Metal Mobility in Embryonic-to-Proto-Ni-Laterite Profiles from Non-Tropical Climates
Abstract
:1. Introduction
2. Geology, Climate Setting, and Field Characteristics of the Weathering Profiles
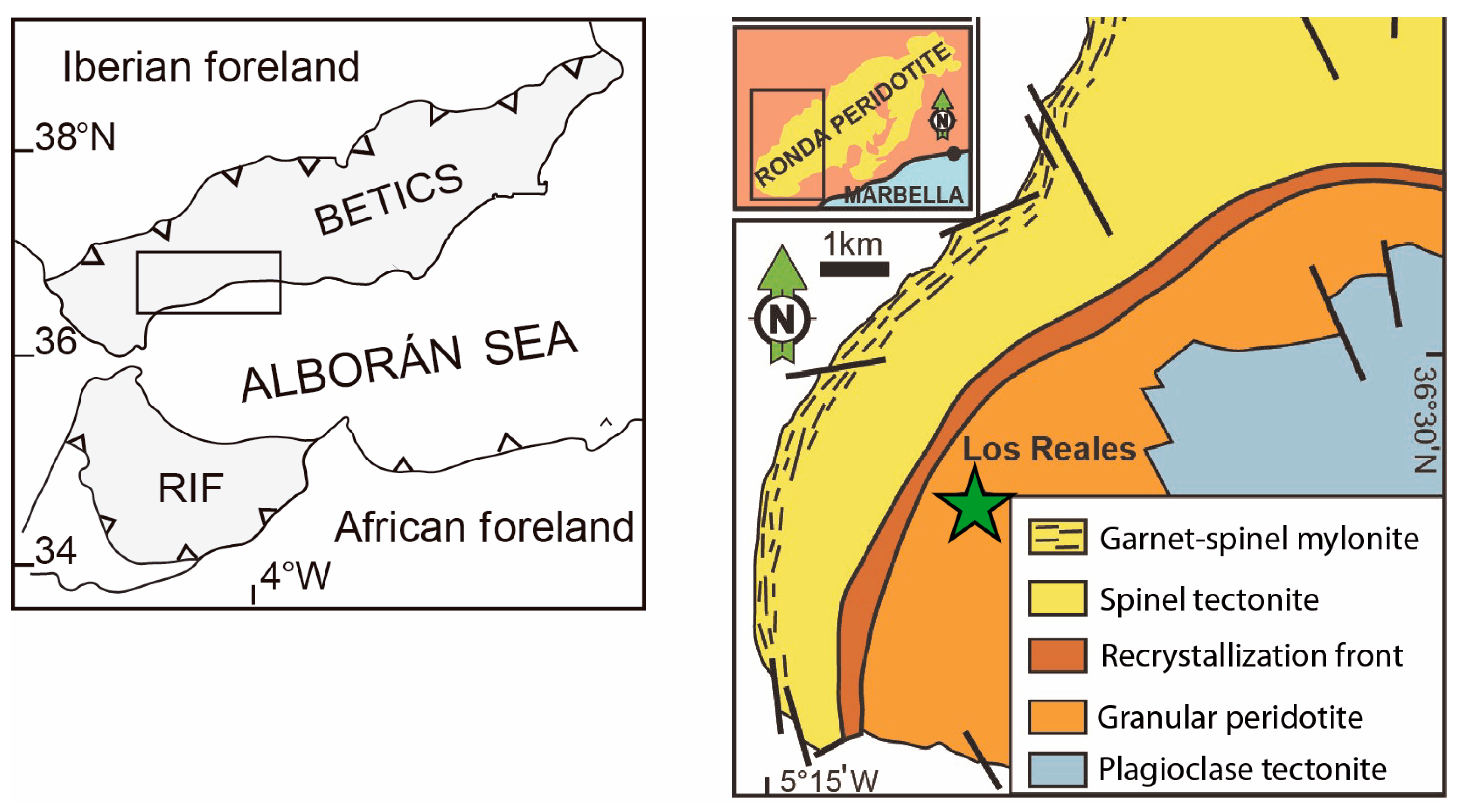
2.1. The Los Reales Range, Serranía de Ronda, in Southwestern Spain
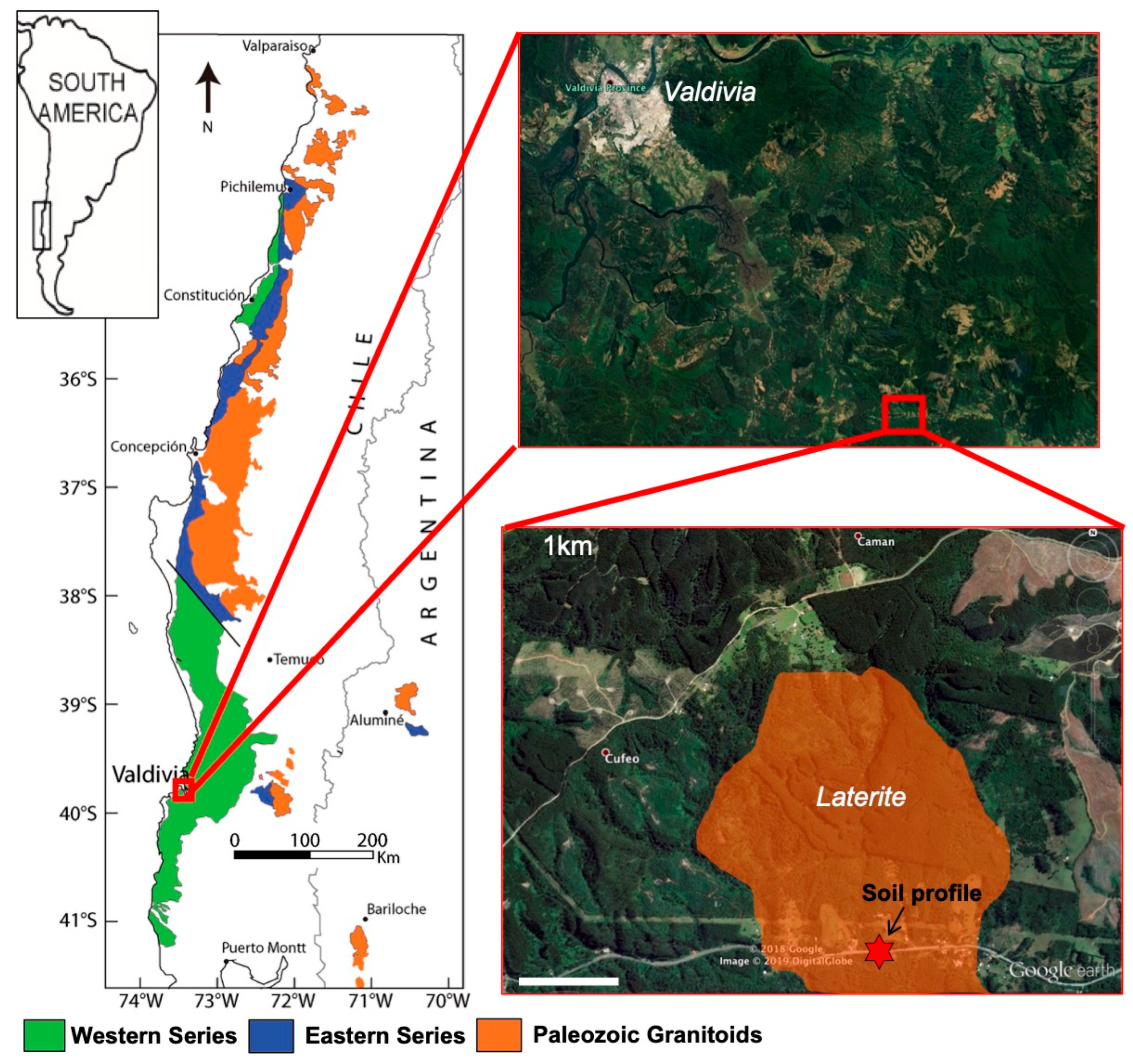
2.2. The Camán Ultramafic Body, Chilean Coastal Cordillera
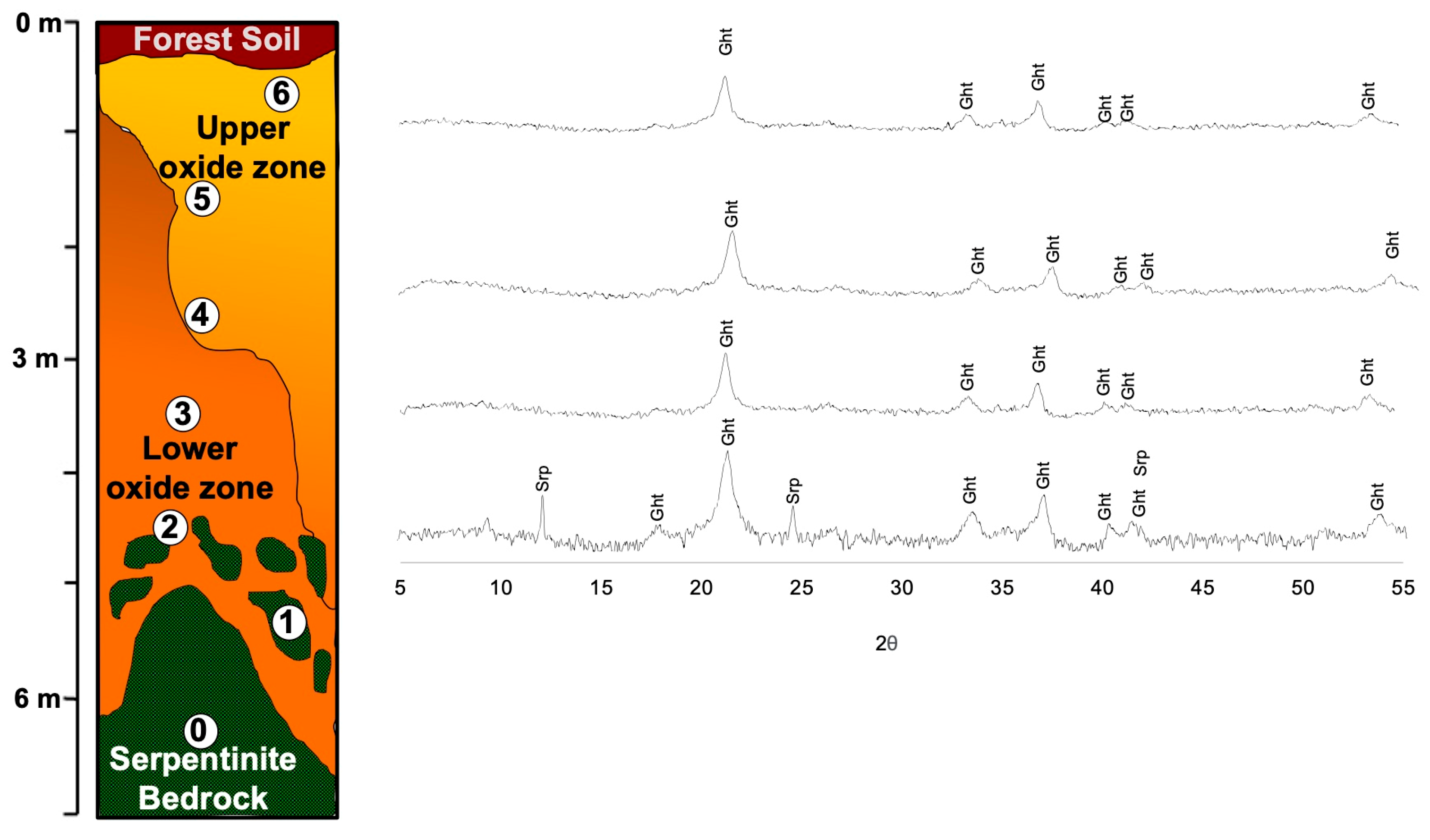
2.3. Local Geology of the Studied Weathering Profiles
3. Materials and Methods
3.1. Sampling
3.2. Geochemistry of Major, Minor, and Trace Elements
3.2.1. Major, Minor, and Trace Elements
3.2.2. Platinum-Group Elements and Gold
3.3. Mineralogy
3.3.1. X-ray Powder Diffraction (XRPD)
3.3.2. Scanning Electron Microscope and Electron Micro Probe Analyzer
4. Results
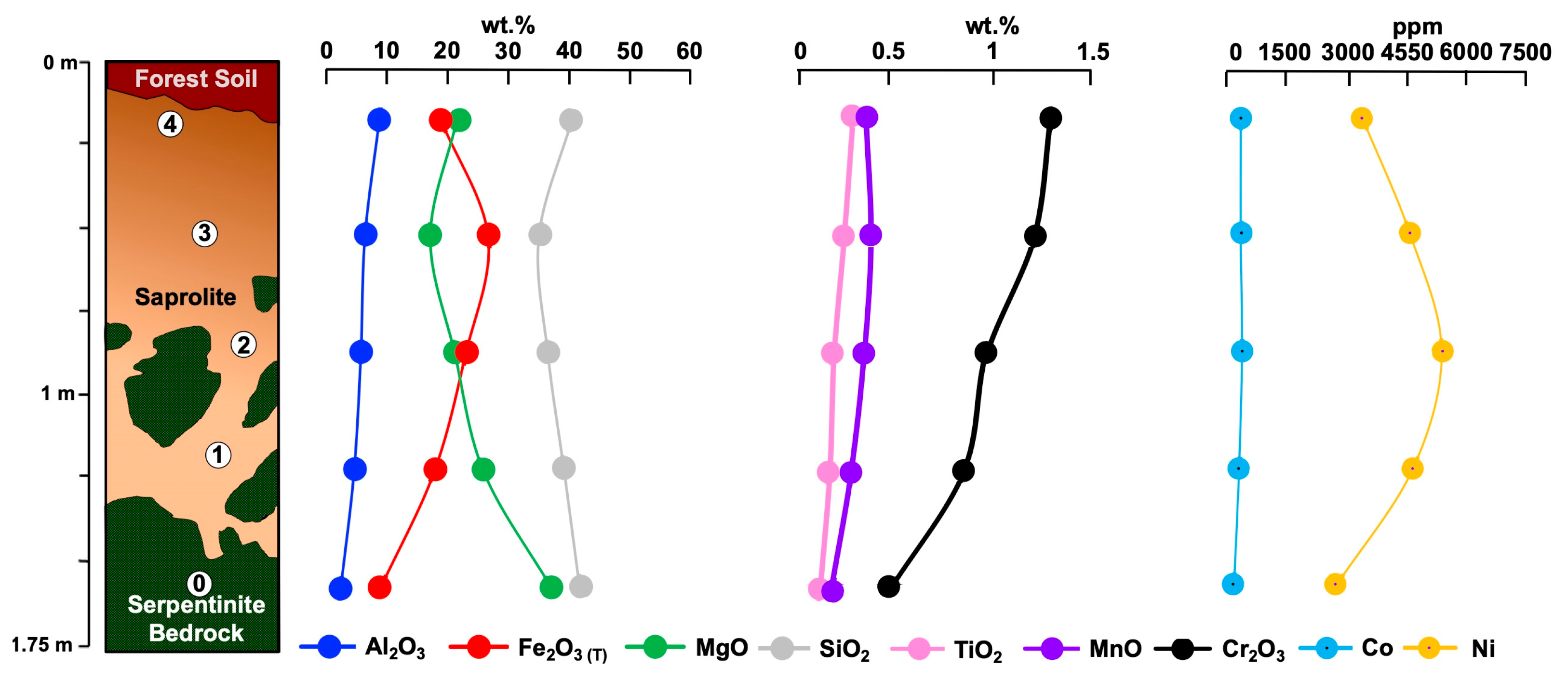
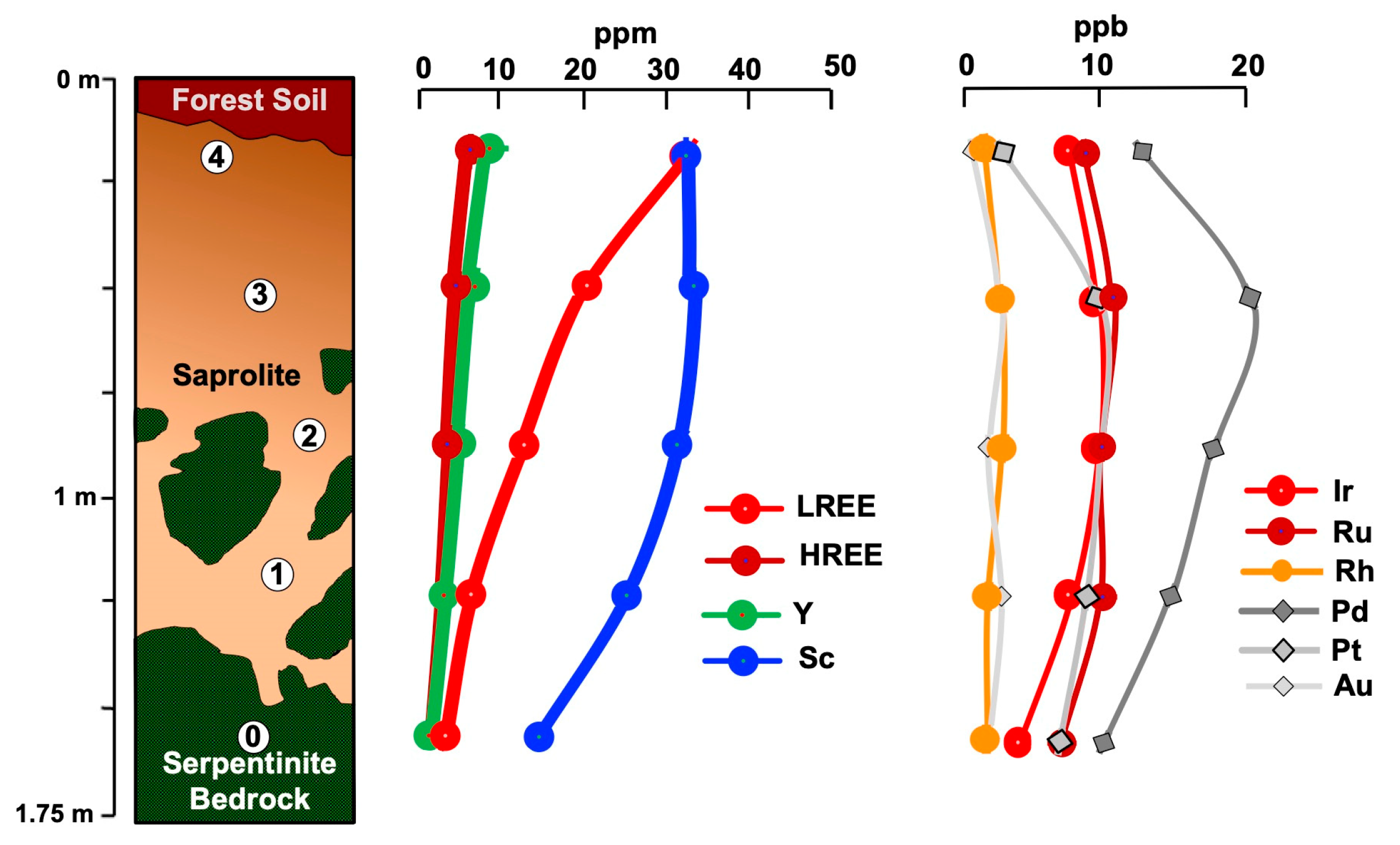
4.1. Los Reales
4.1.1. Geochemistry
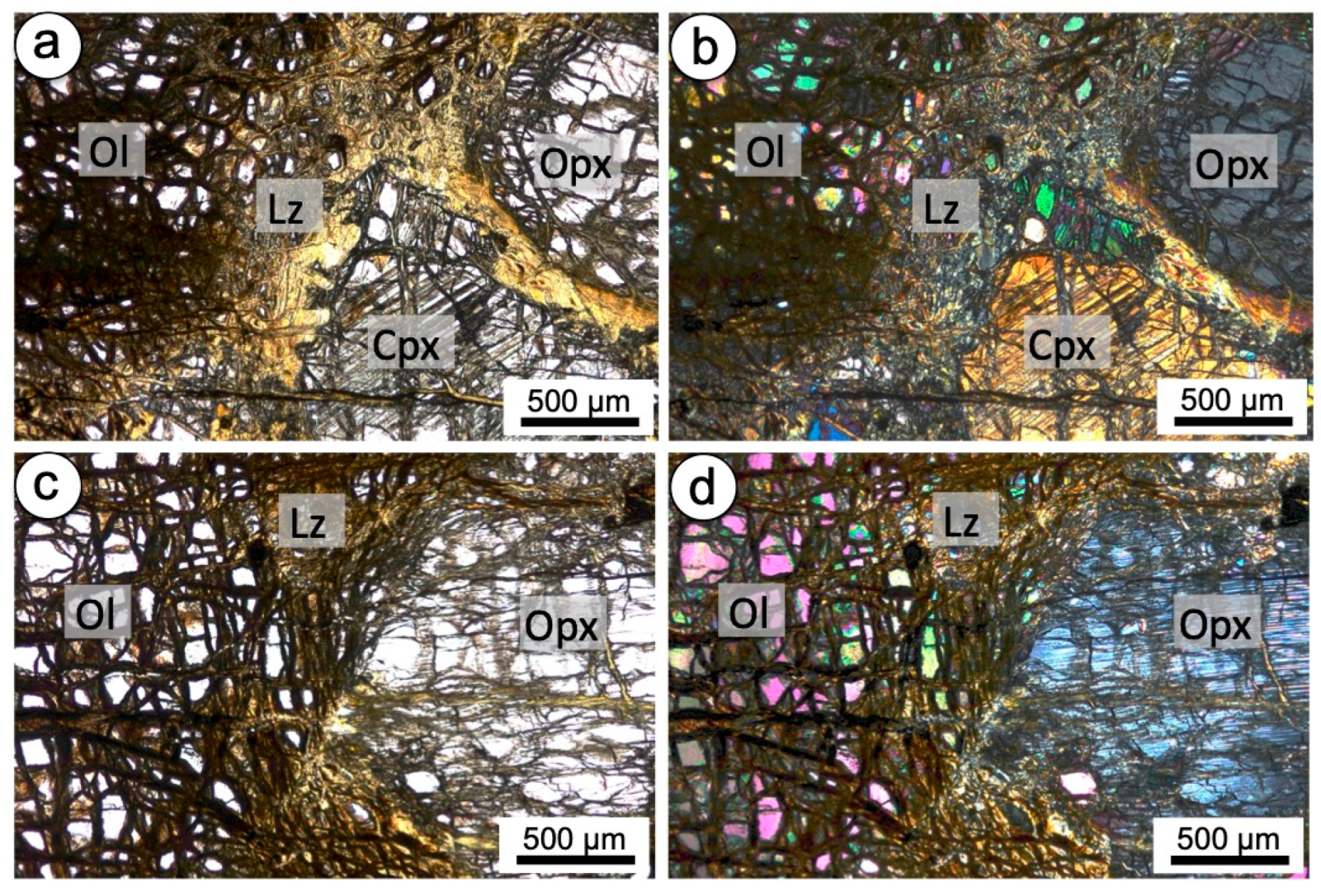
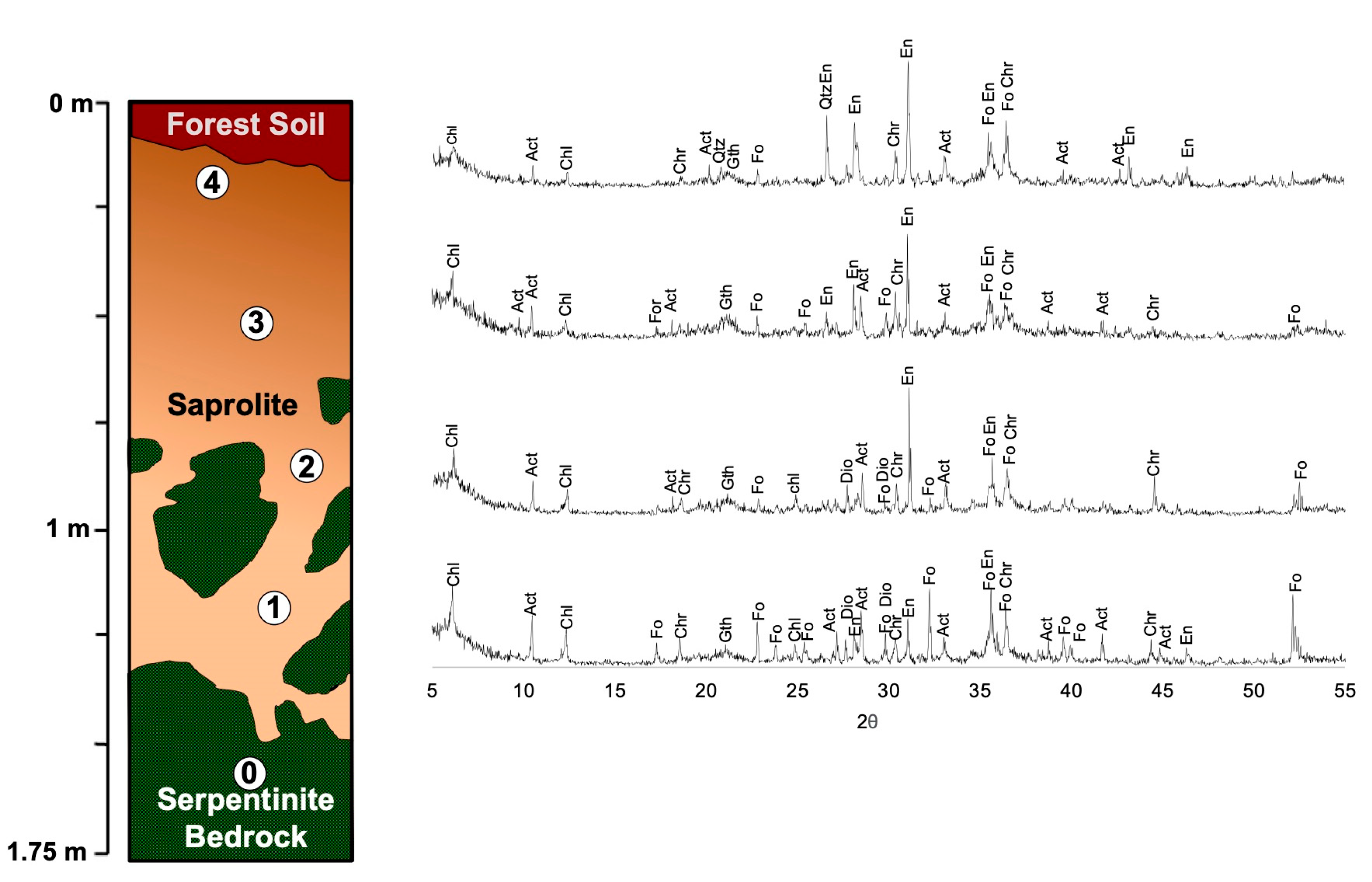
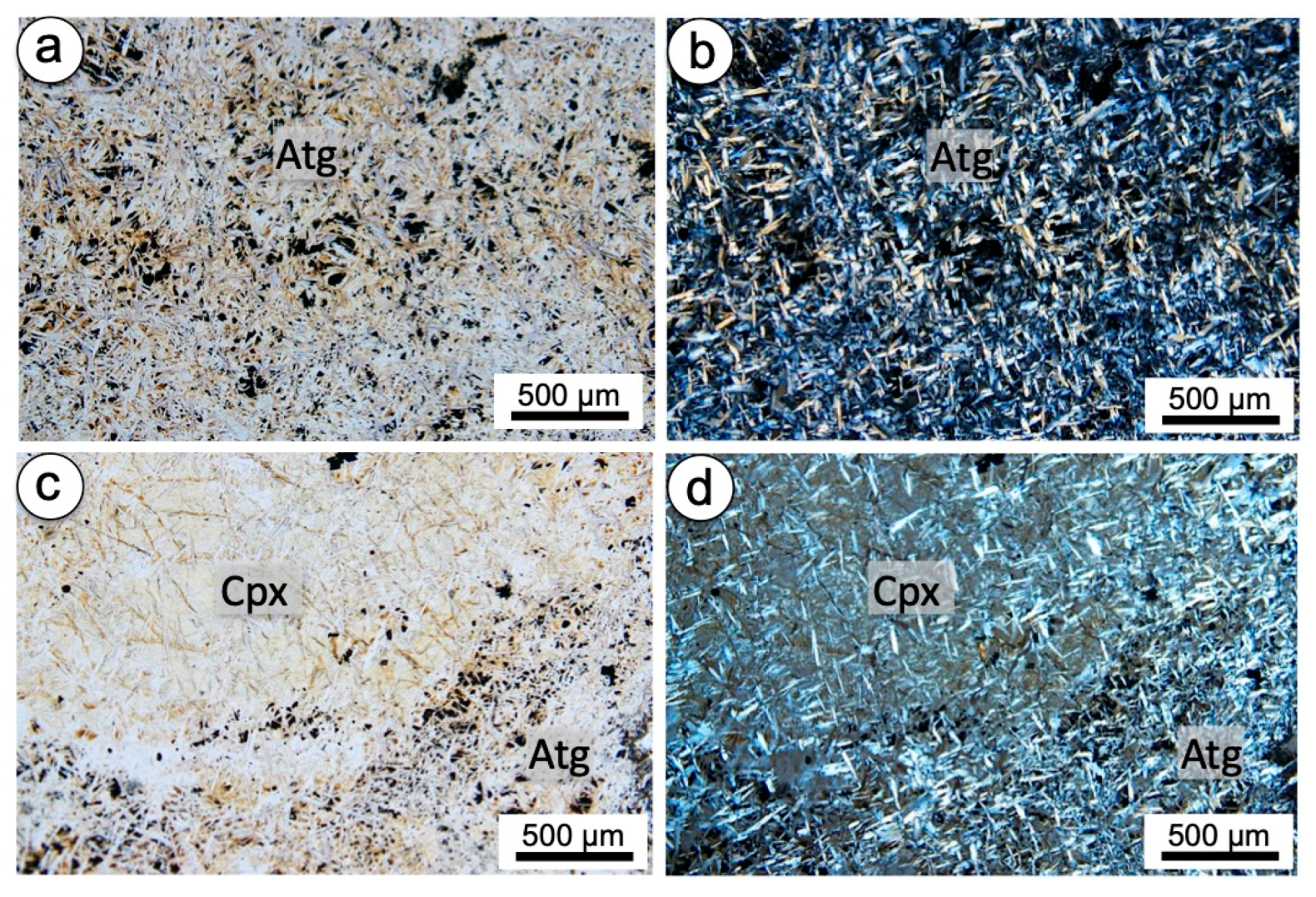
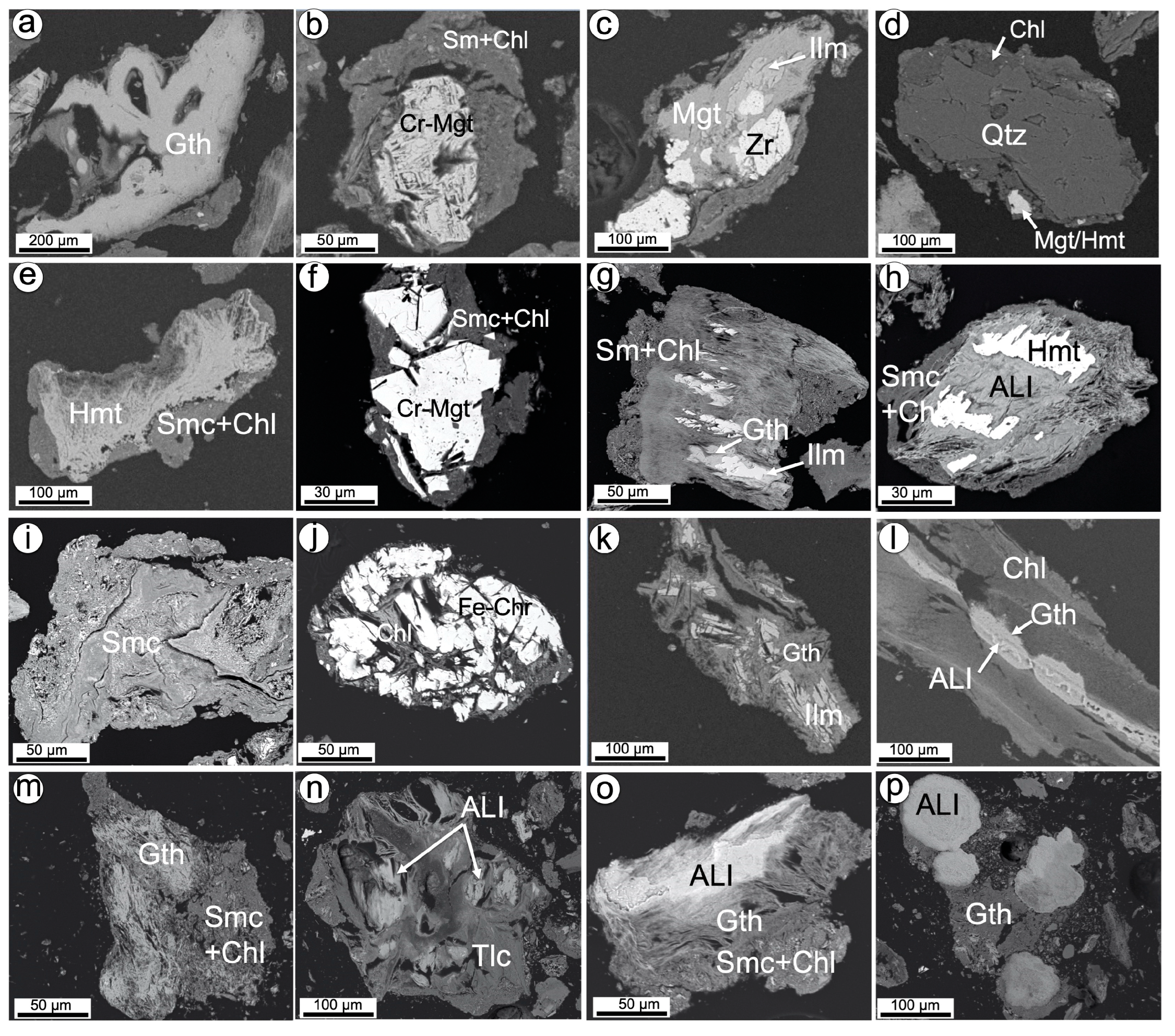
4.1.2. Mineralogy
4.2. Camán
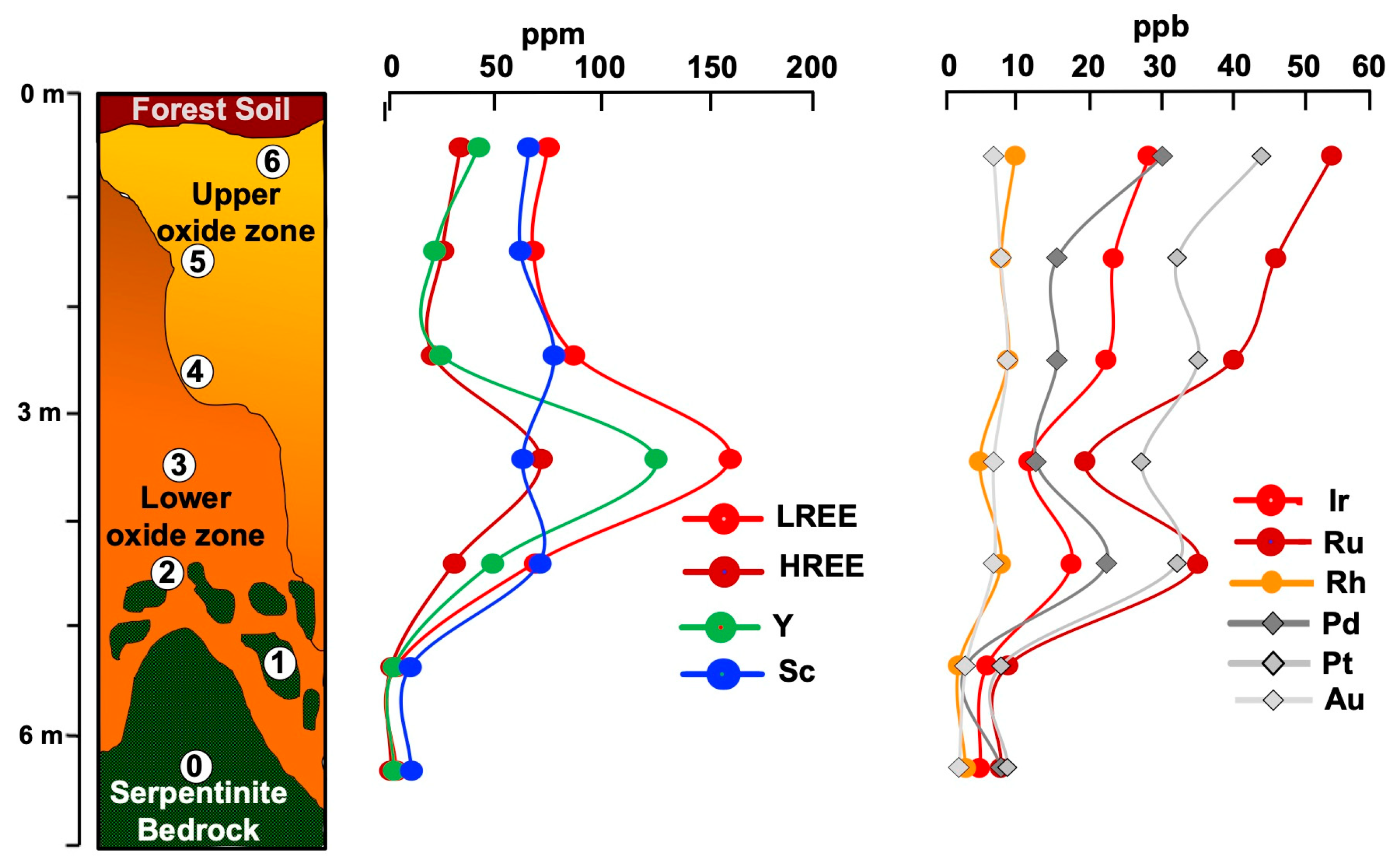
4.2.1. Geochemistry
4.2.2. Mineralogy
5. Discussion
5.1. Maturity of the Ni-Laterite Profiles
5.2. Mineralogical Siting of Economic Metals: Clues on Enrichment Mechanisms
5.2.1. Nickel and Cobalt
5.2.2. Behavior of REE, Sc, and PGE
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://pubs.usgs.gov/periodicals/mcs2023/mcs2023-nickel.pdf (accessed on 1 January 2020).
- Aiglsperger, T.; Proenza, J.A.; Lewis, J.F.; Labrador, M.; Svotjka, M.; Rojas-Purón, A.; Longo, F.; Durišova, J. Critical metals (REE, Sc, PGE) in Ni lateritas from Cuba and the Dominican Republic. Ore Geol. Rev. 2016, 73, 127–147. [Google Scholar] [CrossRef]
