Gravity Separation Tests of a Complex Rutile Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
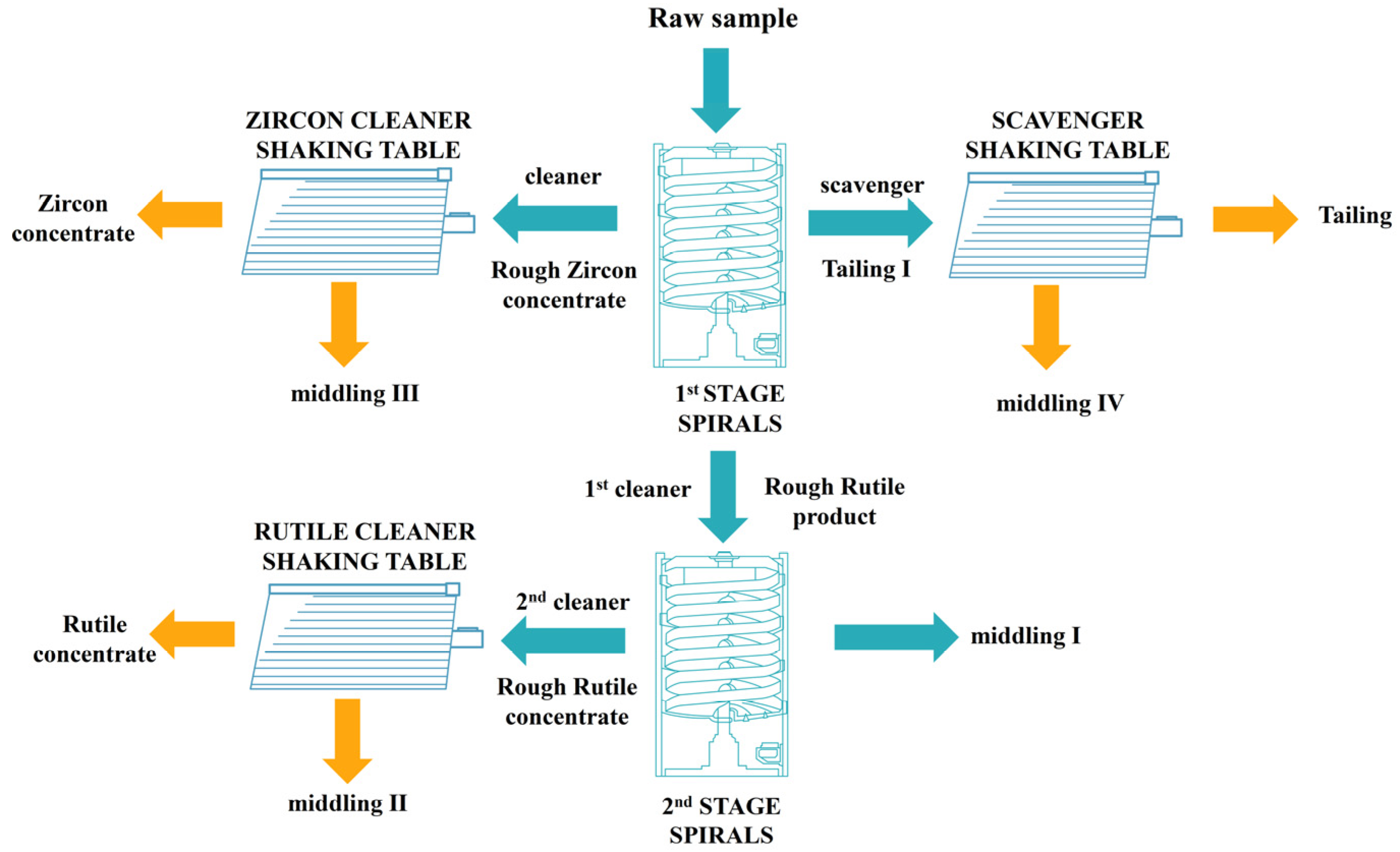
2.2. Experimental Method
2.3. Analytical Techniques
3. Results and Discussion
3.1. Mineralogical Characterization
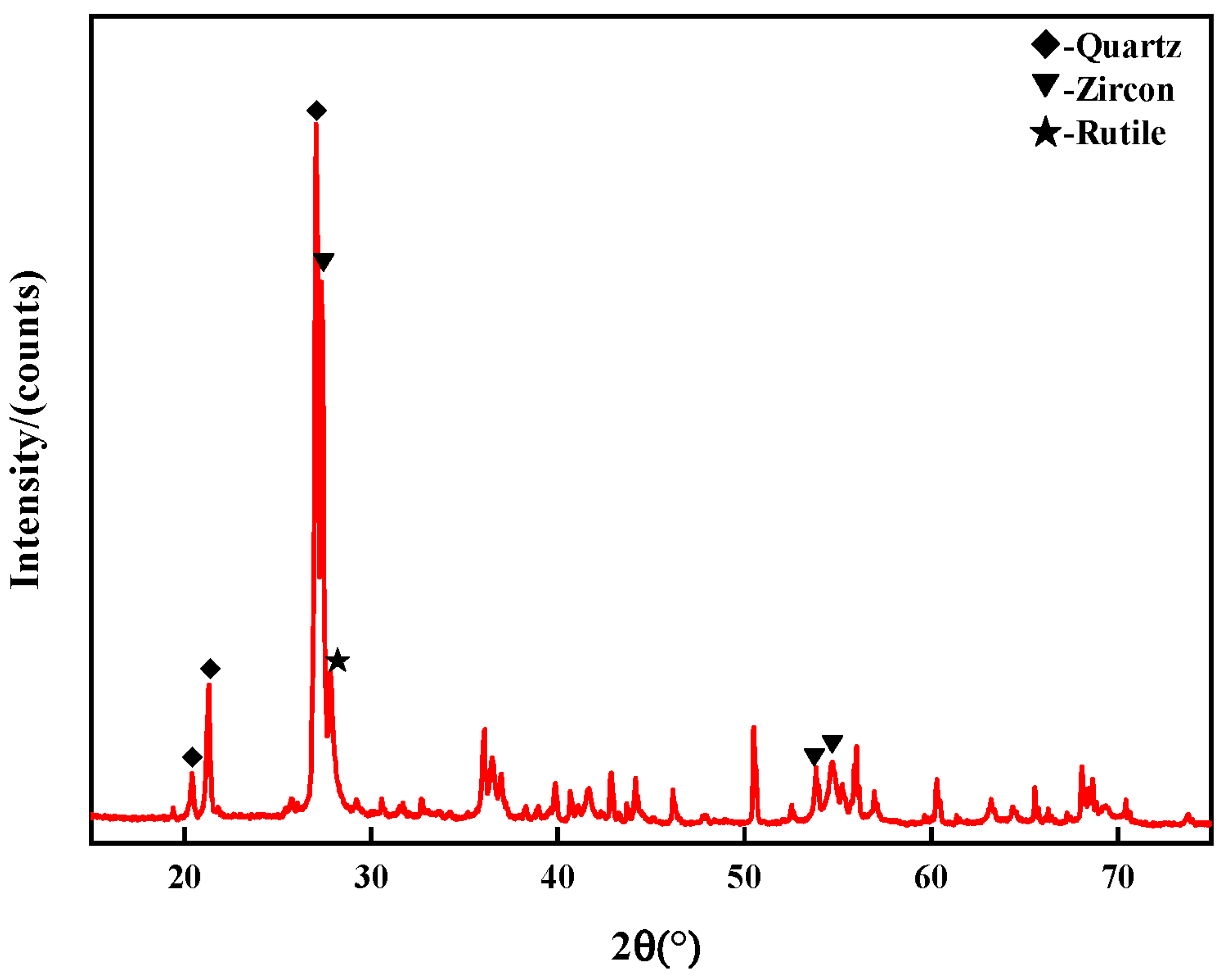
3.1.1. XRD Analyses
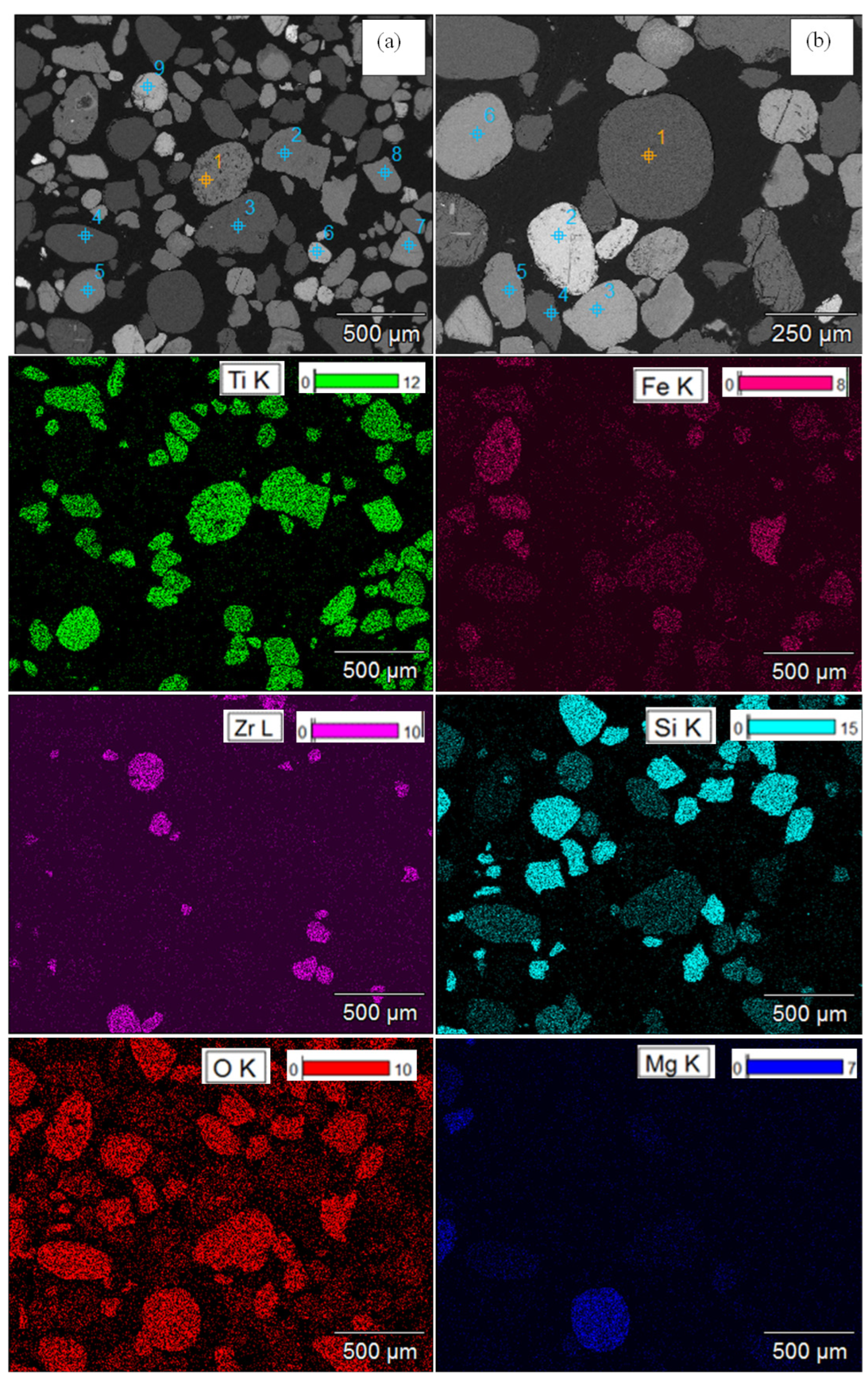
3.1.2. EPMA-EDS Analyses
3.1.3. Size Distribution
