Genesis and Geological Significance of Permian Oilfield Water in the Western Periphery of the Mahu Sag, Junggar Basin, China
Abstract
:1. Introduction
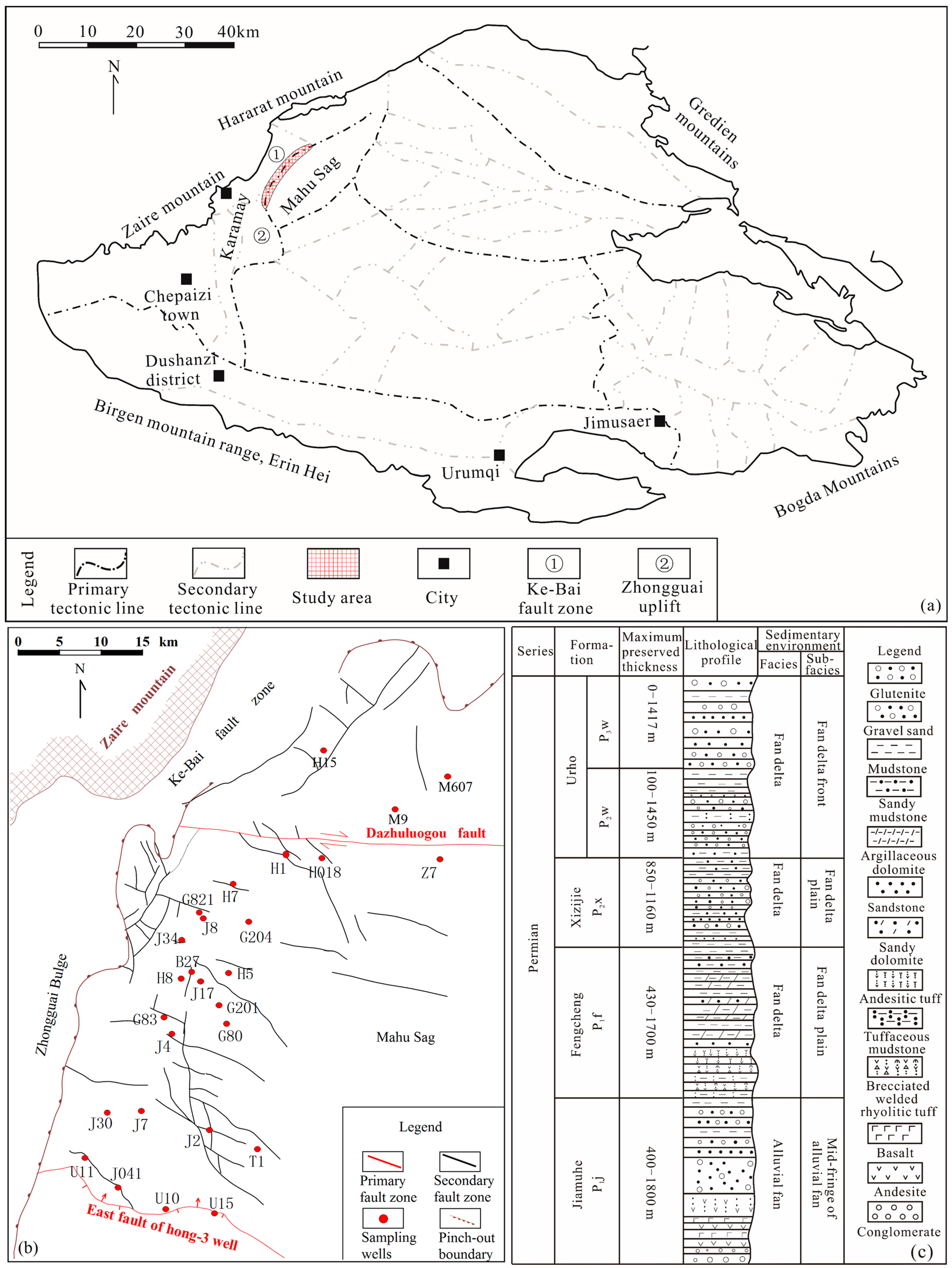
2. Regional Geology
3. Samples and Methods
4. Results
4.1. Total Dissolved Solids
4.2. Oilfield Water Type
4.3. Chemical Parameters of Oilfield Water
4.3.1. Na+ and Cl−
4.3.2. SO42– and rCl–
4.3.3. (HCO3– + CO32–) and Ca2+
4.3.4. Mg2+ and Ca2+
5. Discussion
5.1. Oil and Gas Preservation
5.2. Genesis of Oilfield Water
5.2.1. Tectonic Activity and Paleoclimate
5.2.2. Water–Rock Interactions
6. Geological Significance
6.1. Physical Properties of Reservoirs
6.2. Favourable Areas for Exploration
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewan, M.D. Experiments on the role of water in petroleum formation. Geochim. Cosmochim. Acta 1997, 61, 3691–3723. [Google Scholar] [CrossRef]
- Pan, C.C.; Geng, A.S.; Zhong, N.N.; Liu, J.Z.; Yu, L.P. Kerogen pyrolysis in the presence and absence of water and minerals: Amounts and compositions of bitumen and liquid hydrocarbons. Fuel 2009, 88, 909–919. [Google Scholar] [CrossRef]
- Wei, L.; Gao, Z.Y.; Mastalerz, M.; Schimmelmann, A.; Gao, L.; Wang, X.; Liu, X.X.; Wang, Y.; Qiu, Z. Influence of water hydrogen on the hydrogen stable isotope ratio of methane at low versus high temperatures of methanogenesis. Org. Geochem. 2019, 128, 137–147. [Google Scholar] [CrossRef]
- Bachu, S.; Underschultz, J.R. Hydrogeology of formation waters, northeastern Alberta basin. AAPG Bull. 1993, 77, 1745–1768. [Google Scholar]
- Verweij, J.M. Hydrocarbon Migration Systems Analysis; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Mccaffrey, M.A.; Lazar, B.H.D.H.; Holland, H.D. The evaporation path of seawater and the coprecipitation of Br− and K+ with halite. J. Sediment. Res. 1987, 57, 928–937. [Google Scholar]
- Davisson, M.L.; Criss, R.E. Na-Ca-Cl relations in basinal fluids. Geochim. Cosmochim. Acta 1996, 60, 2743–2752. [Google Scholar] [CrossRef]
