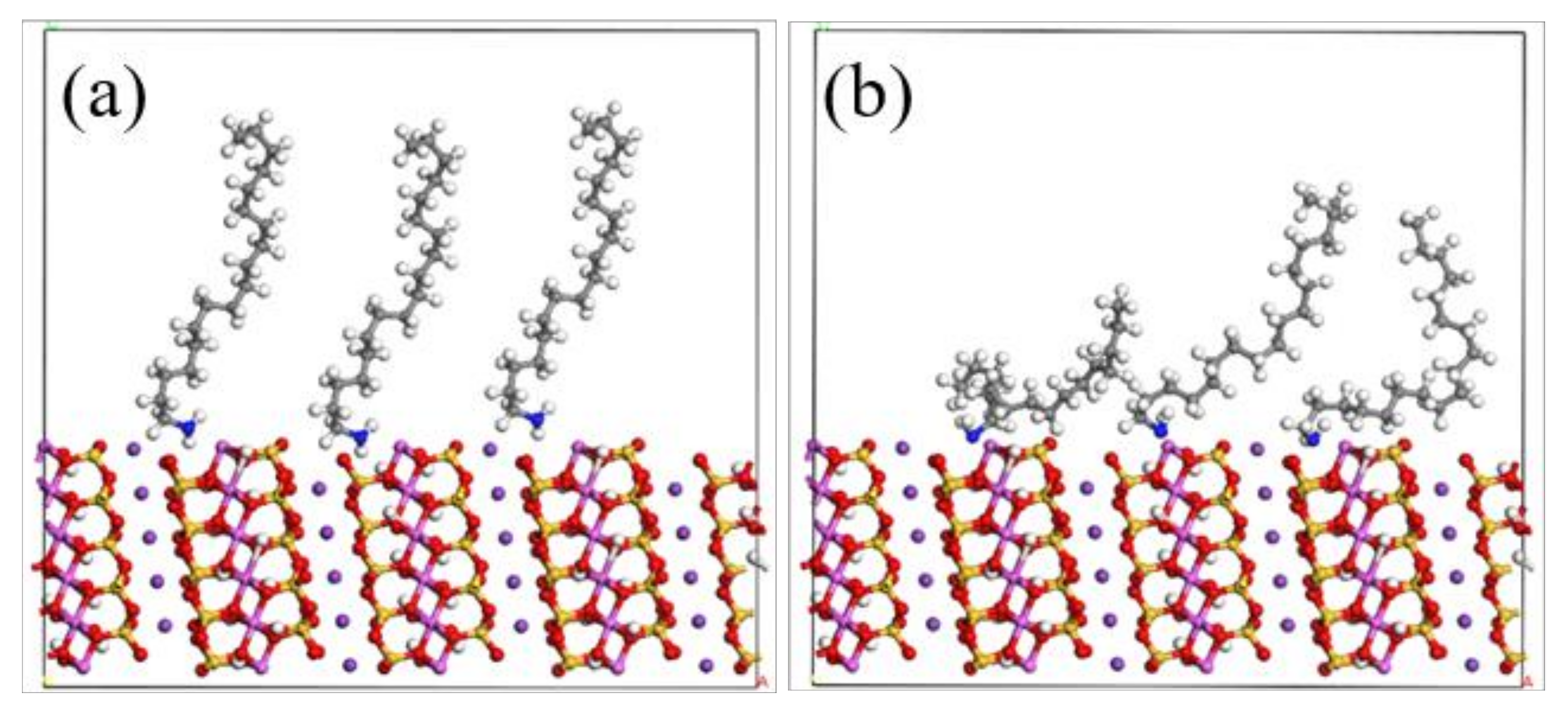
3.4. Adsorption Energy of Muscovite (002) and (131) Crystal Planes
3.5. Adsorption Difference of ODA on (002) and (131) Crystal Planes of Muscovite
3.6. Flotation Test Results and XRD under Different Flotation Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, S.; Lv, J.; Guo, L.K.; Xu, G.Q.; Wang, D.M.; Zheng, Z.X.; Wu, Y.C. Preparation and photocatalytic properties of N-doped nano-TiO2/muscovite composites. Appl. Surf. Sci. 2012, 258, 6136–6141. [Google Scholar] [CrossRef]
- Chen, S.; Chen, F.; Di, Y.; Han, S.; Zhu, X. Preparation and characterisation of exfoliated muscovite/poly(2,3-dimethylaniline) nanocomposites with an enhanced anticorrosive performance. Micro Nano Lett. 2020, 15, 509–513. [Google Scholar] [CrossRef]
- Di, Y.; Jiang, A.; Huang, H.; Deng, L.; Zhang, D.; Deng, W.; Wang, R.; Luo, Q.; Chen, S. Molecular Dynamics Simulation Study on the Interactions of Mixed Cationic/Anionic Collectors on Muscovite (001) Surface in Aqueous Solution. Materials 2022, 15, 3816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gao, Y.; Khoso, A.S.; Ji, W.; Hu, Y. A new approach for characterization of hydrophobization mechanisms of surfactants on muscovite surface. Sep. Purif. Technol. 2019, 209, 936–945. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Liu, J.; Sun, Y.; Sun, W. Flotation and adsorption of muscovite using mixed cationic-nonionic surfactants as collector. Powder Technol. 