Stability of CO2 Fluid in Eclogitic Mantle Lithosphere: Thermodynamic Calculations
Abstract
:1. Introduction
2. Methods
3. Results
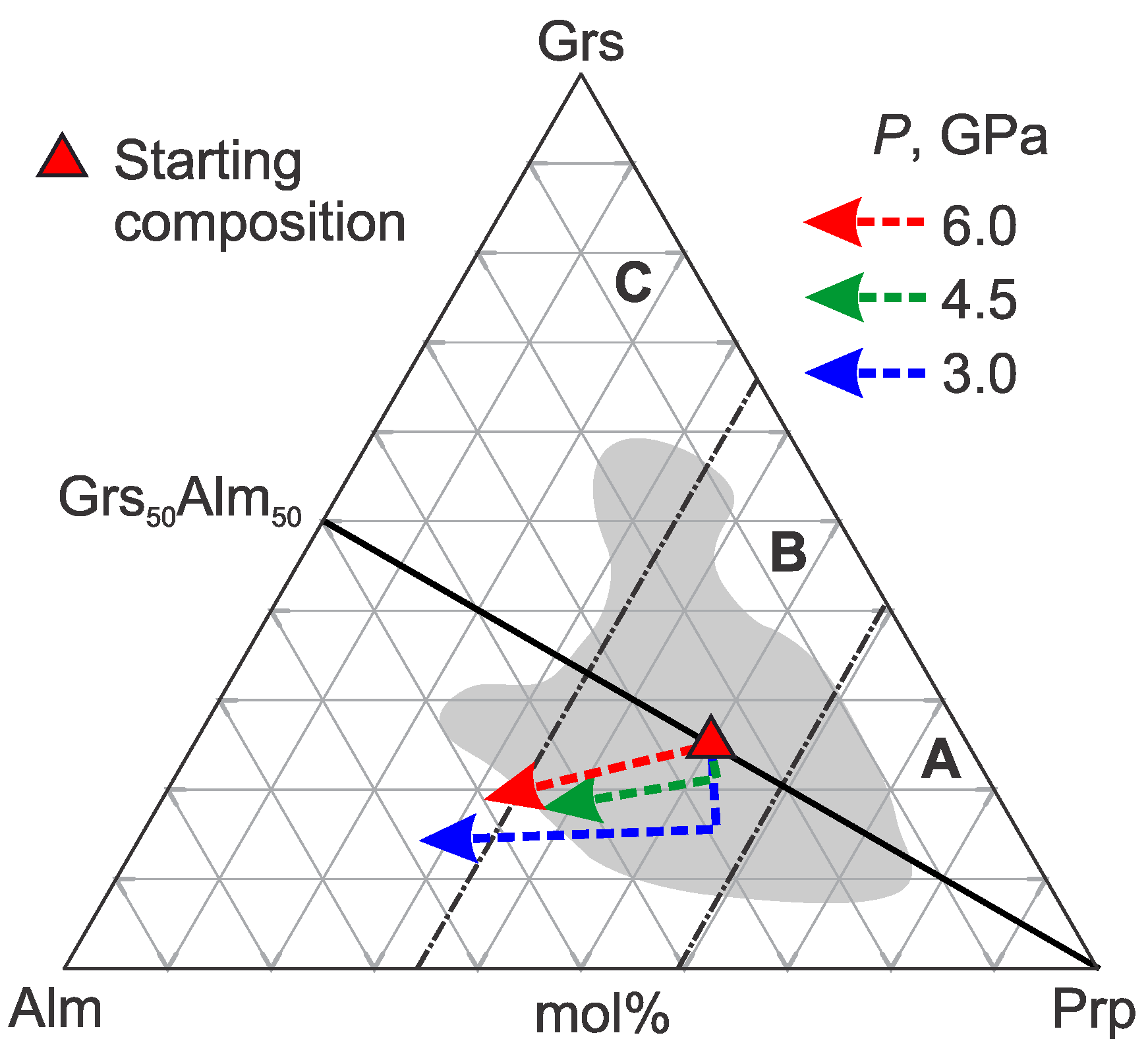
3.1. Pyrope–Grossular
3.2. Pyrope–Almandine–Grossular
3.3. Eclogite–CO2
4. Discussion
5. Conclusions
- (1)
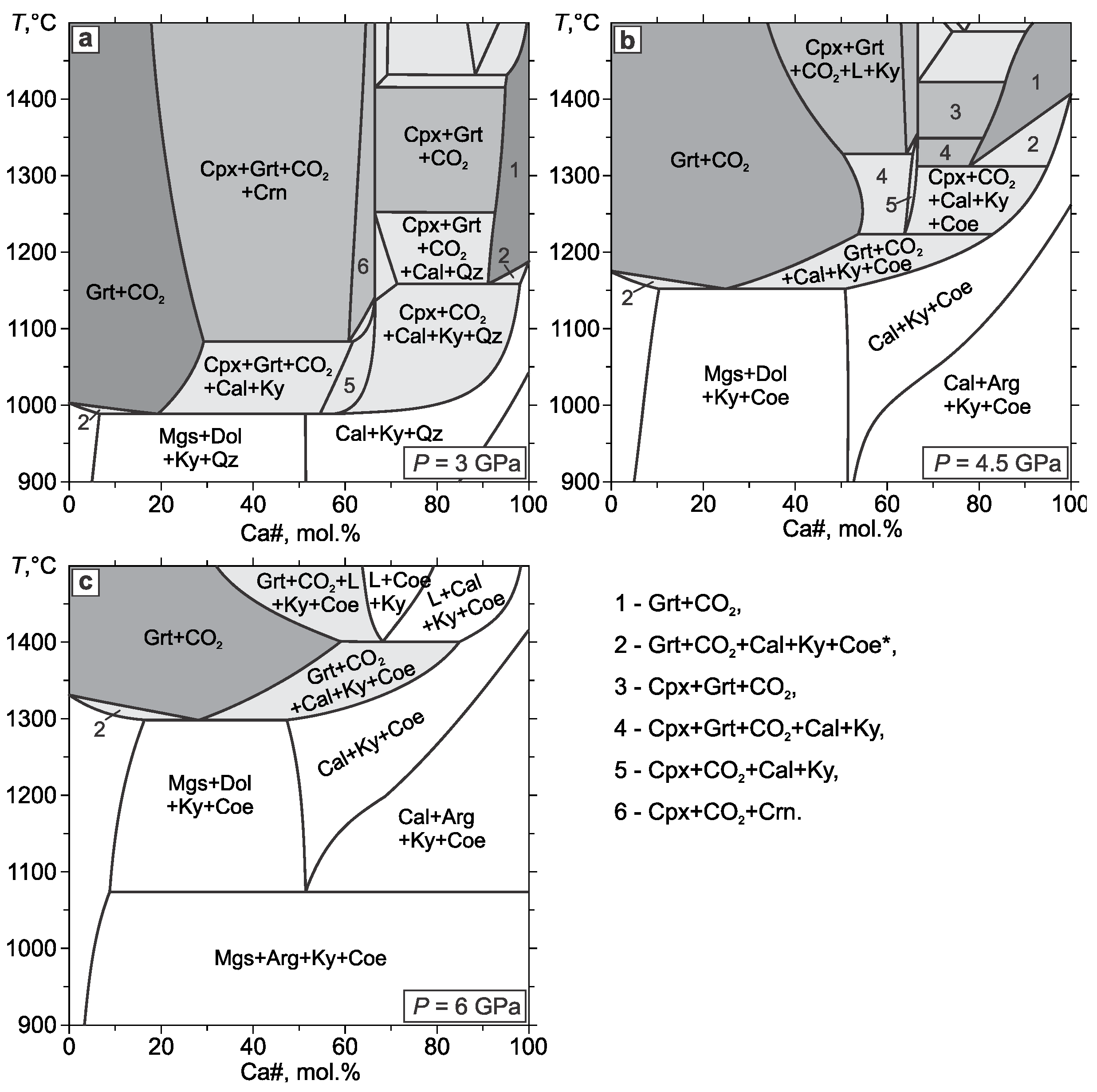
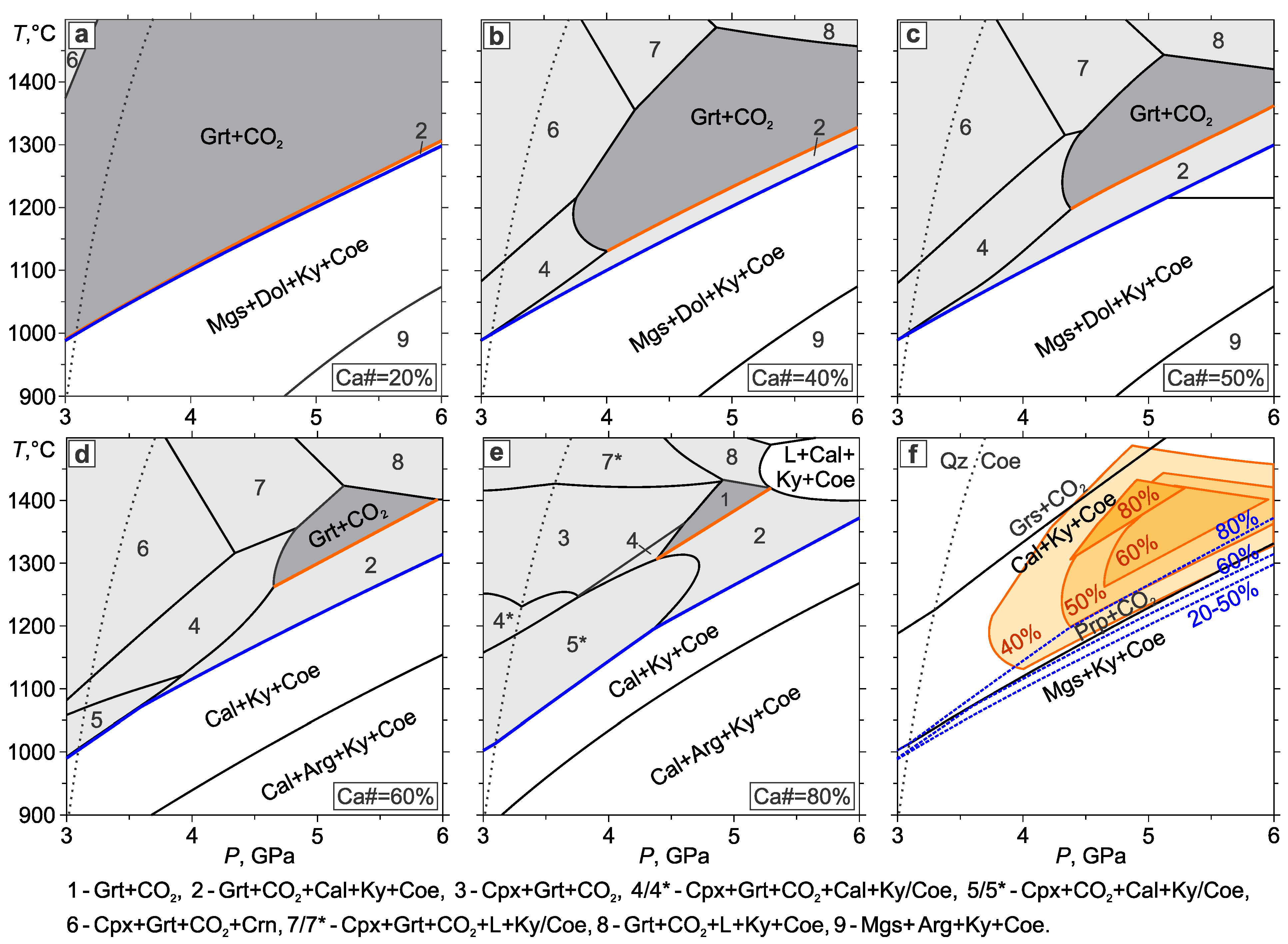
- In the Prp–Grs+3CO2 system, from the low-pressure and high-temperature side, the Grt+CO2 field is limited by the reaction of the partial decomposition of garnet:Grt → Cpx ± Grtnew ± Crn.On the high-pressure and low-temperature side, the Grt+CO2 field is restricted by the reaction of the partial carbonation of garnet:Grt + 3CO2 → Grtnew + CO2 + Cal + Ky + Coe.On the low-pressure and low-temperature side, the field Grt+CO2 is limited by the combination of reactions 2 and 3. On the high-pressure and high-temperature side, the field Grt+CO2 is restricted by the system solidus and the formation of a carbonate melt. We also expect that adding water, which generally lowers the solidus, should restrict the CO2 fluid stability field. At Ca# of 20–30, the reaction lines of the partial and full carbonation of the grant are almost identical. As the Ca# of the system increases above 30, the lower boundary of the Grt+CO2 field shifts towards low pressures and high temperatures. However, because the full carbonation line hardly changes its position, the area where garnet can coexist with CO2 remains almost unchanged when the Ca# changes from 20 to 80.
