Self-Potential as a Tool to Monitor Redox Reactions at an Ore Body: A Sandbox Experiment
Abstract
:1. Introduction
2. Experimental Setup and Results
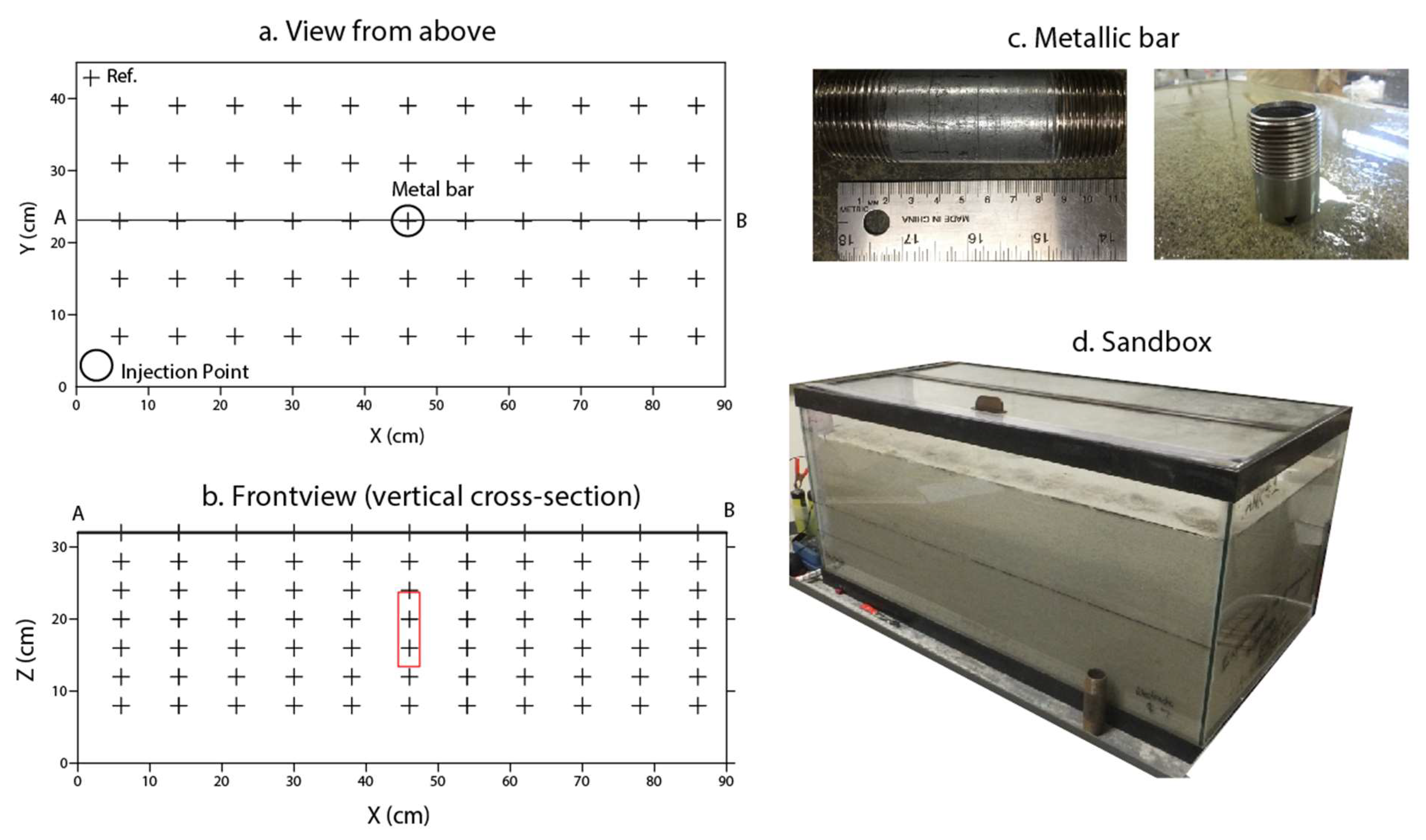
2.1. Sandbox Experiment
2.2. Measurements
2.3. Description of the Experiment
3. Interpretation
3.1. Forward Analysis of the Self-Potential Signals
3.2. Self-Potential Tomography
3.3. Analysis of the 3D Data Sets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, M.; Mooney, H.M. The electrochemical mechanism of sulfide self-potentials. Geophysics 1960, 25, 226–249. [Google Scholar] [CrossRef]
- Castermant, J.; Mendonça, C.A.; Revil, A.; Trolard, F.; Bourrié, G.; Linde, N. Redox potential distribution inferred from self-potential measurements associated with the corrosion of a burden metallic body. Geophys. Prospect. 2008, 56, 269–282. [Google Scholar] [CrossRef]
- Rittgers, J.B.; Revil, A.; Karaoulis, M.; Mooney, M.A.; Slater, L.D.; Atekwana, E.A. Self-potential signals generated by the corrosion of buried metallic objects with application to contaminant plumes. Geophysics 2013, 78, EN65–EN82. [Google Scholar] [CrossRef]
- Constable, S.; Kowalczyk, P.; Bloomer, S. Measuring marine self-potential using an autonomous underwater vehicle. Geophys. J. Int. 2018, 215, 49–60. [Google Scholar] [CrossRef]
- Bigalke, J.; Grabner, E.W. The geobattery model: A contribution to large scale electrochemistry. Electrochim. Acta. 1997, 42, 3443–3452. [Google Scholar] [CrossRef]
- Timm, F.; Möller, P. The relation between electric and redox potential: Evidence from laboratory and field measurements. J. Geochem. Explor. 2001, 72, 115–128. [Google Scholar] [CrossRef]
- Maineult, A. Estimation of the electrical potential distribution along metallic casing from surface self-potential profile. J. Appl. Geophys. 2016, 129, 66–78. [Google Scholar] [CrossRef]
- Revil, A.; Fernandez, P.; Mao, D.; French, H.K.; Bloem, E.; Binley, A. Self-potential monitoring of the enhanced biodegradation of an organic contaminant using a bioelectrochemical cell. Lead. Edge 2015, 34, 198–202. [Google Scholar] [CrossRef]
- Kawada, Y.; Kasaya, T. Marine self-potential survey for exploring seafloor hydrothermal ore deposits. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Kawada, Y.; Kasaya, T. Self-potential mapping using an autonomous underwater vehicle for the Sunrise deposit, Izu-Ogasawara arc, southern Japan. Earth Planets Space 2018, 70, 142. [Google Scholar] [CrossRef]
- Revil, A.; Ehouarne, L.; Thyreault, E. Tomography of self-potential anomalies of electrochemical nature. Geophys. Res. Lett. 2001, 28, 4363–4366. [Google Scholar] [CrossRef]
