Authigenic Fe Mineralization in Shallow to Marginal Marine Environments: A Case Study from the Late Paleocene—Early Eocene Cambay Shale Formation
Abstract
1. Introduction
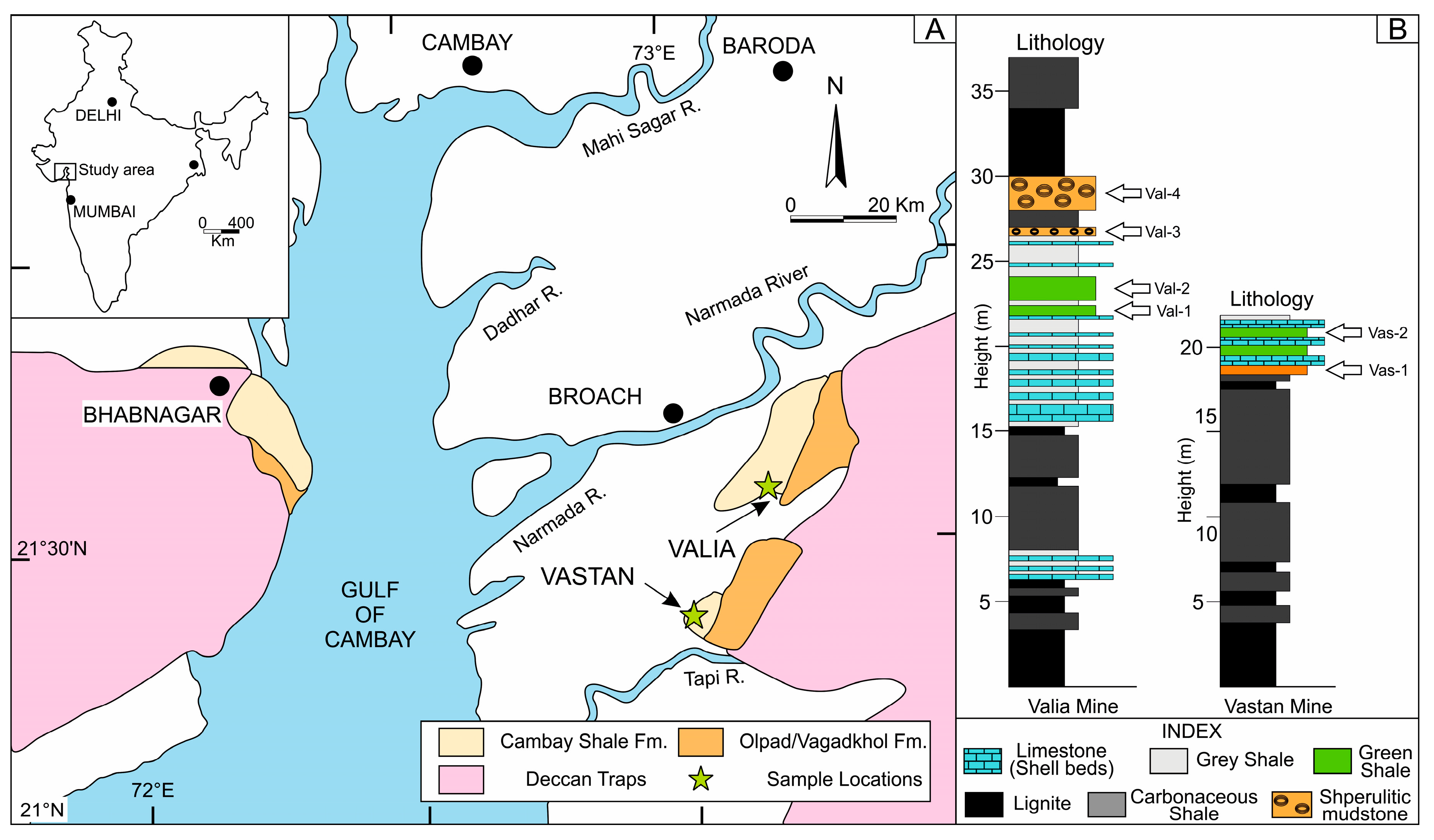
2. Geological Background
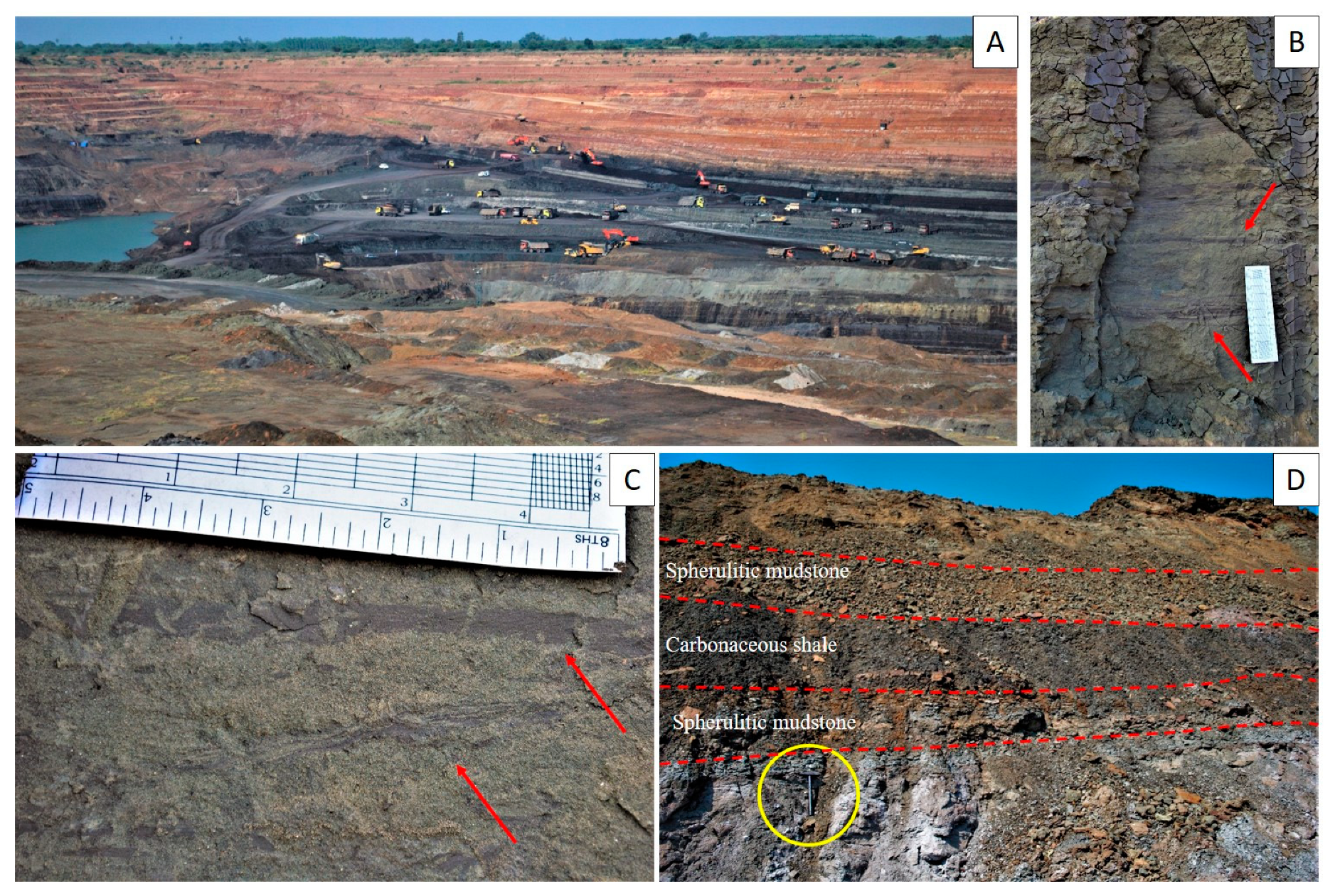
3. Sedimentological Background
4. Materials and Methods
5. Results
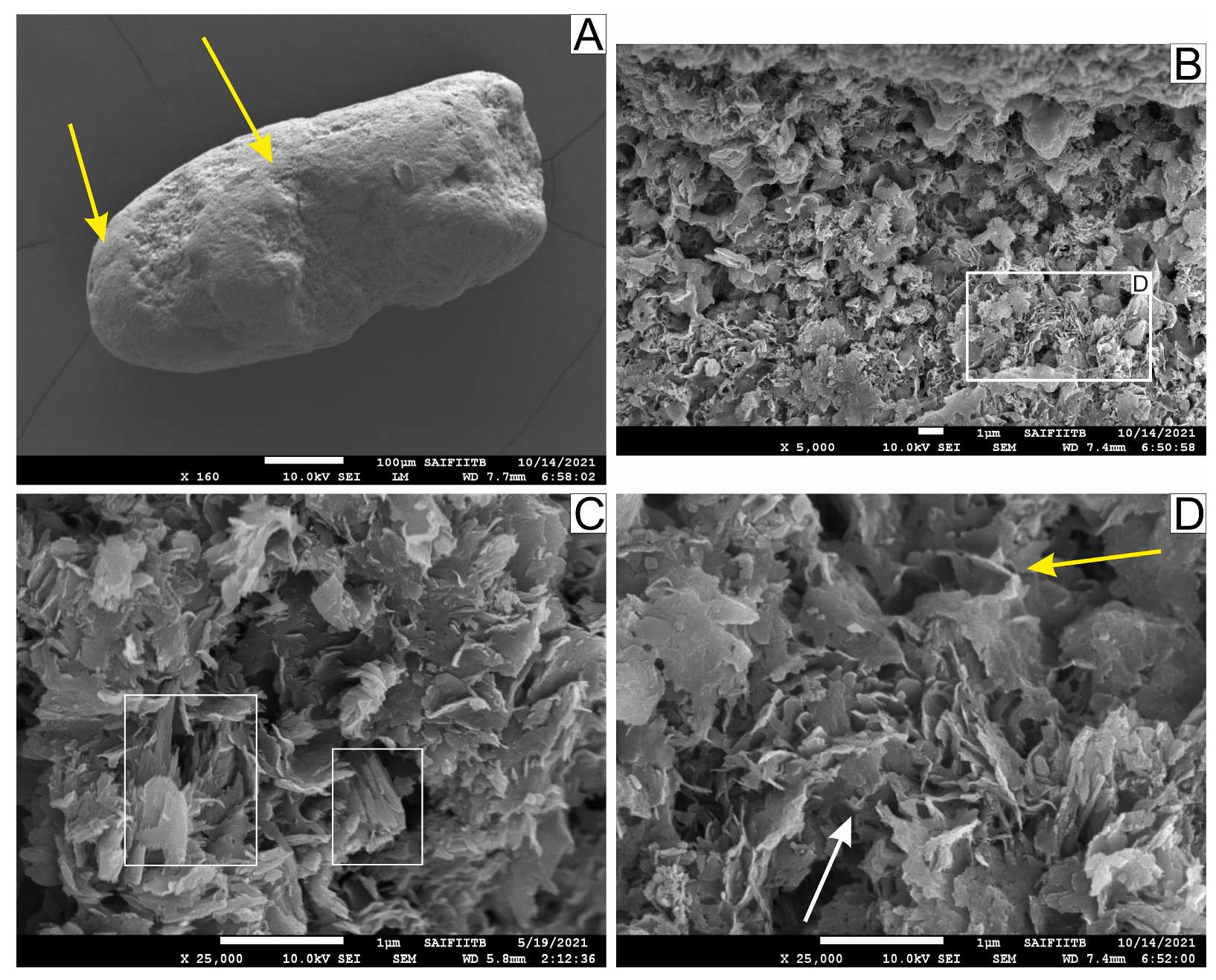
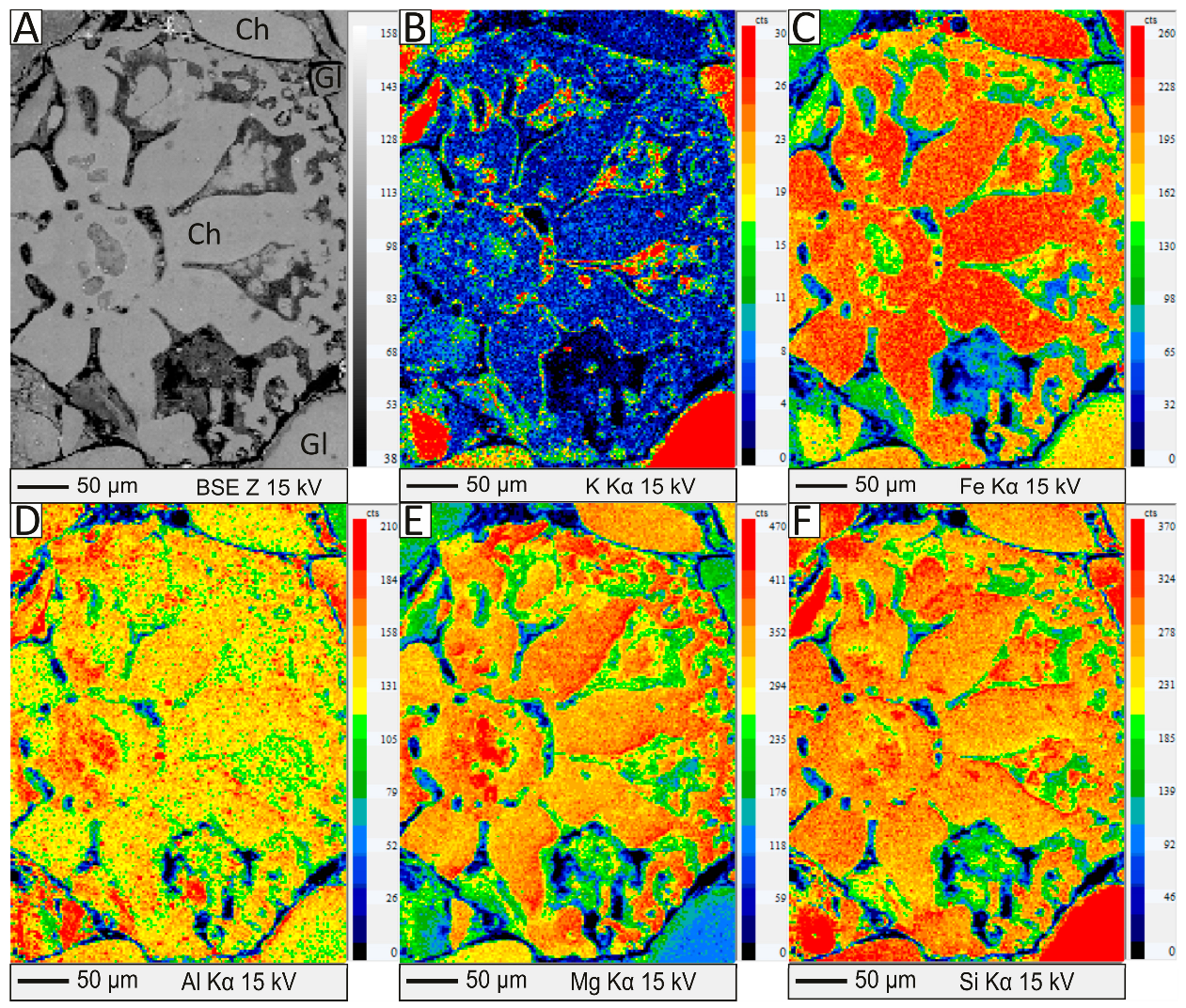
5.1. Petrographic and Micro-Textural Studies of the Green and Brown Pellets and the Spherulites
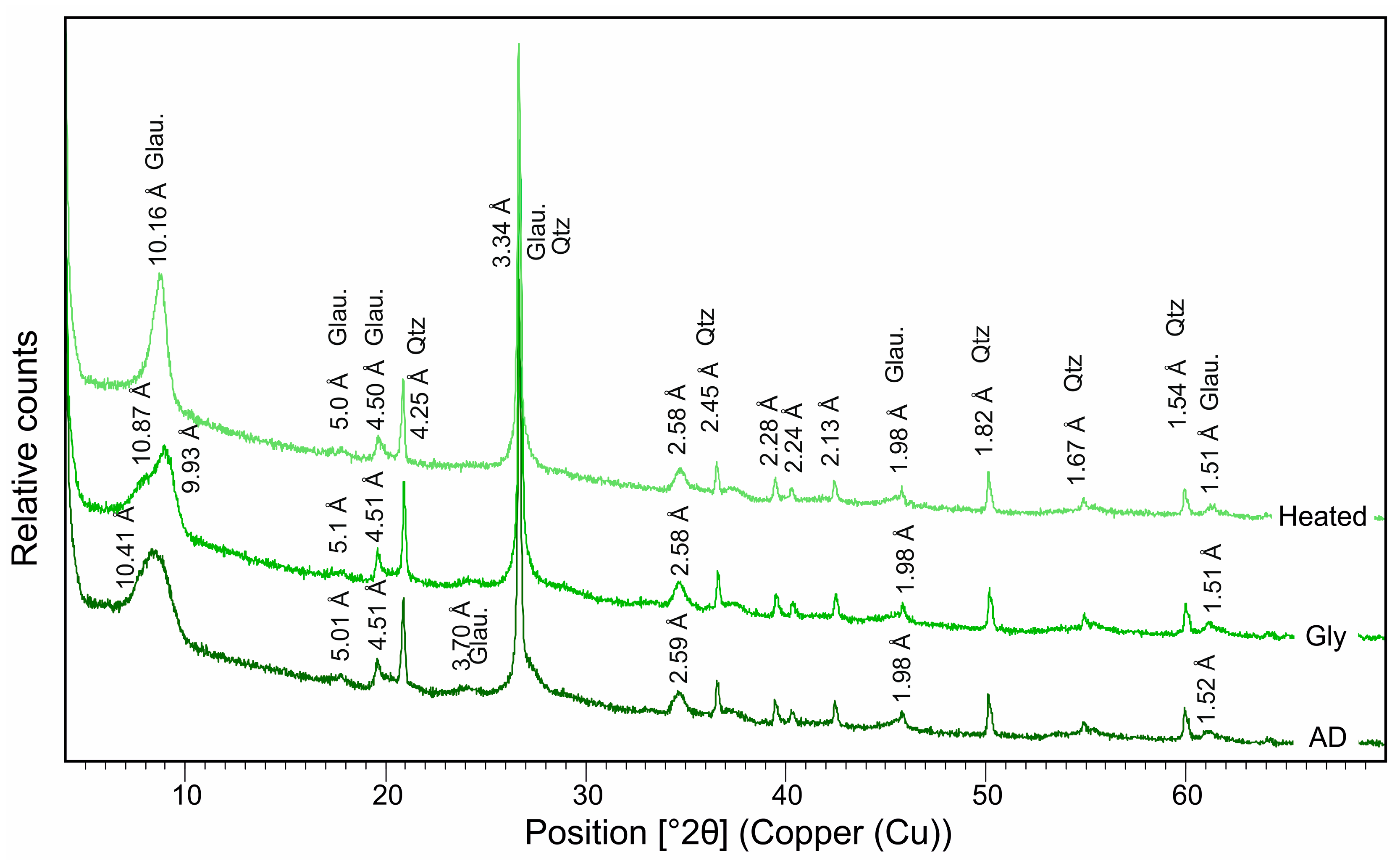
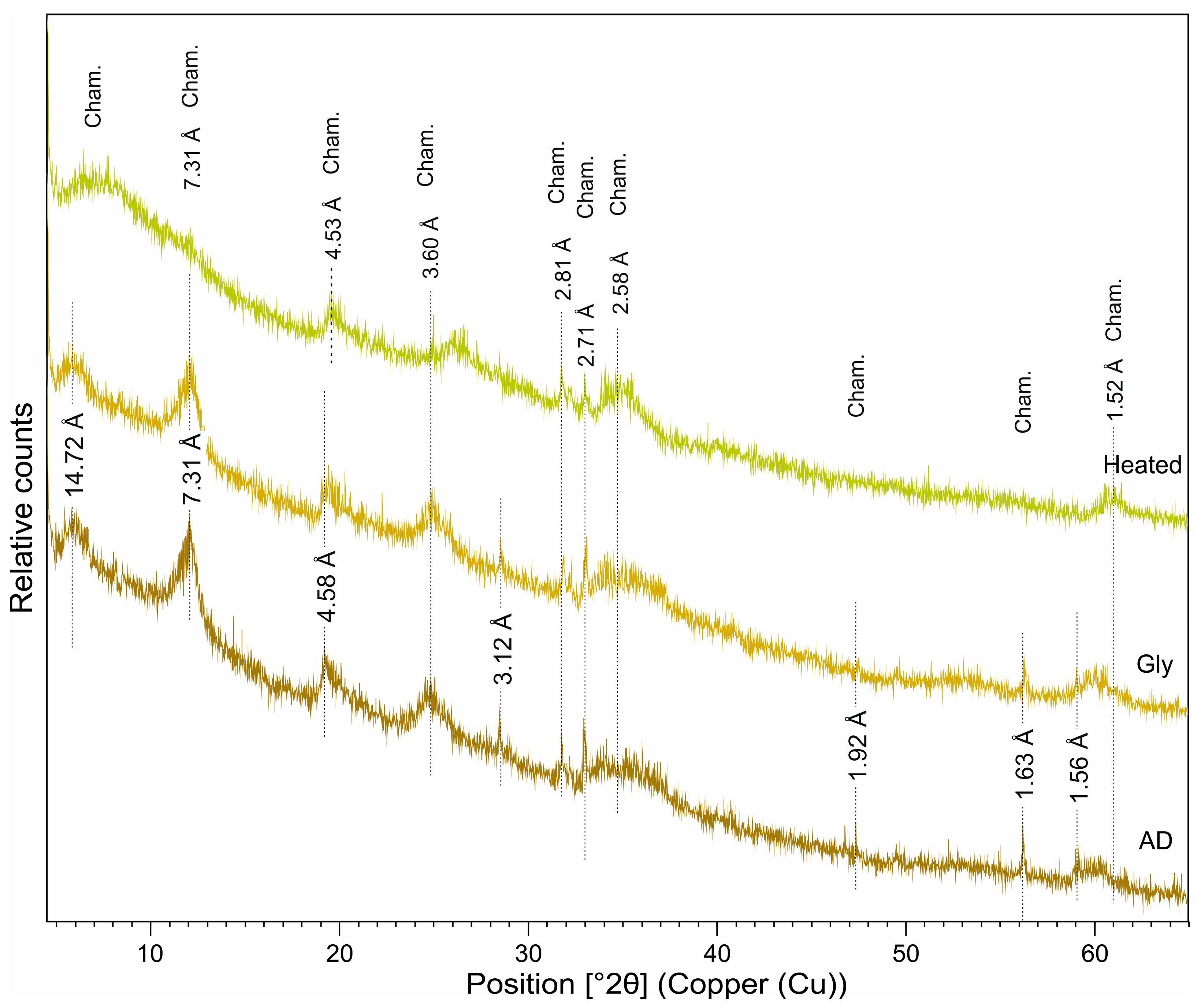
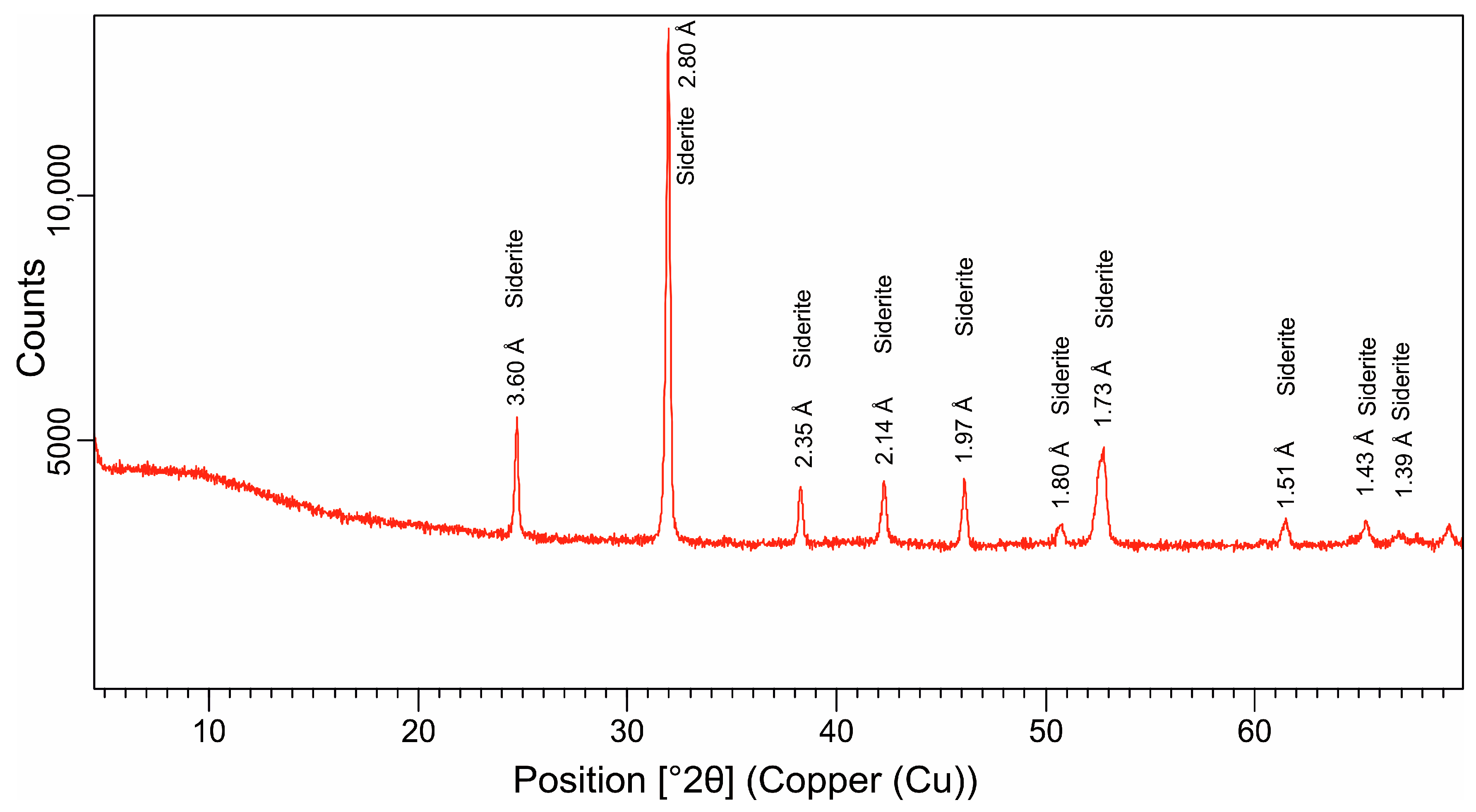
5.2. Mineralogy of the Green and Brown Pellets and the Spherulites
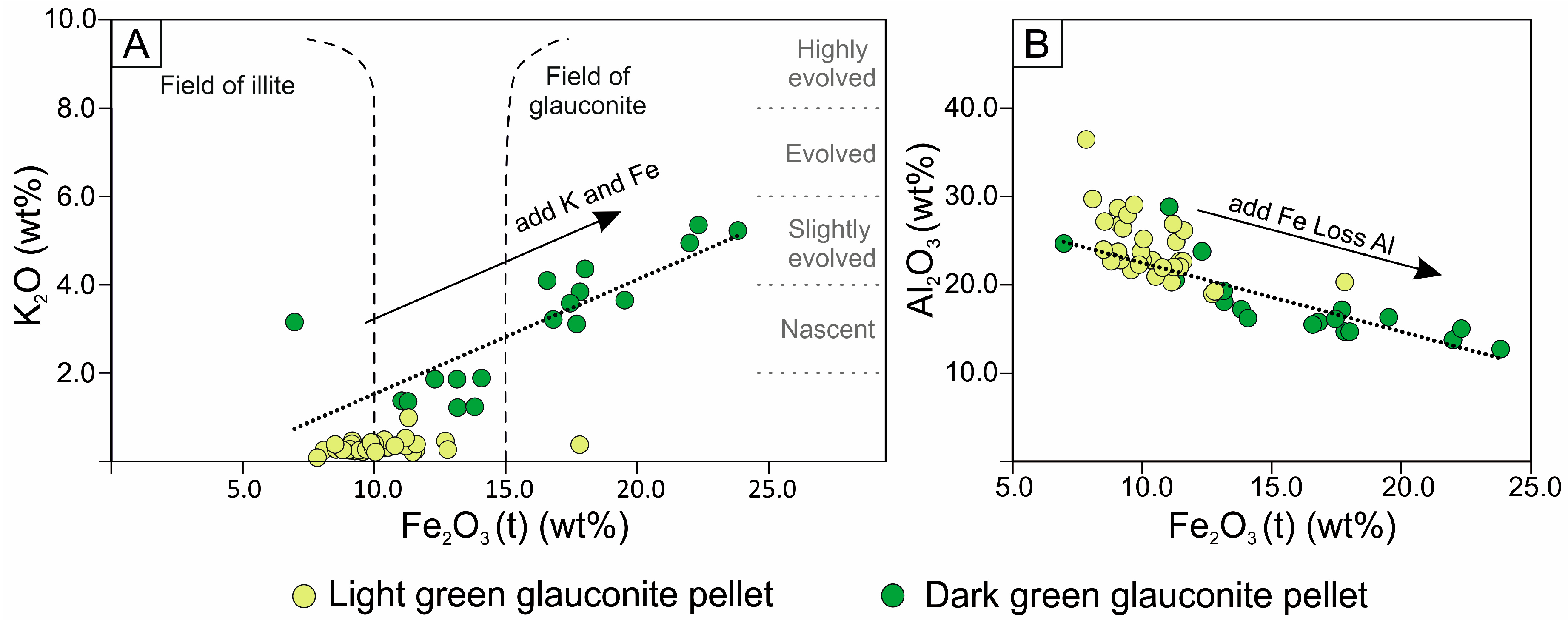
5.3. Major Element Composition of Green and Brown Pellets and the Spherulites
6. Discussion
6.1. Origin of the Glauconite, Chamosite, and Siderite in the Cambay Shale Formation
6.1.1. Glauconite
6.1.2. Chamosite
6.1.3. Siderite
6.2. Stratigraphic Implication of Authigenic Green Clays in Cambay Shale Formation
6.3. Implications of Authigenic Fe Mineralization in Shallow to Marginal Marine Sediments
7. Conclusions
- (A)
- The lignite-bearing marginal marine Cambay Shale Formation hosts episodes of marine incursions, which are reflected in the accumulation of limestone–green shale alternations in the Valia and Vastan mines.
- (B)
- The marine incursions are characterized by authigenic Fe–silicates such as glauconite and chamosite. The overlying regressive deposits exhibit abundant authigenic Fe mineralization in the form of siderite spherulites.
- (C)
- The moderate K2O content and characteristic basal (00l) reflections indicate the ‘slightly evolved’ nature of glauconite in the dark-green pellets in the Cambay Shale Formation. These glauconites are rich in Al2O3 with a moderate-to-high Fe2O3(total) content. The brown pellets with a low SiO2 and negligible K2O content have a (001) reflection at 14.32 Å, which demarcates a chamositic composition. The brown spherulites, on the other hand, are almost entirely composed of FeCO3, exhibit a monomineralic siderite composition, and represent freshwater influence.
- (D)
- The green glauconite pellets of the Valia mine are less matured and contain less K2O, Fe2O3(total), and more Al2O3 compared to the Vastan mine. On the contrary, the Vastan mine contains significant chamosite pellets, suggesting a more anoxic depositional condition at the lower part of the succession. Freshwater influx is the prime reason for the poor maturation of glauconites in the Valia mine.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PETM | Paleocene Eocene Thermal Maximum |
| ETM 2 | Eocene Thermal Maximum 2 |
| SBZ | Shallow Benthic Zone |
| XRD | X-Ray Diffraction |
