Comparative Study on the Effect of Pyrophosphate and Tripolyphosphate on the Flotation Separation of Arsenopyrite and Muscovite
Abstract
1. Introduction
2. Experimental Section
2.1. Minerals and Reagents
2.2. Flotation Tests
2.3. Reflection Polarizing Microscope Tests
2.4. Electrokinetic Potential
2.5. FTIR Measurements
2.6. XPS Detection
3. Results and Discussion
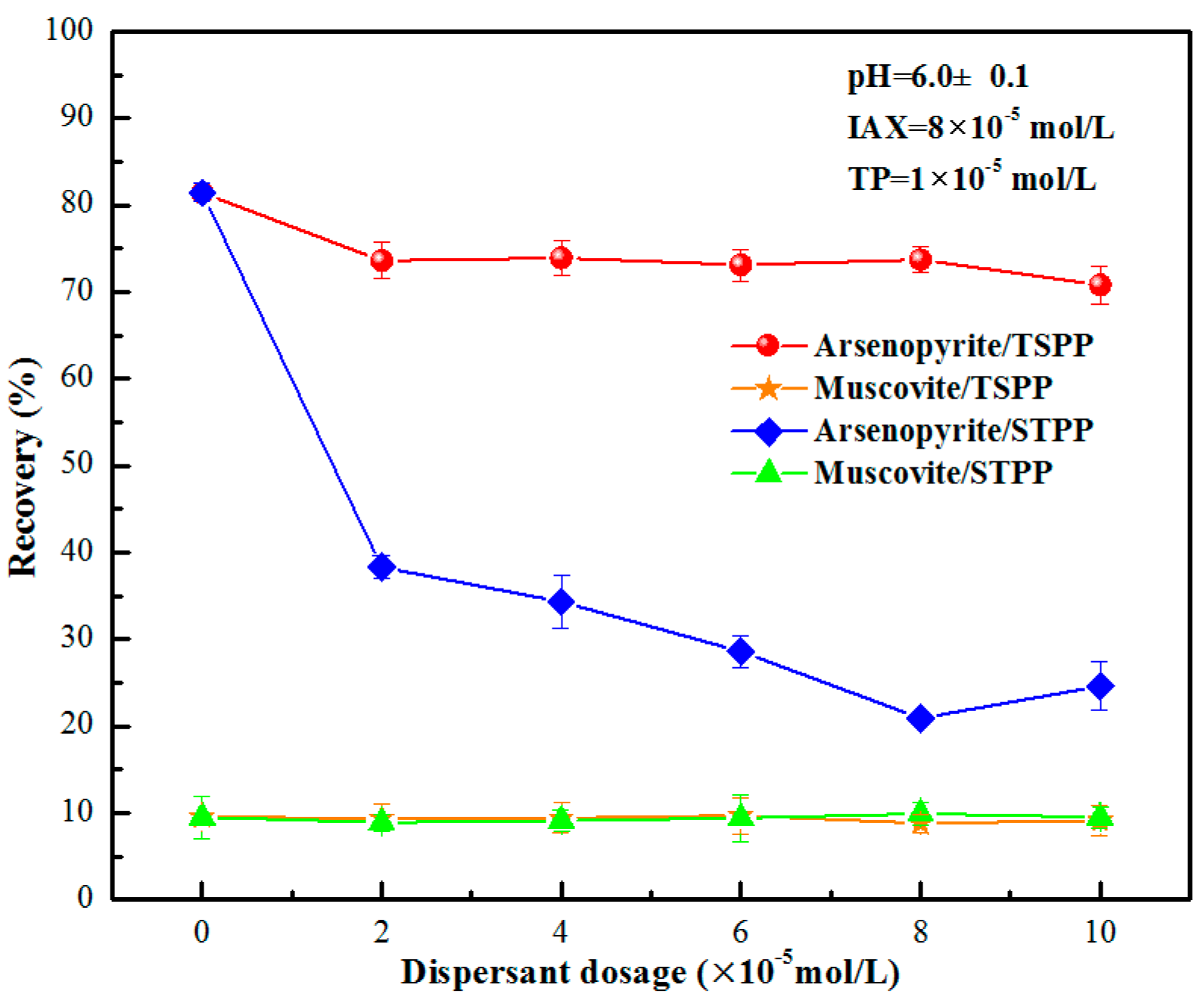
3.1. Single-Mineral Flotation
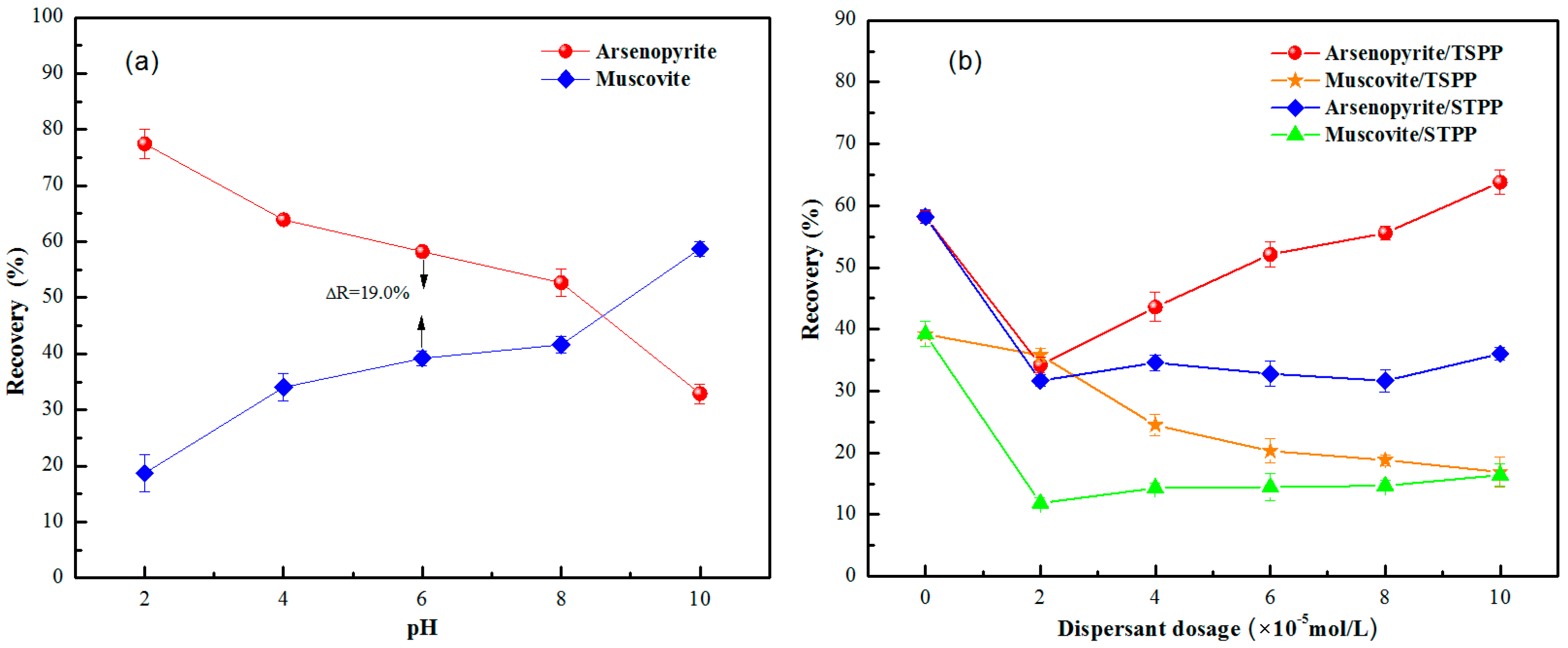
3.2. Artificial Mixed-Mineral Flotation
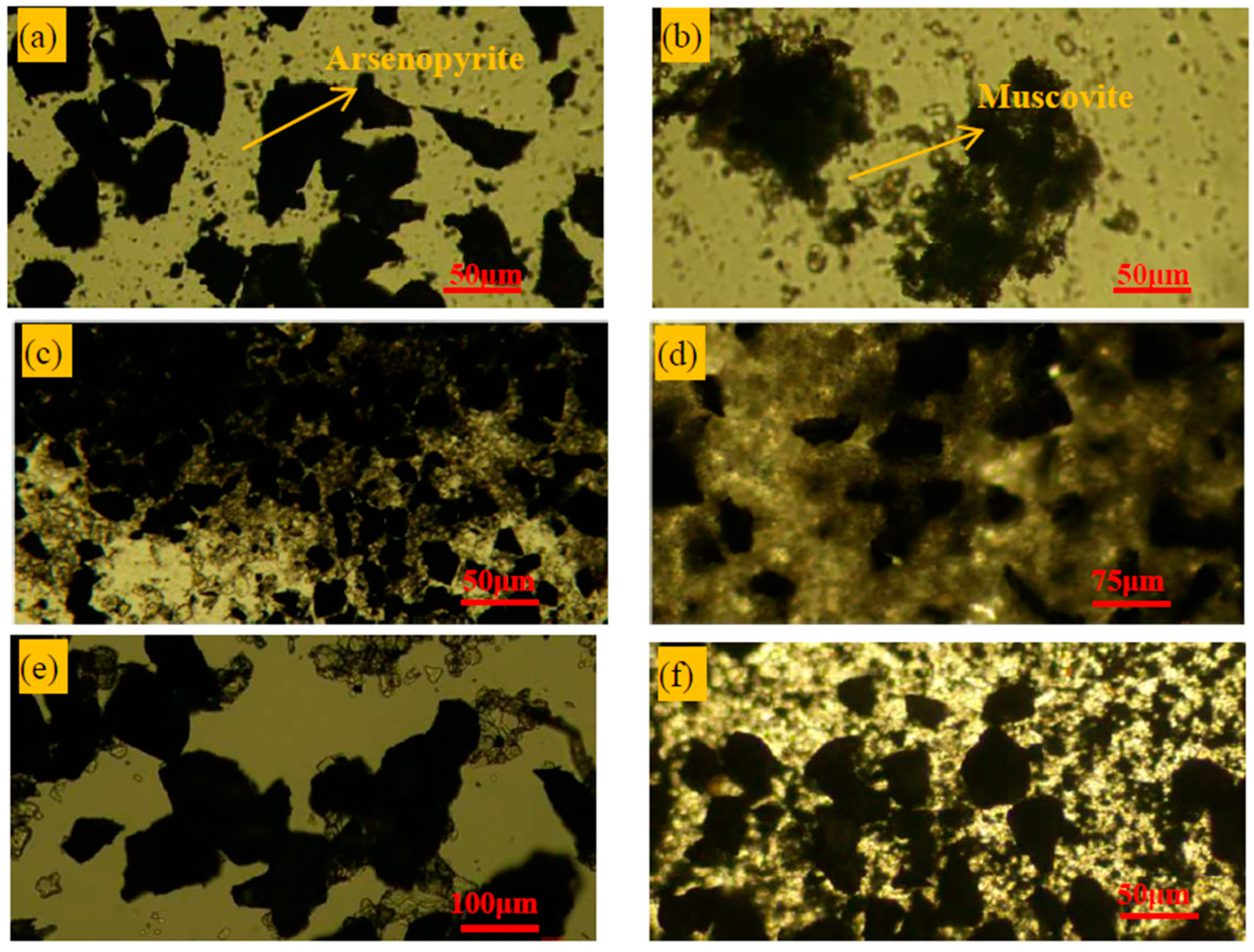
3.3. RPM Detection
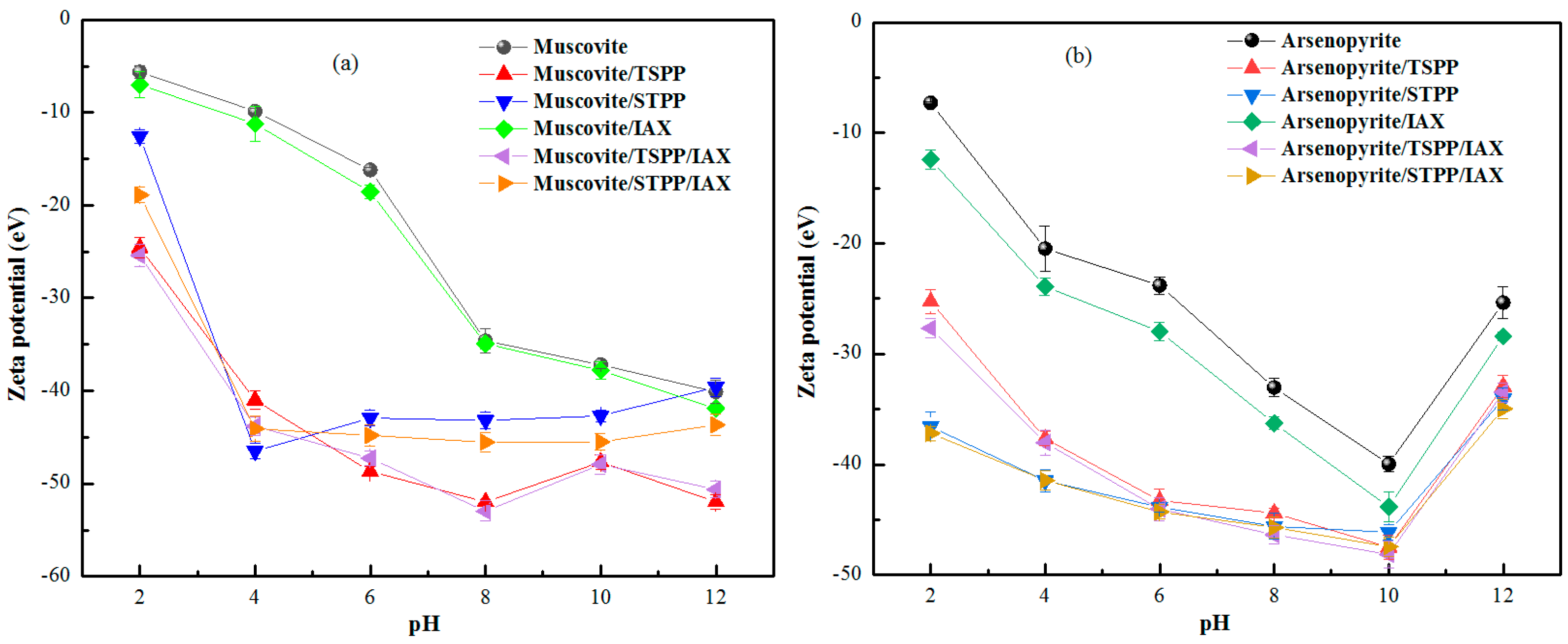
3.4. Electrokinetic Potential
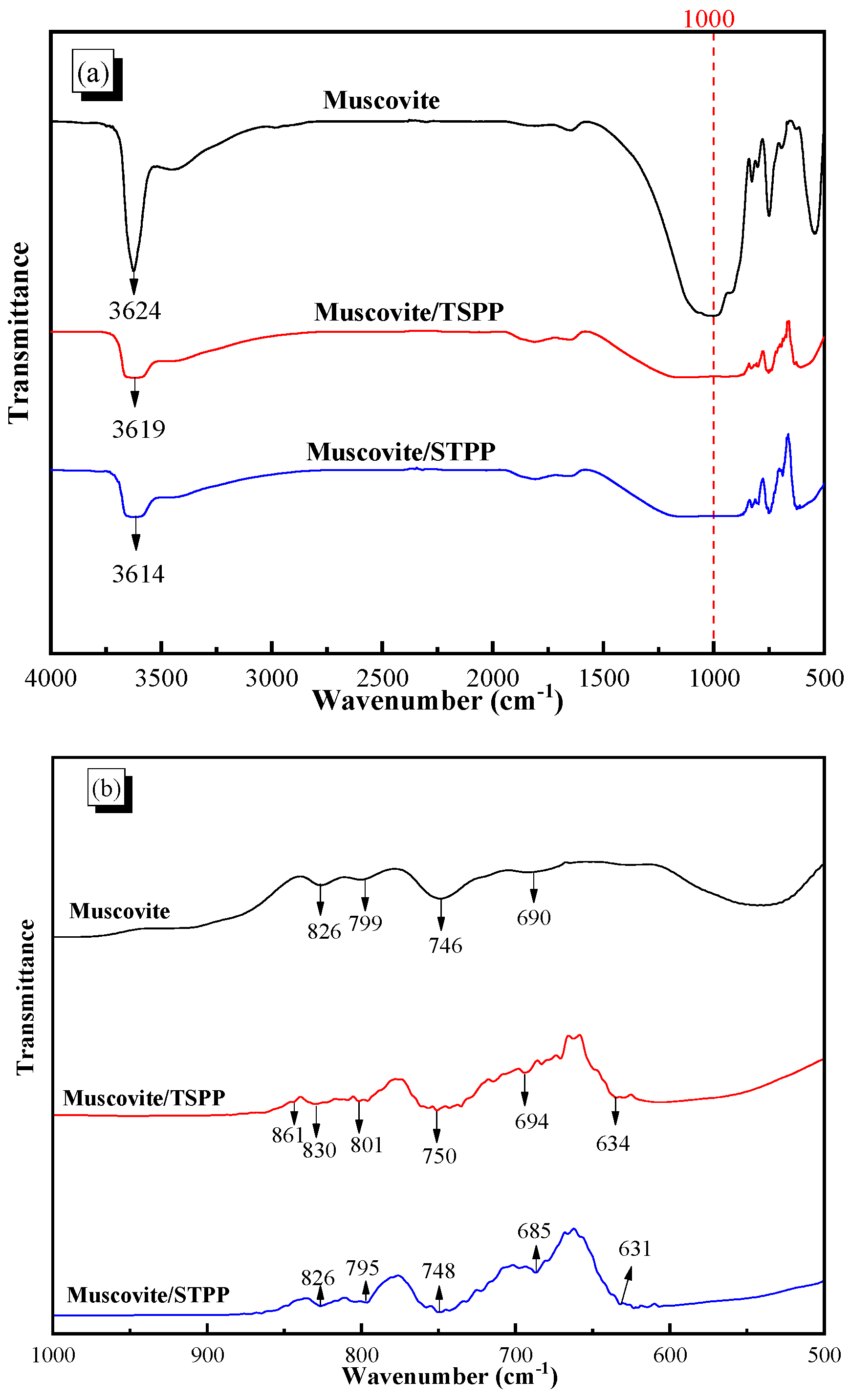
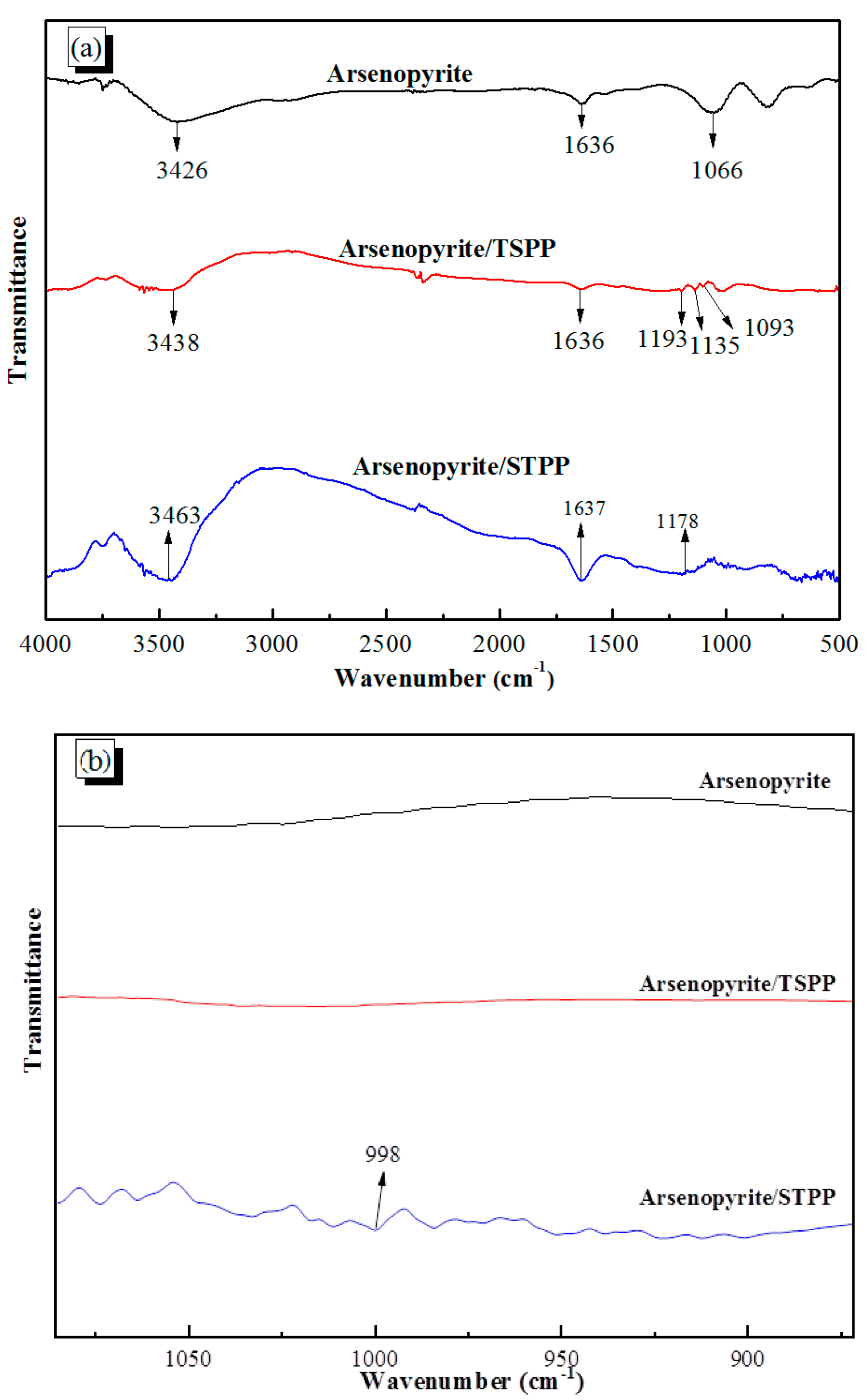
3.5. FTIR Detection
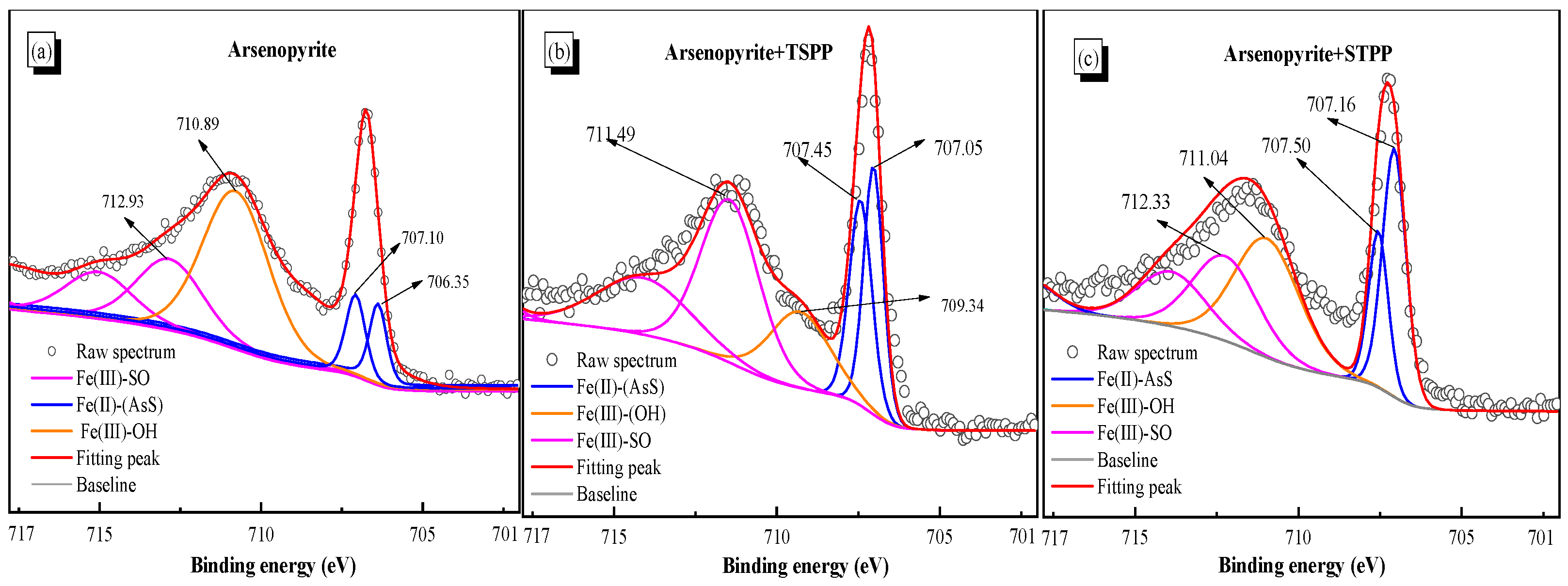
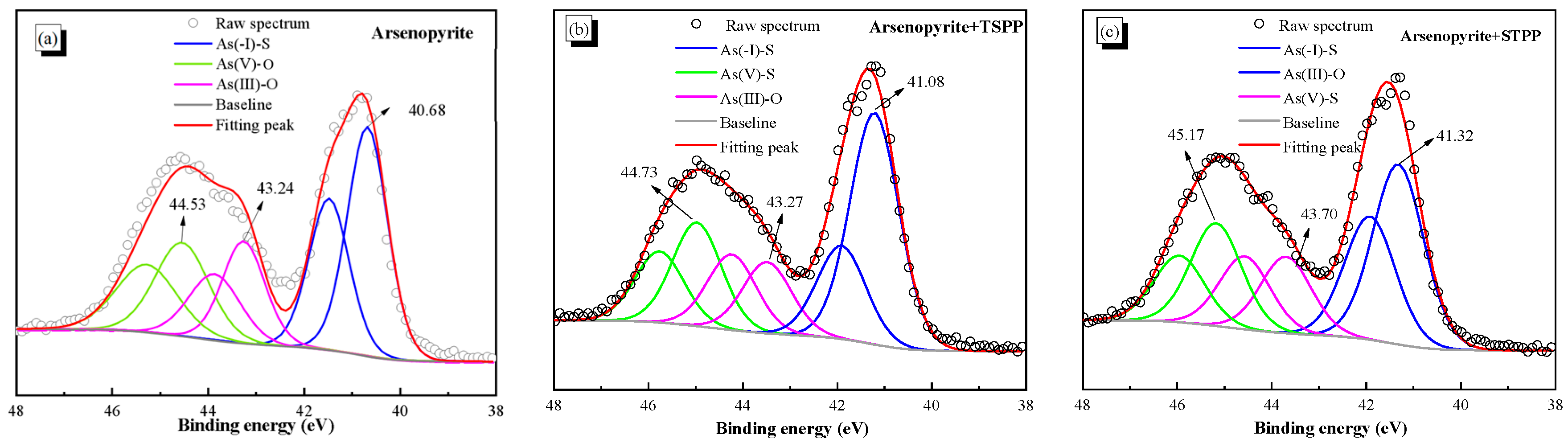
