Rapid Determination of Ti in Quartz Using a Portable/Handheld Laser-Induced Breakdown Spectroscopy (LIBS) Instrumentation: A Case Study on Quartz Veinlets in Hornfels from Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Setup, Spectra Acquisition and Processing
2.3. Calculation of the Plasma Temperature and Electron Density
2.4. Calculation of the Relative Abundance (Concentration) of Ti
3. Results and Discussion
3.1. LIBS Spectra
3.2. Plasma Characterization
3.3. Self-Absorption and Matrix Effects
3.4. Relative Variation in Ti Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wark, D.A.; Watson, E.B. TitaniQ: A titanium-in-quartz geothermometer. Contrib. Mineral. Petrol. 2006, 152, 743–754. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochim. Acta B At. Spectrosc. 2021, 175, 106013. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld LIBS. In Laser-Induced Breakdown Spectroscopy, 2nd ed.; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 537–560. [Google Scholar]
- Harmon, R.S.; Senesi, G.S. Laser-induced breakdown spectroscopy—A geochemical tool for the 21st century. Appl. Geochem. 2021, 128, 104929. [Google Scholar] [CrossRef]
- Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A.; Tognoni, E. New procedure for quantitative elemental analysis by laser-induced plasma spectroscopy. Appl. Spectrosc. 1999, 53, 960–964. [Google Scholar] [CrossRef]
- Corsi, M.; Cristoforetti, G.; Hidalgo, M.; Legnaioli, S.; Palleschi, V.; Salvetti, A.; Tognoni, E.; Vallebona, C. Double pulse, calibration-free laser-induced breakdown spectroscopy: A new technique for in situ standard-less analysis of polluted soils. Appl. Geochem. 2006, 21, 748–755. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G.; Legnaioli, S.; Palleschi, V. Calibration-free laser induced breakdown spectroscopy: State of the art. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 1–14. [Google Scholar] [CrossRef]
- Cavalcanti, G.H.; Teixeira, D.C.; Legnaioli, S.; Lorenzetti, G.; Pardini, L.; Palleschi, V. One-point calibration for calibration-free laser-induced breakdown spectroscopy quantitative analysis. Spectrochim. Acta B At. Spectrosc. 2013, 87, 51–56. [Google Scholar] [CrossRef]
- Brogi, A.; Caggianelli, A.; Liotta, D.; Zucchi, M.; Spina, A.; Capezzuoli, E.; Casini, A.; Buracchi, E. The Gavorrano Monzogranite (Northern Apennines): An Updated Review of Host Rock Protoliths, Thermal Metamorphism and Tectonic Setting. Geosciences 2021, 11, 124. [Google Scholar] [CrossRef]
- Huang, R.; Audetat, A. The titanium-in-quartz (TitaniQ) thermobarometer: A critical examination and re-calibration. Geochim. Cosmochim. Acta 2012, 84, 75–89. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A. Characterization of laser induced plasmas by optical emission spectroscopy: A review of experiments and methods. Spectrochim. Acta B At. Spectrosc. 2008, 63, 893–916. [Google Scholar] [CrossRef]
- Tognoni, E.; Palleschi, V.; Corsi, M.; Cristoforetti, G.; Omenetto, N.; Gorhushkin, I.; Smith, B.W.; Winefordner, J.D. From sample to signal in laser-induced breakdown spectroscopy: A complex route to quantitative analysis. In Laser-Induced Breakdown Spectroscopy (LIBS) Fundamentals and Applications; Miziolek, A.W., Palleschi, V., Schechter, I., Eds.; Cambridge University Press: New York, NY, USA, 2006; pp. 122–170. [Google Scholar]
- Zwicker, H. Evaluation of Plasma Parameters in Optically Thick Plasmas. In Plasma Diagnostics; Lochte Holtgreven, W., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1968; pp. 214–248. [Google Scholar]
- Ma, Q.L.; Motto-Ros, V.; Lei, W.Q.; Boueri, M.; Zheng, L.J.; Zeng, H.P.; Bar-Matthews, M.; Ayalon, A.; Panczer, G.; Yua, J. Multi-elemental mapping of a speleothem using laser-induced breakdown spectroscopy. Spectrochim. Acta B At. Spectrosc. 2010, 65, 707–714. [Google Scholar] [CrossRef]
- NIST Atomic Spectra Database (Version 5.8). Available online: https://physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 17 February 2023).