- Worden, R.H.; Coleman, M.L.; Matray, J.M. Basin scale evolution of formation waters: A diagenetic and formation water study of the Triassic Chaunoy Formation, Paris Basin. Geochim. Cosmochim. Acta 1999, 63, 2513–2528. [Google Scholar] [CrossRef]
- Grassi, S.; Cortecci, G.; Squarci, P. Groundwater resource degradation in coastal plains: The example of the Cecina area (Tuscany–Central Italy). Appl. Geochem. 2007, 22, 2273–2289. [Google Scholar] [CrossRef]
- Li, Q.G.; Ju, Y.W.; Lu, W.Q.; Wang, G.C.; Neupane, B.; Sun, Y. Water-rock interaction and methanogenesis in formation water in the southeast Huaibei coalfield, China. Mar. Pet. Geol. 2016, 77, 435–447. [Google Scholar] [CrossRef]
- Xie, X.N.; Jiao, J.J.; Cheng, J.M. Regional variation of formation water chemistry and diagenesis reaction in underpressured system: Example from Shiwu depression of Songliao basin, NE China. J. Geochem. Explor. 2003, 78, 585–590. [Google Scholar]
- Al-Hajeri, M.M.; Bowden, S.A. Application of formation water geochemistry to assess seal integrity of the Gotnia Formation, Kuwait. Arab. J. Geosci. 2017, 10, 56. [Google Scholar] [CrossRef]
- Cheng, J.M.; McIntosh, J.C.; Xie, X.N.; Jiao, J.J. Hydrochemistry of formation water with implication to diagenetic reactions in Sanzhao depression and Qijia-gulong depression of Songliao Basin, China. J. Geochem. Explor. 2006, 88, 86–90. [Google Scholar] [CrossRef]
- Wang, Q.M.; Hu, Q.H.; Larsen, C.; Zhao, C.; Sun, M.D.; Zhang, Y.X.; Zhang, T. Microfracture-pore structure characterization and water-rock interaction in three lithofacies of the Lower Eagle Ford Formation. Eng. Geol. 2021, 292, 106276. [Google Scholar] [CrossRef]
- Zhang, C.M.; Song, X.M.; Wang, X.J.; Wang, X.L.; Zhao, K.; Shuang, Q.; Li, S.H. Origin and depositional characteristics of supported conglomerates. Pet. Explor. Dev. 2020, 47, 292–305. [Google Scholar] [CrossRef]
- Ma, H.N.; Tang, C.; Qiu, Z.Y.; Xiang, J.L. Reservoir Characteristics and Main Controlling Factors of Lower Wuerhe Formation in Madong Slope Belt of Mahu Sag. Fresenius Environ. Bull. 2021, 30, 11287–11296. [Google Scholar]