3.6. XPS Detection
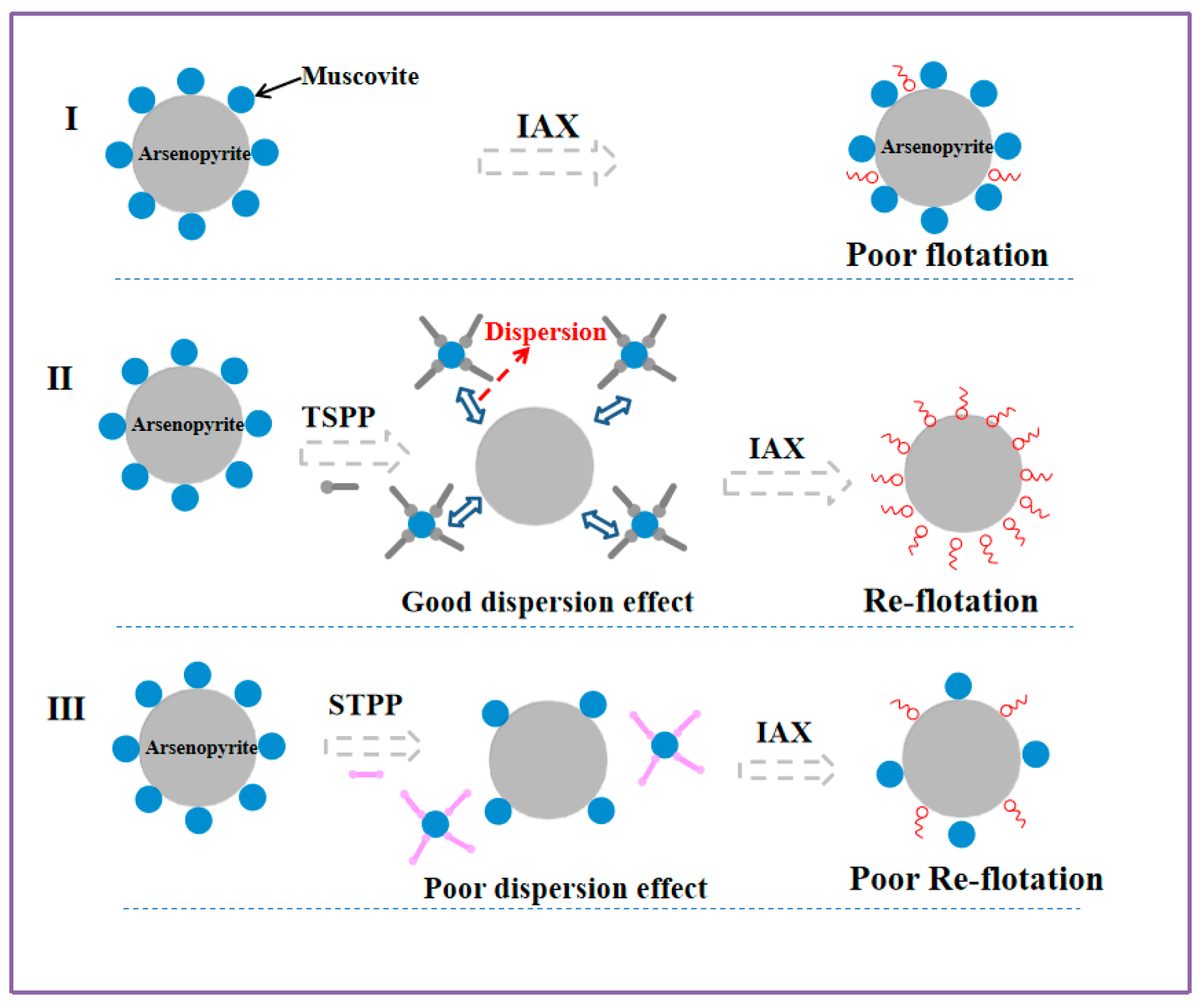
3.7. Suggested Adsorption Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qian, Y.L.; Zou, D.; Chen, R.; Huang, X.; Wang, Z. Improving xanthate flotation of fine pyrite using SPAM and the mechanism. Sep. Sci. Technol. 2023, 58, 2208–2216. [Google Scholar] [CrossRef]
- Basnyaka, L.; Subasinghe, N.; Albijanic, B. Influence of clays on the slurry rheology and flotation of a pyritic gold ore. Appl. Clay Sci. 2017, 136, 230–238. [Google Scholar] [CrossRef]
- Ramirez, A.; Gutierrez, L.; Vega-Garcia, D.; Reyes-Bozo, L. The depressing effect of kaolinite on molybdenite flotation in seawater. Minerals 2020, 10, 578. [Google Scholar] [CrossRef]
- Ming, P.; Xie, Z.; Guan, Y.; Wang, Z.; Li, F.; Xing, Q. The effect of polysaccharide depressant xanthan gum on the flotation of arsenopyrite from chlorite. Miner. Eng. 2020, 157, 106551. [Google Scholar] [CrossRef]
