Genesis of Pyrite in Clastic Rocks of Deep Salt-Related Strata in the Simao Basin and Its Implication for Potash Mineralization: A Case Study of the Well MK-3
Abstract
:1. Introduction
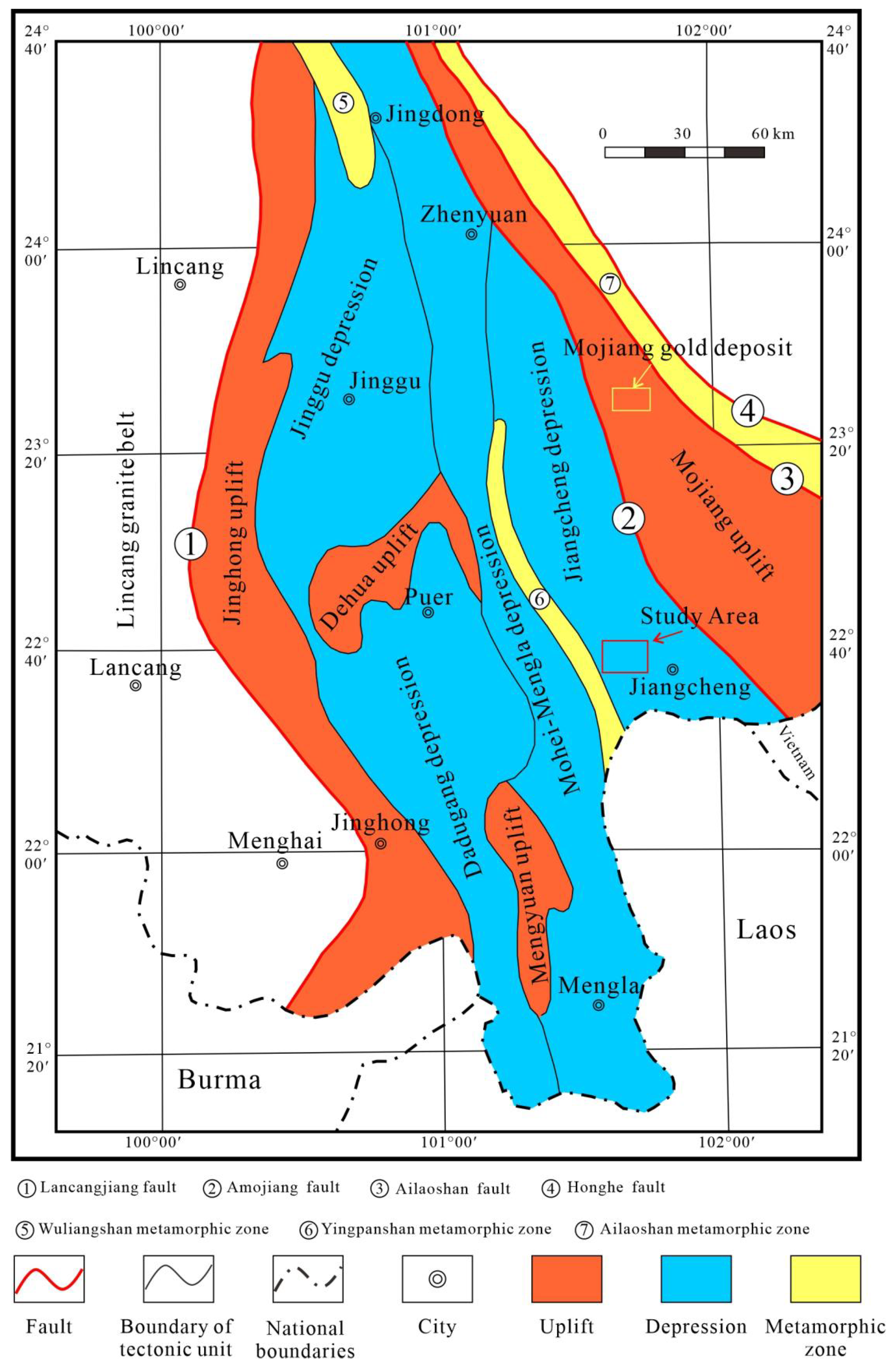
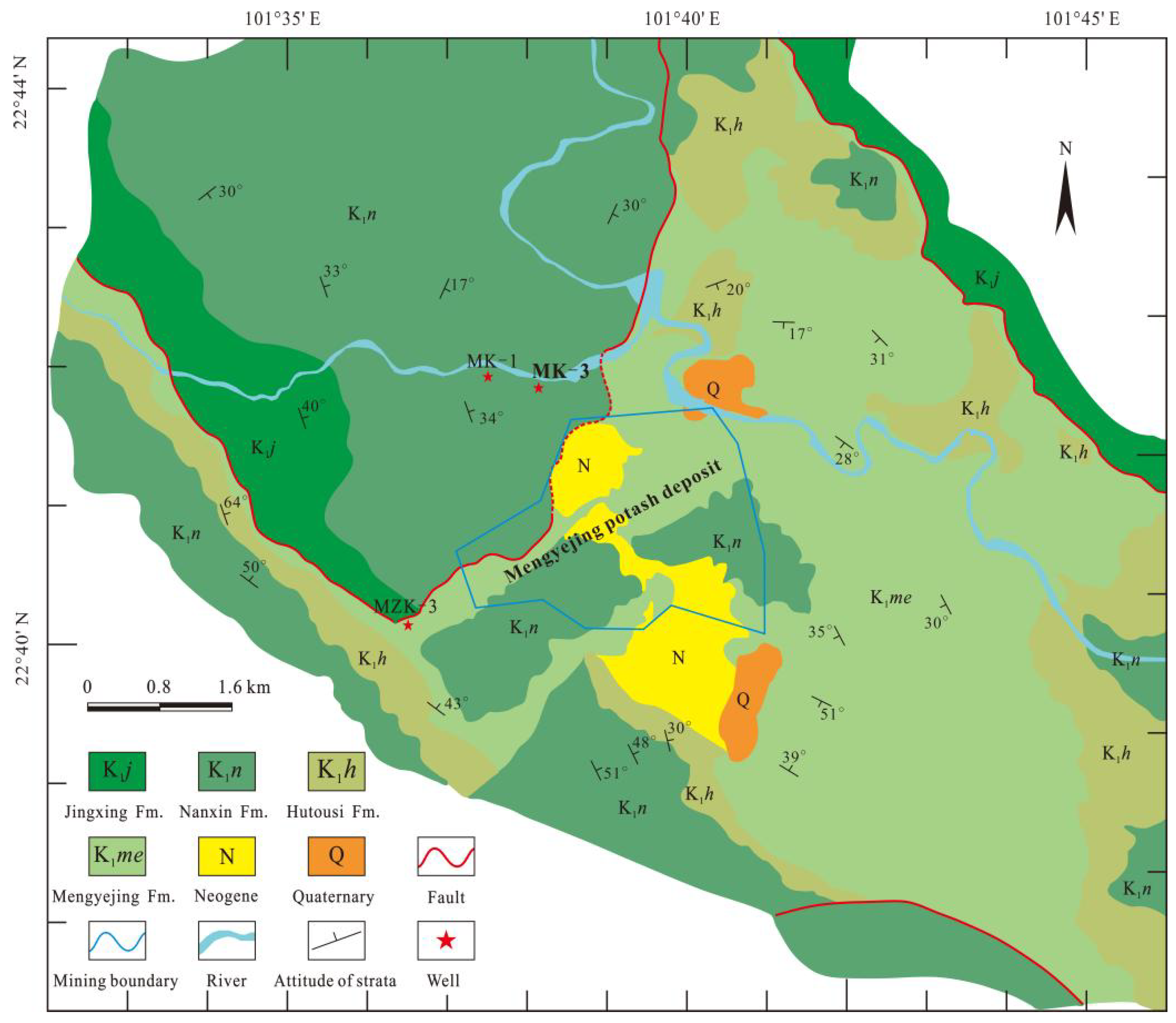
2. Geological Setting
3. Samples and Methods
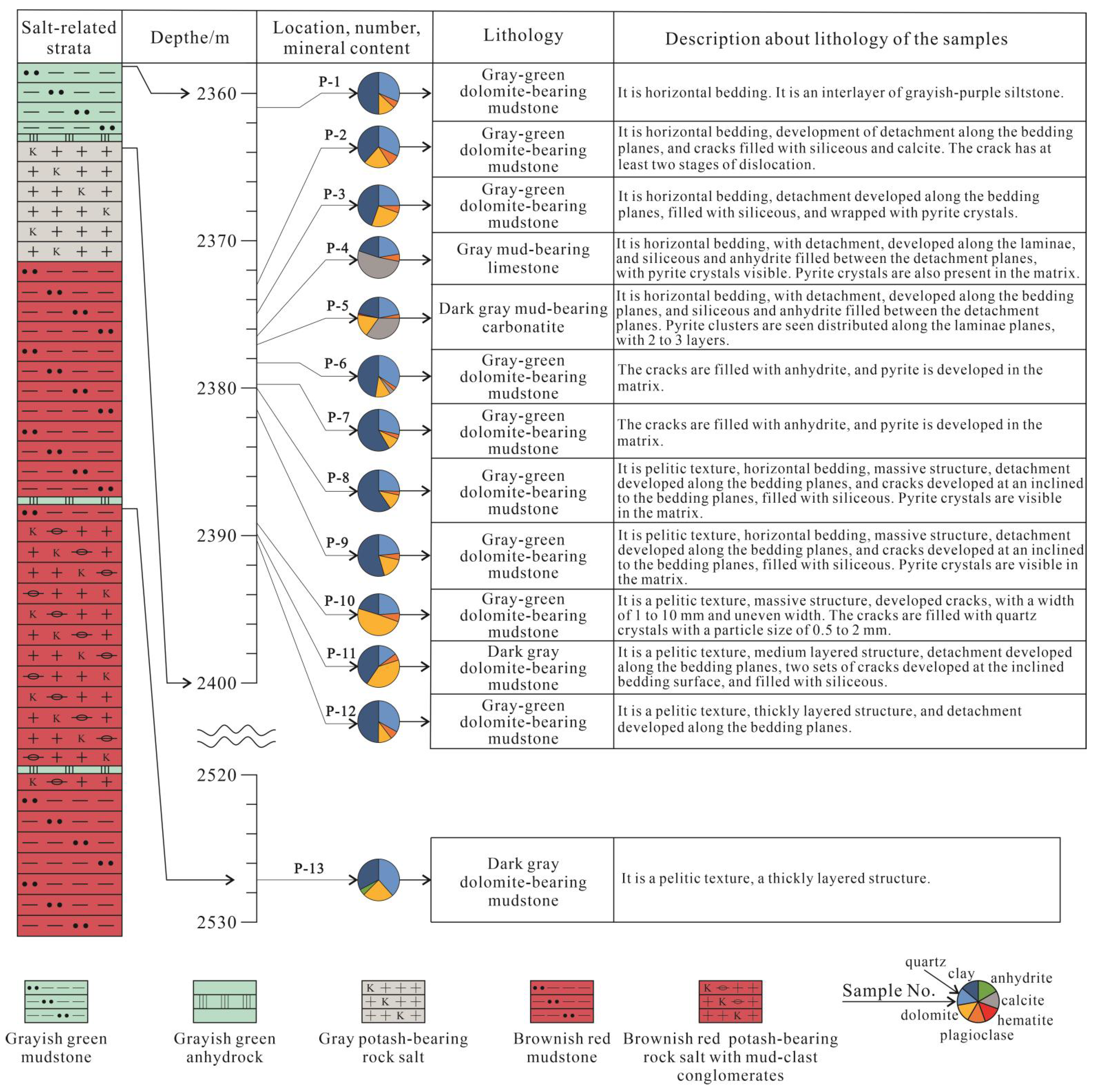
3.1. Samples
3.2. Methods
3.2.1. Thin Section Identification of Whole Rock Samples
3.2.2. Mineral Composition Analysis of Whole Rock Samples
3.2.3. Scanning Electron Microscope and Energy Dispersive Spectrometer
3.2.4. In Situ Analysis of Sulfur Isotopes
4. Results
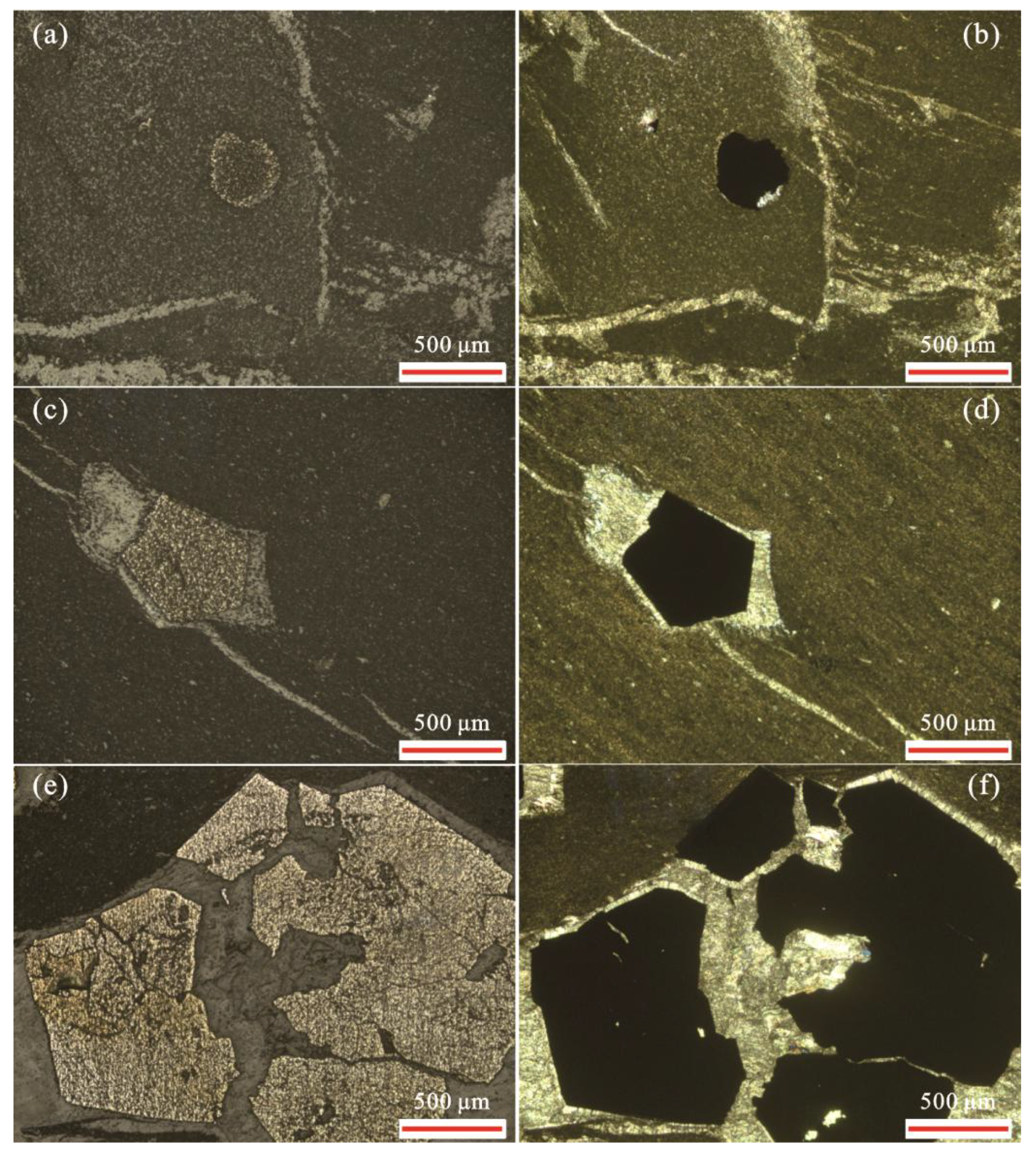
4.1. Petrological Characteristics of the Sample
4.2. Whole Rock Mineral Composition
4.3. Crystal, Elemental, and Sulfur Isotope Characteristics of Pyrite
5. Discussion
5.1. The Origin of Pyrite
5.2. Properties of Hydrothermal Fluids about Pyrite
5.3. Influence of Hydrothermal Activity on Potash Mineralization
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, M.P.; Zhang, Z.; Yin, H.W.; Tan, X.H.; Yu, C.Q.; Shi, L.F.; Zhang, X.F.; Yang, J.X.; Jiao, J.; Wu, G.P. A new viewpoint concerning the formation of the Mengyejing potash deposit in Jiangcheng, Yunnan. Acta Geosci. Sin. 2014, 35, 11–24. [Google Scholar]
- Miao, Z.Y.; Zheng, M.; Lou, P.C.; Wang, D.; Xu, Q.H.; Xu, J.M. A tuff interlayer in deep potash-bearing salt rocks and its implication for potash mineralization in the Simao Basin, southwestern China. Sci. Rep. 2022, 12, 16320. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.Y.; Zheng, M.P.; Li, W.Q.; Xu, Q.H.; Xia, Z.G.; Lou, P.C.; Zhang, X.F.; Chen, J.Y. Sr-S-K isotopes to tracing material sources of the deep potassium-bearing salt body, in the Simao Basin, southwestern China. Acta Geol. Sin. (Chin. Ed.) 2023, 97, 1987–2001. [Google Scholar]
- Du, S.R.; Zheng, M.P.; Lü, Y.Y.; Zhang, Y.S.; Zhang, X.F.; Miao, Z.Y.; Li, M.M. Origin of halite in the Lanping–Simao Basin, Southeastern Tibetan Plateau, China: Evidence from Sr and Cl isotopes. Acta Geol. Sin. (Engl. Transl.) 2022, 96, 2028–2039. [Google Scholar] [CrossRef]
