An Environmentally Friendly Sulfuric Acid Decomposition Strategy for Mixed Rare Earth Concentrate
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.3. Thermodynamic Theory of Ce-, F-, PO4- and SO4-H2O Systems
3. Results and Discussion
3.1. Effect of Sulfuric Acid Concentration on Leaching
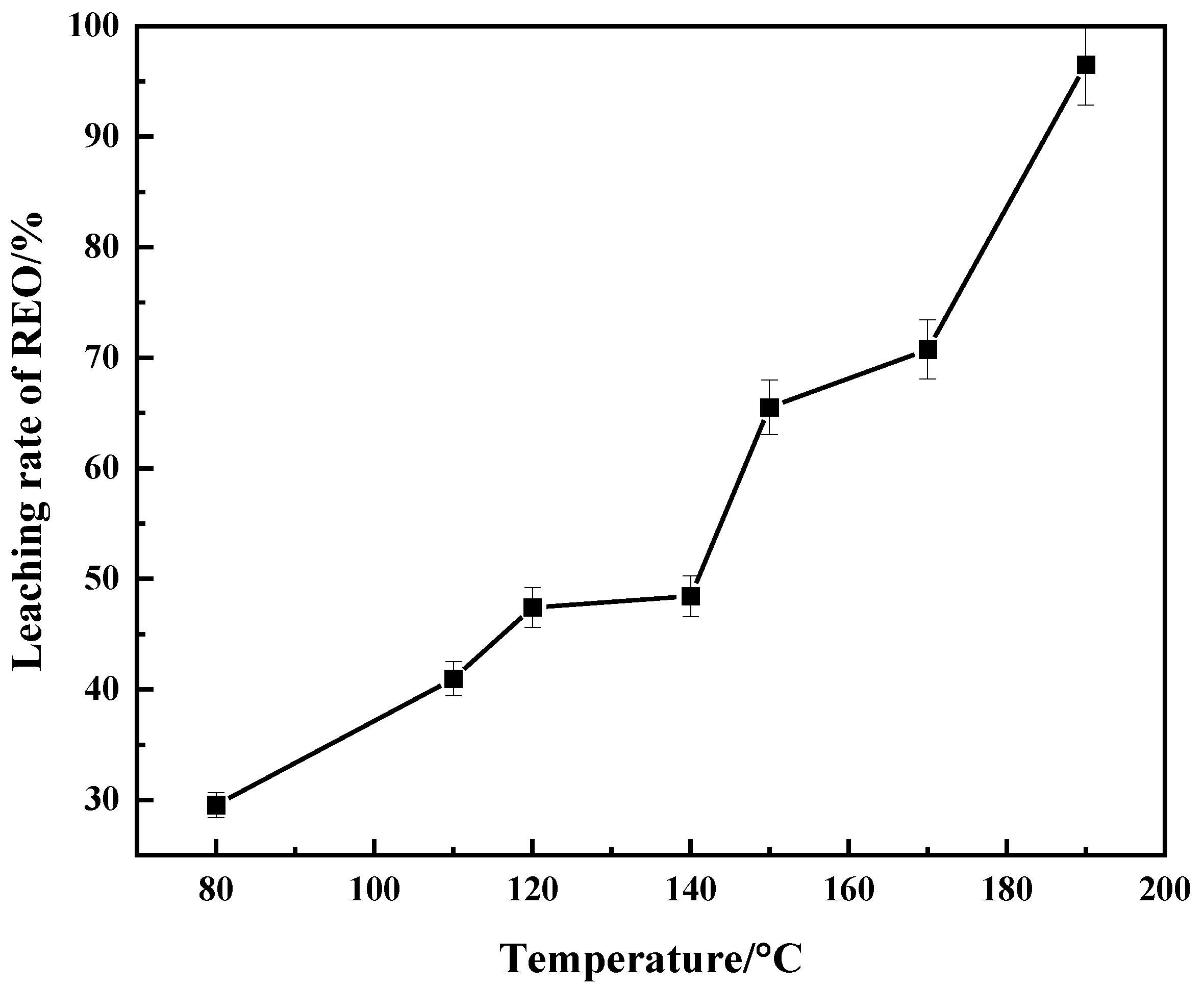
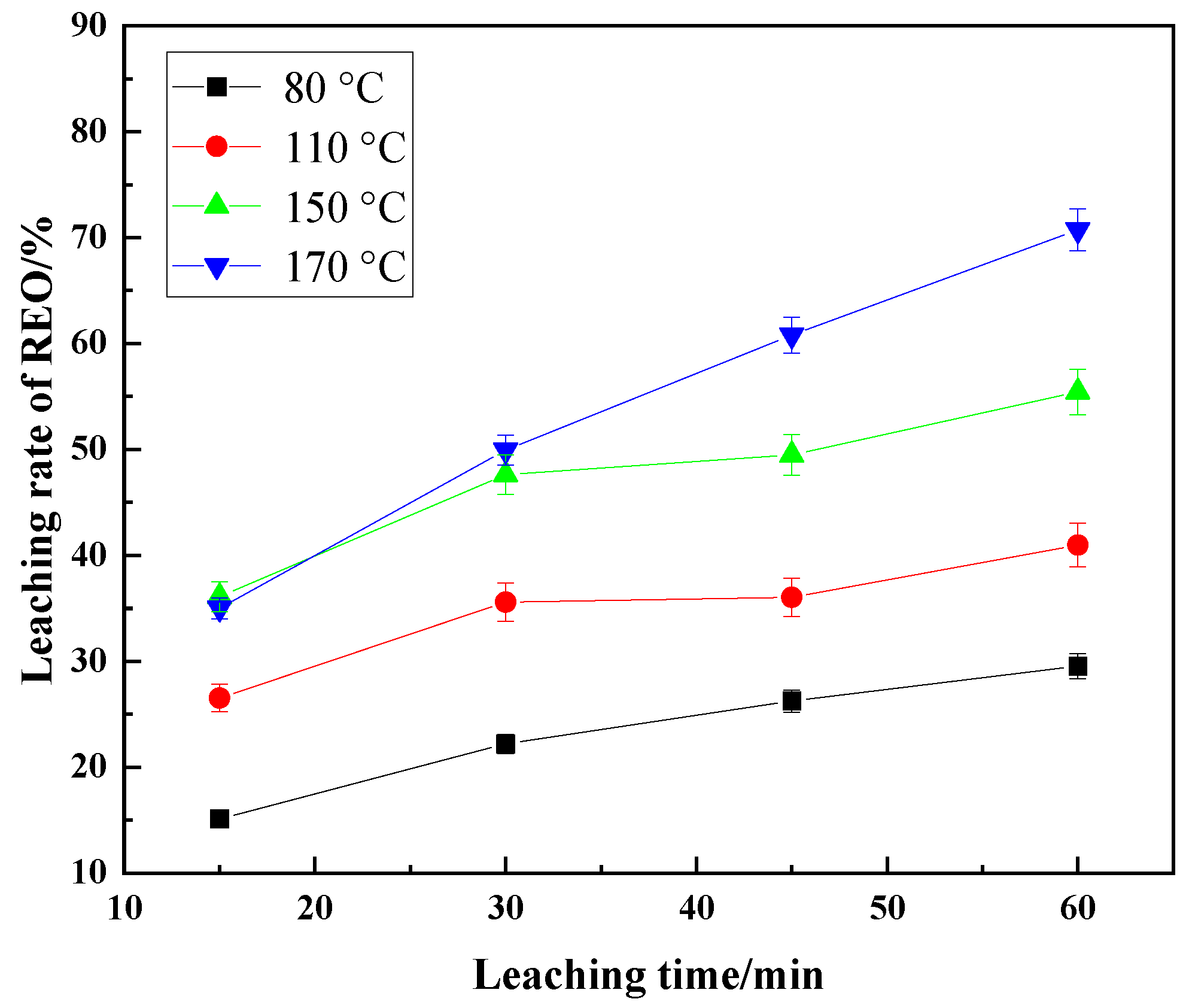
3.2. Effect of Temperature on Leaching
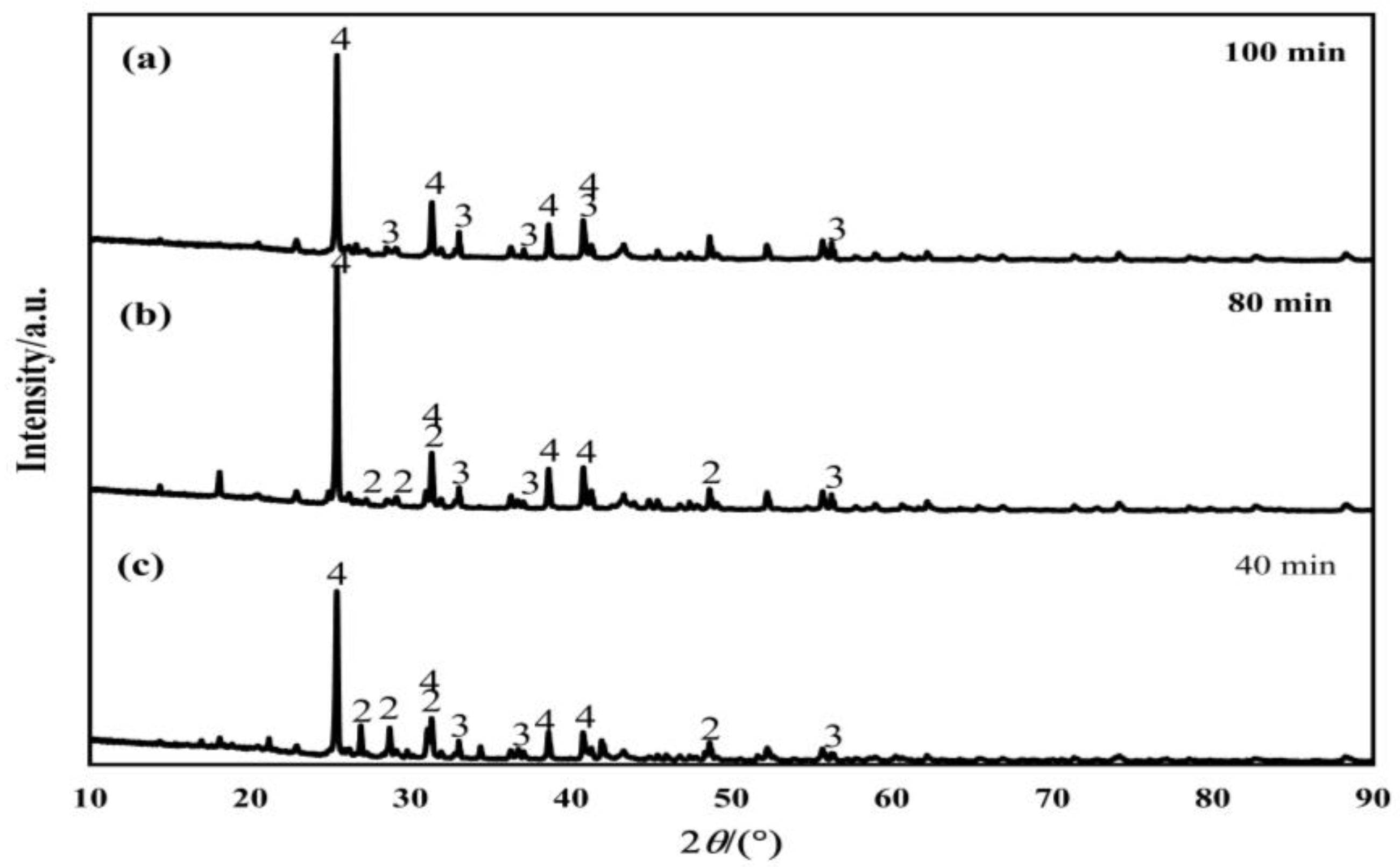
3.3. Effect of Leaching Time on Leaching Rate
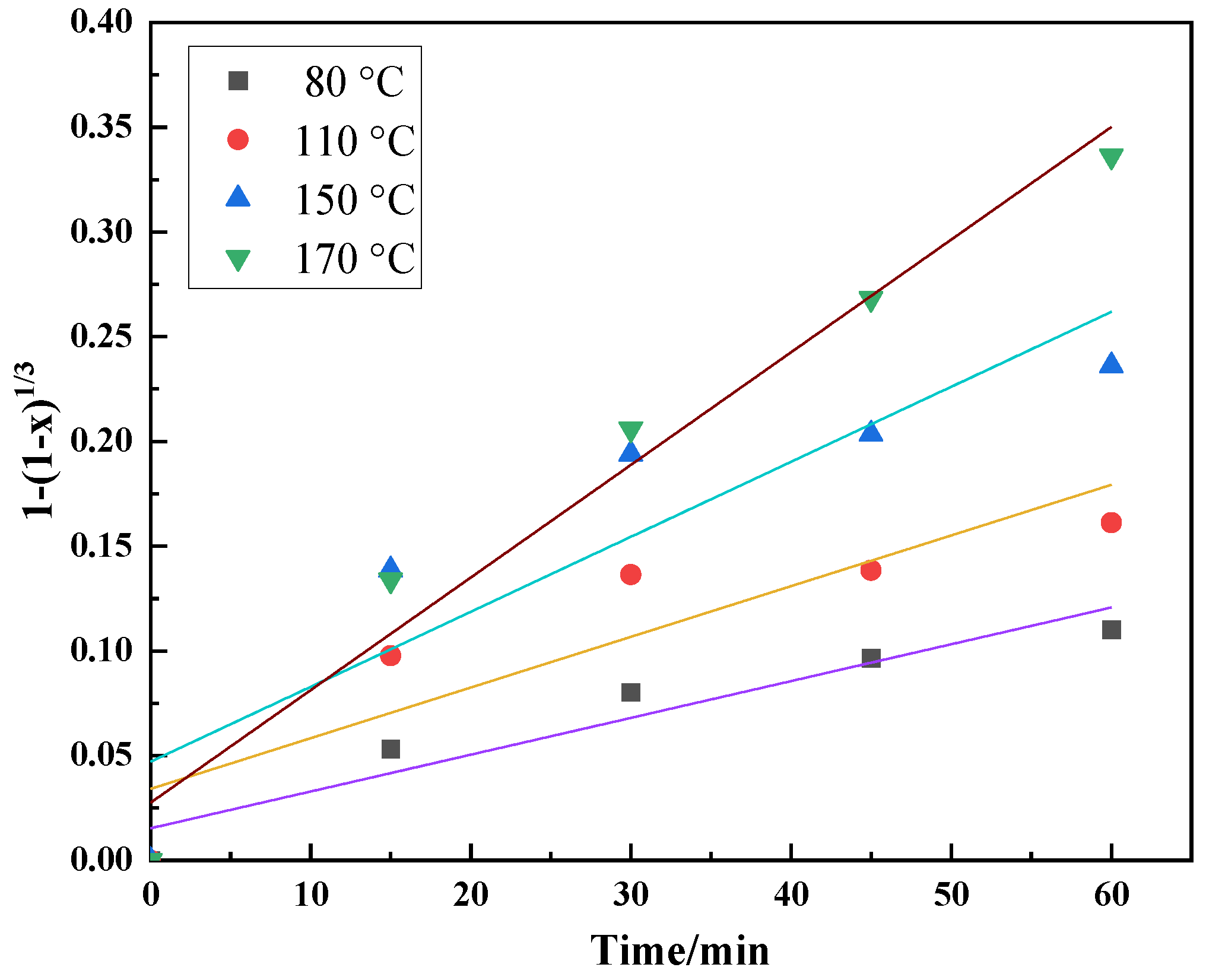
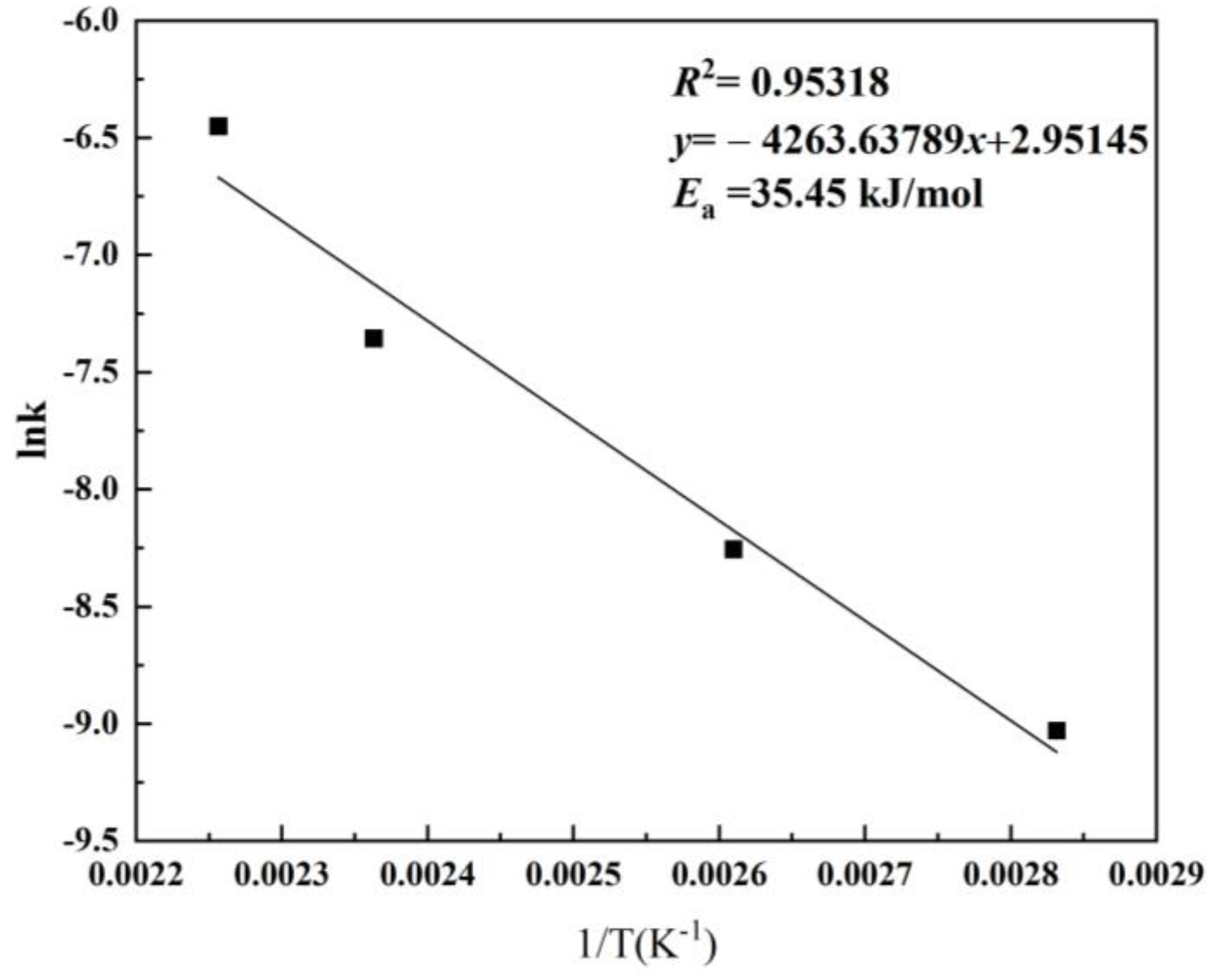
3.4. Kinetics of H2SO4 Leaching Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, H.R.; Yang, K.F.; Hu, F.F.; Liu, S.; Wang, K.Y. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Tian, P.F.; Yang, X.Y.; Yuan, W.M. Formation and preservation of the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia: Insight from evidences of petrogenesis, geochemistry and apatite fission track dating. Solid Earth Sci. 2021, 6, 228–245. