A Collector Promoter for Apatite Flotation in the Serra do Salitre Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mining Process and Mineralogy Characterization
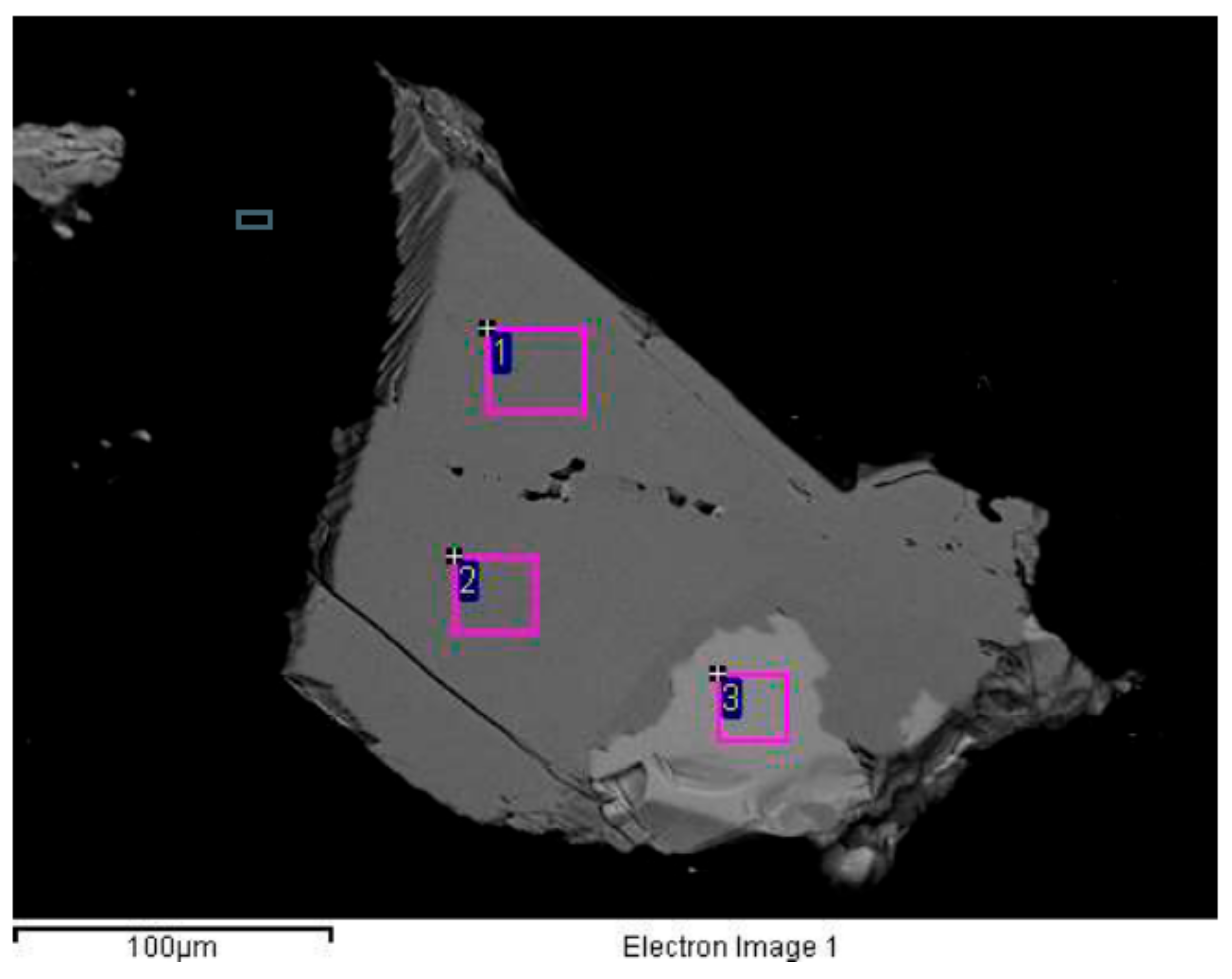
| EDS Region | Element/Mineral Grade (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| F | MgO | SiO2 | P2O5 | CaO | TiO2 | Fe2O3 | |
| 1 | - | 11.4 | 51.4 | - | 22.6 | 0.70 | 11.4 |
| 2 | - | 11.3 | 51.1 | - | 21.7 | 0.51 | 12.9 |
| 3 | 2.85 | - | - | 40.5 | 53.3 | - | - |
2.2. Size Distribution of the Sample
2.3. Soy Oil Fatty Acid (SOFA) Characterization
2.4. Contact Angle Measurements
2.5. Reagents Surface Tension Analysis
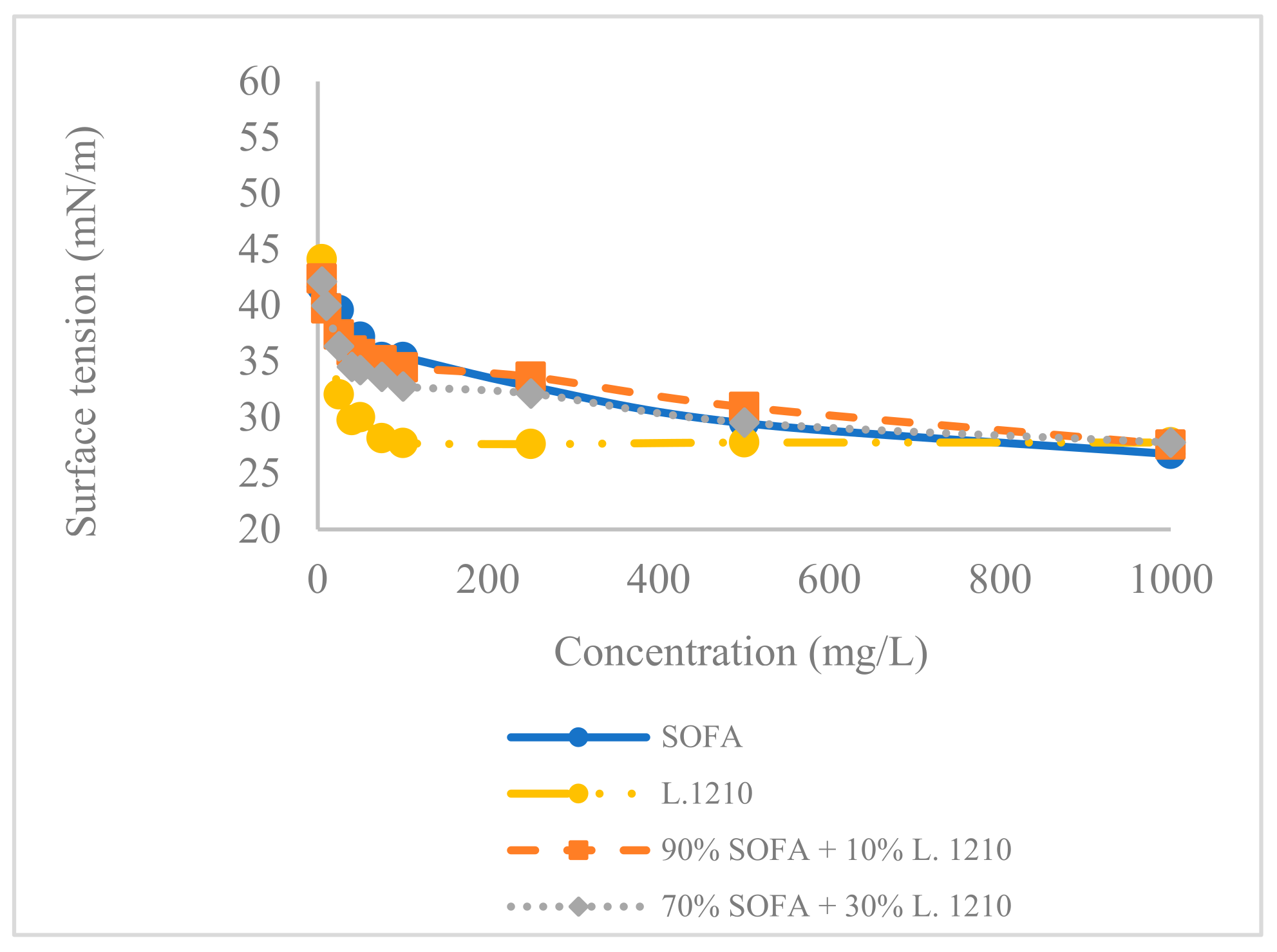
2.6. A Qualitative Trend of Collector Adsorption through Surface Tension