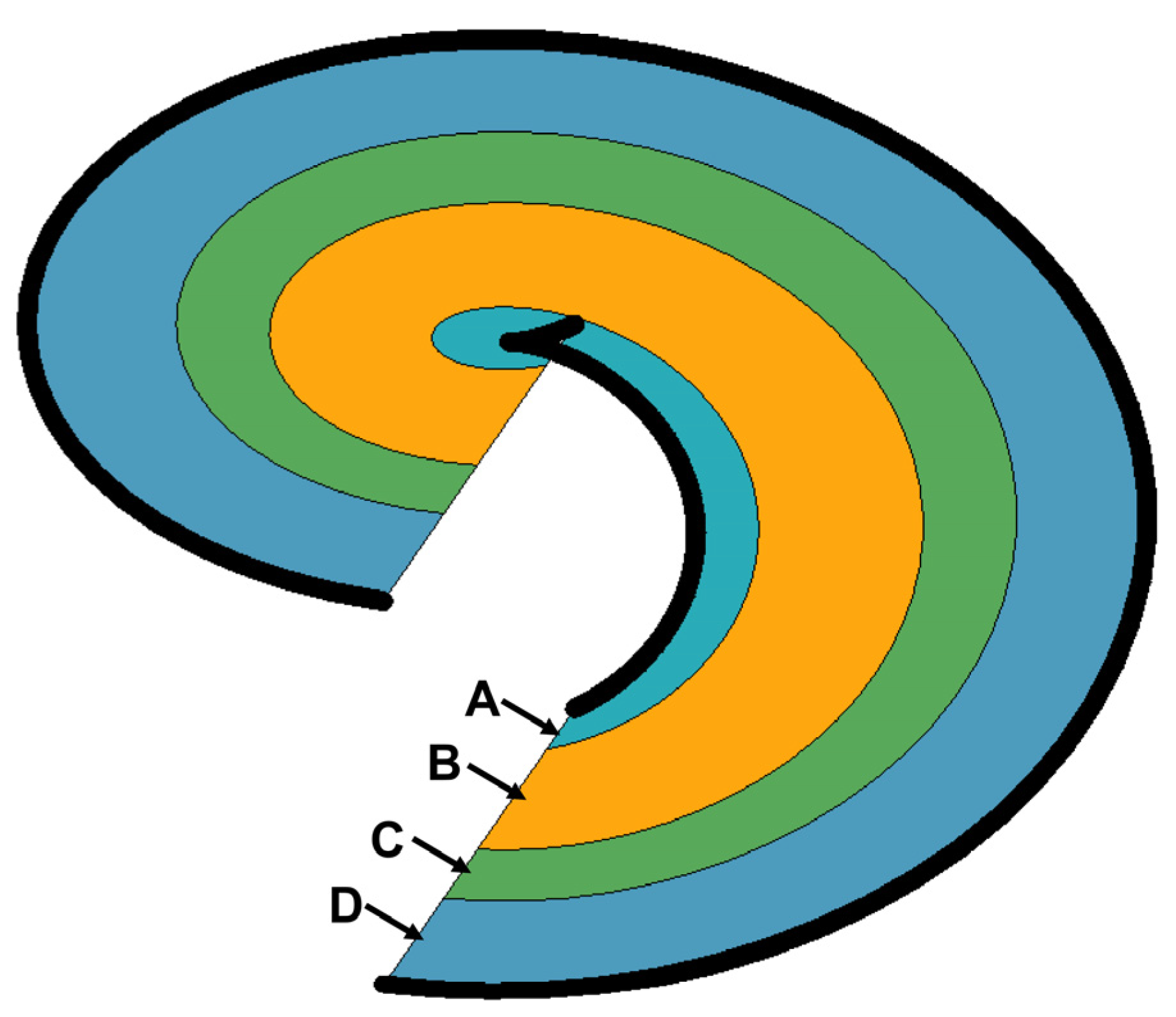
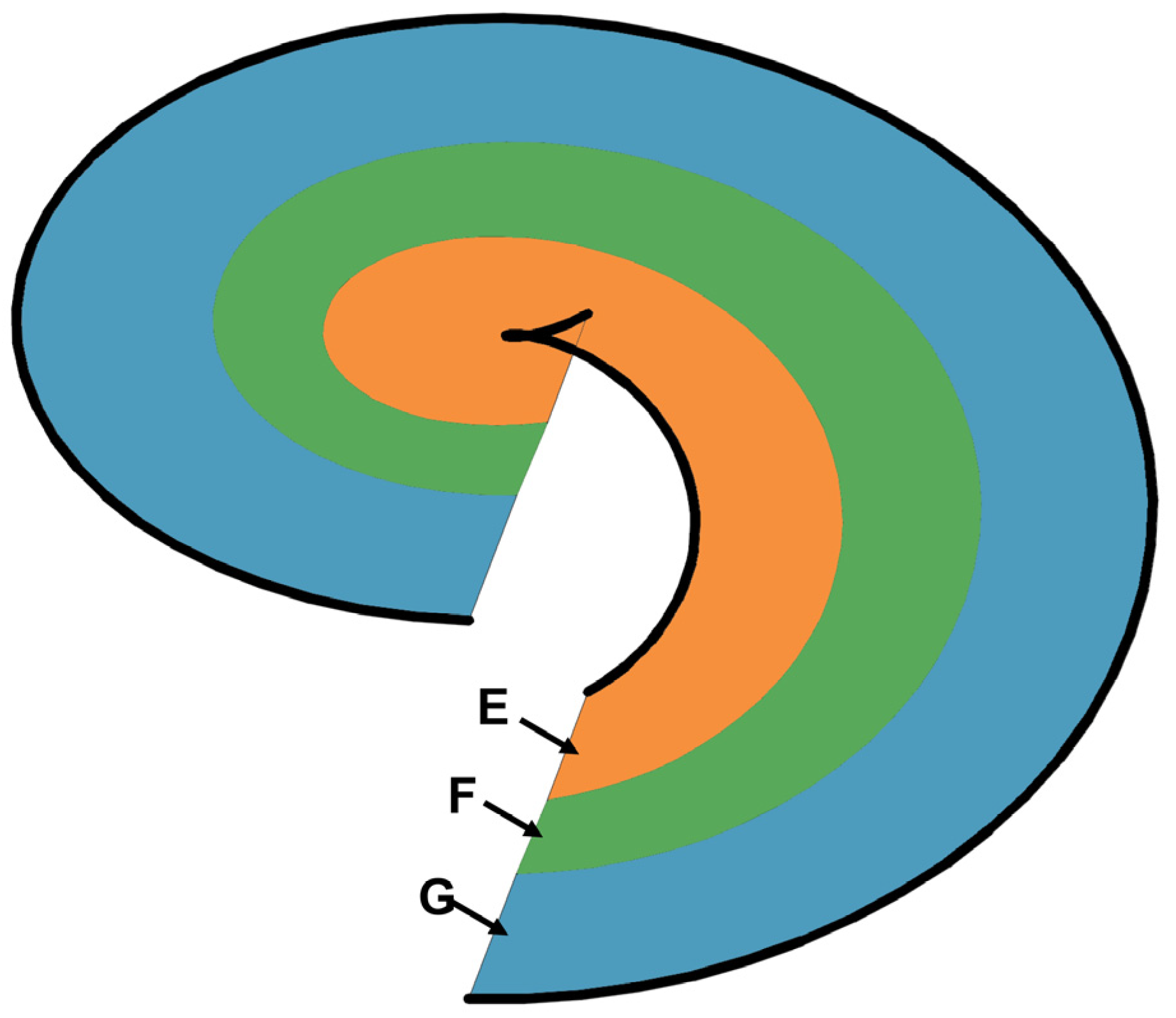
3.2. Spiral Chute Tests
3.2.1. First-Stage Spiral Chute
3.2.2. Second-Stage Spiral Chute
3.3. Shaking Table Tests
3.3.1. Tests for the Rough Zircon Concentrate
3.3.2. Tests for the Rough Rutile Concentrate
3.3.3. Tests for the Tailing Ⅰ
3.4. Products Phase Analyses
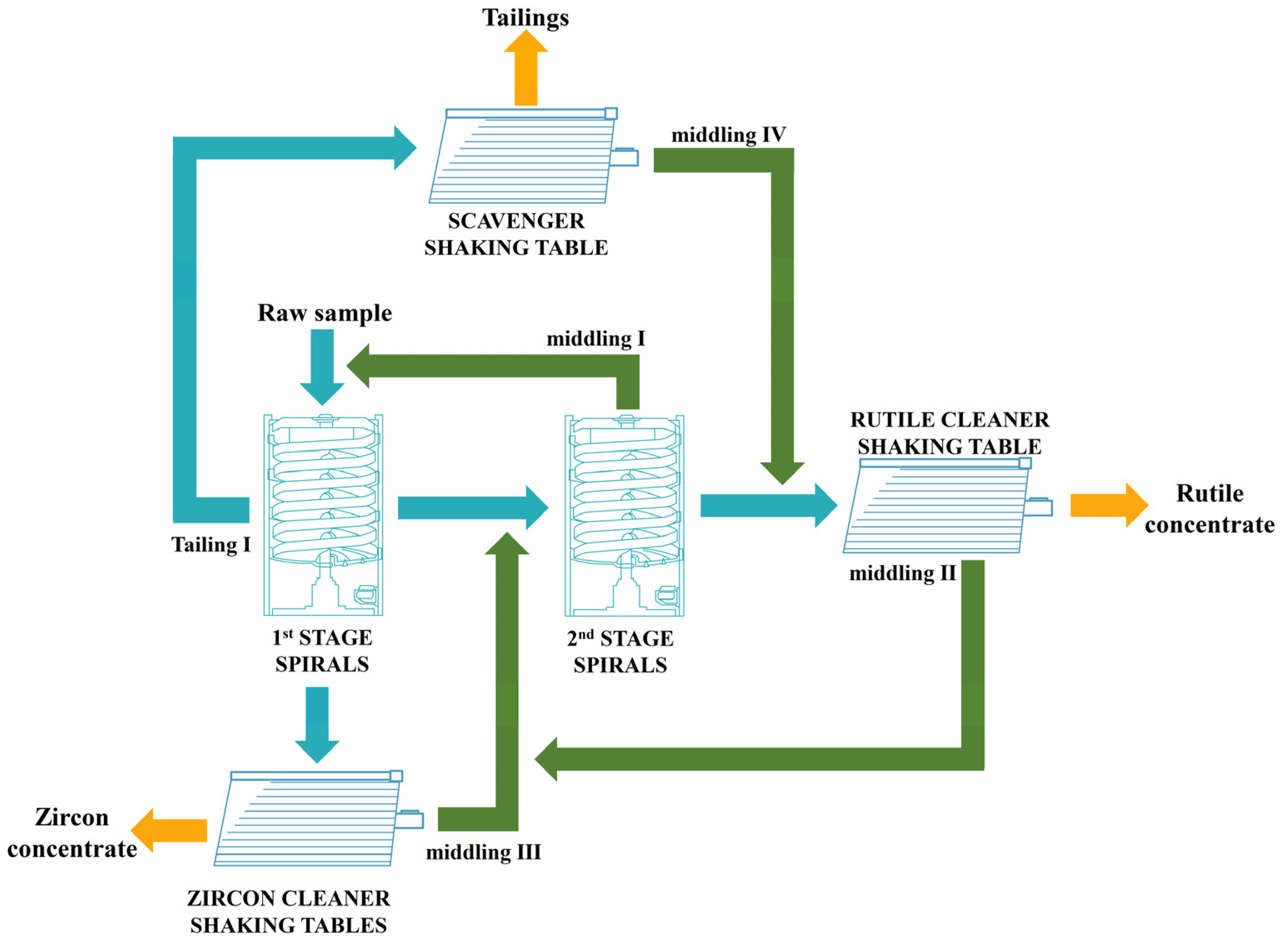