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ling, M.X.; Williams, I.S.; Yang, X.Y.; Wang, C.Y.; Sun, W.D. The formation of the giant Bayan Obo REE-Nb-Fe deposit, North China, Mesoproterozoic carbonatite and overprinted Paleozoic dolomitization. Ore Geol. Rev. 2018, 92, 73–83. [Google Scholar] [CrossRef]
- Drew, J.; Meng, Q.R.; Sun, W.J.; Lithos. The Bayan Obo iron-rare-earth-niobium deposits, Inner Mongolia, China. Lawrence 1990, 26, 43–65. [Google Scholar] [CrossRef]
- Ma, P.Q.; Gao, Y.S.; Xu, Z.L. Comprehensive Utilization and Environmental Protection of Baotou Baiyunbo Resources. Decis.-Mak. Advis. Newsl. China 2009, 20, 88–91. [Google Scholar]
- Cheng, S.K.; Li, W.B.; Han, Y.X.; Sun, Y.S.; Gao, P.; Zhang, X.L. Recent process developments in beneficiation and metallurgy of rare earths: A review. J. Rare Earth 2023, 33, 1–12. [Google Scholar] [CrossRef]
- Nebeal, F.; Rahul, R.; James, T.; Suresh, B.; Scott, M.; Mark, I.P. Application of ferrous pyrometallurgy to the beneficiation of rare earth bearing iron ores-A review. Miner. Eng. 2017, 110, 20–30. [Google Scholar]
- Wang, L.; Wang, P.; Chen, W.Q.; Wang, Q.Q.; Lu, H.S. Environmental impacts of scandium oxide production from rare earths tailings of Bayan Obo Mine. J. Clean. Prod. 2020, 270, 122464. [Google Scholar] [CrossRef]
