Two-Dimensional Hydration and Triple-Interlayer Lattice Structures in Sulfate-Intercalated Graphene Oxide Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GO and s-GO
2.2. Sample Preparation for Characterization
3. Results and Discussion
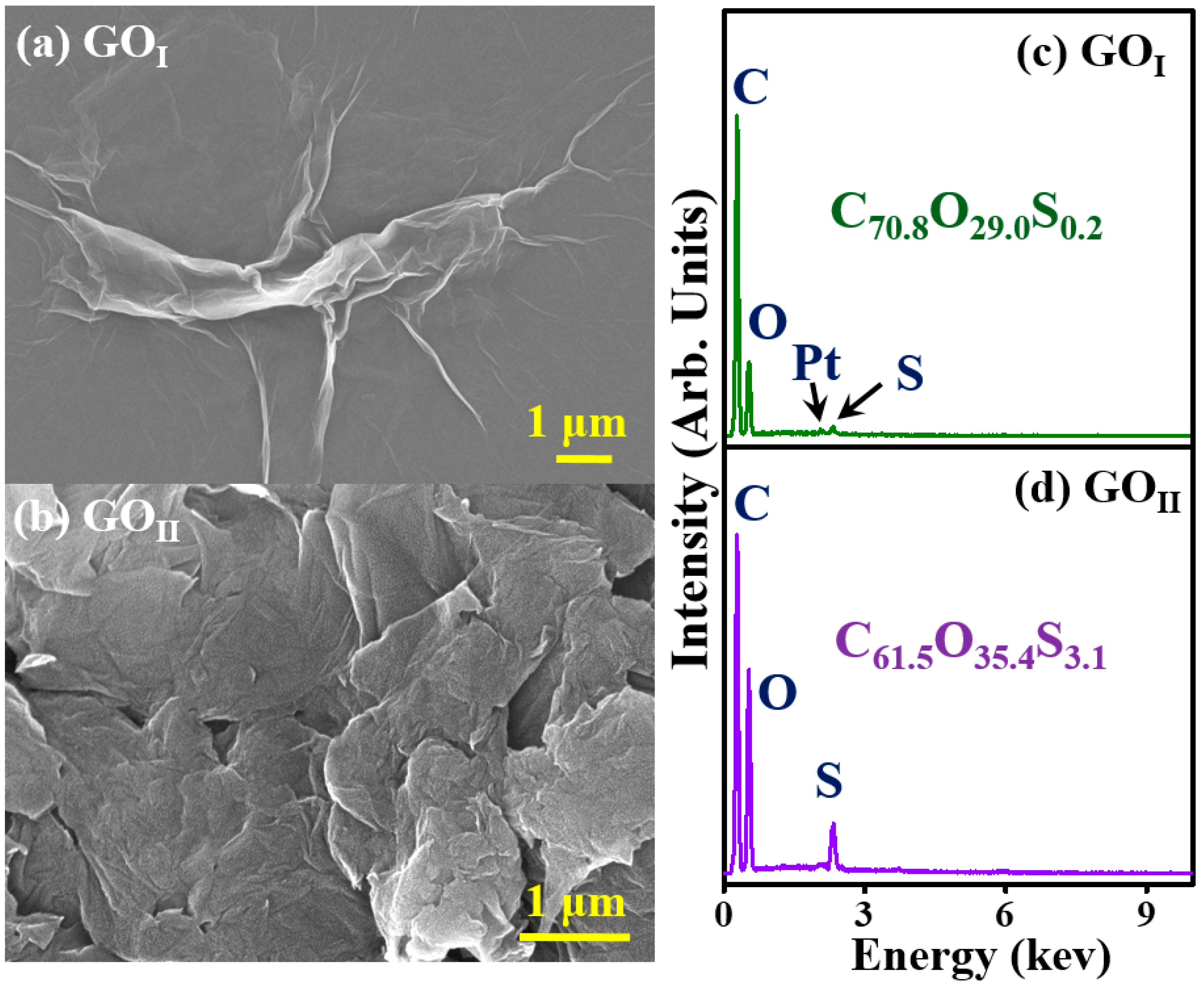
3.1. Morphology and Composition of GOI and GOII
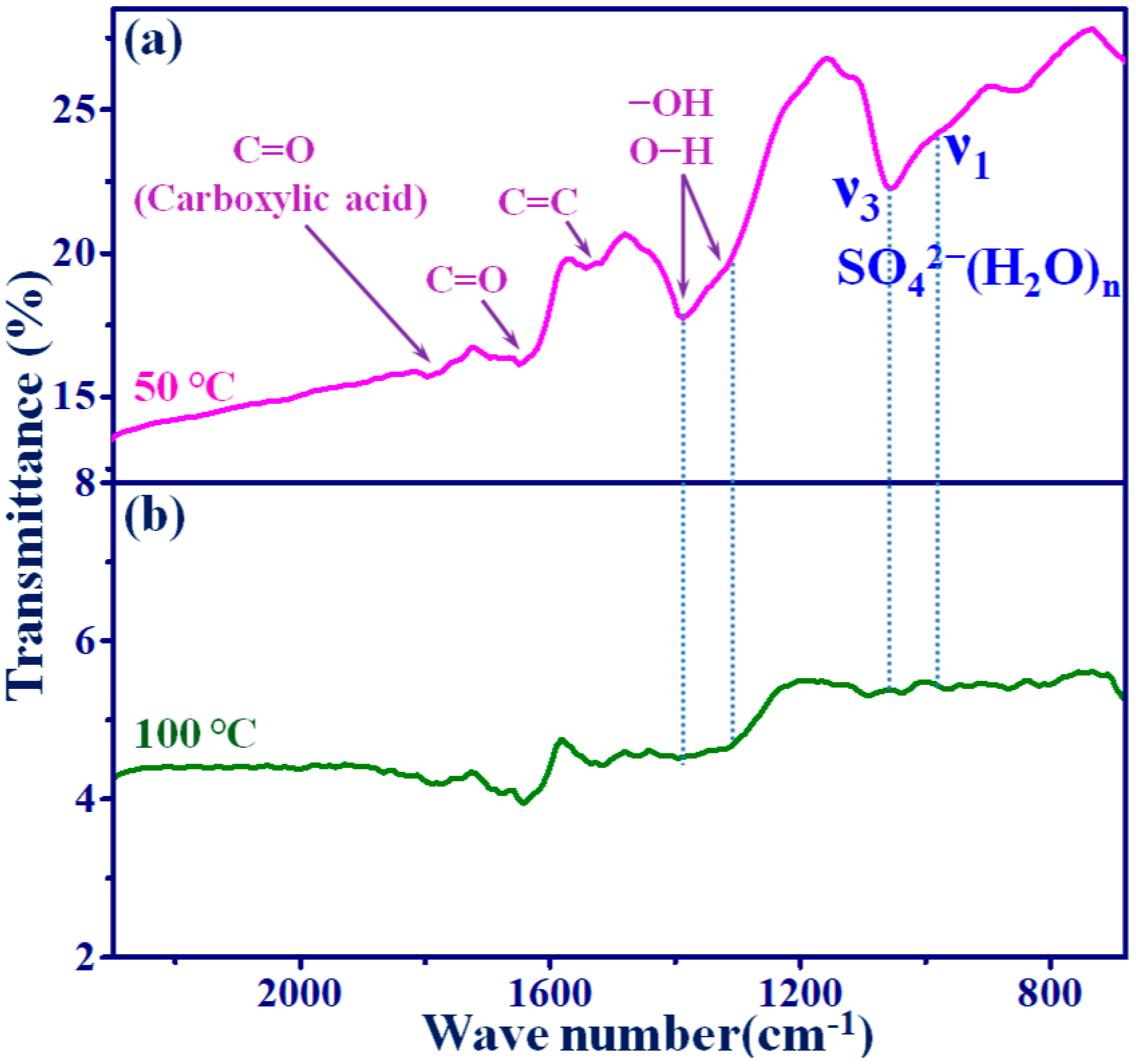
3.2. FT-IR Analysis of GOI and GOII
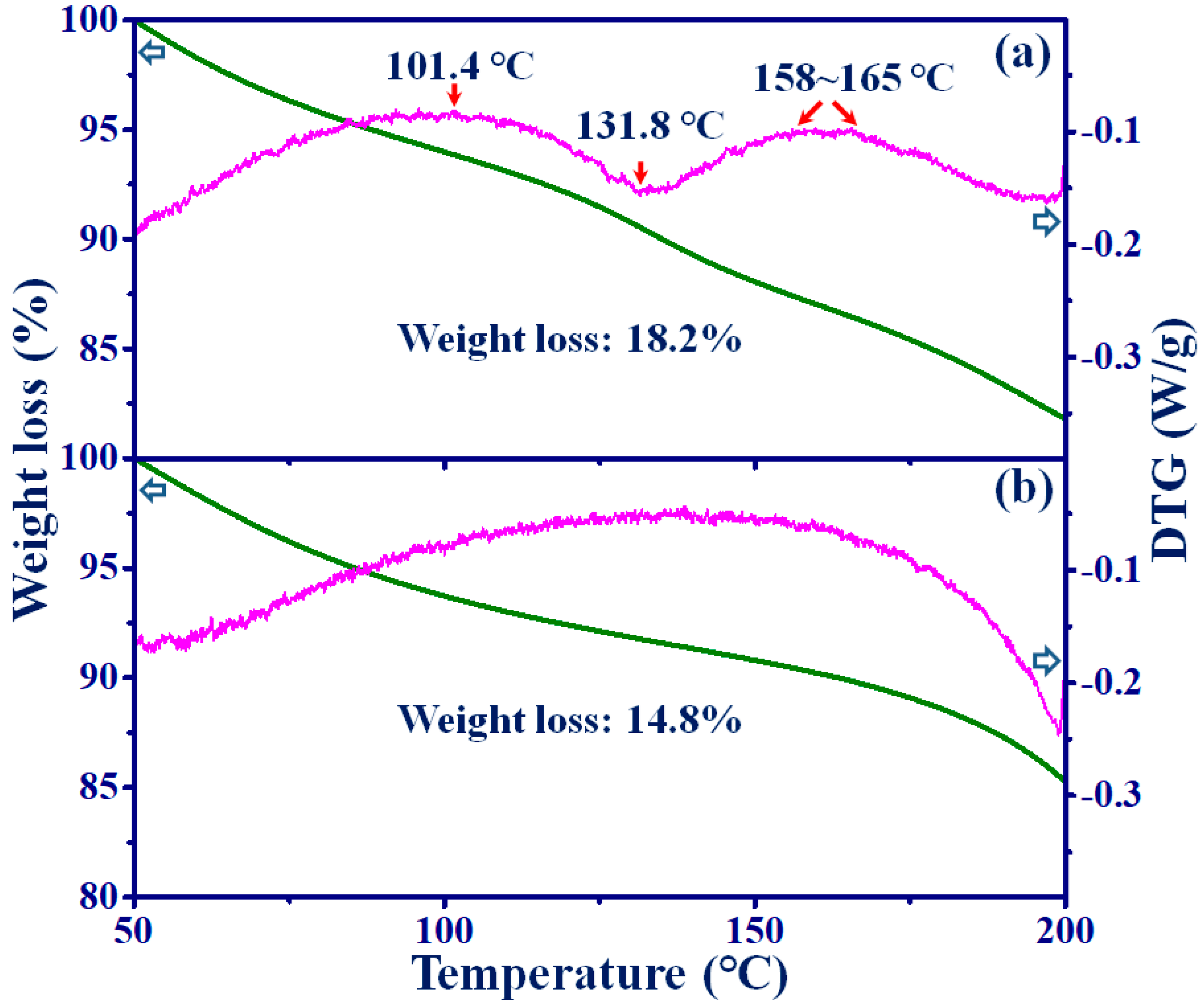
3.3. TGA and XRD Analysis
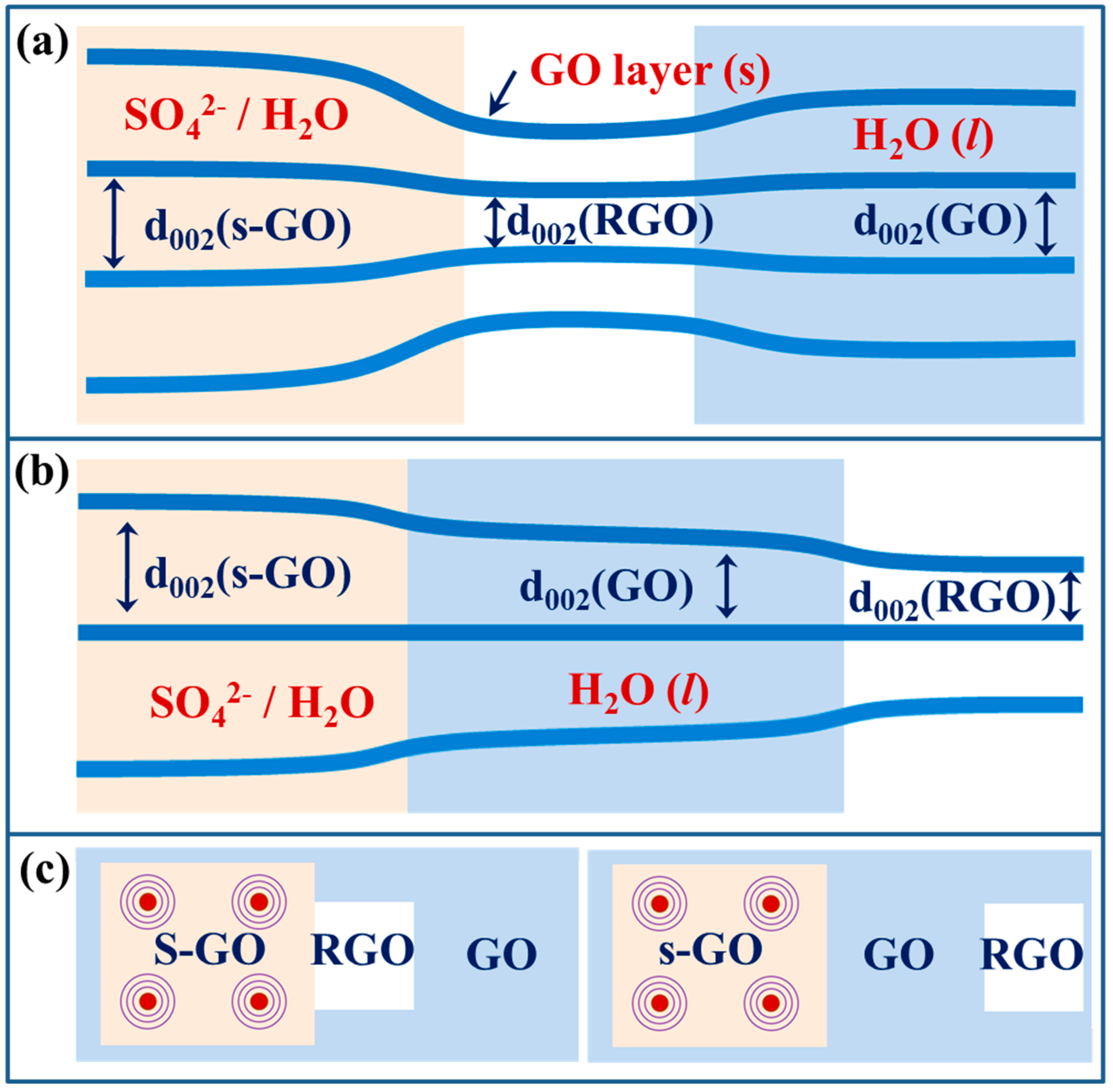
3.4. 2D-Orientated Hydration of Intercalated Sulfate Anions