- Song, G.; Miao, Z.Y.; Du, S.R.; Li, X.M. Geochemical Characteristics of trace elements before and after the upper salt forming period of the MK-3 core, Simao Basin and their paleoenvironmental implications. Acta Geosci. Sin. 2022, 43, 584–592. [Google Scholar]
- Large, R.R.; Maslennikov, V.V.; Robert, F.; Danyushevsky, L.V.; Chang, Z.S. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi log deposit, Lena Gold Province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Murowchick, J.B.; Barnes, H.L. Effects of temperature and degree of supersaturation on pyrite morphology. Am. Mineral. 1987, 72, 1241–1250. [Google Scholar]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Steadman, J.A.; Large, R.R.; Meffre, S.; Olin, P.H.; Danyushevsky, L.V.; Gregory, D.D.; Belousov, I.; Lounejeva, E.; Ireland, T.R.; Holden, P. Synsedimentary to early diagenetic gold in black shale-hosted pyrite nodules at the golden mile deposit, Kalgoorlie, Western Australia. Econ. Geol. 2015, 110, 1157–1191. [Google Scholar] [CrossRef]
- Metcalfe, I. Gondwana dispersion and Asian accretion: Tectonic and palaeogeographic evolution of eastern Tethys. J. Asian Earth Sci. 2013, 66, 1–33. [Google Scholar] [CrossRef]
- Pan, G.T.; Li, X.Z.; Wang, L.Q.; Ding, J.; Chen, Z.L. Preliminary division of tectonic units of the Qinghai-Tibet Plateau and its adjacent regions. Geol. Bull. China 2002, 21, 701–707. [Google Scholar]
- Wu, N.P.; Jiang, S.Y.; Liao, Q.L.; Pan, J.Y.; Dai, B.Z. Lead and sulfur isotope geochemistry and the ore sources of the vein-type copper deposits in Lanping-Simao Basin, Yunnan province. Acta Petrol. Sin. 2003, 19, 799–807. [Google Scholar]
- Tan, F.W. The sedimentary characteristics of Simao Triassic rear arc foreland basin, Yunnan province. Acta Sedimentol. Sin. 2002, 20, 560–567. [Google Scholar]
- Yin, F.G.; Pan, G.T.; Wan, F.; Li, X.Z.; Wang, F.G. Tectonic facies along the Nujiang-Lancangjiang-Jinshajiang orogenic belt in southwestern China. Sediment. Geol. Tethyan Geol. 2006, 26, 33–39. [Google Scholar]