2015, 276, 26–33. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Liu, J.; Tang, H.; Liu, R.; Han, H.; Sun, W.; Hu, Y. Effect of Chain Length Compatibility of Alcohols on Muscovite Flotation by Dodecyl Amine. Minerals 2018, 8, 168. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite-Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Chen, G.; Ren, L.; Zhang, Y.; Bao, S. Improvement of fine muscovite flotation through nanobubble pretreatment and its mechanism. Miner. Eng. 2022, 189, 107868. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.-S.; Yu, J.-G. Effect of Primary Alkylamine Adsorption on Muscovite Hydrophobicity. Acta Phys.-Chim. Sin. 2012, 28, 201–207. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Wu, H.; Tian, J.; Xu, L.; Wang, Z.; Xu, Y.; Gao, Z.; Sun, W.; Hu, Y. Anisotropic surface chemistry properties of salt-type and oxide mineral crystals. Miner. Eng. 2020, 154, 106411. [Google Scholar] [CrossRef]
- Xu, L.; Peng, T.; Tian, J.; Lu, Z.; Hu, Y.; Sun, W. Anisotropic surface physicochemical properties of spodumene and albite crystals: Implications for flotation separation. Appl. Surf. Sci. 2017, 426, 1005–1022. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Fang, S.; Lu, Z.; Ma, C.; Sun, W.; Hu, Y. Anisotropic surface chemistry properties and adsorption behavior of silicate mineral crystals. Adv. Colloid Interface Sci. 2018, 256, 340–351. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Z.; Zeng, W.N. Adhesion between nanobubbles and fine cassiterite particles. Int. J. Min. Sci. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Juarez, F.; Salmazo, D.; Savinova, E.R.; Quaino, P.; Belletti, G.; Santos, E.; Schmickler, W. The initial stage of OH adsorption on Ni(111). J. Electroanal. Chem. 2019, 832, 137–141. [Google Scholar] [CrossRef]
- Loncaric, I.; Alducin, M.; Juaristi, J.I. Molecular dynamics simulation of O-2 adsorption on Ag(110) from first principles electronic structure calculations. Phys. Chem. Chem. Phys. 2016, 18, 27366–27376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, H.; Li, Z.; Qi, S.; Cui, S.; Chen, W.; Wang, S. Molecular dynamics and density functional theory simulations of cesium and strontium adsorption on illite/smectite. J. Radioanal. Nucl. Chem. 2022, 331, 2983–2992. [Google Scholar] [CrossRef]
- Khnifira, M.; Boumya, W.; Attarki, J.; Mahsoune, A.; Abdennouri, M.; Sadiq, M.H.; Kaya, S.; Barka, N. Elucidating the adsorption mechanisms of anionic dyes on chitosan (110) surface in aqueous medium by quantum chemical and molecular dynamics. Mater. Today Commun. 2022, 33, 104488. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, Y.; Feng, Z.; Liu, F.; Zhang, Z. Molecular dynamics simulation of adsorption and diffusion of partially hydrolyzed polyacrylamide on kaolinite surface. J. Mol. Liq. 2022, 367, 120377. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.; Zhao, Y.; Feng, Y.; Liu, J.; Dong, H.; Tang, H.; Wang, W.; Ren, W.; Wang, F.; et al. Molecular dynamics simulation of interface adhesion characteristics between dust suppressant and coal. Mater. Today Commun. 2022, 33, 104487. [Google Scholar] [CrossRef]
- Hajjaoui, H.; Khnifira, M.; Soufi, A.; Abdennouri, M.; Akkaya, S.; Akkaya, R.; Barka, N. Experimental, DFT and MD simulation studies of Mordant Black 11 dye adsorption onto polyaniline in aqueous solution. J. Mol. Liq. 2022, 364, 120045. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.H.; Liu, X.W. Surface energies and appearances of commonly exposed surfaces of scheelite crystal. Trans. Nonferrous Met. Soc. China 2013, 23, 2147–2152. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Tian, J.; Deng, W.; Chen, H. Research Advance in Anisotropy of Phyllosilicate Minerals and Its Relationship with Flotation. Mod. Min. 2018, 34, 46–53. [Google Scholar]
- Wang, J.; Sun, Z.; Bai, J. Research on Crystal Anisotropy and Surface Properties of Smithsonite. Conserv. Util. Miner. Resour. 2021, 41, 1–6. [Google Scholar]
