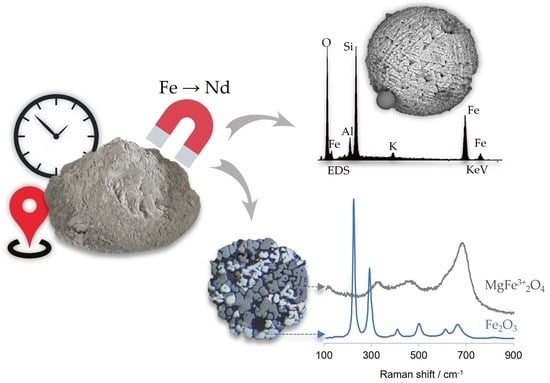
Physicochemical Properties of Fe-Bearing Phases from Commercial Colombian Coal Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analytical Methodologies
2.2.1. Chemical Analysis
2.2.2. X-ray Diffraction (XRD)
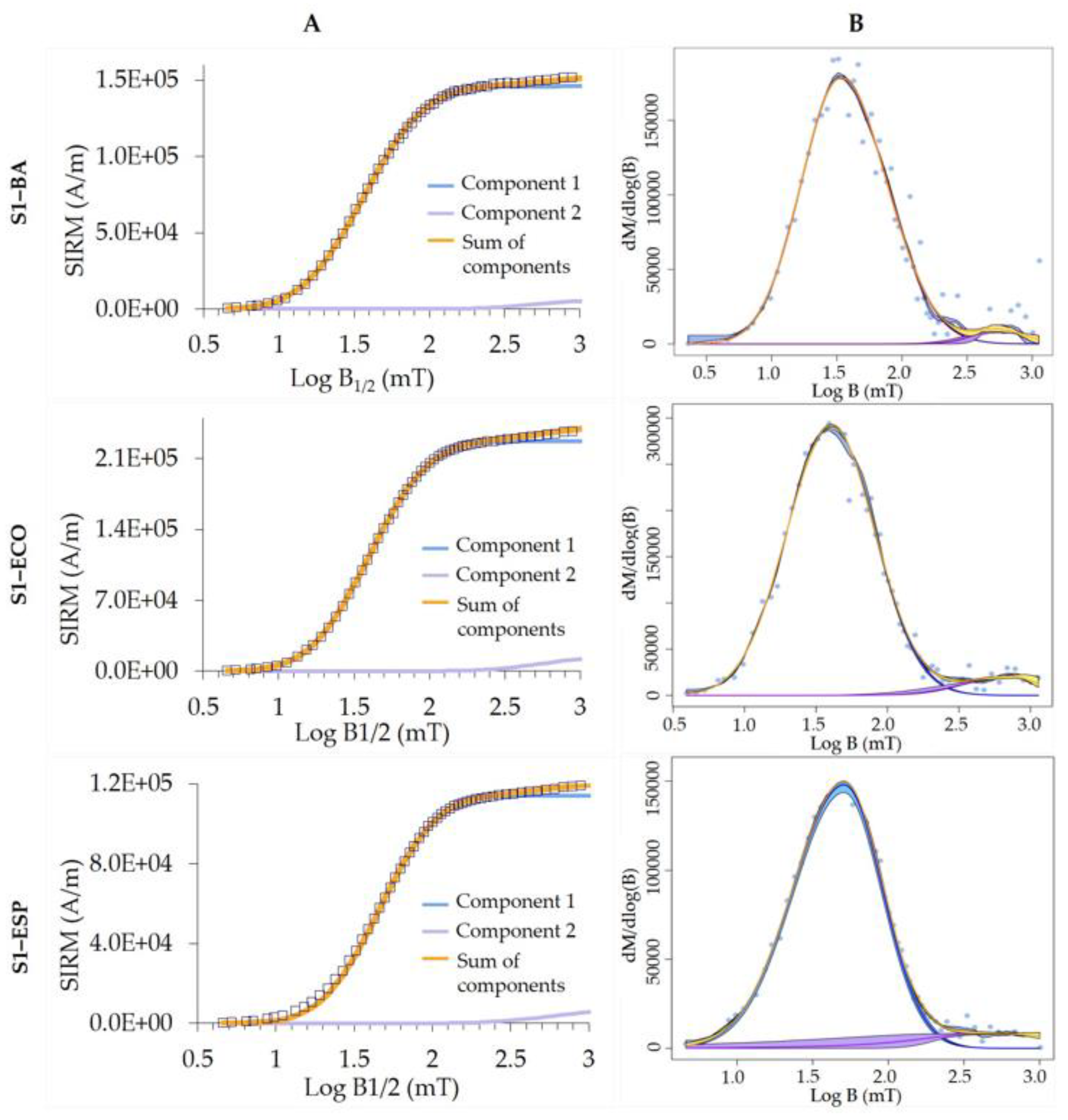
2.2.3. Magnetic Susceptibility and Isothermal Remanent Magnetization (IRM)
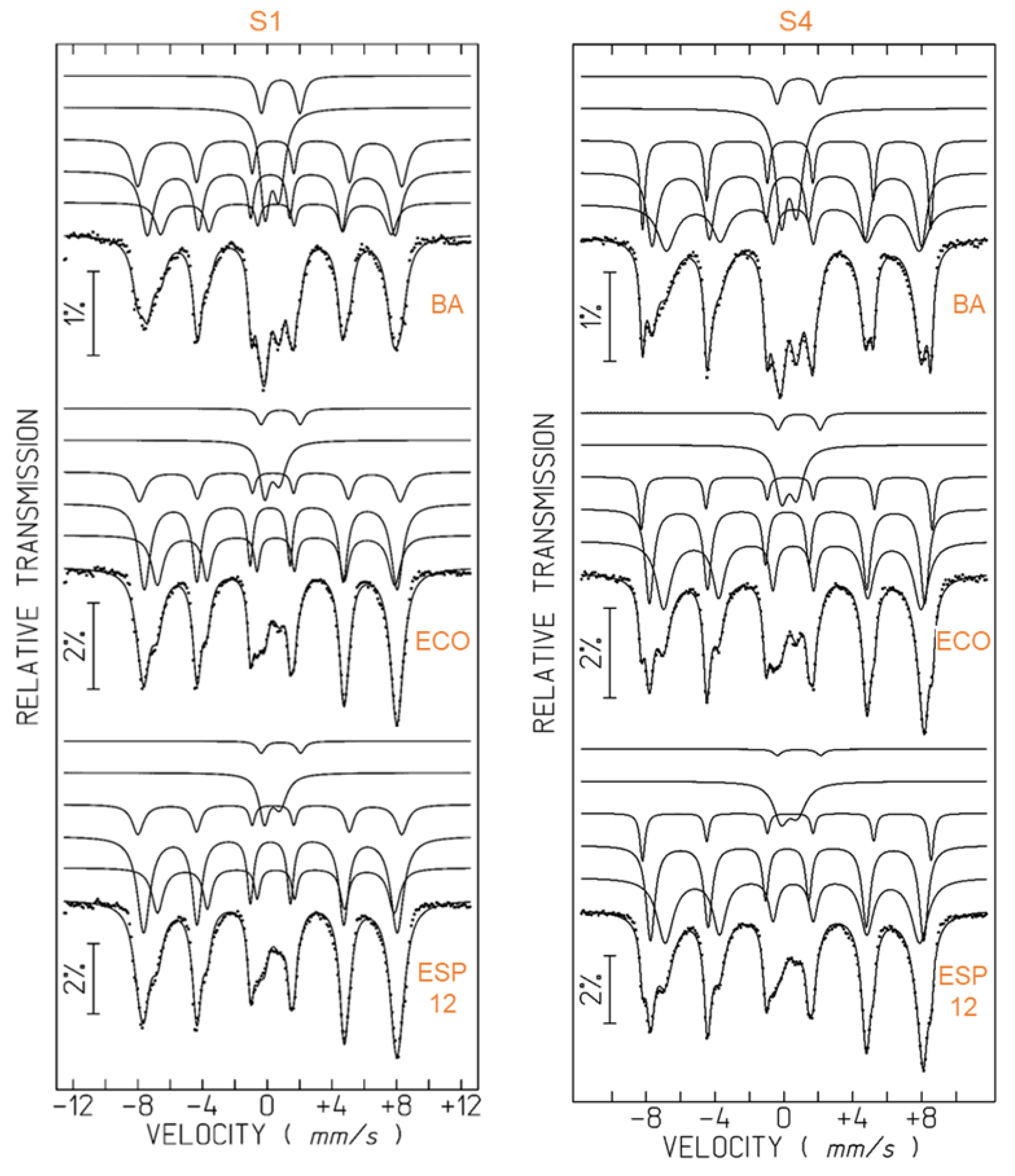
2.2.4. Mössbauer Spectroscopy
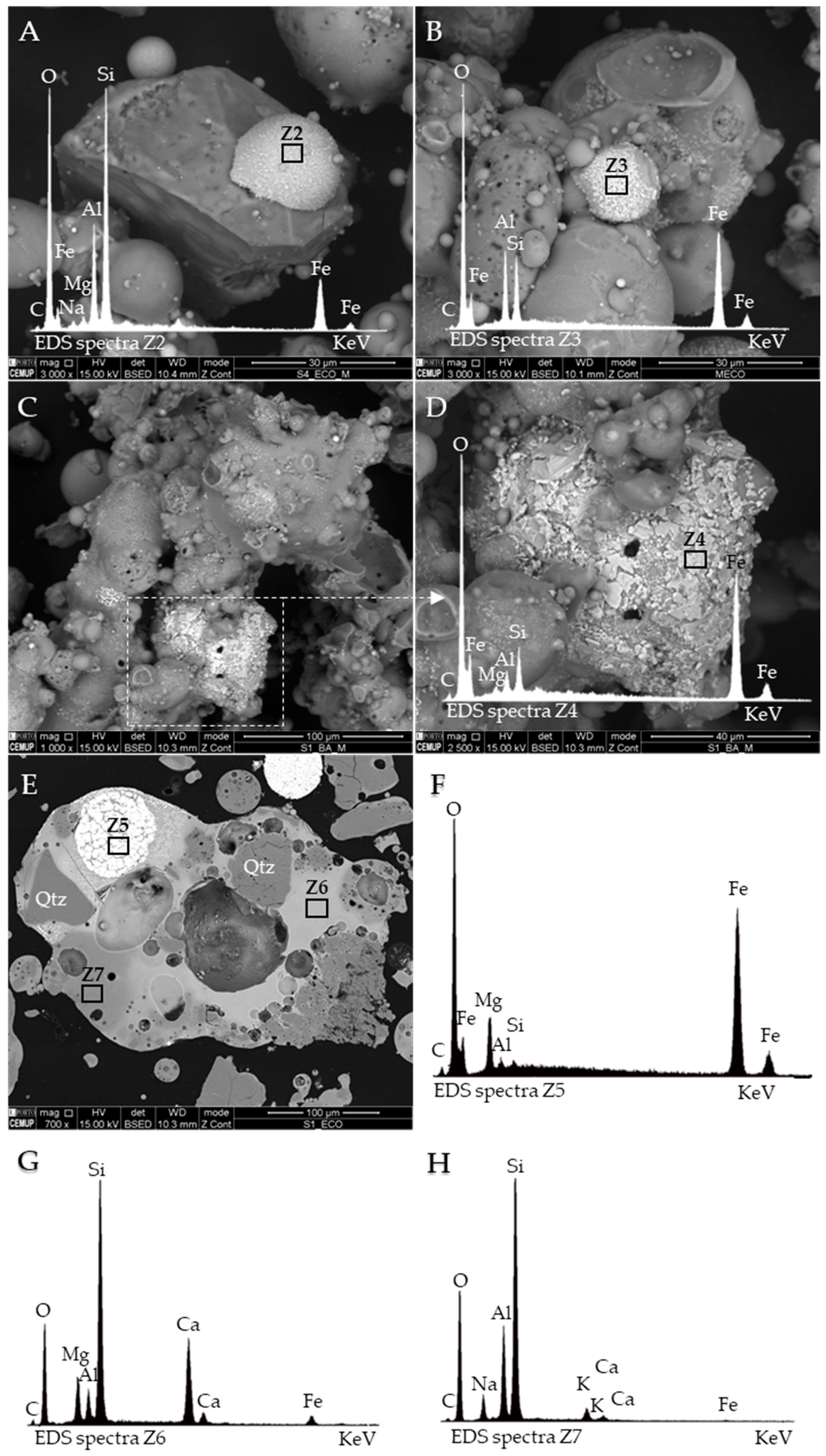
2.2.5. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM/EDS)
2.2.6. Optical Microscopy
2.2.7. Raman Microspectroscopy
3. Results and Discussion
3.1. Magnetic Parameters
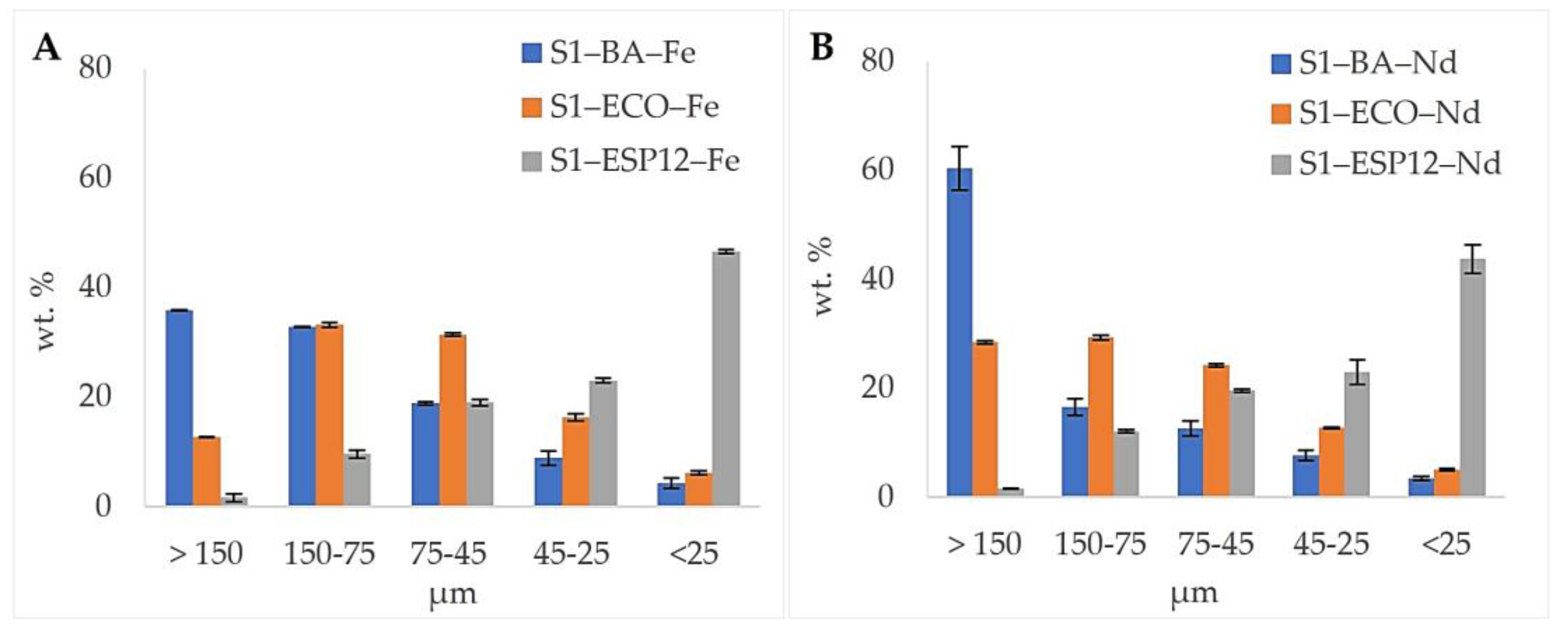
3.2. Yield and Particle-Size Distribution
3.3. Mineralogy and Iron Oxidation State
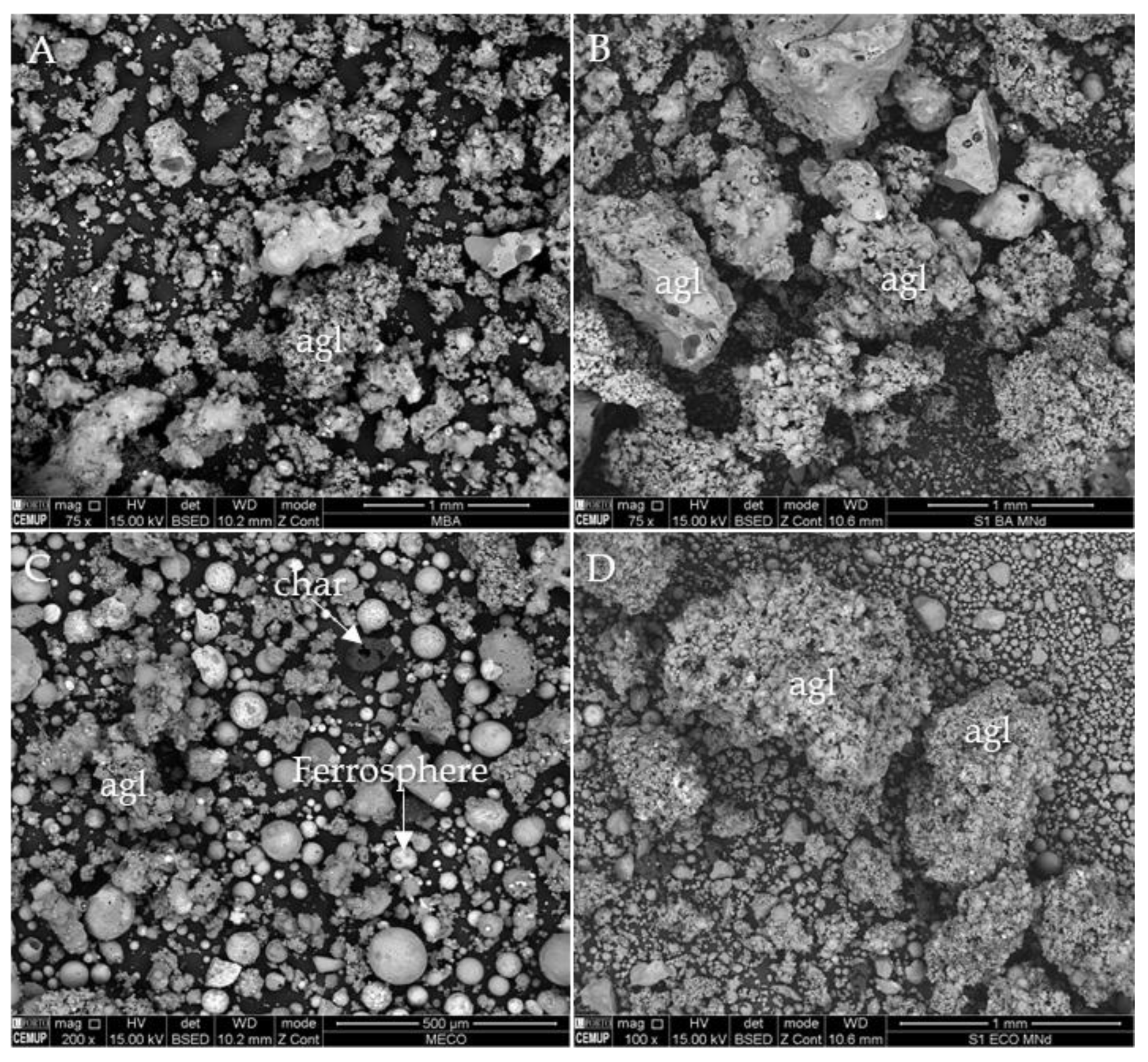
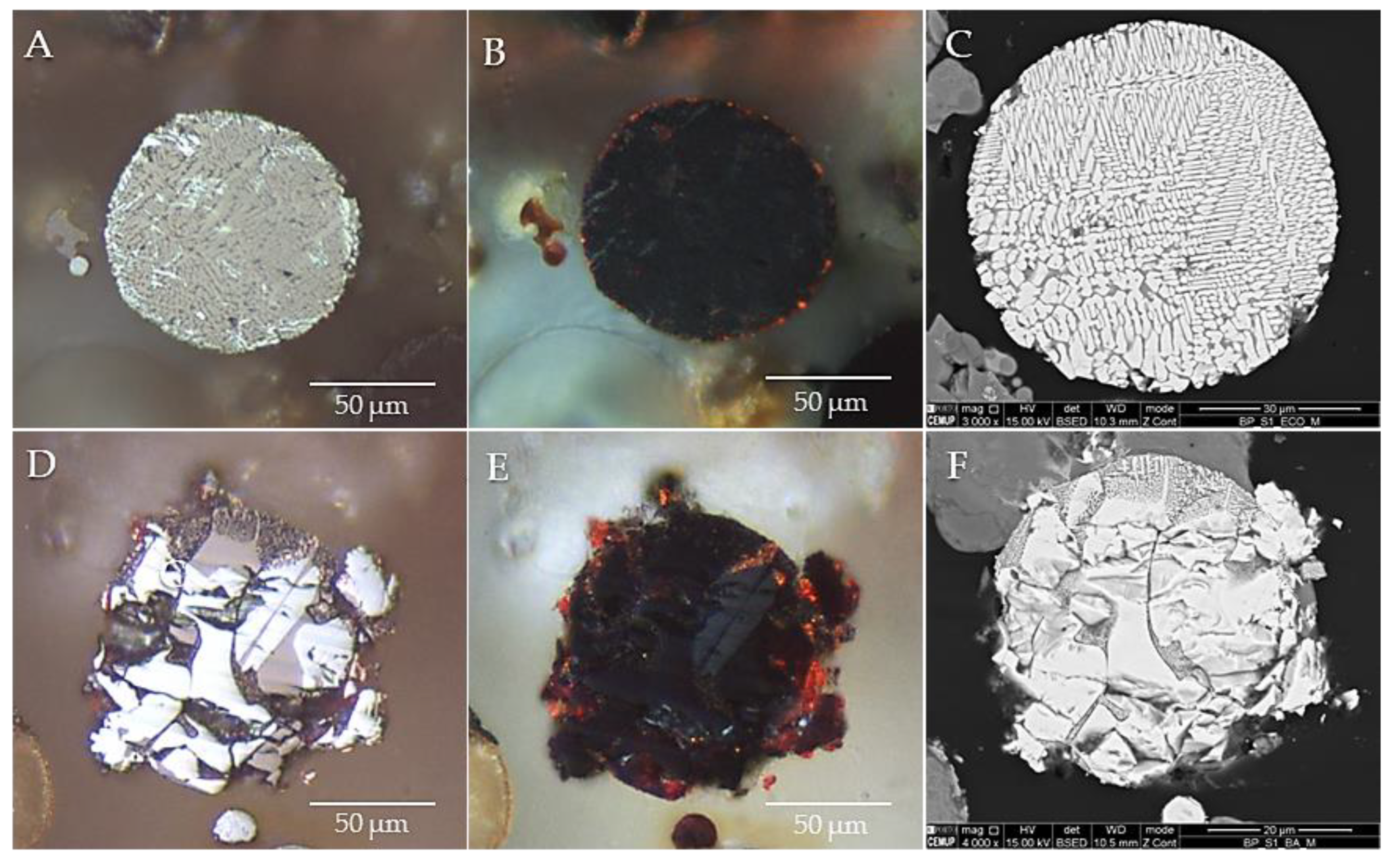
3.4. Occurrence, Morphology and Microtexture
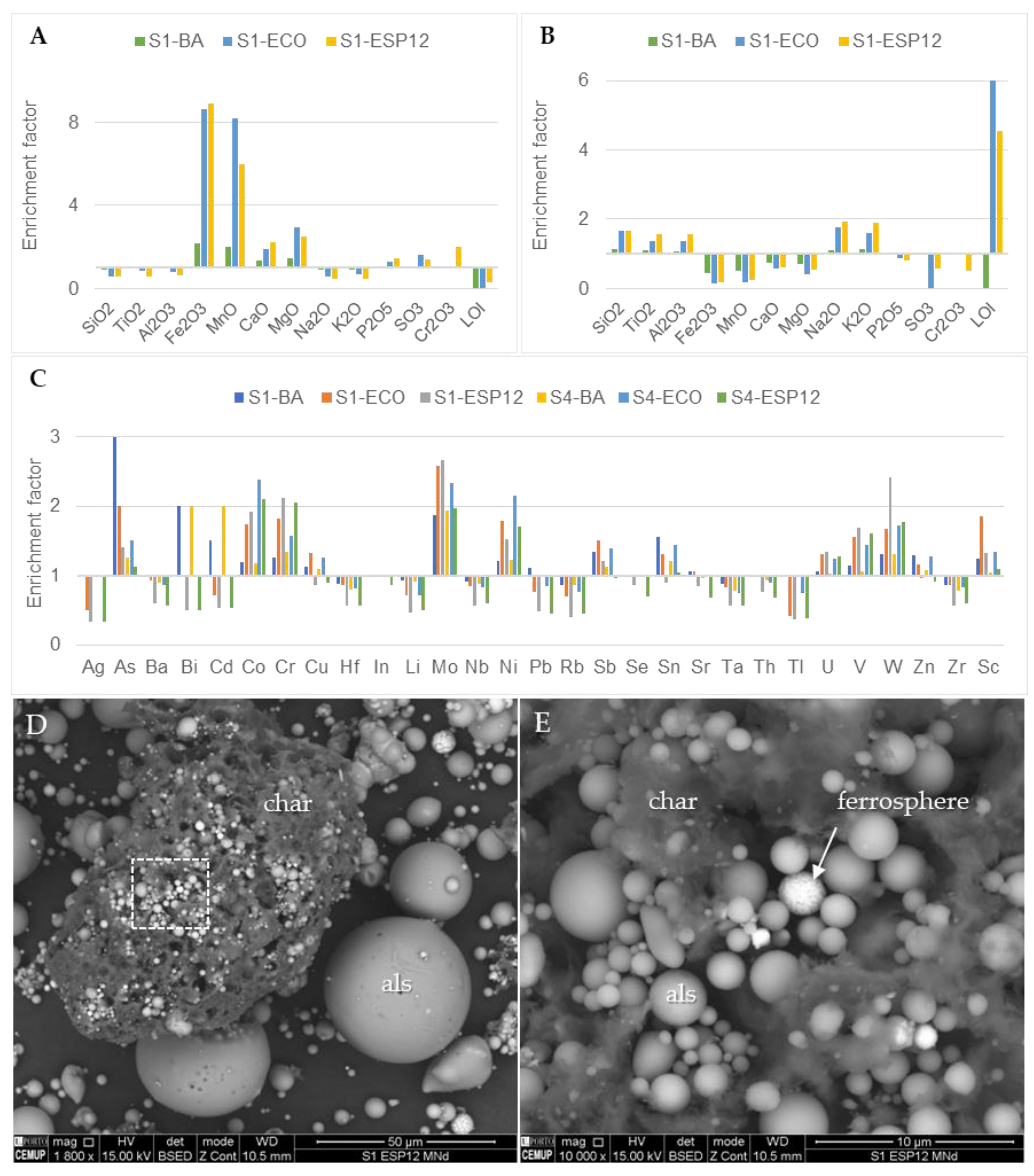
3.5. Chemical Composition
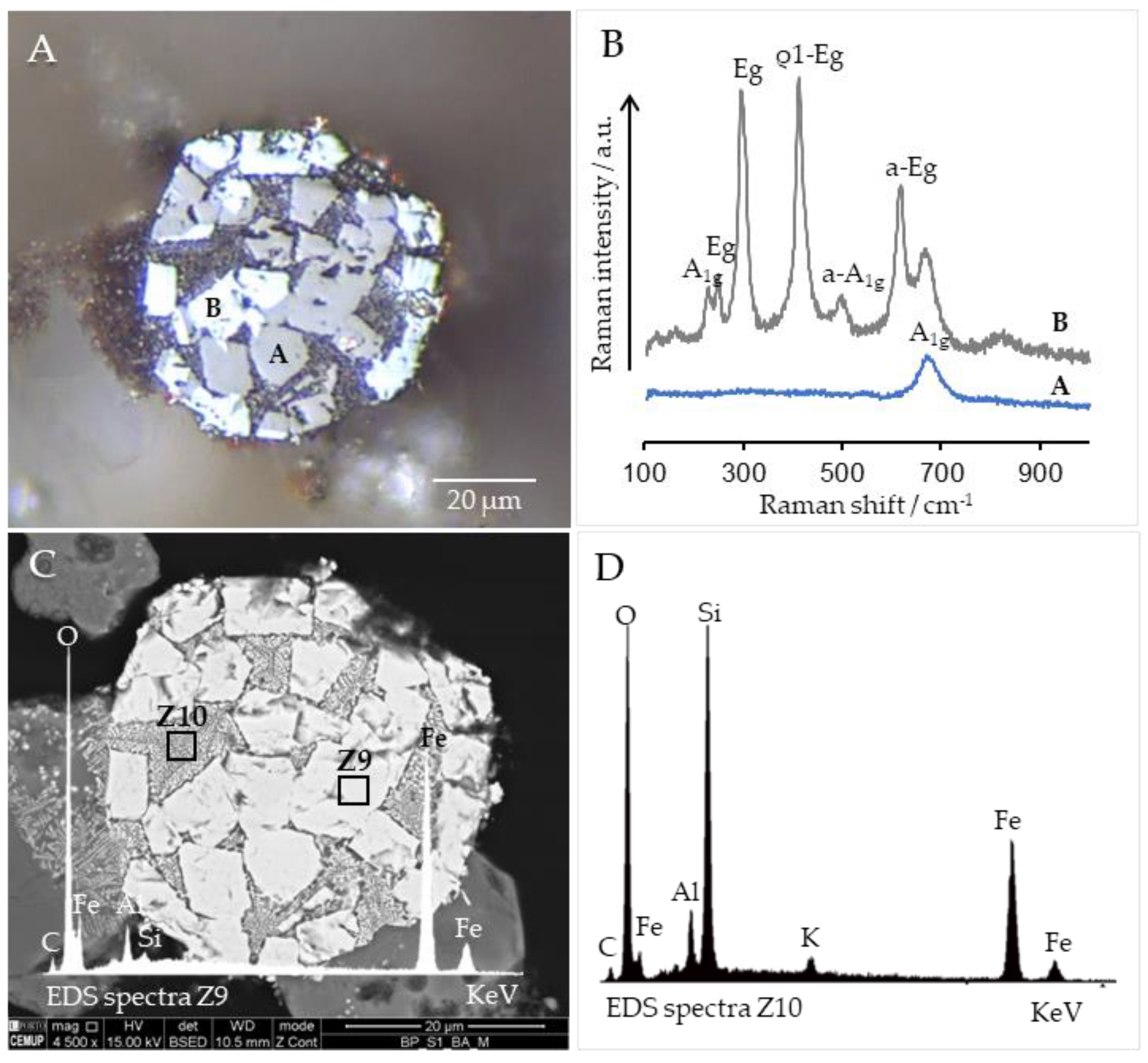
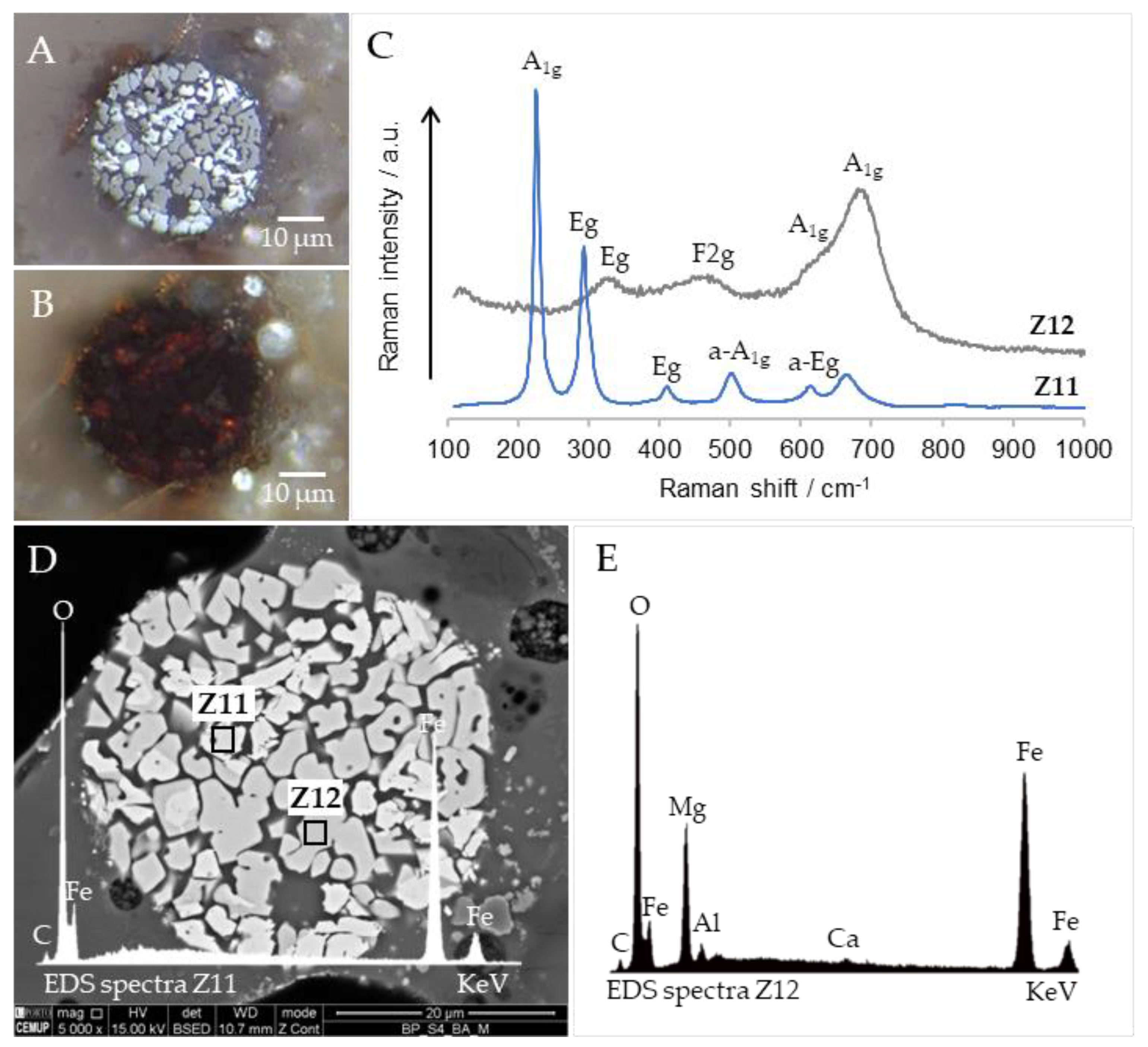
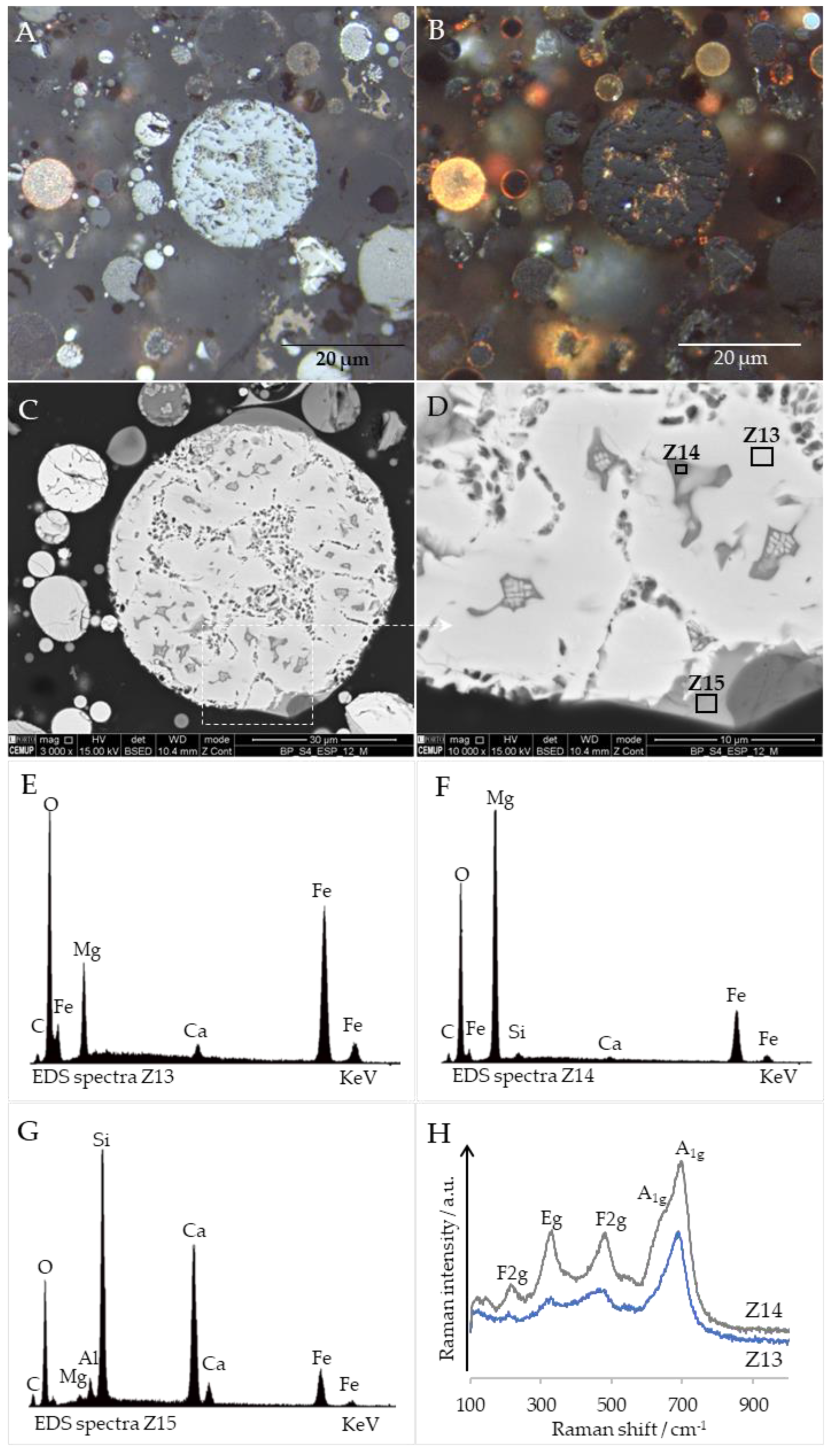
3.6. Cross-Sections Integrated Characterization: Petrography, SEM/EDS, and Raman Microspectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, D.; Feuerborn, J.; Heidrich, C.; Feuerborn Kurzfassung, J. Global Aspects on Coal Combustion Products. In Proceedings of the World of Coal Ash (WOCA), St. Louis, MO, USA, 13 May 2019; Volume 10. [Google Scholar]
- IEA. Coal 2022; IEA: Paris, France, 2022. [Google Scholar]
- Zhou, H.; Bhattarai, R.; Li, Y.; Si, B.; Dong, X.; Wang, T.; Yao, Z. Towards Sustainable Coal Industry: Turning Coal Bottom Ash into Wealth. Sci. Total Environ. 2022, 804, 149985. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Hower, J.C.; Zhang, W.; Luo, G.; Hu, H.; Yao, H. A Review of Rare Earth Elements and Yttrium in Coal Ash: Content, Modes of Occurrences, Combustion Behavior, and Extraction Methods. Prog. Energy Combust. Sci. 2022, 88, 100954. [Google Scholar] [CrossRef]
- Badenhorst, C.; Santos, C.; Lázaro-Martínez, J.; Białecka, B.; Cruceru, M.; Guedes, A.; Guimarães, R.; Moreira, K.; Predeanu, G.; Suárez-Ruíz, I.; et al. Assessment of Graphitized Coal Ash Char Concentrates as a Potential Synthetic Graphite Source. Minerals 2020, 10, 986. [Google Scholar] [CrossRef]
- Fang, Z.; Gesser, H.D. Recovery of Gallium from Coal Fly Ash. Hydrometallurgy 1996, 41, 187–200. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Jiang, J.; Hower, J.C.; Song, X.; Jiang, Y.; Wang, X.; Gornostaeva, T.; Li, X.; et al. Composition and Modes of Occurrence of Minerals and Elements in Coal Combustion Products Derived from High-Ge Coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Valeev, D.; Bobylev, P.; Osokin, N.; Zolotova, I.; Rodionov, I.; Salazar-Concha, C.; Verichev, K. A Review of the Alumina Production from Coal Fly Ash, with a Focus in Russia. J. Clean. Prod. 2022, 363, 132360. [Google Scholar] [CrossRef]
- Sharonova, O.M.; Anshits, N.N.; Fedorchak, M.A.; Zhizhaev, A.M.; Anshits, A.G. Characterization of Ferrospheres Recovered from High-Calcium Fly Ash. Energy Fuels 2015, 29, 5404–5414. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A Comprehensive Review on the Applications of Coal Fly Ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, E.G.; Stiles, D.V. Melanterite-Rozenite Equilibrium. Am. Mineral. 1965, 50, 1457–1458. [Google Scholar]
- Gluskoter, H.J. Inorganic Sulfur in Coal. Energy Sources 1977, 3, 125–131. [Google Scholar] [CrossRef]
- Gruner, D.; Hood, W.C. Three Iron Sulfate Minerals from Coal Mine Refuse Dumps in Perry County, Illinois. Trans. Ill. State Acad. Sci. 1971, 156–158. [Google Scholar]
- Dai, S.; Li, T.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhou, Y.; Zhang, M.; Song, X.; Song, W.; Zhao, C. Origin of Minerals and Elements in the Late Permian Coals, Tonsteins, and Host Rocks of the Xinde Mine, Xuanwei, Eastern Yunnan, China. Int. J. Coal Geol. 2014, 121, 53–78. [Google Scholar] [CrossRef]
