Efficient Extraction of Lithium from Calcined Kaolin Lithium Clay with Dilute Sulfuric Acid
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussion
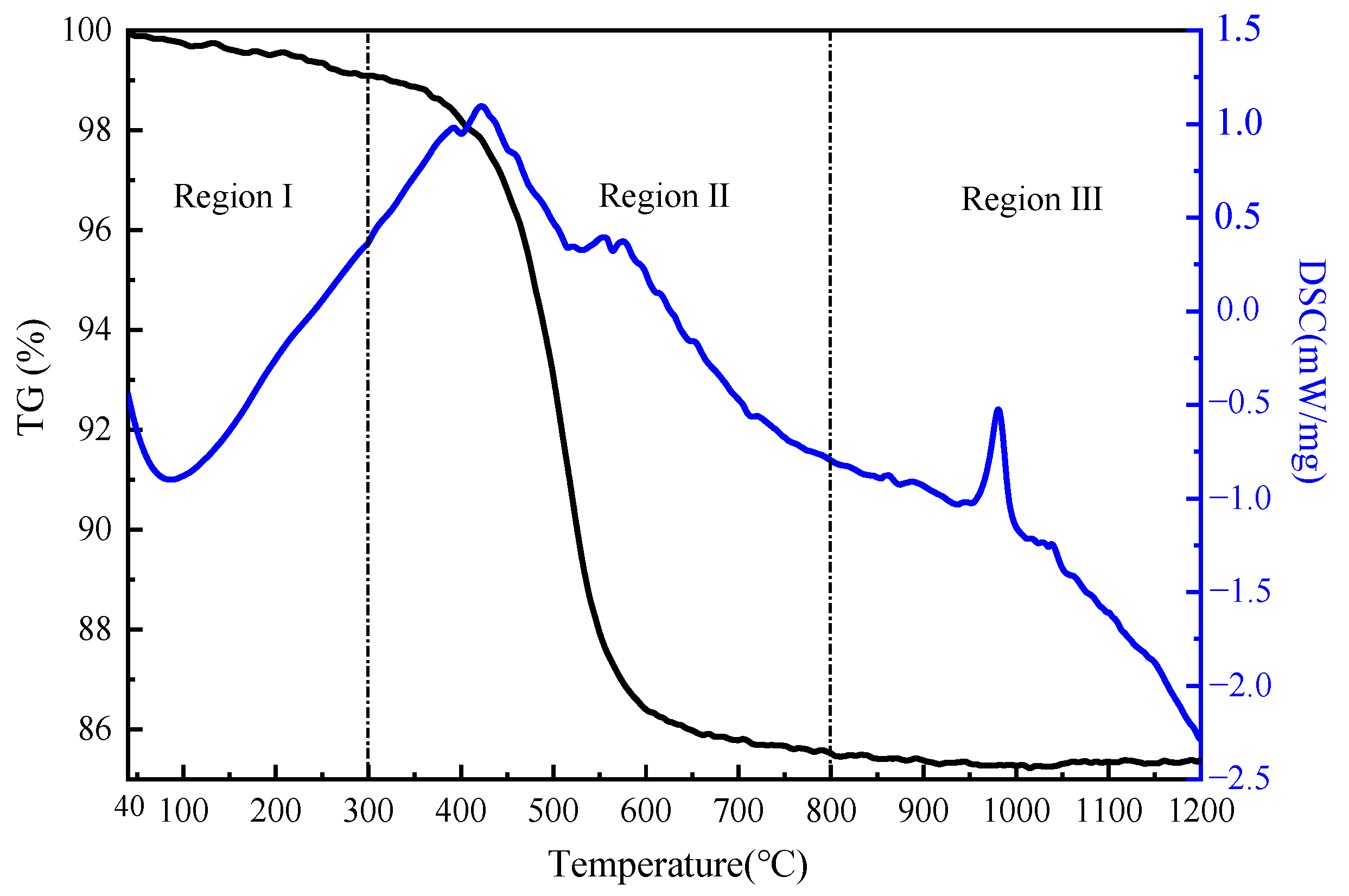
3.1. Composition and Thermodynamic Properties of Kaolin Lithium Clay
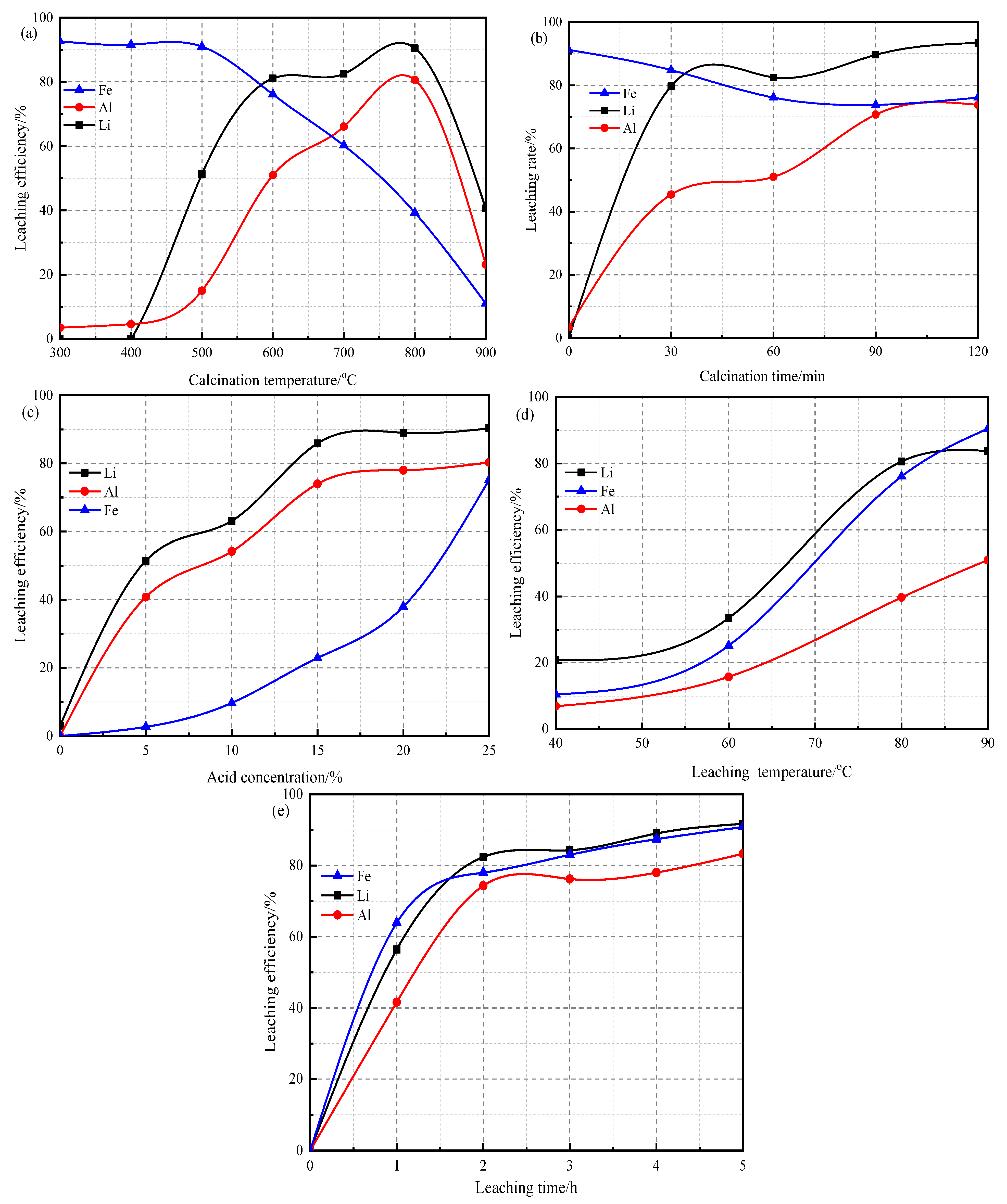
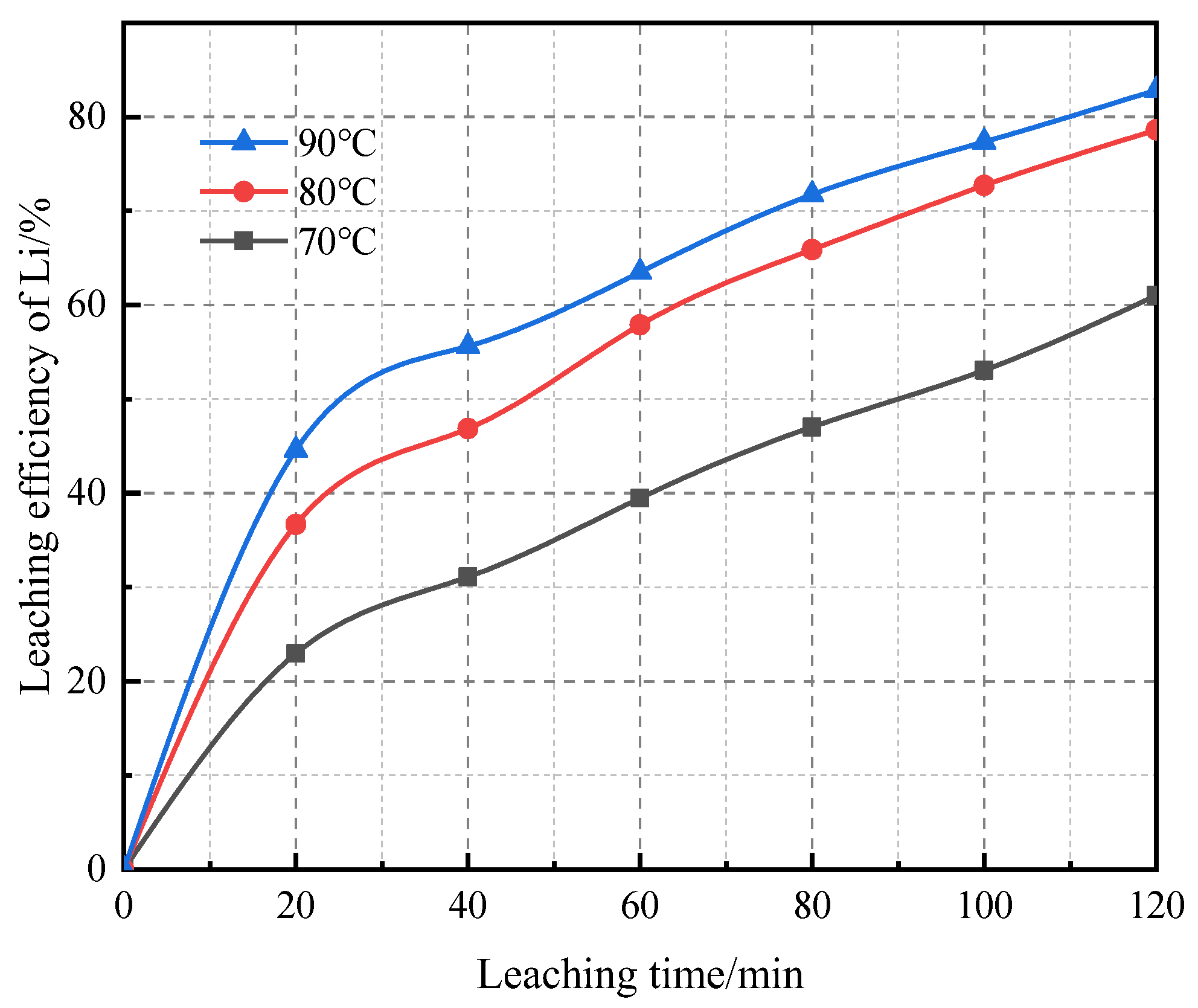
3.2. Lithium Ion Leaching Rate under Different Influencing Factors
3.3. Morphology and Structure of the Residue
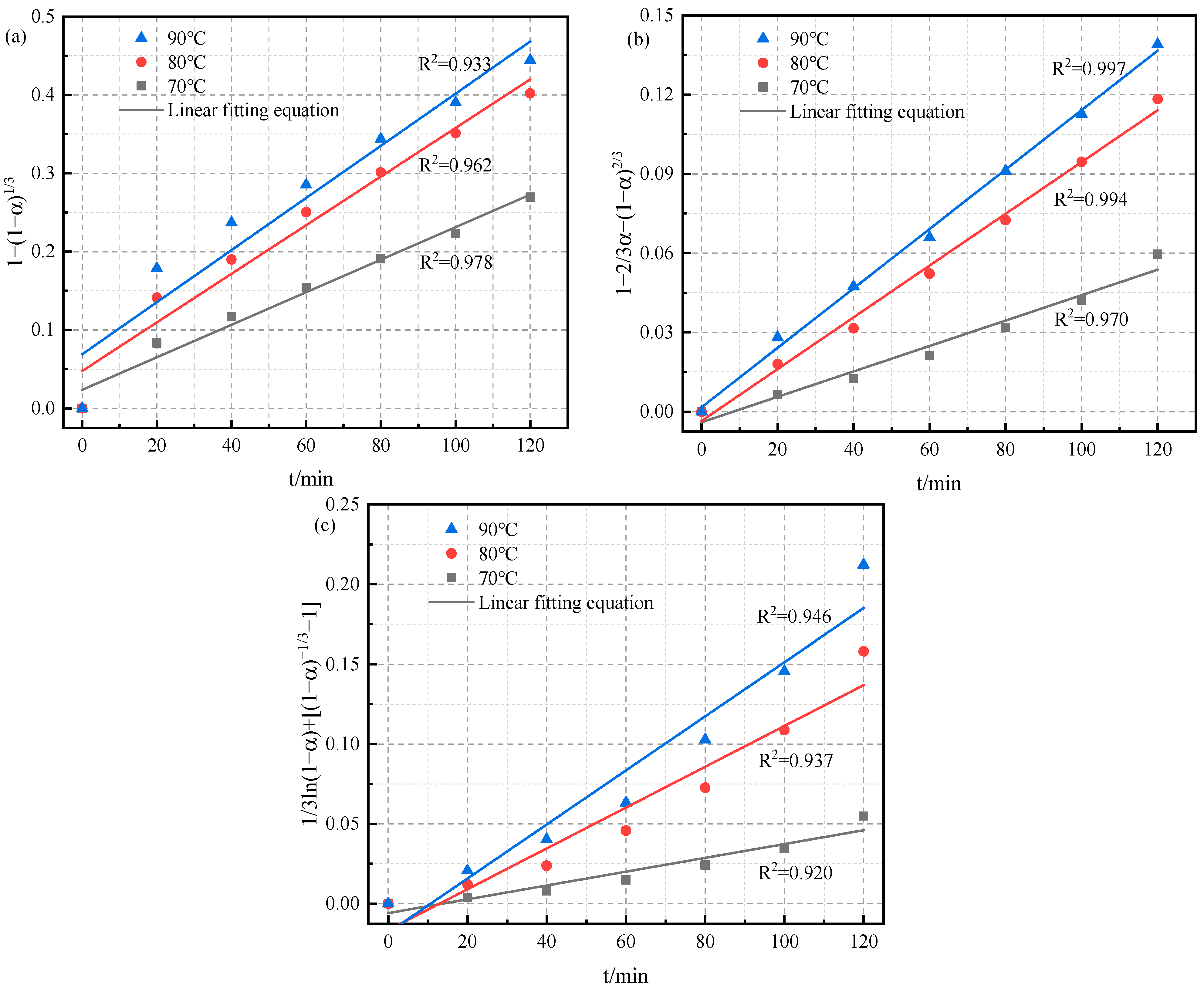
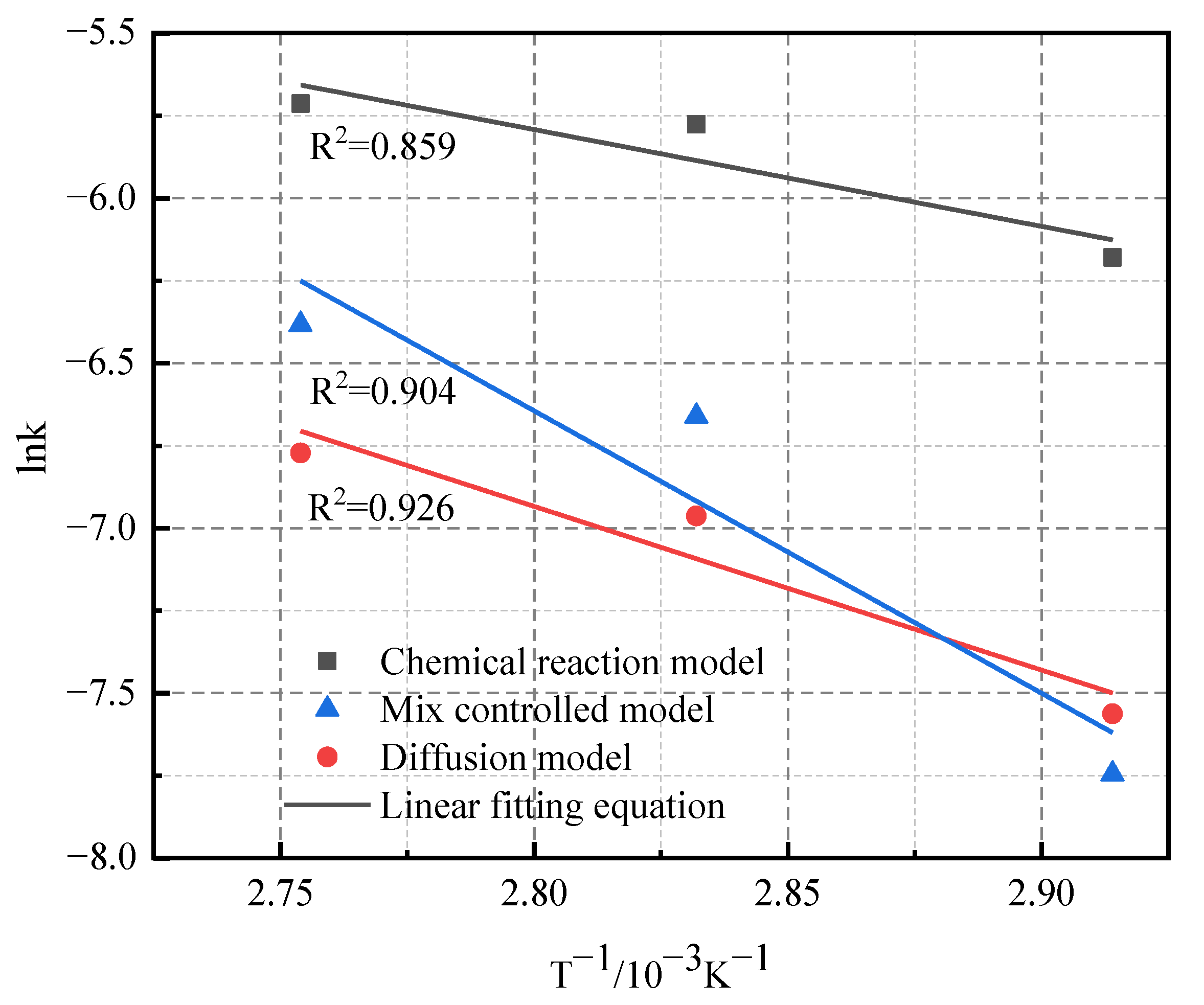
3.4. Kinetics Study
4. Conclusions
- (1)
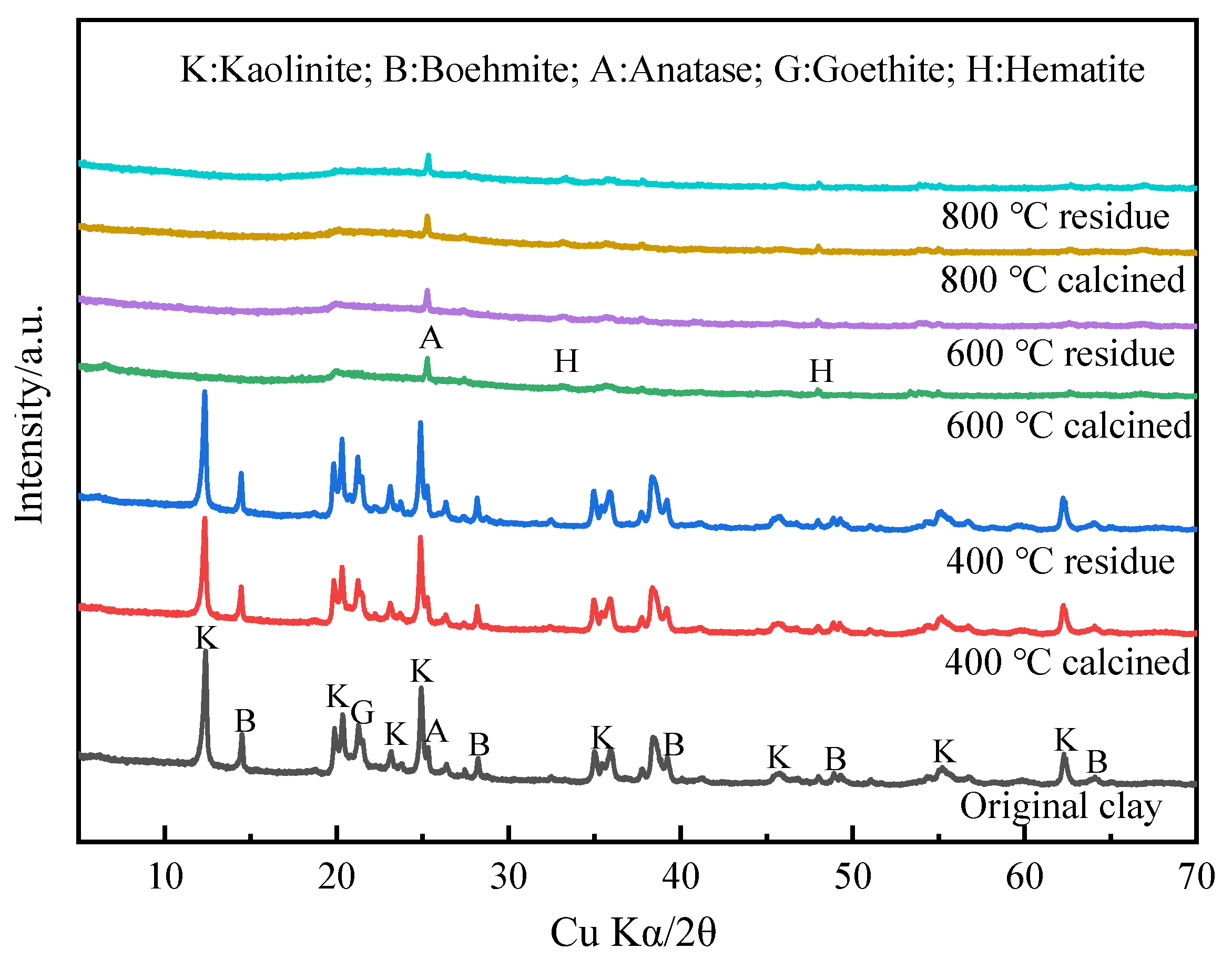
- The main minerals in the sampled clay were kaolinite, boehmite, and goethite, while there was no independent lithium mineral. The lithium content in the clay was 1867 μg/g, which was found to exist in the form of ions in the kaolinite structure.
- (2)
- In the calcination process, the dehydroxylation of kaolinite produced an unstable metakaolinite. In the leaching process, the Al on the mineral surface was dissolved by H2SO4, resulting in the destruction of the Al-O structure of the mineral. The Li+ in the mineral was exchanged by H+ and entered the solution under the action of diffusion.
- (3)
