Abstract
Ferromanganese (Fe-Mn) crusts are potential marine deposits for many high-tech metals and are exciting proxies for recording the oceanic paleoenvironment. During their growth, phosphatization generally occurs, causing the remobilization and reorganization of the elements and minerals in Fe-Mn crusts. Rare earth elements plus yttrium (REY), well-known critical metals for many new and emerging technologies, as well as valuable geological proxies, are the important critical metals in Fe-Mn crusts. The REY occurrence is closely influenced by the phosphatization processes, which still remain discursive. In this study, the textures, structures, and REY geochemistry of the growth of an Fe-Mn crust sample (MP2D32A) from the Line Islands archipelago were analyzed using multiple microanalysis methods. The analyzed Fe-Mn crust is mainly characterized by the presence of laminated and concentric colloforms. Massive fine particles and some veins of carbonate-rich fluorapatite (CFA) were observed in the old part of MP2D32A, demonstrating that this sample underwent phosphatization. The phosphatized and non-phosphatized layers, as well as the CFA veins, display distinctly different PAAS-normalized REY patterns. Higher REY contents in the phosphatized layer than those in the non-phosphatized layer suggest the positive role of phosphatization in REY enrichment. Moreover, the phosphatized layer contains higher REY contents than the CFA, implying that the REY enrichment in the phosphatized layer is not only influenced by CFA and Fe-Mn (oxyhydr)oxides but also other factors, such as the probable PO43− complexation induced by Fe oxyhydroxides. The synergistical sorption of REY(III) and HPO42− ions on Fe oxyhydroxides should facilitate REY enrichment during the phosphatization processes. These fundamental results provide novel insights into the influence of phosphatization in REY geochemical behaviors in the Fe-Mn crust.
1. Introduction
Marine ferromanganese (Fe-Mn) crusts, as well as Fe-Mn nodules, are the most important polymetallic deep-sea Fe-Mn deposits, which are generally composed of nanosized Fe-Mn (oxyhydr)oxides [1,2]. The Fe-Mn crusts are formed globally due to precipitation of these Fe-Mn (oxyhydr)oxides on the surface of seamounts, ridges, and plateaus at a water depth of 400–7000 m [1,3,4]. These nanosized Fe-Mn (oxyhydr)oxides in the Fe-Mn crusts are commonly precipitated from seawater and are clarified into three major types of origin: hydrogenetic, diagenetic, and hydrothermal [5,6,7]. During Fe-Mn crust precipitation, the Mn oxides commonly take negative charges whereas the Fe (oxyhydr)oxides possess positive charges, giving rise to the accumulation of various charged metals (e.g., cobalt (Co), nickel (Ni), rare earth elements plus yttrium (REY)) from the ambient seawater [3,8,9,10]. Therefore, the Fe-Mn crusts are the potential deposits of numerous critical metals for many high- and green-technologies [4,11] and are further regarded as exciting proxies recording paleoenvironmental variability (e.g., chemical and redox environments) [3,12,13,14,15,16].
Generally, most of the Fe-Mn crusts underwent phosphatization [17,18,19,20], causing changes in the elements and minerals of the primary crust precipitate [18], such as the occurrence of carbonate-rich fluorapatite (CFA, Ca9.54Na0.33Mg0.13(PO4)4.8(CO3)1.2F2.48 [21]) in the old phosphatized part of the Fe-Mn crusts [16,18,22,23,24]. The Pacific Ocean is the primary area (e.g., Magellan Seamounts and Line Islands archipelago) for the occurrence of Fe-Mn deposits [3,16,25], where over 80% of Fe-Mn crusts around the world are found [26]. In the Pacific Ocean, there are two major episodes of phosphatization: late Eocene/early Oligocene (39–34 Ma) and late Oligocene/early Miocene (27–21 Ma), as demonstrated by the strontium and oxygen isotope measured in the CFA from nineteen Fe-Mn crust samples collected from the Central Pacific [19,27]. In addition, three minor phosphatization events might also happen at approximately 71, 31, and 15 Ma [19]. The fluctuations in climate, sea level, CO2 fluxes, and bottom water circulation may drive the cycles of enrichment and depletion of the phosphorus reservoir, leading to the phosphatization and CFA precipitation [19]. For example, the phosphatized episode of the late Eocene/early Oligocene (39–34 Ma) was characterized by the transition from a warm equitable climate with sluggish oceanic circulation of the Cretaceous to middle Eocene, to a cool and more arid climate with vigorous oceanic circulation in the Oligocene [19,28]. As a direct result of phosphatization, hiatuses commonly occur during the growth of Fe-Mn crusts [18,19].
The occurrence of REY, which are not only high-tech metals but also useful proxies in geological events [5], could be significantly altered after the Fe-Mn crusts underwent phosphatization [16,18,20]. The phosphatization processes could lead to the remobilization and reorganization of elements (e.g., REY) and minerals (e.g., Fe-Mn (oxyhydr)oxides) in the Fe-Mn crusts [16,18,20]. For example, the suboxic conditions in the phosphate-rich seawater caused greater REY enrichment in the older phosphatized layer compared with the younger non-phosphatized layer in the Fe-Mn crusts [18,27]. Further, the enrichment of heavy rare earth elements (HREE) can be even more notable [20]. However, the opposite conclusion was drawn by [29], who reported a lower content of REY in the old phosphatized layer than that in the young non-phosphatized layer. Hence, the influence of phosphatization on REY occurrence in Fe-Mn crusts needs further clarification.
In this study, the REY geochemical characteristics of the non-phosphatized layer, phosphatized layer, and CFA veins in the Fe-Mn crust (MP2D32A) from the Line Islands archipelago were investigated using an electron probe microanalyzer (EPMA) and laser ablation inductively coupled plasma mass spectrometer (LA-ICP-MS). With the analysis of the textural and structural characteristics of non-phosphatized and phosphatized layers, the influence of phosphatization in REY geochemical characteristics in the MP2D32A sample was discussed. The obtained findings are significant to the occurrence of REY in phosphatized Fe-Mn crusts, the role of phosphatization in REY geochemical behaviors, and the further use of REY in Fe-Mn crusts as proxies to investigate the environmental variability of the ocean over millions of years.
2. Materials and Methods
2.1. Geological Setting and Sample
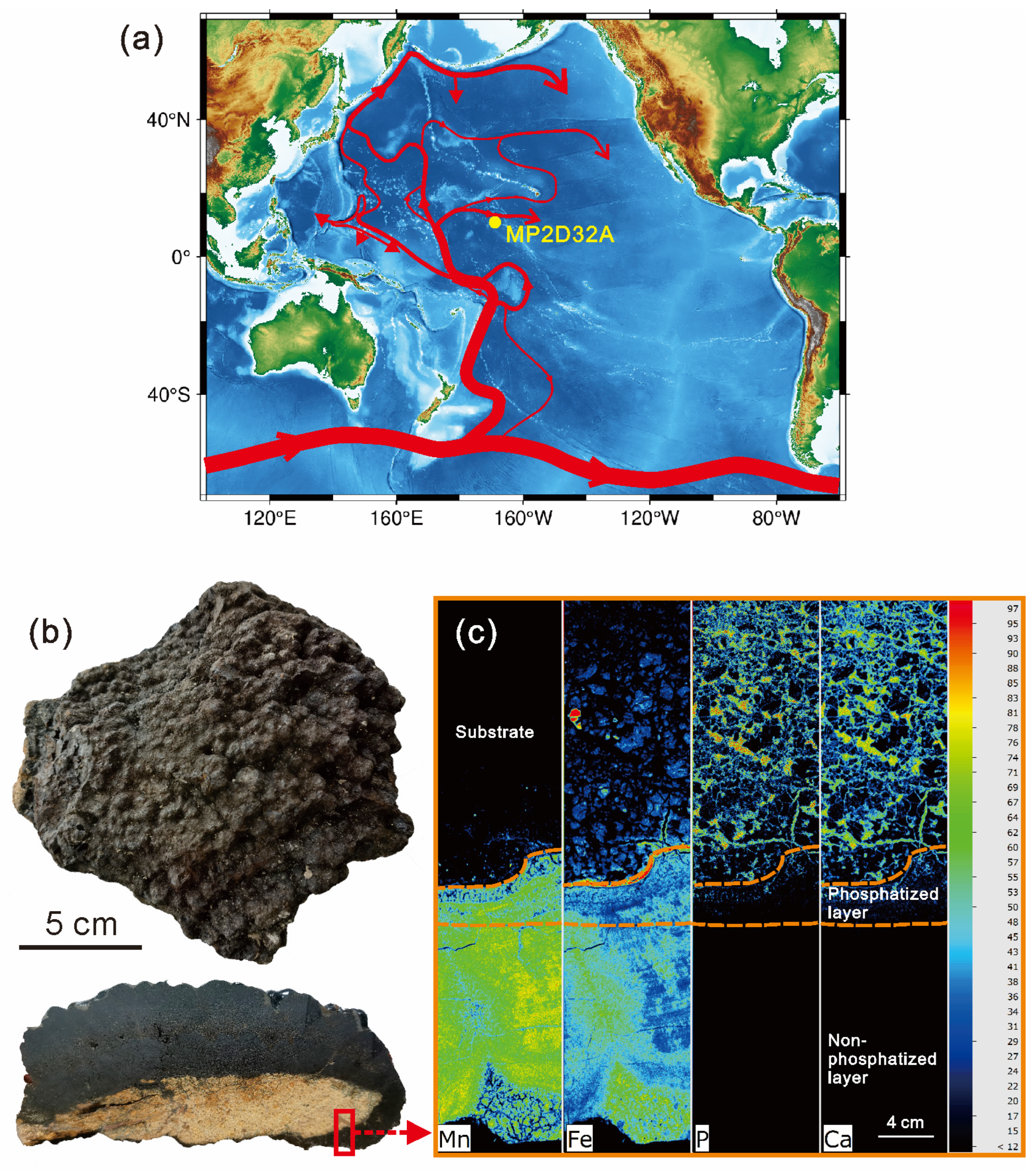
The studied Fe-Mn crust was collected from the Line Islands archipelago (169.62 W, 10.65 N, Figure 1) at a water depth of ~2000 m through the marine survey ship of Guangzhou Marine Geology Bureau. Line Islands archipelago is one of the most widely known areas where Fe-Mn crusts occur [26]. Importantly, Fe-Mn crusts in the Line Islands archipelago are generally strongly influenced by phosphatization [18,19,25,27,30] and are appropriate for investigating the influence of phosphatization in REY enrichment. The Line Islands archipelago with a general northwest–southeast trend along its 4500 km length, which is the result of hotspots (e.g., Crough, Marquesas, Tahiti) [31], is located in the central Pacific Ocean [19,25]. The Line Islands archipelago is a collection of seamounts and volcanic ridges, whose eruption ages generally range from 88 to 50 Ma [31]. The trends of the 87Sr/86Sr versus 206Pb/204Pb indicate a similar mantle source [31,32].
