Provenance of Coastal and Seabed Sediments Relative to Mining and Processing Wastes: The Case of Lavrion, Attiki Peninsula, Greece
Abstract
:1. Introduction
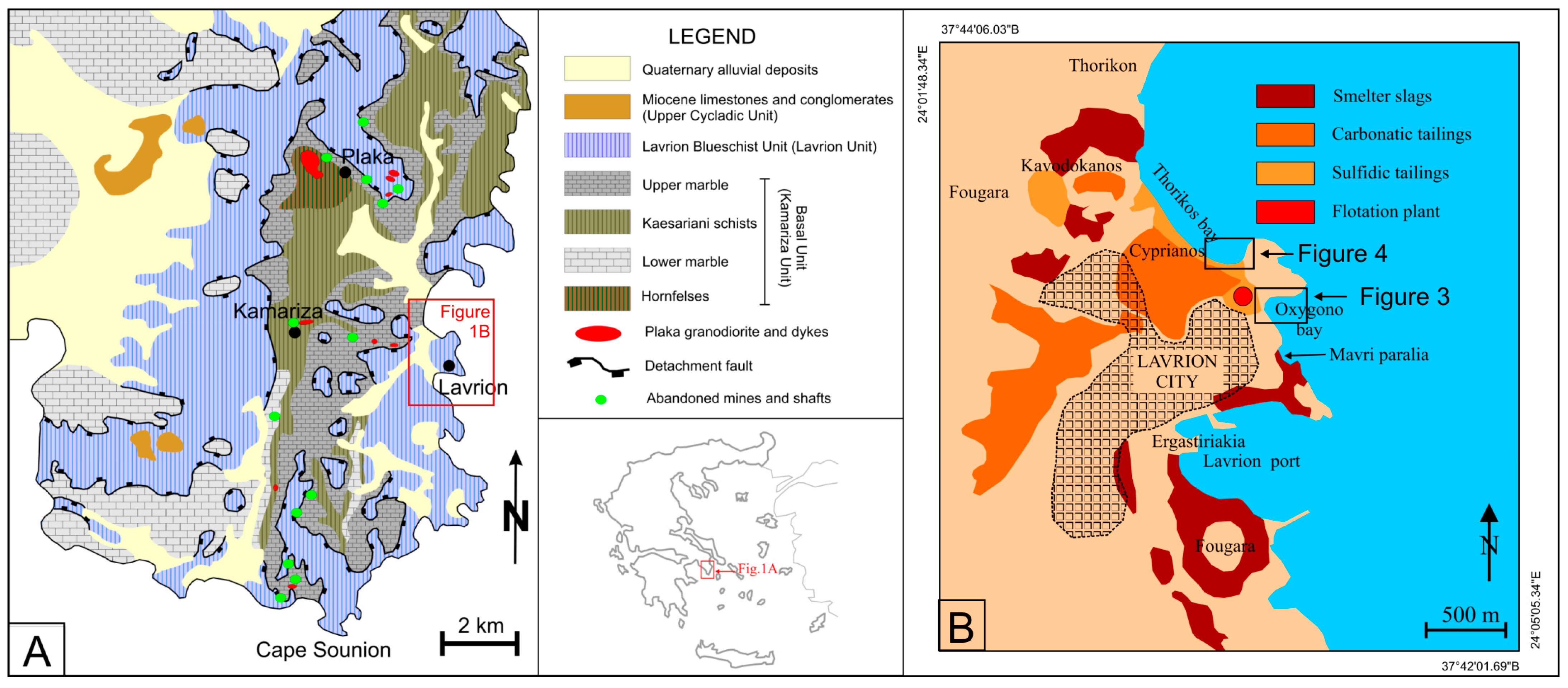
2. Regional Geology, Ore Deposit Types and Historical Flashback of Exploitation
- Smelter slags disposed of close to the former smelting plants, with their total quantity estimated at 7 Mt (Figure 1B). It is worth mentioning that the largest part of the slag has been removed due to the construction of the new Lavrion port in the early 2000s,