- Dai, S.; Chou, C.-L. Occurrence and Origin of Minerals in a Chamosite-Bearing Coal of Late Permian Age, Zhaotong, Yunnan, China. Am. Mineral. 2007, 92, 1253–1261. [Google Scholar] [CrossRef]
- Kossenberg, M.; Cook, A.C. Weathering of Sulphide Minerals in Coal: Production of Ferrous Sulphate Heptahydrate. Mineral. Mag. J. Mineral. Soc. 1961, 32, 829–830. [Google Scholar] [CrossRef]
- Rao, C.P.; Gluskoter, H.J. Occurrence and Distribution of Minerals in Illinois Coals; Illinois State Geological Survey: Urbana, IL, USA, 1973. [Google Scholar]
- Raask, E. Mineral Impurities in Coal Combustion: Behavior, Problems, and Remedial Measures; Hemisphere Pub: Washington, DC, USA, 1985; ISBN 089116362X. [Google Scholar]
- Permana, A.K.; Ward, C.R.; Li, Z.; Gurba, L.W. Distribution and Origin of Minerals in High-Rank Coals of the South Walker Creek Area, Bowen Basin, Australia. Int. J. Coal Geol. 2013, 116–117, 185–207. [Google Scholar] [CrossRef]
- Stach, E.; Mackowsky, M.-T.; Teichmuller, M.; Taylor, G.H.; Chandra, D.; Teichmuller, R. Stach’s Textbook of Coal Petrology; Gebruder Borntraeger: Berlin, Germany, 1982; 535p. [Google Scholar]
- Ward, C.R. Analysis, Origin and Significance of Mineral Matter in Coal: An Updated Review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Çelik, Y.; Karayigit, A.I.; Oskay, R.G.; Kayseri-Özer, M.S.; Christanis, K.; Hower, J.C.; Querol, X. A Multidisciplinary Study and Palaeoenvironmental Interpretation of Middle Miocene Keles Lignite (Harmancık Basin, NW Turkey), with Emphasis on Syngenetic Zeolite Formation. Int. J. Coal Geol. 2021, 237, 103691. [Google Scholar] [CrossRef]
- Hower, J.C.; Rathbone, R.F.; Robertson, J.D.; Peterson, G.; Trimble, A.S. Petrology, Mineralogy, and Chemistry of Magnetically-Separated Sized Fly Ash. Fuel 1999, 78, 197–203. [Google Scholar] [CrossRef]
- Kukier, U.; Ishak, C.F.; Sumner, M.E.; Miller, W.P. Composition and Element Solubility of Magnetic and Non-Magnetic Fly Ash Fractions. Environ. Pollut. 2003, 123, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Querol, X.; Fernández-Turiel, J.; López-Soler, A. Trace Elements in Coal and Their Behaviour during Combustion in a Large Power Station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kalugin, V.M.; Nigmatulina, E.N.; Volkova, N.I.; Frenkel, A.E.; Maksimova, N.V. Ferrospheres from Fly Ashes of Chelyabinsk Coals: Chemical Composition, Morphology and Formation Conditions. Fuel 2002, 81, 867–876. [Google Scholar] [CrossRef]
- Strzałkowska, E. Morphology, Chemical and Mineralogical Composition of Magnetic Fraction of Coal Fly Ash. Int. J. Coal Geol. 2021, 240, 103746. [Google Scholar] [CrossRef]
- Valentim, B.; Białecka, B.; Gonçalves, P.A.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, L.G.; Predeanu, G.; Santos, A.C. Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres. Minerals 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Menendez, R.; Borrego, A.G.; Diaz-Somoano, M.; Rosa Martinez-Tarazona, M. Phase-Mineral and Chemical Composition of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 3. Characterization of Magnetic and Char Concentrates. Fuel 2004, 83, 1563–1583. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Sun, J.; Bai, X.; Zheng, C. Mineralogy, Chemical Composition, and Microstructure of Ferrospheres in Fly Ashes from Coal Combustion. Energy Fuels 2006, 20, 1490–1497. [Google Scholar] [CrossRef]
- Lauf, R.J. Microstructures of Coal Fly Ash Particles. Am. Ceram. Soc. Bull. 1982, 61, 4. [Google Scholar]
- Lauf, R.J.; Harris, L.A.; Rawlston, S.S. Pyrite Framboids as the Source of Magnetite Spheres in Fly Ash. Environ. Sci. Technol. 1982, 16, 218–220. [Google Scholar] [CrossRef]
- Anshits, A.G.; Bayukov, O.A.; Kondratenko, E.V.; Anshits, N.N.; Pletnev, O.N.; Rabchevskii, E.V.; Solovyov, L.A. Catalytic Properties and Nature of Active Centers of Ferrospheres in Oxidative Coupling of Methane. Appl. Catal. A Gen. 2016, 524, 192–199. [Google Scholar] [CrossRef]