| EPMA | Electron Probe Micro Analyzer |
| A.S.T.M. | American Society for Testing and Materials |
References
- Odin, G.S.; Matter, A. De Glauconiarum Origine. Sedimentology 1981, 28. [Google Scholar] [CrossRef]
- Amorosi, A.; Sammartino, I.; Tateo, F. Evolution Patterns of Glaucony Maturity: A Mineralogical and Geochemical Approach. Deep. Res. Part II Top. Stud. Oceanogr. 2007, 54, 1364–1374. [Google Scholar] [CrossRef]
- López-Quirós, A.; Lobo, F.J.; Mendes, I.; Nieto, F. Holocene Glaucony from the Guadiana Shelf, Northern Gulf of Cadiz (SW Iberia): New Genetic Insights in a Sequence Stratigraphy Context. Miner. 2023, 13, 177. [Google Scholar] [CrossRef]
- Clauer, N.; Keppens, E.; Stille, P. Sr Isotopic Constraints on the Process of Glauconitization. Geology 1992, 20, 133–136. [Google Scholar] [CrossRef]
- Baldermann, A.; Dietzel, M.; Mavromatis, V.; Mittermayr, F.; Warr, L.N.; Wemmer, K. The Role of Fe on the Formation and Diagenesis of Interstratified Glauconite-Smectite and Illite-Smectite: A Case Study of Lower Cretaceous Shallow-Water Carbonates. Chem. Geol. 2017, 453, 21–34. [Google Scholar] [CrossRef]
- Banerjee, S.; Bansal, U.; Vilas Thorat, A. A Review on Palaeogeographic Implications and Temporal Variation in Glaucony Composition. J. Palaeogeogr. 2016, 5, 43–71. [Google Scholar] [CrossRef]
- Banerjee, S.; Choudhury, T.R.; Saraswati, P.K.; Khanolkar, S. The Formation of Authigenic Deposits during Paleogene Warm Climatic Intervals: A Review. J. Palaeogeogr. 2020, 9, 27. [Google Scholar]
- Van Houten, F.B.; Bhattacharyya, D.P. Phanerozoic Oolitic Ironstones—Geologic Record and Facies Model. Annu. Rev. Earth Planet. Sci. 1982, 10, 441–457. [Google Scholar] [CrossRef]
- Van Houten, F.B.; Purucker, M.E. Glauconitic Peloids and Chamositic Ooids—Favorable Factors, Constraints, and Problems. Earth Sci. Rev. 1984, 20, 211–243. [Google Scholar] [CrossRef]
- Tang, D.; Shi, X.; Jiang, G.; Zhou, X.; Shi, Q. Ferruginous Seawater Facilitates the Transformation of Glauconite to Chamosite: An Example from the Mesoproterozoic Xiamaling Formation of North China. Am. Mineral. 2017, 102. [Google Scholar] [CrossRef]
- Dasgupta, S.; Chaudhuri, A.K.; Fukuoka, M. Compositional Characteristics of Glauconitic Alterations of K-Feldspar from India and Their Implications. J. Sediment. Res. 1990, 60, 277–281. [Google Scholar] [CrossRef]
- Giresse, P. Quaternary Glauconitization on Gulf of Guinea, Glauconite Factory: Overview of and New Data on Tropical Atlantic Continental Shelves and Deep Slopes. Minerals 2022, 12, 908. [Google Scholar] [CrossRef]
- Porrenga, D.H. Glauconite and Chamosite as Depth Indicators in the Marine Environment. Mar. Geol. 1967, 5, 495–501. [Google Scholar] [CrossRef]