- carbonatic tailings (light) after hydromechanical processing of carbonate ore, estimated at 5 Mt (Figure 1B), and
- sulfidic flotation tailings with a total estimated quantity of ~0.8 Mt (Figure 1B). The quantities disposed of in the southern part of Thorikos Bay reach approximately 120 kt, building a pile of waste with an average height of 3.5 m.
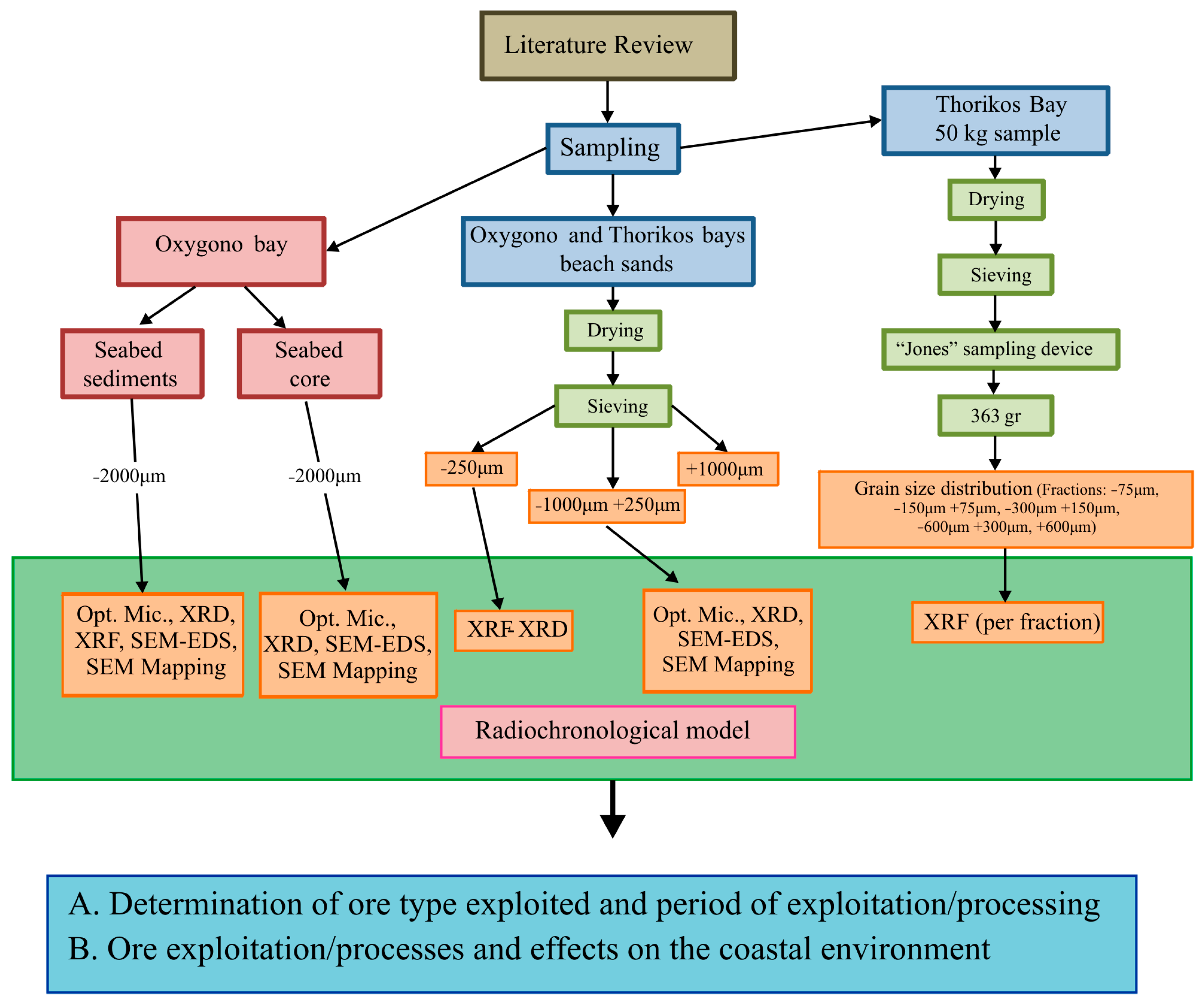
3. Methodology, Sampling, and Analytical Techniques