- Anshits, A.G.; Kondratenko, E.V.; Fomenko, E.V.; Kovalev, A.M.; Anshits, N.N.; Bajukov, O.A.; Sokol, E.V.; Salanov, A.N. Novel Glass Crystal Catalysts for the Processes of Methane Oxidation. Catal. Today 2001, 64, 59–67. [Google Scholar] [CrossRef]
- Vereshchagin, S.N.; Kondratenko, E.V.; Rabchevskii, E.V.; Anshits, N.N.; Solov’ev, L.A.; Anshits, A.G. New Approach to the Preparation of Catalysts for the Oxidative Coupling of Methane. Kinet. Catal. 2012, 53, 449–455. [Google Scholar] [CrossRef]
- Anshits, A.G.; Kondratenko, E.V.; Fomenko, E.V.; Kovalev, A.M.; Bajukov, O.A.; Anshits, N.N.; Sokol, E.V.; Kochubey, D.I.; Boronin, A.I.; Salanov, A.N.; et al. Physicochemical and Catalytic Properties of Glass Crystal Catalysts for the Oxidation of Methane. J. Mol. Catal. A Chem. 2000, 158, 209–214. [Google Scholar] [CrossRef]
- Anshits, A.G.; Sharonova, O.M.; Anshits, N.N.; Vereshchagin, S.N.; Rabchevskii, E.V.; Solovjev, L.A. Ferrospheres from Fly Ashes: Composition and Catalytic Proprieties in High-Temperature Oxidation of Methane. In Proceedings of the World of Coal Ash, Denver, CO, USA, 9–12 May 2011. [Google Scholar]
- Kirik, N.P.; Anshits, N.N.; Rabchevskii, E.V.; Solov’ev, L.A.; Anshits, A.G. Effect of HF Modification on the Catalytic Properties of Ferrospheres in the Oxidative Coupling of Methane. Kinet. Catal. 2019, 60, 196–204. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Santos, A.C.; Jarrais, B.; Valentim, B.; Guedes, A.; Freire, C.; Peixoto, A.F. Application of Fe-Rich Coal Fly Ashes to Enhanced Reduction of 4-Nitrophenol. Clean. Chem. Eng. 2022, 2, 100019. [Google Scholar] [CrossRef]
- Bibby, D. Composition and Variation of Pulverized Fuel Ash Obtained from the Combustion of Sub-Bituminous Coals, New Zealand. Fuel 1977, 56, 427–431. [Google Scholar] [CrossRef]
- Lu, S.G.; Chen, Y.Y.; Shan, H.D.; Bai, S.Q. Mineralogy and Heavy Metal Leachability of Magnetic Fractions Separated from Some Chinese Coal Fly Ashes. J. Hazard. Mater. 2009, 169, 246–255. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fomenko, E.V.; Anshits, A.G. Composition–Structure Relationship and Routes of Formation of Blocklike Ferrospheres Produced by Pulverized Combustion of Two Coal Types. ACS Omega 2021, 6, 26004–26015. [Google Scholar] [CrossRef] [PubMed]
- Anshits, N.N.; Fedorchak, M.A.; Zhizhaev, A.M.; Anshits, A.G. Composition–Structure Relationship of Skeletal–Dendritic Ferrospheres Formed during Industrial Combustion of Lignite and Coal. Energy Fuels 2019, 33, 6788–6796. [Google Scholar] [CrossRef]
- Sharonova, O.M.; Anshits, N.N.; Yumashev, V.V.; Anshits, A.G. Composition and Morphology of Char Particles of Fly Ashes from Industrial Burning of High-Ash Coals with Different Reactivity. Fuel 2008, 87, 1989–1997. [Google Scholar] [CrossRef]
- Sharonova, O.M.; Anshits, N.N.; Solovyov, L.A.; Salanov, A.N.; Anshits, A.G. Relationship between Composition and Structure of Globules in Narrow Fractions of Ferrospheres. Fuel 2013, 111, 332–343. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Fomenko, E.V.; Mazurova, E.V.; Anshits, A.G. Composition, Structure, and Formation Routes of Blocklike Ferrospheres Separated from Coal and Lignite Fly Ashes. Energy Fuels 2020, 34, 3743–3754. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Zhizhaev, A.M.; Sharonova, O.M.; Anshits, A.G. Composition and Structure of Block-Type Ferrospheres Isolated from Calcium-Rich Power Plant Ash. Inorg. Mater. 2018, 54, 187–194. [Google Scholar] [CrossRef]
- Valentim, B.; Shreya, N.; Paul, B.; Gomes, C.S.; Sant’Ovaia, H.; Guedes, A.; Ribeiro, J.; Flores, D.; Pinho, S.; Suárez-Ruiz, I.; et al. Characteristics of Ferrospheres in Fly Ashes Derived from Bokaro and Jharia (Jharkand, India) Coals. Int. J. Coal Geol. 2016, 153, 52–74. [Google Scholar] [CrossRef]
- Santos, A.C.; Guedes, A.; French, D.; Futuro, A.; Valentim, B. Integrative Study Assessing Space and Time Variations with Emphasis on Rare Earth Element (REE) Distribution and Their Potential on Ashes from Commercial (Colombian) Coal. Minerals 2022, 12, 194. [Google Scholar] [CrossRef]