- Berner, R.A. A New Geochemical Classification of Sedimentary Environments. J. Sediment. Res. 1981, 51, 359–365. [Google Scholar] [CrossRef]
- Young, T.P. Phanerozoic Ironstones: An Introduction and Review. Geol. Soc. Spec. Publ. 1989, 46. [Google Scholar] [CrossRef]
- Bekker, A.; Planavsky, N.J.; Krapež, B.; Rasmussen, B.; Hofmann, A.; Slack, J.F.; Rouxel, O.J.; Konhauser, K.O. Iron Formations: Their Origins and Implications for Ancient Seawater Chemistry. Treatise Geochem. Second. Ed. 2014, 9, 561–628. [Google Scholar] [CrossRef]
- Tounekti, A.; Boukhalfa, K.; Choudhury, T.R.; Soussi, M.; Banerjee, S. Global and Local Factors behind the Authigenesis of Fe-Silicates (Glauconite/Chamosite) in Miocene Strata of Northern Tunisia. J. African Earth Sci. 2021, 184, 104342. [Google Scholar] [CrossRef]
- Rudmin, M.; Mazurov, A.; Banerjee, S. Origin of Ooidal Ironstones in Relation to Warming Events: Cretaceous-Eocene Bakchar Deposit, South-East Western Siberia. Mar. Pet. Geol. 2019, 100, 309–325. [Google Scholar] [CrossRef]
- Amorosi, A. Glaucony and Sequence Stratigraphy; a Conceptual Framework of Distribution in Siliciclastic Sequences. J. Sediment. Res. 1995, 65, 419–425. [Google Scholar] [CrossRef]
- Roy Choudhury, T.; Banerjee, S.; Khanolkar, S.; Saraswati, P.K.; Meena, S.S. Glauconite Authigenesis during the Onset of the Paleocene-Eocene Thermal Maximum: A Case Study from the Khuiala Formation in Jaisalmer Basin, India. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 571, 110388. [Google Scholar] [CrossRef]
- Bansal, U.; Banerjee, S.; Pande, K.; Ruidas, D.K. Unusual Seawater Composition of the Late Cretaceous Tethys Imprinted in Glauconite of Narmada Basin, Central India. Geol. Mag. 2020, 157, 233–247. [Google Scholar] [CrossRef]
- Wigley, R.; Compton, J.S. Oligocene to Holocene Glauconite–Phosphorite Grains from the Head of the Cape Canyon on the Western Margin of South Africa. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1375–1395. [Google Scholar] [CrossRef]
- Roy Choudhury, T.; Khanolkar, S.; Banerjee, S. Glauconite Authigenesis during the Warm Climatic Events of Paleogene: Case Studies from Shallow Marine Sections of Western India. Glob. Planet. Chang. 2022, 214, 103857. [Google Scholar] [CrossRef]
- Isson, T.T.; Planavsky, N.J. Reverse Weathering as a Long-Term Stabilizer of Marine PH and Planetary Climate. Nature 2018, 560, 471–475. [Google Scholar] [CrossRef]
- Baldermann, A.; Banerjee, S.; Czuppon, G.; Dietzel, M.; Farkaš, J.; Löhr, S.; Moser, U.; Scheiblhofer, E.; Wright, N.M.; Zack, T. Impact of Green Clay Authigenesis on Element Sequestration in Marine Settings. Nat. Commun. 2022, 13, 1527. [Google Scholar] [CrossRef]
