Molecular Dynamics Study on Crack Angle Effect on Amorphous Silica Fracture Performance
Abstract
:1. Introduction
2. Simulation Methods
2.1. Amorphous SiO2 Model Development
2.2. Amorphous SiO2 Model Parameters Comparison
2.2.1. Specific Surface Energy
2.2.2. Density and Radial Distribution Function (RDF)
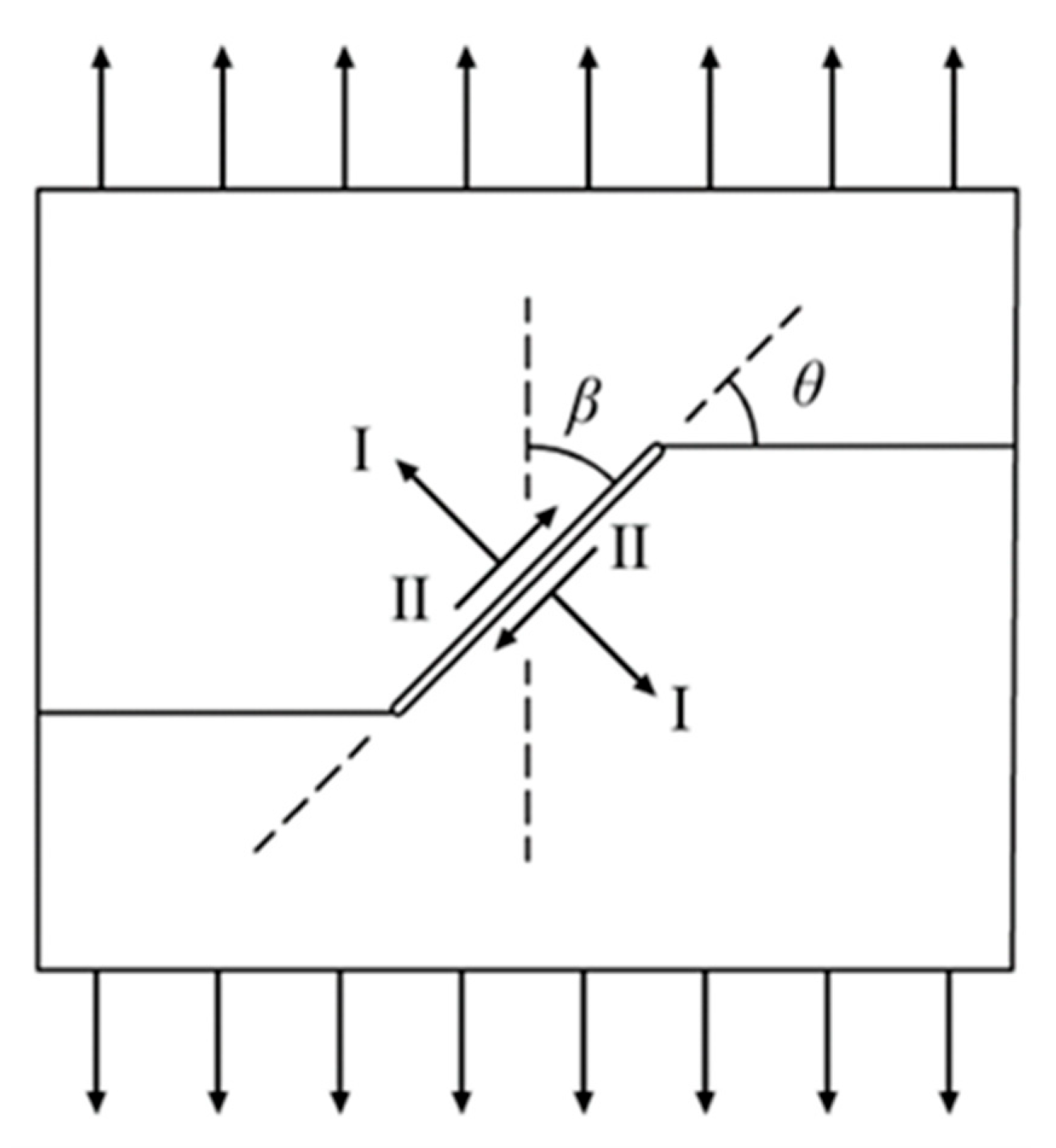
2.3. Crack Model and Tensile Process
3. Results and Discussion
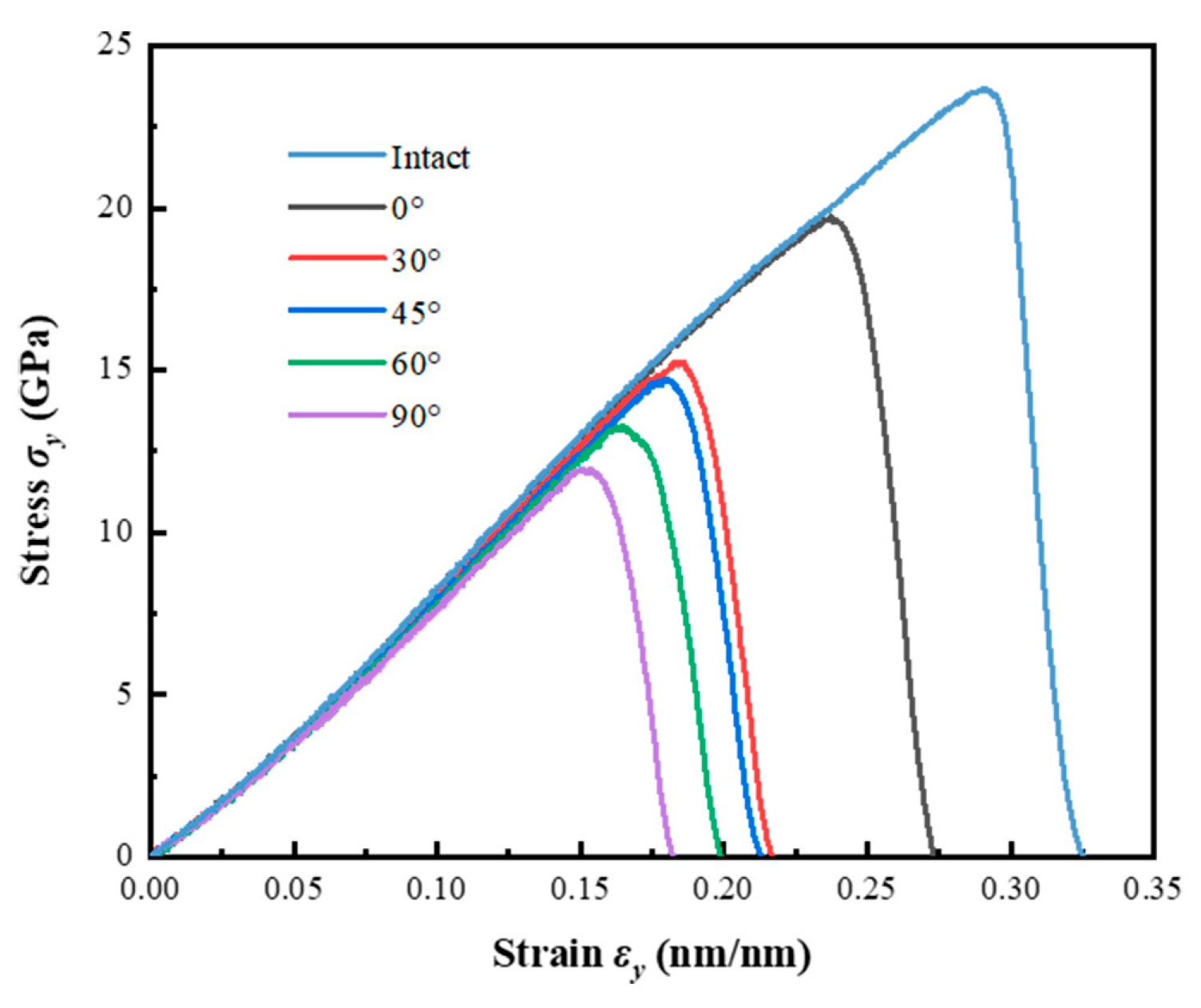
3.1. The Influence of Crack Angle on Mechanical Properties
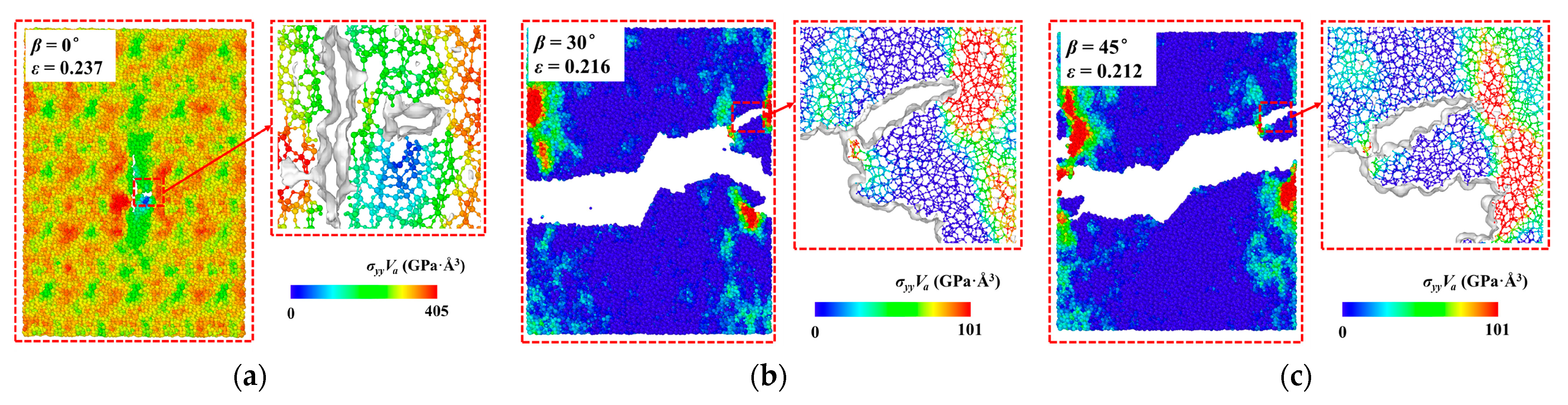
3.2. The Influence of Crack Angle on Crack Propagation
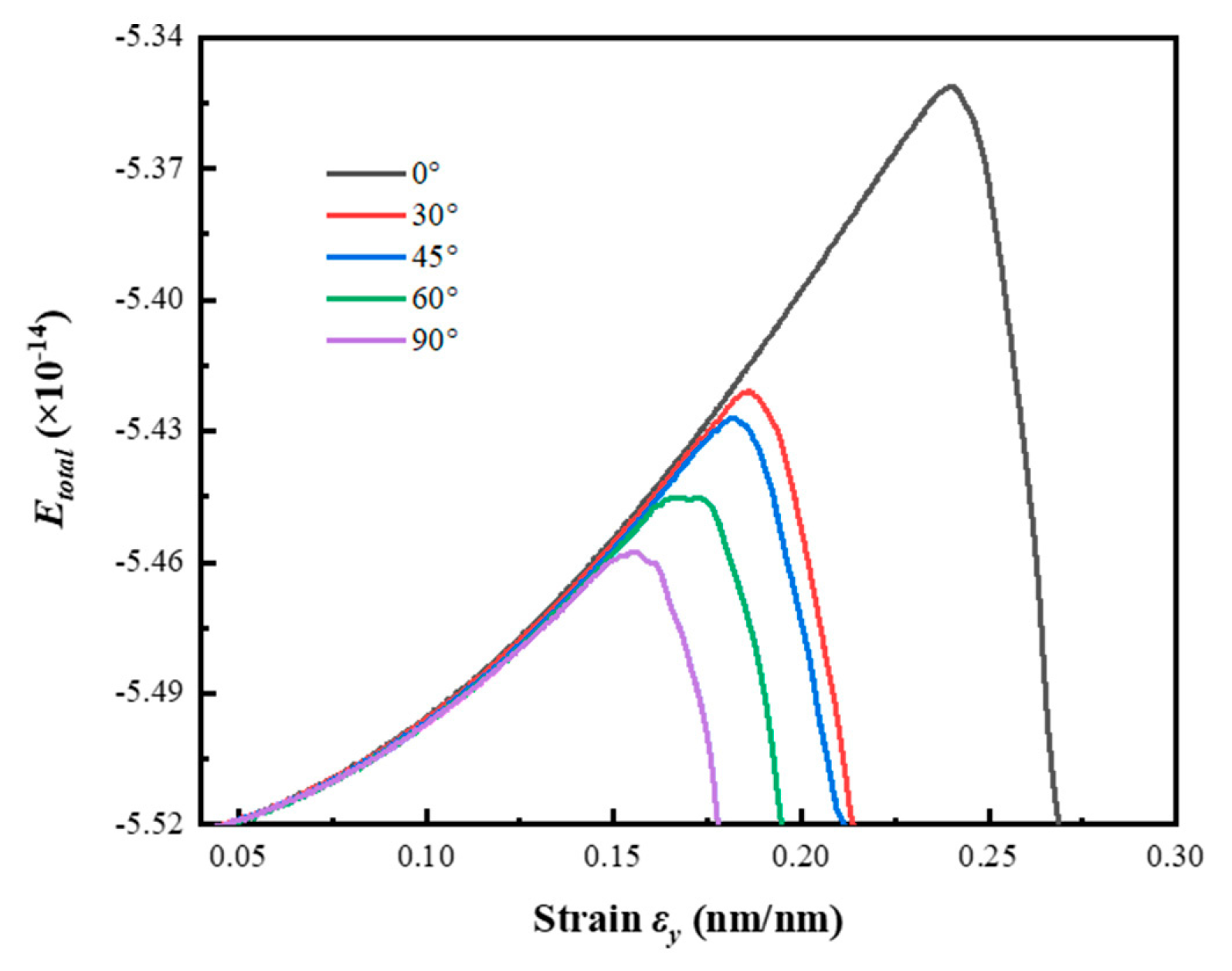
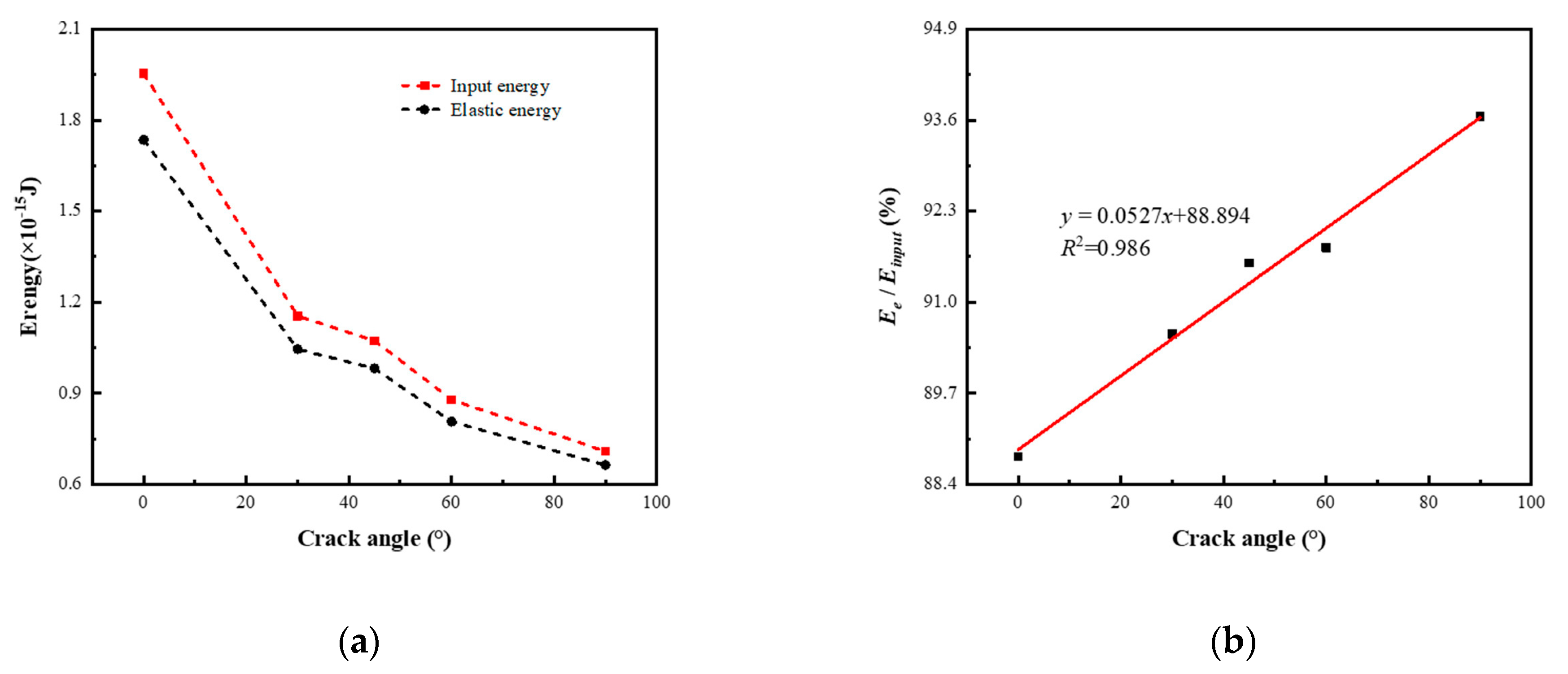
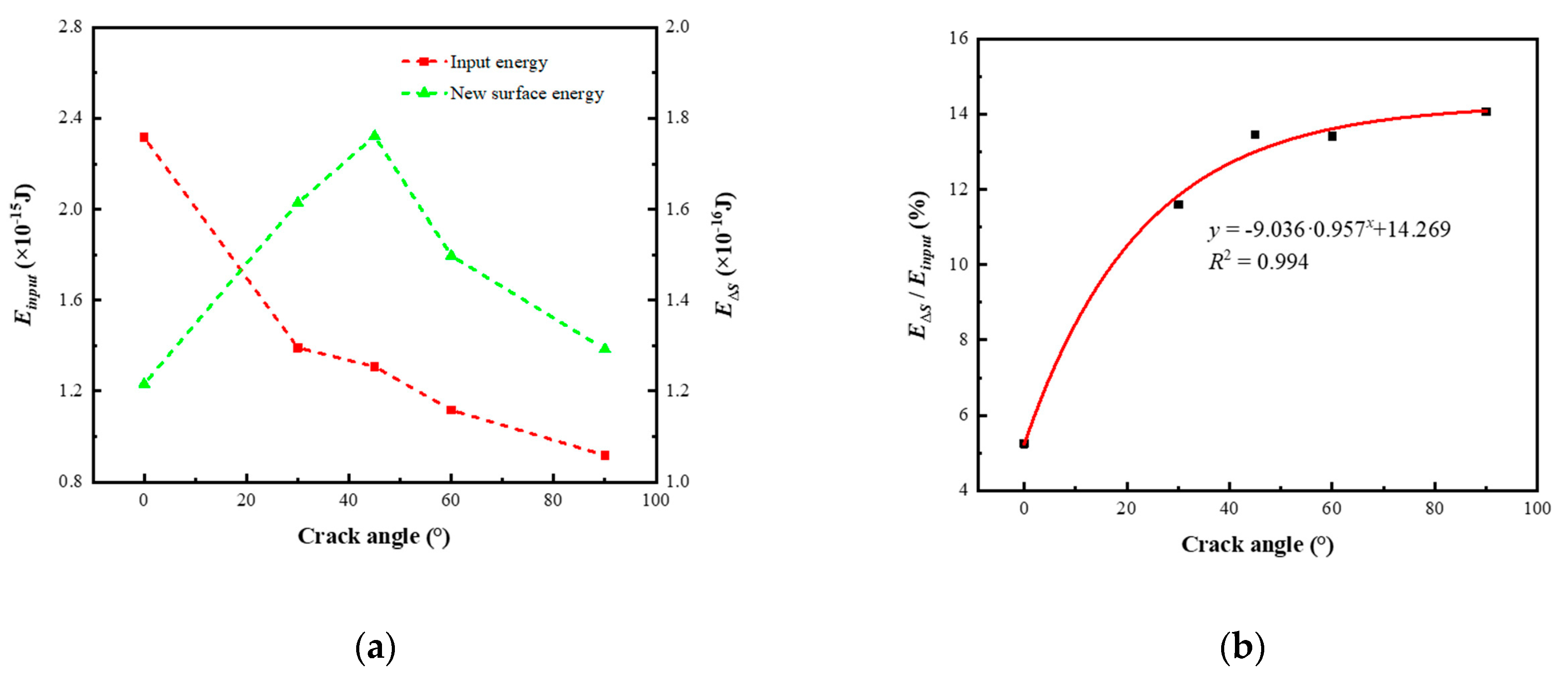
3.3. The Influence of Crack Angle on Energy Evolution
4. Conclusions
- (1)
- As the crack angle increases, the ultimate stress and strain of the model gradually decrease from 19.727 GPa and 0.237 at 0° to 11.971 GPa and 0.150 at 90°. The tensile and shear components around the crack will vary from the change of crack angle.
- (2)
- The crack propagation path in the fracture process exhibits a “Z” shape due to the coupling effect of tensile and shear loads, and the crack branching and healing phenomena occur at crack angles of 30° and 45°. The average bond length and crack angle of the model during the fracture exhibit a nonlinear trend.