- Taylor, J.C. Computer Programs for Standardless Quantitative Analysis of Minerals Using the Full Powder Diffraction Profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Kruiver, P.P.; Dekkers, M.J.; Heslop, D. Quantification of Magnetic Coercivity Components by the Analysis of Acquisition Curves of Isothermal Remanent Magnetisation. Earth Planet. Sci. Lett. 2001, 189, 269–276. [Google Scholar] [CrossRef]
- Maxbauer, D.P.; Feinberg, J.M.; Fox, D.L. MAX UnMix: A Web Application for Unmixing Magnetic Coercivity Distributions. Comput. Geosci. 2016, 95, 140–145. [Google Scholar] [CrossRef]
- Evans, M.E.; Heller, F. Environmental Magnetism: Principles and Applications of Enviromagnetics; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Maher, B.A.; Thompson, R. Quaternary Climates, Environments, and Magnetism; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Thompson, R.; Oldfield, F. Environmental Magnetism; Springer: Dordrecht, The Netherlands, 1986; ISBN 978-94-011-8038-2. [Google Scholar]
- Bloemendal, J.; Lamb, B.; King, J. Paleoenvironmental Implications of Rock-Magnetic Properties of Late Quaternary Sediment Cores from the Eastern Equatorial Atlantic. Paleoceanography 1988, 3, 61–87. [Google Scholar] [CrossRef]
- Long, G.J.; Cranshaw, T.E.; Longworth, G. The Ideal Mössbauer Effect Absorber Thickness. Mössbauer Eff. Ref. Data J. 1983, 43–49. [Google Scholar]
- Waerenborgh, J.C.; Salamakha, P.; Sologub, O.; Gonçalves, A.P.; Cardoso, C.; Sério, S.; Godinho, M.; Almeida, M. Influence of Thermal Treatment and Crystal Growth on the Final Composition and Magnetic Properties of the YFexAl12−x (4 ≤ x ≤ 4.2) Intermetallics. Chem. Mater. 2000, 12, 1743–1749. [Google Scholar] [CrossRef]
- Verosub, K.L.; Roberts, A.P. Environmental Magnetism: Past, Present, and Future. J. Geophys. Res. Solid Earth 1995, 100, 2175–2192. [Google Scholar] [CrossRef]
- Dekkers, M.J. Environmental Magnetism: An Introduction. Geol. Mijnb. (Geol. Min.) 1997, 76, 163–182. [Google Scholar] [CrossRef]
- Veneva, L.; Hoffmann, V.; Jordanova, D.; Jordanova, N.; Fehr, T. Rock Magnetic, Mineralogical and Microstructural Characterization of Fly Ashes from Bulgarian Power Plants and the Nearby Anthropogenic Soils. Phys. Chem. Earth Parts A/B/C 2004, 29, 1011–1023. [Google Scholar] [CrossRef]
- Kapicka, A.; Jordanova, N.; Petrovský, E.; Ustjak, S. Effect of Different Soil Conditions on Magnetic Parameters of Power-Plant Fly Ashes. J. Appl. Geophy. 2001, 48, 93–102. [Google Scholar] [CrossRef]
- Egli, R. Characterization of Individual Rock Magnetic Components by Analysis of Remanence Curves, 1. Unmixing Natural Sediments. Stud. Geophys. Et Geod. 2004, 48, 391–446. [Google Scholar] [CrossRef]
- Abrajevitch, A.; Kodama, K. Diagenetic Sensitivity of Paleoenvironmental Proxies: A Rock Magnetic Study of Australian Continental Margin Sediments. Geochem. Geophys. Geosystems 2011, 12. [Google Scholar] [CrossRef]
- Matjie, R.H.; French, D.; Ward, C.R.; Pistorius, P.C.; Li, Z. Behaviour of Coal Mineral Matter in Sintering and Slagging of Ash during the Gasification Process. Fuel Process. Technol. 2011, 92, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.S. Mineralogical and Chemical Composition of Magnetic Fly Ash Fraction. Environ. Earth Sci. 2014, 71, 1673–1681. [Google Scholar] [CrossRef]
- Sokol, E.V.; Maksimova, N.V.; Volkova, N.I.; Nigmatulina, E.N.; Frenkel, A.E. Hollow Silicate Microspheres from Fly Ashes of the Chelyabinsk Brown Coals (South Urals, Russia). Fuel Process. Technol. 2000, 67, 35–52. [Google Scholar] [CrossRef]
- Hansen, L.D.; Silberman, D.; Fisher, G.L. Crystalline Components of Stack-Collected, Size-Fractionated Coal Fly Ash. Environ. Sci. Technol. 1981, 15, 1057–1062. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Mineralogy of Combustion Wastes from Coal-Fired Power Stations. Fuel Process. Technol. 1996, 47, 261–280. [Google Scholar] [CrossRef]