- (2)
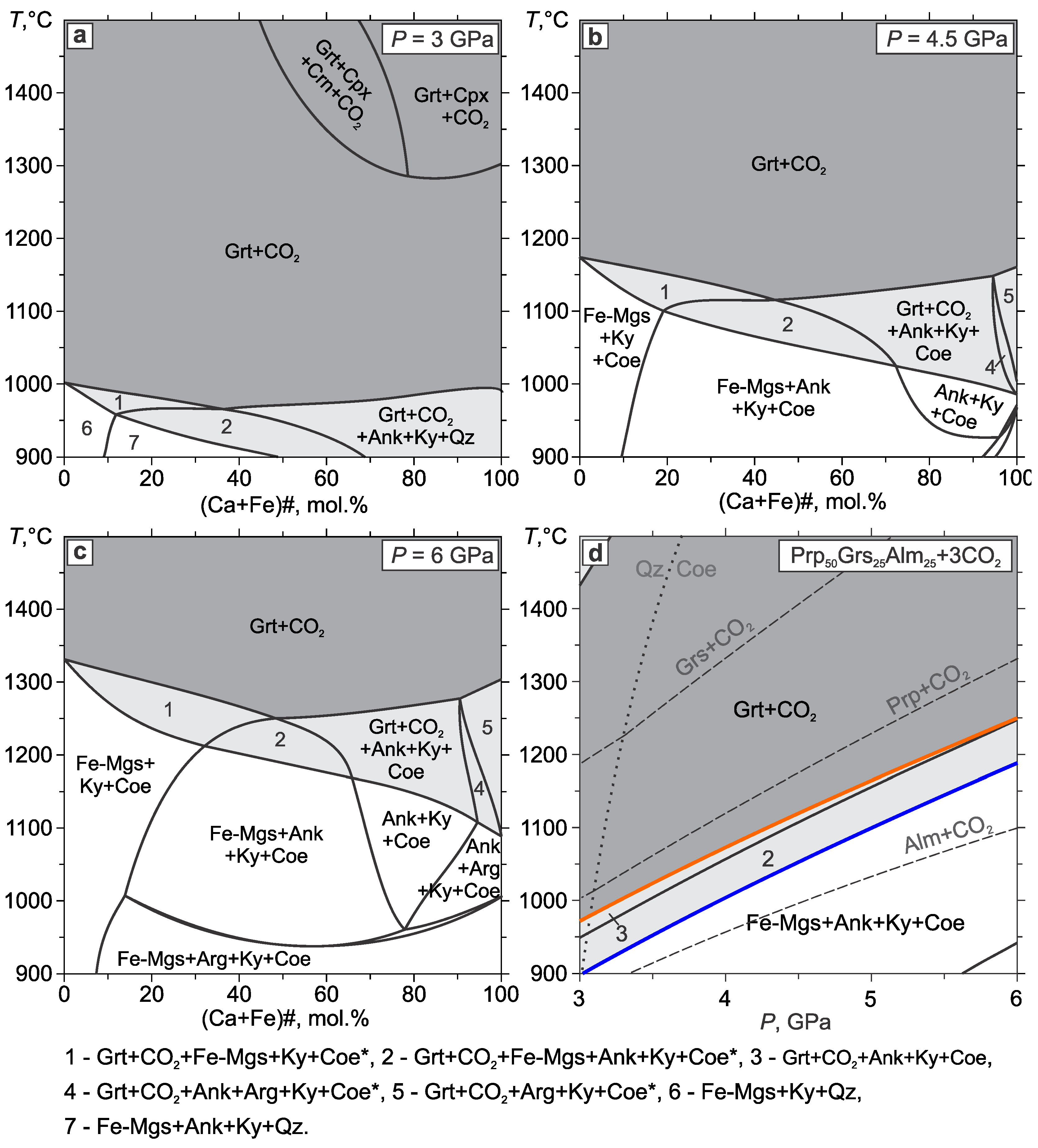
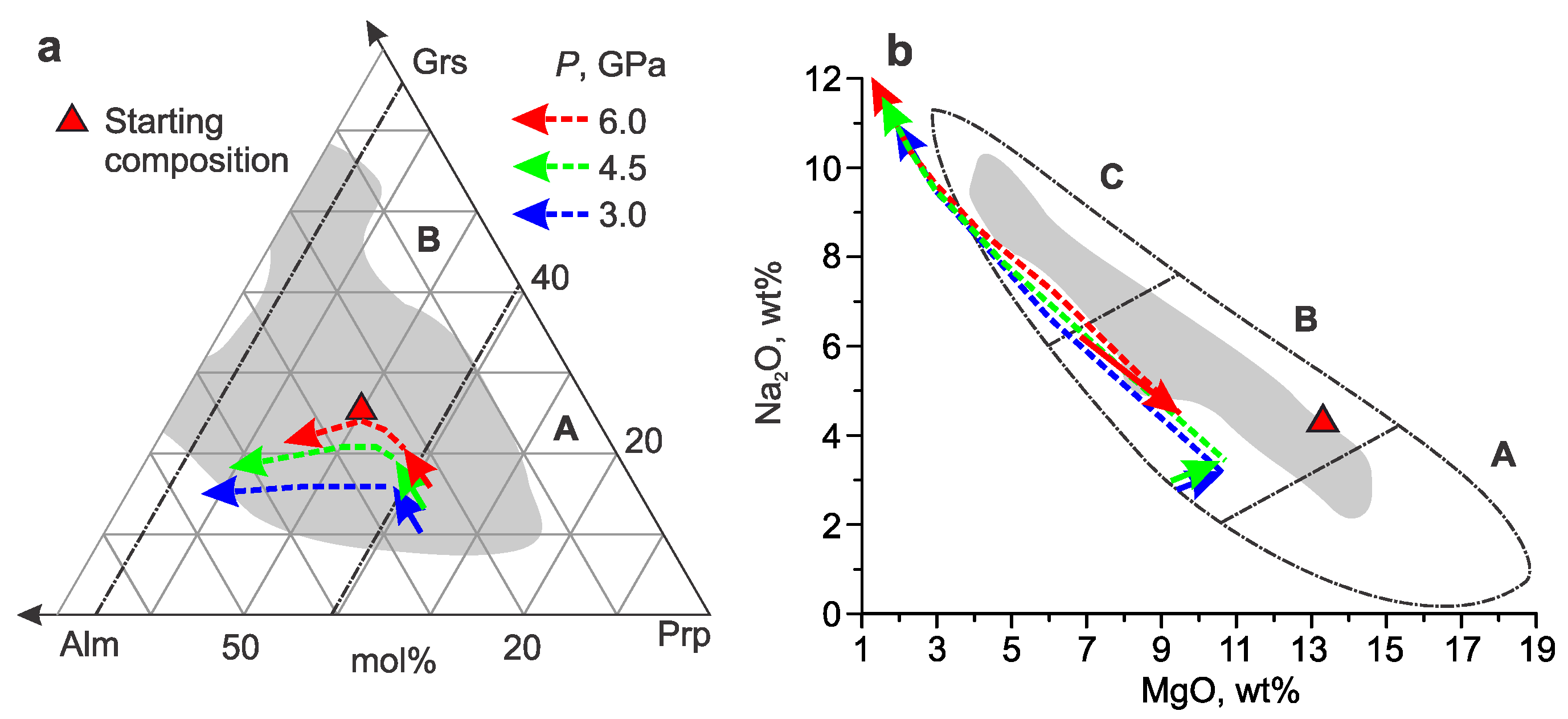
- The addition of almandine to the Prp–Grs+CO2 system shifts the limit of partial carbonation of garnet by 30–70 °C and the full carbonation of garnet by 100–140 °C below the Prp+CO2 line. The Grt+CO2 region appears at high temperatures and is continuous over the entire range of compositions. Along the Prp–Alm50Grs50 join, there is no garnet composition that would react with CO2 to produce the Carb+Ky+Coe assemblage directly. The regions of Grt+CO2 and Carb+Ky+Coe are separated by the Grt+CO2+Carb+Ky+Coe field. The composition of garnet in the field of partial carbonation is richer in the almandine component than that in the Grt+CO2 field.