- Taylor, K.G.; Macquaker, J.H.S. Iron Minerals in Marine Sediments Record Chemical Environments. Elements 2011, 7, 113–118. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, H.; Sloan, L.; Thomas, E.; Billups, K. Trends, Rhythms, and Aberrations in Global Climate 65 Ma to Present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Nicolo, M.J.; Dickens, G.R.; Hollis, C.J.; Zachos, J.C. Multiple Early Eocene Hyperthermals: Their Sedimentary Expression on the New Zealand Continental Margin and in the Deep Sea. Geology 2007, 35, 699–702. [Google Scholar] [CrossRef]
- Clementz, M.; Bajpai, S.; Ravikant, V.; Thewissen, J.G.M.; Saravanan, N.; Singh, I.B.; Prasad, V. Early Eocene Warming Events and the Timing of Terrestrial Faunal Exchange between India and Asia. Geology 2011, 39, 15–18. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Mazurov, A. Compositional Variation of Glauconites in Upper Cretaceous-Paleogene Sedimentary Iron-Ore Deposits in South-Eastern Western Siberia. Sediment. Geol. 2017, 355, 20–30. [Google Scholar] [CrossRef]
- Sluijs, A.; Van Roij, L.; Harrington, G.J.; Schouten, S.; Sessa, J.A.; LeVay, L.J.; Reichart, G.-J.; Slomp, C.P. Warming, Euxinia and Sea Level Rise during the Paleocene–Eocene Thermal Maximum on the Gulf Coastal Plain : Implications for Ocean Oxygenation and Nutrient Cycling. Clim. Past 2014, 10, 1421–1439. [Google Scholar] [CrossRef]
- Anwar, D.; Choudhary, A.K.; Saraswati, P.K. Strontium Isotope Stratigraphy of the Naredi Formation, Kutch Basin, India. India Artic. J. Geol. Soc. India 2013, 1, 298–306. [Google Scholar] [CrossRef]
- Samanta, A.; Bera, M.K.; Ghosh, R.; Bera, S.; Filley, T.; Pande, K.; Rathore, S.S.; Rai, J.; Sarkar, A. Do the Large Carbon Isotopic Excursions in Terrestrial Organic Matter across Paleocene-Eocene Boundary in India Indicate Intensification of Tropical Precipitation? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 387, 91–103. [Google Scholar] [CrossRef]
- Saraswati, P.K.; Khanolkar, S.; Raju, D.S.N.; Dutta, S.; Banerjee, S.; Wang, Y.; Liu, M. Foraminiferal Biostratigraphy of Lignite Mines of Kutch India: Age of Lignite Fossil Vertebrates. J. Palaeogeogr. 2014, 3, 90–98. [Google Scholar]
- Khanolkar, S.; Saraswati, P.K. ECOLOGICAL RESPONSE OF SHALLOW-MARINE FORAMINIFERA TO EARLY EOCENE WARMING IN EQUATORIAL INDIA. J. Foraminifer. Res. 2015, 45, 293–304. [Google Scholar] [CrossRef]
- Roy Choudhury, T.; Banerjee, S.; Khanolkar, S.; Meena, S.S. Paleoenvironmental Conditions during the Paleocene–Eocene Transition Imprinted within the Glauconitic Giral Member of the Barmer Basin, India. Minerals 2022, 12, 56. [Google Scholar] [CrossRef]
- Kumar, S.; Das, S.; Bastia, R.; Ojha, K. Mineralogical and Morphological Characterization of Older Cambay Shale from North Cambay Basin, India: Implication for Shale Oil/Gas Development. Mar. Pet. Geol. 2018, 97, 339–354. [Google Scholar] [CrossRef]