- (3)
- As the crack angle increases, the maximum elastic energy stored in the model at the ultimate strain gradually decreases, with a maximum value of 2.758 times the minimum value. The elastic energy efficiency gradually increases with the increase in crack angle, and the R2 fitted by the linear regression model for its trend is 0.986. As the crack angle increases, the new surface energy at the time of the model completely fractured first increases and then decreases, and its maximum value at 45° is 1.448 times the minimum value at 90°. The new surface energy efficiency gradually increases and stabilizes with the increase in crack angle, and its maximum value is 2.678 times the minimum value. The R2 fitted by the progressive regression model for the new surface energy efficiency is 0.994.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yahyaei, M.; Hilden, M.; Shi, F.; Liu, L.X.; Ballantyne, G.; Palaniandy, S. Comminution. In Production, Handling and Characterization of Particulate Materials; Merkus, H.G., Meesters, G.M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 157–199. [Google Scholar]
- Fuerstenau, D.W.; Abouzeid, A.-Z.M. The energy efficiency of ball milling in comminution. Int. J. Miner. Process. 2002, 67, 161–185. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Ouchterlony, F. Energy Requirement for Rock Breakage in Laboratory Experiments and Engineering Operations: A Review. Rock Mech. Rock Eng. 2021, 55, 629–667. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, Q.; Pan, Y.T.; Zhu, C.Y.; Wei, Y.H. The accurate algorithm of new surface area of single particle comminution, incorporating particle shape and roughness. Physicochem. Probl. Miner. Process. 2021, 57, 259–272. [Google Scholar] [CrossRef]
- Guo, Q.; Pan, Y.T.; Zhou, Q.; Zhang, C.; Bi, Y.K. Kinetic Energy Calculation in Granite Particles Comminution Considering Movement Characteristics and Spatial Distribution. Minerals 2021, 11, 15. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, M.; Hu, N.; Yang, H.R.; Lu, J.F. Calculation model of coal comminution energy consumption. Miner. Eng. 2016, 92, 21–27. [Google Scholar] [CrossRef]
- Nadolski, S.; Klein, B.; Kumar, A.; Davaanyam, Z. An energy benchmarking model for mineral comminution. Miner. Eng. 2014, 65, 178–186. [Google Scholar] [CrossRef]
- Hukki, R.T. Proposal for a solomnic settlement between the theories of von Rittinger, Kick and Bond. Trans. AIME 1961, 220, 403–408. [Google Scholar]
- Martins, S. Size-energy relationship exponents in comminution. Miner. Eng. 2020, 149, 12. [Google Scholar] [CrossRef]