3.5. Recommend Closed-Circuit Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jackson, M.; Dring, K. A review of advances in processing and metallurgy of titanium alloys. Mater. Sci. Technol 2013, 22, 881–887. [Google Scholar] [CrossRef]
- Feng, E.; Gao, D.; Wang, Y.; Yu, F.; Wang, C.; Wen, J.; Gao, Y.; Huang, G.; Xu, S. Sustainable recovery of titanium from secondary resources: A review. J. Environ. Manag. 2023, 339, 117818. [Google Scholar] [CrossRef] [PubMed]
- Meinhold, G. Rutile and its applications in earth sciences. Earth Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Review on machinability of titanium alloys: The process perspective. Rev. Adv. Mater. Sci 2013, 34, 148–164. [Google Scholar]
- Jones, G. Mineral Sands: An Overview of the Industry; Iluka Resources Limited: Capel, Australia, 2009. [Google Scholar]
- Qiu, G.; Guo, Y. Current situation and development trend of titanium metal industry in China. Int. J. Miner. Metall. Mater 2022, 29, 599–610. [Google Scholar] [CrossRef]
- Schulz, K.J.; DeYoung, J.H.; Seal, R.R., II; Bradley, D.C. (Eds.) Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; U.S. Geological Survey: Reston, VA, USA, 2017.