- Yang, J.X.; Yin, H.W.; Zhang, Z.; Zheng, M.P. Geologic settings of the potassium formations in the Lanping-Simao Basin, Yunnan Province. Geotecton. Metallog. 2013, 37, 633–640. [Google Scholar]
- Lou, P.C.; Miao, Z.Y.; Zheng, M.P.; Zhang, X.F.; Ruan, Z.; Xu, Q.H. Paleogeographic characteristics of the Mengyejing formation in the Simao Basin during its depositional period and its indication of potash mineralization: A case study of MZK-3 well. Minerals 2021, 11, 338. [Google Scholar] [CrossRef]
- Lou, P.C.; Miao, Z.Y.; Zheng, M.P.; Ma, N.N.; Xu, Q.H.; Li, X.M. Genetic Model of mud-clast conglomerates in salt rocks and their significance for salt mineralization in Mohei Area, Simao Basin, China: A case study of well L-2. ACS Omega 2022, 7, 15547–15560. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Zheng, M.P.; Zhang, X.F.; Zhang, Z.; Gao, Y.Z. Geochemistry of sulfur isotope in evaporite and its sedimentology significance: A case study from the well MZK-3 in the Simao basin, southwestern China. Acta Geol. Sin. (Chin. Ed.) 2019, 93, 1166–1179. [Google Scholar]
- Li, M.H.; Yan, M.D.; Wang, Z.R.; Liu, X.M.; Fang, X.M.; Li, J. The origins of the Mengye potash deposit in the Lanping–Simao Basin, Yunnan Province, Western China. Ore Geol. Rev. 2015, 69, 174–186. [Google Scholar] [CrossRef]
- Shen, L.J.; Wang, L.C.; Liu, C.L.; Zhao, Y.J. Sr, S, and O isotope compositions of evaporites in the Lanping–Simao Basin, China. Minerals 2021, 11, 96. [Google Scholar] [CrossRef]
- Tan, F.W.; Xu, X.S.; Yin, F.G.; Li, X.Z. Upper carboniferous sediments in the Simao region, Yunnan and their tectonic settings. Sediment. Facies Palaeogeogr. 1999, 19, 26–34. [Google Scholar]
- Mou, C.L.; Tan, Q.Y.; Yu, Q. The permian sequence stratigraphic framework and source-reservoir caprock associations in the Simao Basin, Yunnan. Sediment. Geol. Tethyan Geol. 2005, 25, 33–37. [Google Scholar]