3.5. Triple-Interlayer Lattices of s-GO Nanosheets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, H.; Bi, X.; Liu, C.; Shi, E.; Cao, H.; Xin, Y.; Fu, X.; Qiao, L.; Zhang, J.; Wu, Z.; et al. Spatial distributions and origin of hydrated sulfate minerals at the mineral bowl in Ophir Chasma, Mars. Planet. Space Sci. 2021, 207, 105323. [Google Scholar] [CrossRef]
- Gill, K.K.; Jagniecki, E.A.; Benison, K.C.; Gibson, M.E. A Mars-analog sulfate mineral, mirabilite, preserves biosignatures. Geology 2023, 51, 818–822. [Google Scholar] [CrossRef]
- Perkins, D. Mineralogy, LibreTextsTM; University of North Dakota: Grand Forks, ND, USA, 2023. [Google Scholar]
- Johan, P.R.; Buseck, P.R. Mineralogy and Instrumentation. In Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Tomasz, M.S.; Stawski Alexander, E.S.; Driessche, V. Editorial for Special Issue “Formation of Sulfate Minerals in Natural and Industrial Environments”. Mineral 2022, 12, 299. [Google Scholar] [CrossRef]
- Radha, S.; Kamath, P.V. Polytypism in Sulfate-Intercalated Layered Double Hydroxides of Zn and M(III) (M = Al, Cr): Observation of Cation Ordering in the Metal Hydroxide Layers. Inorg. Chem. 2013, 52, 4834. [Google Scholar] [CrossRef]
- Ida, S.; Shiga, D.; Koinuma, M.; Matsumoto, Y. Synthesis of Hexagonal Nickel Hydroxide Nanosheets by Exfoliation of Layered Nickel Hydroxide Intercalated with Dodecyl Sulfate Ions. J. Am. Chem. Soc. 2008, 130, 14038. [Google Scholar] [CrossRef]
- Jorgensen, S.E.; Fath, B.D. Encyclopedia of Ecology. Sulfur Cycle 2008, 4, 3424–3431. Available online: https://drs.nio.org/drs/handle/2264/1462 (accessed on 10 October 2024).