- Díaz Pace, D.M.; Molina, J.; Rodríguez, C.I. Rapid Assessment of Extractability of Macronutrients from Yerba Mate (Illex paraguariensis) Leaves Based on Laser-Induced Breakdown Spectroscopy. Chemosensors 2024, 12, 18. [Google Scholar] [CrossRef]
- Díaz Pace, D.M.; Gabriele, N.A.; Garcimuño, M.; D’Angelo, C.A.; Bertuccelli, G.; Bertuccelli, D. Analysis of Minerals and Rocks by Laser-Induced Breakdown Spectroscopy. Spectr. Lett. 2011, 44, 399–411. [Google Scholar] [CrossRef]
- Morgan, D.J.; Jollands, M.C.; Lloyd, G.E.; Banks, D. Using titanium-in-quartz geothermometry and geospeedometry to recover temperatures in the aureole of the ballachulish igneous complex, NW Scotland. Geol. Soc. Spec. Publ. 2014, 394, 145–165. [Google Scholar] [CrossRef]
- Voll, G. The setting of the Ballachulish intrusive igneous complex in the Scottish Highlands. In Equilibrium and Kinetics in Contact Metamorphism: The Ballachulish Igneous Complex and Its Aureole; Voll, G., Töpel, J., Pattison, D.R.M., Seifert, F., Eds.; Springer: Heidelberg, Germany, 1991; pp. 3–17. [Google Scholar]

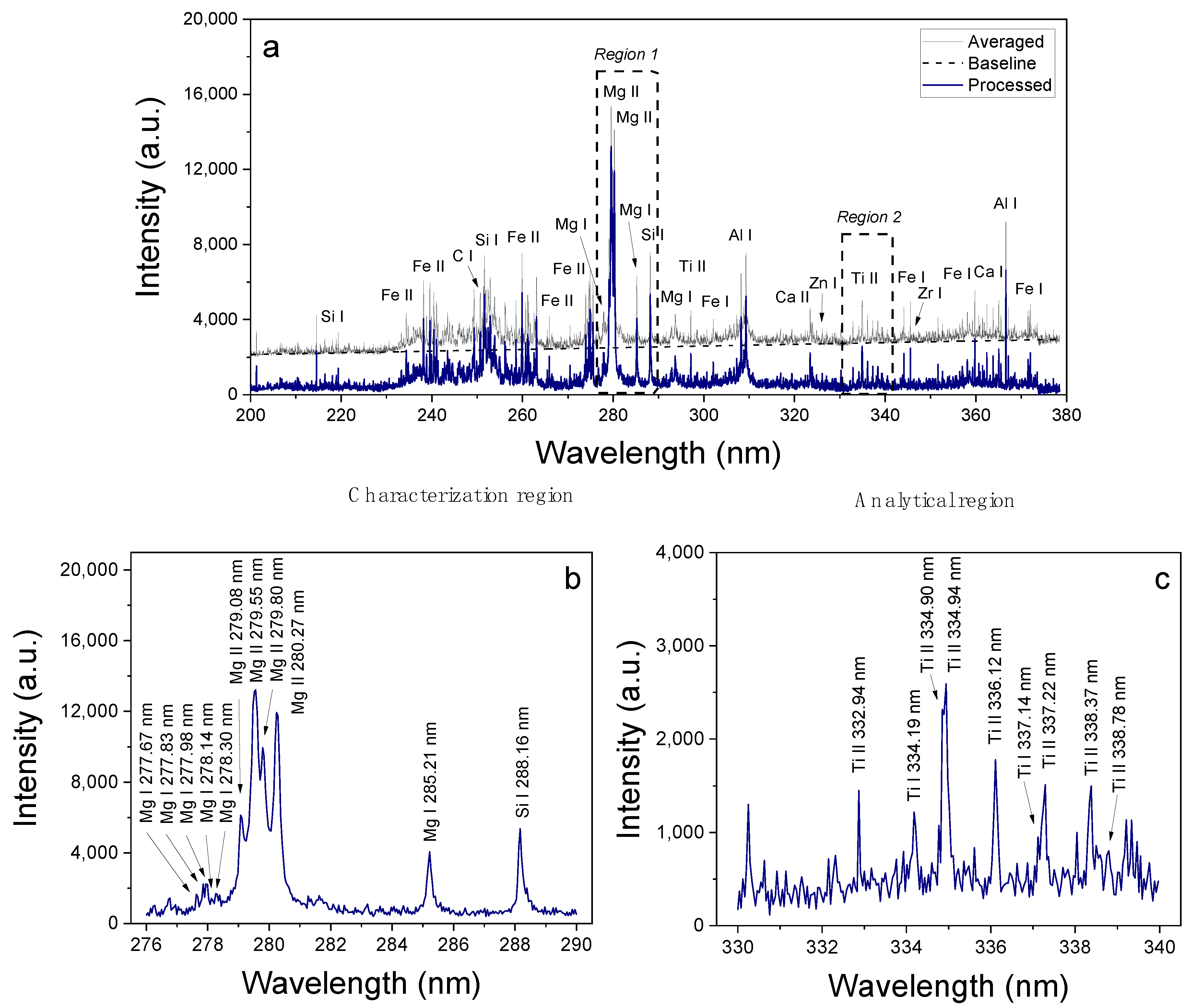
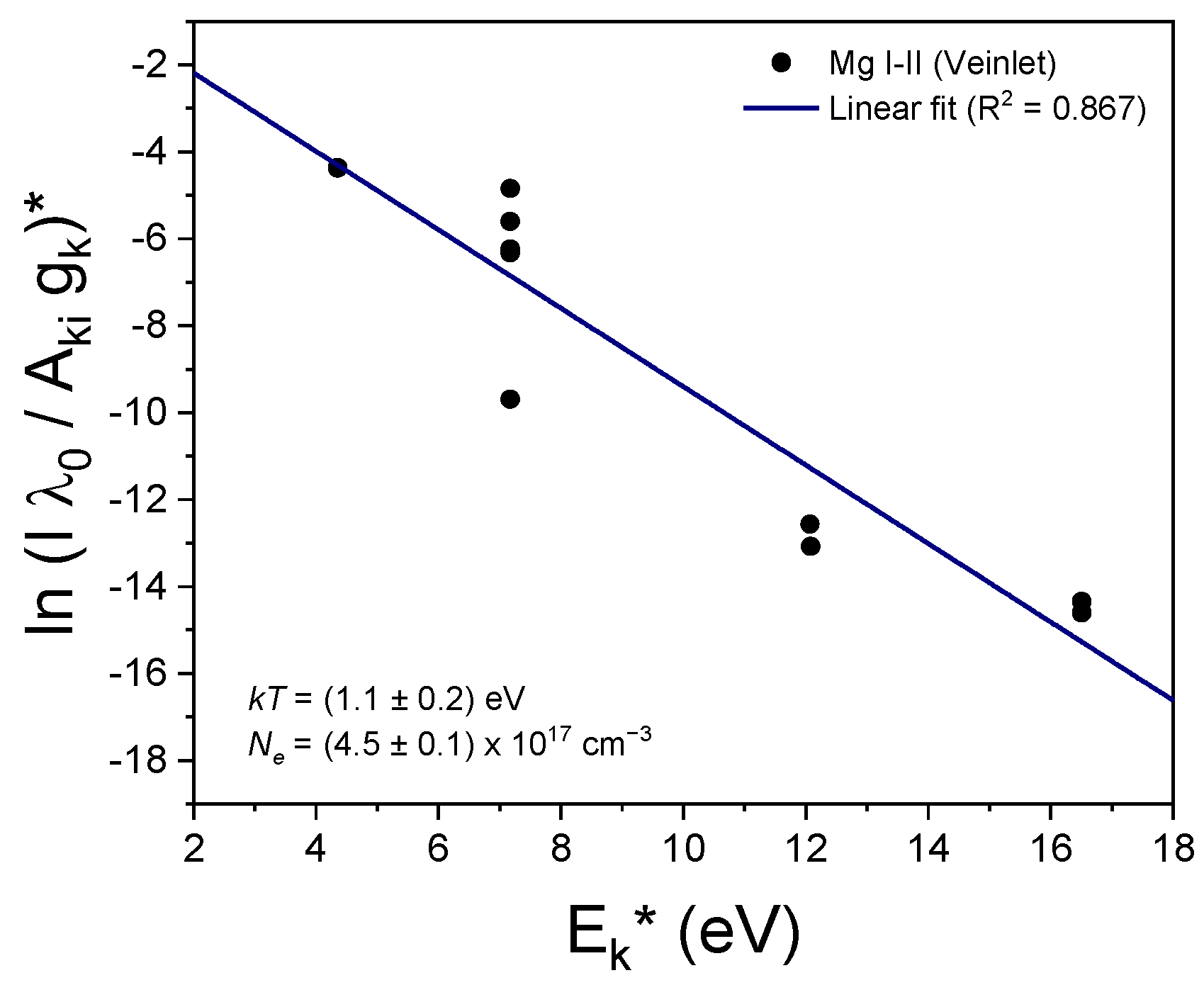
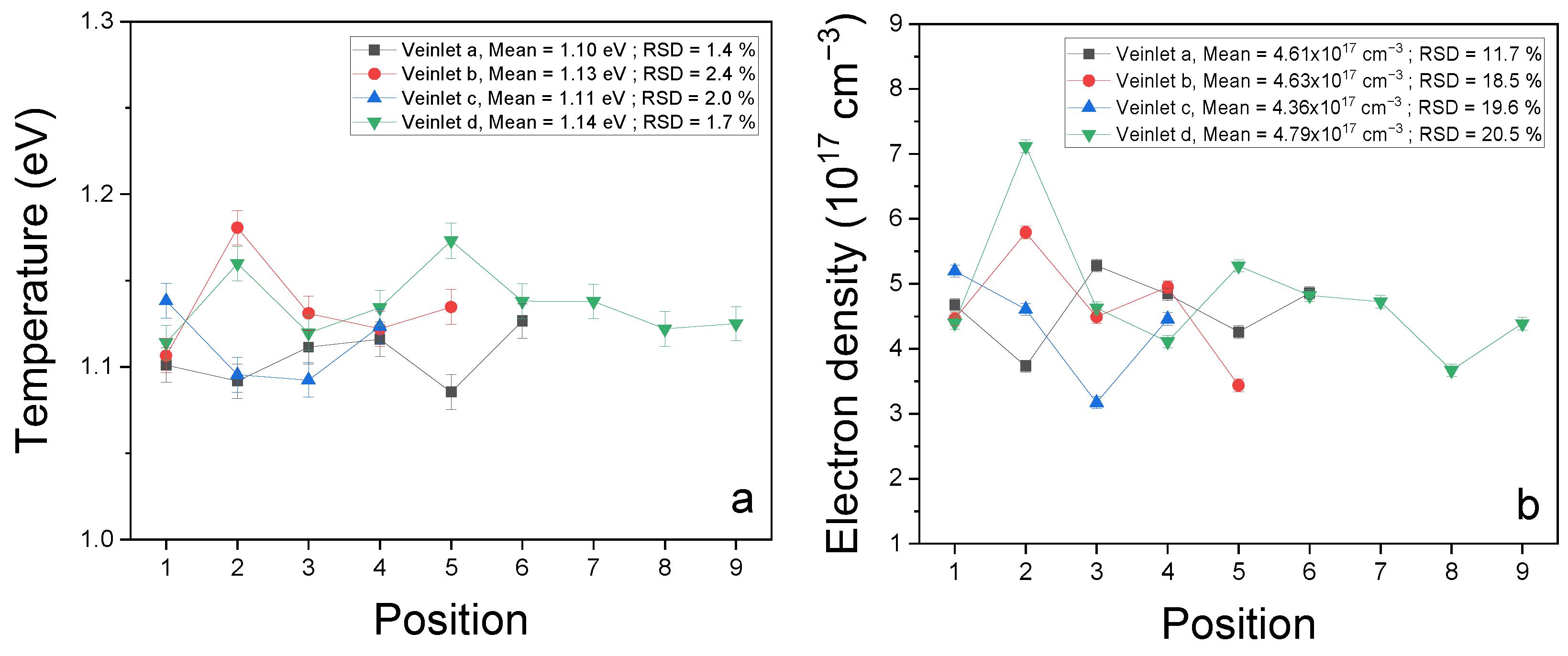


| Element | Ionization State | λ0 (nm) | Aki (108 s−1) | Ei (eV) | Ek (eV) | gi | gk |
|---|---|---|---|---|---|---|---|
| Mg | I | 277.67 | 1.32 | 2.711 | 7.175 | 3 | 5 |
| I | 277.83 | 1.82 | 2.709 | 7.170 | 1 | 3 | |
| I | 277.98 | 1.36 | 2.711 | 7.170 | 3 | 3 | |
| I | 278.14 | 5.43 | 2.711 | 7.168 | 3 | 1 | |
| I | 278.30 | 2.14 | 2.717 | 7.170 | 5 | 3 | |
| I | 285.21 | 4.91 | 0.000 | 4.346 | 1 | 3 | |
| II | 279.08 | 4.01 | 4.422 | 8.864 | 2 | 4 | |
| II | 279.55 | 2.60 | 0.000 | 4.434 | 2 | 4 | |
| II | 279.80 | 4.79 | 4.434 | 8.864 | 4 | 6 | |
| II | 280.27 | 2.57 | 0.000 | 4.422 | 2 | 2 | |