- Qu, Y.H.; Yuan, P.Q.; Shuai, K.Y.; Zhang, Y.; Cai, K.Q.; Jia, S.Y.; Chen, C.D. Potash-Forming Rules and Prospect of Lower Tertiary in Lanping-Simao Basin, Yunnan; Geological Publishing House: Beijing, China, 1998; pp. 1–118. [Google Scholar]
- Yin, J.Y.; Sun, Z.M.; Yang, Z.Y.; Liang, Q.Z. Cretaceous and early tertiary paleomagnetic results from the Lanping basin and its geological implications. Chin. J. Geophys. 1999, 42, 648–659. [Google Scholar]
- Tong, Y.B.; Yang, Z.Y.; Zheng, L.D.; Xu, Y.L.; Wang, H.; Gao, L.; Hu, X.Z. Internal crustal deformation in the northern part of Shan-Thai Block: New evidence from paleomagnetic results of Cretaceous and Paleogene redbeds. Tectonophysics 2013, 608, 1138–1158. [Google Scholar] [CrossRef]
- Tapponnier, P.; Lacassin, R.; Leloup, P.H.; Schärer, U.; Zhong, D.L.; Wu, H.W.; Liu, X.H.; Ji, S.C.; Zhang, L.S.; Zhong, J.Y. The Ailao Shan/Red River metamorphic belt: Tertiary left-lateral shear between Indochina and South China. Nature 1990, 343, 431–437. [Google Scholar] [CrossRef]
- Shen, L.J.; Liu, C.L.; Zhao, J.X.; Feng, Y.X.; Wang, L.C. The remaking of the Mengyejing potash deposit in Yunnan, China: Evidence from Rb-Sr isotopic systematics. Ore Geol. Rev. 2017, 89, 876–886. [Google Scholar] [CrossRef]
- Crowe, D.E.; Vaughan, R.G. Characterization and use of isotopically homogeneous standards for in situ laser microprobe analysis of 34S/32S ratios. Am. Mineral. 1996, 81, 187–193. [Google Scholar] [CrossRef]
- Li, R.C.; Xia, X.P.; Yang, S.H.; Chen, H.Y.; Yang, Q. Off-mount calibration and one new potential pyrrhotite reference material for sulfur isotope measurement by secondary ion mass spectrometry. Geostand. Geoanal. Res. 2019, 43, 177–187. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.S.; Wei, Q.; Wang, X.Q.; Li, Q.; Hu, G.W.; Gao, Y.Y. Study on the authigenic pyrites and their sulfur stable isotopes in recovered sediments during IODP 311 expedition. Geoscience 2008, 22, 402–406. [Google Scholar]
- Hu, Y.L.; Wang, W.; Zhou, C.M. Morphologic and isotopic characteristics of sedimentary pyrite: A case study from deepwater facies, Ediacaran Lantian formation in south China. Acta Sedimentol. Sin. 2020, 38, 138–149. [Google Scholar]