- Jia, H.B.; Ji, H.C.; Wang, L.S.; Gao, Y.; Li, X.W.; Zhou, H. Reservoir quality variations within a conglomeratic fan-delta system in the Mahu sag, northwestern Junggar Basin: Characteristics and controlling factors. J. Pet. Sci. Eng. 2017, 152, 165–181. [Google Scholar] [CrossRef]
- Yu, K.H.; Cao, Y.C.; Qiu, L.W.; Sun, P.P.; Yang, Y.Q.; Qu, C.S.; Lei, D.W.; Jia, X.Y.; Wan, M.; Zhang, Z.J. Unconformity-controlled dissolution contributes to reservoirs in the carbonate-rich Permian Fengcheng Formation, northwestern Junggar Basin, China. Carbonates Evaporites 2020, 35, 26. [Google Scholar] [CrossRef]
- Feng, C.; Ma, M.Z.; He, W.J.; Li, T.; Wu, Q.Y.; Zhang, Z.X.; Zhao, H.Y. Paleoenvironmental changes of source rocks from the Carboniferous to Permian sediments of the Mahu Sag, Junggar Basin, China. Geosystem Eng. 2020, 23, 276–286. [Google Scholar] [CrossRef]
- Xiang, B.L.; Li, E.T.; Gao, X.W.; Wang, M.; Wang, Y.; Xu, H.; Huang, P.; Yu, S.; Liu, J.Z.; Zou, Y.R.; et al. Petroleum generation kinetics for Permian lacustrine source rocks in the Junggar Basin, NW China. Org. Geochem. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Zhang, Y.Y.; Chen, S.; Guo, Z.J.; Tang, W.B. Controls of a strike-slip fault system on the tectonic inversion of the Mahu Sag at the northwestern margin of the Junggar Basin, NW China. J. Asian Earth Sci. 2020, 198, 104229. [Google Scholar] [CrossRef]
- Zhang, C.M.; Yin, T.J.; Tang, Y.; Guo, X.G.; Zhao, K.; Pan, J.; Chen, M.L. Advances in sedimentological reservoir research in Mahu sag and northwest margin ofJunggar Basin. J. Palaeogeogr. 2020, 22, 129–146. [Google Scholar]
- Lian, Y.C. Geologic Structure and Tectonic Evolution of the Karamay-Baikouquan Overthrust Belt at the Northwestern Margin of Junggar Basin. Master’s Thesis, China University of Geosciences, Beijing, China, 2016. [Google Scholar]
- Imin, A.; Zha, M.; Ding, X.J.; Bian, B.L.; Liu, Y.; Zheng, M.L.; Han, C. Identification of a Permian foreland basin in the western Junggar Basin (NW China) and its impact on hydrocarbon accumulation. J. Pet. Sci. Eng. 2020, 187, 106810. [Google Scholar] [CrossRef]
- SY/T 5523-2016; Method for Analysis of Oilfiled Water. Petroleum Industry Press: Beijng, China, 2016.
