Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Methods
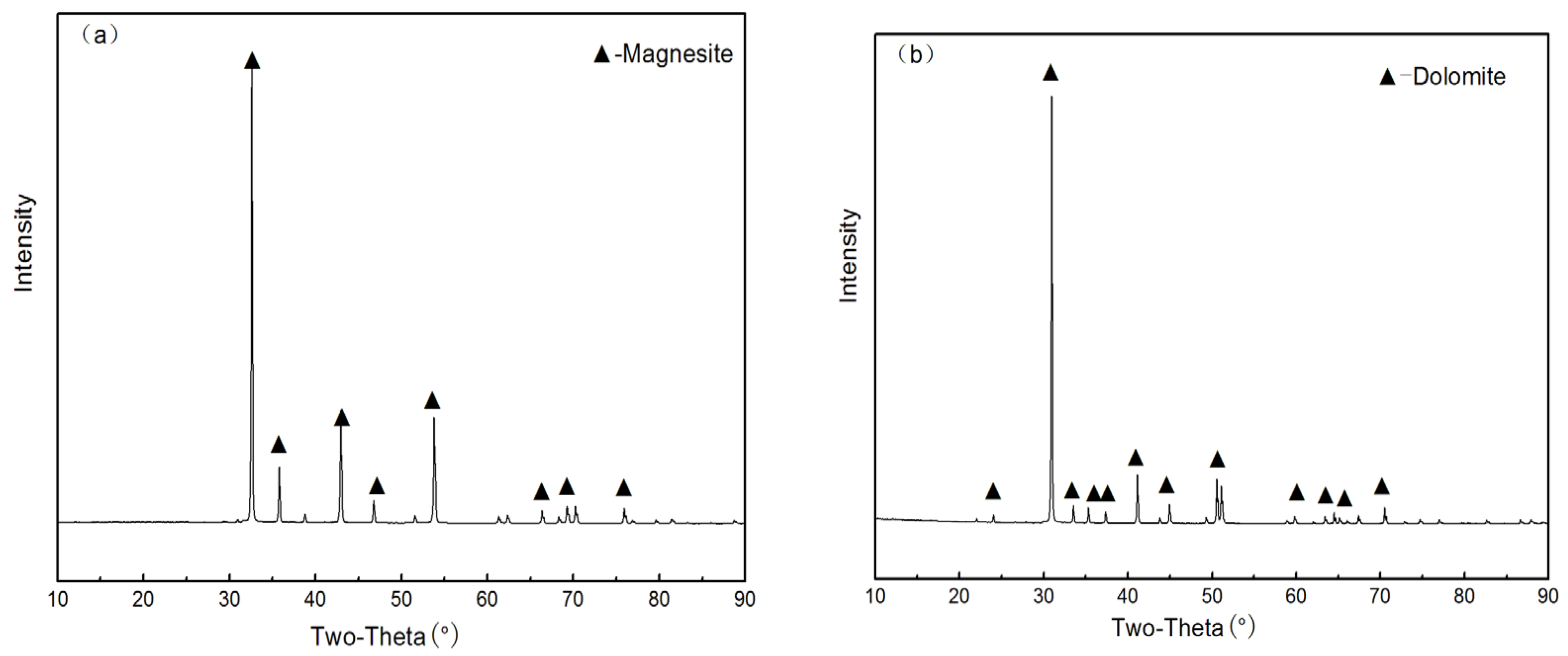
2.2.1. XRD Tests
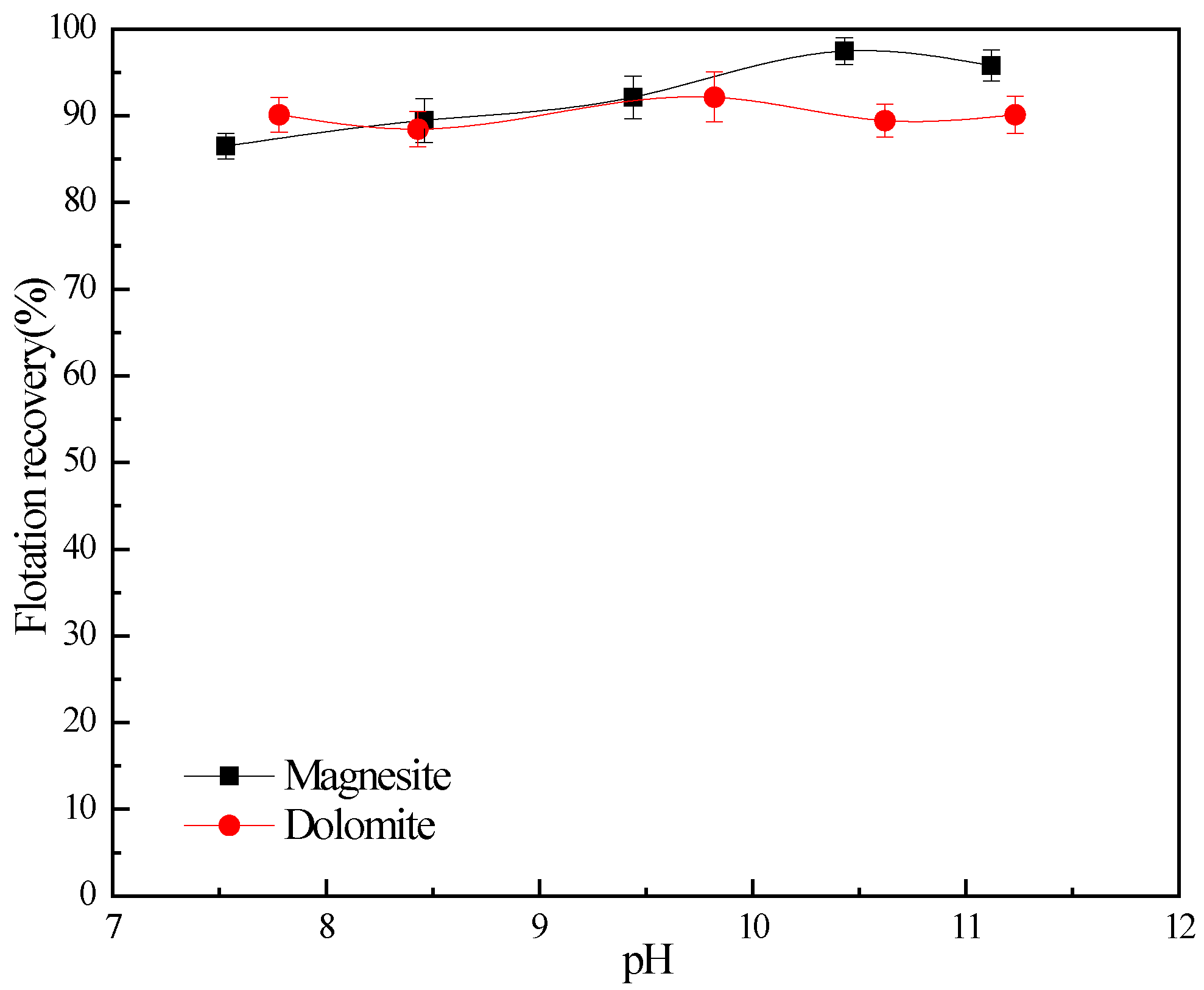
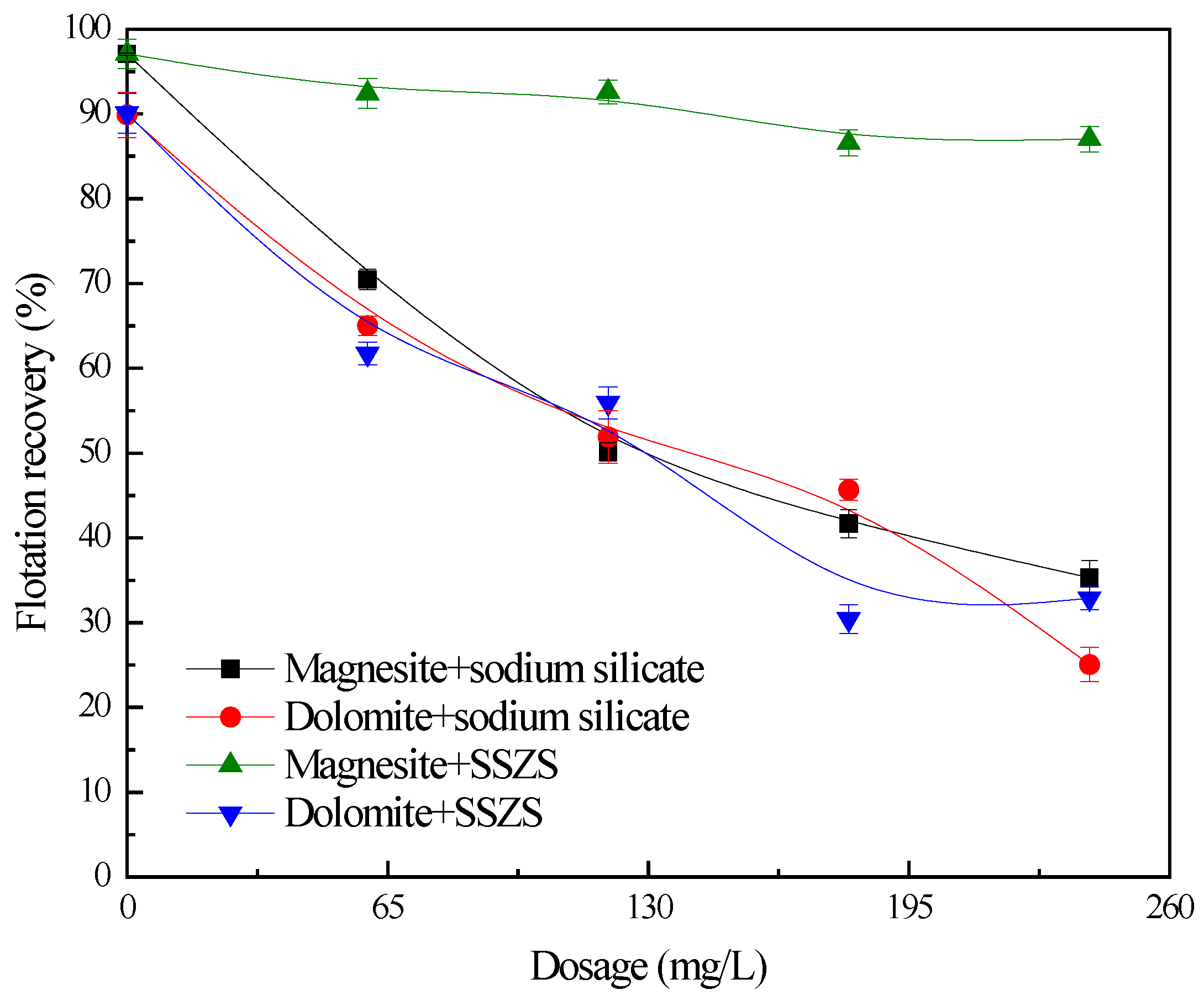
2.2.2. Flotation Tests
2.2.3. Zeta Potential Analysis
2.2.4. Contact Angle Measurements
2.2.5. FT-IR Spectra Analysis
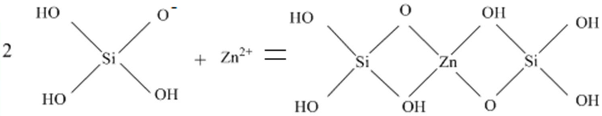
3. Results and Discussion
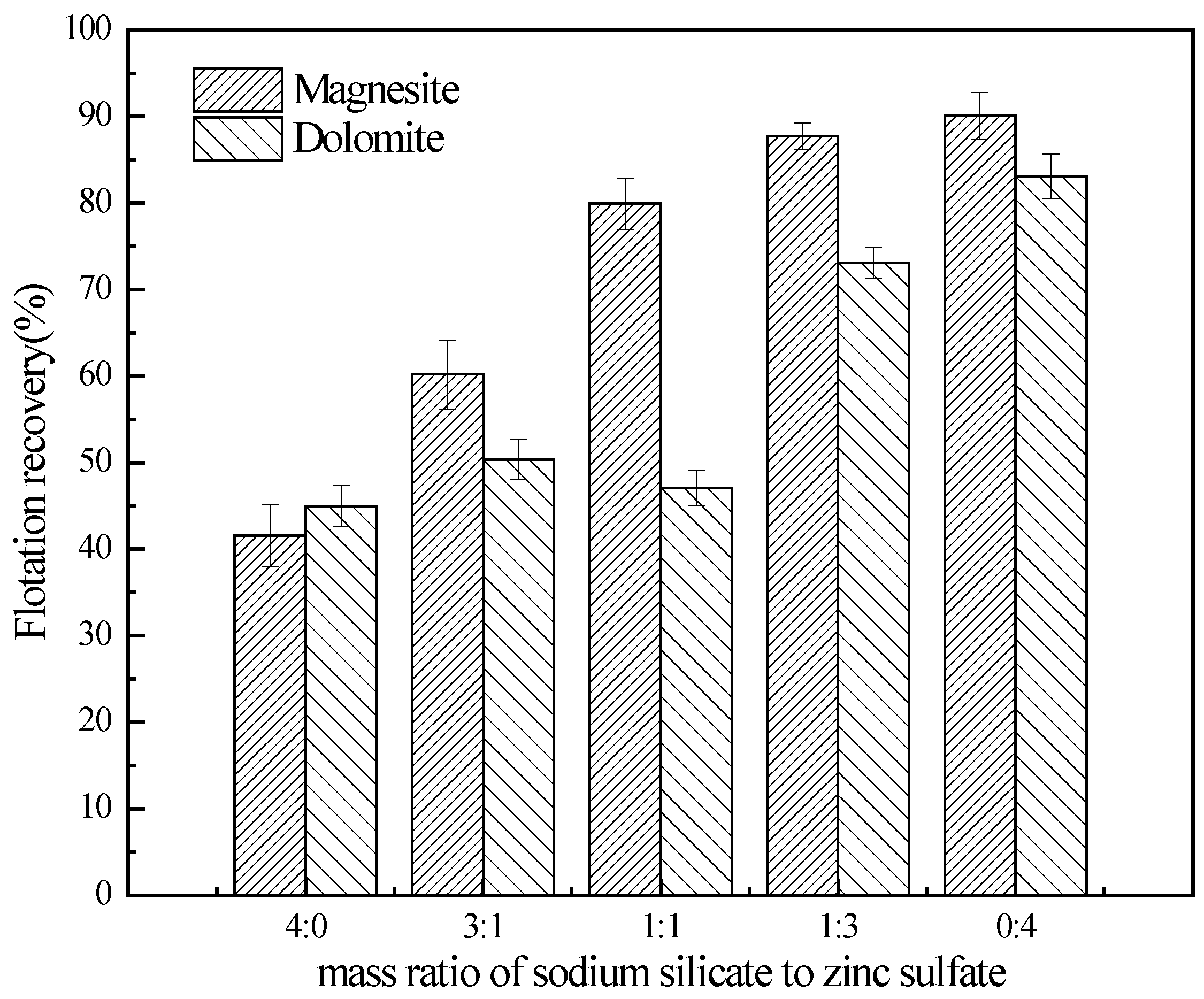
3.1. Flotation Studies
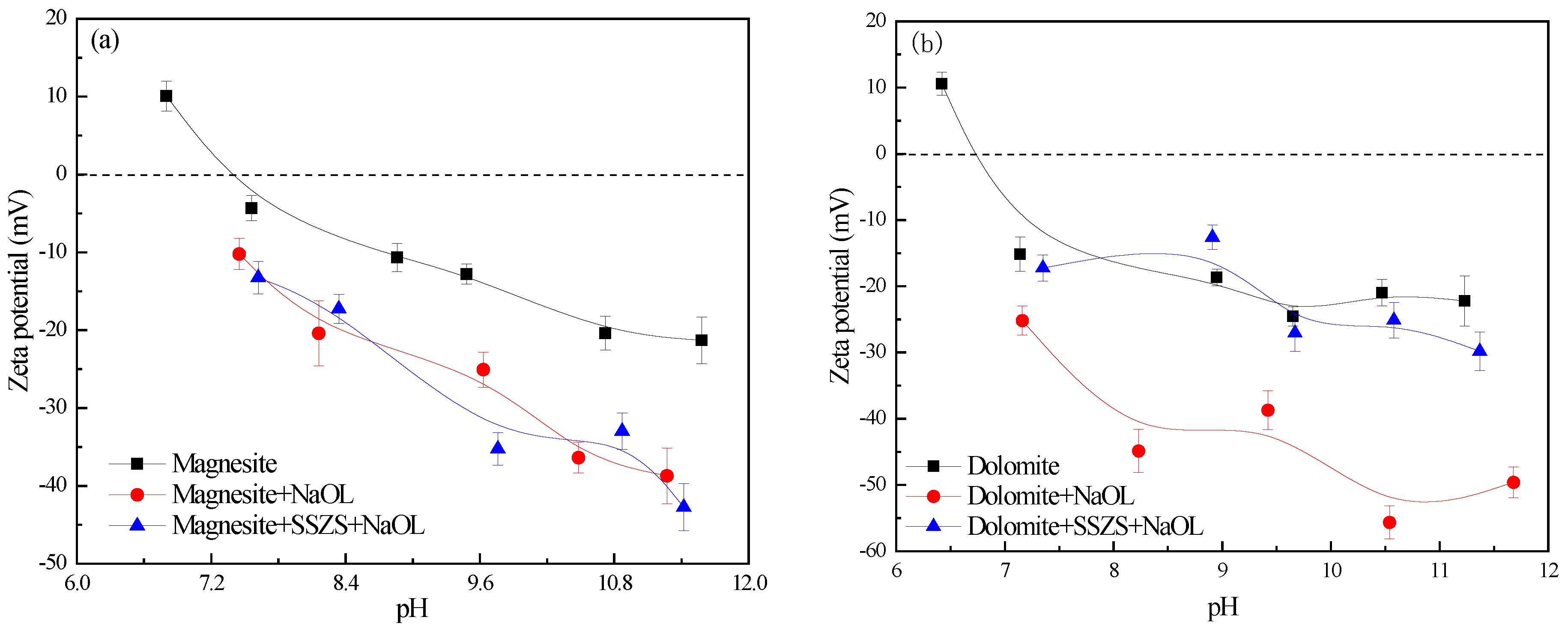
3.2. Zeta Potential Analysis
3.3. Contact Angle Measurements
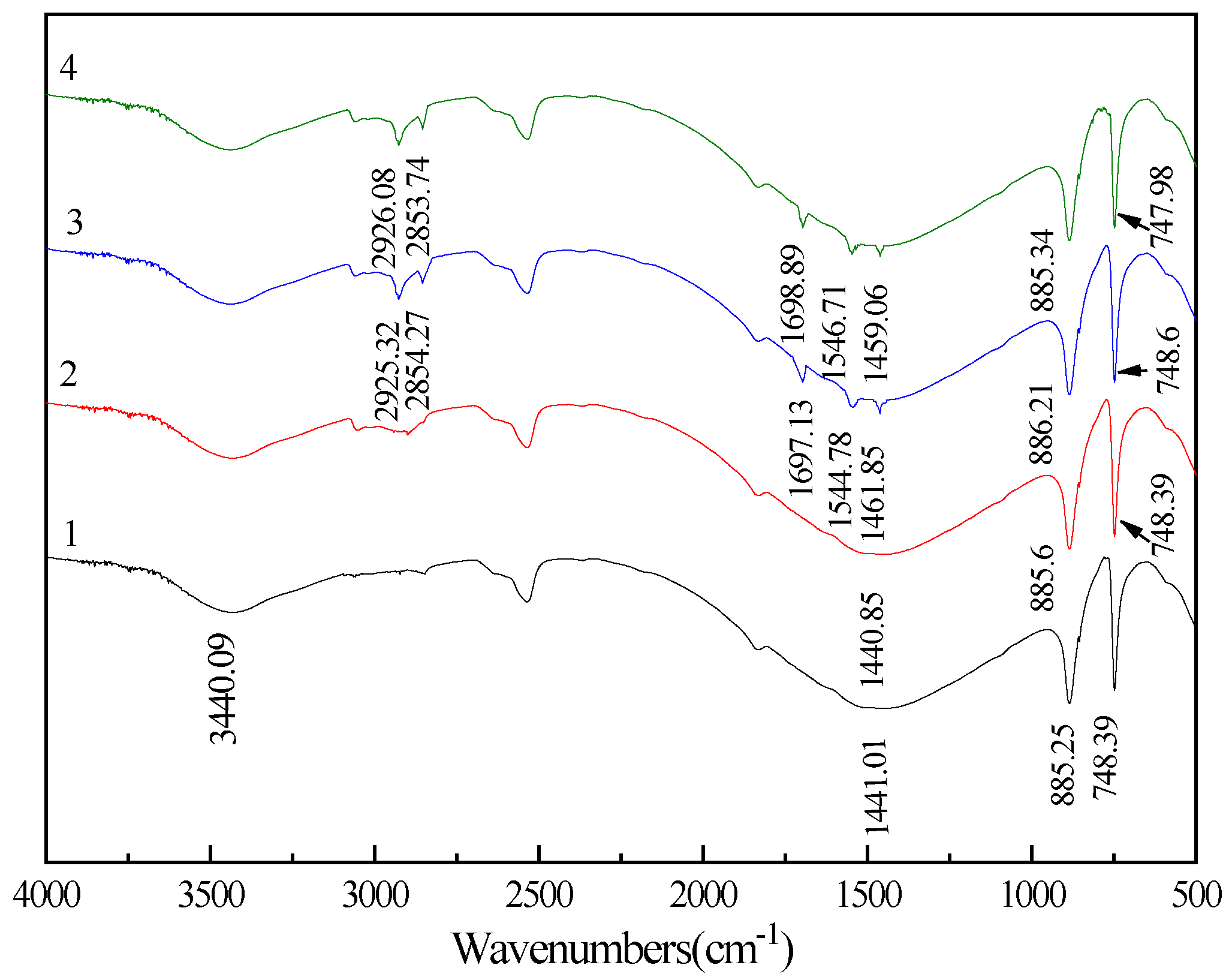
3.4. FT-IR Spectra Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tran, K.T.; Han, K.S.; Kim, S.J.; Kim, M.J.; Tran, T. Recovery of magnesium from Uyuni salar brine as hydrated magnesium carbonate. Hydrometallurgy 2016, 160, 106–114. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Hu, X.; Zhang, X.; Zheng, G. Flotation separation of quartz from magnesite using carboxymethyl cellulose as depressant. Trans. Nonferrous Met. Soc. China 2022, 32, 1623–1637. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research—A review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Zhong, W.; Yin, W.; Wang, Y.; Yao, J. Selective flotation of magnesite from dolomite using α-chloro-oleate acid as collector. Powder Technol. 2020, 373, 147–151. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Peng, X.; Sun, W. Effect and mechanism of depressant amino trimethylene phosphonic acid on flotation separation of magnesite and dolomite. Conserv. Util. Miner. Resour. 2022, 42, 91–99. [Google Scholar]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Luo, N.; Wei, D.; Shen, Y.; Han, C.; Zhang, C. Elimination of the adverse effect of calcium ion on the flotation separation of magnesite from dolomite. Minerals 2017, 7, 150. [Google Scholar] [CrossRef]
- Gence, N. Wetting behavior of magnesite and dolomite surfaces. Appl. Surf. Sci. 2006, 252, 3744–3750. [Google Scholar] [CrossRef]
- Yin, W.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Metall. Mater. 2020, 27, 571–583. [Google Scholar] [CrossRef]
- Sun, W.H.; Liu, W.G.; Dai, S.J.; Yang, T.; Duan, H.; Liu, W.B. Effect of Tween 80 on flotation separation of magnesite and dolomite using NaOL as the collector. J. Mol. Liq. 2020, 315, 113712. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Yin, W.; Chen, X. Influence of sodium phosphate salts with different chain length on the flotation behavior of magnesite and dolomite. Minerals 2020, 10, 1031. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.; Feng, Q.; Deng, H. Effect of solution chemistry on the flotation system of smithsonite and calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Sun, H.; Yin, W.; Yang, B.; Han, F. Simultaneous separation of quartz and dolomite from magnesite using monosodium phosphateas a regulator via reverse flotation. Miner. Eng. 2021, 172, 107185. [Google Scholar] [CrossRef]
- Sun, H.; Han, F.; Yin, W.; Hong, J.; Yang, B. Modification of selectivity in the flotation separation of magnesite from dolomite. Colloids Surf. A 2020, 606, 125460. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P. Anionic flotation of magnesium carbonates by modifiers. Int. J. Miner. Process. 1989, 25, 261–274. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.H.; Sun, W.; Zeng, X.B.; Fang, S.; Han, H.S.; Hong, K. Use of Al2(SO4)3 and acidiffed water glass as mixture depressants in flotation separation of fluorite from calcite and celestite. Miner. Eng. 2019, 137, 160–170. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J.; Zuo, W.; Ma, Y. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Sun, R.F.; Liu, D.; Liu, Y.B.; Wang, D.Q.; Wen, S.M. Pb-water glass as a depressant in the flotation separation of fluorite from calcite. Colloids Surf. A 2021, 629, 127447. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.L.; Zhang, M.; Cui, R.; Shi, J.; Ning, J.F. Effect of Zn2+ and its addition sequence on flotation separation of scheelite from calcite using water glass. Colloids Surf. A 2020, 588, 124394. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Luo, D.; Zeng, Y. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation. Miner. Eng. 2018, 121, 31–38. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.M.; Forssberg, E. Mechanism of oleate interaction on salt-type minerals, part II. Adsorption and electrokinetic studies of apatite in the presence of sodium oleate and sodium metasilicate. Int. J. Miner. Process. 1990, 28, 59–79. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.G.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Qi, G.W.; Parentich, A.; Little, L.H.; Warren, L.J. Selective flotation of apatite from iron oxides. Int. J. Miner. Process. 1992, 34, 83–102. [Google Scholar]
- Ding, K.; Laskowski, J.S. Application of a modified water glass in a cationic flotation of calcite and dolomite. Can. Metall. Quart. 2006, 45, 199–206. [Google Scholar] [CrossRef]
- Jin, J.; Gao, H.; Chen, X.; Peng, Y. The separation of kyanite from quartz by flotation at acidic pH. Miner. Eng. 2016, 92, 221–228. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Zhou, Q.; Lu, S. Acidized sodium silicate-an effective modifier in fluorite flotation. Miner. Eng. 1992, 5, 435–444. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, W.G.; Chen, X.D.; Wang, Z.H.; Liu, W.B.; Sun, W.H.; Shen, Y.B. The role of sodium tripolyphosphate in wet grinding process of magnesite. Colloids Surf. A 2023, 668, 131449. [Google Scholar] [CrossRef]