- Biswas, S.K. Regional Tectonic Framework, Structure and Evolution of the Western Marginal Basins of India. Tectonophysics 1987, 135, 307–327. [Google Scholar] [CrossRef]
- Garg, R.; Ateequzzaman, K.; Prasad, V.; Tripathi, S.K.M.; Singh, I.B.; Jauhri, A.K.; Bajpai, S. Age-Diagnostic Dinoflagellate Cysts from the Lignite-Bearing Sediments of the Vastan Lignite Mine, Surat District, Gujarat, Western India. J. Palaeontol. Soc. India 2008, 53, 99–105. [Google Scholar]
- Phaye, D.K.; Bhattacharya, B.; Chakrabarty, S. Heterogeneity Characterization from Sequence Stratigraphic Analysis of Paleocene-Early Eocene Cambay Shale Formation in Jambusar-Broach Area, Cambay Basin, India. Mar. Pet. Geol. 2021, 128, 104986. [Google Scholar] [CrossRef]
- Pundaree, N.; Krishna, A.K.; Subramanyam, K.S.V.; Sawant, S.S.; Kavitha, S.; Kalpana, M.S.; Patil, D.J.; Dayal, A.M. Early Eocene Carbonaceous Shales of Tadkeshwar Formation, Cambay Basin, Gujarat, India: Geochemical Implications, Petrogenesis and Tectonics. Mar. Pet. Geol. 2015, 68, 258–268. [Google Scholar] [CrossRef]
- Misra, A.A.; Maitra, A.; Sinha, N.; Dey, S.; Mahapatra, S. Syn- to Post-Rift Fault Evolution in a Failed Rift: A Reflection Seismic Study in Central Cambay Basin (Gujarat), India. Int. J. Earth Sci. 2019, 108, 1293–1316. [Google Scholar] [CrossRef]
- Ganguli, S.S.; Sen, S. Investigation of Present-Day in-Situ Stresses and Pore Pressure in the South Cambay Basin, Western India: Implications for Drilling, Reservoir Development and Fault Reactivation. Mar. Pet. Geol. 2020, 118, 104422. [Google Scholar] [CrossRef]
- Punekar, J.; Saraswati, P.K. Age of the Vastan Lignite in Context of Some Oldest Cenozoic Fossil Mammals from India. J. Geol. Soc. India 2010, 76, 63–68. [Google Scholar] [CrossRef]
- Prasad, V.; Singh, I.B.; Bajpai, S.; Garg, R.; Thakur, B.; Singh, A.; Saravanan, N.; Kapur, V.V. Palynofacies and Sedimentology-Based High-Resolution Sequence Stratigraphy of the Lignite-Bearing Muddy Coastal Deposits (Early Eocene) in the Vastan Lignite Mine, Gulf of Cambay, India. Facies 2013, 59, 737–761. [Google Scholar] [CrossRef]
- Khanolkar, S.; Sharma, J. Record of Early to Middle Eocene Paleoenvironmental Changes from Lignite Mines, Western India. J. Micropalaeontol. 2019, 38, 1–24. [Google Scholar] [CrossRef]
- Samanta, A.; Sarkar, A.; Bera, M.K.; Rai, J.; Rathore, S.S. Late Paleocene-Early Eocene Carbon Isotope Stratigraphy from a near-Terrestrial Tropical Section and Antiquity of Indian Mammals. J. Earth Syst. Sci. 2013, 122, 163–171. [Google Scholar] [CrossRef]
- El Albani, A.; Meunier, A.; Fürsich, F. Unusual Occurrence of Glauconite in a Shallow Lagoonal Environment (Lower Cretaceous, Northern Aquitaine Basin, SW France). Terra Nov. 2005, 17, 537–544. [Google Scholar] [CrossRef]