- El Khalloufi, M.; Drevelle, O.; Soucy, G. Titanium: An Overview of Resources and Production Methods. Minerals 2021, 11, 1425. [Google Scholar] [CrossRef]
- Perks, C.; Mudd, G. Titanium, zirconium resources and production: A state of the art literature review. Ore Geol. Rev. 2019, 107, 629–646. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Sparrow, G.J.; Aral, H.; Smith, L.K.; Bruckard, W.J. Recovery and processing of zircon from Murray Basin mineral sand deposits. Miner. Process. Extr. Metall. 2015, 124, 240–253. [Google Scholar] [CrossRef]
- Bedinger, B.G.M. Zirconium and Hafnium; U.S. Geological Survey: Reston, VA, USA, 2017.
- Bisht, A.; Martinez-Alier, J. Coastal sand mining of heavy mineral sands: Contestations, resistance, and ecological distribution conflicts at HMS extraction frontiers across the world. J. Ind. Ecol. 2022, 27, 238–253. [Google Scholar] [CrossRef]
- Zhu, X.; Geng, Y.; Gao, Z.; Tian, X.; Xiao, S.; Houssini, K. Investigating zirconium flows and stocks in China: A dynamic material flow analysis. Resour. Policy 2023, 80, 103139. [Google Scholar] [CrossRef]
- Li, Z.; Chen, C. Development Status of Global Titanium Resources Industry. Acta Geosci. Sin. 2021, 42, 6. [Google Scholar]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P.; Lang, C. Towards sustainable TiO2 production: An investigation of environmental impacts of ilmenite and rutile processing routes in Australia. J. Clean. Prod. 2018, 196, 1016–1025. [Google Scholar] [CrossRef]
- Poon, P.; Graham, I.T.; Liepa, E.A.C.; Cohen, D.R.; Pringle, I.J.; Burkett, D.A.; Privat, K. Mineral distribution and provenance of heavy mineral sands (zircon, ilmenite, rutile) deposits from the NW Murray Basin, far western NSW, Australia. Aust. J. Earth Sci. 2020, 67, 575–590. [Google Scholar] [CrossRef]
- Gonçalves, C.; Braga, P. Heavy Mineral Sands in Brazil: Deposits, Characteristics, and Extraction Potential of Selected Areas. Minerals 2019, 9, 176. [Google Scholar] [CrossRef]
- Laxmi, T.; Srikant, S.S.; Rao, D.S.; Rao, R.B. Beneficiation studies on recovery and in-depth characterization of ilmenite from red sediments of badlands topography of Ganjam District, Odisha, India. Int. J. Min. Sci. Technol. IJMST 2013, 23, 725–731. [Google Scholar] [CrossRef]
- Rao, R.B. Recovery of Ilmenite and Other Heavy Minerals from Teri Sands (Red Sands) of Tamil Nadu, India. J. Miner. Mater. Charact. Eng. 2009, 8, 149–159. [Google Scholar]
- Rejith, R.G.; Sundararajan, M. Combined magnetic, electrostatic, and gravity separation techniques for recovering strategic heavy minerals from beach sands. Mar. Georesour. Geotechnol. 2017, 36, 959–965. [Google Scholar] [CrossRef]
- Dieye, M.; Thiam, M.M.; Geneyton, A.; Gueye, M. Monazite Recovery by Magnetic and Gravity Separation of Medium Grade Zircon Concentrate from Senegalese Heavy Mineral Sands Deposit. J. Miner. Mater. Charact. Eng. 2021, 09, 590–608. [Google Scholar] [CrossRef]
- Fawzy, M.M.; Ghar, M.; Gaafar, I.M.; Shafey, A.; Diab, M.; Hussein, A.W. Recovery of Valuable Heavy Minerals via Gravity and Magnetic Separation Operations from Diit Quaternary Stream Sediments, Southern Coast of the Red Sea, Egypt; IOP Publishing Ltd.: Bristol, UK, 2022. [Google Scholar]
- Bazin, C.; Sadeghi, M.; Renaud, M. An operational model for a spiral classifier. Miner. Eng. 2016, 91, 74–85. [Google Scholar] [CrossRef]