- Cheng, Z.H.; Wang, S.N.; Wang, L.; Cha, M. Characteristics of formation water chemical fields and its petroleum significance of the Neogene in Dongying Sag, Shandong Province. J. Palaeogeogr. 2012, 14, 685–693. [Google Scholar]
- Akstinat, M. Chemical and physicochemical properties of formation waters of the oil and gas industry. J. Hydrol. 2019, 578, 124011. [Google Scholar] [CrossRef]
- Guseva, N.V.; Kopylova, Y.G.; Oidup, C.K.; Arakchaa, K.D.; Rychkova, K.M.; Khvashchevskaya, A.A.; Ayunova, O.D. Formation of the chemical composition of brackish and brine groundwater in the Tuva depression and surrounding areas. Russ. Geol. Geophys. 2018, 59, 135–143. [Google Scholar] [CrossRef]
- Chen, J.; Liu, D.Y.; Hou, X.L.; Fan, Y.K.; Jia, W.L.; Peng, P.A.; Zhang, B.S.; Xiao, Z.Y. Origin and evolution of oilfield waters in the Tazhong oilfield, Tarim Basin, China, and their re-lationship to multiple hydrocarbon charging events. Mar. Pet. Geol. 2018, 98, 554–568. [Google Scholar] [CrossRef]
- Collins, A.G. Geochemistry of Oilfield Waters; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Surin, B.A.; Wang, C.Y. Oilfield Waters in Natural Water Systems; Petroleum Industry Press: Beijng, China, 1956. [Google Scholar]
- Zhang, S.W.; Zhang, L.Y.; Bao, Y.S.; Li, X.Y.; Liu, Q.; Li, J.Y.; Yin, Y.; Zhu, R.F.; Zhang, S.C. Formation fluid characteristics and hydrocarbon accumulation in the Dongying sag, Shengli Oilfield. Pet. Explor. Dev. 2012, 39, 423–435. [Google Scholar] [CrossRef]
- Xie, X.N.; Fan, Z.H.; Liu, X.F.; Lu, Y.C. Geochemistry of formation water and its implication on over pressured fluid flow in the Dongying Depression of the Bohaiwan Basin, China. J. Geochem. Explor. 2006, 89, 432–435. [Google Scholar] [CrossRef]
- Çelik, M.; Sari, A. Geochemistry of formation waters from upper cretaceous calcareous rocks of Southeast Turkey. J. Geol. Soc. India 2002, 59, 419–430. [Google Scholar]
- Tao, H.F.; Qu, Y.Q.; Wu, T.; Liu, B.B. Oilfield water and favorable petroleum exploration targets for the triassic baikouquan formation in the slope of Mahu Sag, junggar basin. Geofluids 2021, 2021, 6699489. [Google Scholar] [CrossRef]
- Yang, C.L.; Xie, Z.Y.; Pei, S.Q.; Guo, J.Y.; Zhang, L.; Dong, C.Y.; Hao, A.S.; Yang, C.X.; Liu, Z.X. Chemical characteristics of Middle Permian formation water and hydrocarbon preservation conditions in Northwest Sichuan. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 600, p. 012041. [Google Scholar]
- Tang, W.B.; Zhang, Y.Y.; Pe-Piper, G.; Piper, D.J.; Guo, Z.J.; Li, W. Permian rifting processes in the NW Junggar Basin, China: Implications for the post-accretionary successor basins. Gondwana Res. 2021, 98, 107–124. [Google Scholar] [CrossRef]
- Tang, W.B.; Zhang, Y.Y.; Pe-Piper, G.; Piper, D.J.; Guo, Z.J.; Li, W. Permian to early Triassic tectono-sedimentary evolution of the Mahu sag, Junggar Basin, western China: Sedimentological implications of the transition from rifting to tectonic inversion. Mar. Pet. Geol. 2021, 123, 104730. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Pe-Piper, G.; Piper, D.J.W.; Guo, Z.; Li, W. Soft-sediment deformation structures in alkaline lake deposits of Lower Permian Fengcheng Formation, Junggar Basin, NW China: Implications for syn-sedimentary tectonic activity. Sediment. Geol. 2020, 406, 105719. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Yuan, X.J.; Wang, M.S.; Zhou, C.M.; Tang, Y.; Chen, X.Y.; Lin, M.J.; Cheng, D.W. Alkaline-lacustrine deposition and paleoenvironmental evolution in Permian Fengcheng Formation at the Mahu sag, Junggar Basin, NW China. Pet. Explor. Dev. 2018, 45, 1036–1049. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.J.; Hu, W.X.; Yao, S.P.; Wang, X.L.; Zhang, Y.Q.; Tang, Y. The Permian hybrid petroleum system in the northwest margin of the Junggar Basin, northwest China. Mar. Pet. Geol. 2005, 22, 331–349. [Google Scholar] [CrossRef]