- Baldermann, A.; Warr, L.N.; Grathoff, G.H.; Dietzel, M. The Rate and Mechanism of Deep-Sea Glauconite Formation at the Ivory Coast-Ghana Marginal Ridge. Clays Clay Miner. 2013, 61, 258–276. [Google Scholar] [CrossRef]
- López-Quirós, A.; Sánchez-Navas, A.; Nieto, F.; Escutia, C. New Insights into the Nature of Glauconite. Am. Mineral. 2020, 105, 674–686. [Google Scholar] [CrossRef]
- Thompson, G.R.; Hower, J. The Mineralogy of Glauconite. Clays Clay Miner. 1975, 23, 289–300. [Google Scholar] [CrossRef]
- Bansal, U.; Banerjee, S.; Nagendra, R. Is the Rarity of Glauconite in Precambrian Bhima Basin in India Related to Its Chloritization? Precambrian Res. 2020, 336, 105509. [Google Scholar] [CrossRef]
- Burst, J.F. Mineral Heterogeneity in “Glauconite” Pellets. Am. Mineral. 1958, 43, 481–497. [Google Scholar]
- Hower, J. Some Factors Concerning the Nature and Origin of Glauconite. Am. Mineral. 1961, 46, 313–334. [Google Scholar]
- Deb, S.P.; Fukuoka, M. Fe-Illites in a Proterozoic Deep Marine Slope Deposit in the Penganga Group of the Pranhita Godavari Valley: Their Origin and Environmental Significance. J. Geol. 1998, 106, 741–749. [Google Scholar] [CrossRef]
- Odin, G.S.; Fullagar, P.D. Chapter C4 Geological Significance of the Glaucony Facies. Dev. Sedimentol. 1988, 45. [Google Scholar] [CrossRef]
- Bansal, U.; Banerjee, S.; Pande, K.; Arora, A.; Meena, S.S. The Distinctive Compositional Evolution of Glauconite in the Cretaceous Ukra Hill Member (Kutch Basin, India) and Its Implications. Mar. Pet. Geol. 2017, 82, 97–117. [Google Scholar] [CrossRef]
- Eder, V.G.; Martín-Algarra, A.; Sánchez-Navas, A.; Zanin, Y.N.; Zamirailova, A.G.; Lebedev, Y.N. Depositional Controls on Glaucony Texture and Composition, Upper Jurassic, West Siberian Basin. Sedimentology 2007, 54, 1365–1387. [Google Scholar] [CrossRef]
- Jach, R.; Starzec, K. Glaucony from the Condensed Lower-Middle Jurassic Deposits of the Krizna Unit, Western Tatra Mountains, Poland. Ann. Soc. Geol. Pol. 2003, 73, 183–192. [Google Scholar]
- Berg-Madsen, V. High-Alumina Glaucony from the Middle Cambrian of Oeland and Bornholm, Southern Baltoscandia. J. Sediment. Res. 1983, 53, 875–893. [Google Scholar] [CrossRef]
- Skiba, M.; Maj-Szeliga, K.; Szymański, W.; Błachowski, A. Weathering of Glauconite in Soils of Temperate Climate as Exemplified by a Luvisol Profile from Góra Puławska, Poland. Geoderma 2014, 235–236, 212–226. [Google Scholar] [CrossRef]
- Fanning, D.S.; Rabenhorst, M.C.; May, L.; Wagner, D.P. Oxidation State of Iron in Glauconite from Oxidized and Reduced Zones of Soil-Geologic Columns. Clays Clay Miner. 1989, 37, 59–64. [Google Scholar] [CrossRef]