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 2019, 10, 809. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze: Zweite Mittheilung. Arch. Exp. Pathol. Pharmacol. 1888, 24, 247. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Prell, J.S.; Bush, M.F.; Williams, E.R. Sulfate ion patterns water at long distance. J. Am. Chem. Soc. 2010, 132, 8248. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, J.; Sun, X.; Li, Z.; Sun, Z. Genesis of Metal Sulfides and Its Significance on Graphite Mineralization in the Huangyangshan Graphite Deposit, East Junggar, Xinjiang Province, China. Minerals 2022, 12, 1450. [Google Scholar] [CrossRef]
- Ferry, J.M. Petrology of graphitic sulfide-rich schists from South-central Maine: An example of desulfidation during prograde regional metamorphism. Am. Min. 1981, 66, 908–930. Available online: https://pubs.geoscienceworld.org/msa/ammin/article/66/9-10/908/41318 (accessed on 10 October 2024).
- Eiger, S. Graphite sulphate—A precursor to graphene. Chem. Commun. 2015, 51, 3162. [Google Scholar] [CrossRef]
- Ng, Y.Z.; Beh, K.P.; Suhaimi, F.H.A.; Abdalraheem, R.; Lim, H.S.; Jafri, M.Z.M.; Yam, F.K. Effect of the Sulfate Concentration on the Graphene Film Produced by Electrochemical Exfoliation. Solid State Phenomena 2019, 290, 127–133. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Jin, X. Sulfuric Acid Intercalated Graphite Oxide for Graphene Preparation. Sci. Rep. 2013, 3, 3439. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Hsu, J.H.; Yu, C. Intercalated graphene oxide for flexible and practically large thermoelectric voltage generation and simultaneous energy storage. Nano Energy 2018, 48, 582. [Google Scholar] [CrossRef]
- DiTucci, M.J.; Stachl, C.N.; Williams, E.R. Long distance ion-water interactions in aqueous sulfate nanodrops persist to ambient temperature in the upper atmosphere. Chem. Sci. 2018, 9, 3970. [Google Scholar] [CrossRef] [PubMed]
- Kulichenko, M.; Fedik, N.; Bozhenko, K.V.; Boldyrev, A.I. Hydrated Sulfate Cluster SO42− (H2O)n (n = 1−40): Charge Distribution Throught, Solvation Shells and Stabilization. J. Phys. Chem. B 2019, 123, 4065. [Google Scholar] [CrossRef]