- Cocker, M. 48th Forum on the Geology of Industrial Minerals, 9th ed.Arizona Geological Survey Special Paper; Conway, F.M., Ed.; Arizona Geological Survey: Tucson, AZ, USA, 2014; Chapter: 4; pp. 1–8. [Google Scholar]
- Thorne, R.L.; Herrington, R.; Roberts, S. Climate change and the formation of nickel laterite deposits. Geology 2012, 40, 331–334. [Google Scholar] [CrossRef]
- Berger, V.I.; Singer, D.A.; Bliss, J.D.; Moring, B.C. Ni-Co laterite deposits of the world—Database and grade and tonnage models: U.S. Geol. Surv. Open File Rep. 2011, 2011–1058, 26p. Available online: http://pubs.usgs.gov/of/2011/1058/ (accessed on 1 January 2020).
- Rivera, J.; Reich, M.; Scoeneberg, R.; González-Jiménez, J.M.; Barra, F.; Aiglsperger, T.; Proenza, J.A.; Carretier, S. Platinum-group element and gold enrichment in soils monitorted by chromium stable isotopes during weathering of ultramafic rocks. Chem. Geol 2018, 499, 84–99. [Google Scholar] [CrossRef]
- Maurizot, P.; Sevin, B.; Iseppi, M.; Giband, T. Nickel-Bearing Laterite Deposits in Accretionary Context and the Case of New Caledonia: From the Large-Scale Structure of Earth to Our Everyday Appliances. GSA Today 2019, 29, 4–10. [Google Scholar] [CrossRef]
- Yusta, A.; Berahona, E.; Huertas, F.; Reyes, E.; Yáñez, J.; Linares, J. Geochemistry of soils from peridotite in Los Reales, Málaga. Acta Miner. Petrogr. 1985, 29, 439–498. [Google Scholar]
- Zamarsky, V.V.; Salmon, H.O.; Tabok, M. Estudio geoquímico de los productos de intemperismo de las rocas ultrabásicas (serpentinitas) en la provincia de Valdivia, Chile. Rev. Geológica De Chile: Int. J. Andean Geol. 1974, 1, 81–102. [Google Scholar]
- Salinas, S. Formación de la Laterita de Camán XIV Región Chile. Master’s Thesis, Univeridad de Chile, Santiago, Chile, 2016. [Google Scholar]