- Zou, D.; Wang, Z.; Zhao, K.; Xu, Y. Dispersion of sodium phytate on muscovite and the implications for arsenopyrite flotation. Physicochem. Probl. Miner. Process. 2022, 58, 154951. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Shi, Q.; Liu, D. Effect of chlorite on the flotation of pyrrhotite and its implications for elimination by different methods. Sep. Sci. Technol. 2019, 54, 1411–1419. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, Z.; Han, X.; Wang, K.; Fang, C.; Zhao, Z.; Tang, W. Ultrasonication Improves the Flotation of Coal Gasification Fine Slag Residue. Minerals 2024, 14, 363. [Google Scholar] [CrossRef]
- Ann Bazar, J.; Rahimi, M.; Fathinia, S.; Jafari, M.; Chipakwe, V.; Chehreh Chelgani, S. Talc flotation—An overview. Minerals 2021, 11, 662. [Google Scholar] [CrossRef]
- Yepsen, R.; Roa, J.; Toledo, P.G.; Gutiérrez, L. Chalcopyrite and molybdenite flotation in seawater: The use of inorganic dispersants to reduce the depressing effects of micas. Minerals 2021, 11, 539. [Google Scholar] [CrossRef]
- Chen, J.; Min, F.F.; Liu, L.Y.; Jia, F.F. Adsorption of methylamine cations on kaolinite basal surfaces: A DFT study. Physicochem. Probl. Miner. Process. 2020, 56, 338–349. [Google Scholar] [CrossRef]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Selim, K.H.A.; Rostom, M.; Youssef, M.A.; Abdel-Khalek, N.A.; Abdel-Khalek, M.A.; Hassan, E.S.R. Surface modified bentonite mineral as a sorbent for Pb2+ and Zn2+ ions removal from aqueous solutions. Physicochem. Probl. Miner. Process. 2020, 56, 145–157. [Google Scholar]