- Li, W.B.; Chen, J.J.; Zhou, W.T.; Han, Y.X.; Shan, Y. Effect of bastnaesite as reductant on hematite reduction during in-situ suspension magnetization roasting of refractory iron ore under neutral atmosphere. Int. J. Min. Sci. Techno. 2022, 32, 877–886. [Google Scholar] [CrossRef]
- Li, L.C. Extraction and Separation of Rare Earths; Inner Mongolia Science & Technology Press: Chifeng, China, 2011; pp. 20–25. [Google Scholar]
- Ma, R.F.; Li, J.F.; Zhang, X.W.; Jia, P.J.; Liu, Z.G.; Wu, J.X.; Feng, F.S.; Xin, W.B. Decomposition of monazite in Bayan Obo rare earth ore by roasting of Na2CO3. Pellets. J. Rare Earth. 2023, 33, 10–21. [Google Scholar] [CrossRef]
- Zhao, J.; Li, B.; Wei, X.L. Dry processing technology of exhaust gas emitted by roasting of rare earth concentrates with concentrated sulfuric acid. J. Clean. Prod. 2021, 327, 129489. [Google Scholar] [CrossRef]
- Xu, G.X. Rare Earths; Metallurgical Industry Press: Beijing, China, 1995; p. 46. [Google Scholar]