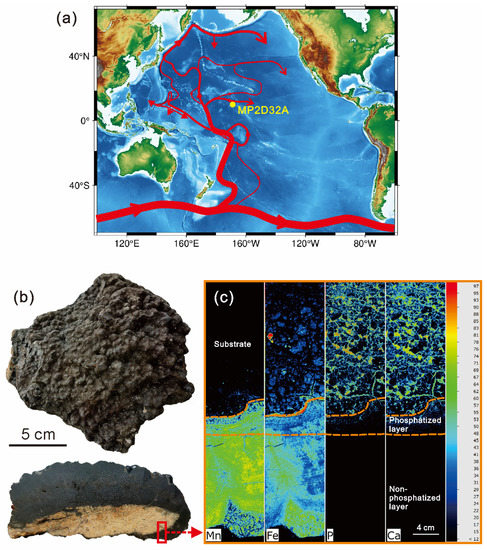
Figure 1.
(a) Sampling location. The red line in (a) indicates the Lower Circumpolar Deep Water (LCDW) after [33]; (b) photographs of the sample and its cross-section; (c) elemental mapping of Mn, Fe, P, and Ca within the red rectangle in (b).
The phosphatization occurs in the old part of MP2D32A (Figure 1c), as demonstrated by the elemental mapping using micro X-ray fluorescence (µ-XRF) and the observation of a scanning electron microscope (SEM). Massive fine particles and some veins of CFA were found in the old part of MP2D32A. Hence, a polished thin cross-section of MP2D32A from the indicated rectangular area was analyzed using the SEM, EMPA, and LA-ICP-MS, to obtain the characteristics of textures and structures as well as the chemical contents of major elements and REY.
2.2. Methods
The elemental mappings of Mn, Fe, P, and Ca shown by red rectangle in Figure 1 were conducted using an M4 Plus µ-XRF instrument equipped with two XFlash Silicon Drift Detectors and a current of 300 µA. The analyzed spot size was 20 µA with 3 mbar vacuum applied.
The backscattered electron (BSE) images were obtained using TESCAN MIRA 3LMH field-emission scanning electron microscope with an accelerating voltage of 15 kV.
Line profiles of chemical compositions of the major elements and REY were obtained by EPMA and LA-ICP-MS. A total 59 points (65 including CFA vein) were selected in the line with an average interval of ~230 µm. The analyzed spots are shown in Figure S1. The chemical composition of major elements was analyzed with a JEOL JXA-iSP-100 EMPA equipped with five wavelength-dispersive spectrometers (WDS). Operating conditions for quantitative WDS analyses were an accelerating voltage of 15 kV, a beam current of 10 nA, and a spot size of 5 µm. Calibration was achieved by using natural and synthetic minerals and oxides standards (e.g., Si, Mg, and Ca: diopside; Al: pyrope; K: orthoclase; Na: jadetite; Ti: rutile; Mn: rhodonite; Fe: hematite; Co: CoO (synthetic)) and ZAF correction scheme. The overall analytical uncertainty (including instrumental repeatability and calibration errors) was typically <3% relative to elements at concentration levels of >3% m/m oxide. The REY contents were measured by LA-ICP-MS with an NWR 193 laser ablation system for laser sampling. An iCAP RQ ICP-MS instrument was used to acquire ion-signal intensities. Helium was applied as a carrier gas. Argon was the make-up gas and mixed with the carrier gas via a Y-connector before entering the ICP. The spot size and frequency of the laser in the measurement were set to 30 µm and 6 Hz, respectively. The analysis was conducted at or near the EMPA spot locations. In the calibration, SRM610, SRM612, and BHVO-2G were applied as external standards, while the elemental total contents obtained by EPMA were used as the internal standards. The EMPA data were only used for the calibration of LA-ICP-MS data and were not discussed in this study.
Fourier transform infrared spectroscopy (FTIR) of the non-phosphatized layer in MP2D32A was recorded by a Bruker Vertex 70 IR spectrometer. The specimens were prepared by mixing 0.9 mg of powder sample and 80 mg of KBr, followed by pressing the mixtures into pellets. A pure KBr pellet was measured as the background. All spectra were collected over 64 scans in the range of 4000–400 cm−1 at a resolution of 4 cm−1.