- Chen, S.P.; Zhang, Y.W.; Tang, L.J.; Bai, G.P. Tectonic Evolution of the Junggar Foreland Basin in the Late Carboniferous-Permian. Acta Geol. Sin. Engl. Ed. 2001, 75, 398–408. [Google Scholar]
- Chen, Y.B.; Cheng, X.G.; Zhang, H.; Li, C.Y.; Ma, Y.P.; Wang, G.D. Fault characteristics and control on hydrocarbon accumulation of middle-shallow layers in the slope zone of Mahu sag, Junggar Basin, NW China. Pet. Explor. Dev. 2018, 45, 1050–1060. [Google Scholar] [CrossRef]
- Li, H.X.; Cai, C.F. Origin and evolution of formation water from the Ordovician carbonate reservoir in the Tazhong area, Tarim Basin, NW China. J. Pet. Sci. Eng. 2017, 148, 103–114. [Google Scholar] [CrossRef]
- Yang, L.J.; Hou, D.J.; Chen, X.D. Chemical characteristics and geological significance of Palaeogene formation water in central Xihu Depression, East China Sea Basin. Nat. Gas Geosci. 2018, 29, 559–571. [Google Scholar]
- An, T.; Yu, B.S.; Wang, Y.S.; Ruan, Z.; Meng, W.; Feng, Y.L. Water-rock interactions and origin of formation water in the Bohai Bay Basin: A case study of the Cenozoic Formation in Bonan Sag. Interpretation 2021, 9, T475–T493. [Google Scholar] [CrossRef]
- Wang, J.; Lou, Z.H.; Zhu, R.; Jin, A.M. The migration of hydrocarbons: A case from the Shahejie formation of the Wenliu area of Bohai Bay Basin, China. Pet. Sci. Technol. 2019, 37, 38–49. [Google Scholar] [CrossRef]
- Bozau, E.; Sattler, C.D.; Berk, W. Hydrogeochemical classification of deep formation waters. Appl. Geochem. 2015, 52, 23–30. [Google Scholar] [CrossRef]
- Wu, J.B.; Yang, S.L.; Gan, B.W.; Cao, Y.S.; Zhou, W.; Kou, G.; Wang, Z.Q.; Li, Q.; Dong, W.G.; Zhao, B.B. Pore Structure and Movable Fluid Characteristics of Typical Sedimentary Lithofacies in a Tight Conglomerate Reservoir, Mahu Sag, Northwest China. ACS Omega 2021, 6, 23243–23261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, W.; Zhou, Q.; Wu, R.B.; Yang, Z.B.; Wang, Y.T.; Peng, X.H.; Liang, T. Study on Permian Sedimentary Environment and Reservoir Characteristics in Mahu Sag, Junggar Basin, China. Fresenius Environ. Bull. 2021, 30, 8886–8898. [Google Scholar]
- Kong, R.S. Research on the Mechanism of Low-Salinity Waterflooding. Master’s Thesis, China University of Geosciences, Beijing, China, 2017. (in Chinese). [Google Scholar]
- Birkle, P.; García, B.M.; Padrón, C.M.M.; Eglington, B.M. Origin and evolution of formation water at the Jujo–Tecominoacán oil reservoir, Gulf of Mexico. Part 2: Isotopic and field-production evidence for fluid connectivity. Appl. Geochem. 2009, 24, 555–573. [Google Scholar] [CrossRef]
- Birkle, P.; García, B.M.; Padrón, C.M.M. Origin and evolution of formation water at the Jujo–Tecominoacán oil reservoir, Gulf of Mexico. Part 1: Chemical evolution and water–rock interaction. Appl. Geochem. 2009, 24, 543–554. [Google Scholar] [CrossRef]








| Types | g L−1 | Current Seawater [26] g L−1 | Current River [26] g L−1 | ||
|---|---|---|---|---|---|
| Max | Min | Avg | |||