| Si | I | 288.16 | 2.17 | 0.781 | 5.082 | 5 | 3 |
| Ti | I | 334.19 | 0.65 | 0.000 | 3.709 | 5 | 7 |
| I | 337.14 | 0.72 | 0.048 | 3.724 | 9 | 11 | |
| II | 332.94 | 0.41 | 0.135 | 3.858 | 8 | 8 | |
| II | 334.90 | 1.61 | 0.0607 | 4.308 | 8 | 10 | |
| II | 334.94 | 1.68 | 0.049 | 3.749 | 10 | 12 | |
| II | 336.12 # | 1.58 | 0.028 | 3.715 | 8 | 10 | |
| II | 337.22 | 0.06 | 0.607 | 4.283 | 8 | 8 | |
| II | 338.37 | 1.39 | 0.000 | 3.363 | 4 | 6 | |
| II | 338.78 | 0.28 | 0.028 | 3.687 | 8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz Pace, D.; Caggianelli, A.; De Pascale, O.; Senesi, G.S. Rapid Determination of Ti in Quartz Using a Portable/Handheld Laser-Induced Breakdown Spectroscopy (LIBS) Instrumentation: A Case Study on Quartz Veinlets in Hornfels from Italy. Minerals 2024, 14, 1257. https://doi.org/10.3390/min14121257
Díaz Pace D, Caggianelli A, De Pascale O, Senesi GS. Rapid Determination of Ti in Quartz Using a Portable/Handheld Laser-Induced Breakdown Spectroscopy (LIBS) Instrumentation: A Case Study on Quartz Veinlets in Hornfels from Italy. Minerals. 2024; 14(12):1257. https://doi.org/10.3390/min14121257
Chicago/Turabian StyleDíaz Pace, Diego, Alfredo Caggianelli, Olga De Pascale, and Giorgio S. Senesi. 2024. "Rapid Determination of Ti in Quartz Using a Portable/Handheld Laser-Induced Breakdown Spectroscopy (LIBS) Instrumentation: A Case Study on Quartz Veinlets in Hornfels from Italy" Minerals 14, no. 12: 1257. https://doi.org/10.3390/min14121257
APA StyleDíaz Pace, D., Caggianelli, A., De Pascale, O., & Senesi, G. S. (2024). Rapid Determination of Ti in Quartz Using a Portable/Handheld Laser-Induced Breakdown Spectroscopy (LIBS) Instrumentation: A Case Study on Quartz Veinlets in Hornfels from Italy. Minerals, 14(12), 1257. https://doi.org/10.3390/min14121257