- Cao, Z.F.; Chen, P.; Yang, F.; Wang, S.; Zhong, H. Transforming structure of dolomite to enhance its ion-exchange capacity for copper(II). Colloids Surf. A 2018, 593, 201–208. [Google Scholar] [CrossRef]
- Sun, H.R.; Yin, W.Z. Selective flotation separation of magnesite from quartz by palmitoyl trimethylammonium chloride. Sep. Purif. Technol. 2022, 295, 121201. [Google Scholar] [CrossRef]
- Wright, K.; Cygan, R.T.; Slater, B. Structure of the (1014) surfaces of calcite, dolomite, and magnesite under wet and dry conditions. Phys. Chem. Chem. Phys. 2001, 3, 839–844. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, H.Q.; Xu, Y.B.; Shu, K.Q.; Fang, S.; Xu, L.H. The effect of dissolved calcite species on the flotation of bastnaesite using sodium oleate. Miner. Eng. 2020, 145, 106095. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G. Electrokinetic and flotation behaviors of hemimorphite in the presence of sodium oleate. Miner. Eng. 2015, 84, 74–76. [Google Scholar] [CrossRef]
- Solotchina, È.P.; Solotchin, P.A. Composition and structure of low-temperature natural carbonates of the calcite-dolomite series. J. Struct.Chem. 2014, 55, 779–785. [Google Scholar] [CrossRef]
- El-Nahrawy, A.M.; Ali, A.I.; Abou Hammad, A.B.; Youssef, A.M. Influences of Ag-NPs doping chitosan/calcium silicate nanocomposites for optical and antibacterial activity. Int. J. Biol. Macromol. 2016, 93, 267–275. [Google Scholar] [CrossRef]
- Spiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnasite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Liao, R.P.; Wen, S.M.; Liu, J.; Zuo, Q.; Zheng, Y.X.; Luo, D.Q. Flotation behavior and mechanism of ilmenite using ferrate as activator. Miner. Eng. 2022, 178, 107400. [Google Scholar] [CrossRef]

| Samples | MgO | CaO | SiO2 | Al2O3 | FeO |
|---|---|---|---|---|---|
| Magnesite | 47.24 | 0.17 | 0.19 | / | 0.17 |
| Dolomite | 21.52 | 30.13 | 0.21 | 0.10 | / |
| SSZS Dosage (mg/L) | Product | Weight Recovery (%) | Magnesite | Dolomite | ||
|---|---|---|---|---|---|---|
| Grade (%) | Recovery (%) | Grade (%) | Recovery (%) | |||
| 0 | concentrate | 97.45 | 49.85 | 97.43 | 50.15 | 97.46 |
| tailing | 2.55 | 50.19 | 2.57 | 49.81 | 2.54 | |