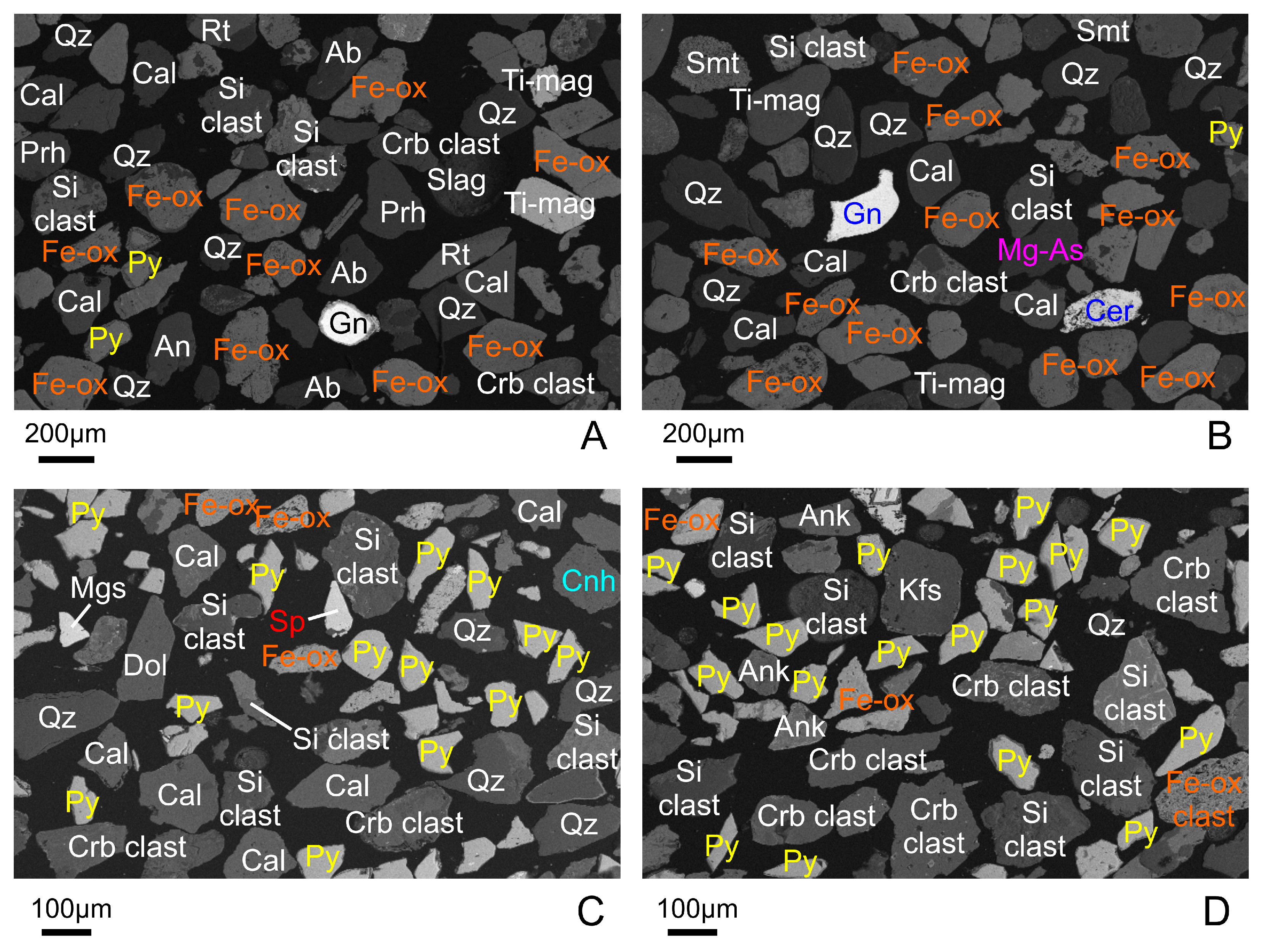
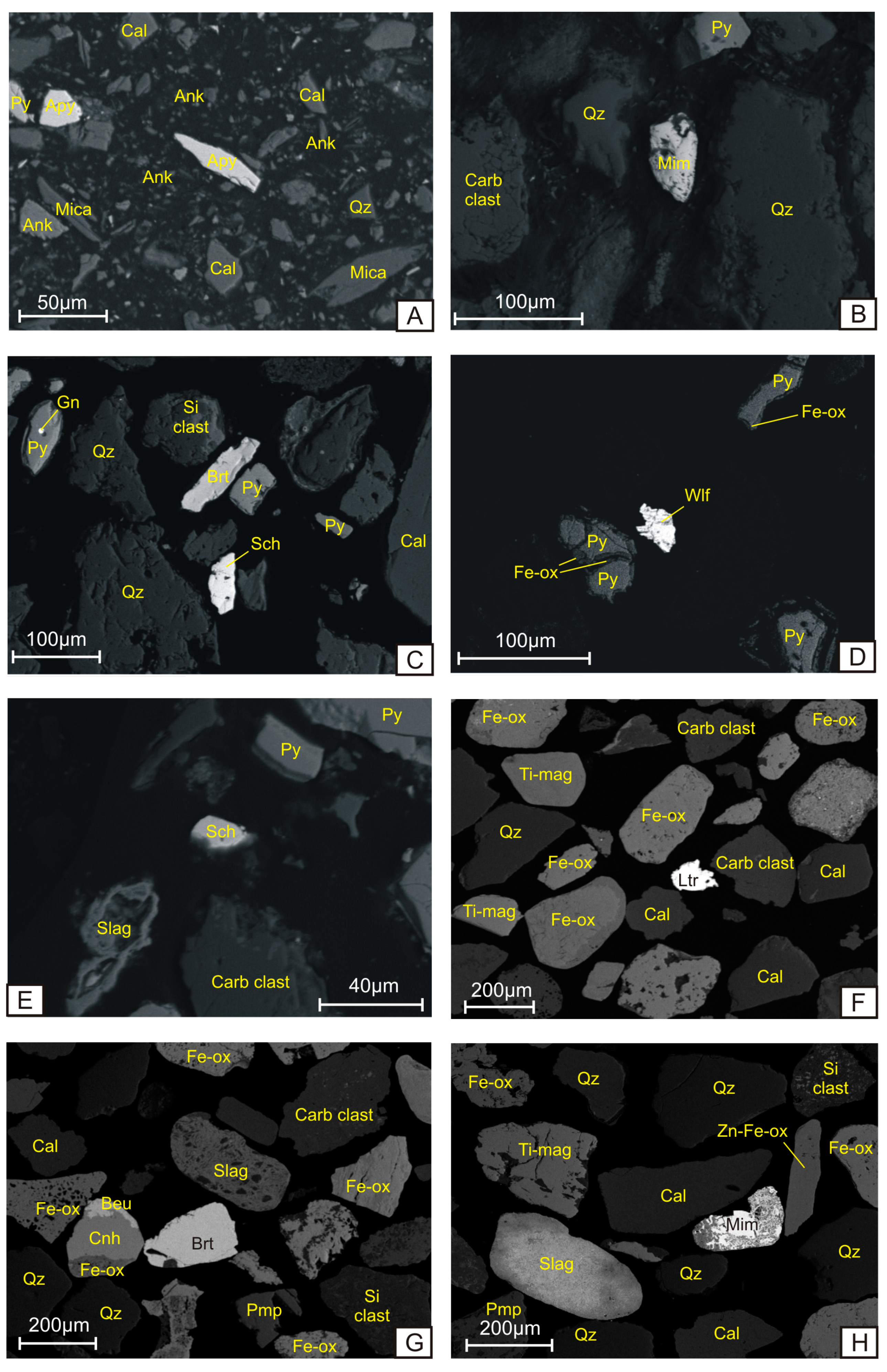
4. Results
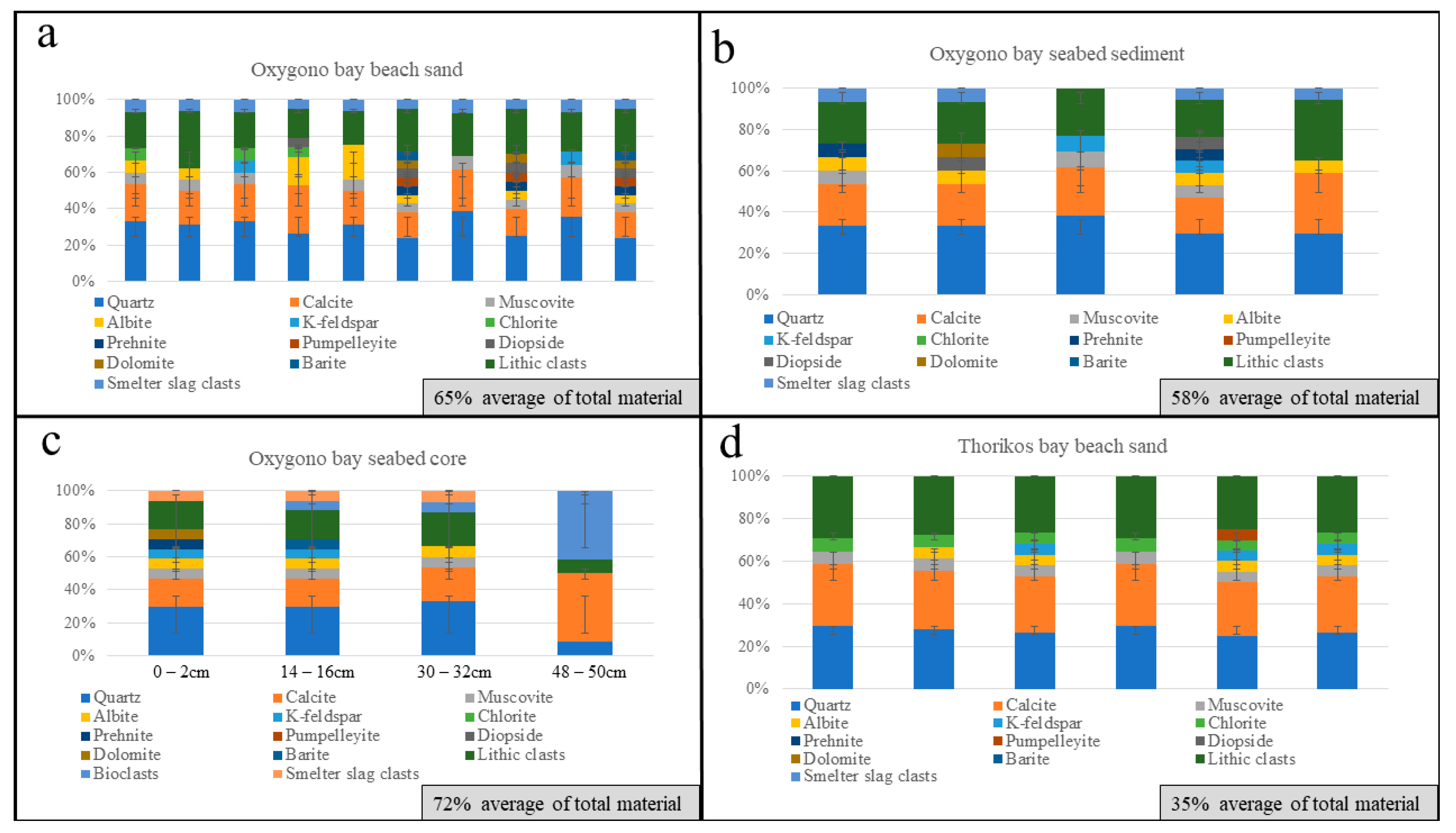
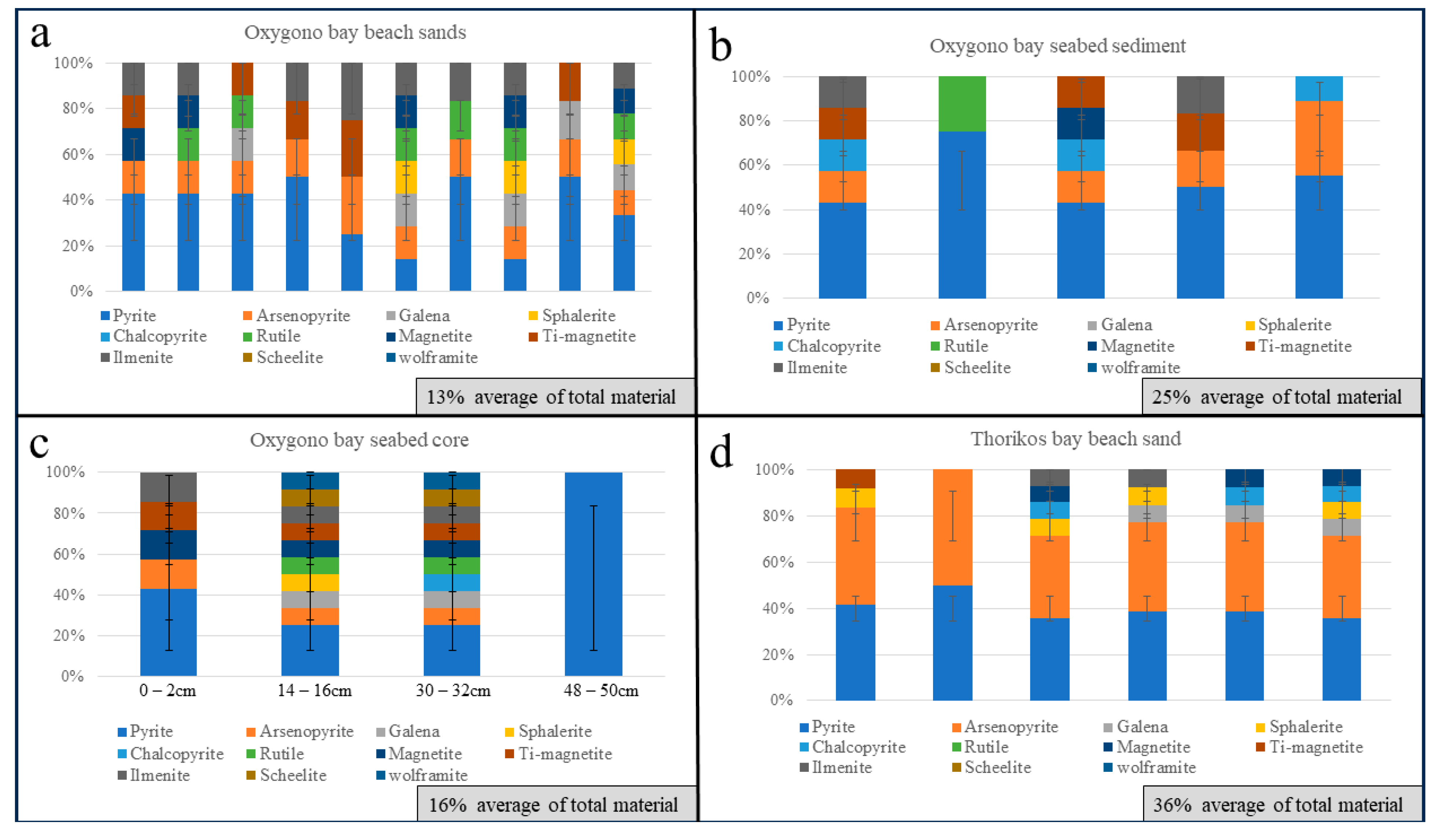
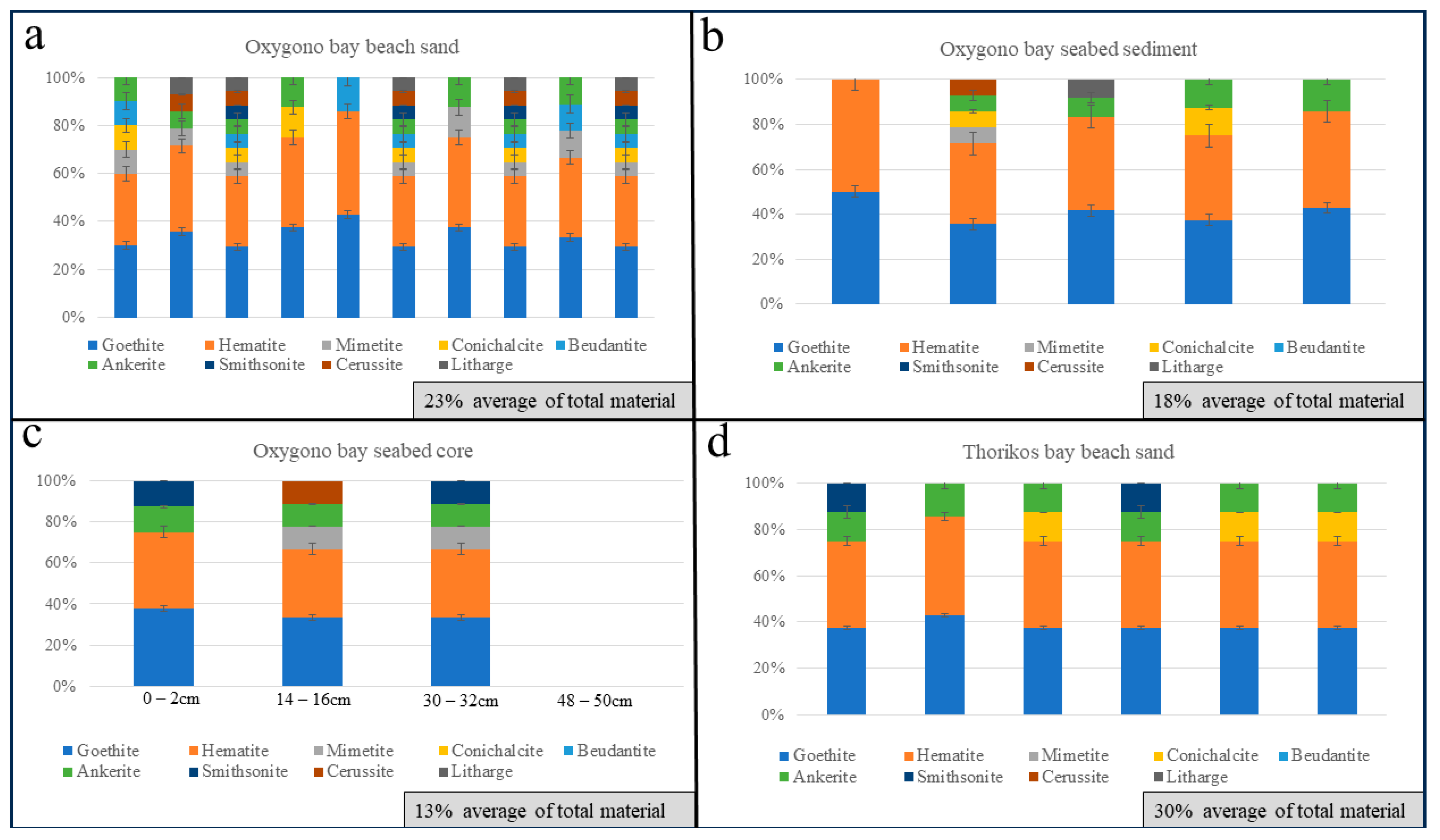
4.1. Oxygono Bay
4.1.1. Beach Sands
4.1.2. Seabed Sediments–Seabed Cores
4.2. Southern Thorikos Bay Beach Sands
5. Discussion
5.1. Correlation between Coastal Detritus and Ore Type
- Large size material (pebbles and boulders) originating from nearby lithologies and related to natural weathering/erosion and transportation of lithic clasts to lower stratigraphic levels (rounded-subrounded material).
- Circulation and transportation of ancient and recent smelter slag material, since Oxygono Bay is very close to areas where smelter slags were disposed (e.g., Mavri paralia is largely composed of smelter slags; Figure 1B).
- Mining and ore processing wastes characterising the various ore types and processing technologies.