- Liu, S.; Chen, X.; Lauten, R.A.; Peng, Y.; Liu, Q. Mitigating the negative effects of clay minerals on gold flotation by a lignosulfonate-based biopolymer. Miner. Eng. 2018, 126, 9–15. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wen, K.; Dang, W.; Xun, J. Strengthening the inhibition effect of sodium silicate on muscovite by electrochemical modification. Miner. Eng. 2021, 161, 106731. [Google Scholar] [CrossRef]
- Zhang, L.; Khoso, S.A.; Tian, M.; Sun, W. Cassiterite recovery from a sulfide ore flotation tailing by combined gravity and flotation separations. Physicochem. Probl. Miner. Process. 2021, 57, 206–215. [Google Scholar] [CrossRef]
- Ren, J.; Xu, Y.; Huang, J.; Wang, Y.; Jia, Z. Gradation optimization and strength mechanism of aggregate structure considering macroscopic and mesoscopic aggregate mechanical behaviour in porous asphalt mixture. Constr. Build. Mater. 2021, 300, 124262. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.; Li, S.; Deng, J.; Luo, B.; Lai, H. Depression mechanism involving Fe3+ during arsenopyrite flotation. Sep. Purif. Technol. 2019, 222, 109–116. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Y.; Bao, S. The influence of roasting temperature on the flotation properties of muscovite. Minerals 2016, 6, 53. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Vink, S. Diagnosis of the surface chemistry effects on fine coal flotation using saline water. Energy Fuels 2013, 27, 4869–4874. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Hou, L.; Gu, S.Y.; Hou, C.H. Optimization of preparation process of sodium pyrophosphate chelates zinc by response surface analysis. Inorg. Chem. Ind. 2020, 52, 30–35. [Google