| K+ + Na+ | 95.91 | 0.83 | 11.14 | 11.04 | 0.006 |
| Ca2+ | 9.46 | 0.00 | 1.60 | 0.42 | 0.015 |
| Mg2+ | 0.17 | 0.00 | 0.02 | 1.32 | 0.004 |
| HCO3− | 47.99 | 0.07 | 4.56 | 0.14 | 0.059 |
| SO42− | 5.51 | 0.00 | 0.71 | 2.69 | 0.011 |
| Cl− | 58.39 | 1.21 | 12.61 | 19.32 | 0.003 |
| CO32− | 68.35 | 0.00 | 7.55 | 0.15 | 0.059 |
| TDS | 241.18 | 2.84 | 40.91 | 35.00 | 0.098 |
| Water Type | Classification Coefficient | Setting | ||
|---|---|---|---|---|
| rNa/rCl | rNa−Cl/2rSO4 | rCl−Na/2rMg | ||
| NaHCO3 | >1 | <1 | <0 | Continental environment |
| Na2SO4 | >1 | >1 | <0 | |
| MgCl2 | <1 | <0 | <1 | Marine environment |
| CaCl2 | <1 | <0 | >1 | Deep burial environment |
| Type | Concentration/Unit | Total | Hydrogeological Interpretation | ||||
|---|---|---|---|---|---|---|---|
| P3w | P2w | P2x | P1f | P1j | |||
| NaHCO3 | - | 1 | 3 | 9 | 1 | 14 | Hydrogeological active area, which has a destructive effect on oil and gas |
| CaCl2 | 15 | 6 | 4 | 4 | 12 | 41 | The formation has good sealing properties, which are favorable for oil and gas preservation |
| Na2SO4 | 1 | - | - | 1 | - | 2 | Affected by surface water; the formation has poor oil and gas preservation potential |
| No. | Well | Formation | Deep (m) | Oil Production (m3/day) | Gas Production (104 m3/day) |
|---|---|---|---|---|---|
| 1 | H014 | P3w2 | 3737~3844 | 138.29 | 0.86 |
| 2 | H015 | P3w1 | 3681~3702 | 7.40 | |
| P3w2 | 3614~3649 | 26.20 | 0.18 | ||
| T1b | 3496~3551 | 6.20 | |||
| 3 | H10 | P3w2 | 3570~3573 | 6.30 | |
| 4 | H11 | P3w | 3364~3396 | 8.70 | |
| T1b | 3230~3264 | 8.00 | |||
| 5 | H14 | P2w | 4100~4179 | 18.80 | |
| 6 | H18 | P3w1 | 3741~3755 | 6.85 | |
| P3w2 | 3642~3682 | 21.70 | 0.33 | ||
| 7 | H21 | P2w | 3578~3652 | 7.30 | |
| 8 | H22 | P2w | 3586~3668 | 25.60 | |
| 9 | H23 | P3w1 | 4283~4316 | 11.62 | |
| 10 | H24 | P3w2 | 3182~3205 | 16.64 | |
| 11 | H27 | P2w | 3281~3285 | 10.20 | 0.68 |
| 12 | H29 | P3w2 | 3242~3245 | 22.20 | 0.23 |
| 13 | Z7 | P2w | 4466~4563 | 18.62 | |
| 14 | H016 | P2w | 3724~3748 | 5.70 | |
| P3w21 | 3563~3568.5 | 12.30 | |||
| T1b | 3454~3495 | 12.50 | |||
| 15 | H017 | P2w | 3624.5~3646.5 | 20.50 | |
| P3w12 | 3537~3554 | 36.20 | |||
| P3w11 | 3494~3505.5 | 27.80 | |||
| 16 | H018 | P2w | 3672~3684 | 6.20 | |
| P3w12 | 3640~3658 | 7.40 | |||
| 17 | H402 | T1b2 | 3411~3434 | 9.40 | |
| 18 | G891 | P3w | 3072~3078 | 6.00 | |
| 19 | G204 | P3w22 | 3575~3584 | 6.50 | |
| 20 | G205 | P2x | 4339~4352 | 22.20 | |
| 4268~4286 | 12.40 | ||||
| 21 | L47 | P3w2 | 3225~3254 | 6.90 | 0.85 |
| 22 | M901 | T1b | 3521.5~3529 | 6.50 | |
| 23 | G811 | T1b | 3220~3227 | 8.80 | |
| 24 | J225 | P3w | 3461~3489 | 6.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, S.; Jin, J.; Jin, A.; Lou, Z.; Zhu, R. Genesis and Geological Significance of Permian Oilfield Water in the Western Periphery of the Mahu Sag, Junggar Basin, China. Minerals 2023, 13, 1043. https://doi.org/10.3390/min13081043
Li J, Zhang S, Jin J, Jin A, Lou Z, Zhu R. Genesis and Geological Significance of Permian Oilfield Water in the Western Periphery of the Mahu Sag, Junggar Basin, China. Minerals. 2023; 13(8):1043. https://doi.org/10.3390/min13081043
Chicago/Turabian StyleLi, Jiasi, Shuncun Zhang, Jun Jin, Aimin Jin, Zhanghua Lou, and Rong Zhu. 2023. "Genesis and Geological Significance of Permian Oilfield Water in the Western Periphery of the Mahu Sag, Junggar Basin, China" Minerals 13, no. 8: 1043. https://doi.org/10.3390/min13081043