- Martins, S. Size-energy relationship in comminution, incorporating scaling laws and heat. Int. J. Miner. Process. 2016, 153, 29–43. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ma, C.D.; Wei, X.A.; Xie, W.B. Experimental study on mechanical properties and failure modes of pre-existing cracks in sandstone during uniaxial tension/compression testing. Eng. Fract. Mech. 2021, 255, 16. [Google Scholar] [CrossRef]
- Li, X.W.; Yao, Z.S.; Liu, X.H.; Huang, X.W. Energy Evolution and Damage Mechanism of Fractured Sandstone with Different Angles. Energies 2022, 15, 16. [Google Scholar] [CrossRef]
- Lv, H.Y.; Wang, D.F.; Cheng, Z.B.; Zhang, Y.N.; Zhou, T. Study on Mechanical Characteristics and Failure Modes of Coal-Mudstone Combined Body with Prefabricated Crack. Mathematics 2022, 10, 177. [Google Scholar] [CrossRef]
- Fang, T.H.; Shen, C.Y.; Fan, Y.C.; Chang, W.J. Fracture characteristics of silicene nanosheet with a crack under tension estimated using molecular dynamics simulation. Superlattices Microstruct. 2019, 129, 124–129. [Google Scholar] [CrossRef]
- Chowdhury, S.C.; Wise, E.A.; Ganesh, R.; Gillespie, J.W. Effects of surface crack on the mechanical properties of Silica: A molecular dynamics simulation study. Eng. Fract. Mech. 2019, 207, 99–108. [Google Scholar] [CrossRef]