Scholar]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Ran, Y.; Liu, D. Mechanism of sodium tripolyphosphate inhibiting the syneresis of HPAM hydrogel. RSC Adv. 2015, 5, 84872–84878. [Google Scholar] [CrossRef]
- Lu, J.; Tong, Z.; Yuan, Z.; Li, L. Investigation on flotation separation of chalcopyrite from arsenopyrite with a novel collector: N-Butoxycarbonyl-O-Isobutyl Thiocarbamate. Miner. Eng. 2019, 137, 118–123. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, Y.; Han, Y.; Gao, P.; Gu, X.; Sun, Y. Chemical oxidation of arsenopyrite using a novel oxidant—Chlorine dioxide. Miner. Eng. 2019, 139, 105863. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Zhou, M.; Zhang, Y.; Safarov, S.; Wang, Z. Comparative Study on the Effect of Pyrophosphate and Tripolyphosphate on the Flotation Separation of Arsenopyrite and Muscovite. Minerals 2024, 14, 785. https://doi.org/10.3390/min14080785
Qian Y, Zhou M, Zhang Y, Safarov S, Wang Z. Comparative Study on the Effect of Pyrophosphate and Tripolyphosphate on the Flotation Separation of Arsenopyrite and Muscovite. Minerals. 2024; 14(8):785. https://doi.org/10.3390/min14080785
Chicago/Turabian StyleQian, Yunlou, Mengyao Zhou, Yongde Zhang, Sayfidin Safarov, and Zhen Wang. 2024. "Comparative Study on the Effect of Pyrophosphate and Tripolyphosphate on the Flotation Separation of Arsenopyrite and Muscovite" Minerals 14, no. 8: 785. https://doi.org/10.3390/min14080785
APA StyleQian, Y., Zhou, M., Zhang, Y., Safarov, S., & Wang, Z. (2024). Comparative Study on the Effect of Pyrophosphate and Tripolyphosphate on the Flotation Separation of Arsenopyrite and Muscovite. Minerals, 14(8), 785. https://doi.org/10.3390/min14080785