- Wang, M.; Huang, X.W.; Feng, Z.Y.; Long, Z.Q.; Peng, X.L. Progress and trend of green technology in hydrometallurgy separation of Baotou Mixed rare earth concentrate. Chin. J. Rare Met. 2019, 43, 1131–1141. [Google Scholar]
- He, F.; Luo, W.; Wang, G.H.; Zhu, Z.H.; Dai, Y.D. The migration of U, Th, Ra during the smelting process of rare earth. Chin. Rare Earths 2018, 39, 32–39. [Google Scholar]
- Li, N.; Ren, C.H.; Hou, L.M.; Jiao, K.L.; Wu, W.F. Effects and mechanism of Fe loading and sulfuric acid concentration on selective catalytic activity of SO42−-CeCO3F-CePO4. J. Taiwan Inst. Chem. E. 2023, 152, 105164. [Google Scholar] [CrossRef]
- John, D.; Elizabeth, H.; Karin, S.; Gamini, S.; Inna, K.; Gamini, S. Beneficial effect of iron oxide/hydroxide minerals on sulfuric acid baking and leaching of monazite. Hydrometallurgy 2022, 211, 105864. [Google Scholar]
- Xu, Y.H.; Liu, H.J.; Meng, Z.J.; Cui, J.G.; Zhao, W.Y.; Li, L.C. Decomposition of bastnasite and monazite mixed rare earth minerals calcined by alkali liquid. J. Rare Earths 2012, 30, 155–158. [Google Scholar] [CrossRef]