| raw ore | 100 | 49.86 | 100 | 50.14 | 100 | |
| 120 | concentrate | 68.12 | 62.13 | 83.63 | 37.87 | 52.23 |
| tailing | 31.88 | 25.99 | 16.37 | 74.01 | 47.77 | |
| raw ore | 100 | 50.61 | 100 | 49.39 | 100 | |
| 180 | concentrate | 59.13 | 75.21 | 87.99 | 24.79 | 29.64 |
| tailing | 40.87 | 14.85 | 12.01 | 85.15 | 70.36 | |
| raw ore | 100 | 50.54 | 100 | 49.46 | 100 | |
| Test System | Magnesite | Dolomite |
|---|---|---|
| Without reagent | 34.8° | 31.5° |
| With NaOL | 68.8° | 60.2° |
| With SSZS + NaOL | 67.4° | 33.5° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, N.; Shi, J.; Yan, B.; Wang, X. Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant. Minerals 2024, 14, 355. https://doi.org/10.3390/min14040355
Luo N, Shi J, Yan B, Wang X. Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant. Minerals. 2024; 14(4):355. https://doi.org/10.3390/min14040355
Chicago/Turabian StyleLuo, Na, Jingyang Shi, Baobao Yan, and Xiaoping Wang. 2024. "Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant" Minerals 14, no. 4: 355. https://doi.org/10.3390/min14040355