3. Results
3.1. Textural and Structural Characteristics
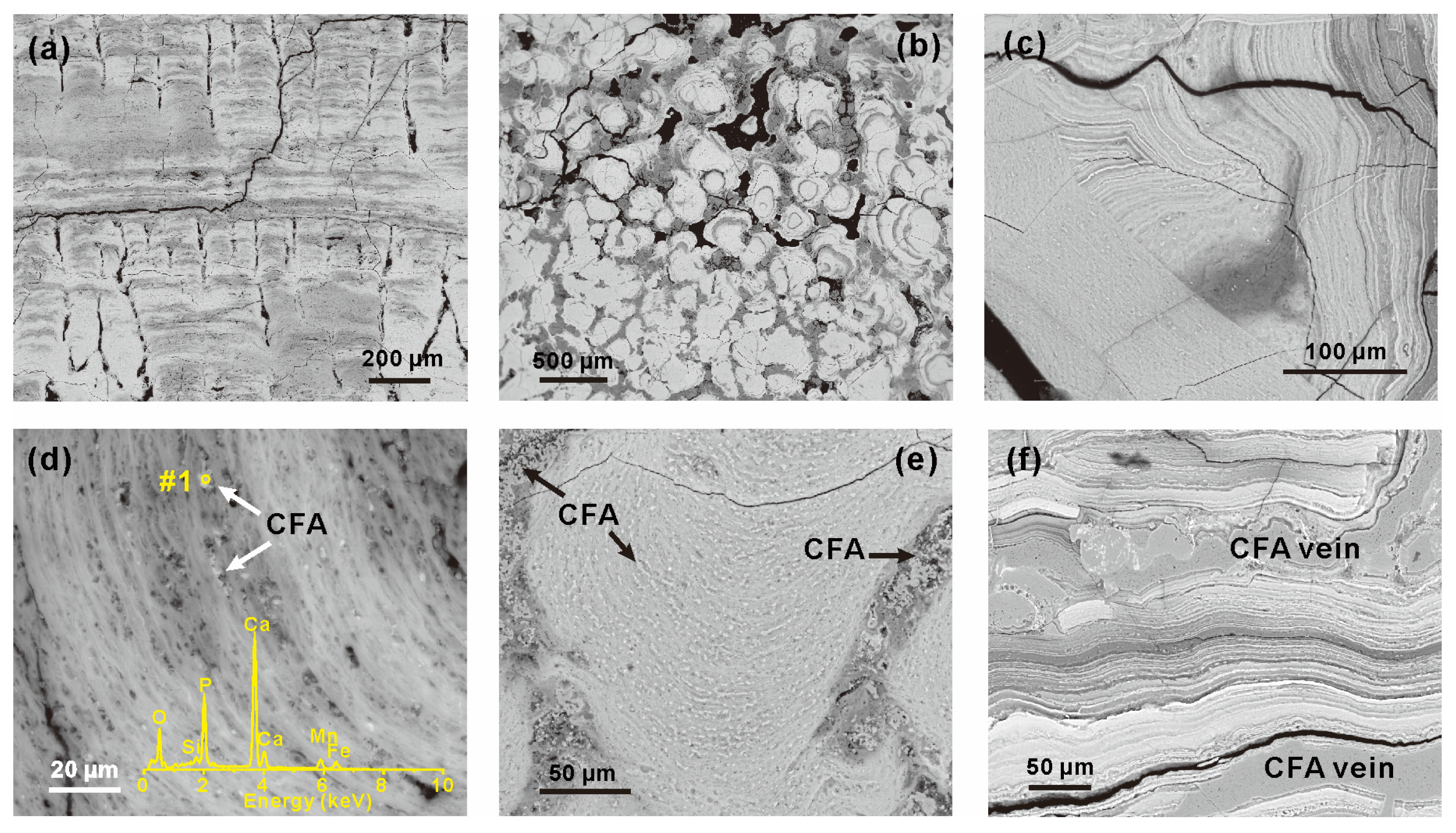
Textural and structural characteristics in the Fe-Mn crust and its substrate of MP2D32A are shown in Figure 2 and Figure S2, respectively. The Fe-Mn (oxyhydr)oxides in MP2D32A mainly occur as horizontally laminated (Figure 2a) and concentric (Figure 2b) colloforms. At the bottom of the Fe-Mn crust, the structures are complex, such as the parallel unconformity in Figure 2c. The bottom of the Fe-Mn crust displays alternating dark and bright layers with a thickness of submicron to microns, as identified by the high contrast in BSE images (Figure 2c). In addition to these distinctly dark and bright submicron layers (SMLs), the crust also exhibits indistinct growth submicron layers (Figure 2a,b,d). In these indistinct SMLs, there are many CFA particles protruding from empty spaces and pores (Figure 2d) and their interlayer (Figure 2e). Besides, many CFA particles and aluminosilicate minerals exist between different structures altogether (Figure 2e). Moreover, CFA veins were also observed (Figure 2f). These fine particles and veins of CFA are distributed at the bottom of MP2D32A, indicating that the old part of MP2D32A underwent phosphatization processes. The phosphatized layer is mainly composed of distinct and indistinct SMLs, whereas the non-phosphatized layer primarily consists of columnar structures (Figure 2a) that are constitutive of indistinct SMLs (Figure 2a,b). In addition, the substrate was primarily composed of aggregations of platy clay minerals (e.g., smectite), rodlike phillipsite, and CFA (Figure S2). The occurrence of smectite and phillipsite suggests the possible volcanic tuffs as parent rocks of the substrate.
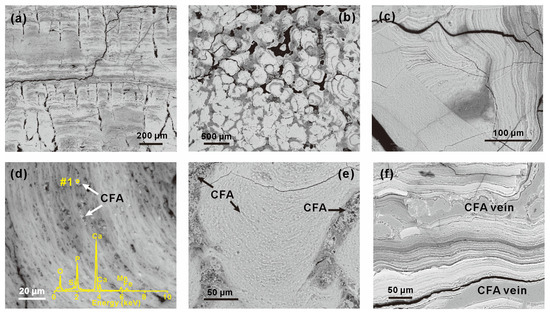
Figure 2.
BSE images of structures identified in MP2D32A. (a) Laminated group of colloforms; (b) concentric colloforms; (c) massive laminated colloforms with visible internal cracking; (d) indistinct colloforms protruded by CFA particles; the insert is the EDS spectrum of spot #1; (e) fine CFA particles in groups of colloforms and holes between different structures; (f) CFA veins in the bottom of sample analyzed.
3.2. REY Geochemistry
The chemical composition of MP2D32A from surface to bottom was compiled in Table S1, and the average contents of various elements in non-phosphatized and phosphatized layers are shown in Table 1. The weight contents of Mn and Fe in the non-phosphatized layer are 19.65–32.50 wt% and 7.34–19.65 wt%, respectively (Table S1). Their corresponding average contents are 27.22 wt% and 12.33 wt%, respectively (Table 1). In the phosphatized layer, the Mn contents range from 19.58 wt% to 28.06 wt%, with an average of 23.60 wt%, which is slightly lower compared with the non-phosphatized layer; the Fe contents vary from 4.93 wt% to 14.09 wt% with an average of 10.48 wt%. Similarly, the phosphatized layer shows lower Fe contents than the non-phosphatized layer (Table 1).

Table 1.
Mean contents of major elements and REY in non-phosphatized layer, phosphatized layer, and CFA vein in MP2D32A sample (LA-ICP-MS data in Table S1).
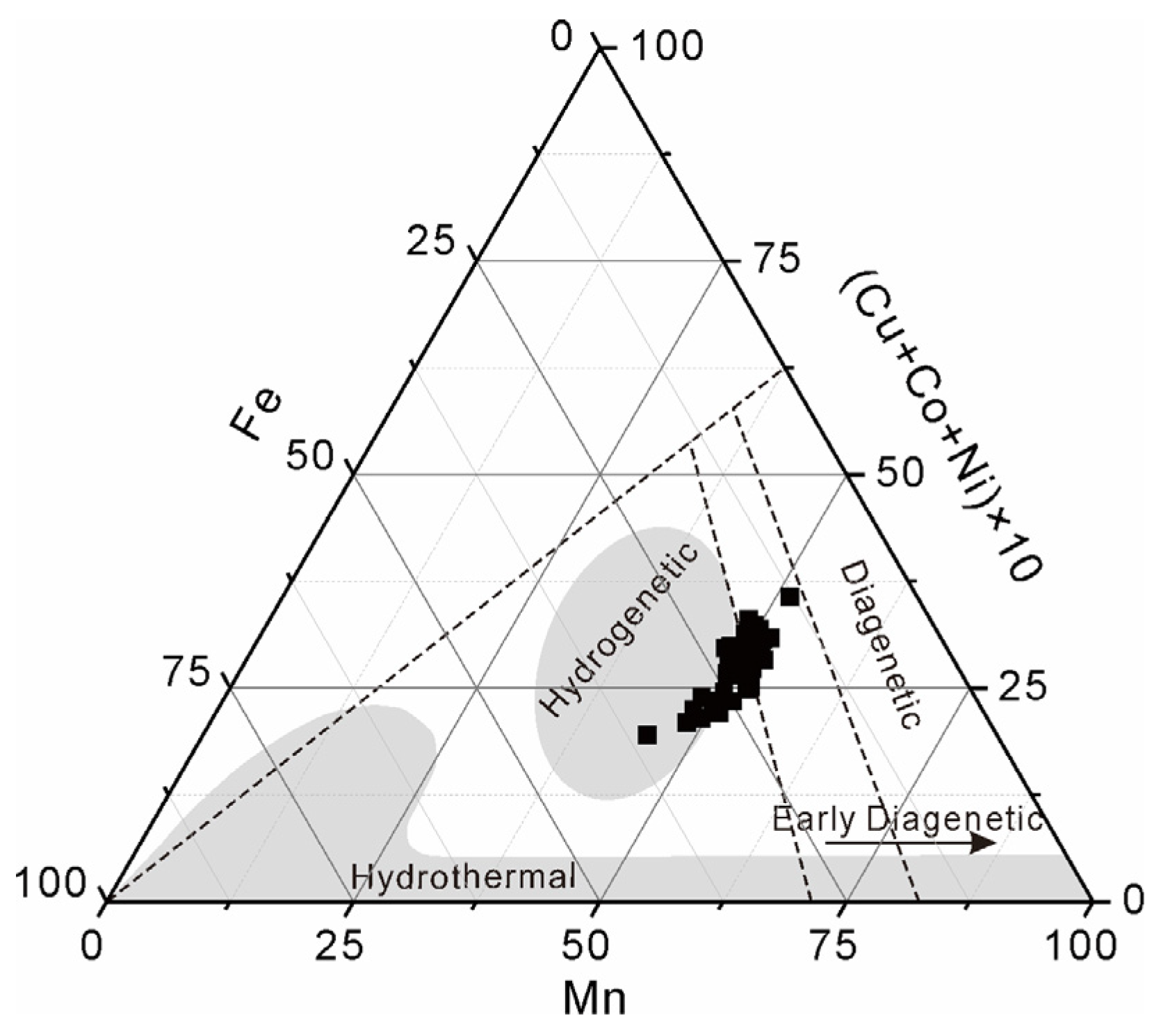
The average contents of Co, Ni, and Cu in the non-phosphatized layer of MP2D32A are 0.85 wt%, 0.56 wt%, and 0.08 wt%, respectively (Table 1). Based on the contents of Mn, Fe, and Ni-Co-Cu (Table S1), a traditional ternary discrimination diagram was used to identify the general formation mechanisms of Fe-Mn crusts [34,35,36,37]. This traditional ternary discrimination diagram of MP2D32A is shown in Figure 3, which suggests that the MP2D32A is primarily formed through hydrogenetic process.