- Feng, X.L.; Long, Z.Q.; Cui, D.L.; Wang, L.S.; Huang, X.W.; Zhang, G.C. Kinetics of rare earth leaching from roasted ore of bastnaesite with sulfuric acid. Trans. Nonferrous Met. Soc. China 2013, 23, 849–854. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Top, S.; Hussaini, S.; Gokcen, H.S.; Kaya, M. Extraction of lanthanum and cerium from a bastnasite ore by direct acidic leaching. Bilimsel Madencilik Derg. 2020, 59, 58–92. [Google Scholar] [CrossRef]
- John, D.; Elizabeth, H.; Karin, S.; Gamini, S. The sulfuric acid bake and leach route for processing of rare earth ores and concentrates: A review. Hydrometallurgy 2019, 188, 123–139. [Google Scholar]
- John, D.; Elizabeth, H.; Karin, S.; Gamini, S. Sulfuric acid baking and leaching of rare earth elements, thorium and phosphate from a monazite concentrate: Effect of bake temperature from 200 to 800 °C. Hydrometallurgy 2018, 179, 254–267. [Google Scholar]
- Ariuntuya, B.; Altansukh, B.; Ariunbolor, N.; Kazutoshi, H.; Yasushi, W.; Atsushi, S. Recovery of light and heavy rare earth elements from apatite ore using sulphuric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2018, 179, 100–109. [Google Scholar]