- Mendonça, C.A. Forward and inverse self-potential modeling in mineral exploration. Geophysics 2007, 73, F33–F43. [Google Scholar] [CrossRef]
- Su, Z.; Tao, C.; Shen, J.; Revil, A.; Zhu, Z.; Deng, X.; Nie, Z.; Li, Q.; Liu, L.; Wu, T. 3D self-potential tomography of seafloor massive sulfide deposits using an autonomous underwater vehicle. Geophysics 2022, 87, B255–B267. [Google Scholar] [CrossRef]
- Su, Z.; Tao, C.; Zhu, Z.; Revil, A.; Shen, J.; Nie, Z.; Li, Q.; Deng, X.; Zhou, J.; Liu, L. Joint interpretation of marine self-potential and transient electromagnetic survey for seafloor massive sulfide (SMS) deposits: Application at TAG hydrothermal mound, mid-Atlantic ridge. J. Geophys. Res. Solid Earth 2022, 127, e2022JB024496. [Google Scholar] [CrossRef]
- Zhu, Z.; Tao, C.; Shen, J.; Revil, A.; Deng, X.; Liao, S.; Zhou, J.; Wang, W.; Nie, Z.; Yu, J. Self-potential tomography of a deep-sea polymetallic sulfide deposit on Southwest Indian Ridge. J. Geophys. Res. Solid Earth 2020, 125, e2020JB019738. [Google Scholar] [CrossRef]
- Zhu, Z.; Shen, J.; Tao, C.; Deng, X.; Wu, T.; Nie, Z.; Wang, W.; Su, Z. Autonomous-underwater-vehicle-based marine multicomponent self-potential method: Observation scheme and navigational correction. Geosci. Instrum. Methods Data Syst. 2021, 10, 35–43. [Google Scholar] [CrossRef]
- Eppelbaum, L.V. Review of processing and interpretation of self-potential anomalies: Transfer of methodologies developed in magnetic prospecting. Geosciences 2021, 11, 194. [Google Scholar] [CrossRef]
- Minsley, B.J.; Sogade, J.; Morgan, F.D. Three-dimensional self-potential inversion for subsurface DNAPL contaminant detection at the Savannah River Site, South Carolina. WRR 2007, 43, W04429. [Google Scholar] [CrossRef]
- Abdelrahman, E.M.; Essa, K.S.; Abo-Ezz, E.R.; Soliman, K.S. Self-potential data interpretation using standard deviations of depths computed from moving-average residual anomalies. Geophys. Prospect. 2006, 54, 409–423. [Google Scholar] [CrossRef]
- Abdelrahman, E.M.; Saber, H.S.; Essa, K.S.; Fouda, M.A. A least-squares approach to depth determination from numerical horizontal self-potential gradients. Pure Appl. Geophys. 2004, 161, 399–411. [Google Scholar] [CrossRef]
- Essa, K.S.; Diab, Z.E.; Mehanee, S.A. Self-potential data inversion utilizing the Bat optimizing algorithm (BOA) with various application cases. Acta Geophys. 2023, 71, 567–586. [Google Scholar] [CrossRef]