- The results of t leaching test showed that the calcination temperature had the greatest influence on the leaching of Li. After calcination at 600 °C for 1 h, then using 15.0% sulfuric acid at 80 °C for 2 h, the optimal leaching rate of lithium was 81.1%. The main ions in the leaching solution were Al3+ (12,696 mg/L), Fe2+ (3983 mg/L), and Li+ (336 mg/L).
- (4)
- The results of leaching kinetics analysis showed that the leaching of lithium was controlled by a diffusion model, with a special model ion exchange. The activation energy (Ea) of the process was 41.3 kJ/mol.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rosales, G.; Resentera, A.; Gonzalez, J.; Wuilloud, R.; Rodriguez, M. Efficient extraction of lithium from spodumene by direct roasting with NaF and leaching. Chem. Eng. Res. Des. 2019, 150, 320–326. [Google Scholar] [CrossRef]
- Benson, T.; Coble, M.; Rytuba, J.; Mahood, G. Lithium enrichment in intracontinental rhyolite magmas leads to Li deposits in caldera basins. Nat. Commun. 2017, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, Y.; Du, B.; Zhao, Y.; Wang, M. Recovery of both magnesium and lithium from high Mg/Li ratio brines using a novel process. Hydrometallurgy 2018, 175, 102–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, H.; Luo, C.; Xu, L. Geochemical characteristics and indicative significance of clay lithium carbonate deposits in Yunnan-Guizhou area. Earth-Sci. Rev. 2019, 189, 159–176. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Liu, X.; Zhou, G.; Xu, H.; Zhu, Y. Provenance and oreforming process of Permian lithium rich bauxite in central Yunnan, SW China. Ore Geol. Rev. 2022, 145, 104862. [Google Scholar] [CrossRef]
- Yang, J.; Luo, C.; Du, S.; Yu, W.; Yang, Y.; Wen, H. Discussion on the applicability of paleo environmental indexes of sedimentary rocks with high clay content. Acta Mineral. Sin. 2020, 40, 723–733. [Google Scholar] [CrossRef]
- Vigier, N.; Decarreau, A.; Millot, R.; Carignan, J.; Petit, S.; France, C. Quantifying Li isotope fractionation during smectite formation and implications for the Li cycle. Geochim. Cosmochim. Acta. 2008, 72, 780–792. [Google Scholar] [CrossRef]
- He, M.; Fang, Z.; Zhang, P. Atomic and electronic structures of montmorillonite in soft rock. Chin. Phys. B 2009, 18, 2933. [Google Scholar] [CrossRef]
- Shaifei, L.; Adhikari, P.; Ching, W. DFT Study of Electronic Structure and Optical Properties of Kaolinite, Muscovite, and Montmorillonite. Crystals 2021, 11, 618. [Google Scholar] [CrossRef]
- Wen, H.; Luo, C.; Du, S.; Yu, W.; Gu, H.; Ling, K.; Cui, Y.; Li, Y.; Yang, J. Discovery and significance of carbonate clay lithium resources. Sci. Bull. 2020, 65, 53–59. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Caldwell, T.B.; Thurston, A.M. Selective Extraction of Lithium from Clay Minerals. U.S. Patent 17/136,701, 29 December 2020. [Google Scholar]