- Courbe, C.; Velde, B.; Meunier, A. Weathering of Glauconites: Reversal of the Glauconitization Process in a Soil Profile in Western France. Clay Miner. 1981, 16, 231–243. [Google Scholar] [CrossRef]
- Giresse, P.; Odin, G.S. Nature Mineralogique et Origine Des Glauconies Du Plateau Continental Du Gabon et Du Congo. Sedimentology 1973, 20, 457–488. [Google Scholar] [CrossRef]
- Andrade, G.R.P.; de Azevedo, A.C.; Cuadros, J.; Souza, V.S.; Furquim, S.A.C.; Kiyohara, P.K.; Vidal-Torrado, P. Transformation of Kaolinite into Smectite and Iron-Illite in Brazilian Mangrove Soils. Soil Sci. Soc. Am. J. 2014, 78, 655–672. [Google Scholar] [CrossRef]
- Dai, S.; Chou, C.L. Occurrence and Origin of Minerals in a Chamosite-Bearing Coal of Late Permian Age, Zhaotong, Yunnan, China. Am. Mineral. 2007, 92, 1253–1261. [Google Scholar] [CrossRef]
- Stonecipher, S.A. Genetic Characteristics of Glauconite and Siderite: Implications for the Origin of Ambiguos Isolated Marine Sandbodies. SEPM Spec. Publ. 1999, 64, 191–204. [Google Scholar]
- Passey, S.R. The Habit and Origin of Siderite Spherules in the Eocene Coal-Bearing Prestfjall Formation, Faroe Islands. Int. J. Coal Geol. 2014, 122, 76–90. [Google Scholar] [CrossRef]
- Mozley, P.S. Relation between Depositional Environment and the Elemental Composition of Early Diagenetic Siderite. Geology 1989, 17, 704–706. [Google Scholar] [CrossRef]
- Serra-Kiel, J.; Hottinger, L.; Schaub, H.; Sirel, E.; Strougo, A.; Tambareau, Y.; Tosquella, J.; Zakrevskaya, E.; Caus, E.; Drobne, K.; et al. Larger Foraminiferal Biostratigraphy of the Tethyan Paleocene and Eocene. Bull. la Société Géologique Fr. 1998, 169, 281–299. [Google Scholar]
- Stassen, P.; Thomas, E.; Speijer, R.P. Paleocene-Eocene Thermal Maximum Environmental Change in the New Jersey Coastal Plain: Benthic Foraminiferal Biotic Events. Mar. Micropaleontol. 2015, 115, 1–23. [Google Scholar] [CrossRef]
- John, C.M.; Bohaty, S.M.; Zachos, J.C.; Sluijs, A.; Gibbs, S.; Brinkhuis, H.; Bralower, T.J. North American Continental Margin Records of the Paleocene-Eocene Thermal Maximum: Implications for Global Carbon and Hydrological Cycling. Paleoceanography 2008, 23, PA2217. [Google Scholar] [CrossRef]
- Carmichael, M.J.; Inglis, G.N.; Badger, M.P.S.; Naafs, B.D.A.; Behrooz, L.; Remmelzwaal, S.; Monteiro, F.M.; Rohrssen, M.; Farnsworth, A.; Buss, H.L.; et al. Hydrological and Associated Biogeochemical Consequences of Rapid Global Warming during the Paleocene-Eocene Thermal Maximum. Glob. Planet. Chang. 2017, 157, 114–138. [Google Scholar] [CrossRef]
- Bolle, M.-P.; Adatte, T. Palaeocene- Early Eocene Climatic Evolution in the Tethyan Realm: Clay Mineral Evidence. Clay Miner. 2001, 36, 249–261. [Google Scholar] [CrossRef]