- (3)
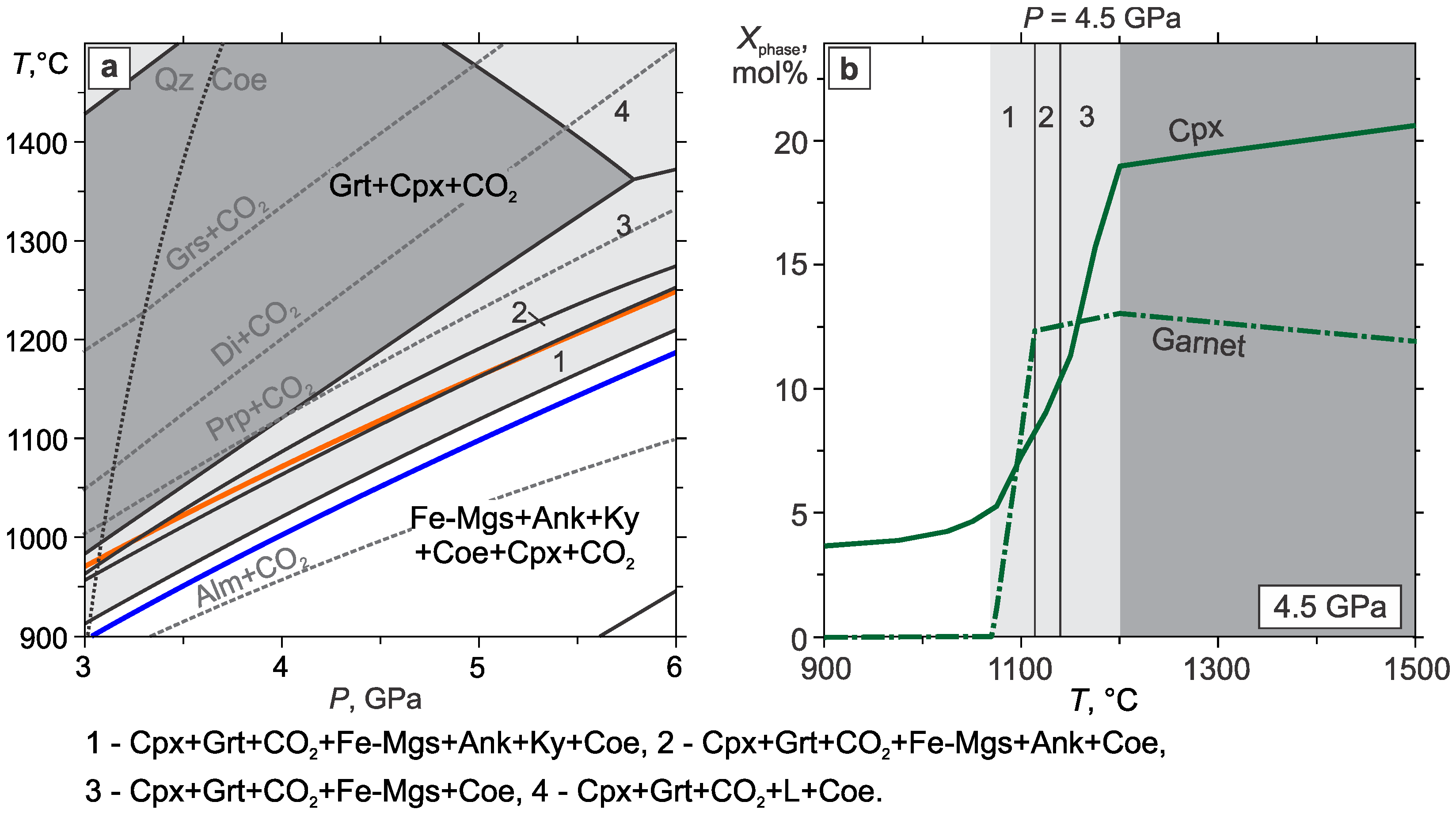
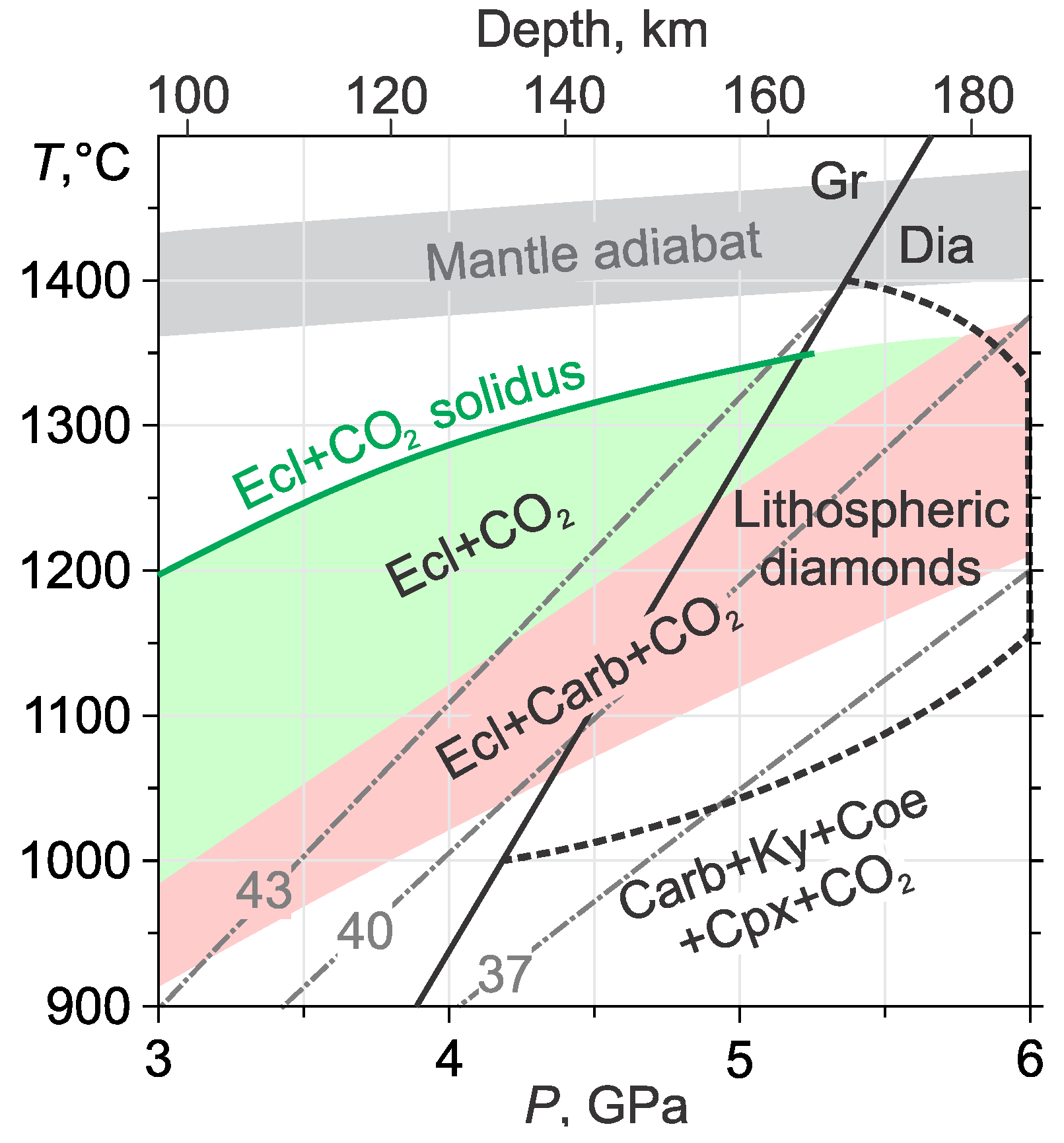
- In the eclogite–CO2 system, the lower boundary of the Grt+Cpx+CO2 assemblage almost coincides with the pyrope carbonation reaction. In the field of partial carbonation, the garnet and omphacite react with CO2 to form carbonate, kyanite, coesite, garnet, and clinopyroxene with a new composition. Due to the carbonation of clinopyroxene, the temperature of the partial carbonation of eclogite is significantly higher than that of garnet, which restricts the eclogite + CO2 stability field to pressures lower than the diamond stability field. The partial carbonation of the eclogite stabilizes the carbonate, kyanite, and coesite, wherein, the garnet composition shifts toward more almandine, while the clinopyroxene composition evolves to jadeite. This would extend the CO2 stability field in the eclogitic suite to lower temperatures. Yet, taking into account the fact that CO2 is in short supply under natural conditions, it should be completely spent on the carbonation of the eclogite just below the eclogite + CO2 field.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ank | ankerite |
| Alm | almandine |
| Arg | aragonite |
| Cal | calcite |