- Navarro-Hasse, E. Análisis Mineralógico y Concentración de Cromo, Níquel y Cobalto en el Suelo Serpentinitico del Sector de Camán, Región de los Ríos, Chile. Master’s Thesis, Universidad Austral de Chile, Valdivia, Chile, 2020. [Google Scholar]
- Lenoir, X.; Garrido, C.J.; Bodinier, J.-L.; Dautria, J.-M.; Gervilla, F. The recrystallization front of the Ronda peridotite: Evidence for melting and thermal erosion of subcontinental lithospheric mantle beneath the Alborán basin. J. Petrol. 2001, 42, 141–158. [Google Scholar] [CrossRef]
- Bucher, K.; Stober, I.; Muller-Sigmund, H. Weathering crusts on peridotite. Contrib. Miner. Petrol. 2015, 169, 52. [Google Scholar] [CrossRef]
- Faundez, R. Distribución de Metales Nobles en Rocas Ultramáficas Serpentinizadas del Centro-Sur de Chile. Master’s Thesis, Univeridad de Chile, Santiago, Chile, 2016. [Google Scholar]
- Hidas, K.; Booth-Rea, G.; Garrido, C.J.; Martínez-Martínez, J.M.; Padrón-Navarta, J.A.; Konc, Z.; Giaconia, F.; Frets, E.; Marchesi, C. Back-arc basin inversion and subcontinental mantle emplacement in the crust: Kilometre-scale folding and shearing at the base of the proto-Alborán lithospheric mantle (Betic Cordillera, southern Spain). J. Geol. Soc. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Garrido, C.J.; Gueydan, F.; Booth-Rea, G.; Precigout, J.; Hidas, K.; Padron-Navarta, J.A.; Marchesi, C. Garnet lherzolite and garnet-spinel mylonite in the Ronda peridotite: Vestiges of Oligocene backarc mantle lithospheric extension in the western Mediterranean. Geology 2011, 39, 927–930. [Google Scholar] [CrossRef] [Green Version]
- Luque del Villar, F.J.; Rodas-González, M.; Doval-Montoya, D. Mineralogía y génesis de los yacimientos de vermiculita del macizo de Ojén (Serranía de Ronda, Málaga). Boletín De La Soc. Española De Mineral. 1985, 8, 229–238. [Google Scholar]