- Bush, M.F.; Saykally, R.J.; Williams, E.R. Evidence for Water Ring in the Hexahydrated Sulfate Dianion from IR Spectroscopy. J. Am. Chem. Soc. 2007, 129, 2220–2221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Nicholas, J.B.; Wang, L.S. Electronic Instability of Isolated SO42− and its Solvation Stabilization. J. Chem. Phys. 2000, 113, 10837–10840. [Google Scholar] [CrossRef]
- Thaunay, F.; Clavaguéra, C.; Ohanessian, G. Hydration of the sulfate dianion in cold nanodroplets: SO42−(H2O)12 and SO42−(H2O)13. Phys. Chem. Chem. Phys. 2015, 17, 25935. [Google Scholar] [CrossRef]
- DiTucci, M.J.; Williams, E.R. Nanometer patterning of water by tetraanionic ferrocyanide stabilizied in aqueous nanodrops. Chem. Sci. 2017, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Fan, Y.; Song, Z.; Chen, J. Effects of interlayer spacing and oxidation degree of graphene oxide nanosheets on water permeation: A molecular dynamics study. J. Mol. Model. 2022, 28, 57. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 1988, 88, 1475. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen bond networks in graphene oxide composite paper: Structure and mechanical properties. ACS Nano 2010, 4, 2300. [Google Scholar] [CrossRef] [PubMed]
- Thaunay, F.; Hassan, A.A.; Cooper, R.J.; Williams, E.R.; Clavaguéra, C.; Ohanessian, G. Hydration of the sulfate dianion in size-selected water clusters: From SO42−(H2O)9 to SO42−(H2O)13. Int. J. Mass Spec. 2017, 418, 15. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Cole, W.T.S.; Saykally, R.J. The water dimer I: Experimental characterization. Chem. Phys. Lett. 2015, 633, 13–26. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X.; Bocquet, M.L. Structure and chemistry of graphene oxide in liquid water from first principles. Nat. Comm. 2020, 11, 1566. [Google Scholar] [CrossRef]
- Rezania, B.; Severin, N.; Talyzin, A.V.; Rabe, J.P. Hydration of bilayered graphene oxide. Nano Lett. 2014, 14, 3993. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255. [Google Scholar] [CrossRef]
- Surekha, G.; Krishnaiah, K.V.; Ravi, N.; Suvarna, R.P. FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. J. Phys. Conf. Ser. 2020, 1495, 012012. [Google Scholar] [CrossRef]