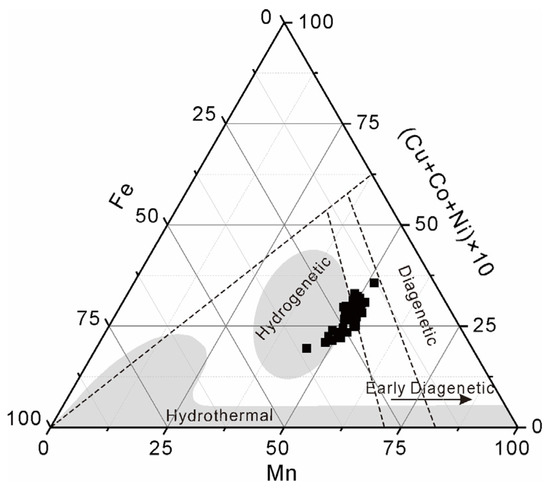
Figure 3.
Ternary discrimination diagram of Mn versus Fe versus (Ni-Co-Cu) × 10 in non-phosphatized layer of MP2D32A sample (LA-ICP-MS data in Table S1).
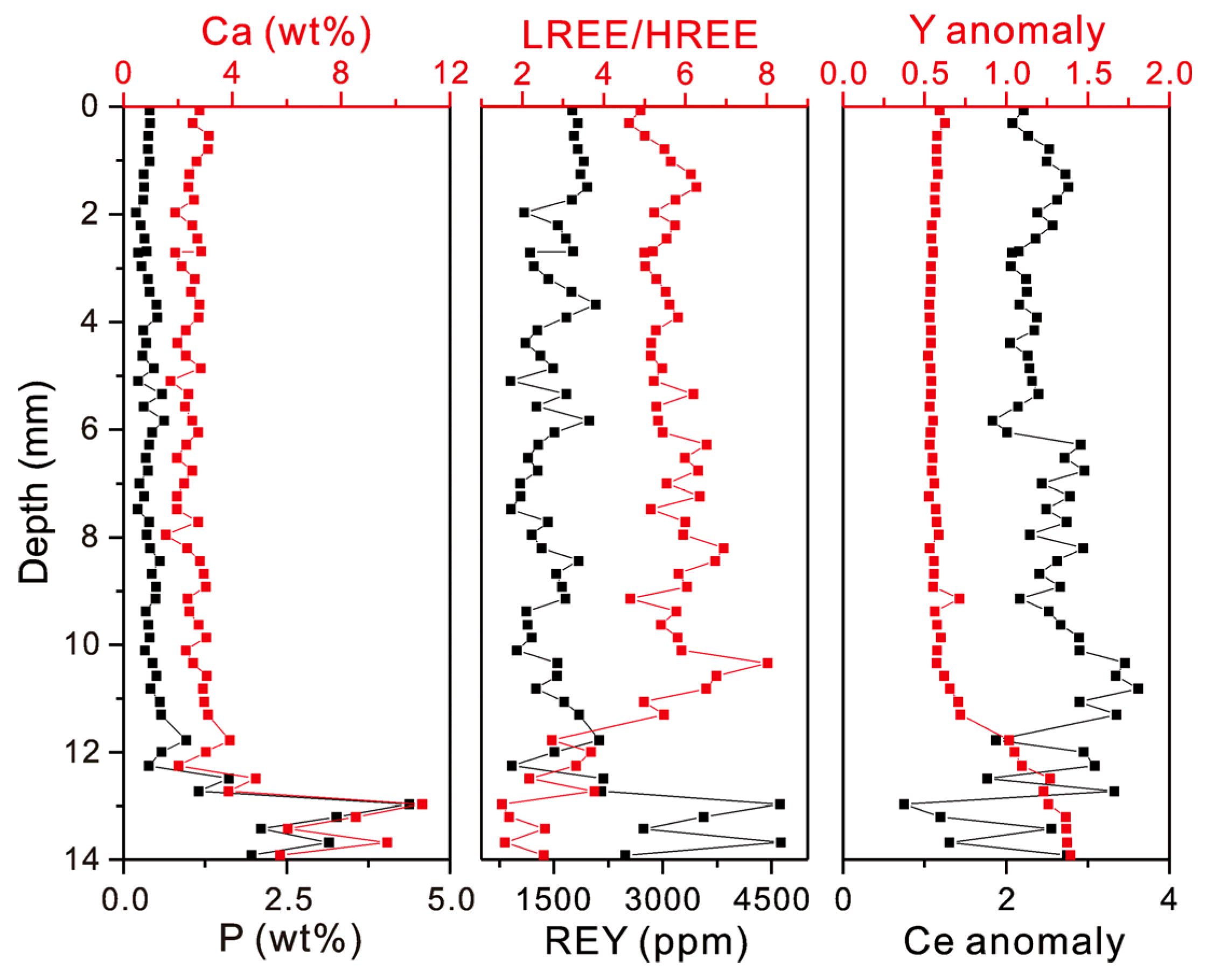
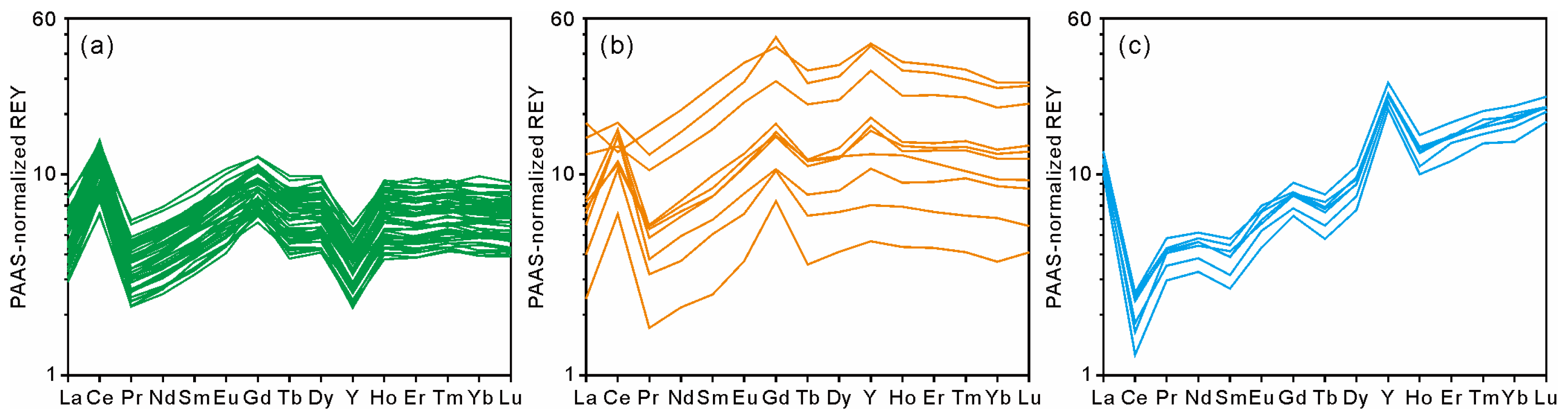
The profiles of weight contents of P, Ca, and REY, as well as the LREE/HREE ratios and Ce and Y anomalies, in the MP2D32A show that the phosphatization occurred at the bottom part of the Fe-Mn crust with a depth of ~12–14 mm (Figure 4). The average contents of P and Ca in the non-phosphatized layer are 0.38 wt% and 2.51 wt%, respectively. In the phosphatized layer, the average contents of P and Ca increase to 1.96 wt% and 5.87 wt% (Table 1), respectively. Similarly, the REY content, LREE/HREE ratio, CeN/CeN*, and YN/YN* also display distinct differences between the non-phosphatized and phosphatized layers (Figure 4). The phosphatized layer shows higher REY contents and YN/YN* and lower LREE/HREE ratios and CeN/CeN* than the non-phosphatized layer. It should be noted that the phosphatized region was ascertained not only according to P and Ca contents but also the Y and Ce anomalies in this study. The PAAS-normalized REY patterns demonstrate that CFA displays positive Y and negative Ce anomalies and Fe-Mn (oxyhydr)oxides exhibit negative Y and positive Ce anomalies (Figure 5). Although P contents at a depth of 11.77 to 12.25 mm are low (Table S1), the distinct changes in Y and Ce anomalies (i.e., decreasing CeN/CeN* value and increasing YN/YN* value) at the depth of 11.77 mm indicate that phosphatized region is at the depth from 11.77 mm to 13.91 mm.
