Evolution of Pore Spaces in Marine Organic-Rich Shale: Insights from Multi-Scale Analysis of a Permian–Pennsylvanian Sample
Abstract
:1. Introduction
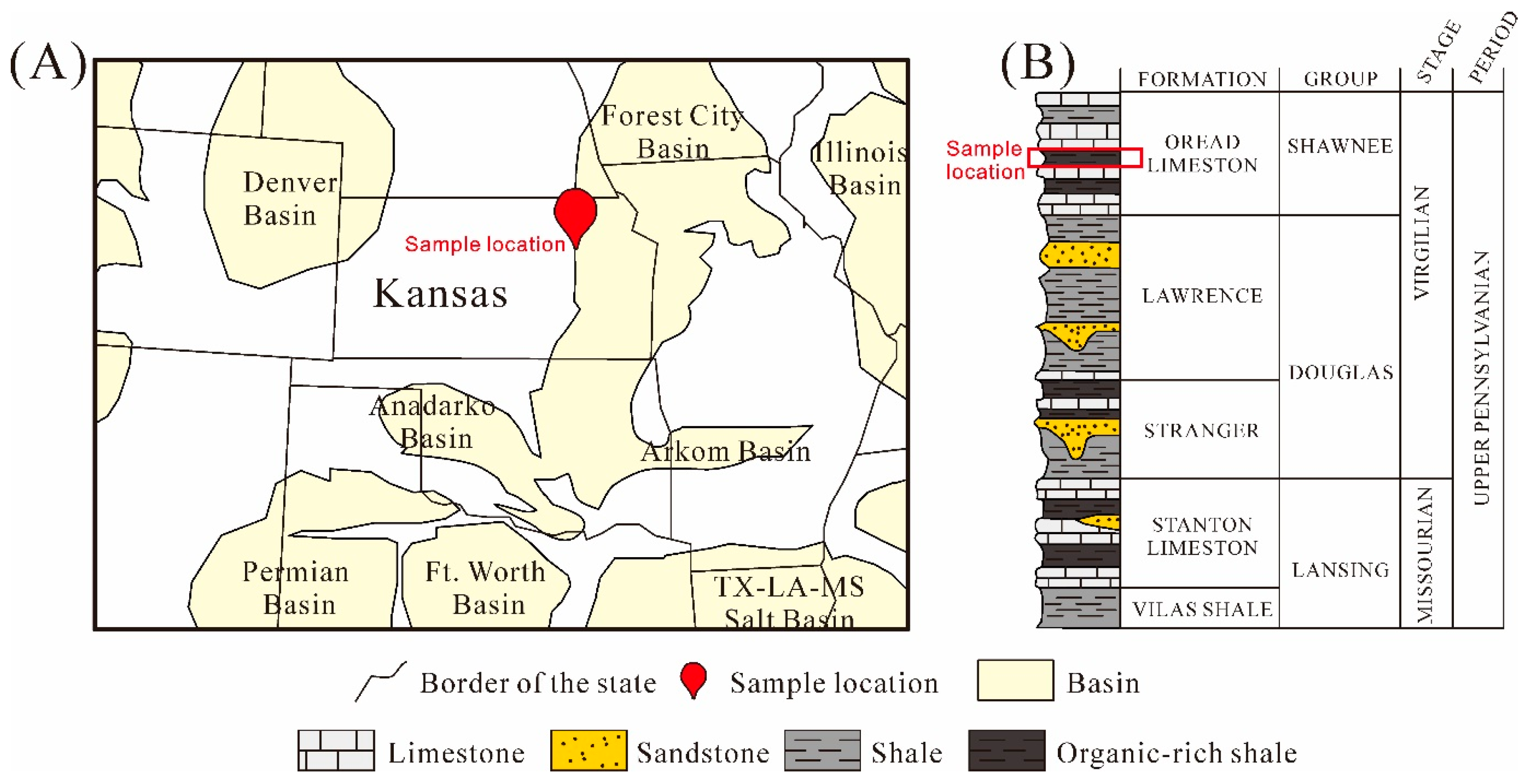
2. Geological Setting
3. Methods
3.1. Thermal Simulation Experiment
3.2. Vitrinite Reflectance (Ro)
3.3. X-ray Diffraction
3.4. Pore-Size Characterization Methods
3.5. SEM and IRT
4. Results
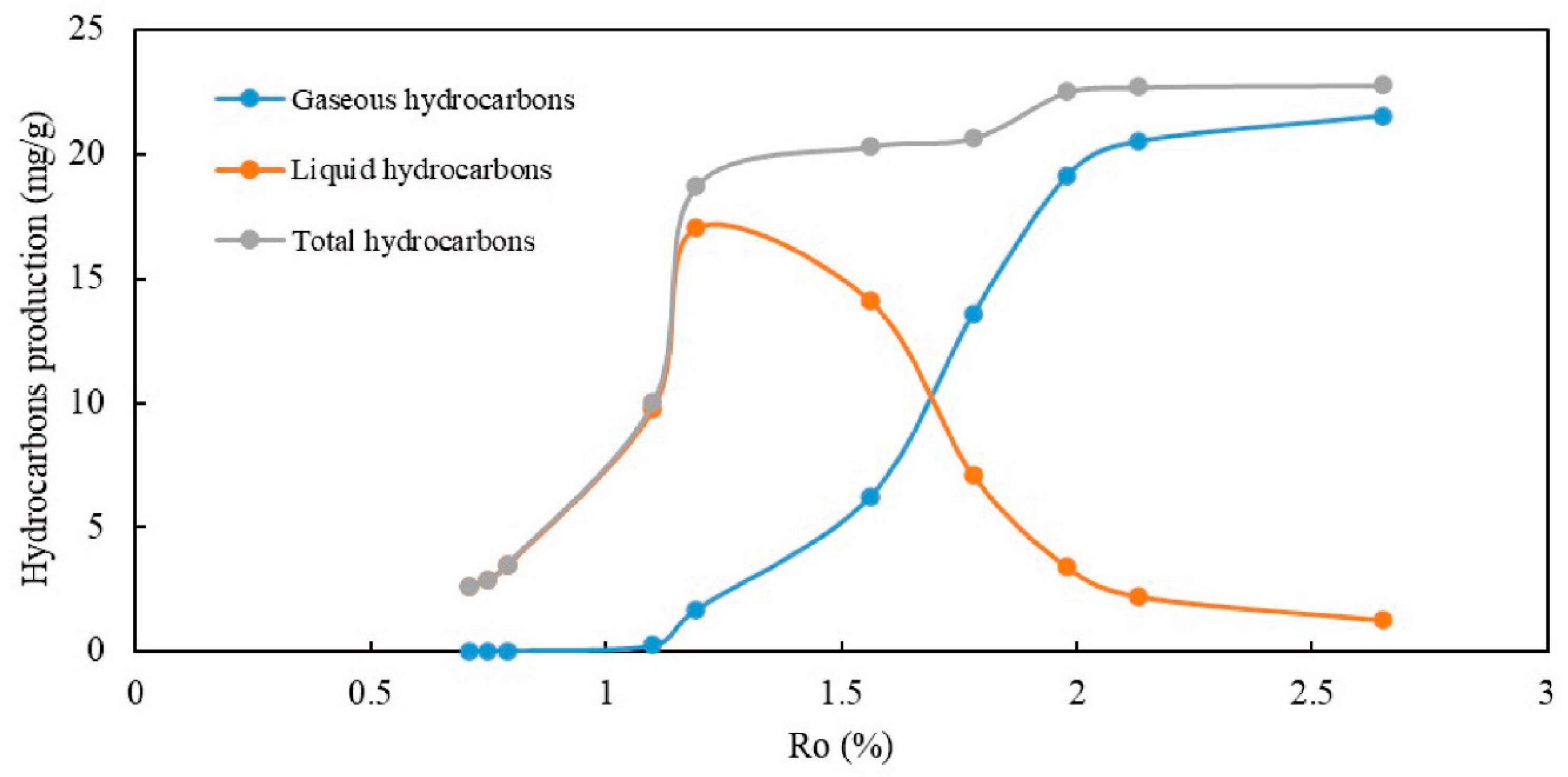
4.1. Maturity and Hydrocarbon Yield
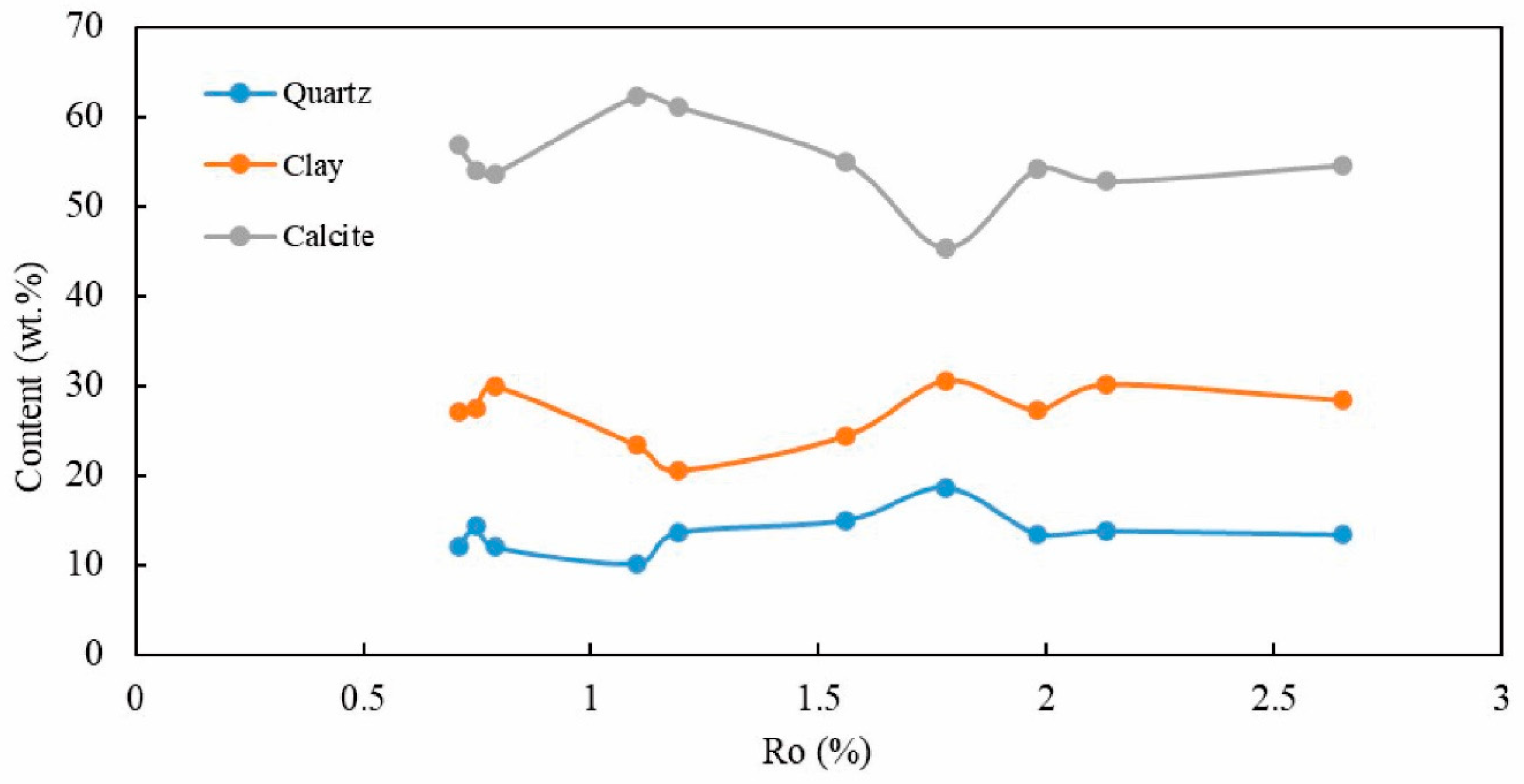
4.2. Whole-Rock Mineral Composition
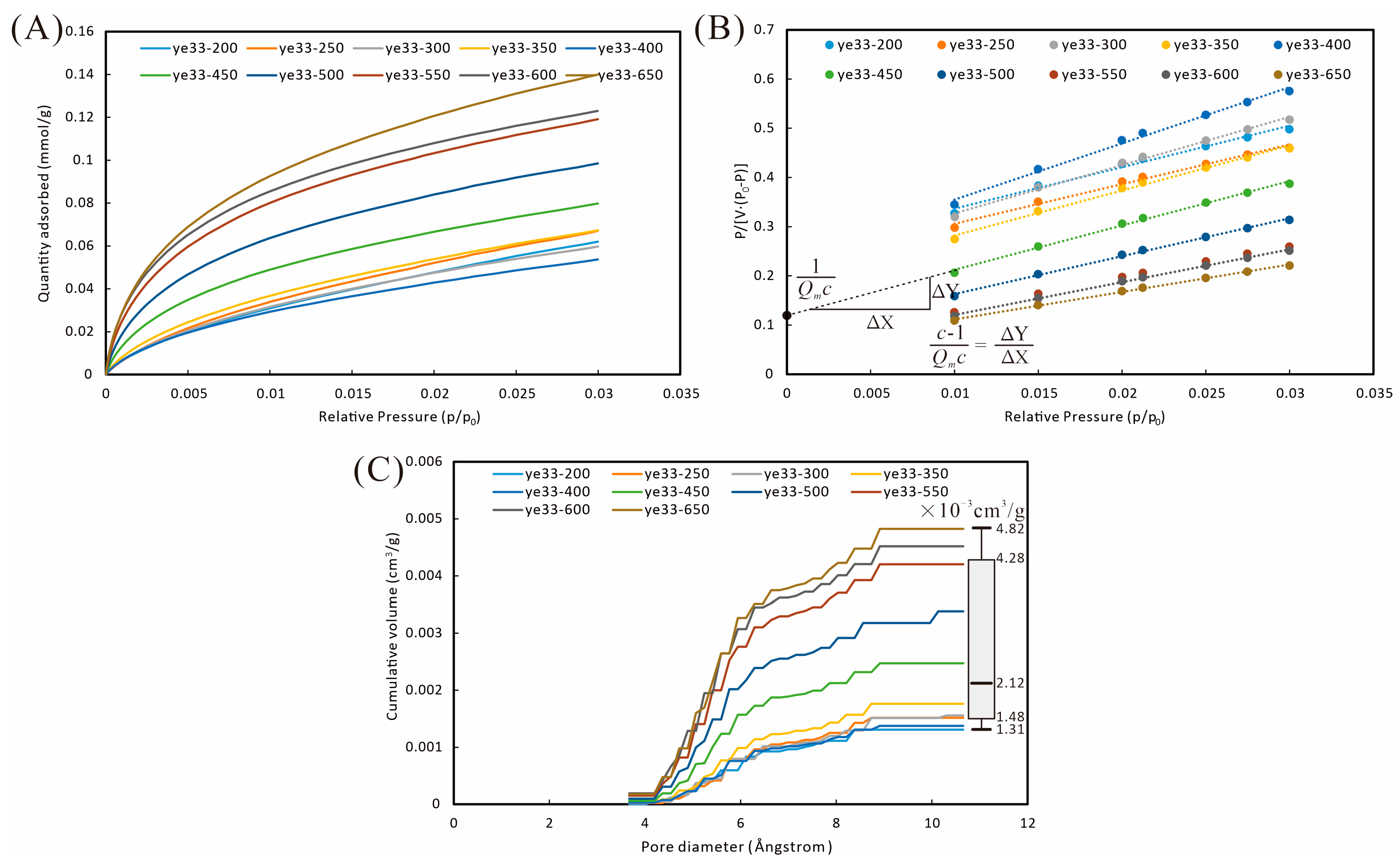