- Zhu, L.; Gu, H.; Wen, H.; Yang, Y. Lithium extraction from clay-type lithium resource using ferric sulfate solutions via an ion-exchange leaching process. Hydrometallurgy 2021, 206, 105759. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wu, L.; Tan, L.; Xie, F.; Cheng, J. Extraction of lithium and aluminium from bauxite mine tailings by mixed acid treatment without roasting. J. Hazard. Mater. 2021, 404, 124044. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Guo, T.; Wen, H.; Luo, C.; Cui, Y.; Du, S.; Wang, N. Leaching efficiency of sulfuric acid on selective lithium leach ability from lithium clay. Miner. Eng. 2020, 145, 106076. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, C.; Xu, L.; Zhang, H.; Deng, M.; Gu, H.; Meng, Y.; Qin, C.; Wen, H. Weathering origin and enrichment of lithium in clay rocks of the Jiujialu Formation, central Guizhou Province, Southwest China, Bulletin of Mineralogy. Earth-Sci. Rev. 2018, 37, 696–704. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Geng, J. Microstructural characterization of limestone exposed to heat with XRD, SEM and TG-DSC. Mater. Charact. 2017, 134, 285–295. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S. Reexamining calcination of kaolinite for the synthesis of metakaolin geopolymers roles of dehydroxylation and recrystallization. Non-Cryst. Solids 2017, 460, 74–80. [Google Scholar] [CrossRef]
- Chen, J.; Yan, C.; Tu, J. The structure and property of Suzhou kaolin at different temperature. Non-Met. Mines 2019, 32, 21–24. [Google Scholar] [CrossRef]
- Heller, K.L. Thermally modifified clay minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kassa, A.; Shibeshi, N.; Tizazu, B. Kinetic analysis of dehydroxylation of Ethiopian kaolinite during calcination. J. Therm. Anal. Calorim. 2022, 147, 12837–12853. [Google Scholar] [CrossRef]
- Lin, Z.; Natoli, J.; Picuri, J.; Shaw, S.; Bowyer, W. Replication of the conversion of goethite to hematite to make pigments in both furnace and campfire. J. Archaeol. Sci. 2021, 39, 103314. [Google Scholar] [CrossRef]
- Huestis, P.; Graham, T.; Mergelsberg, S.; LaVerne, J. Identification of radiolytically-active thermal transition phases in boehmite. Thermochim. Acta 2020, 689, 178611. [Google Scholar] [CrossRef]
- Drits, V.; Derkowski, A.; Sakharov, B.; Zviagina, B. Experimental evidence of the formation of intermediate phases during transition of kaolinite into metakaolinite. Am. Miner. 2016, 101, 2331–2346. [Google Scholar] [CrossRef]