- Gómez-Zotano, J.A.; Cunill-Artigas, R.; Martínez-Ibarra, E. Descubrimiento y caracterización geográfica de una depresión ultramáfica en Sierra Bermeja: Nuevos datos geomorfoedáficos, fitogeográficos y paleoecológicos. Pirineos 2017, 172, 26. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Hernández, O.; Cámara Artigas, R.; García, L.V. Regeneración de los pinsapares béticos. Análisis de tendencia interanual y estacional del NDVI. Pirineos 2018, 173, e035. [Google Scholar] [CrossRef] [Green Version]
- Willner, A.P.; Thomson, S.N.; Kröner, A.; Wartho, J.A.; Wijbrans, J.R.; Hervé, F. Time markers for the evolution and exhumation history of a Late Palaeozoic paired metamorphic belt in North–Central Chile (34–35 30′ S). J. Petrol. 2005, 46, 1835–1858. [Google Scholar] [CrossRef] [Green Version]
- Deckart, K.; Hervé, F.; Fanning, M.; Ramírez, V.; Calderón, M.; Godoy, E. U-Pb geochronology and Hf-O isotopes of zircons from the Pennsylvanian coastal batholith, south central Chile. Andean Geol. 2014, 41, 49–82. [Google Scholar] [CrossRef] [Green Version]
- González-Jimenez, J.M.; Plissart, G.; Garrido, L.; Padrón-Navarta, J.; Aiglsperger, T.; Romero, R.; Marchesi, C.; Moreno-Abril, A.; Reich, M.; Barra, F.; et al. Ti-clinohumite and Ti-chondrodite in antigorite serpentinites from Central Chile: Evidence for deep and cold subduction. Eur. J. Miner. 2017, 29, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://explorador.cr2.cl (accessed on 1 January 2020).
- Hoffman, E.L.; Dunn, B. Sample preparation and bulk analytical methods for PGE. In The Geology, Geochemistry and Mineral Beneficiation of Platinum Group Elements; Cabri, L.J., Ed.; Canadian Institute of Mining, Metallurgyand Petroleum: Montreal, QC, Canada, 2002; Volume 54, pp. 1–11. [Google Scholar]