- Rattana; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.Y.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene oxide liquid crystals. Angew. Chem. Int. Edgl. 2011, 50, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Moissette, A.; Fuzellier, H.; Burneau, A.; Dubessy, J.; Lelaurain, M. Uranyl-sulfate graphite intercalation compounds–I. Their synthesis and stability. Carbon 1995, 33, 809–815. [Google Scholar] [CrossRef]
- Huh, S.H. Thermal Reduction of Graphene Oxide. Physics and Applications of Graphene—Experiments. Chapter 2011, 5, 73. [Google Scholar] [CrossRef]
- Ju, H.M.; Huh, S.H.; Choi, S.H.; Lee, H.L. X-ray Diffraction Patterns of Thermally-reduced Graphenes. Mater. Lett. 2010, 64, 1649–1652. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Pardal, J.M.; Tavares, S.S.M.; Fonseca, M.P.C. Study of the austenite quantification by X-ray diffraction in the 18Ni-CO-Mo maraging 300 steel. J. Mater. Sci. 2006, 41, 2301–2307. [Google Scholar] [CrossRef]
- Kiefer, J.; Stärk, A.; Kiefer, A.L.; Glade, H. Infrared Spectroscopic Analysis of the Inorganic Deposits from Water in Domestic and Technical Heat Exchangers. Energies 2018, 11, 798. [Google Scholar] [CrossRef]
- Zhou, J.; Santambrogio, G.; Brümmer, M.; Moore, D.T.; Wöste, L.; Meijer, G.; Neumark, D.M.; Asmis, K.R. Infrared spectroscopy of hydrated sulfate dianions. J. Chem. Phys. 2006, 125, 111102. [Google Scholar] [CrossRef]
- Vasu1, K.S.; Prestat, E.; Abraham, J.; Dix, J.; Kashtiban, R.J.; Beheshtian, J.; Sloan, J.; Carbone, P.; Amal, M.N.; Haigh, S.J.; et al. Van der Waals pressure and its effect on trapped interlayer molecules. Nat. Commun. 2016, 7, 12168. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, H.; Wu, H.; Jiu, J.; Zhao, J. Nanoconfined Crystal Growth of Copper-Intercalated Graphene Oxide Interlayers. J. Phys. Chem. C 2022, 126, 17344–17352. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, Q.; Wang, M.; Zhang, J.; Wang, P.; Ge, Y. Molecular dynamics study on water and ions on the surface of graphene oxide sheet: Effects of functional groups. Comput. Mater. Sci. 2019, 167, 237–247. [Google Scholar] [CrossRef]