- Wilkin, R.T.; Arthur, M.A.; Dean, W.E. History of water-column anoxia in the Black Sea indicated by pyrite framboid size distributions. Earth Planet. Sci. Lett. 1997, 148, 517–525. [Google Scholar] [CrossRef]
- Sun, X.M.; Xiong, D.X.; Wang, S.W.; Shi, G.Y.; Zhai, W. Platinum group elements (PGE) geochemistry of Mojiang Au-Ni deposit and its constraint on ore genesis. Miner. Deposits 2006, 25, 438–446. [Google Scholar]
- Xianwu, B.; Ruizhong, H. The mechanism for formation and evolution of ore fluids of the Mojiang gold deposit, Yunnan. Geol. Rev. 1997, 43, 381–387. [Google Scholar]
- Li, Y.; Jin, S.C.; Yu, G.J. Isotope geochemistry and genesis of Mojiang gold deposit, Yunnan. Geol. -Geochem. 1998, 26, 15–20. [Google Scholar]
- Zhou, K.; Zhang, H.R.; Chai, P.; Zhang, H.C.; Cheng, X.F.; Yang, S. On the occurrence and genesis of gold and nickel in Jinchang deposit, Mojiang county, Yunnan province. Miner. Deposits 2020, 39, 97–110. [Google Scholar]
- Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposits. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Woodruff, L.G.; Shanks Iii, W.C. Sulfur isotope study of chimney minerals and vent fluids from 21°N, East Pacific Rise: Hydrothermal sulfur sources and disequilibrium sulfate reduction. J. Geophys. Res. 1988, 93, 4562–4572. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Meng, Q.Q. Genesis of pyrite in Ordovician carbonate of the Tarim basin. Acta Petrol. Mineral. 2010, 29, 516–524. [Google Scholar]
- Kemp, A.L.W.; Thode, H.G. The mechanism of the bacterial reduction of sulphate and of sulphite from isotope fractionation studies. Geochim. Cosmochim. Acta 1968, 32, 71–91. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B. The production of 34S-depleted sulfide during bacterial disproportionation of elemental sulfur. Science 1994, 266, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Sawlowicz, Z. Pyrite framboids and their development: A new conceptual mechanism. Geol. Rundsch. 1993, 82, 148–156. [Google Scholar] [CrossRef]