- Hu, Q.; Yong, Q.; He, L.; Gu, Y.; Zeng, J. Structural evolution of kaolinite in muddy intercalation under microwave heating Materials Research Express. Therm. Anal. Calorim. 2021, 8, 105503. [Google Scholar] [CrossRef]
- Wang, N.; Gu, H.; Wen, H.; Liu, S. Enrichment of niobium and titanium from kaolin using an acid-alkali leaching process. Metall. Mater. Trans. B 2018, 49, 3552–3558. [Google Scholar] [CrossRef]
- MacDonald, B.; Fox, W.; Dubreuilc, L.; Beddardc, J.; Pidruczny, A. Iron oxide geochemistry in the Great Lakes Region (North America): Implications for ochre provenance studies. J. Archaeol. Sci. 2018, 19, 476–490. [Google Scholar] [CrossRef]
- Stevenson, C.; Gurnick, M. Structural collapse in kaolinite, montmorillonite and illite clay and its role in the ceramic rehydroxylation dating of low fired earthenware. J. Archaeol. Sci. 2016, 69, 54–63. [Google Scholar] [CrossRef]
- Li, H.; Kuang, G.; Hu, S.; Guo, H.; Jin, R.; Vekariya, R. Removal of aluminum from leaching solution of lepidolite by adding ammonium. JOM 2016, 68, 2653–2658. [Google Scholar] [CrossRef]
- Lin, G.; Li, J.; Zeng, B.; Wang, W.; Li, C.; Zhang, L. Leaching of rubidium from biotite ore by chlorination roasting and ultrasonic enhancement. Arab. J. Chem. 2022, 15, 104275. [Google Scholar] [CrossRef]