- Roqué-Rosell, J.; Mosselmans, J.F.W.; Proenza, J.A.; Labrador, M.; Galí, S.; Atkinson, K.D.; Quinn, P.D. Sorption of Ni by “lithiophorite–asbolane” intermediates in Moa Bay lateritic deposits, eastern Cuba. Chem. Geol. 2010, 275, 9–18. [Google Scholar] [CrossRef]
- Lambiv Dzemua, G.; Gleeson, S.A.; Schofield, P.F. Mineralogical characterization of the Nkamouna Co-Mn laterite ore, southeast Cameroon. Miner. Depos. 2013, 48, 155–171. [Google Scholar] [CrossRef]
- Manceau, A.; Llorca, S.; Calas, G. Crystal chemistry of cobalt and nickel in lithiophorite and asbolane from New Caledonia. Geochim. Cosmochim. Acta 1987, 51, 105–113. [Google Scholar] [CrossRef]
- Putzolu, F.; Boni, M.; Mondillo, N.; Maczurad, M.; Pirajno, F. Ni-Co enrichment and High-Tech metals geochemistry in the Wingellina Ni-Co oxide-type laterite deposit (Western Australia). J. Geochem. Explor. 2019, 196, 282–296. [Google Scholar] [CrossRef]
- Brand, N.W.; Butt, C.R.M.; Elias, M. Nickel laterites: Classification and features. AGSO J. Aust. Geol. Geoph. 1998, 17, 81–88. [Google Scholar]
- Ulrich, M.; Cathelineau, M.; Muñoz, M.; Boiron, M.C.; Teitler, Y.; Karpoff, A.M. The relative distribution of critical (Sc, REE) and transition metals (Ni, Co, Cr, Mn, V) in some Ni-laterite deposits of New Caledonia. J. Geochem. Explor. 2019, 197, 93–113. [Google Scholar] [CrossRef]
- Brand, N.W.; Butt, C.R.M. Weathering, element distribution and geochemical dispersion at Mt Keith, Western Australia: Implication for nickel sulphide exploration. Geochem. Explor. Environ. Anal. 2001, 1, 391–407. [Google Scholar] [CrossRef]