- Boucher, D.; Deng, Z.; Leadbeater, T.W.; Langlois, R.; Waters, K.E. Speed analysis of quartz and hematite particles in a spiral concentrator by PEPT. Miner. Eng. 2016, 91, 86–91. [Google Scholar] [CrossRef]
- Yıldırım Gülsoy, Ö.; Gülcan, E. A new method for gravity separation: Vibrating table gravity concentrator. Sep. Purif. Technol. 2019, 211, 124–134. [Google Scholar] [CrossRef]
- Peng, H.; Li, G.; Hu, H.; Zhou, Z. Research Actuality and Prospect of Spiral Chute. Jiangxi Nonferrous Met. 2009, 23, 26–29. [Google Scholar]
- Galvin, K.P.; Iveson, S.M. New challenges for gravity concentration and classification of fine particles. Miner. Eng. 2022, 190, 107888. [Google Scholar] [CrossRef]
- Bing, Y. Industrial Production Application Research and Development on YunTin YXB New Type Fine Sand Table Concentrator. Yunnan Metall. 2020, 49, 27–30. [Google Scholar]
- Nayak, A.; Jena, M.S.; Mandre, N.R. Application of Enhanced Gravity Separators for Fine Particle Processing: An Overview. J. Sustain. Metall. 2021, 7, 315–339. [Google Scholar] [CrossRef]
- Izerdem, D.; Ergun, S.L. Investigation of the effects of particle size on the performance of classical gravity concentration equipment. Miner. Process. Extr. Metall. Rev. 2022, 1–18. [Google Scholar] [CrossRef]
- Manser, R.; Barley, R.; Wills, B. The shaking table concentrator—The influence of operating conditions and table parameters on mineral separation—The development of a mathematical model for normal operating conditions. Miner. Eng. 1991, 4, 369–381. [Google Scholar] [CrossRef]

| O | Na | Mg | Al | Si | P | S | Cl |
| 38.36 | 0.05 | 0.25 | 2.75 | 15.26 | 0.02 | 0.04 | 0.02 |
| Pb | U | Ca | Ti | Cr | Mn | Fe | Zn |
| 0.03 | 0.01 | 0.10 | 23.78 | 0.07 | 0.12 | 5.62 | 0.04 |
| Th | Zr | Nb | La | Ce | Nd | Hf | Y |
| 0.14 | 5.24 | 0.07 | 0.24 | 0.47 | 0.20 | 0.17 | 0.07 |
| TiO2 | ZrO2 | Fe | Al2O3 | SiO2 |
| 33.78 | 5.56 | 5.72 | 7.97 | 34.74 |
| Point | O | Mg | Al | Si | Ti | Mn | Fe | Zr |
|---|---|---|---|---|---|---|---|---|
| 1 | 36.97 | - | 0.29 | - | 61.55 | - | 1.19 | - |
| 2 | 35.78 | - | 0.87 | - | 60.91 | - | 1.46 | - |
| 3 | 44.73 | 1.49 | 28.48 | 13.44 | 0.33 | - | 11.52 | - |
| 4 | 48.13 | 3.01 | 18.93 | 18.13 | 0.28 | - | 9.46 | - |
| 5 | 35.10 | - | - | - | 61.80 | - | 2.43 | - |
| 6 | 32.30 | - | - | 15.70 | - | - | - | 52.00 |
| 7 | 33.04 | - | - | - | 38.05 | 2.18 | 26.73 | - |
| 8 | 30.72 | - | - | - | 69.28 | - | - | - |
| 9 | 31.26 | - | - | 15.62 | - | - | 53.12 |
| Point | O | Mg | Al | Si | Ti | Mn | Fe | Zr |
|---|---|---|---|---|---|---|---|---|
| 1 | 40.87 | 17.19 | 39.92 | - | - | - | 2.02 | - |
| 2 | 32.32 | - | - | 15.46 | - | - | - | 52.22 |
| 3 | 30.55 | - | - | - | 39.39 | 1.65 | 28.41 | - |
| 4 | 50.78 | - | - | 49.22 | - | - | - | - |
| 5 | 37.78 | - | - | - | 64.47 | - | 0.75 | - |
| 6 | 31.38 | - | - | - | 66.21 | - | 2.41 | - |
| Particle Size/mm | Yield/% | Grade/% | Distribution/% | ||
|---|---|---|---|---|---|
| TiO2 | ZrO2 | TiO2 | ZrO2 | ||
| +0.25 | 1.63 | 2.29 | 0.46 | 0.11 | 0.11 |
| −0.25~+0.18 | 3.22 | 11.30 | 0.99 | 1.08 | 0.45 |
| −0.18~+0.15 | 5.84 | 21.07 | 1.15 | 3.64 | 0.95 |
| −0.15~+0.106 | 33.96 | 26.98 | 1.90 | 27.11 | 11.77 |
| −0.106~+0.074 | 30.15 | 40.31 | 6.55 | 35.97 | 34.47 |
| −0.074 | 25.19 | 43.03 | 13.55 | 32.08 | 52.25 |
| Feeding | 100.00 | 33.78 | 6.15 | 100.00 | 100.00 |