- Nada, A.; Imam, N.; Aassy, I.; Ghanem, A. Effect of different concentrations of sulfuric acid on leaching of radionuclide isotopes in sedimentary rock samples. J. Radioanal. Nucl. Chem. 2019, 322, 347–359. [Google Scholar] [CrossRef]
- Hui, X.; Zhang, J.; Liang, Y.; Chang, Y.; Zhang, W.; Zhang, G. Comparison and evaluation of vanadium extraction from the calcification roasted vanadium slag with carbonation leaching and sulfuric acid leaching. Sep. Purif. Technol. 2022, 297, 121466. [Google Scholar] [CrossRef]
- Lv, Z.; Pan, X.; Geng, X.; Yu, H. Synergistic removal of calcium and iron impurities from calcium-rich and high-alumina fly ash by acid leaching control. J. Environ. Chem. Eng. 2022, 10, 107268. [Google Scholar] [CrossRef]
- Dhawan, N.; Safarzadeh, M.S.; Birinci, M. Kinetics of hydrochloric acid leaching of smithsonite. Russ. J. Non-Ferr. Met. 2021, 52, 209–216. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Yang, K.; Yang, Y.; Zhou, X.; Lu, C.; Xiao, J.; Liu, F.; Zhang, X. Recovery of rare earths from weathered crust elution-deposited rare earth ore without ammonia-nitrogen pollution: I. Leaching with magnesium sulfate. Hydrometallurgy 2015, 439, 58–60. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Xie, F.; Lu, D.; Jiang, K. Effect of temperature, oxygen partial pressure and calcium lignosulphonate on chalcopyrite dissolution in sulfuric acid solution. Trans. Non-Ferr. Met. Soc. China 2022, 32, 1650–1663. [Google Scholar] [CrossRef]