- Smirnov, V.A.; Vasil’ev, V.P.; Denisov, N.N.; Baskakova, Y.V.; Dubovitskii, V.A. Electric behavior of interlayer water in graphene oxide films. Chem. Phys. Lett. 2016, 648, 87–90. [Google Scholar] [CrossRef]


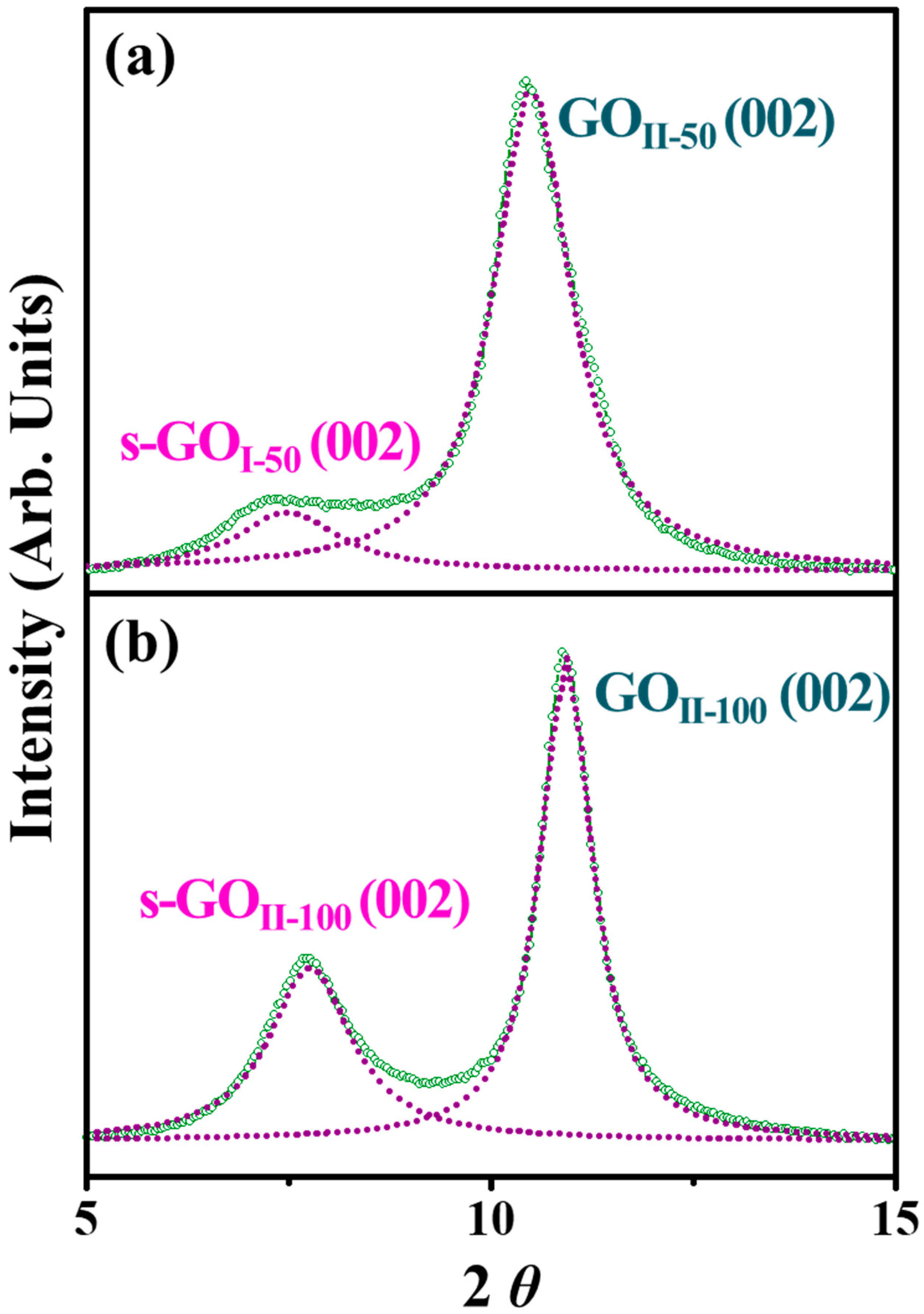
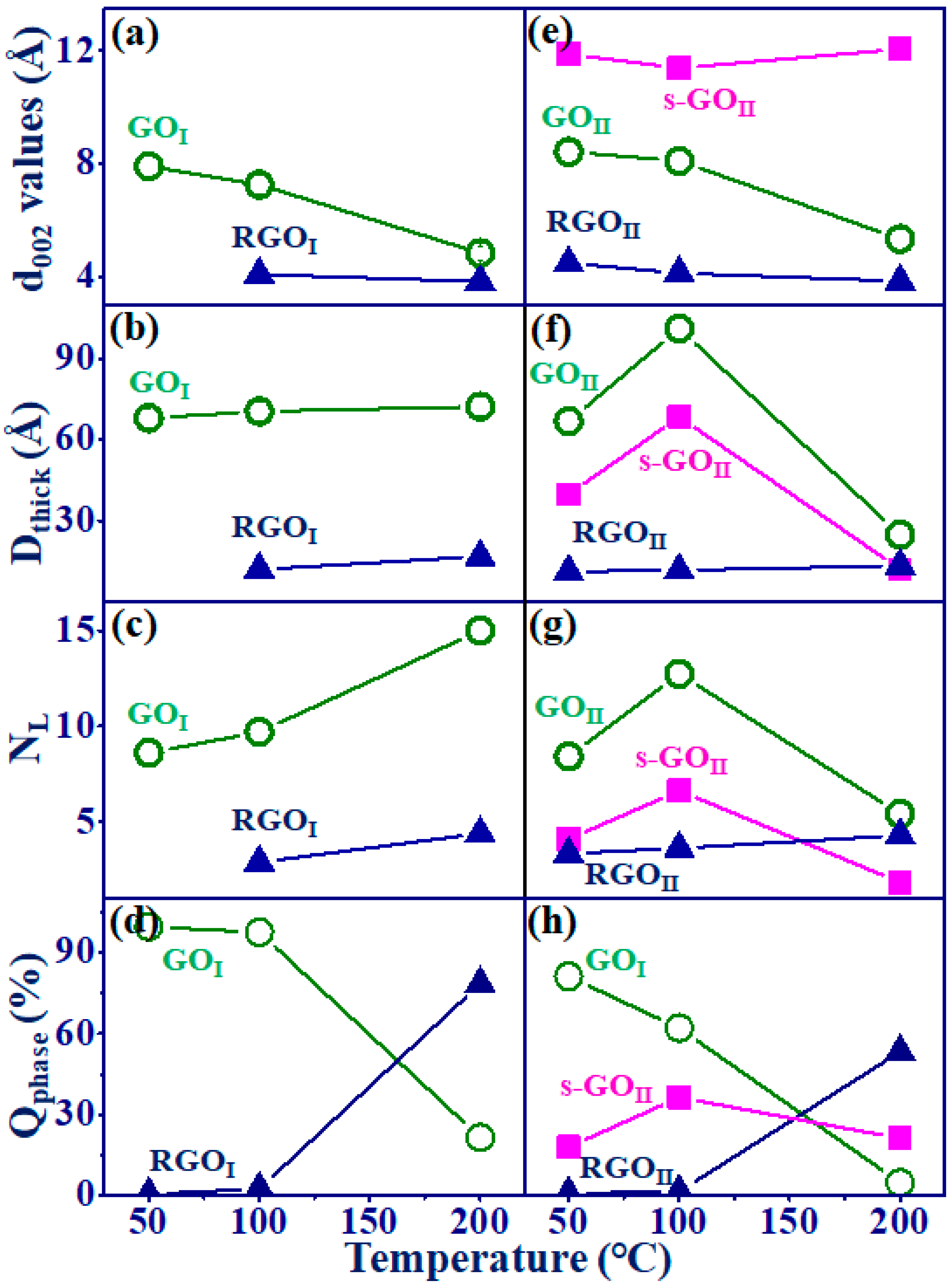

| T (°C) | 2D Materials | a 2θ (°) | b FWHM (°) | c d002 (Å) | d Dthick (Å) | e NL | f Qphase (%) |
|---|---|---|---|---|---|---|---|
| 50 | GOI-50 | 11.194 | 1.16 | 7.904 | 68.0 | 8.6 | 99.5 |
| 100 | GOI-100 | 12.202 | 1.12 | 7.253 | 70.5 | 9.7 | 97.5 |
| RGOI-100 | 21.765 | 6.69 | 4.083 | 12.0 | 2.9 | 2.5 | |
| 200 | GOI-200 | 18.340 | 1.10 | 4.837 | 72.3 | 15.0 | 21.5 |
| RGOI-200 | 23.239 | 4.80 | 3.828 | 16.7 | 4.4 | 78.5 | |
| 50 | s-GOII-50 | 7.441 | 1.98 | 11.880 | 39.8 | 3.3 | 18.0 |
| GOII-50 | 10.506 | 1.18 | 8.420 | 66.9 | 7.9 | 81.2 | |
| RGOII-50 | 19.838 | 7.14 | 4.475 | 11.2 | 2.5 | 0.8 | |
| 100 | s-GOII-100 | 7.767 | 1.15 | 11.382 | 68.5 | 6.0 | 36.4 |
| GOII-100 | 10.930 | 0.78 | 8.095 | 101.2 | 12.5 | 62.1 | |
| RGOII-100 | 21.522 | 6.90 | 4.129 | 11.6 | 2.8 | 1.5 | |
| 200 | s-GOII-200 | 7.300 | 7.20 | 12.050 | 12.0 | 0.9 | 21.2 |
| GOII-200 | 16.623 | 3.20 | 5.333 | 24.8 | 4.7 | 15.4 | |
| RGOII-200 | 23.166 | 6.02 | 3.839 | 13.3 | 3.5 | 53.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.J.; Kim, S.J.; Kim, H.G.; Jee, Y.; Huh, S.H. Two-Dimensional Hydration and Triple-Interlayer Lattice Structures in Sulfate-Intercalated Graphene Oxide Nanosheets. Minerals 2024, 14, 1030. https://doi.org/10.3390/min14101030
Ahn HJ, Kim SJ, Kim HG, Jee Y, Huh SH. Two-Dimensional Hydration and Triple-Interlayer Lattice Structures in Sulfate-Intercalated Graphene Oxide Nanosheets. Minerals. 2024; 14(10):1030. https://doi.org/10.3390/min14101030
Chicago/Turabian StyleAhn, Hae Jun, Sun Jie Kim, Hyun Goo Kim, Youngho Jee, and Seung Hun Huh. 2024. "Two-Dimensional Hydration and Triple-Interlayer Lattice Structures in Sulfate-Intercalated Graphene Oxide Nanosheets" Minerals 14, no. 10: 1030. https://doi.org/10.3390/min14101030
APA StyleAhn, H. J., Kim, S. J., Kim, H. G., Jee, Y., & Huh, S. H. (2024). Two-Dimensional Hydration and Triple-Interlayer Lattice Structures in Sulfate-Intercalated Graphene Oxide Nanosheets. Minerals, 14(10), 1030. https://doi.org/10.3390/min14101030