- Liu, J.; Dou, Z.H.; Zhang, T.A. Leaching of rare earths from mechanochemically decomposed bastnaesite. Miner. Eng. 2020, 145, 106052. [Google Scholar] [CrossRef]
- Philippe, R.; Gauthier, J.P.D.; Simon, B.B.; Laetitia, L.; Ludovic, D.; Cédric, M.; Bruno, C.; Valérie, W.; Denis, B. Leaching of niobium and REE-bearing iron ores: Significant reduction of H2SO4 consumption using SO2 and activated carbon. Sep. Purif. Technol. 2017, 189, 1–10. [Google Scholar]
- Li, M.; Li, J.F.; Zhang, D.L.; Gao, K.; Wang, H.H.; Xu, W.; Geng, J.L.; Zhang, X.Y.; Ma, X.F. Decomposition of mixed rare earth concentrate by NaOH roasting and kinetics of hydrochloric acid leaching process. J. Rare Earth 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, J.M.; Li, M.; Fan, H.L.; Yang, Q.S. Decomposition reaction kinetics of Baotou RE concentrate with concentrated sulfuric acid at low temperature. Rare Met. 2010, 29, 121–125. [Google Scholar] [CrossRef]
- Zhang, J.; Edwards, C. Mineral decomposition and leaching processes for treating rare earth ore concentrates. Can. Metall. Q. 2013, 52, 243–248. [Google Scholar] [CrossRef]