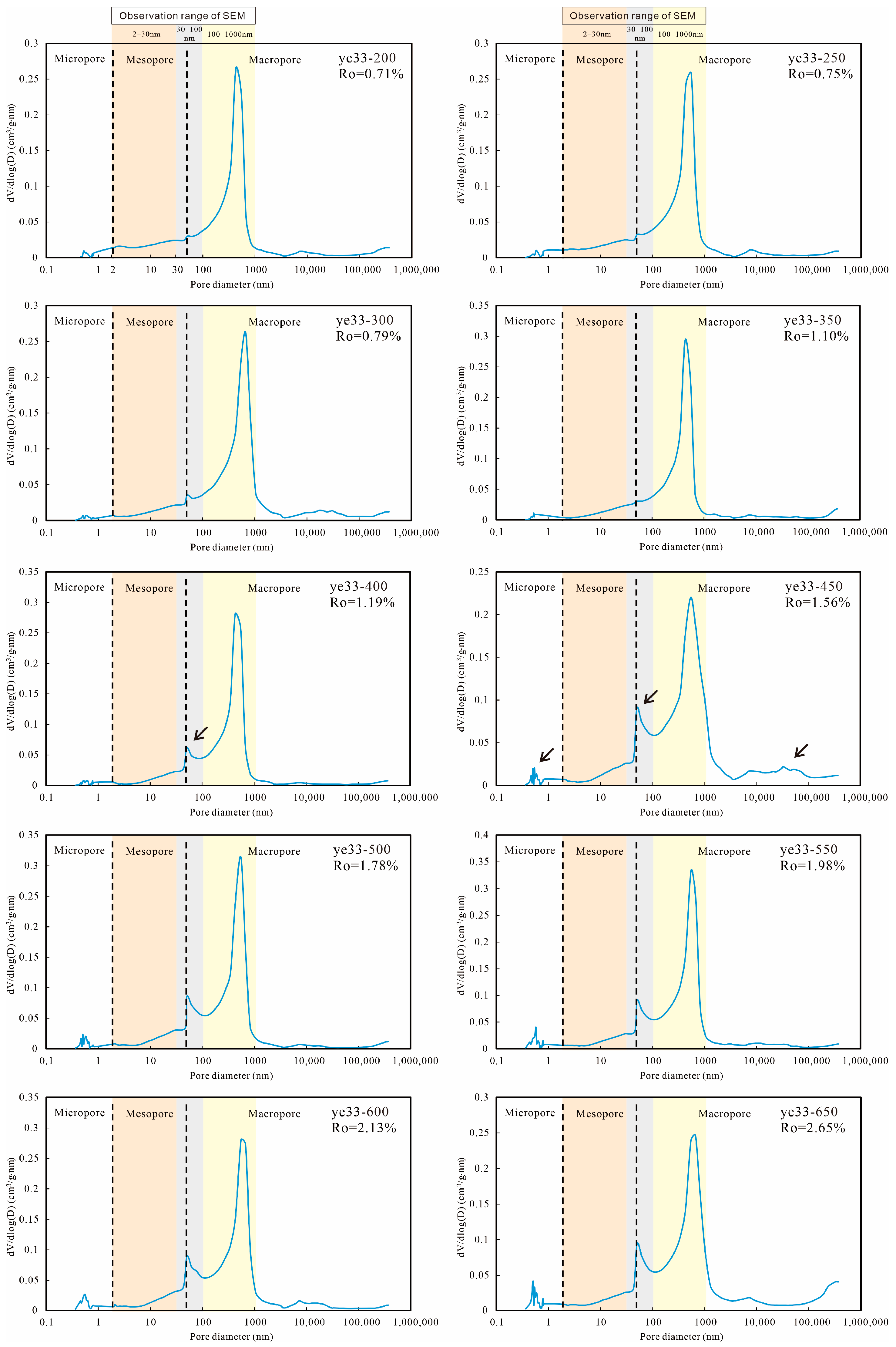
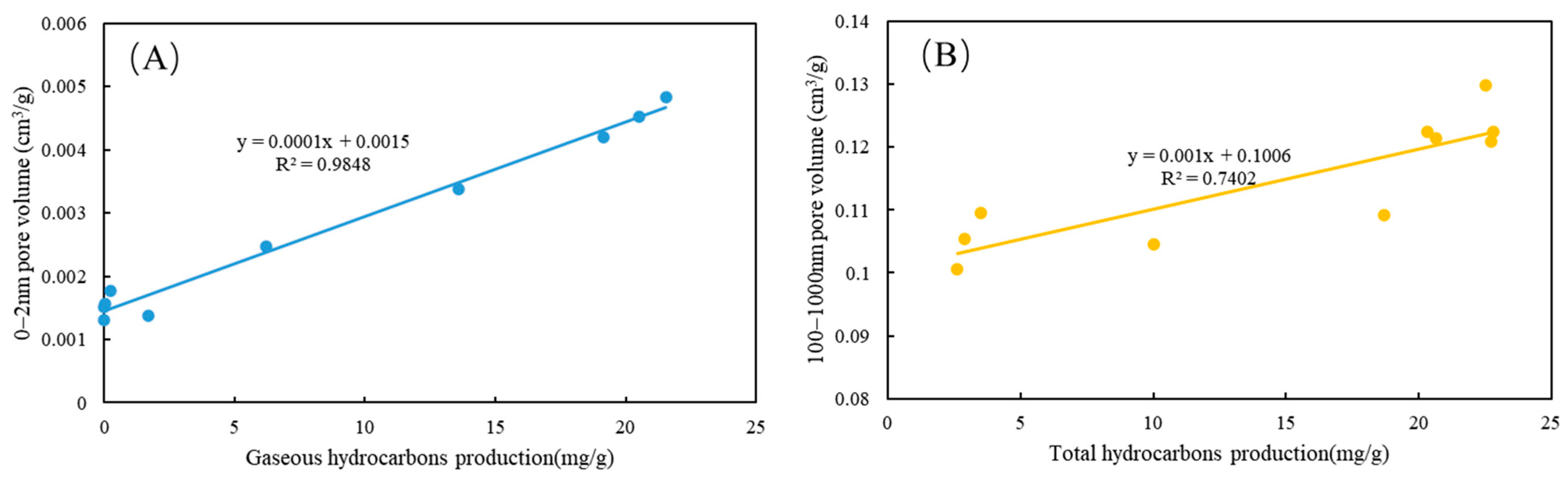
4.3. Pore Volume and Structure Characterization
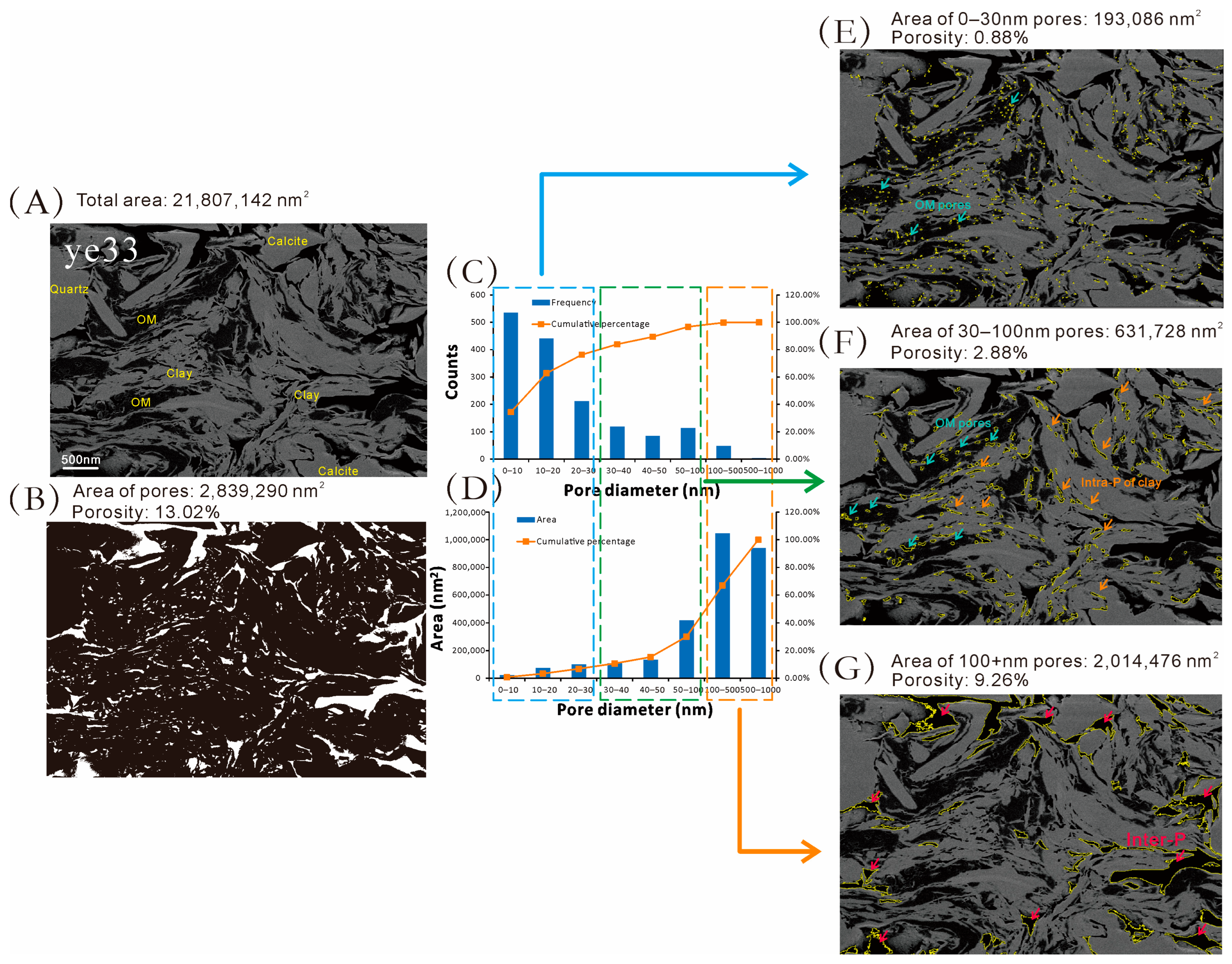
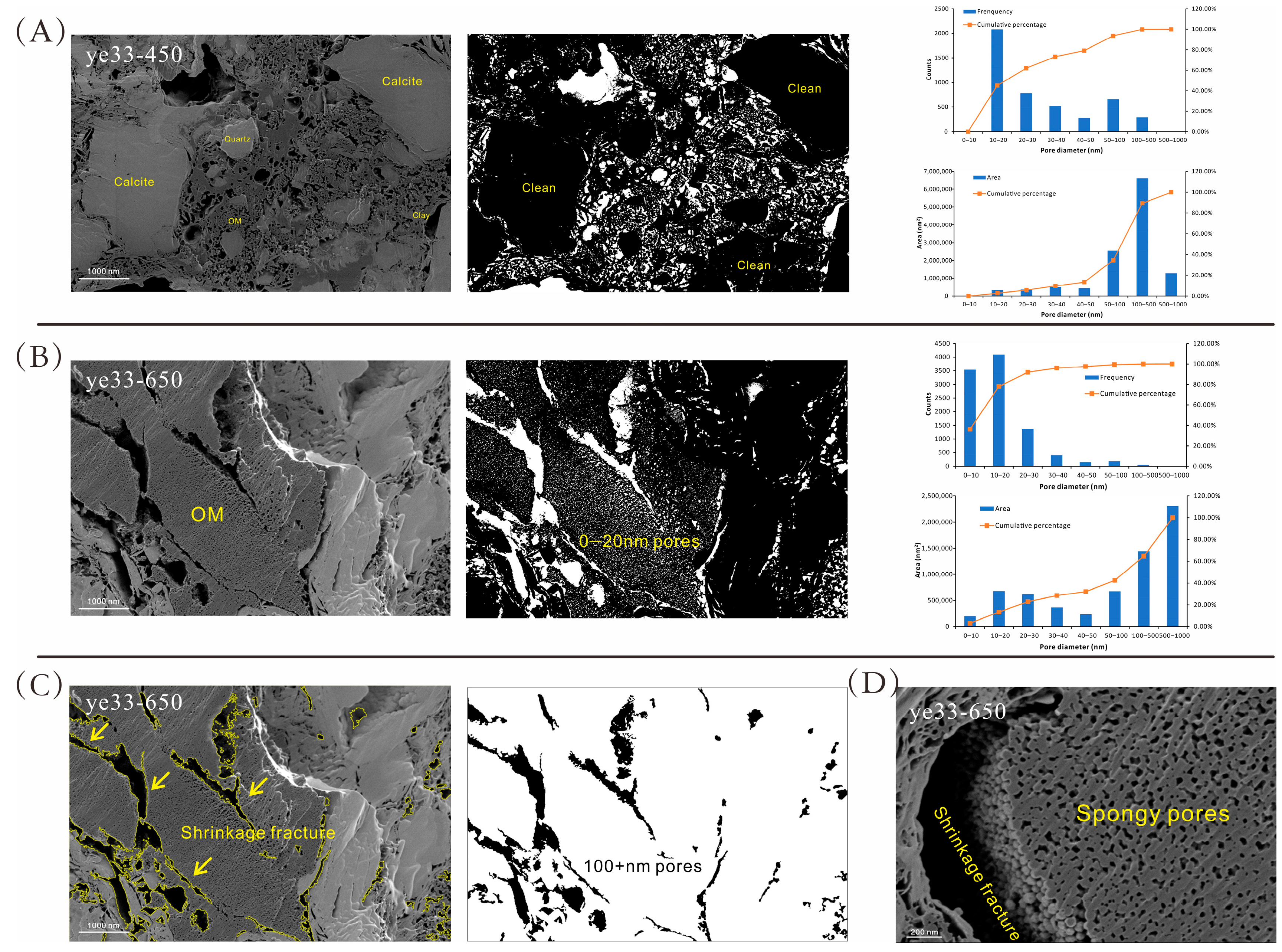
4.4. Pore Characteristics from SEM Imaging
5. Discussion
5.1. Pore-Size Polarization during Thermal Maturation
5.1.1. Evidence from SEM and IRT
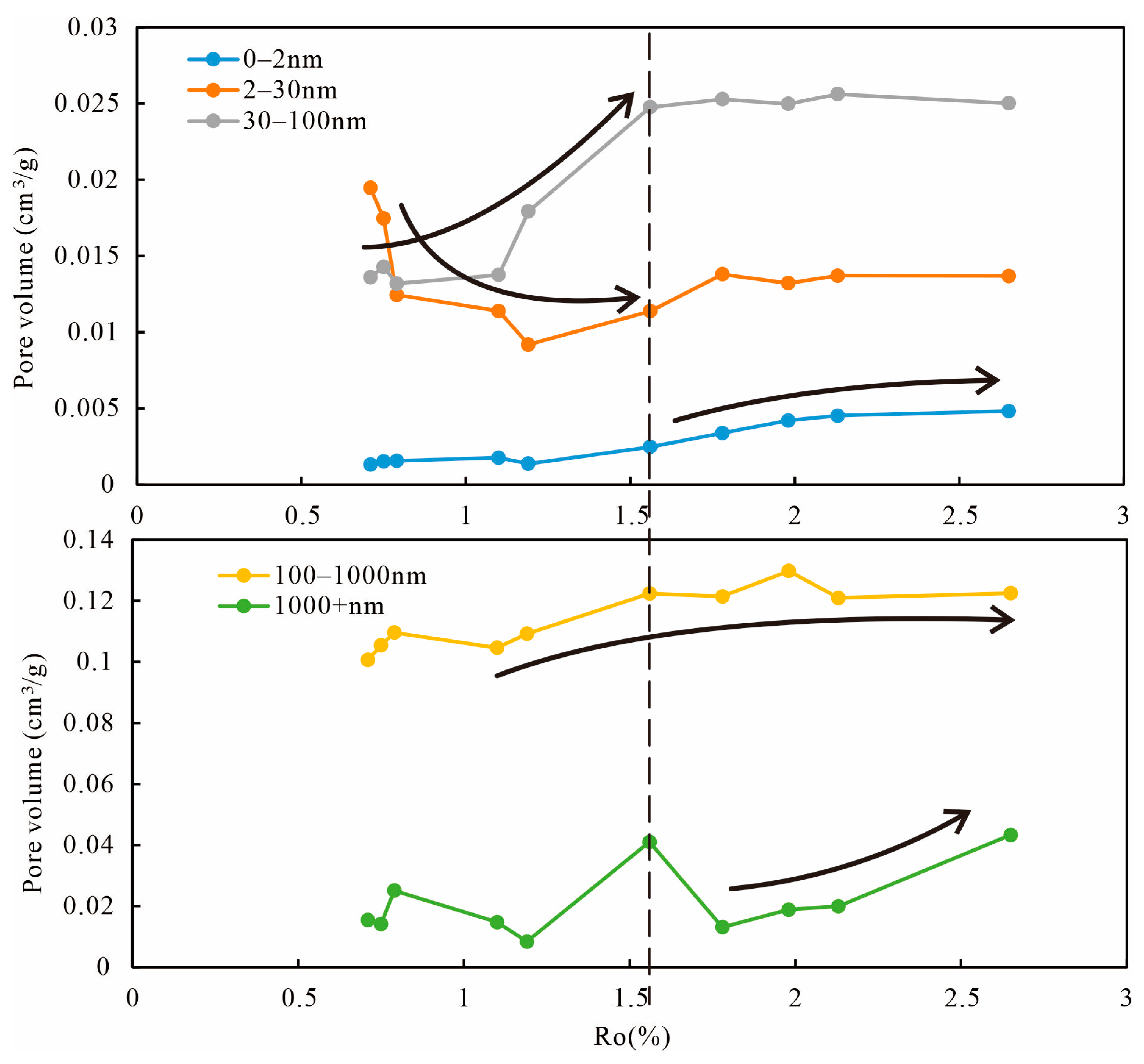
5.1.2. Evidence from LTGA and MIP
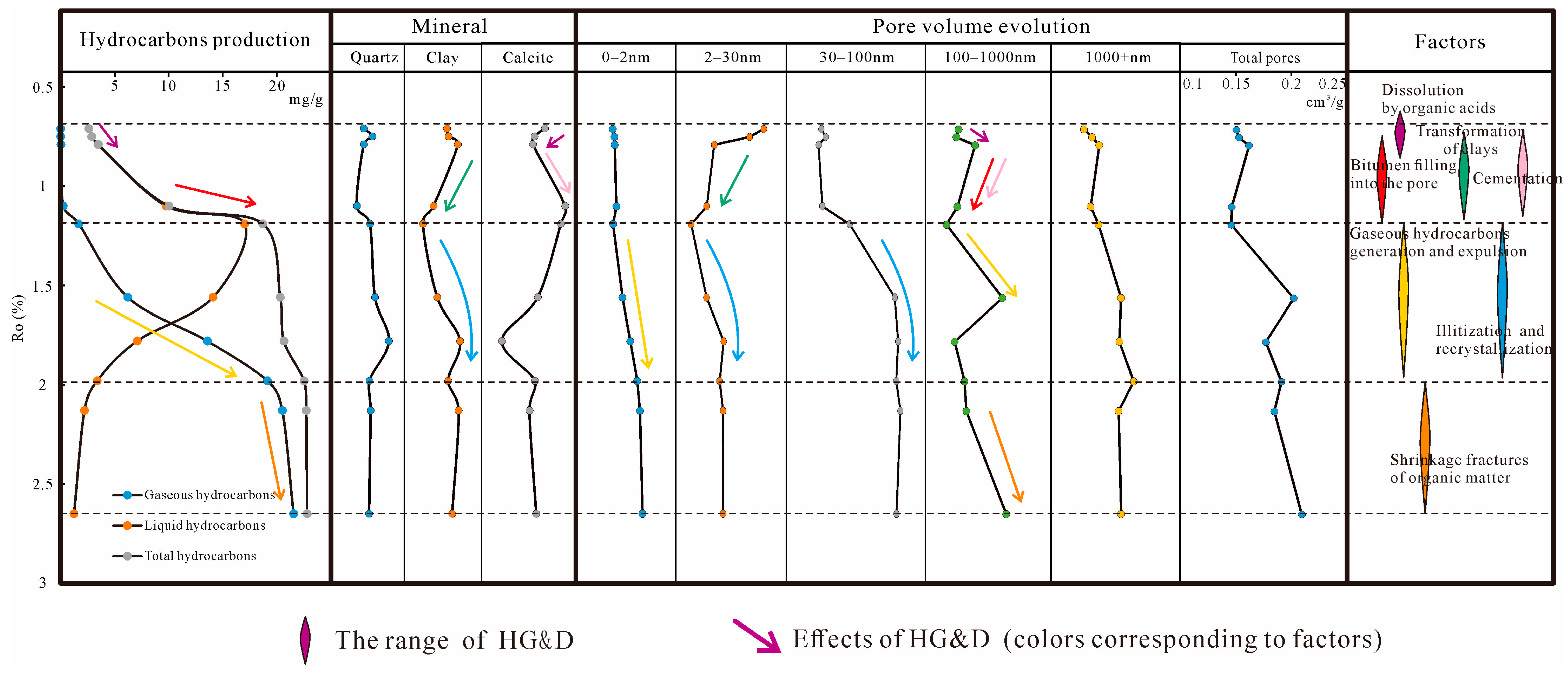
5.2. Effects of Hydrocarbon Generation and Diagenesis
- (1)