- Murad, E. Clays and Clay Minerals: What Can Mössbauer Spectroscopy Do to Help Understand Them? Hyperfine Interact 1998, 117, 39–70. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Barrero, C.A.; da Costa, G.M.; van San, E.; de Grave, E. Mössbauer Characterization of Iron Oxides and (Oxy)Hydroxides: The Present State of the Art. Hyperfine Interact 2000, 126, 247–259. [Google Scholar] [CrossRef]
- de Grave, E.; Vanleerberghe, R.; Dauwe, C.; de Sitter, J.; Govaert, A. Magnetic Hyperfine Field Distributions in Ferrimagnetic Spinels Fe2(1-y)Mg1+yTiyO4 with y ≤ 0.5. J. Phys. Colloq. 1976, 37, C6-97–C6-100. [Google Scholar] [CrossRef]
- Waerenborgh, J.C.; Figueiredo, M.O.; Cabral, J.M.P.; Pereira, L.C.J. Powder XRD Structure Refinements and 57Fe Mössbauer Effect Study of Synthetic Zn1−XFexAl2O4 (0 < x ≦ 1) Spinels Annealed at Different Temperatures. Phys. Chem. Min. 1994, 21, 460–468. [Google Scholar] [CrossRef]
- Jayasuriya, K.D.; O’Neill, H.S.C.; Berry, A.J.; Campbell, S.J. A Mössbauer Study of the Oxidation State of Fe in Silicate Melts. Am. Mineral. 2004, 89, 1597–1609. [Google Scholar] [CrossRef]
- Ferreira, N.M.; Kovalevsky, A.V.; Valente, M.A.; Waerenborgh, J.C.; Frade, J.R.; Costa, F.M. Structural and Redox Effects in Iron-Doped Magnesium Aluminosilicates. J. Cryst. Growth 2017, 457, 19–23. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; de Resende, V.G.; de Grave, E. Mössbauer Effect Study of Fly and Bottom Ashes from an Electric Generating Plant. In ISIAME 2008; Springer: Berlin/Heidelberg, Germany, 2009; pp. 341–346. [Google Scholar]
- Szumiata, T.; Brzózka, K.; Górka, B.; Gawroński, M.; Gzik-Szumiata, M.; Świetlik, R.; Trojanowska, M. Iron Speciation in Coal Fly Ashes—Chemical and Mössbauer Analysis. Hyperfine Interact 2014, 226, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.L.; Chang, D.P.Y.; Brummer, M. Fly Ash Collected from Electrostatic Precipitators: Microcrystalline Structures and the Mystery of the Spheres. Science 1976, 192, 553–555. [Google Scholar] [CrossRef]
- Miller, S.F.; Schobert, H.H. Effect of Fuel Particle and Droplet Size Distribution on Particle Size Distribution of Char and Ash during Pilot-Scale Combustion of Pulverized Coal and Coal-Water Slurry Fuels. Energy Fuels 1993, 7, 520–531. [Google Scholar] [CrossRef]
- Miller, S.F.; Schobert, H.H. Effect of the Occurrence and Composition of Silicate and Aluminosilicate Compounds on Ash Formation in Pilot-Scale Combustion of Pulverized Coal and Coal-Water Slurry Fuels. Energy Fuels 1994, 8, 1197–1207. [Google Scholar] [CrossRef]
- Kutchko, B.; Kim, A. Fly Ash Characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Valentim, B.; Hower, J.C.; Guedes, A.; Flores, D. Scanning Electron Miccroscopy and Energy-Dispersive X-Ray Spectroscopy of Low-Sulfur Coal Fly Ash. Int. J. Energy A Clean Environ. 2009, 10, 147–166. [Google Scholar] [CrossRef]
- Ramsden, A.R.; Shibaoka, M. Characterization and Analysis of Individual Fly-Ash Particles from Coal-Fired Power Stations by a Combination of Optical Microscopy, Electron Microscopy and Quantitative Electron Microprobe Analysis. Atmos. Environ. 1982, 16, 2191–2206. [Google Scholar] [CrossRef]
- Blaha, U.; Sapkota, B.; Appel, E.; Stanjek, H.; Rosler, W. Micro-Scale Grain-Size Analysis and Magnetic Properties of Coal-Fired Power Plant Fly Ash and Its Relevance for Environmental Magnetic Pollution Studies. Atmos. Environ. 2008, 42, 8359–8370. [Google Scholar] [CrossRef]
- Hulett, L.D.; Weinberger, A.J.; Northcutt, K.J.; Ferguson, M. Chemical Species in Fly Ash from Coal-Burning Power Plants. Science 1980, 210, 1356–1358. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous Biolithes: World Averages for Trace Element Contents in Black Shales and Coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- European Commission Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Economic and Social Committee: Brussels, Belgium, 2020.