- Li, M.; Lian, D.; Li, H.; Chen, W.; Hu, Y.; Gao, X.; Tong, X. Research on Anisotropy of Chlorite and Its Relationship with Floatability. Met. Mine 2020, 2020, 56–61. [Google Scholar]
- King, R.P. Butterworth-Heinemann Ltd. Modeling and Simulation of Mineral Processing Systems; Society for Mining, Metallurgy & Exploration, Incorporated: Englewood, CO, USA, 2001; pp. 127–212. [Google Scholar]
- Xue, X.; Xu, Z.; Pedruzzi, I.; Ping, L.; Yu, J. Interaction between low molecular weight carboxylic acids and muscovite: Molecular dynamic simulation and experiment study. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 559, 8–17. [Google Scholar] [CrossRef]
- Hasan, M.; Hossain, A. First-principles calculations to investigate the structural, electronic, optical anisotropy, and bonding properties of a newly synthesized ThRhGe equiatomic ternary intermetallic superconductor. Results Phys. 2022, 42, 106004. [Google Scholar] [CrossRef]
- Kilic, K.I.; Dauskardt, R.H. Mechanically reliable hybrid organosilicate glasses for advanced interconnects. J. Vac. Sci. Technol. B 2020, 38, 060601. [Google Scholar] [CrossRef]
- Meiser, J.; Urbassek, H.M. Influence of the Crystal Surface on the Austenitic and Martensitic Phase Transition in Pure Iron. Crystals 2018, 8, 469. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Wang, D.; Qin, J.; Wan, Z.; Zhong, Y.; Hu, C.; Zhou, H. First-Principles Investigation of Atomic Hydrogen Adsorption and Diffusion on/into Mo-doped Nb (100) Surface. Appl. Sci. 2018, 8, 2466. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Li, B.; Liu, S.P.; Liu, X.; Jiang, Q. Determining the adsorption energies of small molecules with the intrinsic properties of adsorbates and substrates. Nat. Commun. 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Allan, H.; Graeme, W.; Sadia, K.; Hyunjung. Prediction of grade and recovery in flotation from physicochemical and operational aspects using machine learning models. Miner. Eng. 2022, 183, 107627. [Google Scholar] [CrossRef]
- Kyuhyeong, P.; Junhyun, C.; Gomez-Flores, A.; Hyunjung, K. Flotation Behavior of Arsenopyrite and Pyrite, and Their Selective Separation. Mater. Trans. 2015, 56, 435–440. [Google Scholar] [CrossRef]

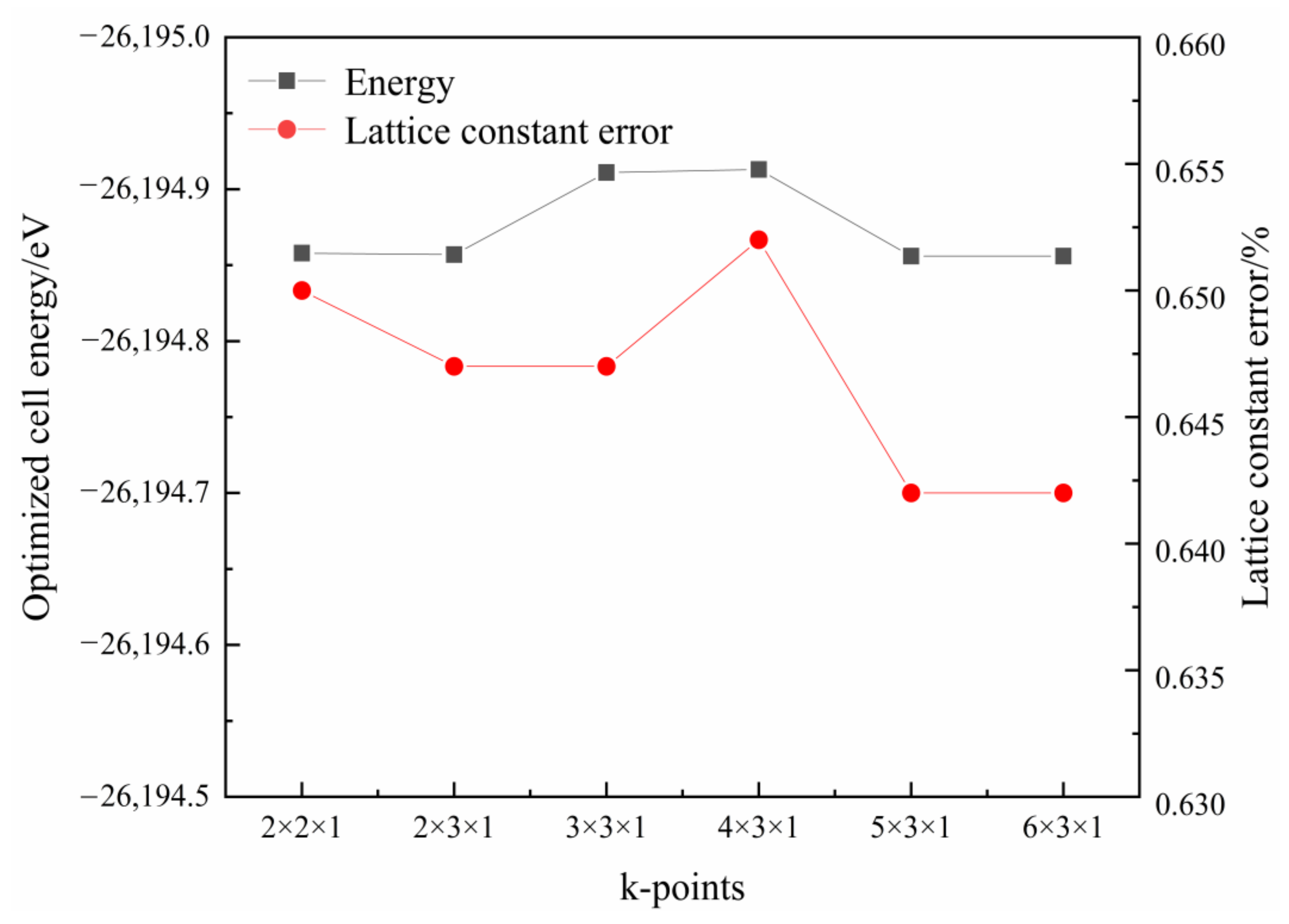
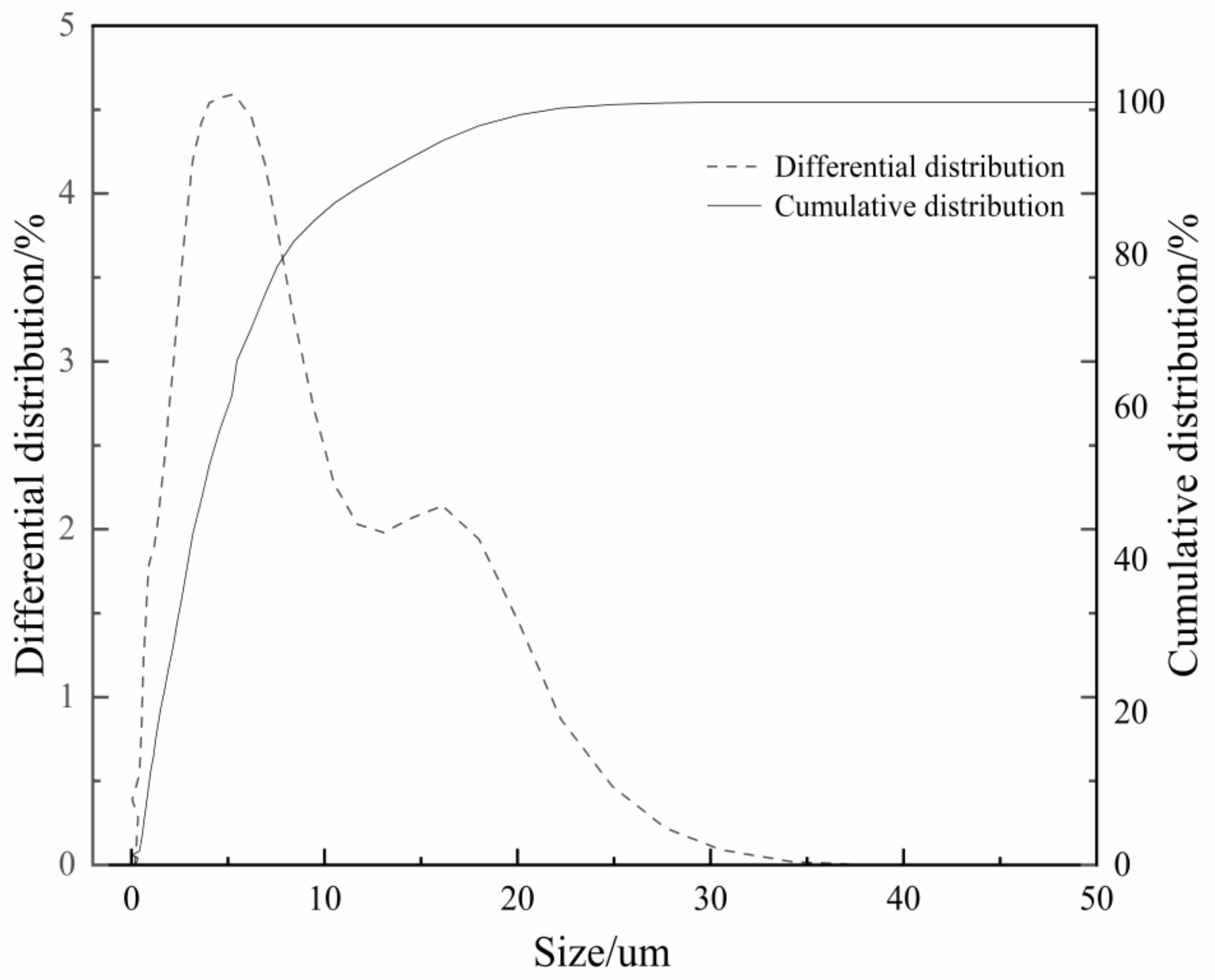
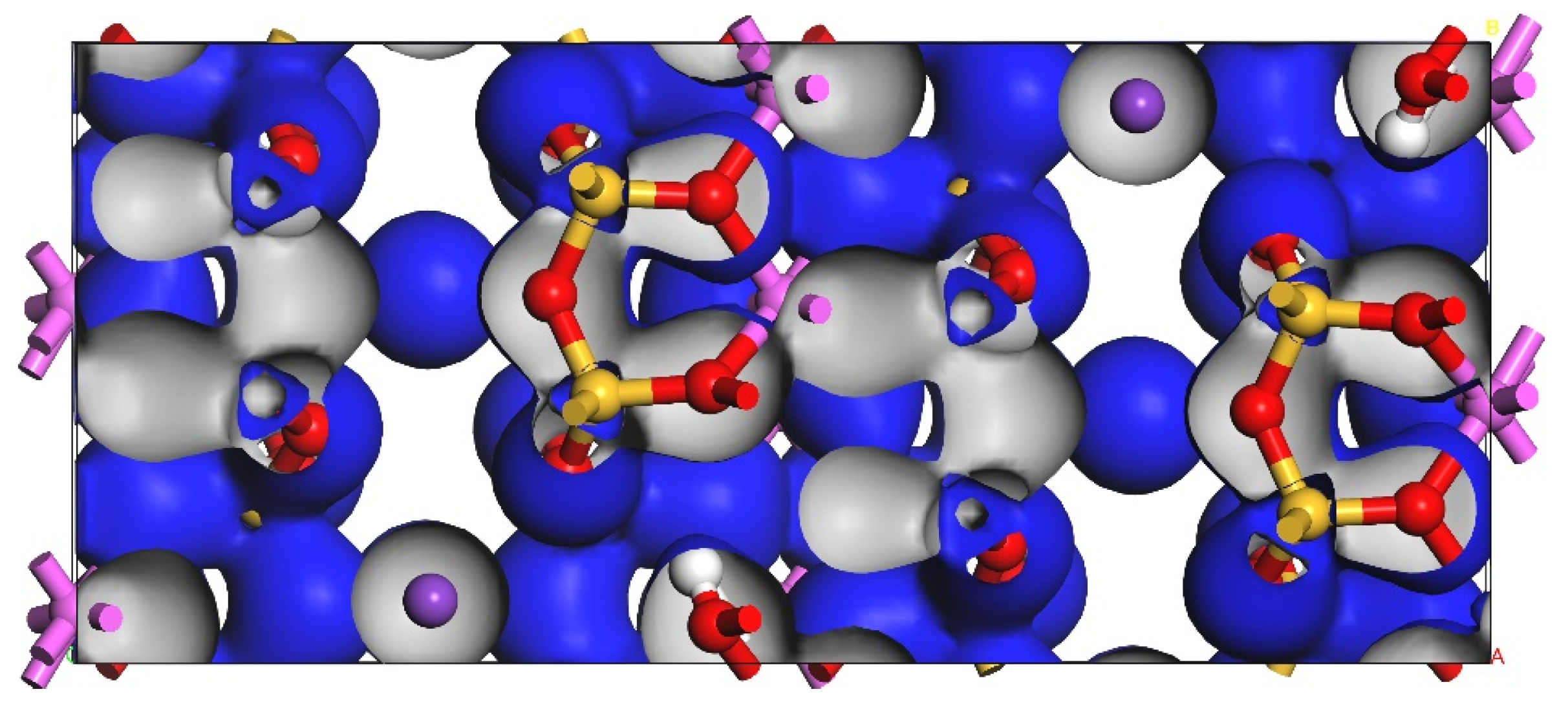
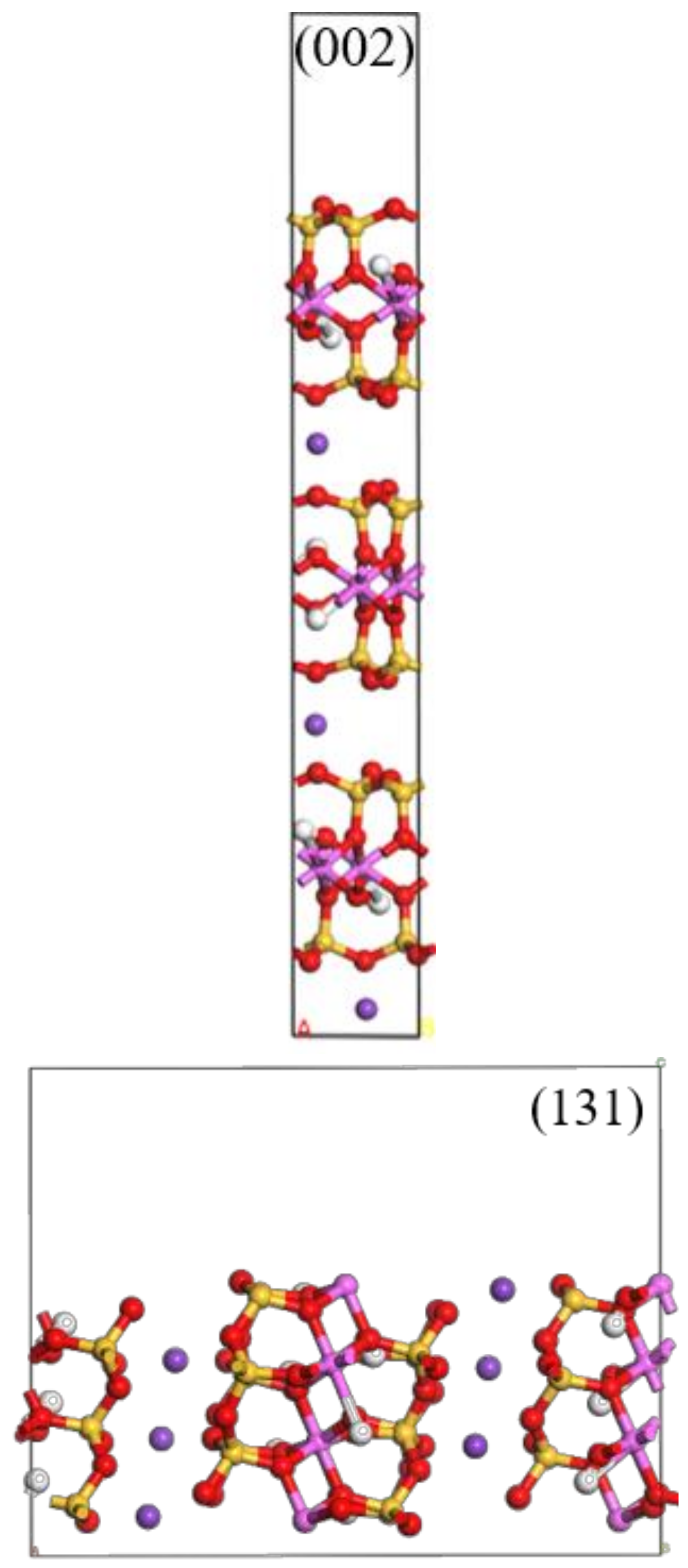
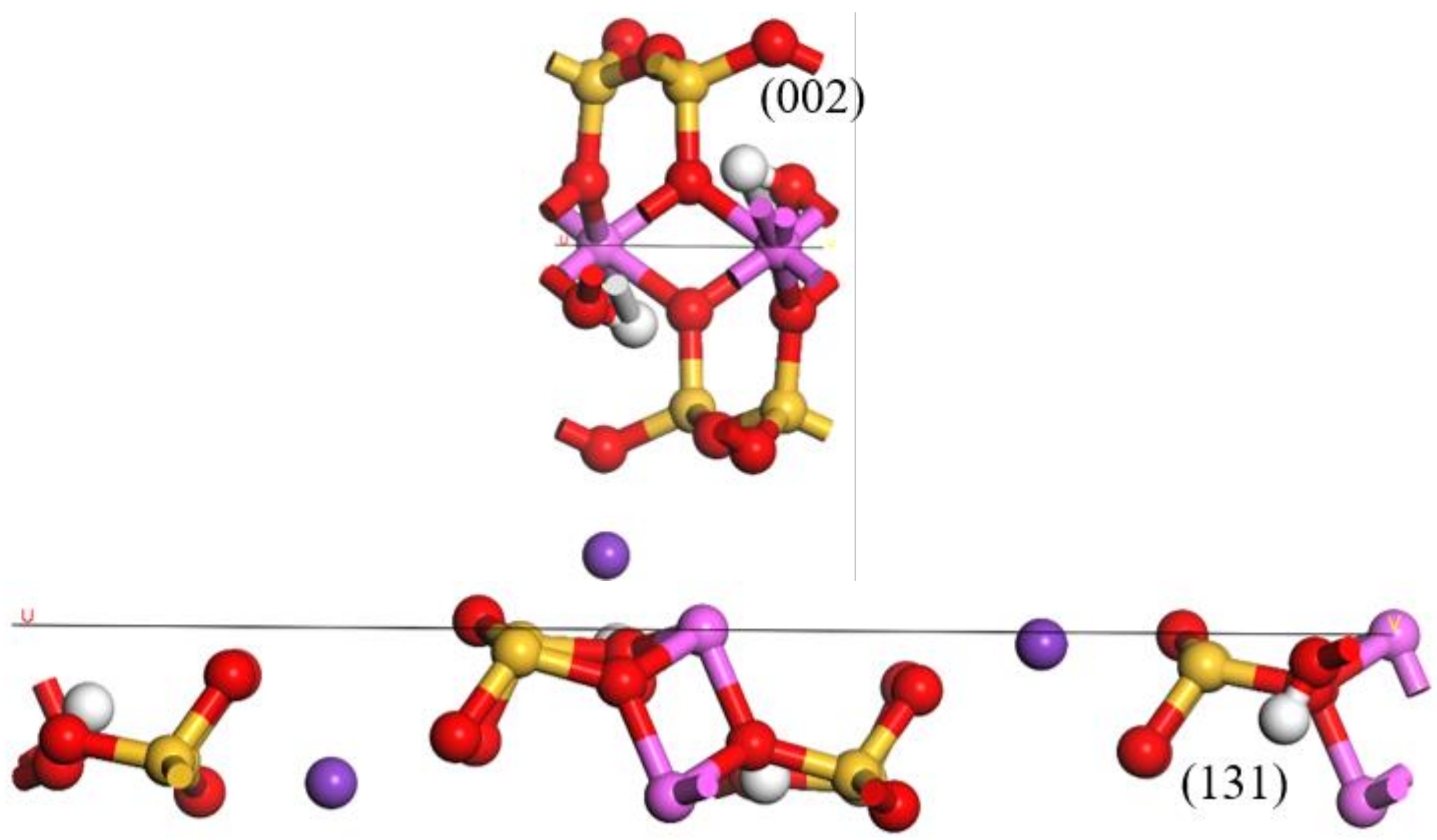



| Function | a/nm | b/nm | c/nm | β/° | Energy/eV | Maximum Error/% |
|---|---|---|---|---|---|---|
| GGA-PBE | 5.241 | 9.073 | 20.934 | 95.423 | −26,269.677 | 4.232 |
| GGA-PBESOL | 5.197 | 8.989 | 20.603 | 95.389 | −26,315.327 | 2.584 |
| GGA-WC | 5.200 | 8.997 | 20.696 | 95.289 | −26,228.042 | 3.047 |
| GGA-PW91 | 5.232 | 9.058 | 20.764 | 95.430 | −26,300.069 | 3.386 |
| GGA-RPBE | 5.287 | 9.162 | 21.292 | 95.667 | −26,294.096 | 6.015 |