- Ro at 0.7%–1.2%, in the initial stage, kerogen decarboxylated and formed organic acids [54], calcite was dissolved in the acidic environment, and the Inter-P (100–1000 nm) increased. Subsequently, high-calcium fluids were redeposited in the Inter-P to produce calcium cementation [55], resulting in a decrease in Inter-P (100–1000 nm). The space of loose clay minerals (mainly kaolinite) is compressed, leading to a reduction in the Intra-P of clay minerals (2–30 nm). As the OM matures, the OM begins to generate large amounts of liquid hydrocarbons and 30–100 nm OM inside pores (30–100 nm) [56,57]. However, the generated liquid hydrocarbons and bitumen entered and blocked the Inter-P [58,59], resulting in a large reduction of 100–1000 nm pores. Some microcrystalline authigenic quartz generation also has a negative effect on the pores [60,61].
- (2)
- At 1.2%–2.0%, many gaseous hydrocarbons were generated, bitumen and OM shrank, spongy pores were formed in OM [62,63], and the OM pore size started to polarize, generating a large number of micropores (<2 nm) and a large volume of macropores (100–1000 nm). Illitization and recrystallization [64,65] of clay minerals increased the Inter-P and Intra-P of clay minerals, while gaseous hydrocarbons entered the pores, and high fluid pressure made the pores of clay larger, so the volume of 30–100 nm pores increased considerably.
- (3)
- When Ro was higher than 2.0%, the yield of hydrocarbons gradually decreased, so the change in the 0–100 nm pore volume was small. Most hydrocarbons have been expelled, and the density of the reduced OM resembles a sponge, with many pores developed in it. The OM then continued to shrink, forming shrinkage fractures at the perimeter of the organic matter [29,66], resulting in a continuous increase in the 100–1000 nm pores.