- Merinero, R.; Lunar, R.; Martínez-Frías, J.; Somoza, L.; Díaz-Del-Río, V. Iron oxyhydroxide and sulphide mineralization in hydrocarbon seep- related carbonate submarine chimneys, Gulf of Cadiz (SW Iberian Peninsula). Mar. Petrol. Geol. 2008, 25, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.S.; Wu, C.D.; Liang, J.Q.; Wang, Y.Z.; Ye, Y.T.; Fang, Y.X.; Ma, J.; Zhai, L.N. Characterstics and formation mechanism of pyrite tubes in sediments from Xisha trough in northern South China Sea. J. Palaeogeogr. 2020, 22, 175–192. [Google Scholar]
- Xie, G.Q.; Hu, R.Z.; Ni, P.; Su, W.C. Geochemical characteristics of fluid inclusions in gold-bearing quartz veins in the Mojiang gold deposit and their implications. Acta Mineral. Sin. 2001, 21, 613–618. [Google Scholar]
- Xiong, Y.Q.; Yang, L.Q.; Shao, Y.J.; Zhao, K.; Li, P.; Lu, Y.G.; Du, D.Y. Metallogenic process in Jinchang gold-nickel deposit, Mojiang County, SW Yunnan, China: Constraints from occurrence of gold and nickel. Acta Petrol. Sin. 2015, 31, 3309–3330. [Google Scholar]
- Fan, W.M.; Wang, Y.J.; Zhang, A.M.; Zhang, F.F.; Zhang, Y.Z. Permian arc-back-arc basin development along the Ailaoshan tectonic zone: Geochemical, isotopic and geochronological evidence from the Mojiang volcanic rocks, Southwest China. Lithos 2010, 119, 553–568. [Google Scholar] [CrossRef]
- Xie, G.Q.; Hu, R.Z.; Mao, J.W.; Fang, W.X.; Li, R.L. Discussion on metallogenic ages of Mojiang gold deposit in Yunnan province. Miner. Deposits 2004, 23, 253–260. [Google Scholar]
- Wang, Q.W.; Morse, J.W. Pyrite formation under conditions approximating those in anoxic sediments I. Pathway and morphology. Mar. Chem. 1996, 52, 99–121. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, L.Q.; Li, P.; Xiong, Y.Q. Morphology and chemistry composition of pyrite in the Laowangzhai gold deposit, Ailaoshan orogenic belt, SW China. Acta Petrol. Sin. 2013, 29, 3937–3948. [Google Scholar]