- Dai, S.; Finkelman, R.B. Coal as a Promising Source of Critical Elements: Progress and Future Prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
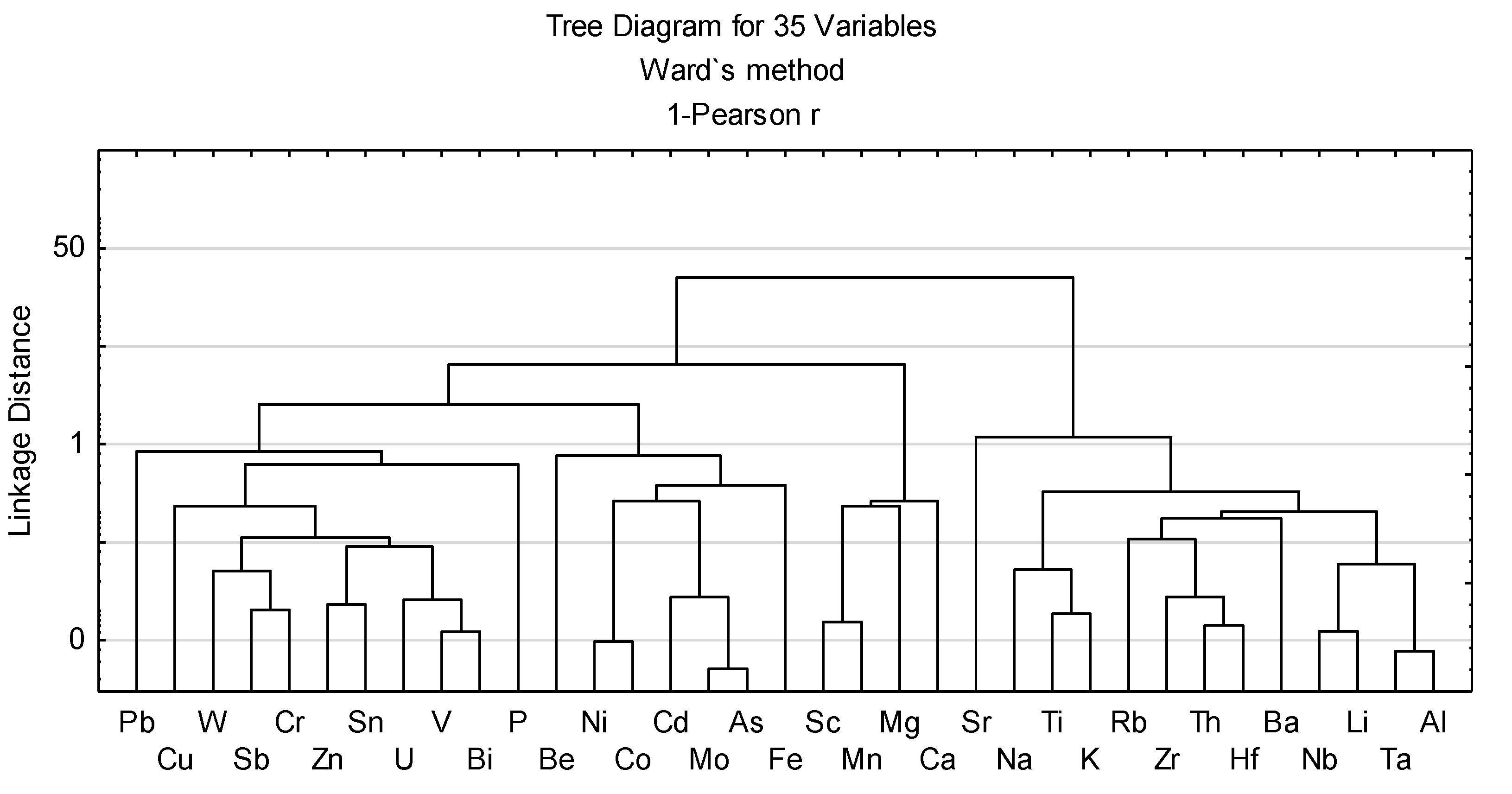
- Xu, N.; Xu, C.; Finkelman, R.B.; Engle, M.A.; Li, Q.; Peng, M.; He, L.; Huang, B.; Yang, Y. Coal Elemental (Compositional) Data Analysis with Hierarchical Clustering Algorithms. Int. J. Coal Geol. 2022, 249, 103892. [Google Scholar] [CrossRef]
- Xu, N.; Finkelman, R.B.; Dai, S.; Xu, C.; Peng, M. Average Linkage Hierarchical Clustering Algorithm for Determining the Relationships between Elements in Coal. ACS Omega 2021, 6, 6206–6217. [Google Scholar] [CrossRef] [PubMed]
- Eminagaoglu, M.; Oskay, R.G.; Karayigit, A.I. Evaluation of Elemental Affinities in Coal Using Agglomerative Hierarchical Clustering Algorithm: A Case Study in a Thick and Mineable Coal Seam (KM2) from Soma Basin (W. Turkey). Int. J. Coal Geol. 2022, 259, 104045. [Google Scholar] [CrossRef]
- Eskanazy, G.; Finkelman, R.B.; Chattarjee, S. Some Considerations Concerning the Use of Correlation Coefficients and Cluster Analysis in Interpreting Coal Geochemistry Data. Int. J. Coal Geol. 2010, 83, 491–493. [Google Scholar] [CrossRef]
- Gürdal, G. Abundances and Modes of Occurrence of Trace Elements in the Çan Coals (Miocene), Çanakkale-Turkey. Int. J. Coal Geol. 2011, 87, 157–173. [Google Scholar] [CrossRef]
- Zhao, L.; Ward, C.R.; French, D.; Graham, I. Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China. Minerals 2015, 5, 870–893. [Google Scholar] [CrossRef]
- Sharonova, O.M.; Fedorchak, M.A.; Zhizhaev, A.M.; Mazurova, E.V.; Anshits, A.G. Composition of Individual Ferrospheres of Different Morphological Types. Inorg. Mater. 2015, 51, 1143–1150. [Google Scholar] [CrossRef]
- Orberger, B.; Wagner, C.; Tudryn, A.; Wirth, R.; Morgan, R.; Fabris, J.D.; Greneche, J.M.; Rosière, C. Micro- to Nano-Scale Characterization of Martite from a Banded Iron Formation in India and a Lateritic Soil in Brazil. Phys. Chem. Min. 2014, 41, 651–667. [Google Scholar] [CrossRef] [Green Version]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Sanz, A.; Flores, D.; Noronha, F. Characterization of Fly Ash from a Power Plant and Surroundings by Micro-Raman Spectroscopy. Int. J. Coal Geol. 2008, 73, 359–370. [Google Scholar] [CrossRef]
- Owens, F.J.; Orosz, J. Effect of Nanosizing on Lattice and Magnon Modes of Hematite. Solid State Commun. 2006, 138, 95–98. [Google Scholar] [CrossRef]
- Minitti, M.E.; Lane, M.D.; Bishop, J.L. A New Hematite Formation Mechanism for Mars. Meteorit. Planet. Sci. 2005, 40, 55–69. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Lopes, F.N. Heated Goethite and Natural Hematite: Can Raman Spectroscopy Be Used to Differentiate Them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular Composition of Iron Oxide Nanoparticles, Precursors for Magnetic Drug Targeting, as Characterized by Confocal Raman Microspectroscopy. Analyst 2005, 130, 1395. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Zhao, D.; Zhang, X.J.; Jin, W.T.; Kashkarov, P.; Zhang, H. Synthesis and Characterization of Single-Crystalline α-Fe2O3 Nanoleaves. Phys. E Low Dimens. Syst. Nanostruct. 2009, 41, 806–811. [Google Scholar] [CrossRef]
- Lopez, L.B.M.; Pasteris, J.D.; Biswas, P. Sensitivity of Micro-Raman Spectrum to Crystallite Size of Electrospray-Deposited and Post-Annealed Films of Iron-Oxide Nanoparticle Suspensions. Appl. Spectrosc. 2009, 63, 627–635. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella Jr, M.F.; Madden, A.S. Size-Dependent Structural Transformations of Hematite Nanoparticles. 1. Phase Transition. Phys. Chem. Chem. Phys. 2007, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, V.; Andreozzi, G.B.; Bersani, D.; Lottici, P.P. Raman Fingerprint of Chromate, Aluminate and Ferrite Spinels. J. Raman Spectrosc. 2015, 46, 1255–1264. [Google Scholar] [CrossRef]
- Nakagomi, F.; da Silva, S.W.; Garg, V.K.; Oliveira, A.C.; Morais, P.C.; Franco, A. Influence of the Mg-Content on the Cation Distribution in Cubic Mg Fe3O4 Nanoparticles. J. Solid State Chem. 2009, 182, 2423–2429. [Google Scholar] [CrossRef]
- Yang, B.L.; Cheng, D.S.; Lee, S.B. Effect of Steam on the Oxidative Dehydrogenation of Butene over Magnesium Ferrites with and without Chromium Substitution. Appl. Catal. 1991, 70, 161–173. [Google Scholar] [CrossRef]


| S1-C | S4-C1 | S4-C2 | |
|---|---|---|---|
| Proximate analysis | |||
| Ad | 3.28 | 10.1 | 9.01 |
| Vdaf | 39.82 | 41.7 | 40.44 |
| Ultimate analysis | |||
| Sd | 0.52 | 0.73 | 0.72 |
| Cdaf | 80.34 | 78.53 | 80.3 |
| Hdaf | 6.2 | 6.07 | 6.08 |
| Ndaf | 1.64 | 1.6 | 1.57 |
| Odaf | 11.28 | 12.98 | 11.25 |
| GCVd | 29.19 | 29.66 | 29.78 |
| Mineralogy | |||
| Quartz | 31 | 38.7 | n.d. |
| Albite | 0.6 | 0.7 | n.d. |
| Hornblende | 0.8 | 0.4 | n.d. |
| Muscovite | 4 | 8.4 | n.d. |
| Illite | 4.2 | 7.9 | n.d. |
| Kaolinite | 45.5 | 25.6 | n.d. |
| Montmorillonite | 0.3 | 0 | n.d. |
| Chlorite | 0.3 | 0.3 | n.d. |
| Anatase | 0.3 | 0.7 | n.d. |
| Boehmite | 0.2 | 0.1 | n.d. |
| Calcite | 0 | 0 | n.d. |
| Siderite | 0.3 | 0 | n.d. |
| Bassanite | 0.7 | 1.2 | n.d. |
| Gypsum | 3.4 | 3.5 | n.d. |
| Hexahydrite | 2.3 | 4.2 | n.d. |
| Jarosite | 1.1 | 2.3 | n.d. |
| Alunogen | 3.3 | 4 | n.d. |
| Tschermigite | 0 | 0 | n.d. |
| Apatite | 0.6 | 0.5 | n.d. |
| Pyrite | 1.2 | 1.7 | n.d. |
| X (×10−8 m3/kg) | Method | S Ratio | Component | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | |||||||||||||
| SIRM (A/m) | B1/2 (mT) | DP | Cont. (%) | S | SIRM (A/m) | B1/2 (mT) | DP | Cont. (%) | S | |||||
| S1 | BA | 872.79 | CLG | 0.943 | 146,000 | 36 | 0.32 | 96 | n.a. | 6000 | 501 | 0.28 | 4 | n.a. |
| Max UnMix | n.a. | n.a. | 37 | 0.33 | 97 | 1.09 | n.a. | 578 | 0.21 | 3 | 1.11 | |||