- Freyssinet, P.; Butt, C.R.M.; Morris, R.C. Ore-forming processes related to lateritic weathering. Econ. Geol. One Hundredth Anniv. Volume 2005, 681–722. [Google Scholar] [CrossRef]
- Golightly, J.P. Progress in understading the evolution of nickel laterites. Econ. Geol. Spec. Pub. 2010, 15, 451–485. [Google Scholar]
- Golightly, J.P.; Arancibia, O.N. The chemical composition and infrared spectrum of nickel- and iron-substituted serpentine form a nickeliferous laterite profile, Soroako, Indonesia. Can. Miner. 1979, 17, 719–728. [Google Scholar]
- Villanova-de-Benavent, C.; Domènech, C.; Tauler, E.; Galí, S.; Tassara, S.; Proenza, J.A. Fe-Ni-bearing serpenties from the saprolite horizon of Caribbean Ni-laterite deposits: New insights from thermodynamic calculations. Miner. Depos. 2017, 52, 979–992. [Google Scholar] [CrossRef]
- Beukes, J.P.; Giesekke, E.W.; Elliot, W. Nickel retention by goethite and haematite. Miner. Eng. 2000, 13, 1573–1579. [Google Scholar] [CrossRef]
- Singh, B.; Sherman, D.M.; Gilkes, R.J.; Wells, M.A.; Mosselmans, J.E.W. Incorporation of Cr, Mn and Ni into goethite (aFeOOH): Mechanism from extended X-ray absorption fine structure spectroscopy. Clay Min. 2002, 37, 639–649. [Google Scholar] [CrossRef]
- Trivedi, P.; Axe, L. Ni and Zn sorption to amorphous versus crystalline iron oxides: Macroscopic studies. J. Coll. Int. Sci. 2001, 244, 221–229. [Google Scholar] [CrossRef]
- Ostwald, J. Two varieties of lithiophorite in some Australian deposits. Miner. Mag. 1984, 48, 383–388. [Google Scholar] [CrossRef]
- Roberts, D.R.; Scheidegger, A.M.; Sparks, D.L. Kinetics of mixed Ni-Al precipitate formation on a soil clay fraction. Environ. Sci. Technol. 1999, 33, 3749–3754. [Google Scholar] [CrossRef]
- Peacock, C.L. Physiochemical controls on the crystal-chemistry of Ni in birnessite: Genetic implications for ferro-manganese precipitates. Geochim. Cosmochim. Acta 2009, 73, 3568–3578. [Google Scholar] [CrossRef]