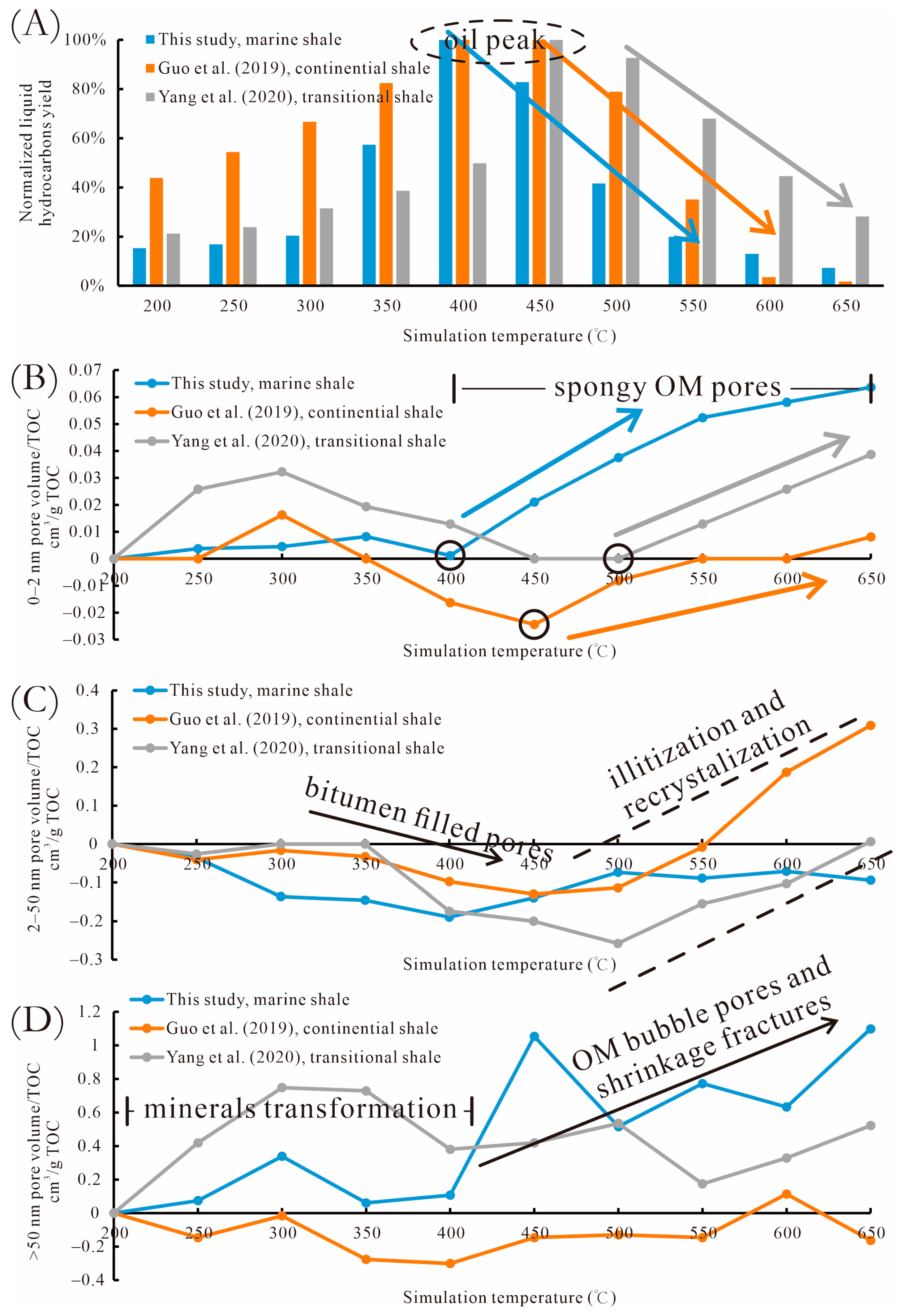
5.3. Compared to Continental and Transitional Shales
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, L.; Liu, B.; Yang, J.; Tian, S.; Wang, B.; Huang, S. Differences in hydrocarbon composition of shale oils in different phase states from the Qingshankou Formation, Songliao Basin, as determined from fluorescence experiments. Front. Earth Sci. 2021, 15, 438–456. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Q.; Long, S.; Zhao, J.; Peng, N.; Wang, H.; Lin, X.; Sun, M. Mineral-controlled nm-μm-scale pore structure of saline lacustrine shale in Qianjiang Depression, Jianghan Basin, China. Mar. Pet. Geol. 2019, 99, 347–354. [Google Scholar] [CrossRef]
- Owusu, E.B.; Tsegab, H.; Sum, C.W.; Padmanabhan, E. Organic geochemical analyses of the Belata black shale, Peninsular Malaysia; implications on their shale gas potential. J. Nat. Gas Sci. Eng. 2019, 69, 102945. [Google Scholar] [CrossRef]
- Tan, J.; Horsfield, B.; Fink, R.; Krooss, B.; Schulz, H.-M.; Rybacki, E.; Zhang, J.; Boreham, C.J.; Van Graas, G.; Tocher, B.A. Shale gas potential of the major marine shale formations in the Upper Yangtze Platform, south China, Part III: Mineralogical, lithofacial, petrophysical, and rock mechanical properties. Energy Fuels 2014, 28, 2322–2342. [Google Scholar] [CrossRef]
- Enomoto, C.B.; Hackley, P.C.; Valentine, B.J.; Rouse, W.A.; Dulong, F.T.; Lohr, C.D.; Hatcherian, J.J. Geologic Characterization of the Hydrocarbon Resource Potential of the Upper Cretaceous Tuscaloosa Marine Shale in Mississippi and Louisiana, USA; United States Geological Survey: Reston, VA, USA, 2017.
- Zou, C.; Zhu, R.; Chen, Z.-Q.; Ogg, J.G.; Wu, S.; Dong, D.; Qiu, Z.; Wang, Y.; Wang, L.; Lin, S. Organic-matter-rich shales of China. Earth-Sci. Rev. 2019, 189, 51–78. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Hu, Q.; Liu, L.; Hao, L. Diagenesis and pore evolution for various lithofacies of the Wufeng-Longmaxi shale, southern Sichuan Basin, China. Mar. Pet. Geol. 2021, 133, 105251. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, Z.; Liu, K. A new meshless method to solve the two-phase thermo-hydro-mechanical multi-physical field coupling problems in shale reservoirs. J. Nat. Gas Sci. Eng. 2022, 105, 104683. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X. Evolution of nanoporosity in organic-rich shales during thermal maturation. Fuel 2014, 129, 173–181. [Google Scholar] [CrossRef]
- Sun, L.; Tuo, J.; Zhang, M.; Wu, C.; Wang, Z.; Zheng, Y. Formation and development of the pore structure in Chang 7 member oil-shale from Ordos Basin during organic matter evolution induced by hydrous pyrolysis. Fuel 2015, 158, 549–557. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents. Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Yang, J.; Little, E.; Zhou, Y. Deep learning-based method for SEM image segmentation in mineral characterization, an example from Duvernay Shale samples in Western Canada Sedimentary Basin. Comput. Geosci. 2020, 138, 104450. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, L.; Hu, Q.; Pan, Z.; Yu, B.; Sun, L.; Bai, L.; Fu, H.; Zhang, Y.; Zhang, C. Multiscale connectivity characterization of marine shales in southern China by fluid intrusion, small-angle neutron scattering (SANS), and FIB-SEM. Mar. Pet. Geol. 2020, 112, 104101. [Google Scholar] [CrossRef]
- Tahmasebi, P.; Javadpour, F.; Sahimi, M. Three-dimensional stochastic characterization of shale SEM images. Transp. Porous Media 2015, 110, 521–531. [Google Scholar] [CrossRef]
- Yang, R.; He, S.; Yi, J.; Hu, Q. Nano-scale pore structure and fractal dimension of organic-rich Wufeng-Longmaxi shale from Jiaoshiba area, Sichuan Basin: Investigations using FE-SEM, gas adsorption and helium pycnometry. Mar. Pet. Geol. 2016, 70, 27–45. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, G.; Xue, H.; Guo, W.; Li, X. 2D and 3D nanopore characterization of gas shale in Longmaxi formation based on FIB-SEM. Mar. Pet. Geol. 2016, 73, 174–180. [Google Scholar] [CrossRef]
- Bai, L.-H.; Liu, B.; Du, Y.-J.; Wang, B.-Y.; Tian, S.-S.; Wang, L.; Xue, Z.-Q. Distribution characteristics and oil mobility thresholds in lacustrine shale reservoir: Insights from N2 adsorption experiments on samples prior to and following hydrocarbon extraction. Pet. Sci. 2022, 19, 486–497. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the Barnett, Woodford, Haynesville, Marcellus, and Doig units. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar]
- Dong, T.; Harris, N.B.; Ayranci, K.; Twemlow, C.E.; Nassichuk, B.R. Porosity characteristics of the Devonian Horn River shale, Canada: Insights from lithofacies classification and shale composition. Int. J. Coal Geol. 2015, 141, 74–90. [Google Scholar] [CrossRef]
- Gao, F.; Song, Y.; Li, Z.; Xiong, F.; Chen, L.; Zhang, X.; Chen, Z.; Moortgat, J. Quantitative characterization of pore connectivity using NMR and MIP: A case study of the Wangyinpu and Guanyintang shales in the Xiuwu basin, Southern China. Int. J. Coal Geol. 2018, 197, 53–65. [Google Scholar] [CrossRef]
- Li, Y.; Schieber, J.; Fan, T.; Wei, X. Pore characterization and shale facies analysis of the Ordovician-Silurian transition of northern Guizhou, South China: The controls of shale facies on pore distribution. Mar. Pet. Geol. 2018, 92, 697–718. [Google Scholar] [CrossRef]
- Connan, J. Time-temperature relation in oil genesis: Geologic notes. AAPG Bull. 1974, 58, 2516–2521. [Google Scholar]
- Waples, D.W. Time and temperature in petroleum formation: Application of Lopatin’s method to petroleum exploration: Reply. AAPG Bull. 1982, 66, 1152. [Google Scholar]
- Sweeney, J.J.; Burnham, A.K. Evaluation of a simple model of vitrinite reflectance based on chemical kinetics. AAPG Bull. 1990, 74, 1559–1570. [Google Scholar]
- Songtao, W.; Rukai, Z.; Jinggang, C.; Jingwei, C.; Bin, B.; Zhang, X.; Xu, J.; Desheng, Z.; Jianchang, Y.; Xiaohong, L. Characteristics of lacustrine shale porosity evolution, Triassic Chang 7 member, Ordos Basin, NW China. Pet. Explor. Dev. 2015, 42, 185–195. [Google Scholar]
- Wang, F.; Guo, S. Influential factors and model of shale pore evolution: A case study of a continental shale from the Ordos Basin. Mar. Pet. Geol. 2019, 102, 271–282. [Google Scholar] [CrossRef]
- Guo, S.; Mao, W. Division of diagenesis and pore evolution of a Permian Shanxi shale in the Ordos Basin, China. J. Pet. Sci. Eng. 2019, 182, 106351. [Google Scholar] [CrossRef]