- Shao, D.W.; Song, J.; Du, X.B.; Deng, Y.C.; Xue, Z.Y.; Yu, H.D.; Qi, T. Leaching characteristics and kinetics of scandium from Sc-concentrate of Bayan Obo rare earth tailings in sulfuric acid solution. J. Environ. Chem. Eng. 2023, 11, 111037. [Google Scholar] [CrossRef]
- Markku, L.; Irina, M.; Tuomo, S.; Eveliina, R. Selective acid leaching of rare earth elements from roasted NdFeB magnets. Sep. Purif. Technol. 2021, 278, 119571. [Google Scholar]
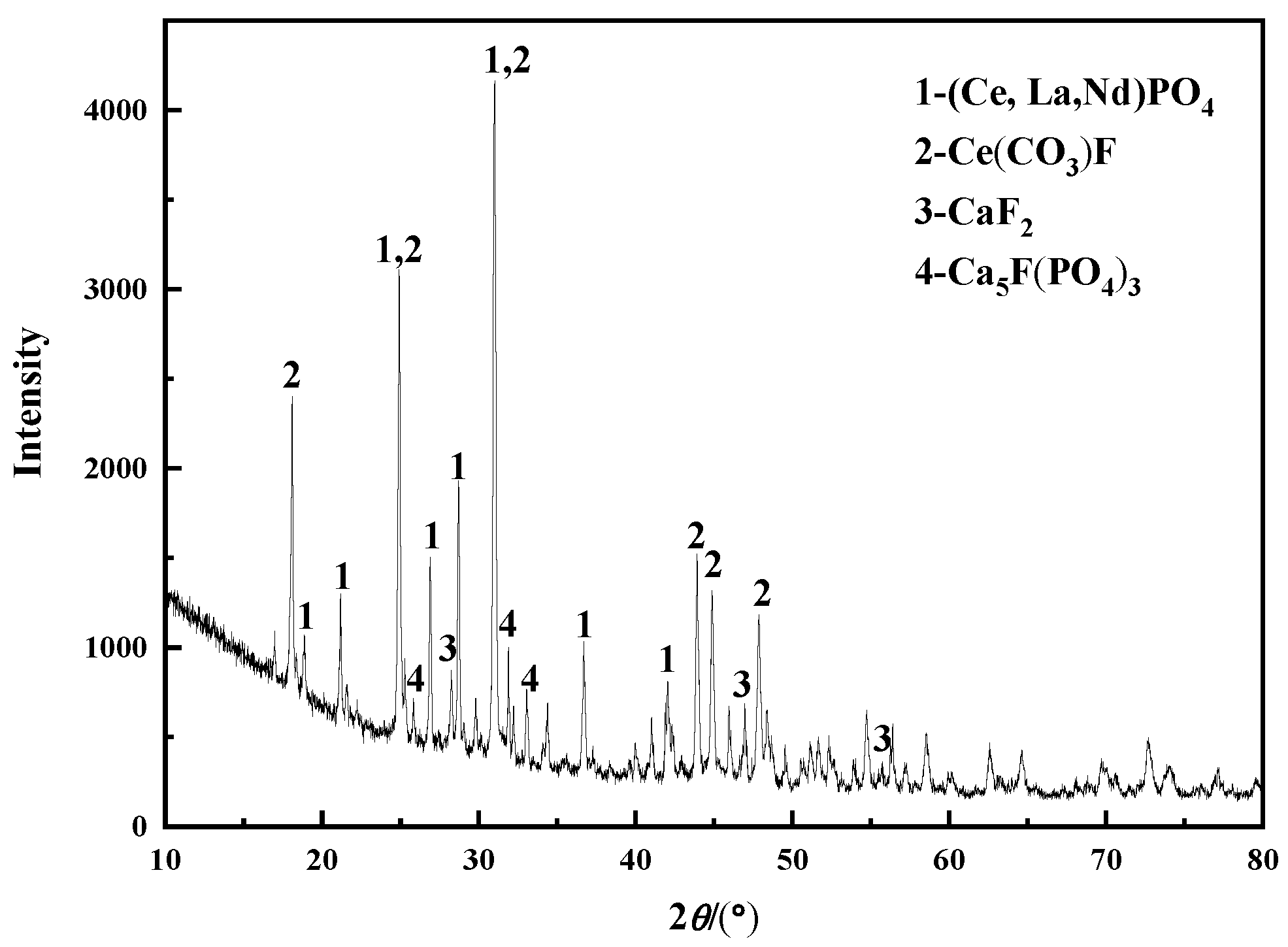
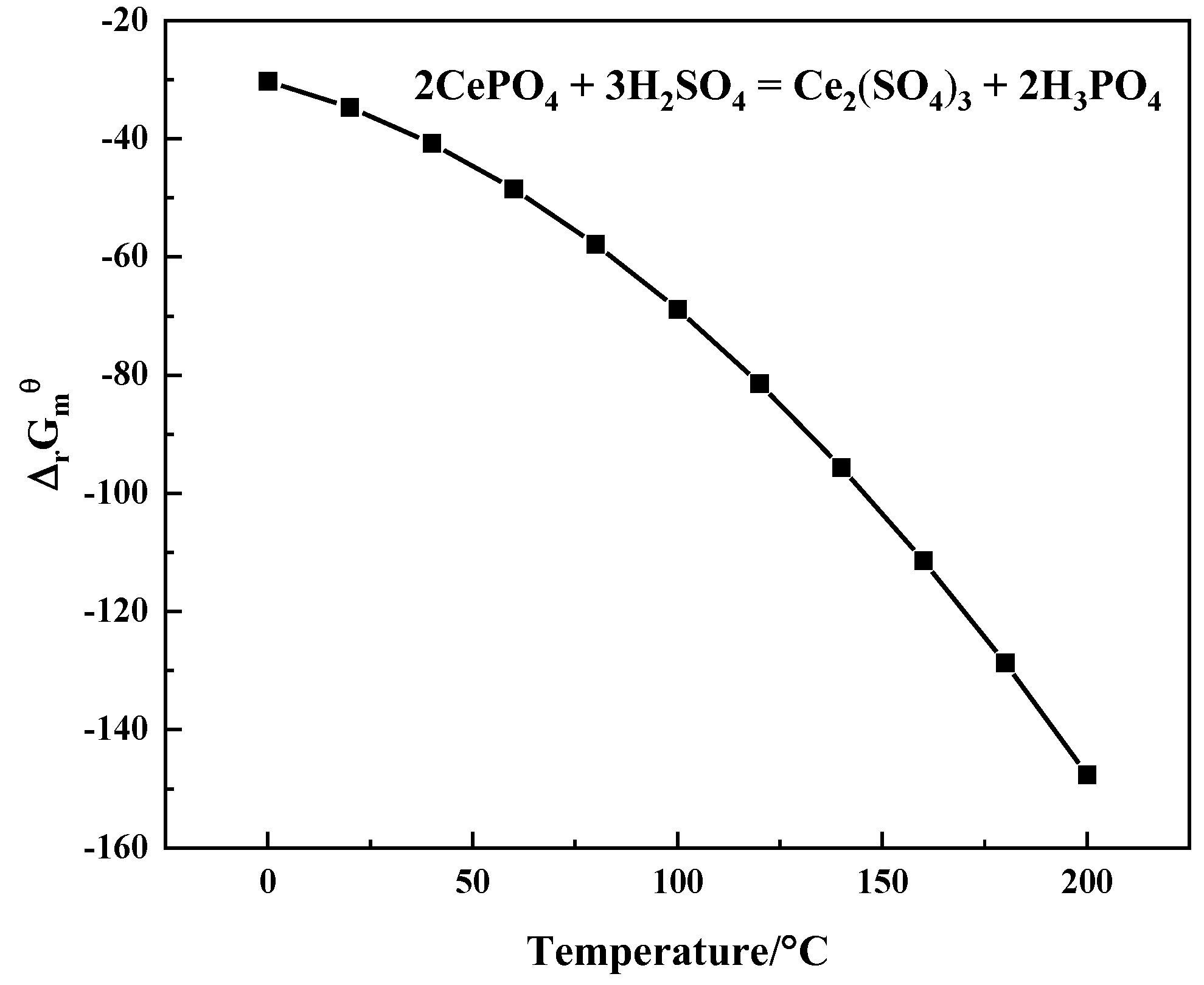
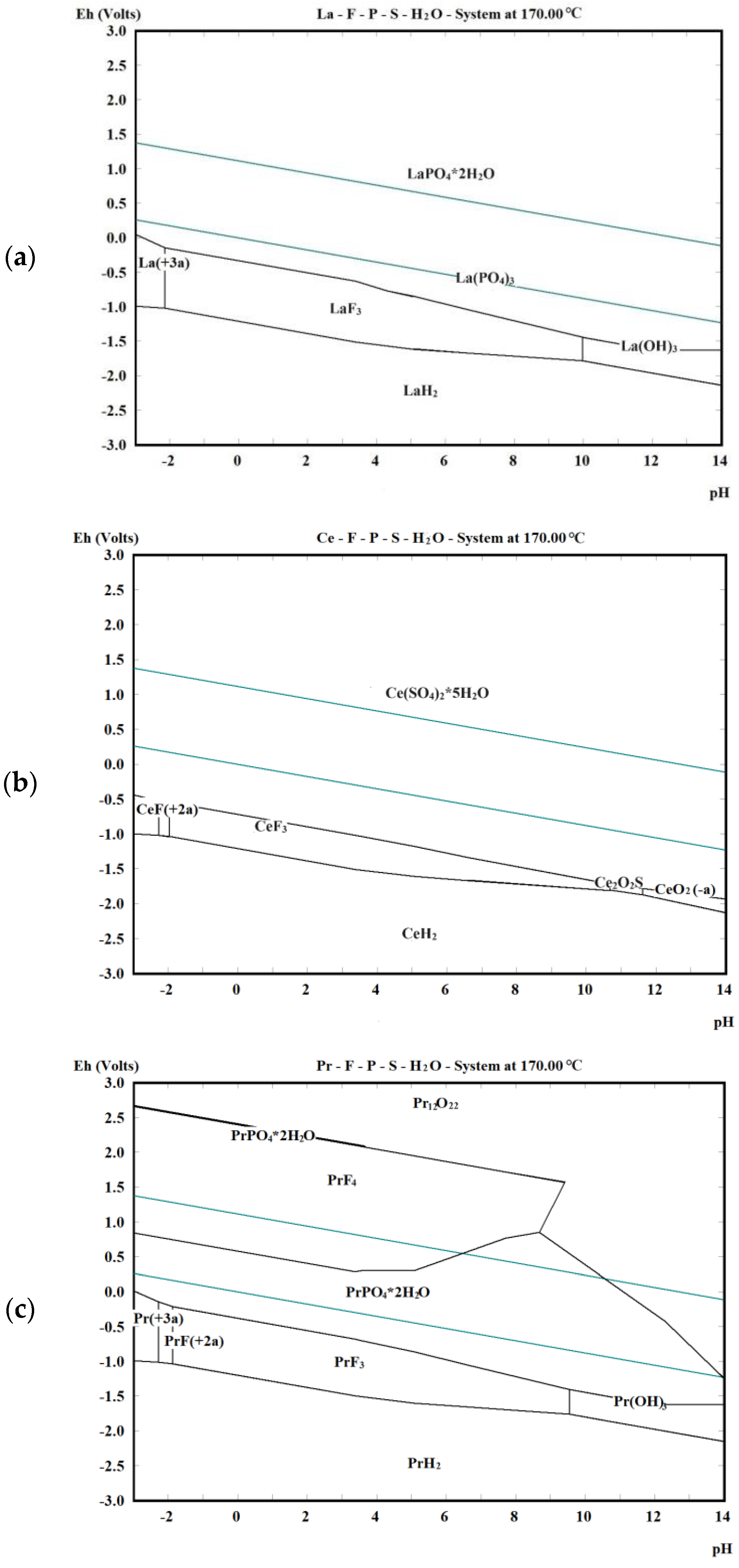
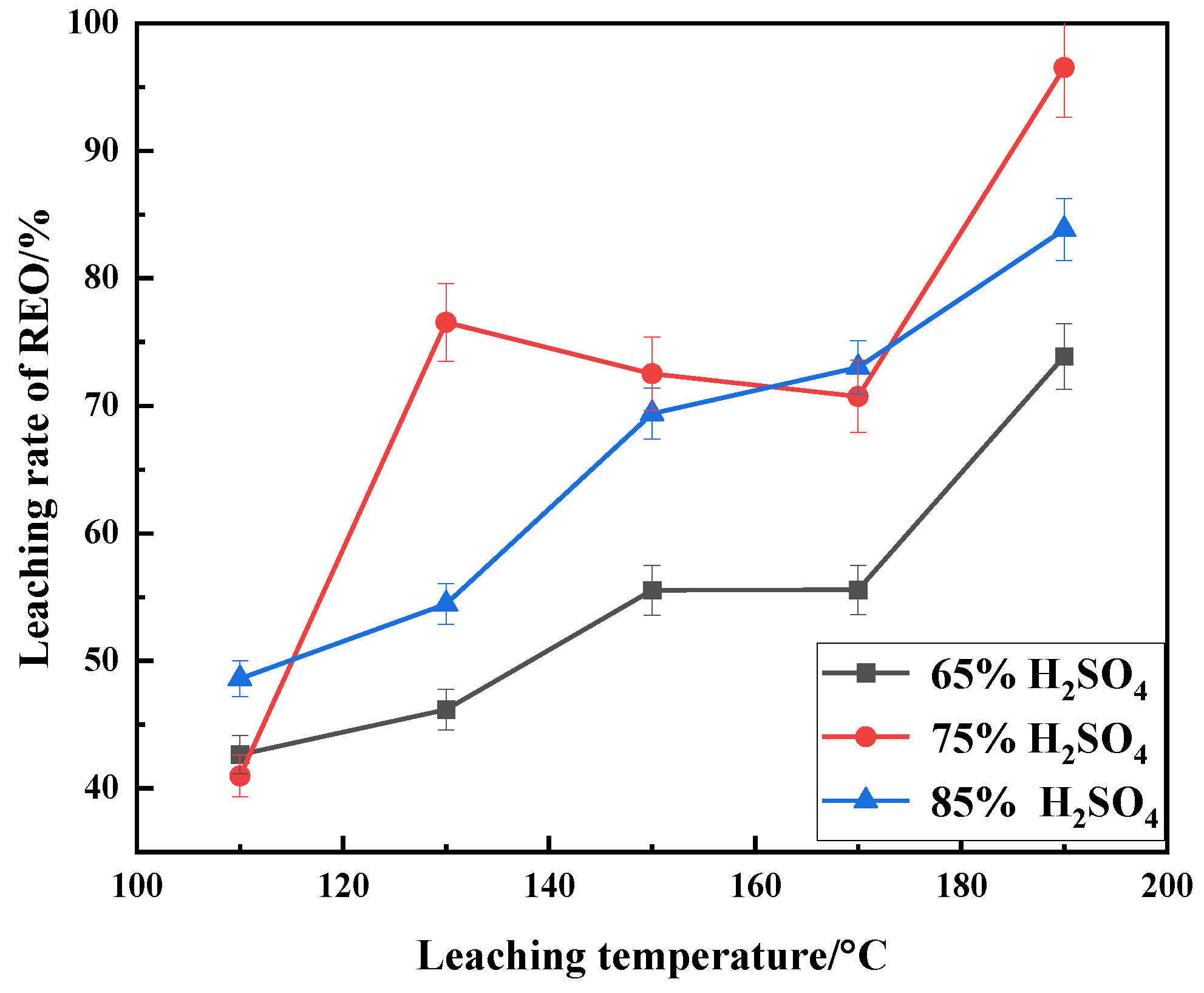

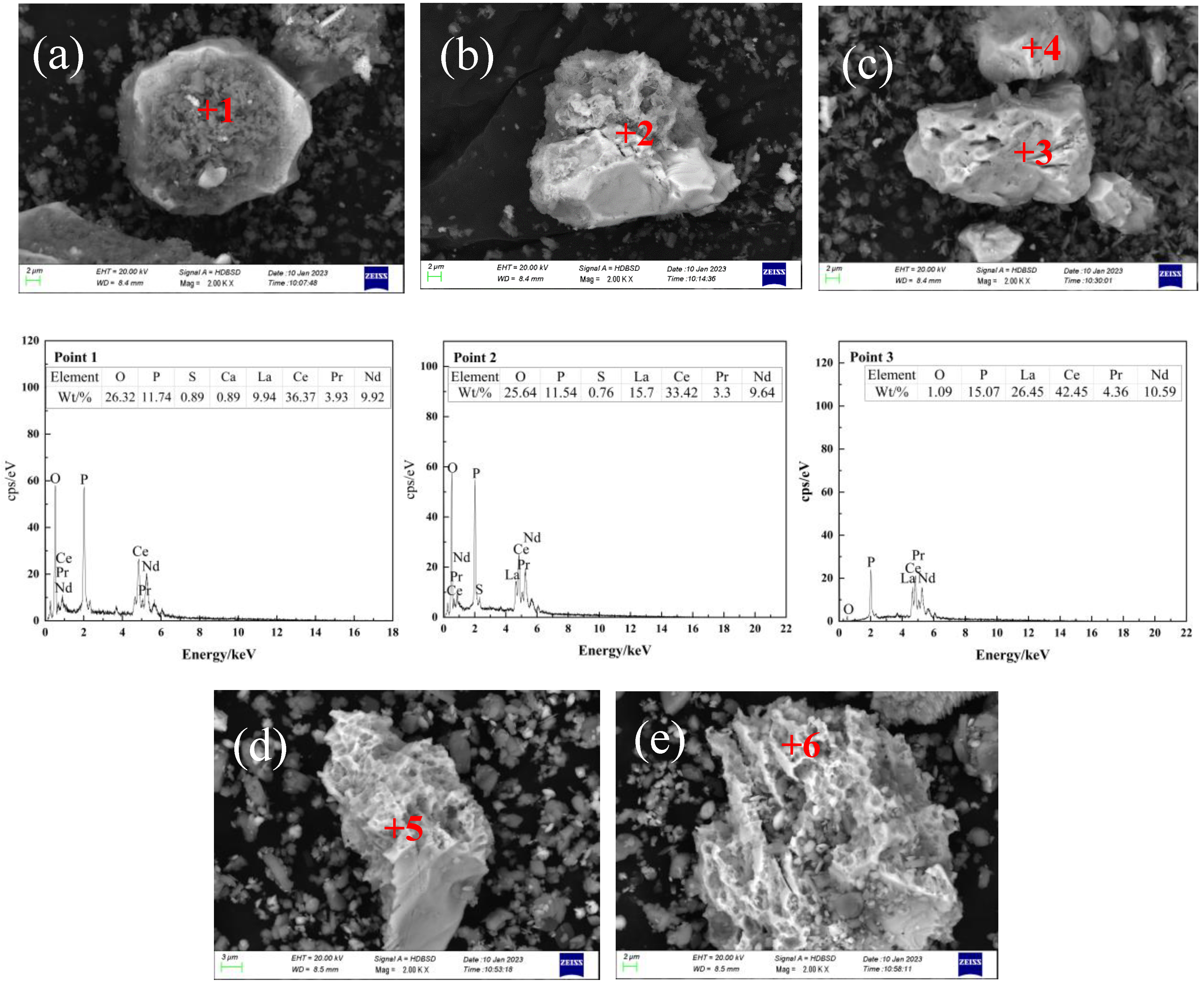
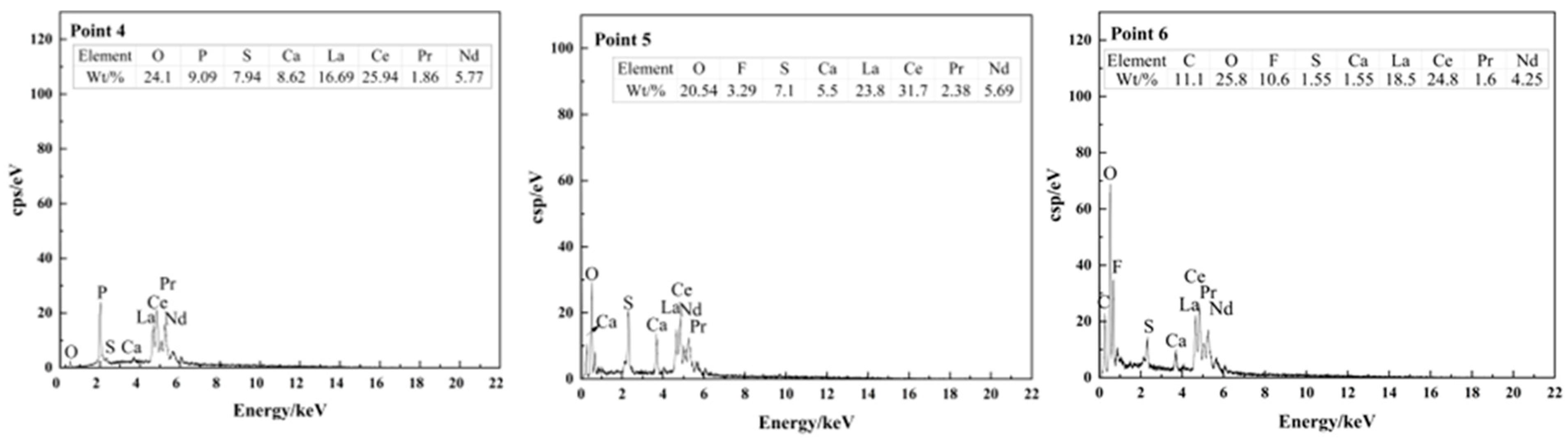






| Composition | Na2O | K2O | MgO | CaO | BaO | MnO2 | SiO2 | TiO2 | ThO2 |
|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 0.29 | 0.010 | 0.18 | 8.59 | 0.94 | 0.13 | 0.69 | 0.11 | 0.21 |
| Al2O3 | Sc2O3 | REO | P2O5 | Nb2O5 | F | S | TFe | / | |
| Wt.% | 0.074 | <0.0050 | 59.05 | 12.92 | 0.054 | 5.88 | 1.66 | 2.77 | / |
| Y2O3 | La2O3 | Ce2O3 | Pr6O11 | Nd2O3 | Sm2O3 | Eu2O3 | Gd2O3 |
|---|---|---|---|---|---|---|---|