| ECO | 2375.18 | CLG | 0.921 | 227,000 | 40 | 0.31 | 94 | n.a. | 15,000 | 562 | 0.30 | 6 | n.a. | |
| Max UnMix | n.a. | n.a. | 40 | 0.31 | 95 | 0.99 | n.a. | 573 | 0.27 | 5 | 0.77 | |||
| ESP12 | 1164.99 | CLG | 0.923 | 114,000 | 46 | 0.29 | 94 | n.a. | 7000 | 562 | 0.30 | 6 | n.a. | |
| Max UnMix | n.a. | n.a. | 44 | 0.30 | 93 | 0.87 | n.a. | 527 | 0.57 | 7 | 1.09 | |||
| S4 | BA | 834.79 | CLG | 0.925 | 133,000 | 34 | 0.33 | 93 | n.a. | 9500 | 355 | 0.30 | 7 | n.a. |
| Max UnMix | n.a. | n.a. | 35 | 0.35 | 95 | 1.11 | n.a. | 460 | 0.28 | 5 | 0.92 | |||
| ECO | 2024.94 | CLG | 0.909 | 176,000 | 47 | 0.31 | 92 | n.a. | 15,000 | 562 | 0.29 | 8 | n.a. | |
| Max UnMix | n.a. | n.a. | 47 | 0.32 | 94 | 0.97 | n.a. | 632 | 0.25 | 6 | 1.14 | |||
| ESP12 | 1325.87 | CLG | 0.944 | 145,500 | 49 | 0.28 | 96 | n.a. | 6000 | 562 | 0.28 | 4 | n.a. | |
| Max UnMix | n.a. | n.a. | 48 | 0.29 | 96 | 0.85 | n.a. | 422 | 0.22 | 4 | 0.94 | |||
| S1–BA | S1–ECO | S1–ESP-12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk * | Fe | Nd | Bulk * | Fe | Nd | Bulk * | Fe | Nd | |
| Quartz (SiO2) | 11.5 | 17.4 | 18.1 | 24.8 | 11.2 | 29.6 | 12.5 | 5.5 | 11.4 |
| Cristobalite (SiO2) | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0 | 0 | 0.1 |
| Mullite (Al6Si2O13) | 6.6 | 14.2 | 13.2 | 8.3 | 7.6 | 11.4 | 4.7 | 6.2 | 5.4 |
| Cordierite (Mg2Al4Si5O18) | 0.2 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0 |
| Albite (NaAlSi3O8) | 0 | 1.3 | 1.6 | 1.4 | 1.4 | 0.9 | 0.9 | 2.4 | 2.0 |
| Diopside ((Ca,Mg,Fe)2Si2O6)) | 0.1 | 0.9 | 0.3 | 0.7 | 3.9 | 0.9 | 0.8 | 5.5 | 0 |
| Calcium aluminate (Ca3Al2O6) | 0 | 0.5 | 0.2 | 0.5 | 0.2 | 0.0 | 0.1 | 0 | 0.3 |
| Rutile (TiO2) | 0 | 0.7 | 0.3 | 0.5 | 0.8 | 0.3 | 0.3 | 0.4 | 0.5 |
| Hematite (Fe2O3) | 0.7 | 2.0 | 0.5 | 0.6 | 5.0 | 0.6 | 0.7 | 5.1 | 0.7 |
| Maghemite (Fe2O3) | 0.9 | 2.3 | 0.7 | 1.2 | 0 | 0.6 | 0.2 | 1.9 | 0.8 |
| Magnetite (Fe3O4) | 0 | 4.4 | 0.8 | 2.0 | 8.6 | 0.4 | 0.9 | 19.9 | 0.7 |
| Magnesioferrite (MgFe2O4) | 0 | 0.8 | 0 | 0.2 | 8.3 | 0 | 0.4 | 5.5 | 0 |
| Hercynite (FeAl2O4) | 0 | 0.7 | 0.2 | 0.4 | 0.7 | 0 | 0 | 0 | 0.4 |
| Calcite (CaCO3) | 0 | 0.2 | 0.1 | 0.1 | 0.7 | 0.2 | 0.1 | 0.7 | 0.6 |
| Gypsum (CaSO4·2H2O) | 1.4 | 0.2 | 0.5 | 0.4 | 0.6 | 0 | 0.3 | 0.5 | 0.1 |
| Anhydrite (CaSO4) | 0 | 0 | 0 | 0.1 | 0 | 0 | 0.1 | 0.2 | 0.1 |
| Amorphous | 78.3 | 54.1 | 63.4 | 58.5 | 50.8 | 54.9 | 78.0 | 46.2 | 77.1 |
| Fe-MC | Fe Species | IS, mm/s | QS, ε, mm/s | Bhf, Tesla | I |
|---|---|---|---|---|---|
| S1–BA | Fe2.5+ magnetite | 0.65 | 0 | 44.2 | 15% |
| Fe3+ magnetite + maghemite | 0.33 | 0.06 | 47.5 | 35% | |
| Fe3+ hematite | 0.37 | −0.2 | 50.6 | 23% | |
| Fe3+ aluminosilicate phases | 0.41 | 0.87 | - | 21% | |
| Fe2+ aluminosilicate phases | 0.94 | 2.38 | - | 6% | |
| S1–ECO | Fe2.5+ magnetite | 0.65 | −0.02 | 45.5 | 26% |
| Fe3+ magnetite + maghemite | 0.31 | 0.04 | 48.5 | 44% | |
| Fe3+ hematite | 0.37 | −0.2 | 50 | 15% | |
| Fe3+ aluminosilicate phases | 0.41 | 0.91 | - | 12% | |
| Fe2+ aluminosilicate phases | 0.94 | 2.42 | - | 3% | |
| S1–ESP12 | Fe2.5+ magnetite | 0.65 | 0 | 45.3 | 23% |
| Fe3+ magnetite + maghemite | 0.31 | 0 | 48.6 | 49% | |
| Fe3+ hematite | 0.37 | −0.2 | 50.4 | 17% | |
| Fe3+ aluminosilicate phases | 0.49 | 0.93 | - | 8% | |
| Fe2+ aluminosilicate phases | 0.95 | 2.56 | - | 3% | |
| S4–BA | Fe2.5+ magnetite | 0.65 | −0.03 | 45.5 | 29% |
| Fe3+ magnetite + maghemite | 0.3 | −0.03 | 48.5 | 31% | |
| Fe3+ hematite | 0.37 | −0.2 | 51.9 | 16% | |
| Fe3+ aluminosilicate phases | 0.41 | 0.87 | - | 20% | |
| Fe2+ aluminosilicate phases | 0.96 | 2.47 | - | 4% | |
| S4–ECO | Fe2.5+ magnetite | 0.63 | −0.05 | 46.3 | 39% |
| Fe3+ magnetite + maghemite | 0.3 | −0.01 | 49.6 | 35% | |
| Fe3+ hematite | 0.38 | −0.2 | 52.4 | 10% | |
| Fe3+ aluminosilicate phases | 0.42 | 0.88 | - | 13% | |
| Fe2+ aluminosilicate phases | 0.99 | 2.44 | - | 3% | |
| S4–ESP12 | Fe2.5+ magnetite | 0.62 | −0.04 | 45.8 | 43% |
| Fe3+ magnetite + maghemite | 0.3 | −0.01 | 49.2 | 37% | |
| Fe3+ hematite | 0.38 | −0.19 | 50.4 | 9% | |
| Fe3+ aluminosilicate phases | 0.42 | 0.95 | - | 10% | |
| Fe2+ aluminosilicate phases | 0.99 | 2.61 | - | 1% |
| S1–BA | S1–ECO | S1–ESP12 | S4–BA | S4–ECO | S4–ESP12 | |
|---|---|---|---|---|---|---|
| Fe fraction in Fe oxides (MgFe2O4 included when present) | 73% | 85% | 89% | 76% | 84% | 89% |
| Fe3+/total Fe | 86.5% | 84% | 85.5% | 81.5% | 77.5% | 77.5% |
| Yield wt.% | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | CaO | MgO | Na2O | K2O | P2O5 | SO3 | Cr2O3 | LOI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1–BA | Bulk | 66.07 | 0.81 | 18.28 | 7.95 | 0.08 | 1.50 | 1.87 | 1.04 | 2.11 | 0.14 | 0.03 | 0.02 | 0.10 | ||
| Fe (mm) | MC | 5.4 | 58.85 | 0.79 | 17.73 | 14.87 | 0.14 | 1.92 | 2.59 | 0.96 | 1.97 | 0.15 | <0.002 | 0.02 | 0.00 | |
| >500 | 9.0 | 65.57 | 0.84 | 18.80 | 7.78 | 0.07 | 1.62 | 1.93 | 1.09 | 2.13 | 0.15 | <0.002 | 0.01 | 0.00 | ||
| 500–150 | 26.8 | 63.43 | 0.84 | 18.68 | 9.38 | 0.10 | 1.91 | 2.18 | 1.07 | 2.11 | 0.16 | <0.002 | 0.04 | 0.10 | ||
| 150–75 | 32.9 | 60.65 | 0.81 | 18.05 | 12.77 | 0.13 | 1.89 | 2.49 | 0.99 | 2.04 | 0.16 | <0.002 | 0.01 | 0.00 | ||
| 75–45 | 21.7 | 57.09 | 0.81 | 17.91 | 16.45 | 0.15 | 1.75 | 2.75 | 0.92 | 1.99 | 0.16 | <0.002 | 0.02 | 0.00 | ||
| <45 | 16.4 | 52.50 | 0.79 | 17.95 | 21.00 | 0.16 | 1.61 | 2.91 | 0.86 | 1.94 | 0.15 | <0.002 | 0.02 | 0.10 | ||
| TL | 94.2 | 66.85 | 0.83 | 18.60 | 6.88 | 0.07 | 1.46 | 1.77 | 1.06 | 2.13 | 0.14 | <0.002 | 0.02 | 0.20 | ||
| Nd (mm) | MC | 38.3 | 66.15 | 0.87 | 19.25 | 6.74 | 0.07 | 1.45 | 1.81 | 1.08 | 2.23 | 0.15 | <0.002 | 0.02 | 0.20 | |
| >2000 | 20.9 | 65.69 | 0.86 | 19.43 | 7.24 | 0.07 | 1.48 | 1.86 | 1.07 | 2.23 | 0.15 | <0.002 | 0.02 | −0.10 | ||
| 2000–500 | 20.0 | 65.04 | 0.88 | 19.78 | 7.35 | 0.07 | 1.54 | 1.92 | 1.09 | 2.27 | 0.15 | <0.002 | 0.02 | −0.10 | ||