2.7. Bench Scale Column Flotation Tests
3. Results and Discussion
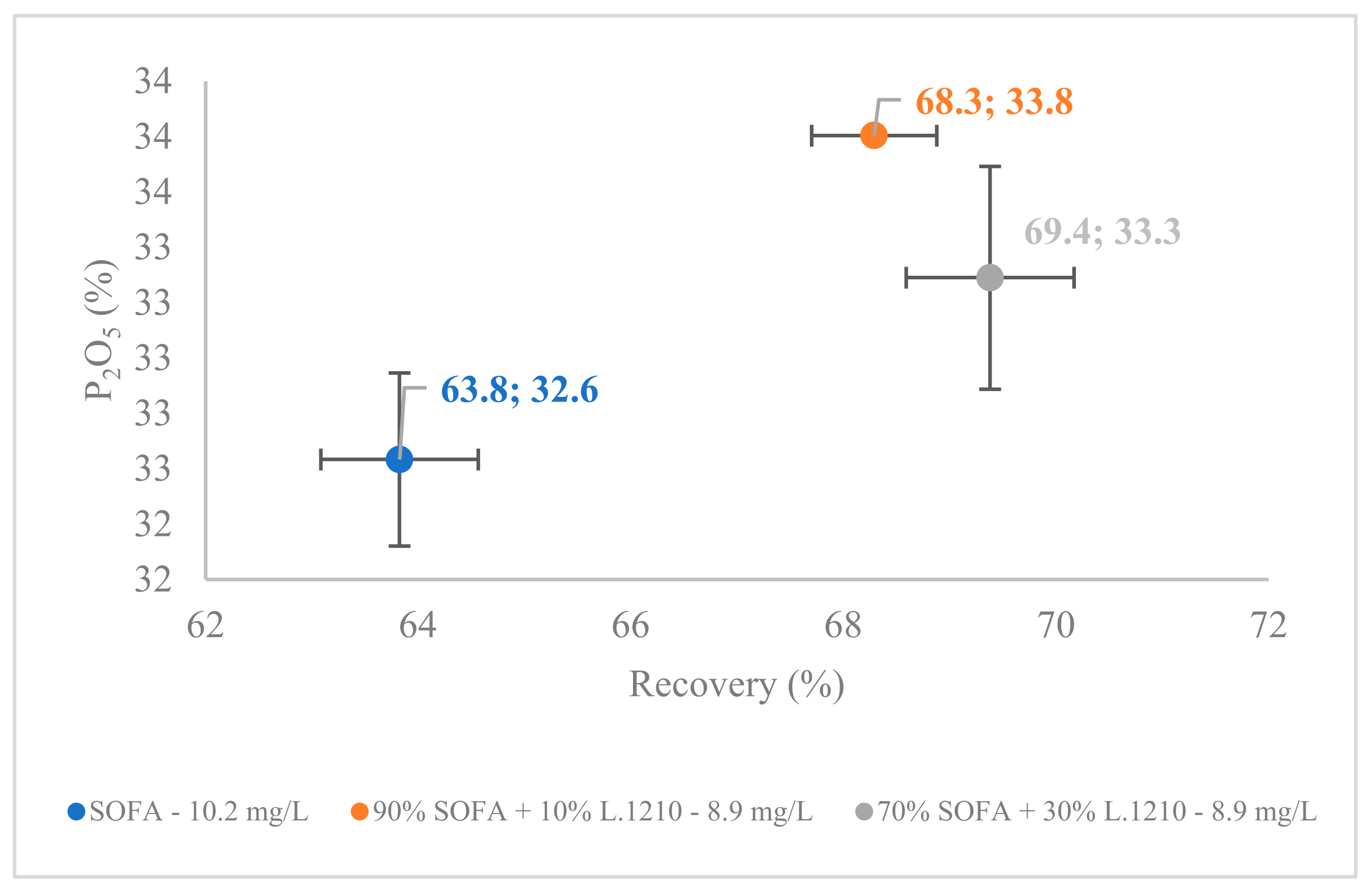
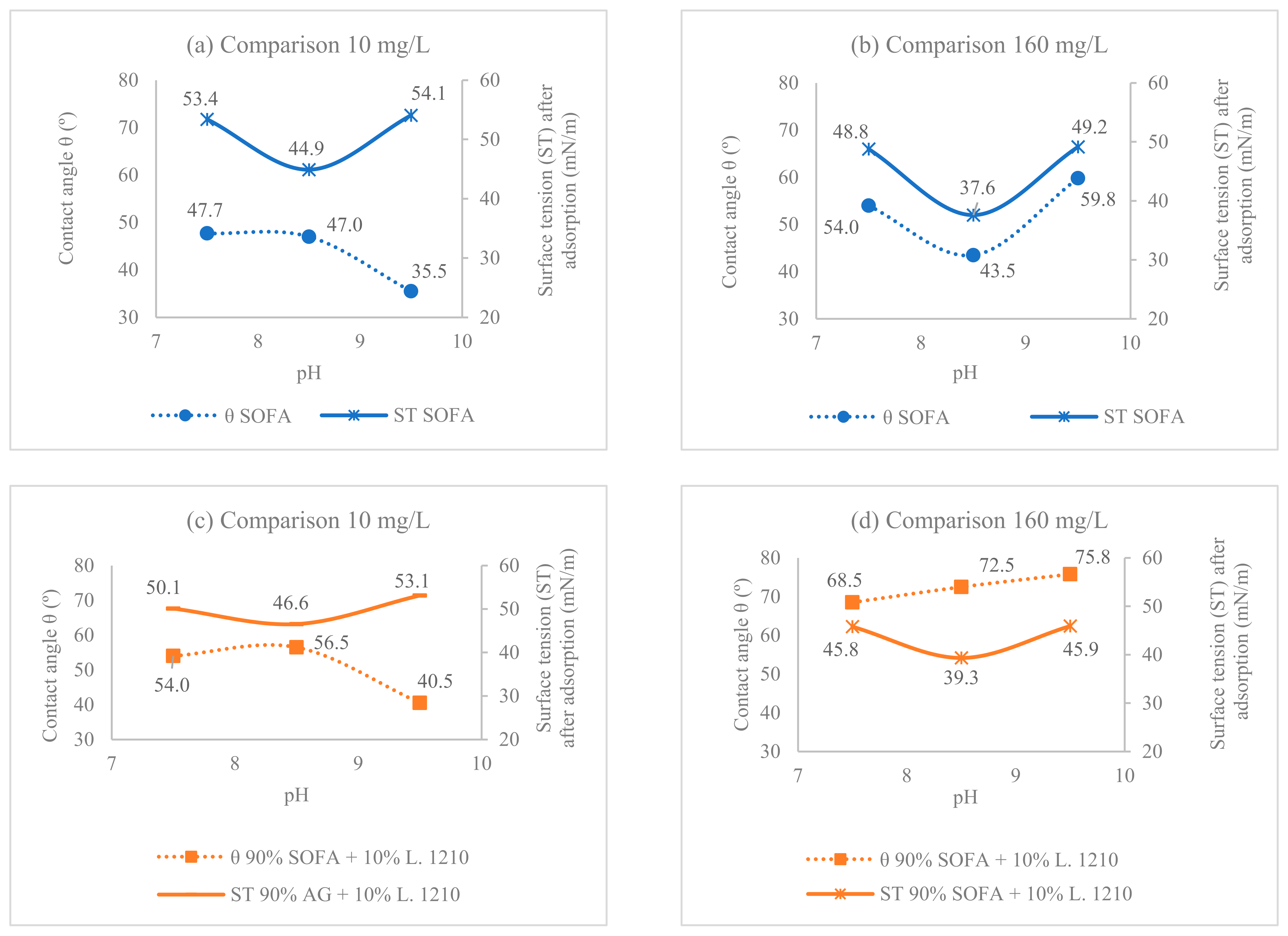
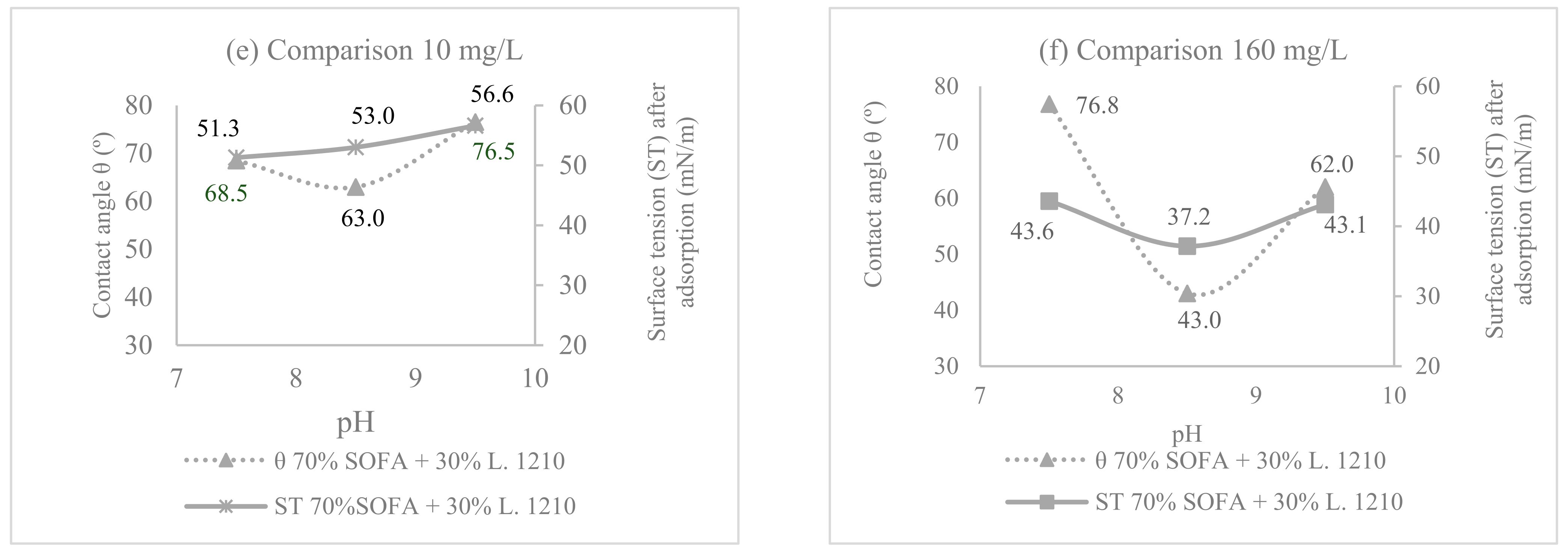
3.1. Comparison between SOFA and Collectors’ Mixture Composition
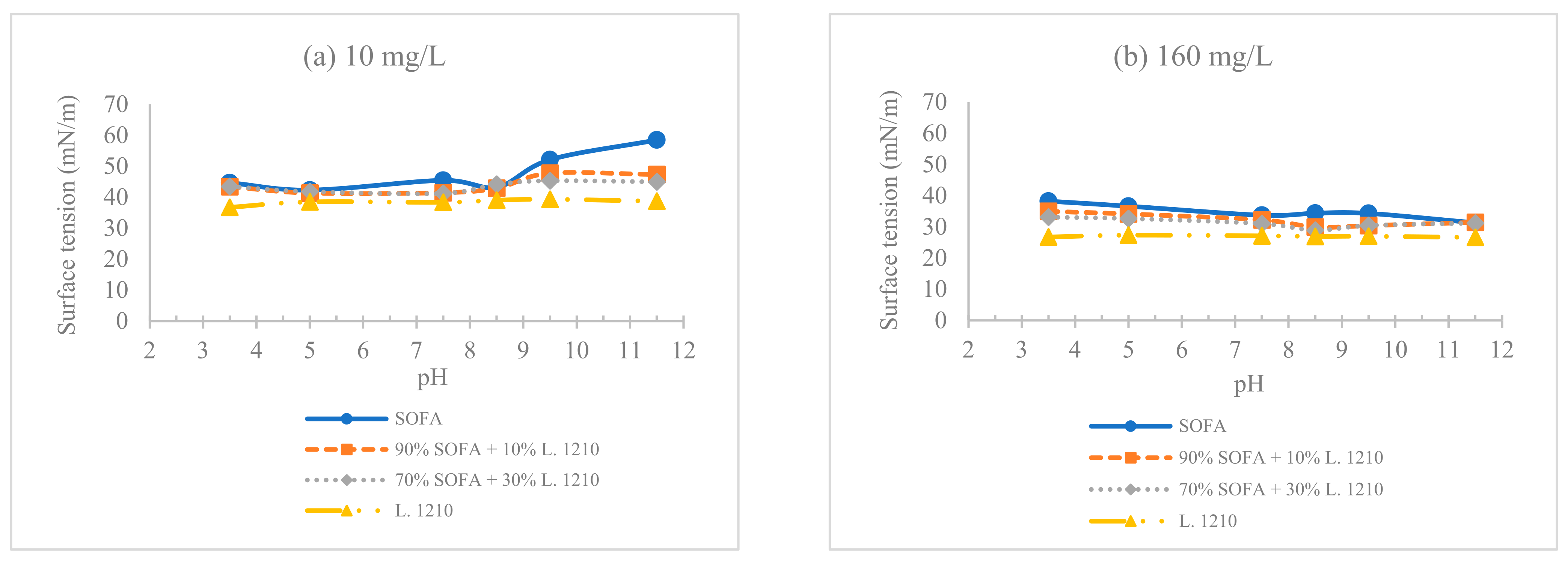
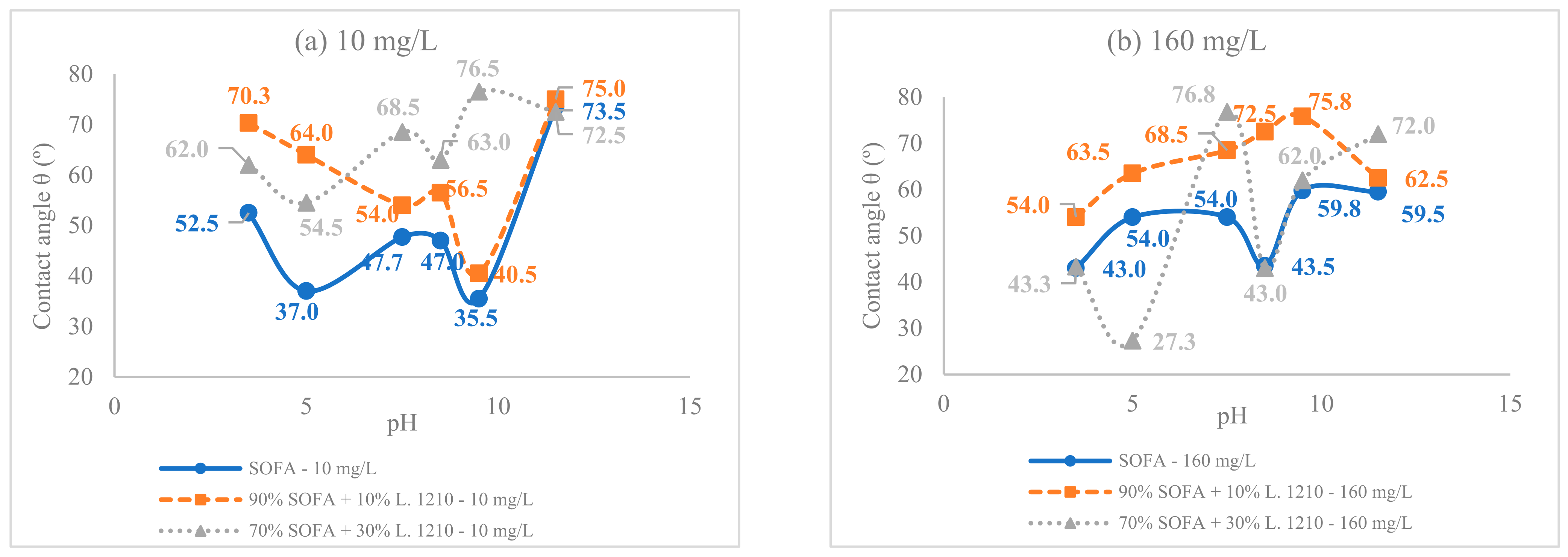
3.2. pH Effect on the Surface Tension and Contact Angle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, C.; Dutrow, B. Manual of Mineral Science; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Hanna, H.S.; Somasundaran, P. Flotation of salt-type minerals. In Flotation—A.M. Gaudin Memorial; Fuerstenau, M.C., Ed.; SME/AIME: New York, NY, USA, 1976; pp. 197–271. [Google Scholar]