- Ballester, A.; González, F.; Blázquez, M.; Gómez, C.; Mier, J. The use of catalytic ions in bioleaching. Hydrometallurgy 1992, 29, 145–160. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of microbial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef]
- Ogram, A.; Sayler, G.S.; Barkay, T. The extraction and purification of microbial DNA from sediments. J. Microbiol. Methods 1987, 7, 57–66. [Google Scholar] [CrossRef]
- Lever, M.A.; Torti, A.; Eickenbusch, P.; Michaud, A.B.; Šantl-Temkiv, T.; Jørgensen, B.B. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Front. Microbiol. 2015, 6, 476. [Google Scholar] [CrossRef] [PubMed]
- Revil, A.; Finizola, A.; Gresse, M. Self-potential as a tool to assess groundwater flow in hydrothermal systems: A review. J. Volcanol. Geotherm. Res. 2023, 437, 107788. [Google Scholar] [CrossRef]
- Tikhonov, A.N.; Arsenin, V.Y. Solutions of Ill-Posed Problems; John Wiley & Sons: Hoboken, NJ, USA, 1977. [Google Scholar]
- Michael, Z.; Ekaterina, T. Minimum support nonlinear parametrization in the solution of a 3D magnetotelluric inverse problem. Inverse. Probl. 2004, 20, 937. [Google Scholar]
- Jardani, A.; Revil, A.; Bolève, A.; Dupont, J.P. Three-dimensional inversion of self-potential data used to constrain the pattern of groundwater flow in geothermal fields. J. Geophys. Res. Solid Earth 2008, 113, B09204. [Google Scholar] [CrossRef]
- Revil, A.; Jardani, A. The Self-Potential Method: Theory and Applications in Environmental Geosciences; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Mao, D.; Revil, A. Induced polarization response of porous media with metallic particles—Part 3: A new approach to time-domain induced polarization tomography. Geophysics 2016, 81, D345–D357. [Google Scholar] [CrossRef]
- Hattori, S.; Galushko, A.S.; Kamagata, Y.; Schink, B. Operation of the CO dehydrogenase/acetyl coenzyme A pathway in both acetate oxidation and acetate formation by the syntrophically acetate-oxidizing bacterium Thermacetogenium phaeum . J. Bacteriol. 2005, 187, 3471–3476. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revil, A.; Su, Z.; Zhu, Z.; Maineult, A. Self-Potential as a Tool to Monitor Redox Reactions at an Ore Body: A Sandbox Experiment. Minerals 2023, 13, 716. https://doi.org/10.3390/min13060716
Revil A, Su Z, Zhu Z, Maineult A. Self-Potential as a Tool to Monitor Redox Reactions at an Ore Body: A Sandbox Experiment. Minerals. 2023; 13(6):716. https://doi.org/10.3390/min13060716
Chicago/Turabian StyleRevil, André, Zhaoyang Su, Zhongmin Zhu, and Alexis Maineult. 2023. "Self-Potential as a Tool to Monitor Redox Reactions at an Ore Body: A Sandbox Experiment" Minerals 13, no. 6: 716. https://doi.org/10.3390/min13060716