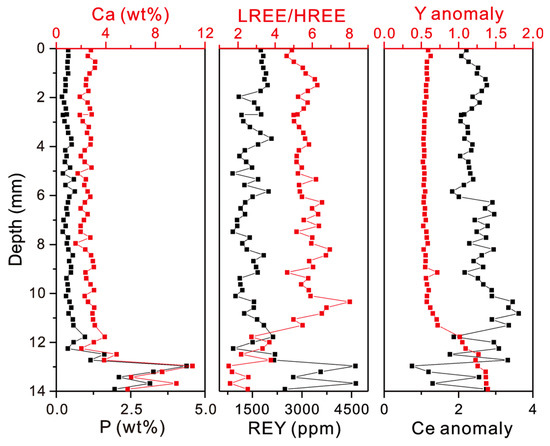
Figure 4.
Profiles of weight contents of Ca, P, and REY, LREE/HREE ratios, and Ce and Y anomalies.
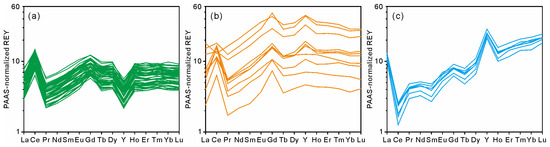
Figure 5.
PAAS-normalized REY patterns of non-phosphatized crust layer (a), phosphatized crust layer (b), and CFA veins (c).
The REY patterns of MP2D32A normalized by the Post Archean Australian Shale (PAAS) are shown in Figure 5. The PAAS-normalized REY patterns in the non-phosphatized layer are similar (Figure 5a), which display the positive Ce anomaly with the average CeN/CeN* value of 2.52 and the negative Y anomaly with the average YN/YN* value of 0.57 (Table 1). Compared with the non-phosphatized layer, the phosphatized layer has different PAAS-normalized REY patterns (Figure 5b), which displays the positive Y anomaly with the average YN/YN* value of 1.24 (Table 1). Although most of the data from the phosphatized layer show a positive Ce anomaly (Figure 5b), the CeN/CeN* ratios decrease with the increasing REY and P contents (Figure 4). Moreover, the measured plot with the highest REY content shows a negative Ce anomaly (Figure 4 and Figure 5b). In addition, both non-phosphatized and phosphatized layers exhibit slight negative Eu anomalies with similar average EuN/EuN* values of 0.96 and 0.91, respectively. The PAAS-normalized REY patterns of the CFA veins (Figure 5c) exhibit HREE enrichment, likely causing the decreasing LREE/HREE ratios in the phosphatized layer (Figure 4).
4. Discussion
4.1. Chemical Species of Phosphorus in MP2D32A Sample
P is an important element composing Fe-Mn crusts. Many Fe-Mn crusts commonly underwent phosphatization [17,18,19,20] and generally contain CFA minerals, whose content could be up to 30% in their old phosphatized parts [16]. Many fine particles and some veins of CFA were observed in the phosphatized layer (Figure 2), revealing that authigenic CFA is one type of P chemical species (i.e., mineral phase) in the MP2D32A sample.
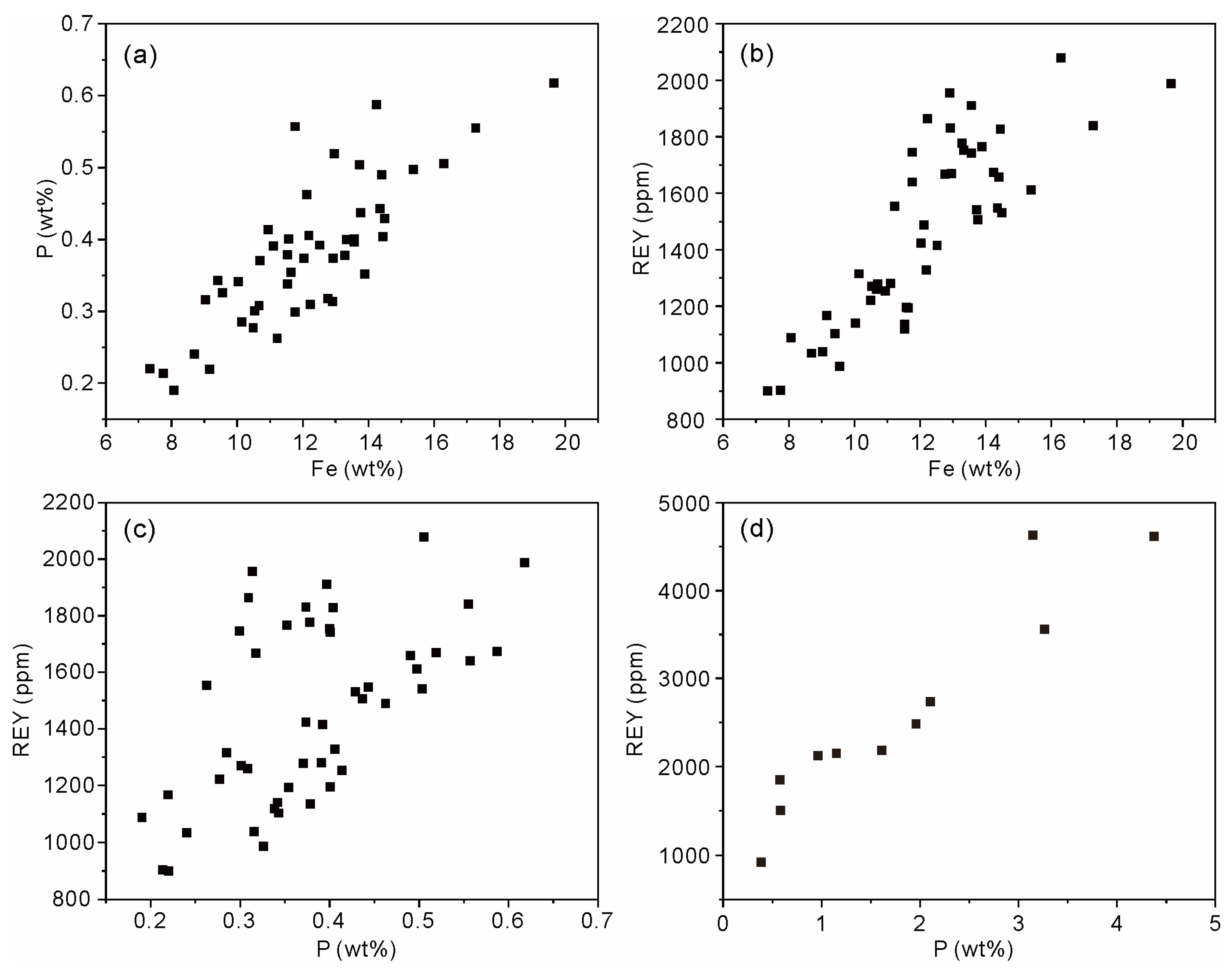
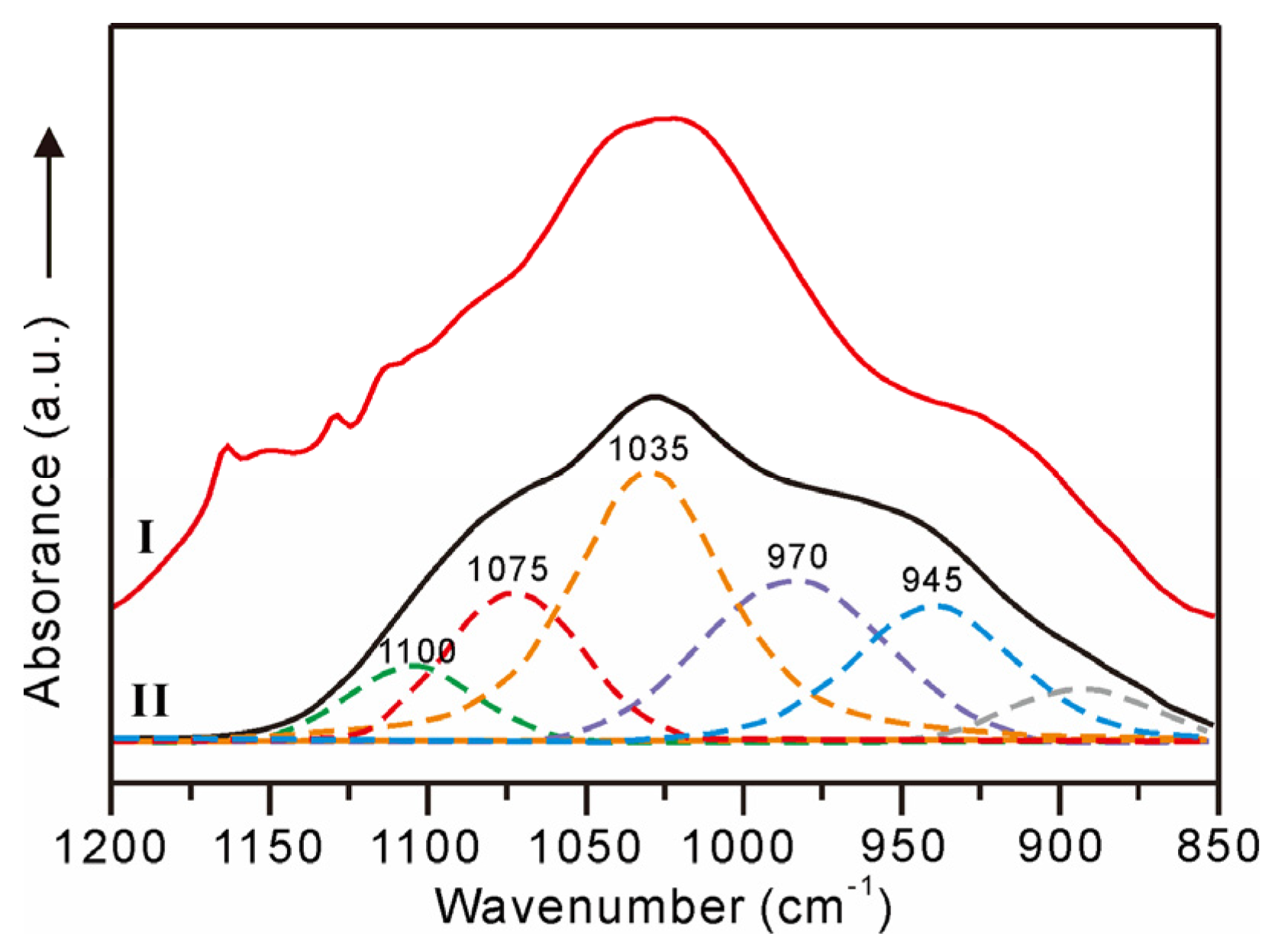
Apart from the CFA, Fe-bound P is the principal carrier of solid phase P in oceans [38]. In seawater, the major species of dissolved P, i.e., HPO42− ions [38], can be effectively adsorbed by the Fe oxyhydroxides through electrostatic attraction and surface complexation (e.g., monoprotonated bidentate, non-protonated bidentate, and outer-sphere complexes) [39,40,41]. In the non-phosphatized layer of MP2D32A, there is no CFA mineral, whereas the weight content of P ranges from 0.19% to 0.62% with an average of 0.38%. Meanwhile, the weight contents of P and Fe display