- Cui, H.; You, L.; Feng, X.; Tan, W.; Qiu, G.; Liu, F. Factors governing the formation of lithiophorite at atmospheric pressure. Clays Clay Miner. 2009, 57, 353–360. [Google Scholar] [CrossRef]
- McKenzie, R.M. Manganese oxides and hydroxides. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; SSSA: Madison, WI, USA, 1989; pp. 439–465. [Google Scholar]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Geochemical fractionation of lanthanides in soils and rocks: A review of publications. Eurasian Soil Sci. 2012, 45, 56–67. [Google Scholar] [CrossRef]
- Audet, M.A. Le Massif du Koniambo-Nouvelle Calédonie: Formation et Obduction d’un Complexe Ophiolitique de Type SSZ. Enrichissement en Nickel, Cobalt et Scandium Dans les Profils Résiduels. Ph.D. Thesis, Université de Nouvelle Calédonie, Nouméa, France, 2008; p. 327. (In French). [Google Scholar]
- Tobón, M.; Weber, M.; Proenza, J.A.; Aiglsperger, T.; Betancur, S.; Farré-de-Pablo, J.; Ramírez, C.; Pujol-Solà, N. Geochemistry of Platinum-Group Elements (PGE) in Cerro Matoso and Planeta Rica Ni-Laterite deposits, Northern Colombia. Boletín De La Soc. Geológica Mex. 2020, 72, A201219. [Google Scholar] [CrossRef]
- Al-Khirbash, S.A.; Ahmed, H. Distribution and mobility of platinum-group elements in the Late Cretaceous Ni-laterite iin the Northern Oman Mountains. JGR Solid Earth 2021, 126, e2021JB022363. [Google Scholar] [CrossRef]
- Gervilla, F.; González-Jiménez, J.M.; Hidas, K.; Marchesi, C.; Piña, R. Geology and Metallogeny of the Upper Mantle Rocks from the 939 Serranía de Ronda. Mineral. Span. Soc. Ronda 2019, 122. Available online: https://digital.csic.es/handle/10261/206926 (accessed on 1 January 2020).