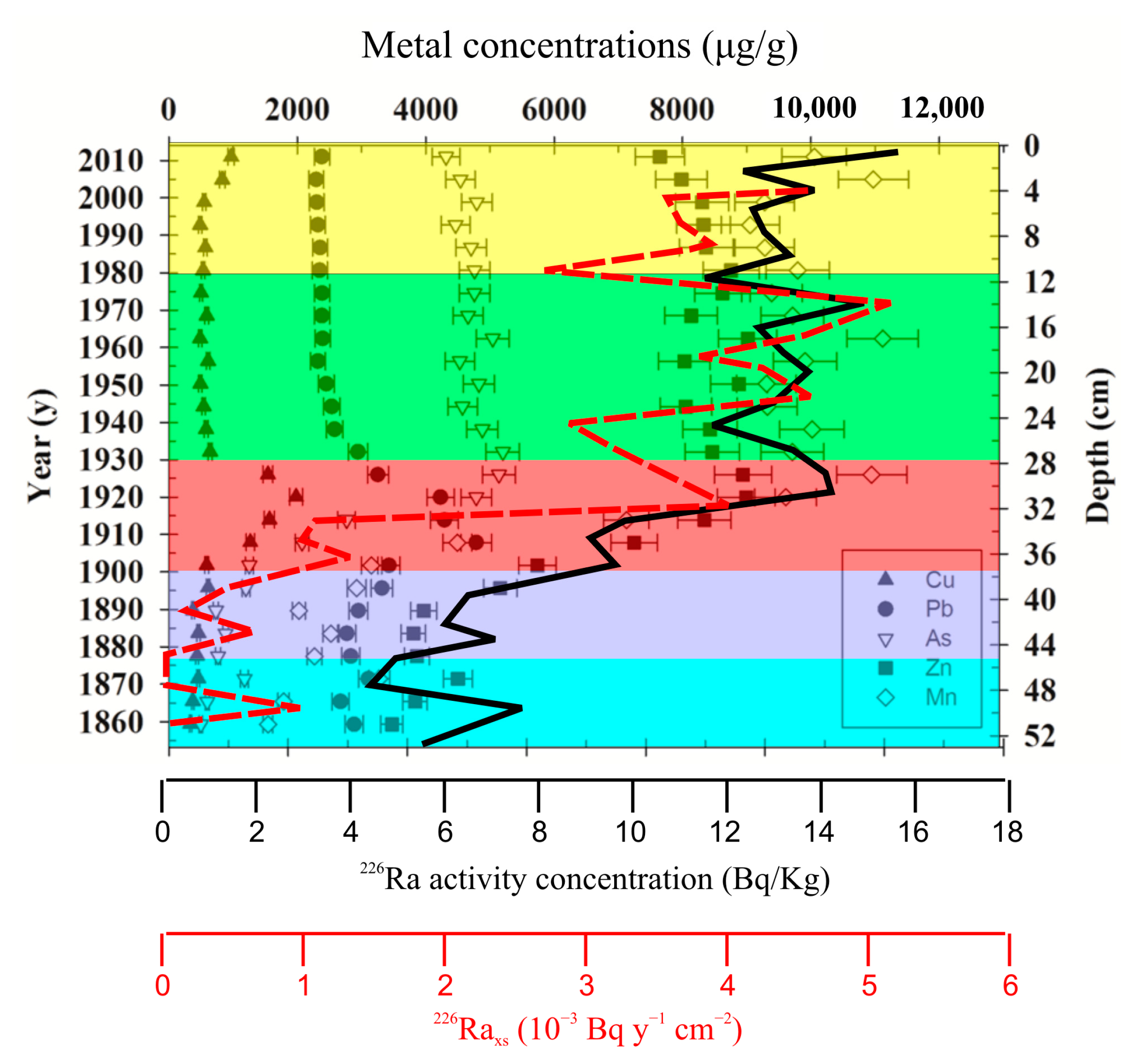
5.2. Oxygono Bay—Periods of Exploitation
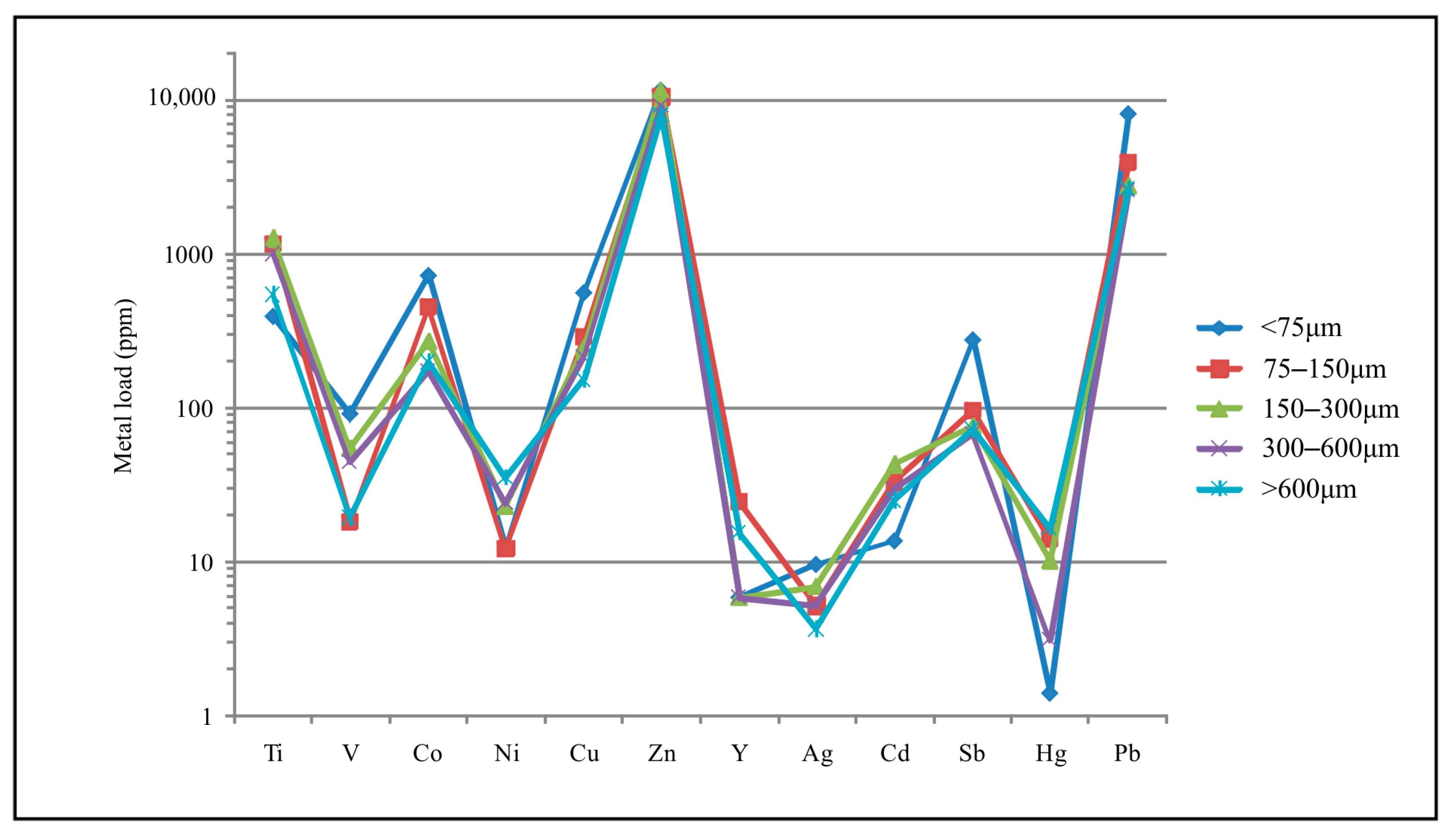
5.3. Effect of Mining and Processing Wastes in the Lavrion Coastal Environment
6. Conclusions
- 1860–1875 A.D. (46–52 cm depth), with primary focus on the ancient smelter slags scattered in the broader Lavrion area.
- 1875–1900 A.D. (37.5–46 cm depth), where exploitation of the ancient smelter slags continues and, at the same time, the exploitation of underground sulfide ore commences (gradual increase in radionuclide content).
- 1900–1930 A.D. (28–37.5 cm depth), where the replacement type sulfide ore was heavily mined, and production reached its peak due to the implementation of a “Water-jacket” type furnace (steep increase in radionuclide content).