- Rao, K.H.; Britt-Marie, A.; Forssberg, E. Mechanism of Interaction on Salt-Type Minerals, Part II. Adsorption and Electrokinetic Studies of Apatite in the Presence of Sodium Oleate and Sodium Metasilicate. Int. J. Miner. Process. 1990, 28, 59–79. [Google Scholar] [CrossRef]
- Finkelstein, N.P. Review of interactions in flotation of sparingly soluble calcium minerals with anionic collectors. Miner. Process Extr. Metall. 1989, 98, 157–178. [Google Scholar]
- Sis, H.; Chander, S. Adsorption and contact angle of single and binary mixtures of surfactants on apatite. Miner. Eng. 2003, 16, 839–848. [Google Scholar] [CrossRef]
- Daltin, D. Tensoativos: Química, Propriedades e Aplicações; Blucher: São Paulo, Brasil, 2011. [Google Scholar]
- Martins, M. Molhabilidade de Apatita e Sua Influência na Flotação. Ph.D. Thesis, Escola Politécnica, Universidade de São Paulo, São Paulo, Brasil, 2009. [Google Scholar]
- Zisman, W.A. Relation of the equilibrium contact angle to liquid and solid constitution. Adv. Chem. 1964, 43, 1–51. [Google Scholar]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Barros, L.A.F.; Ferreira, E.E.; Peres, A.E.C. Floatability of apatites and gangue minerals of an igneous phosphate ore. Miner. Eng. 2008, 21, 994–999. [Google Scholar] [CrossRef]
- Kelly, E.G.; Spottiswood, D.J. Introduction to Mineral Processing; Willey Interscience: New York, NY, USA, 1982. [Google Scholar]