- Vo, T.; He, B.; Blum, M.; Damone, A.; Newell, P. Molecular scale insight of pore morphology relation with mechanical properties of amorphous silica using ReaxFF. Comput. Mater. Sci. 2020, 183, 10. [Google Scholar] [CrossRef]
- Mei, H.; Yang, Y.J.; van Duin, A.C.T.; Sinnott, S.B.; Mauro, J.C.; Liu, L.S.; Fu, Z.Y. Effects of water on the mechanical properties of silica glass using molecular dynamics. Acta Mater. 2019, 178, 36–44. [Google Scholar] [CrossRef]
- Lawn, B. Fracture of Brittle Solids, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar] [CrossRef]
- Erdogan, F.; Sih, G.C. On the Crack Extension in Plates Under Plane Loading and Transverse Shear. J. Basic Eng. 1963, 85, 519–525. [Google Scholar] [CrossRef]
- Fogarty, J.C.; Aktulga, H.M.; Grama, A.Y.; van Duin, A.C.T.; Pandit, S.A. A reactive molecular dynamics simulation of the silica-water interface. J. Chem. Phys. 2010, 132, 10. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.F.; Feng, F.; Li, Y.J.; Hu, Z.Q.; Shao, J.L.; Mei, Y. Mechanical and microstructural response of densified silica glass under uniaxial compression: Atomistic simulations. Chin. Phys. B 2020, 29, 8. [Google Scholar] [CrossRef]
- Chowdhury, S.C.; Haque, B.Z.; Gillespie, J.W. Molecular dynamics simulations of the structure and mechanical properties of silica glass using ReaxFF. J. Mater. Sci. 2016, 51, 10139–10159. [Google Scholar] [CrossRef]