| Species | Ion | s/e | p/e | d/e | f/e | Total/e | Charge/e |
|---|---|---|---|---|---|---|---|
| H | 1 | 0.59 | 0.00 | 0.00 | 0.00 | 0.59 | 0.41 |
| H | 2 | 0.60 | 0.00 | 0.00 | 0.00 | 0.60 | 0.40 |
| O | 1 | 1.84 | 5.25 | 0.00 | 0.00 | 7.09 | −1.09 |
| O | 2 | 1.85 | 5.29 | 0.00 | 0.00 | 7.15 | −1.15 |
| O | 3 | 1.84 | 5.16 | 0.00 | 0.00 | 7.00 | −1.00 |
| O | 4 | 1.86 | 5.30 | 0.00 | 0.00 | 7.16 | −1.16 |
| O | 5 | 1.83 | 5.31 | 0.00 | 0.00 | 7.14 | −1.14 |
| O | 6 | 1.84 | 5.29 | 0.00 | 0.00 | 7.13 | −1.13 |
| O | 30 | 1.87 | 5.31 | 0.00 | 0.00 | 7.18 | −1.18 |
| Al | 1 | 0.50 | 0.81 | 0.00 | 0.00 | 1.31 | 1.69 |
| Al | 2 | 0.47 | 0.85 | 0.00 | 0.00 | 1.33 | 1.67 |
| Si | 1 | 0.67 | 1.34 | 0.00 | 0.00 | 2.01 | 1.99 |
| Si | 5 | 0.68 | 1.36 | 0.00 | 0.00 | 2.04 | 1.96 |
| Si | 6 | 0.66 | 1.31 | 0.00 | 0.00 | 1.97 | 2.03 |
| K | 1 | 2.02 | 5.48 | 0.00 | 0.00 | 7.50 | 1.50 |
| K | 3 | 2.02 | 5.49 | 0.00 | 0.00 | 7.51 | 1.49 |
| Bond | Population | Length/Å |
|---|---|---|
| Al-Al | −0.37 | 2.9872 |
| Si-Si | −0.17 | 2.9872 |
| O-O | −0.03 | 2.9902 |
| O-K | −0.04 | 2.9660 |
| H-Si | −0.01 | 2.9480 |
| Al-Si | −0.19 | 2.9379 |
| H-Al | −0.02 | 2.8398 |
| H-O | −0.01 | 2.8384 |
| O-Al | 0.30 | 1.9618 |
| O-Si | 0.61 | 1.5213 |
| Crystal Planes | Surface Energy/J·m−2 | Fracture Bond Density/nm2 |
|---|---|---|
| (002) | 1.349 | 8.581 |