| 500–150 | 19.3 | 65.66 | 0.85 | 18.91 | 7.19 | 0.07 | 1.69 | 1.89 | 1.09 | 2.18 | 0.15 | <0.002 | 0.02 | 0.30 | ||
| 150–75 | 16.6 | 69.17 | 0.78 | 17.52 | 5.92 | 0.06 | 1.46 | 1.63 | 1.06 | 2.04 | 0.14 | <0.002 | 0.01 | 0.20 | ||
| 75–45 | 13.6 | 68.49 | 0.84 | 18.87 | 5.27 | 0.05 | 1.20 | 1.58 | 1.05 | 2.20 | 0.14 | <0.002 | 0.01 | 0.30 | ||
| <45 | 13.8 | 65.85 | 0.95 | 20.53 | 5.58 | 0.05 | 1.08 | 1.64 | 1.08 | 2.40 | 0.13 | <0.002 | 0.02 | 0.70 | ||
| TL | 54.5 | 66.78 | 0.79 | 18.06 | 7.41 | 0.07 | 1.45 | 1.77 | 1.05 | 2.05 | 0.13 | 0.31 | 0.02 | 0.10 | ||
| S1–ECO | Bulk | 67.77 | 0.66 | 15.15 | 8.13 | 0.08 | 1.81 | 1.86 | 1.10 | 1.72 | 0.13 | 0.07 | 0.02 | 1.51 | ||
| Fe (μm) | MC | 11.6 | 41.40 | 0.57 | 12.59 | 35.49 | 0.33 | 3.03 | 4.37 | 0.66 | 1.23 | 0.17 | 0.05 | 0.02 | 0.10 | |
| >150 | 12.8 | 58.86 | 0.82 | 18.66 | 10.90 | 0.11 | 2.22 | 2.48 | 1.11 | 2.08 | 0.18 | 0.03 | 0.02 | 2.54 | ||
| 150–75 | 33.2 | 48.97 | 0.60 | 13.59 | 26.42 | 0.29 | 3.06 | 3.81 | 0.81 | 1.42 | 0.17 | 0.04 | 0.01 | 0.80 | ||
| 75–45 | 31.5 | 43.89 | 0.58 | 13.16 | 32.96 | 0.30 | 2.84 | 4.21 | 0.69 | 1.29 | 0.16 | 0.01 | 0.01 | −0.10 | ||
| <45µm | 22.5 | 41.27 | 0.65 | 14.43 | 34.44 | 0.26 | 2.56 | 4.23 | 0.67 | 1.37 | 0.20 | 0.01 | 0.02 | −0.10 | ||
| TL | 87.7 | 71.05 | 0.68 | 15.63 | 4.11 | 0.04 | 1.61 | 1.49 | 1.14 | 1.77 | 0.13 | 0.03 | 0.02 | 2.30 | ||
| Nd (μm) | MC | 53.4 | 68.67 | 0.78 | 17.22 | 5.62 | 0.06 | 1.79 | 1.85 | 1.15 | 1.98 | 0.15 | <0.002 | 0.02 | 0.69 | |
| >150 | 28.5 | 64.66 | 0.88 | 19.57 | 6.80 | 0.07 | 1.65 | 1.99 | 1.18 | 2.22 | 0.16 | <0.002 | 0.02 | 0.81 | ||
| 150–75 | 29.3 | 74.52 | 0.56 | 13.14 | 4.70 | 0.06 | 1.89 | 1.64 | 1.14 | 1.53 | 0.12 | <0.002 | 0.01 | 0.69 | ||
| 75–45 | 24.3 | 69.94 | 0.76 | 16.81 | 5.05 | 0.06 | 1.85 | 1.82 | 1.11 | 1.95 | 0.15 | <0.002 | 0.01 | 0.50 | ||
| <45 | 17.9 | 64.39 | 0.97 | 20.47 | 5.92 | 0.06 | 1.78 | 2.05 | 1.14 | 2.34 | 0.17 | <0.002 | 0.02 | 0.70 | ||
| TL | 38.0 | 75.46 | 0.56 | 13.21 | 2.01 | 0.02 | 1.30 | 0.97 | 1.15 | 1.50 | 0.12 | 0.01 | 0.00 | 3.70 | ||
| S1–ESP12 | Bulk | 59.65 | 0.88 | 20.22 | 6.36 | 0.06 | 1.34 | 1.83 | 1.07 | 2.37 | 0.16 | 0.13 | 0.02 | 5.89 | ||
| Fe (μm) | MC | 4.5 | 34.03 | 0.55 | 12.71 | 41.95 | 0.30 | 2.49 | 4.24 | 0.50 | 1.13 | 0.23 | 0.14 | 0.04 | 1.69 | |
| >75 | 11.3 | 44.52 | 0.58 | 14.58 | 21.75 | 0.24 | 2.74 | 3.12 | 0.74 | 1.56 | 0.16 | 0.43 | 0.01 | 9.58 | ||
| 75–45 | 19.0 | 42.13 | 0.57 | 13.56 | 32.59 | 0.27 | 2.51 | 3.73 | 0.62 | 1.31 | 0.16 | 0.15 | 0.02 | 2.39 | ||
| 45–25 | 23.0 | 36.78 | 0.55 | 12.76 | 40.59 | 0.27 | 2.40 | 4.03 | 0.49 | 1.11 | 0.18 | 0.10 | 0.03 | 0.71 | ||
| <25 | 46.6 | 36.21 | 0.62 | 14.63 | 38.78 | 0.26 | 2.24 | 4.21 | 0.56 | 1.29 | 0.31 | 0.15 | 0.05 | 0.69 | ||
| TL | 94.1 | 60.63 | 0.91 | 21.01 | 4.71 | 0.05 | 1.11 | 1.69 | 1.07 | 2.46 | 0.16 | 0.10 | 0.02 | 6.07 | ||
| Nd (μm) | MC | 8.9 | 56.66 | 0.85 | 19.82 | 7.70 | 0.07 | 1.52 | 2.24 | 0.96 | 2.17 | 0.18 | 0.08 | 0.02 | 7.72 | |
| >75 | 13.7 | 53.46 | 0.69 | 16.11 | 5.56 | 0.07 | 1.43 | 1.76 | 0.97 | 1.84 | 0.14 | <0.002 | 0.01 | 17.95 | ||
| 75−45 | 19.6 | 56.71 | 0.75 | 17.41 | 5.20 | 0.06 | 1.46 | 1.82 | 0.94 | 1.96 | 0.15 | <0.002 | 0.02 | 13.52 | ||
| 45–25 | 23.0 | 59.35 | 0.86 | 19.61 | 6.47 | 0.07 | 1.82 | 2.10 | 0.96 | 2.15 | 0.18 | <0.002 | 0.02 | 6.40 | ||
| <25 | 43.7 | 57.23 | 0.98 | 22.30 | 9.34 | 0.09 | 1.52 | 2.69 | 1.03 | 2.45 | 0.21 | 0.01 | 0.03 | 2.11 | ||
| TL | 82.9 | 61.17 | 0.93 | 21.21 | 3.88 | 0.04 | 1.05 | 1.59 | 1.09 | 2.49 | 0.15 | 0.02 | 0.01 | 6.37 | ||
| S4–BA | Bulk | 66.56 | 0.79 | 17.60 | 9.01 | 0.07 | 1.56 | 1.65 | 0.94 | 1.88 | 0.10 | 0.03 | 0.02 | −0.20 | ||
| Fe | MC | 5.5 | 58.99 | 0.74 | 16.47 | 16.88 | 0.14 | 1.97 | 2.24 | 0.84 | 1.69 | 0.12 | 0.01 | 0.02 | −0.10 | |
| TL | 94.1 | 67.20 | 0.80 | 17.73 | 8.16 | 0.06 | 1.52 | 1.59 | 0.95 | 1.87 | 0.10 | <0.002 | 0.02 | 0.00 | ||
| S4–ECO | Bulk | 63.11 | 0.70 | 16.23 | 8.37 | 0.07 | 1.76 | 1.63 | 0.87 | 1.70 | 0.11 | 0.05 | 0.01 | 5.38 | ||
| Fe | MC | 13.1 | 40.98 | 0.57 | 12.96 | 35.37 | 0.26 | 2.44 | 2.99 | 0.58 | 1.20 | 0.13 | 0.12 | 0.02 | 2.39 | |
| TL | 85.7 | 65.77 | 0.71 | 16.68 | 4.35 | 0.04 | 1.58 | 1.40 | 0.90 | 1.74 | 0.10 | 0.08 | 0.02 | 6.61 | ||
| S4–ESP 12 | Bulk | 58.71 | 0.83 | 18.97 | 7.82 | 0.06 | 1.57 | 1.76 | 1.06 | 2.08 | 0.13 | 0.34 | 0.02 | 6.66 | ||
| Fe | MC | 5.9 | 33.68 | 0.51 | 11.99 | 44.19 | 0.25 | 2.16 | 3.15 | 0.49 | 1.00 | 0.17 | 0.10 | 0.03 | 2.29 | |
| TL | 93.2 | 60.39 | 0.86 | 19.76 | 4.75 | 0.05 | 1.33 | 1.65 | 1.08 | 2.18 | 0.13 | 0.13 | 0.02 | 7.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.C.; Cruz, C.; Font, E.; French, D.; Guedes, A.; Moreira, K.; Sant’Ovaia, H.; Vieira, B.J.C.; Waerenborgh, J.C.; Valentim, B. Physicochemical Properties of Fe-Bearing Phases from Commercial Colombian Coal Ash. Minerals 2023, 13, 1055. https://doi.org/10.3390/min13081055
Santos AC, Cruz C, Font E, French D, Guedes A, Moreira K, Sant’Ovaia H, Vieira BJC, Waerenborgh JC, Valentim B. Physicochemical Properties of Fe-Bearing Phases from Commercial Colombian Coal Ash. Minerals. 2023; 13(8):1055. https://doi.org/10.3390/min13081055
Chicago/Turabian StyleSantos, Ana Cláudia, Cláudia Cruz, Eric Font, David French, Alexandra Guedes, Karen Moreira, Helena Sant’Ovaia, Bruno J. C. Vieira, João C. Waerenborgh, and Bruno Valentim. 2023. "Physicochemical Properties of Fe-Bearing Phases from Commercial Colombian Coal Ash" Minerals 13, no. 8: 1055. https://doi.org/10.3390/min13081055
APA StyleSantos, A. C., Cruz, C., Font, E., French, D., Guedes, A., Moreira, K., Sant’Ovaia, H., Vieira, B. J. C., Waerenborgh, J. C., & Valentim, B. (2023). Physicochemical Properties of Fe-Bearing Phases from Commercial Colombian Coal Ash. Minerals, 13(8), 1055. https://doi.org/10.3390/min13081055