- Aiglsperger, T.; González-Jiménez, J.M.; Proenza, J.A.; Galí, S.; Longo, F.; Griffin, W.L.; O’Reilly, S.Y. Open System Re-OsIsotope Behavior in Platinum-Group Minerals during Laterization? Minerals 2021, 11, 1083. [Google Scholar] [CrossRef]
- Sassani, D.C.; Shock, E.L. Solubility and transport of platinum-group elements in supercritical fluids: Summary and estimates of thermodynamic properties for ruthenium, rhodium, palladium, and platinum solids, aqueous ions, and complexes to 1000 degrees C and 5 kbar. Geochim. Cosmochim. Acta 1998, 62, 2643–2671. [Google Scholar] [CrossRef]
- Colombo, C.; Oates, C.J.; Monhemius, A.J.; Plant, J.A. Complexation of platinum, palladium and rhodium with inorganic ligands in the environment. Geochem. Explor. Environ. Anal. 2008, 8, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Reith, F.; Campbell, S.G.; Ball, A.S.; Pring, A.; Southam, G. Platinum in Earth surface environments. Earth-Sci. Rev. 2014, 131, 1–21. [Google Scholar] [CrossRef]
- Fuchs, W.A.; Rose, A.W. The geochemical behavior of platinum and palladium in the weathering cycle in the Stillwater Complex, Montana. Econ. Geol. 1974, 69, 332–346. [Google Scholar] [CrossRef]
- Locmelis, M.; Melcher, F.; Oberthür, T. Platinum-group element distribution in the oxidized main sulfide zone, Great Dyke, Zimbabwe. Miner. Depos. 2010, 45, 93–109. [Google Scholar] [CrossRef]
- Oberthür, T. The fate of platinum-group minerals in the exogenic environment—From sulfide ores via oxidized ores into placers: Case studies bushveld complex, South Africa, and Great Dyke, Zimbabwe. Minerals 2018, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Junge, M.; Oberthür, T.; Kraemer, D.; Melcher, F.; Piña, R.; Derrey, I.T.; Manyeruke, T.; Strauss, H. Distribution of platinum-group elements in pristine and near-surface oxidized Platreef ore and the variation along strike, northern Bushveld Complex, South Africa. Miner. Depos. 2019, 54, 885–912. [Google Scholar] [CrossRef]
- Korges, M.; Junge, M.; Borg, G.; Oberthür, T. Supergene mobilization and redistribution of platinum-group elements in the Merensky Reef, eastern Bushveld Complex, South Africa. Can. Miner. 2021, 59, 1381–1396. [Google Scholar] [CrossRef]
- Bowles, J.F.W. The development of platinum-group minerals in laterites. Econ. Geol. 1986, 81, 1278–1285. [Google Scholar] [CrossRef]
- Cabral, A.R.; Radtke, M.; Munnik, F.; Lehmann, B.; Reinholz, U.; Riesemeier, H.; Tupinambá, M.; Kwitko-Ribeiro, R. Iodine in alluvial platinum-palladium nuggets: Evidence for biogenic precious-metal fixation. Chem. Geol. 2011, 281, 125–132. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Fortygin, A.V.; Lobov, S.G.; Koscheeva, I.Y.; Tyutyunnik, O.A.; Mironenko, M.V. Migration of platinum, palladium, and gold in the water systems of platinum deposits. Geochem. Int. 2011, 49, 1072–1084. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Zaccarini, F.; Lewis, J.F.; Garuti, G.; Labrador, M.; Longo, F. Platinum group minerals (PGM) in the Falcondo Ni-laterite deposit, Loma Caribe peridotite (Dominican Republic). Miner. Depos. 2015, 50, 105–123. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Galí, S.; Longo, F.; Roqué-Rosell, J. Geochemical and mineralogical survey of critical elements (PGE, REE, Sc and Co) in Ni laterites from the Caribbean. EGU Gen. Assem. 2019, 21, 1. [Google Scholar]
- Aiglsperger, T.; Proenza, J.A.; Font-Bardia, M.; Baurier-Amat, S.; Galí, S.; Lewis, J.F.; Longo, F. Supergene neoformation of Pt-Ir-Fe-Ni alloys: Multistage grains explain nugget formation in Ni-laterites. Miner. Depos. 2017, 52, 1069–1083. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Jiménez, J.M.; Villanova-de-Benavent, C.; Yesares, L.; Marchesi, C.; Cartwright, D.; Proenza, J.A.; Monasterio-Guillot, L.; Gervilla, F. Metal Mobility in Embryonic-to-Proto-Ni-Laterite Profiles from Non-Tropical Climates. Minerals 2023, 13, 844. https://doi.org/10.3390/min13070844
González-Jiménez JM, Villanova-de-Benavent C, Yesares L, Marchesi C, Cartwright D, Proenza JA, Monasterio-Guillot L, Gervilla F. Metal Mobility in Embryonic-to-Proto-Ni-Laterite Profiles from Non-Tropical Climates. Minerals. 2023; 13(7):844. https://doi.org/10.3390/min13070844
Chicago/Turabian StyleGonzález-Jiménez, José María, Cristina Villanova-de-Benavent, Lola Yesares, Claudio Marchesi, David Cartwright, Joaquín A. Proenza, Luis Monasterio-Guillot, and Fernando Gervilla. 2023. "Metal Mobility in Embryonic-to-Proto-Ni-Laterite Profiles from Non-Tropical Climates" Minerals 13, no. 7: 844. https://doi.org/10.3390/min13070844