- Doveton, J.; Merriam, D. Borehole petrophysical chemostratigraphy of Pennsylvanian black shales in the Kansas subsurface. Chem. Geol. 2004, 206, 249–258. [Google Scholar] [CrossRef]
- Hakes, W.G. Trace Fossils from Brackish-Marine Shales, Upper Pennsylvanian of Kansas, USA; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1984. [Google Scholar]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Dong, T.; Harris, N.B. The effect of thermal maturity on porosity development in the Upper Devonian–Lower Mississippian Woodford Shale, Permian Basin, US: Insights into the role of silica nanospheres and microcrystalline quartz on porosity preservation. Int. J. Coal Geol. 2020, 217, 103346. [Google Scholar] [CrossRef]
- Kibria, M.G.; Hu, Q.; Liu, H.; Zhang, Y.; Kang, J. Pore structure, wettability, and spontaneous imbibition of Woodford shale, Permian Basin, West Texas. Mar. Pet. Geol. 2018, 91, 735–748. [Google Scholar] [CrossRef]
- Jia, B.; Xian, C.; Tsau, J.-S.; Zuo, X.; Jia, W. Status and Outlook of Oil Field Chemistry-Assisted Analysis during the Energy Transition Period. Energy Fuels 2022, 36, 12917–12945. [Google Scholar] [CrossRef]
- Yang, X.G.; Guo, S.B. Porosity model and pore evolution of transitional shales: An example from the Southern North China Basin. Pet. Sci. 2020, 17, 1512–1526. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Solano, N.; Bustin, R.M.; Bustin, A.; Chalmers, G.R.; He, L.; Melnichenko, Y.B.; Radliński, A.; Blach, T.P. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Chen, Y. Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bull. 2013, 97, 1621–1643. [Google Scholar] [CrossRef]
- Drummond, C.; Israelachvili, J. Surface forces and wettability. J. Pet. Sci. Eng. 2002, 33, 123–133. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273. [Google Scholar] [CrossRef]
- Choma, J.; Jaroniec, M.; Kloske, M. Improved pore-size analysis of carbonaceous adsorbents. Adsorpt. Sci. Technol. 2002, 20, 307–315. [Google Scholar] [CrossRef]
- Tian, S.; Bowen, L.; Liu, B.; Zeng, F.; Xue, H.; Erastova, V.; Greenwell, H.C.; Dong, Z.; Zhao, R.; Liu, J. A method for automatic shale porosity quantification using an Edge-Threshold Automatic Processing (ETAP) technique. Fuel 2021, 304, 121319. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kapur, J.N.; Sahoo, P.K.; Wong, A.K. A new method for gray-level picture thresholding using the entropy of the histogram. Comput. Vis. Graph. Image Process. 1985, 29, 273–285. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size heterogeneity and the carbon slit pore: A density functional theory model. Langmuir 1993, 9, 2693–2702. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Pan, Z.; Niu, X.; Yu, Y.; Meng, S. Pore structure and its fractal dimensions of transitional shale: A cross-section from east margin of the Ordos Basin, China. Fuel 2019, 241, 417–431. [Google Scholar] [CrossRef]
- Gregg, S. Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1982; Volume 10, pp. 153–164. [Google Scholar]
- Yang, F.; Ning, Z.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Al Hinai, A.; Rezaee, R.; Esteban, L.; Labani, M. Comparisons of pore size distribution: A case from the Western Australian gas shale formations. J. Unconv. Oil Gas Resour. 2014, 8, 1–13. [Google Scholar] [CrossRef]
- Borjigin, T.; Longfei, L.; Lingjie, Y.; Zhang, W.; Anyang, P.; Baojian, S.; Ye, W.; Yunfeng, Y.; Zhiwei, G. Formation, preservation and connectivity control of organic pores in shale. Pet. Explor. Dev. 2021, 48, 798–812. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, J.; Wang, X.; Jiang, Z.; Song, Y.; Jiang, L.; Jiang, S.; Xue, Z.; Wen, M.; Li, X. Effect of organic maturity on shale gas genesis and pores development: A case study on marine shale in the upper Yangtze region, South China. Open Geosci. 2020, 12, 1617–1629. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Fu, C.; Zhu, Y.; Zuo, Z. Micro and nano-size pores of clay minerals in shale reservoirs: Implication for the accumulation of shale gas. Sediment. Geol. 2016, 342, 180–190. [Google Scholar] [CrossRef]
- Yang, X.; Guo, S. Comparative analysis of shale pore size characterization methods. Pet. Sci. Technol. 2020, 38, 793–799. [Google Scholar] [CrossRef]
- Nie, H.; Sun, C.; Liu, G.; Du, W.; He, Z. Dissolution pore types of the Wufeng Formation and the Longmaxi Formation in the Sichuan Basin, south China: Implications for shale gas enrichment. Mar. Pet. Geol. 2019, 101, 243–251. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Song, G.; Xuejun, W.; Teng, J.; Wang, M.; Bao, Y.; Yao, S.; Wang, W.; Zhang, S. Effect of shale diagenesis on pores and storage capacity in the Paleogene Shahejie Formation, Dongying Depression, Bohai Bay Basin, east China. Mar. Pet. Geol. 2019, 103, 738–752. [Google Scholar] [CrossRef]
- Bernard, S.; Horsfield, B.; Schulz, H.-M.; Wirth, R.; Schreiber, A.; Sherwood, N. Geochemical evolution of organic-rich shales with increasing maturity: A STXM and TEM study of the Posidonia Shale (Lower Toarcian, northern Germany). Mar. Pet. Geol. 2012, 31, 70–89. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef]
- Löhr, S.; Baruch, E.; Hall, P.; Kennedy, M. Is organic pore development in gas shales influenced by the primary porosity and structure of thermally immature organic matter? Org. Geochem. 2015, 87, 119–132. [Google Scholar] [CrossRef]
- Wei, L.; Mastalerz, M.; Schimmelmann, A.; Chen, Y. Influence of Soxhlet-extractable bitumen and oil on porosity in thermally maturing organic-rich shales. Int. J. Coal Geol. 2014, 132, 38–50. [Google Scholar] [CrossRef]
- Emmings, J.F.; Dowey, P.J.; Taylor, K.G.; Davies, S.J.; Vane, C.H.; Moss-Hayes, V.; Rushton, J.C. Origin and implications of early diagenetic quartz in the Mississippian Bowland Shale Formation, Craven Basin, UK. Mar. Pet. Geol. 2020, 120, 104567. [Google Scholar] [CrossRef]
- van de Kamp, P.C. Smectite-illite-muscovite transformations, quartz dissolution, and silica release in shales. Clay Clay Miner. 2008, 56, 66–81. [Google Scholar] [CrossRef]
- Huo, Z.; Peng, J.; Zhang, J.; Tang, X.; Li, P.; Ding, J.; Li, Z.; Liu, Z.; Dong, Z.; Lei, Y. Factors influencing the development of diagenetic shrinkage macro-fractures in shale. J. Pet. Sci. Eng. 2019, 183, 106365. [Google Scholar] [CrossRef]
- Meng, Q.; Hao, F.; Tian, J. Origins of non-tectonic fractures in shale. Earth-Sci. Rev. 2021, 222, 103825. [Google Scholar] [CrossRef]