- Bolle, M.P.; Tantawy, A.A.; Pardo, A.; Adatte, T.; Burns, S.; Kassab, A. Climatic and Environmental Changes Documented in the Upper Paleocene to Lower Eocene of Egypt. Eclogae Geol. Helv. 2000, 93, 33–51. [Google Scholar]
- Rudmin, M.; Banerjee, S.; Abdullayev, E.; Ruban, A.; Filimonenko, E.; Lyapina, E.; Kashapov, R.; Mazurov, A. Ooidal Ironstones in the Meso-Cenozoic Sequences in Western Siberia: Assessment of Formation Processes and Relationship with Regional and Global Earth Processes. J. Palaeogeogr. 2020, 9, 1–21. [Google Scholar] [CrossRef]
- Todd, S.E.; Pufahl, P.K.; Murphy, J.B.; Taylor, K.G. Sedimentology and Oceanography of Early Ordovician Ironstone, Bell Island, Newfoundland: Ferruginous Seawater and Upwelling in the Rheic Ocean. Sediment. Geol. 2019, 379, 1–15. [Google Scholar] [CrossRef]
- Kharkwal, A.D. Glauconite in the Subathu Beds (Eocene) of the Simla Hills of India. Nature 1966, 211, 615–616. [Google Scholar] [CrossRef]
- Chowns, T.M. Depositional Environment of the Cleveland Ironstone Series. Nature 1966, 211, 1286–1287. [Google Scholar] [CrossRef]
- Choi, K.S.; Khim, B.K.; Woo, K.S. Spherulitic Siderites in the Holocene Coastal Deposits of Korea (Eastern Yellow Sea): Elemental and Isotopic Composition and Depositional Environment. Mar. Geol. 2003, 202, 17–31. [Google Scholar] [CrossRef]
- Lim, D.I.; Jung, H.S.; Yang, S.Y.; Yoo, H.S. Sequential Growth of Early Diagenetic Freshwater Siderites in the Holocene Coastal Deposits, Korea. Sediment. Geol. 2004, 169, 107–120. [Google Scholar] [CrossRef]
- Postma, D. Pyrite and Siderite Formation in Brackish and Freshwater Swamp Sediments. Am. J. Sci. 1982, 282, 1151–1183. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy Choudhury, T.; Singh, P.; Chakraborty, A.; Banerjee, S. Authigenic Fe Mineralization in Shallow to Marginal Marine Environments: A Case Study from the Late Paleocene—Early Eocene Cambay Shale Formation. Minerals 2023, 13, 646. https://doi.org/10.3390/min13050646
Roy Choudhury T, Singh P, Chakraborty A, Banerjee S. Authigenic Fe Mineralization in Shallow to Marginal Marine Environments: A Case Study from the Late Paleocene—Early Eocene Cambay Shale Formation. Minerals. 2023; 13(5):646. https://doi.org/10.3390/min13050646
Chicago/Turabian StyleRoy Choudhury, Tathagata, Pragya Singh, Arpita Chakraborty, and Santanu Banerjee. 2023. "Authigenic Fe Mineralization in Shallow to Marginal Marine Environments: A Case Study from the Late Paleocene—Early Eocene Cambay Shale Formation" Minerals 13, no. 5: 646. https://doi.org/10.3390/min13050646
APA StyleRoy Choudhury, T., Singh, P., Chakraborty, A., & Banerjee, S. (2023). Authigenic Fe Mineralization in Shallow to Marginal Marine Environments: A Case Study from the Late Paleocene—Early Eocene Cambay Shale Formation. Minerals, 13(5), 646. https://doi.org/10.3390/min13050646