- Vo, T.; Reeder, B.; Damone, A.; Newell, P. Effect of Domain Size, Boundary, and Loading Conditions on Mechanical Properties of Amorphous Silica: A Reactive Molecular Dynamics Study. Nanomaterials 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Hao, Y.L.; Chen, S.H.; Cheng, M.; Li, C.L.; Sun, S.Q.; Hu, S.Q. DFT study of imidazoles adsorption on the grain boundary of Cu (100) surface. Corros. Sci. 2018, 137, 33–42. [Google Scholar] [CrossRef]
- Rimsza, J.M.; Jones, R.E.; Criscenti, L.J. Surface Structure and Stability of Partially Hydroxylated Silica Surfaces. Langmuir 2017, 33, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Wiederhorn, S.M. Fracture Surface Energy of Glass. J. Am. Ceram. Soc. 1969, 52, 99–105. [Google Scholar] [CrossRef]
- Rouxel, T. Fracture surface energy and toughness of inorganic glasses. Scr. Mater. 2017, 137, 109–113. [Google Scholar] [CrossRef]
- Mozzi, R.L.; Warren, B.E. The structure of vitreous silica. J. Appl. Crystallogr. 1969, 2, 164–172. [Google Scholar] [CrossRef]
- Tsai, D.H. The virial theorem and stress calculation in molecular dynamics. J. Chem. Phys. 1979, 70, 1375–1382. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 7. [Google Scholar] [CrossRef]
- Rimsza, J.M.; Jones, R.E.; Criscenti, L.J. Chemical Effects on Subcritical Fracture in Silica from Molecular Dynamics Simulations. J. Geophys. Res. Solid Earth 2018, 123, 9341–9354. [Google Scholar] [CrossRef]