a distinct positive correlation (Figure 6a), indicating the possible PO43− complexed with Fe oxyhydroxides in the non-phosphatized layer, which is demonstrated by the FTIR spectrum. The FTIR spectra of the non-phosphatized layer and ferrihydrite complexed with PO43− ions at pH 7 are similar (Figure 7). The HPO42− ions in seawater are adsorbed on ferrihydrite through the inner-sphere complex with C2v symmetry (1075, 1035, and 945 cm−1) and outer-sphere complex with C3v symmetry (1100 and 970 cm−1) [40]. Therefore, the PO43− complexed with ferrihydrite should be the main P species in the non-phosphatized layer. Given the high HPO42− ionic concentration in seawater during the phosphatization process [18], PO43− complexed with Fe (oxyhydr)oxides, as well as CFA, should be one type of P species in the phosphatized layer of the MP2D32A sample.
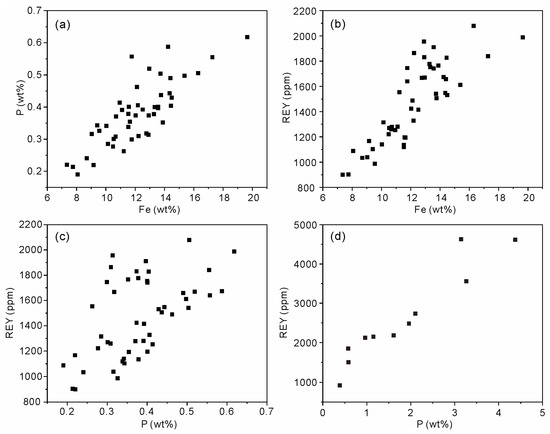
Figure 6.
Content correlation of P versus Fe (a), REY versus Fe (b), and REY versus P (c) of the non-phosphatized layer, and REY versus P (d) of the phosphatized layer in MP2D32A sample.
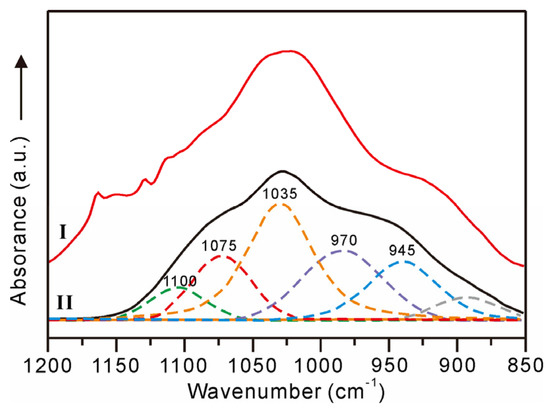
Figure 7.
FTIR spectra of non-phosphatized layer (I) and ferrihydrite complexed with PO43– ions (II) [41].
4.2. Influence of Phosphatization in REY Enrichment
During the process of phosphatization, the suboxic P-rich seawater infiltrated the crusts, leading to a partial redissolution of Fe-Mn (oxyhydr)oxides with the mobilization of associated elements [18]. Meanwhile, the phosphatization processes also form vast CFA particles (Figure 2d,e), which could enrich REY [42] and further influence the REY occurrence in the Fe-Mn crusts. The higher REY contents in the phosphatized layer than those in the non-phosphatized layer of MP2D32A (Table 1 and Figure 3) indicate that the phosphatization facilitated the enrichment of REY.
Previous studies have shown that REY could be more enriched in the old phosphatized crust layer than in the young non-phosphatized crust layer [18,27], corresponding to the results of this study. The positive role of phosphatization in REY enrichment is generally due to the presence of CFA, which generally occurs as holocrystalline aggregates, phosphatized biogenic carbonates, apatite interlayers and veinlets, and fine-dispersed particles [42,43,44,45]. For example, the total REY content could reach up to 6016 ppm in the CFA vein in Fe-Mn crusts from the western Pacific Ocean [42]. However, the total REY contents of CFA veins are lower than those in the phosphatized layer in MP2D32A (Table 1), implying that the CFA seems to have no effect on REY enrichment in MP2D32A.