- Oberthür, T.; Cabri, L.J.; Weiser, T.W.; Mcmahon, G.; Müller, P. Pt, Pd and other trace elements in sulfides of the main sulfide zone, Great Dyke, Zimbabwe: A reconnaissance study. Can. Mineral. 1997, 35, 597–609. [Google Scholar]
- Gao, X.; Fang, Q.F.; Yao, W.; Peng, Q.; Dong, J.; Qin, H.; Di, Y.W. Genesis of the Mengyejing potash deposit in Lanping-Simao basin, Yunnan: Indication from the components of the deposit. Acta Geosci. Sin. 2013, 34, 529–536. [Google Scholar]
- Shen, L.J.; Liu, C.L.; Zhao, J.X.; Feng, Y.X.; Wang, L.C. The formation process of the Mengyejing potash deposit, Yunnan, China: Evidence from geochemical and petrological characteristics. Geosci. J. 2021, 25, 619–633. [Google Scholar] [CrossRef]






| S.N. | No. | Depth/m | Lithology | Relative Mineral Content/wt% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qtz. | Pl. | Cal. | Dol. | Hem. | Anh. | C.M. | ||||
| 1 | P-1 | 2360.8 | D.B.M. | 31.5 | 5.4 | / | 13.0 | / | / | 50.1 |
| 2 | P-2 | 2373.2 | D.B.M. | 32.2 | 8.9 | / | 20.5 | / | / | 38.4 |
| 3 | P-3 | 2374.9 | D.M. | 24.8 | 5.6 | / | 25.0 | / | / | 44.6 |
| 4 | P-4 | 2376.4 | A.L. | 22.4 | 5.7 | 52.0 | / | / | / | 19.9 |
| 5 | P-5 | 2377.2 | A.C.R. | 22.1 | 3.0 | 34.8 | 17.7 | 1.1 | / | 21.3 |
| 6 | P-6 | 2378.2 | D.B.M. | 34.1 | 2.9 | 3.8 | 11.7 | / | / | 47.5 |
| 7 | P-7 | 2379.7 | D.B.M. | 28.3 | 3.5 | / | 9.7 | / | / | 58.5 |
| 8 | P-8 | 2380.0 | D.B.M. | 25.4 | 2.9 | / | 12.2 | / | / | 59.5 |
| 9 | P-9 | 2381.4 | D.B.M. | 23.4 | 5.0 | / | 17.1 | / | / | 54.5 |
| 10 | P-10 | 2388.9 | D.M. | 23.9 | 6.6 | / | 49.3 | / | / | 20.2 |
| 11 | P-11 | 2389.7 | D.M. | 14.8 | 5.2 | / | 39.5 | / | / | 40.5 |
| 12 | P-12 | 2390.2 | D.B.M. | 33.1 | 6.5 | / | 10.6 | / | / | 49.8 |
| 13 | P-13 | 2527.3 | D.B.M. | 38.4 | / | / | 24.1 | / | 4.8 | 32.7 |
| S.N. | No. | Crystal Type | Grain Size/μm | S/Fe | δ34SV-CDT/‰ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | S.D. | M.A. | |||||
| 1 | P-1 | - | - | - | 0.79 | 7.35 | 4.36 | 3.58 | 3 |
| 2 | P-2 | - | - | - | −2.36 | 6.97 | 1.28 | 3.00 | 15 |
| 3 | P-3 | I.G. | 50–180 | 1.97 | −15.65 | 11.41 | 5.7 | 4.90 | 30 |