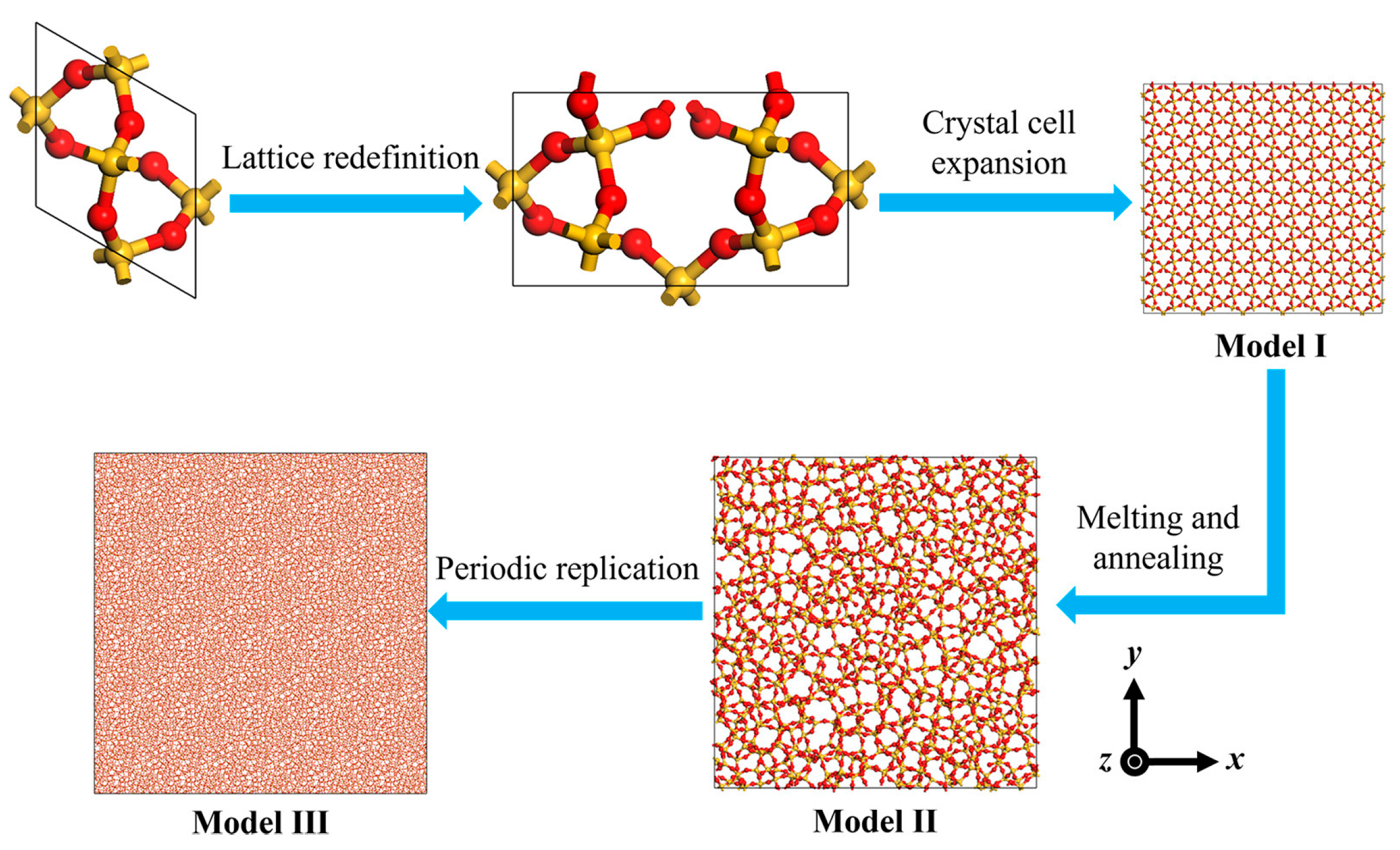





| Model | Dimensions LX × LY × LZ (nm) | Density (g/cm3) | Number of Atoms |
|---|---|---|---|
| I | 5.10 × 4.91 × 1.08 | 2.65 | 2160 |
| II | 5.24 × 5.38 × 1.18 | 2.16 | 2160 |
| III | 26.21 × 26.93 × 1.18 | 2.16 | 54,000 |
| Slicing Position | (nm2) | (×10−15) | (J/m2) |
|---|---|---|---|
| x0.25 | 31.633 | 0.144 | 2.277 |
| x0.50 | 31.633 | 0.140 | 2.211 |
| x0.75 | 31.633 | 0.158 | 2.491 |
| y0.25 | 30.905 | 0.139 | 2.252 |
| y0.50 | 30.905 | 0.154 | 2.483 |
| y0.75 | 30.905 | 0.132 | 2.136 |
| z0.25 | 705.534 | 3.532 | 2.503 |
| z0.50 | 705.534 | 3.323 | 2.355 |
| z0.75 | 705.534 | 3.229 | 2.288 |
| Structural Parameters | Our Simulation Results | Previous Simulation Results [20,24] | Experimental Results [29] |
|---|---|---|---|
| Density (g/cm3) | 2.16 | 2.14 | 2.20 |
| Si-Si RDF 1st peak position (nm) | 0.3075 | 0.3071 | 0.3077 |
| Si-Si RDF 2nd peak position (nm) | 0.5075 | 0.5193 | 0.5182 |
| O-O RDF 1st peak position (nm) | 0.2525 | 0.2538 | 0.2626 |
| O-O RDF 2nd peak position (nm) | 0.4925 | 0.4896 | 0.5097 |
| Si-O RDF 1st peak position (nm) | 0.1575 | 0.1633 | 0.1608 |
| Si-O RDF 2nd peak position (nm) | 0.3875 | 0.3969 | 0.4061 |
| Crack Angle | (nm/nm) | (GPa) | (GPa) |
|---|---|---|---|