In the older phosphatized layer of MP2D32A, the Fe-Mn (oxyhydr)oxides, as well as the CFA particles, are the main carriers of REY. In general, the dominant control factors for the accumulating REY by Fe-Mn (oxyhydr)oxides are the mineral surface charge, concentration, and chemical speciation of REY(III) ions, the stability of surface complexes, the surface oxidation reactions, the specific-surface area, and growth rates [17]. In seawater, the REY(III) ions generally exist as carbonate complexes [46]. For example, a second-order carbonate complex and a first-order carbonate complex are the predominant forms of HREE(III) ions (i.e., HREE(CO3)2–) and LREE(III) ions (i.e., LREE(CO3)+), respectively [46]. The nanosized Fe oxyhydroxides (e.g., ferrihydrite and feroxyhyte) with a higher isoelectric point than the pH value of seawater take positive charges [1,17], while the nanosized Mn oxides take negative charges [1]. Hence, the two types of REY(III) complexes with opposite charges, i.e., HREE(CO3)2–) and LREE(CO3)+, would mainly interact with Fe oxyhydroxides and Mn oxides, respectively [8]. Nevertheless, the average REY concentration (1470.82 ppm) in the non-phosphatized crust layer is lower than that (2690.88 ppm) in the phosphatized crust layer (Table 1), suggesting other factors, in addition to CFA and Fe-Mn (oxyhydr)oxides, in REY enrichment in the phosphatized layer of MP2D32A.
Both in the non-phosphatized and phosphatized layers of MP2D32A, the REY contents increase with increasing P contents (Figure 6c,d). The positive correlation between REY and P suggests that the PO43– complexed with Fe oxyhydroxides, as well as CFA, may play some role in REY accumulation in the Fe-Mn crust. In seawater, the major species of dissolved P, i.e., HPO42– ions [38], can be effectively adsorbed by the Fe oxyhydroxides through electrostatic attraction and surface complexation (e.g., monoprotonated bidentate, non-protonated bidentate, and outer-sphere complexes) [39,40,41]. Previous studies demonstrated the cooperative adsorption of cations and HPO42− ions on Fe oxyhydroxides to facilitate cation accumulation [40,41], suggesting a high HPO42− ionic concentration and positively charged REY(III) (e.g., LREE(CO3)+) ions in the seawater could be adsorbed on Fe oxyhydroxides synergistically during the phosphatization process. This synergistic adsorption process seems to be able to further facilitate the accumulation of REY(III) ions. Additionally, the surface charge of Fe oxyhydroxides could decrease, retarding the accumulation of negatively charged HREE(CO3)2– ions. Given the multiple chemical species of REY(III) ions with different charges in seawater, the complexation of HPO42− ions with Fe oxyhydroxides probably results in REY fractionation in Fe-Mn crusts.
In comparison with LREE/HREE ratios, the Ce anomaly is strongly influenced by phosphatization. The Ce/Ce* ratios decrease from the non-phosphatized layer to the phosphatized layer and display a negative correlation with the REY concentration in the phosphatized layer (Figure 4). Because Fe-Mn oxyhydroxides can adsorb the Ce(III) ions and oxidize to Ce(IV) [47,48], the non-phosphatized layer generally shows positive Ce anomalies (Figure 4). Nevertheless, the CFA and Fe oxyhydroxides-complexed PO43–, both of which likely possess similar REY geochemical characteristics with seawater (Figure 5), display a strongly negative Ce anomaly. Hence, the Ce/Ce* ratios would decrease with the increasing content of P and REY (Figure 4).
5. Conclusions
In this study, the REY geochemical characteristics of the non-phosphatized and phosphatized layer in the Fe-Mn crust (MP2D32A) from the Line Islands archipelago were investigated. The obtained results show that the crust is characterized by laminated and concentric groups of colloforms, which are composed of distinct and indistinct growth layers with a thickness of submicron to microns. Massive fine particles and veins of CFA demonstrate that the MP2D32A underwent phosphatization processes. The REY content and the PAAS normalized patterns between the phosphatized and non-phosphatized layers are distinctly different, indicating that phosphatization influences REY enrichment in MP2D32A. Higher REY contents in the phosphatized layer than those in the non-phosphatized layer and the CFA suggest the positive role of phosphatization in REY enrichment and imply that the REY enrichment in the phosphatized layer is not only controlled by CFA and Fe-Mn (oxyhydr)oxides but also other factors, such as the probable Fe oxyhydroxides complexed PO43−. The synergistical sorption of REY(III) and HPO42− ions on Fe oxyhydroxides likely facilitate REY enrichment during the phosphatization processes. These fundamental results provide novel insights into the influence of phosphatization in REY geochemical behaviors in the Fe-Mn crust.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min13050647/s1, Table S1: Chemical contents of major elements and REY in MP2D32A. Figure S1: Analyzed spots of LA-ICP-MS in the photograph obtained by optical microscope. Figure S2: BSE images of smectite (a), phillipsite (b), and CFA (c) in the substrate of MP2D32A.
Author Contributions
Conceptualization, J.Z. and S.Y.; validation, J.Z.; formal analysis, J.Z. and J.C.; investigation, J.Z.; resources, Z.W.; writing—original draft preparation, J.Z.; writing—review and editing, S.Y., Y.L., D.T., and G.H.; supervision, S.Y.; project administration, J.Z. and Y.D.; funding acquisition, J.Z. and S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Project of Guangdong Province (2020B1111510001), the China Postdoctoral Science Foundation (2022M720888), the National Natural Science Foundation of China (U2244224 and U2244222), and the China Geological Survey (DD20221718).
Data Availability Statement
The data presented in this study are available within the article and Table S1.
Acknowledgments
We are grateful to the Guangzhou Marine Geological Survey for supplying the samples. We thank Guangzhou Tuoyan Analytical Technology Co., Ltd., Guangzhou, China for the measurement of LA-ICP-MS. Finally, we thank anonymous reviewers for their thoughtful and thorough suggestions/reviews that greatly assisted in the improvement of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halbach, P.E.; Jahn, A.; Cherkashov, G. Marine Co-Rich Ferromanganese Crust Deposits: Description and Formation, Occurrences and Distribution, Estimated World-wide Resources. In Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations; Sharma, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 65–141. [Google Scholar]
- Glasby, G.P. Manganese: Predominant Role of Nodules and Crusts. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 371–427. [Google Scholar]
- Koschinsky, A.; Hein, J.R. Marine Ferromanganese Encrustations: Archives of Changing Oceans. Elements 2017, 13, 177–182. [Google Scholar] [CrossRef]
- Toro, N.; Robles, P.; Jeldres, R.I. Seabed mineral resources, an alternative for the future of renewable energy: A critical review. Ore Geol. Rev. 2020, 126, 103699. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Josso, P.; Pelleter, E.; Pourret, O.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Bollinger, C. A new discrimination scheme for oceanic ferromanganese deposits using high field strength and rare earth elements. Ore Geol. Rev. 2017, 87, 3–15. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, P.; Yang, C.; Liu, S.; Luo, W.; Nie, X. Geochemical characteristics and genesis of ferromanganese nodules and crusts from the Central Rift Seamounts Group of the West Philippine Sea. Ore Geol. Rev. 2022, 145, 104923. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Guan, Y.; Xiao, Z.; Liu, Y.; Liao, J.; Guo, Z. Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe-Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study. Minerals 2019, 9, 8. [Google Scholar] [CrossRef]
- Kuhn, T.; Wegorzewski, A.; Rühlemann, C.; Vink, A. Composition, Formation, and Occurrence of Polymetallic Nodules. In Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations; Sharma, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–63. [Google Scholar]