- 1930–1980 A.D. (11.5–28 cm depth), where the replacement type carbonate hosted ore is nearly depleted, and the implementation of flotation-type processing assisted in the exploitation of other poor sulfide ore types, such as the skarn and porphyry type ores. The presence of tungstenates (hubnerite, scheelite, and ferberite) in the seabed material and the highest radionuclide content among all periods support this hypothesis.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A. Human Development and Natural Resources; Sapur and Sons: New Delhi, India, 2006; 263p. [Google Scholar]
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Queensland, Australia, 2010; 400p. [Google Scholar]
- Lambert, J.B. Traces of the Past. Unraveling the Secrets of Archaeology through Chemistry; Basic Books: Reading, MA, USA, 1997; 336p. [Google Scholar]
- Kat, W. Exploitation of Natural Resources Man Systematic Exploitation. J. Bioremediat. Biodegrad. 2021, 12, e009. [Google Scholar]
- Ripley, E.A.; Redmann, R.E.; Crowder, A.A. Environmental Effects of Mining; St Lucie Press: Delray Beach, FL, USA, 1996; 356p. [Google Scholar]
- Shotyk, W.; Cheburkin, A.K.; Appleby, P.G.; Fankhauser, A.; Kramers, J.D. Two thousand years of atmospheric arsenic, antimony, and lead deposition recorded in an ombrotrophic peat bog profile, Jura Mountains, Switzerland. Earth Planet. Sci. Lett. 1996, 145, E1–E7. [Google Scholar] [CrossRef]
- Ernst, W.H.O. The origin and ecology of contaminated, stabilized and non-pristine soils. In Metal Contaminated Soils: In Situ Inactivation and Phytorestoration; Vangronsveld, J., Cunningham, S., Eds.; Springer: Heidelberg, Germany, 1998; pp. 17–29. [Google Scholar]
- Bell, F.G.; Donnelly, L.J. Mining and Its Impact on the Environment; Taylor Francis: London, UK, 2006. [Google Scholar]
- Plumlee, G.S.; Morman, S.A. Mine wastes and human health. Elements 2011, 7, 399–404. [Google Scholar] [CrossRef]
- Agricola, G. De Re Metallica (1556); Hoover, H.C.; Hoover, L.H., Translators; Dover Pub. Inc.: New York, NY, USA, 1950; 638p. [Google Scholar]
- Alpers, C.N.; Blowes, D.W. Environmental Geochemistry of Sulfide Oxidation; American Chemical Society Symposium Series; American Chemical Society: Washington, DC, USA, 1994; p. 550. [Google Scholar]
- Evangelou, V.P. Pyrite Oxidation and Its Control; CRC Press: Boca Raton, FL, USA, 1995; 293p. [Google Scholar]
- Skousen, J.G.; Ziemkiewicz, P.F. Acid Mine Drainage Control and Prevention, 2nd ed.; West Virginia University and the National Mine Land Reclamation Center: Morgantown, WV, USA, 1996; 362p. [Google Scholar]
- Conophagos, C. The Lavrion and the Ancient Greek Techniques for Silver Production; Ekdotiki Athinon: Athens, Greece, 1980; 458p. (In Greek) [Google Scholar]
- Tzeferis, P.; Bitzios, D. Lavreotiki: The chronicle of a unique world heritage of more than 6000 years old. In The Lavrion “Argyritis Land” and the Victorious Naval Battle of Salamis; Tzeferis, P., Bitzios, D., Eds.; Municipality of Lavreotiki: Lavrion, Greece, 2023; pp. 4–31, (English and Greek text). [Google Scholar]
- Renzi, M.; Montero-Ruiz, I.; Bode, M. Non-ferrous metallurgy from the Phoenician site of La Fonteta (Alicante, Spain): A study of provenance. J. Archeol. Sci. 2009, 36, 2584–2596. [Google Scholar] [CrossRef]
- Chiarantini, L.; Benvenuti, M.; Costagliola, P.; Dini, A.; Firmati, M.; Guideri, S.; Villa, I.M.; Corretti, A. Copper metallurgy in ancient Etruria (southern Tuscany, Italy) at the Bronze-Iron Age transition: A lead isotope provenance study. J. Archeol. Sci. Rep. 2018, 19, 11–23. [Google Scholar] [CrossRef]
- Garcia de Madinabeitia, S.; Ibarguchi, J.I.G.; Zalduegui, J.F.S. IBERLID: A lead isotope database and tool for metal provenance and ore deposits research. Ore Geol. Rev. 2021, 137, 104279. [Google Scholar] [CrossRef]
- Vaxevanopoulos, M.; Blichert-Toft, J.; Davis, G.; Albarède, F. New findings of ancient Greek silver sources. J. Archeol. Sci. 2022, 137, 105474. [Google Scholar] [CrossRef]
- Pappa, F.K.; Tsabaris, C.; Patiris, D.L.; Androulakaki, E.G.; Eleftheriou, G.; Betsou, C.; Michalopoulou, V.; Kokkoris, M.; Vlastou, R. Historical trends and assessment of radionuclides and heavy metals in sediments near an abadonded mine, Lavrio, Greece. Environ. Sci. Pollut. Res. 2018, 25, 30084–30100. [Google Scholar] [CrossRef]
- Pappa, F.K. Study and Dispersion of Radionuclides and Heavy Metals in Coastal Areas of Greece, Characterized by Active and Past Mining Activities. Ph.D. Thesis, School of Applied Mathematics and Physical Sciences, National Technical University of Athens, Athens, Greece, 2018; 188p. [Google Scholar]
- Lister, G.S.; Banga, G.; Feenstra, A. Metamorphic core complexes of the Cordilleran type in the Cyclades, Aegean Sea, Greece. Geology 1984, 12, 221–225. [Google Scholar] [CrossRef]
- Avigad, D.; Garfunkel, Z.; Jolivet, L.; Azanón, J.M. Back arc extension and denudation of Mediterranean eclogites. Tectonics 1997, 16, 924–941. [Google Scholar] [CrossRef]
- Liati, A.; Skarpelis, N.; Pe-Piper, G. Late Miocene magmatic activity in the Attic- Cycladic Belt of the Aegean (Lavrion Se Attica, Greece): Implications for the geodynamic evolution and timing of ore deposition. Geol. Mag. 2009, 146, 732–742. [Google Scholar] [CrossRef]
- Van Hinsbergen, D.J.J.; Schmid, S.M. Map view restoration of Aegean–West Anatolian accretion and extension since the Eocene. Tectonics 2012, 31, 1–40. [Google Scholar] [CrossRef]
- Papanikolaou, D.J. Tectonic evolution of the Cycladic blueschist belt (Aegean Sea, Greece). In Chemical Transport in Metasomatic Processes; Helgeson, H.C., Ed.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1987; Volume 218, pp. 429–450. [Google Scholar]
- Faure, M.; Bonneau, M.; Pons, J. Ductile deformation and syntectonic granite emplacement during the late Miocene extension of the Aegean (Greece). BSGF-Earth Sci. Bull. 1991, 162, 3–11. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.W. The Igneous Rocks of Greece. The Anatomy of an Orogen; Schweitzerbart: Berlin-Stuttgart, Germany, 2002; 573p. [Google Scholar]