| Intact | 0.291 | 23.705 | 73.981 |
| 0° | 0.237 | 19.727 | 73.586 |
| 30° | 0.185 | 15.245 | 72.488 |
| 45° | 0.180 | 14.722 | 70.674 |
| 60° | 0.165 | 13.275 | 70.489 |
| 90° | 0.150 | 11.971 | 70.082 |
| Crack Angle | ε, nm/nm | Average Bond Length, Å |
|---|---|---|
| 0° | 0.237 | 1.630 |
| 0.252 | 1.620 | |
| 0.273 | 1.582 | |
| 30° | 0.185 | 1.612 |
| 0.200 | 1.602 | |
| 0.217 | 1.583 | |
| 45° | 0.180 | 1.610 |
| 0.195 | 1.602 | |
| 0.213 | 1.584 | |
| 60° | 0.165 | 1.606 |
| 0.180 | 1.602 | |
| 0.199 | 1.583 | |
| 90° | 0.150 | 1.602 |
| 0.165 | 1.598 | |
| 0.182 | 1.582 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Pan, Y.; Zhang, C.; Bi, Y.; Liu, P.; Wang, C.; Tang, C. Molecular Dynamics Study on Crack Angle Effect on Amorphous Silica Fracture Performance. Minerals 2023, 13, 1068. https://doi.org/10.3390/min13081068
Cao X, Pan Y, Zhang C, Bi Y, Liu P, Wang C, Tang C. Molecular Dynamics Study on Crack Angle Effect on Amorphous Silica Fracture Performance. Minerals. 2023; 13(8):1068. https://doi.org/10.3390/min13081068
Chicago/Turabian StyleCao, Xingjian, Yongtai Pan, Chuan Zhang, Yankun Bi, Pengfei Liu, Congcong Wang, and Chenjie Tang. 2023. "Molecular Dynamics Study on Crack Angle Effect on Amorphous Silica Fracture Performance" Minerals 13, no. 8: 1068. https://doi.org/10.3390/min13081068