- Lusty, P.A.J.; Murton, B.J. Deep-Ocean Mineral Deposits: Metal Resources and Windows into Earth Processes. Elements 2018, 14, 301–306. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Frank, M.; O’Nions, R.K.; Hein, J.R.; Banakar, V.K. 60 Myr records of major elements and Pb-Nd isotopes from hydrogenous ferromanganese crusts: Reconstruction of seawater paleochemistry. Geochim. Cosmochim. Acta 1999, 63, 1689–1708. [Google Scholar] [CrossRef]
- Josso, P.; van Peer, T.; Horstwood, M.S.A.; Lusty, P.; Murton, B. Geochemical evidence of Milankovitch cycles in Atlantic Ocean ferromanganese crusts. Earth Planet. Sci. Lett. 2021, 553, 116651. [Google Scholar] [CrossRef]
- Sutherland, K.M.; Wostbrock, J.A.G.; Hansel, C.M.; Sharp, Z.D.; Hein, J.R.; Wankel, S.D. Ferromanganese crusts as recorders of marine dissolved oxygen. Earth Planet. Sci. Lett. 2020, 533, 116057. [Google Scholar] [CrossRef]
- Schier, K.; Ernst, D.M.; de Sousa, I.M.C.; Garbe-Schönberg, D.; Kuhn, T.; Hein, J.R.; Bau, M. Gallium-aluminum systematics of marine hydrogenetic ferromanganese crusts: Inter-oceanic differences and fractionation during scavenging. Geochim. Cosmochim. Acta 2021, 310, 187–204. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-ocean ferromanganese crusts and nodules. Treatise Geochem. 2014, 13, 273–291. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.A.; Frank, M.; Christl, M.; Sager, W.W. Copper-nickel-rich, amalgamated ferromanganese crust-nodule deposits from Shatsky Rise, NW Pacific. Geochem. Geophys. Geosyst. 2012, 13, Q10022. [Google Scholar] [CrossRef]
- Koschinsky, A.; Stascheit, A.; Bau, M.; Halbach, P. Effects of phosphatization on the geochemical and mineralogical composition of marine ferromanganese crusts. Geochim. Cosmochim. Acta 1997, 61, 4079–4094. [Google Scholar] [CrossRef]
- Hein, J.R.; Yeh, H.-W.; Gunn, S.H.; Sliter, W.V.; Benninger, L.M.; Wang, C.-H. Two Major Cenozoic Episodes of Phosphogenesis Recorded in Equatorial Pacific Seamount Deposits. Paleoceanography 1993, 8, 293–311. [Google Scholar] [CrossRef]
- Josso, P.; Lusty, P.; Chenery, S.; Murton, B. Controls on metal enrichment in ferromanganese crusts: Temporal changes in oceanic metal flux or phosphatisation? Geochim. Cosmochim. Acta 2021, 308, 60–74. [Google Scholar] [CrossRef]
- Jahnke, R.A. The Synthesis And Solubility Of Carbonate Fluorapatite. Am. J. Sci. 1984, 284, 58–78. [Google Scholar] [CrossRef]
- Josso, P.; Rushton, J.; Lusty, P.; Matthews, A.; Chenery, S.; Holwell, D.; Kemp, S.J.; Murton, B. Late Cretaceous and Cenozoic paleoceanography from north-east Atlantic ferromanganese crust microstratigraphy. Mar. Geol. 2020, 422, 106122. [Google Scholar] [CrossRef]
- Ortiz Kfouri, L.; Millo, C.; Estela de Lima, A.; Silveira, C.S.; Sant’Anna, L.G.; Marino, E.; González, F.J.; Sayeg, I.J.; Hein, J.R.; Jovane, L.; et al. Growth of ferromanganese crusts on bioturbated soft substrate, Tropic Seamount, northeast Atlantic ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2021, 175, 103586. [Google Scholar] [CrossRef]
- Yang, K.; Park, H.; Son, S.K.; Baik, H.; Park, K.; Kim, J.; Yoon, J.; Park, C.H.; Kim, J. Electron microscopy study on the formation of ferromanganese crusts, western Pacific Magellan Seamounts. Mar. Geol. 2019, 410, 32–41. [Google Scholar] [CrossRef]
- Aplin, A.C.; Cronan, D.S. Ferromanganese oxide deposits from the Central Pacific Ocean, I. Encrustations from the Line Islands Archipelago. Geochim. Cosmochim. Acta 1985, 49, 427–436. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Yao, H.; Yang, Y.; Ren, J.; Guo, L.; Mei, Y. Global distribution characteristics of seafloor cobalt-rich encrustation resources. Miner. Depos. 2013, 32, 1275–1284. [Google Scholar]
- Ren, J.; He, G.; Yao, H.; Deng, X.; Zhu, K.; Yang, S. The Effects of phosphatization on the REY of Co-rich Fe-Mn crusts. Mar. Geol. Quat. Geol. 2017, 37, 33–43. [Google Scholar]
- Miller, K.G. 8. Middle Eocene to Oligocene Stable Isotopes, Climate, and Deep-Water History: The Terminal Eocene Event? In Eocene-Oligocene Climatic and Biotic Evolution; Donald, R.P., William, A.B., Eds.; Princeton University Press: Princeton, NJ, USA, 1992; pp. 160–177. [Google Scholar]
- Xiao, J.; He, J.; Yang, H.; Wu, C. Comparison between Datangpo-type manganese ores and modern marine ferromanganese oxyhydroxide precipitates based on rare earth elements. Ore Geol. Rev. 2017, 89, 290–308. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, N. The relationship between the growth discontinuity of polymetallic crusts and phosphatization events. Acta Oceanol. Sin. 2021, 43, 102–109. [Google Scholar]
- Pockalny, R.; Barth, G.; Eakins, B.; Kelley, K.A.; Wertman, C. Multiple melt source origin of the Line Islands (Pacific Ocean). Geology 2021, 49, 1358–1362. [Google Scholar] [CrossRef]
- Konter, J.G.; Hanan, B.B.; Blichert-Toft, J.; Koppers, A.A.P.; Plank, T.; Staudigel, H. One hundred million years of mantle geochemical history suggest the retiring of mantle plumes is premature. Earth Planet. Sci. Lett. 2008, 275, 285–295. [Google Scholar] [CrossRef]
- Kawabe, M.; Fujio, S. Pacific Ocean circulation based on observation. J. Oceanogr. 2010, 66, 389–403. [Google Scholar] [CrossRef]
- Bonatti, E.; Kraemer, T.; Harold, R. Classification and genesis of submarine iron-manganese deposits. In Ferromanganese Deposits on the Ocean Floor, 1st ed.; Horn, D.R., Ed.; IDOE Publ: Washington, DC, USA, 1972; pp. 149–166. [Google Scholar]
- Jiang, X.D.; Zhao, X.; Zhao, X.Y.; Chou, Y.M.; Roberts, A.P.; Hein, J.R.; Yu, J.M.; Sun, X.M.; Shi, X.F.; Cao, W.; et al. Abyssal Manganese Nodule Recording of Global Cooling and Tibetan Plateau Uplift Impacts on Asian Aridification. Geophys. Res. Lett. 2022, 49, e2021GL096624. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, X.; Zhao, X.; Chou, Y.-M.; Hein, J.R.; Sun, X.; Zhong, Y.; Ren, J.; Liu, Q. A magnetic approach to unravelling the paleoenvironmental significance of nanometer-sized Fe hydroxide in NW Pacific ferromanganese deposits. Earth Planet. Sci. Lett. 2021, 565, 116945. [Google Scholar] [CrossRef]
- Zawadzki, D.; Maciag, L.; Kotlinski, R.A.; Kozub-Budzyn, G.A.; Piestrzynski, A.; Wrobel, R. Geochemistry of cobalt-rich ferromanganese crusts from the Perth Abyssal Plain (E Indian Ocean). Ore Geol. Rev. 2018, 101, 520–531. [Google Scholar] [CrossRef]
- Haese, R.R. The Biogeochemistry of Iron. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 241–270. [Google Scholar]
- Ler, A.; Stanforth, R. Evidence for surface precipitation of phosphate on goethite. Environ. Sci. Technol. 2003, 37, 2694–2700. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.L.; Liang, X.L.; Ma, L.Y.; Lin, X.J.; Zhu, J.X.; He, H.P.; Parker, S.C.; Molinari, M. Synergistic adsorption of Cd(II) with sulfate/phosphate on ferrihydrite: An in situ ATR-FTIR/2D-COS study. Chem. Geol. 2018, 477, 12–21. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.L.; Ma, L.Y.; Fu, H.Y.; Lin, X.J.; Parker, S.C.; Molinari, M. Adsorption of phosphate and cadmium on iron (oxyhydr)oxides: A comparative study on ferrihydrite, goethite, and hematite. Geoderma 2021, 383, 114799. [Google Scholar] [CrossRef]
- Jiang, X.D.; Sun, X.M.; Chou, Y.M.; Hein, J.R.; He, G.W.; Fu, Y.; Li, D.F.; Liao, J.L.; Ren, J.B. Geochemistry and origins of carbonate fluorapatite in seamount Fe-Mn crusts from the Pacific Ocean. Mar. Geol. 2020, 423, 106135. [Google Scholar] [CrossRef]
- Baturin, G.N.; Bezrukov, P.L. Phosphorites On The Sea-Floor And Their Origin. Mar. Geol. 1979, 31, 317–332. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchu, V. Microstructures Of Agulhas Bank Phosphorites. Mar. Geol. 1974, 16, M63–M70. [Google Scholar] [CrossRef]
- Baturin, G.N.; Yushina, I.G. Rare earth elements in phosphate-ferromanganese crusts on Pacific seamounts. Lithol. Miner. Resour. 2007, 42, 101–117. [Google Scholar] [CrossRef]
- Schijf, J.; Byrne, R.H. Speciation of yttrium and the rare earth elements in seawater: Review of a 20-year analytical journey. Chem. Geol. 2021, 584, 120479. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. REE(III) adsorption onto Mn dioxide (delta-MnO2) and Fe oxyhydroxide: Ce(III) oxidation by delta-MnO2. Geochim. Cosmochim. Acta 2001, 65, 695–703. [Google Scholar] [CrossRef]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).