| Adjustable Splitter Position | Products | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|---|
| TiO2 | ZrO2 | TiO2 | ZrO2 | |||
| Position Ⅰ width of discharge port (5 cm; 9.5 cm) | A | 6.50 | 24.04 | 33.62 | 4.75 | 37.48 |
| B | 70.25 | 40.50 | 2.55 | 86.55 | 30.77 | |
| Tailing Ⅰ | 23.25 | 12.30 | 7.96 | 8.70 | 31.74 | |
| Feeding | 100.00 | 32.88 | 5.82 | 100.00 | 100.00 | |
| Position Ⅱ width of discharge port (7.2 cm; 4.5 cm) | A | 10.74 | 27.13 | 33.18 | 8.72 | 62.43 |
| B | 55.40 | 43.52 | 3.71 | 72.16 | 36.04 | |
| Tailing Ⅰ | 33.86 | 18.86 | 0.26 | 19.11 | 1.52 | |
| Feeding | 100.00 | 33.56 | 5.72 | 100.00 | 100.00 | |
| Position Ⅲ width of discharge port (8.7 cm; 3.5 cm) | A | 16.68 | 36.13 | 26.14 | 17.98 | 78.79 |
| B | 49.26 | 43.76 | 2.24 | 64.30 | 19.96 | |
| Tailing Ⅰ | 34.06 | 17.45 | 0.20 | 17.73 | 1.25 | |
| Feeding | 100.00 | 33.53 | 5.54 | 100.00 | 100.00 | |
| Adjustable Splitter Position | Products | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|---|
| TiO2 | ZrO2 | TiO2 | ZrO2 | |||
| Position Ⅰ width of discharge port (7.5 cm) | E | 67.91 | 51.12 | 4.53 | 77.82 | 84.01 |
| Middling Ⅰ | 32.09 | 27.92 | 1.65 | 22.18 | 15.99 | |
| Feeding | 100.00 | 43.68 | 3.54 | 100.00 | 100.00 | |
| Position Ⅱ width of discharge port (5 cm) | E | 47.64 | 56.77 | 4.64 | 63.65 | 63.23 |
| Middling Ⅰ | 52.36 | 31.30 | 2.46 | 34.45 | 36.77 | |
| Feeding | 100.00 | 43.43 | 3.50 | 100.00 | 100.00 | |
| Products | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|
| TiO2 | ZrO2 | TiO2 | ZrO2 | ||
| Concentrate 1 | 13.91 | 5.76 | 40.47 | 2.96 | 17.04 |
| Concentrate 2 | 32.69 | 14.02 | 43.58 | 16.89 | 43.13 |
| Mixed concentrate | 46.60 | 11.55 | 42.65 | 19.85 | 60.17 |
| Tailing (Middling III) | 53.40 | 40.72 | 24.64 | 80.15 | 39.83 |
| Feeding | 100.00 | 27.13 | 33.03 | 100.00 | 100.00 |
| Products | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|
| TiO2 | ZrO2 | TiO2 | ZrO2 | ||
| Concentrate | 58.65 | 53.76 | 6.99 | 66.80 | 83.28 |
| Tailing (Middling II) | 41.35 | 46.20 | 1.99 | 33.20 | 16.72 |
| Feeding | 100.00 | 51.50 | 4.92 | 100.00 | 100.00 |
| Concentrate | 57.78 | 61.75 | 7.01 | 63.65 | 81.75 |
| Tailing (Middling II) | 42.22 | 48.27 | 2.14 | 34.35 | 18.25 |
| Feeding | 100.00 | 56.06 | 4.95 | 100.00 | 100.00 |
| Products | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|
| TiO2 | ZrO2 | TiO2 | ZrO2 | ||
| Concentrate (Middling Ⅳ) | 28.72 | 57.50 | 0.72 | 89.22 | 80.56 |
| Tailing | 71.28 | 3.29 | 0.07 | 12.43 | 19.44 |
| Feeding | 100.00 | 18.86 | 0.26 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zheng, Y.; Huang, X.; Wang, X.; Peng, J.; Dai, Z. Gravity Separation Tests of a Complex Rutile Ore. Minerals 2024, 14, 68. https://doi.org/10.3390/min14010068
Wang Z, Zheng Y, Huang X, Wang X, Peng J, Dai Z. Gravity Separation Tests of a Complex Rutile Ore. Minerals. 2024; 14(1):68. https://doi.org/10.3390/min14010068
Chicago/Turabian StyleWang, Zhenxing, Yongxing Zheng, Xiang Huang, Xiangding Wang, Jieli Peng, and Zhe Dai. 2024. "Gravity Separation Tests of a Complex Rutile Ore" Minerals 14, no. 1: 68. https://doi.org/10.3390/min14010068
APA StyleWang, Z., Zheng, Y., Huang, X., Wang, X., Peng, J., & Dai, Z. (2024). Gravity Separation Tests of a Complex Rutile Ore. Minerals, 14(1), 68. https://doi.org/10.3390/min14010068