| 4 | P-4 | I.G. | 50–100 | 1.87 | −3.9 | 11.57 | 6.62 | 3.27 | 30 |
| 5 | P-5 | P.D. | 100–120 | 2.11 | 3.89 | 11.03 | 8.2 | 2.26 | 15 |
| I.G. | 100 | 2.03 | |||||||
| 6 | P-6 | I.G. | 100–160 | 1.84 | −3.2 | 7.26 | 3.53 | 3.36 | 15 |
| 7 | P-7 | P.D. | 60–100 | 2.32 | −0.82 | 4.47 | 2.25 | 1.36 | 15 |
| 8 | P-8 | I.G. | 100–200 | 2.05 | −3.49 | 8.12 | 4.43 | 2.68 | 30 |
| Cuboidal | 90–120 | 1.89 | |||||||
| P.D. | 90 | 2.02 | |||||||
| 9 | P-9 | I.G. | 120–220 | 2.01 | 1.72 | 7.99 | 4.17 | 1.47 | 30 |
| Cuboidal | 160–200 | 1.85 | |||||||
| P.D. | 90 | 2.27 | |||||||
| 10 | P-10 | I.G. | 120–300 | 1.98 | −0.83 | 6.96 | 4.29 | 1.93 | 30 |
| 11 | P-11 | - | - | - | −13.74 | 11.81 | 0.79 | 10.30 | 30 |
| 12 | P-12 | I.G. | 90–120 | 1.91 | −2.87 | 6.48 | 2.08 | 2.87 | 30 |
| 13 | P-13 | Octahedral | 50–100 | 1.87 | −15.02 | −6.36 | −10.66 | 2.66 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Z.; Zheng, M.; Lou, P.; Xu, Q.; Liu, Y. Genesis of Pyrite in Clastic Rocks of Deep Salt-Related Strata in the Simao Basin and Its Implication for Potash Mineralization: A Case Study of the Well MK-3. Minerals 2023, 13, 949. https://doi.org/10.3390/min13070949
Miao Z, Zheng M, Lou P, Xu Q, Liu Y. Genesis of Pyrite in Clastic Rocks of Deep Salt-Related Strata in the Simao Basin and Its Implication for Potash Mineralization: A Case Study of the Well MK-3. Minerals. 2023; 13(7):949. https://doi.org/10.3390/min13070949
Chicago/Turabian StyleMiao, Zhongying, Mianping Zheng, Pengcheng Lou, Qihui Xu, and Yuanying Liu. 2023. "Genesis of Pyrite in Clastic Rocks of Deep Salt-Related Strata in the Simao Basin and Its Implication for Potash Mineralization: A Case Study of the Well MK-3" Minerals 13, no. 7: 949. https://doi.org/10.3390/min13070949
APA StyleMiao, Z., Zheng, M., Lou, P., Xu, Q., & Liu, Y. (2023). Genesis of Pyrite in Clastic Rocks of Deep Salt-Related Strata in the Simao Basin and Its Implication for Potash Mineralization: A Case Study of the Well MK-3. Minerals, 13(7), 949. https://doi.org/10.3390/min13070949