| Component | Al2O3 | SiO2 | TFe | Li2O | MgO | CaO |
|---|---|---|---|---|---|---|
| wt% | 41.02 | 36.99 | 3.01 | 0.40 | 0.22 | 0.074 |
| Leaching Process | Linear Fitting Equation | Average R2 | Ea |
|---|---|---|---|
| Chemical reaction model | y = −2.932x + 2.417 | 0.908 | 24.4 kJ/mol |
| Mix controlled model | y = −8.555x + 17.311 | 0.919 | 71.1 kJ/mol |
| Diffusion model | y = −4.961x + 6.955 | 0.957 | 41.3 kJ/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Yang, L.; Rao, F.; Tong, L.; Feng, H. Efficient Extraction of Lithium from Calcined Kaolin Lithium Clay with Dilute Sulfuric Acid. Minerals 2024, 14, 359. https://doi.org/10.3390/min14040359
Zhong W, Yang L, Rao F, Tong L, Feng H. Efficient Extraction of Lithium from Calcined Kaolin Lithium Clay with Dilute Sulfuric Acid. Minerals. 2024; 14(4):359. https://doi.org/10.3390/min14040359
Chicago/Turabian StyleZhong, Wenlin, Lang Yang, Feng Rao, Liangzhou Tong, and Haipeng Feng. 2024. "Efficient Extraction of Lithium from Calcined Kaolin Lithium Clay with Dilute Sulfuric Acid" Minerals 14, no. 4: 359. https://doi.org/10.3390/min14040359