- Jolivet, L.; Brun, J.P. Cenozoic geodynamic evolution of the Aegean. Int. J. Earth Sci. 2010, 99, 109–138. [Google Scholar] [CrossRef]
- Berger, A.; Schneider, D.A.; Grasemann, B.; Stockli, D. Footwall mineralization during Late Miocene extension along the West Cycladic Detachment System, Lavrion, Greece. Terra Nova 2013, 25, 181–191. [Google Scholar] [CrossRef]
- Baltatzis, E. Blueschist-to-greenschist transition and the P–T path of prasinites from the Lavrion area, Greece. Min. Mag. 1996, 60, 551–561. [Google Scholar] [CrossRef]
- Marinos, G.; Petrascheck, W.E. Lavrion: Geological and geophysical research. Inst. Geol. Subsurf. Res. 1956, 4, 246. [Google Scholar]
- Skarpelis, N. The Lavrion deposit (SE Attica, Greece): Geology, mineralogy and minor elements chemistry. Neues Jahrb. Mineral. Abhandlungen 2007, 183, 227–249. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Bonsall, T.; Tarkian, M.; Economou-Eliopoulos, M. Mineralogical and fluid inclusion constraints on the evolution of the Plaka intrusion-related ore system, Lavrion, Greece. Mineral Petrol 2008, 93, 79–110. [Google Scholar] [CrossRef]
- Bonsall, T.A.; Spry, P.G.; Voudouris, P.; Tombros, S.; Seymour, K.; Melfos, V. The Geochemistry of Carbonate-Replacement Pb-Zn-Ag Mineralization in the Lavrion District, Attica, Greece: Fluid Inclusion, Stable Isotope, and Rare Earth Element Studies. Econ. Geol. 2011, 106, 619–651. [Google Scholar] [CrossRef]
- Kontopoulos, A.; Komnitsas, K.; Xenidis, A.; Papassiopi, N. Environmental characterisation of the sulphidic tailings in Lavrion. Miner. Eng. 1995, 8, 1209–1219. [Google Scholar] [CrossRef]
- Xenidis, A.; Papassiopi, N.; Komnitsas, K. Carbonate-rich mining tailings in Lavrion: Risk assessment and proposed rehabilitation schemes. Adv. Environ. Res. 2003, 7, 479–494. [Google Scholar] [CrossRef]
- Leleu, M.; Morikis, A.; Picot, P. Sur des mineralisations de type skarn au Laurium (Grèce). Miner Deposita 1973, 36, 477–489. [Google Scholar]
- Economou, M.; Skounakis, S.; Papathanasiou, C. Magnetite deposits of skarn type from the Plaka area of Laurium, Greece. Chem. Erde 1981, 40, 241–252. [Google Scholar]
- Economou, M.; Sideris, C. A mineralized brecciated granodiorite porphyry in the Laurium mines, Greece. Neues Jahrb. Mineral. Abhandlungen 1976, 128, 209–218. [Google Scholar]
- Voudouris, P. A Field Guide on the Geology and Mineralogy of Lavrion, Attica, Greece; Department of Mineralogy and Petrology, Faculty of Geology and Geoenvironment, National Kapodistrian University of Athens: Athens, Greece, 2018; 21p. [Google Scholar]
- Scheffer, C.; Vanderhaeghe, O.; Lanari, P.; Tarantola, A.; Ponthus, L.; Photiades, A.; France, L. Syn-to post-orogenic exhumation of metamorphic nappes: Structure and thermobarometry of the western Attic-Cycladic metamorphic complex (Lavrion, Greece). J. Geodyn. 2016, 96, 174–193. [Google Scholar] [CrossRef]
- Skarpelis, N.; Argyraki, A. Geology and origin of the supergene ore at the Lavrion Pb-Ag-Zn deposit, Attica, Greece. Resour. Geol. 2009, 59, 1–14. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Mavrogonatos, C.; Photiades, A.; Moraiti, E.; Rieck, B.; Kolitsch, U.; Tarantola, A.; Scheffer, C.; Morin, D.; et al. The Lavrion Mines: A Unique Site of Geological and Mineralogical Heritage. Minerals 2021, 11, 76. [Google Scholar] [CrossRef]
- Jaxel, R.; Gelaude, P. New mineral occurrences from the Laurion slags. Mineral Rec. 1986, 17, 183–190. [Google Scholar]
- Morin, D. Silver from the Abyss: Mining techniques and deep mining in Laurion, Greece. In The Lavrion “Argyritis Land” and the Victorious Naval Battle of Salamis; Tzeferis, P., Bitzios, D., Eds.; Municipality of Lavreotiki: Lavrion, Greece, 2023; pp. 146–161, (English and Greek text). [Google Scholar]
- Ross, J.; Voudouris, P.; Melfos, V.; Vaxevanopoulos, M.; Soukis, K.; Merigot, K. The Lavrion silver district: Reassessing its ancient mining history. Geoarchaeology 2021, 36, 617–642. [Google Scholar] [CrossRef]
- Tsaimou, C. Metals in Ancient Times. The Ancient Mining and Metallurgic Technology, 1st ed.; Symeon Publications: Athens, Greece, 1997; 237p. (In Greek) [Google Scholar]
- Markouli, A. 19th/20th Cent. Mining and metallurgy in Lavrion. In Proceedings of the International Conference “Ari and the Laurion from Prehistoric to Modern Times”, Ruhr-Universität Bochum, Bochum, Germany, 11 January 2019; p. 14. [Google Scholar]
- Kypritidou, Z.; Kourgia, P.M.; Argyraki, A.; Demetriades, A. Do humans take good care of their offspring as animals do…! The Lavreotiki and Lavrion ’sagas’, Hellenic Republic—Part 1: Historical outline and mapping of lead contamination. Environ. Geochem. Health 2021, 45, 1107–1116. [Google Scholar] [CrossRef]
- Komnitsas, K.; Xenidis, A.; Adam, K. Oxidation of pyrite and arsenopyrite in sulphidic spoils in Lavrion. Miner. Eng. 1995, 8, 1443–1454. [Google Scholar] [CrossRef]
- Theodoratos, P.; Papassiopi, N.; Xenidis, A. Evaluation of monobasic calcium phosphate for the immobilization of heavy metals in contaminated soils from Lavrion. J. Haz. Mater. 2002, B94, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, I.; Karayannidis, A.; Adam, K.; Aravossis, K. Application of risk management techniques for the remediation of an old mining site in Greece. Waste Manag. 2009, 29, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, A.; Li, X.; Ramsey, M.H.; Thornton, I. Chemical speciation and bioaccessibility of lead in surface soil and house dust, Lavrion urban area, Attiki, Hellas. Environ. Geochem. Health 2010, 32, 529–552. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Min. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Alnour, I.A.; Wagiran, H.; Ibrahim, N.; Laili, Z.; Omar, M.; Hamzah, S.; Idi, B.Y. Natural radioactivity measurements in the granite rock of quarry sites, Johor, Malaysia. Radiat. Phys. Chem. 2012, 81, 1842–1847. [Google Scholar] [CrossRef]