| 0.24 | 28.16 | 50.93 | 4.76 | 14.19 | 1.03 | 0.20 | 0.36 |
| Tb4O7 | Dy2O3 | Ho2O3 | Er2O3 | Tm2O3 | Yb2O3 | Lu2O3 | / |
| <0.10 | 0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Zhang, B.; Li, W.; Zhao, T.; Da, Z.; Liu, C. An Environmentally Friendly Sulfuric Acid Decomposition Strategy for Mixed Rare Earth Concentrate. Minerals 2024, 14, 185. https://doi.org/10.3390/min14020185
Hou S, Zhang B, Li W, Zhao T, Da Z, Liu C. An Environmentally Friendly Sulfuric Acid Decomposition Strategy for Mixed Rare Earth Concentrate. Minerals. 2024; 14(2):185. https://doi.org/10.3390/min14020185
Chicago/Turabian StyleHou, Shaochun, Bo Zhang, Wenjun Li, Tuo Zhao, Zongyang Da, and Chenghong Liu. 2024. "An Environmentally Friendly Sulfuric Acid Decomposition Strategy for Mixed Rare Earth Concentrate" Minerals 14, no. 2: 185. https://doi.org/10.3390/min14020185
APA StyleHou, S., Zhang, B., Li, W., Zhao, T., Da, Z., & Liu, C. (2024). An Environmentally Friendly Sulfuric Acid Decomposition Strategy for Mixed Rare Earth Concentrate. Minerals, 14(2), 185. https://doi.org/10.3390/min14020185