- Gontijo, C.F.; Fornasiero, D.; Ralston, J. The limits of fine and coarse flotation. Can. J. Chem. Eng. 2007, 85, 739–747. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Hicks, M. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature 1973, 243, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.H. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: A hypothesis. Proc. Natl. Acad. Sci. USA 1983, 80, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, M.L.; Robinson, B.H.; Bucak, S.; Walde, P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf. B 2006, 48, 24–34. [Google Scholar] [CrossRef] [PubMed]




| P2O5 | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | BaO | PF | CaO/P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 4.51 | 14.6 | 32.1 | 4.43 | 19.3 | 7.21 | 8.62 | <0.10 | 5.28 | 3.24 |
| Mineral | % | Mineral | % |
|---|---|---|---|
| Hydrobiotite | 31.0 | Granate | 6.1 |
| Pyroxene | 18.0 | Perovskite | 3.4 |
| Apatite | 8.1 | Other oxides | 3.0 |
| Magnetite | 8.1 | K-feldspar | 2.8 |
| Titanite | 7.5 | Quartz | 1.5 |
| Clay minerals 12–15 A | 6.6 | Others | 4.8 |
| Density at 20 °C (g/cm3) | 0.91 |
| Density at 50 °C (g/cm3) | 0.89 |
| Acidic index (mgKOH/g) | 154.11 |
| Iodine index (gI2/100 g) | 112.75 |
| Saponification index (mgKOH/g) | 196.13 |
| Free fatty acid (%) | 78.58 |
| Chain distribution (%) | |
| C6 | 0.18 |
| C8 | 0.20 |
| C10 | 0.14 |
| C12 | 0.16 |
| C14 | 0.38 |
| C16 | 16.48 |
| C18 | 5.38 |
| C18:1 | 28.64 |
| C18:2 | 44.17 |
| C18:3 | 4.26 |
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, F.V.; Budemberg, G.; Cruz, S.H.; Braga, A.S. A Collector Promoter for Apatite Flotation in the Serra do Salitre Complex. Minerals 2023, 13, 599. https://doi.org/10.3390/min13050599
de Lima FV, Budemberg G, Cruz SH, Braga AS. A Collector Promoter for Apatite Flotation in the Serra do Salitre Complex. Minerals. 2023; 13(5):599. https://doi.org/10.3390/min13050599
Chicago/Turabian Stylede Lima, Franciele Vanessa, Gabriela Budemberg, Santiago Henrique Cruz, and André Soares Braga. 2023. "A Collector Promoter for Apatite Flotation in the Serra do Salitre Complex" Minerals 13, no. 5: 599. https://doi.org/10.3390/min13050599