- Popa, R.; Kinkle, B.; Badescu, A. Pyrite Framboids as Biomarkers for Iron-Sulfur Systems. Geomicrobiol. J. 2004, 21, 193–206. [Google Scholar] [CrossRef]
- Folk, R.L. Nannobacteria and the formation of framboidal pyrite: Textural evidence. J. Earth Syst. Sci. 2005, 114, 369–374. [Google Scholar] [CrossRef]
- Maravelias, C.; Hatzakis, A.; Katsouyianni, K.; Trichopoulos, D.; Koutselinis, A.; Ewers, U.; Brockhaus, A. Exposure to lead and cadmium of children living near a lead smelter at Lavrion, Greece. Sci. Total Environ. 1989, 84, 61–70. [Google Scholar] [CrossRef]



| Location | Mineral | Na2O | CaO | CuO | PbO | CdO | MnO | FeO | As2O5 | P2O5 | SO3 | WO3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core 1 14–16 cm depth | Scheelite | n.d. | 19.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 80.31 | 99.65 |
| Core 1 14–16 cm depth | Hubnerite | n.d. | n.d. | n.d. | n.d. | n.d. | 21.39 | 0.77 | n.d. | n.d. | n.d. | 77.01 | 99.17 |
| Core 1 30–32 cm depth | Scheelite | n.d. | 17.67 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 81.47 | 99.14 |
| Core 1 14–16 cm depth | Scheelite | n.d. | 18.67 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 81.87 | 100.54 |
| Core 1 14–16 cm depth | Ferberite | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 22.29 | n.d. | n.d. | n.d. | 78.60 | 100.89 |
| Core 1 30–32 cm depth | Ferberite | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 22.29 | n.d. | n.d. | n.d. | 77.08 | 99.37 |
| Core 1 14–16 cm depth | Scheelite | n.d. | 18.90 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 82.35 | 101.25 |
| OB 6 | Beudantite | n.d. | n.d. | n.d. | 34.81 | n.d. | n.d. | 35.18 | 15.44 | n.d. | 8.51 | n.d. | 93.94 |
| OB 6 | Beudantite | 1.62 | 6.21 | n.d. | 34.96 | 2.61 | n.d. | 36.57 | 13.39 | 1.97 | n.d. | n.d. | 97.33 |
| OB 6 | Conichalcite | n.d. | 23.48 | 30.02 | n.d. | n.d. | n.d. | n.d. | 43.40 | n.d. | n.d. | n.d. | 96.90 |
| OB 6 | Conichalcite | n.d. | 21.64 | 34.88 | n.d. | n.d. | n.d. | n.d. | . | 1.09 | n.d. | n.d. | 97.10 |
| Core 1 14–16 cm depth | Mimetite | n.d. | 3.88 | n.d. | 69.22 | n.d. | n.d. | n.d. | 23.54 | n.d. | n.d. | n.d. | 96.64 |
| Core 1 30–32 cm depth | Mimetite | n.d. | n.d. | n.d. | 72.56 | n.d. | n.d. | n.d. | 23.22 | n.d. | n.d. | n.d. | 95.78 |
| Core 1 30–32 cm depth | Mimetite | n.d. | n.d. | n.d. | 72.36 | n.d. | n.d. | n.d. | 24.08 | n.d. | n.d. | n.d. | 96.44 |
| Sample | Fe * | As | Mn * | Bi | Co | Cu | Mo | Pb | Sb | Zn * | Sn | WO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxygono Bay, N1 | 1.27 | 0.55 | 0.93 | b.d.l. | b.d.l. | 0.02 | 0.03 | 0.26 | b.d.l. | 0.72 | 0.02 | b.d.l. | 11.30 |
| Oxygono Bay, N2 | 1.24 | 0.58 | 0.75 | b.d.l. | b.d.l. | 0.01 | 0.03 | 0.32 | b.d.l. | 0.74 | 0.02 | b.d.l. | 14.77 |
| Oxygono Bay, N3 | 0.78 | 0.26 | 0.42 | b.d.l. | b.d.l. | 0.01 | b.d.l | 0.32 | b.d.l. | 0.57 | 0.01 | b.d.l. | 23.36 |
| Oxygono Bay, N4 | 0.8 | 0.26 | 0.5 | b.d.l. | b.d.l. | 0.01 | b.d.l | 0.23 | b.d.l. | 0.49 | 0.01 | b.d.l. | 15.12 |
| Oxygono Bay, N5 | 2.06 | 1.00 | 1.13 | b.d.l. | 0.01 | 0.02 | 0.07 | 0.41 | b.d.l. | 1.25 | 0.02 | 0.01 | 11.12 |
| Oxygono Bay, OB4 | n.a. | 1.02 | n.a. | 0.01 | 0.01 | 0.02 | 0.09 | 0.49 | b.d.l. | n.a. | 0.02 | 0.01 | 12.75 |
| Thorikos Bay, TB4 | n.a. | 2.30 | n.a. | 0.02 | 0.01 | 0.04 | 0.16 | 0.49 | 0.01 | n.a. | 0.01 | 0.02 | 23.36 |
| Thorikos Bay, TB5 | n.a. | 0.60 | n.a. | b.d.l. | b.d.l. | 0.05 | 0.05 | 0.49 | b.d.l. | n.a. | 0.02 | 0.01 | 12.43 |
| Standard deviation (S.D.): | - | 0.3% | - | <0.1% | <0.1% | <0.1% | <0.1% | 0.1% | <0.1% | - | <0.1% | <0.1% | 1.2% |
| Fraction | |||||
|---|---|---|---|---|---|
| −75 μm | +150 − 75 μm | −300 + 150 μm | −600 + 300 μm | +600 μm | |
| Major elements | |||||
| Si | 3.59 | 8.5 | 7.26 | 6.08 | 3.38 |
| Al | 1.29 | 2.66 | 2.51 | 2.24 | 1.65 |
| Mg | 0.74 | 1.6 | 1.58 | 1.37 | 1.81 |
| K | 0.48 | 0.52 | 0.57 | 0.56 | 0.31 |
| Ca | 12.3 | 11.59 | 16.78 | 16.11 | 17.26 |
| Fe | 31.03 | 22.62 | 10.6 | 8.33 | 10.18 |
| S | 9.8 | 8.83 | 4.32 | 4.25 | 11.46 |
| Trace elements | |||||
| As | >0.5 | >0.5 | >0.5 | >0.5 | >0.5 |
| Ti | 392 | 1143 | 1251 | 986 | 536 |
| V | 90 | b.d.l. | 54 | 44 | 19.1 |
| Cr | 32.8 | 104.2 | 106 | 237 | 118 |
| Co | 723 | 452 | 267 | 170 | 195 |
| Ni | b.d.l. | 12.1 | 22.6 | 23.7 | 34.8 |
| Cu | 552 | 288.1 | 245.4 | 212.9 | 151.2 |
| Zn | 11,490 | 10,390 | 11,520 | 9126 | 7884 |
| Br | 88 | 17.2 | 8 | 10.4 | 14.7 |
| Rb | b.d.l. | 18.3 | 28.5 | 28.8 | 17.7 |
| Sr | 76.3 | 47.9 | 76.3 | 83.1 | 145.1 |
| Y | b.d.l. | 24.4 | b.d.l. | b.d.l. | 15.2 |
| Ag | 9.6 | 5.1 | 6.9 | 5.2 | b.d.l. |
| Cd | 13.5 | 32.8 | 42.5 | 29.6 | 24.7 |
| Sb | 273 | 95.2 | 74.9 | 66.7 | 70.9 |
| Ba | 233 | 296 | 160 | 178 | 238 |
| Hg | b.d.l. | 14 | 10.1 | 3.1 | 16.5 |
| Pb | 7978 | 3910 | 2737 | 2568 | 2607 |
| Mass (g) | 1.7 | 1.5 | 324.9 | 27.9 | 2.1 |
| % total mass | 0.47 | 0.42 | 90.73 | 7.79 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllidis, S.S.; Anastasakis, G.; Papanastasiou, A.; Stylianou, C.; Kavros, N.; Pappa, F.K.; Tombros, S.F.; Fitros, M.; Skliros, V. Provenance of Coastal and Seabed Sediments Relative to Mining and Processing Wastes: The Case of Lavrion, Attiki Peninsula, Greece. Minerals 2024, 14, 33. https://doi.org/10.3390/min14010033
Triantafyllidis SS, Anastasakis G, Papanastasiou A, Stylianou C, Kavros N, Pappa FK, Tombros SF, Fitros M, Skliros V. Provenance of Coastal and Seabed Sediments Relative to Mining and Processing Wastes: The Case of Lavrion, Attiki Peninsula, Greece. Minerals. 2024; 14(1):33. https://doi.org/10.3390/min14010033
Chicago/Turabian StyleTriantafyllidis, Stavros Savvas, Georgios Anastasakis, Anastasios Papanastasiou, Charalambos Stylianou, Nikolaos Kavros, Filothei K. Pappa, Stylianos Fotios Tombros, Michalis Fitros, and Vasilios Skliros. 2024. "Provenance of Coastal and Seabed Sediments Relative to Mining and Processing Wastes: The Case of Lavrion, Attiki Peninsula, Greece" Minerals 14, no. 1: 33. https://doi.org/10.3390/min14010033