| (131) | 1.426 | 8.642 |
| Crystal Plane | Adsorbed Complex Energy /kJ·mol−1 | Energy at the Bottom of the Crystal Plane /kJ·mol−1 | Energy of Agent /kJ·mol−1 | Adsorption Energy/kJ·mol−1 |
|---|---|---|---|---|
| (002) | 86,164.948 | 85,905.960 | 391.248 | −132.259 |
| (131) | 49,649.868 | 50,185.722 | 22.052 | −557.906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Jiao, Z.; Zhang, Y.; Bao, S. Adsorption Difference of Octadecylamine on (002) and (131) Crystal Planes of Fine Muscovite and Its Guidance on Fine Muscovite Flotation. Minerals 2023, 13, 519. https://doi.org/10.3390/min13040519
Ren L, Jiao Z, Zhang Y, Bao S. Adsorption Difference of Octadecylamine on (002) and (131) Crystal Planes of Fine Muscovite and Its Guidance on Fine Muscovite Flotation. Minerals. 2023; 13(4):519. https://doi.org/10.3390/min13040519
Chicago/Turabian StyleRen, Liuyi, Ziwei Jiao, Yimin Zhang, and Shenxu Bao. 2023. "Adsorption Difference of Octadecylamine on (002) and (131) Crystal Planes of Fine Muscovite and Its Guidance on Fine Muscovite Flotation" Minerals 13, no. 4: 519. https://doi.org/10.3390/min13040519
APA StyleRen, L., Jiao, Z., Zhang, Y., & Bao, S. (2023). Adsorption Difference of Octadecylamine on (002) and (131) Crystal Planes of Fine Muscovite and Its Guidance on Fine Muscovite Flotation. Minerals, 13(4), 519. https://doi.org/10.3390/min13040519