- Berger, G.; Lacharpagne, J.-C.; Velde, B.; Beaufort, D.; Lanson, B. Kinetic constraints on illitization reactions and the effects of organic diagenesis in sandstone/shale sequences. Appl. Geochem. 1997, 12, 23–35. [Google Scholar] [CrossRef]
- Cuadros, J. Modeling of smectite illitization in burial diagenesis environments. Geochim. Cosmochim. Acta 2006, 70, 4181–4195. [Google Scholar] [CrossRef]
- Guo, K.; Song, H.; Chen, X.; Du, X.; Zhong, L. Graphene oxide as an anti-shrinkage additive for resorcinol–formaldehyde composite aerogels. Phys. Chem. Chem. Phys. 2014, 16, 11603–11608. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Xian, C.-G. Permeability measurement of the fracture-matrix system with 3D embedded discrete fracture model. Pet. Sci. 2022, 19, 1757–1765. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Meilijson, A.; Finkelman-Torgeman, E.; Bialik, O.M.; Boudinot, F.G.; Steinberg, J.; Waldmann, N.D.; Benjamini, C.; Vinegar, H.; Makovsky, Y. Significance to hydrocarbon exploration of terrestrial organic matter introduced into deep marine systems: Insights from the lower Cretaceous in the Levant Basin. Mar. Pet. Geol. 2020, 122, 104671. [Google Scholar] [CrossRef]
- Agrawal, V.; Sharma, S. Molecular characterization of kerogen and its implications for determining hydrocarbon potential, organic matter sources and thermal maturity in Marcellus Shale. Fuel 2018, 228, 429–437. [Google Scholar] [CrossRef]
- Wang, G.-C.; Sun, M.-Z.; Gao, S.-F.; Tang, L. The origin, type and hydrocarbon generation potential of organic matter in a marine-continental transitional facies shale succession (Qaidam Basin, China). Sci. Rep. 2018, 8, 6568. [Google Scholar] [CrossRef]
- Xia, L.-W.; Cao, J.; Wang, M.; Mi, J.-L.; Wang, T.-T. A review of carbonates as hydrocarbon source rocks: Basic geochemistry and oil–gas generation. Pet. Sci. 2019, 16, 713–728. [Google Scholar] [CrossRef]
- Zhu, X.; Cai, J.; Wang, X.; Zhang, J.; Xu, J. Effects of organic components on the relationships between specific surface areas and organic matter in mudrocks. Int. J. Coal Geol. 2014, 133, 24–34. [Google Scholar] [CrossRef]
- Wu, J.; Liang, C.; Hu, Z.; Yang, R.; Xie, J.; Wang, R.; Zhao, J. Sedimentation mechanisms and enrichment of organic matter in the Ordovician Wufeng Formation-Silurian Longmaxi Formation in the Sichuan Basin. Mar. Pet. Geol. 2019, 101, 556–565. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.; Liang, L.; Zeng, Q. Methane adsorption on carbon models of the organic matter of organic-rich shales. Energy Fuels 2017, 31, 1489–1501. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Milliken, K.L.; Olson, T. Silica diagenesis, porosity evolution, and mechanical behavior in siliceous mudstones, Mowry Shale (Cretaceous), Rocky Mountains, USA. J. Sediment. Res. 2017, 87, 366–387. [Google Scholar] [CrossRef]
- Liang, C.; Cao, Y.; Liu, K.; Jiang, Z.; Wu, J.; Hao, F. Diagenetic variation at the lamina scale in lacustrine organic-rich shales: Implications for hydrocarbon migration and accumulation. Geochim. Cosmochim. Acta 2018, 229, 112–128. [Google Scholar] [CrossRef]



| Sample ID | Ro (%) | TOC (wt.%) | S2 (mg/g) | Kerogen Type |
|---|---|---|---|---|
| ye33 | 0.65 | 5.52 | 22.32 | II2 |
| Sample ID | Final Temperature | Ro | Gaseous Hydrocarbons | Liquid Hydrocarbons | Total Hydrocarbons |
|---|---|---|---|---|---|
| (°C) | (%) | mg/g | mg/g | mg/g | |
| ye33-200 | 200.0 | 0.71 | 0.00 | 2.62 | 2.62 |
| ye33-250 | 250.0 | 0.75 | 0.00 | 2.88 | 2.88 |
| ye33-300 | 300.0 | 0.79 | 0.03 | 3.47 | 3.50 |
| ye33-350 | 350.0 | 1.1 | 0.25 | 9.77 | 10.02 |
| ye33-400 | 400.0 | 1.19 | 1.68 | 17.02 | 18.70 |
| ye33-450 | 450.0 | 1.56 | 6.21 | 14.11 | 20.31 |
| ye33-500 | 500.0 | 1.78 | 13.57 | 7.08 | 20.65 |
| ye33-550 | 550.0 | 1.98 | 19.13 | 3.39 | 22.52 |
| ye33-600 | 600.0 | 2.13 | 20.51 | 2.21 | 22.72 |
| ye33-650 | 650.0 | 2.65 | 21.55 | 1.25 | 22.80 |
| Sample ID | Whole-Rock Mineral Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Clay | Quartz | Potassium Feldspar | Plagioclase | Calcite | Dolomite | Pyrite | Siderite | |
| ye33-200 | 27 | 12 | 0.4 | 1.7 | 56.9 | 0.9 | 0.4 | 0.7 |
| ye33-250 | 27.4 | 14.3 | 0.8 | 1.4 | 54.1 | 0.6 | 0.7 | 0.7 |
| ye33-300 | 29.9 | 12 | 0.5 | 1.3 | 53.7 | 1 | 0.7 | 0.9 |
| ye33-350 | 23.4 | 10.1 | 0.4 | 1.1 | 62.3 | 1.1 | 0.7 | 0.9 |
| ye33-400 | 20.6 | 13.6 | 0.8 | 1.6 | 61.2 | 1 | 0.3 | 0.9 |
| ye33-450 | 24.4 | 15 | 0.5 | 1.6 | 55 | 1.2 | 0.7 | 1.6 |
| ye33-500 | 30.5 | 18.7 | 0.9 | 1.4 | 45.4 | 1.8 | 0.3 | 1 |
| ye33-550 | 27.3 | 13.5 | 0.8 | 1.5 | 54.2 | 1.4 | 0.3 | 1 |
| ye33-600 | 30.1 | 13.8 | 0.5 | 1 | 52.8 | 0.8 | 0.1 | 0.9 |
| ye33-650 | 28.4 | 13.4 | 0.5 | 0.9 | 54.6 | 1 | 0.3 | 0.9 |
| Scheme 2 | CO2 | N2 | MIP | |||||
|---|---|---|---|---|---|---|---|---|
| Qm (mmol/g) | c | Q (mmol/g) | Stagnant N2 (cm3/g STP) | Q (cm3/g STP) | Retention Efficiency (%) | Breakthrough Pressure (MPa) | Injection Volume (cm3/g) | |
| ye33-200 | 0.115 | 34.47 | 0.062 | 0.40 | 25.60 | 64.30 | 2.26 | 0.17 |
| ye33-250 | 0.121 | 36.74 | 0.067 | 0.29 | 24.54 | 62.94 | 2.26 | 0.14 |
| ye33-300 | 0.100 | 43.91 | 0.060 | 0.26 | 21.09 | 69.21 | 1.85 | 0.18 |
| ye33-350 | 0.107 | 48.98 | 0.067 | 0.12 | 21.95 | 60.27 | 2.27 | 0.17 |
| ye33-400 | 0.086 | 48.55 | 0.054 | 0.00 | 22.47 | 60.56 | 2.26 | 0.18 |
| ye33-450 | 0.109 | 74.80 | 0.080 | 0.26 | 25.52 | 60.57 | 1.19 | 0.24 |
| ye33-500 | 0.128 | 91.06 | 0.099 | 0.73 | 30.98 | 55.52 | 2.26 | 0.20 |
| ye33-550 | 0.149 | 107.64 | 0.119 | 0.70 | 27.48 | 60.26 | 1.85 | 0.22 |
| ye33-600 | 0.149 | 123.77 | 0.123 | 0.66 | 34.55 | 61.56 | 1.85 | 0.21 |
| ye33-650 | 0.178 | 100.48 | 0.140 | 0.57 | 28.61 | 74.82 | 1.53 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, X.; Guo, S. Evolution of Pore Spaces in Marine Organic-Rich Shale: Insights from Multi-Scale Analysis of a Permian–Pennsylvanian Sample. Minerals 2024, 14, 392. https://doi.org/10.3390/min14040392
Wang Z, Yang X, Guo S. Evolution of Pore Spaces in Marine Organic-Rich Shale: Insights from Multi-Scale Analysis of a Permian–Pennsylvanian Sample. Minerals. 2024; 14(4):392. https://doi.org/10.3390/min14040392
Chicago/Turabian StyleWang, Zilong, Xiaoguang Yang, and Shaobin Guo. 2024. "Evolution of Pore Spaces in Marine Organic-Rich Shale: Insights from Multi-Scale Analysis of a Permian–Pennsylvanian Sample" Minerals 14, no. 4: 392. https://doi.org/10.3390/min14040392