| Carb | carbonate |
| Coe | coesite |
| Cpx | clinopyroxene |
| Crn | corundum |
| Di | diopside |
| Dol | dolomite |
| Ecl | eclogite |
| Grs | grossular |
| Grt | garnet |
| Jd | jadeite |
| Ky | kyanite |
| Mgs | magnesite |
| Qz | quartz |
| Prp | pyrope |
References
- Wyllie, P.J.; Huang, W.L. Inflence of mantle CO2 ingeneration of carbonatites and kimberlites. Nature 1975, 257, 297–299. [Google Scholar] [CrossRef]
- Kerrick, D.M.; Connolly, J.A.D. Metamorphic devolatilization of subducted marine sediments and the transport of volatiles into the Earth’s mantle. Nature 2001, 411, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kerrick, D.M.; Connolly, J.A.D. Metamorphic devolatilization of subducted oceanic metabasalts: Implications for seismicity, arc magmatism and volatile recycling. Earth Planet. Sci. Lett. 2001, 189, 19–29. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Experimental demonstration of refractory carbonate-bearing eclogite and siliceous melt in the subduction regime. Earth Planet. Sci. Lett. 1994, 128, 313–325. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Withers, A.C. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 2004, 227, 73–85. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Origin of K-rich diamond-forming immiscible melts and CO2 fluid via partial melting of carbonated pelites at a depth of 180-200 km. Gondwana Res. 2019, 75, 154–171. [Google Scholar] [CrossRef]
- Shatsky, V.; Ragozin, A.; Zedgenizov, D.; Mityukhin, S. Evidence for multistage evolution in a xenolith of diamond-bearing eclogite from the Udachnaya kimberlite pipe. Lithos 2008, 105, 289–300. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Griffin, W.L. Diamond formation during metasomatism of mantle eclogite by chloride-carbonate melt. Contrib. Mineral. Petrol. 2018, 173, 84. [Google Scholar] [CrossRef]
- Roedder, E. Liquid CO2 inclusions in olivine-bearing nodules and phenocrysts from basalts. Am. Mineral. J. Earth Planet. Mater. 1965, 50, 1746–1782. [Google Scholar]
- Kennedy, G.C.; Nordlie, B.E. The genesis of diamond deposits. Econ. Geol. 1968, 63, 495–503. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Solid carbon dioxide in natural diamond. Nature 1993, 365, 42–44. [Google Scholar] [CrossRef]
- Chinn, I.L. A Study of Unusual Diamonds from the George Creek K1 Kimberlite Dyke, Colorado. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1995. [Google Scholar]
- Barannik, E.P.; Shiryaev, A.A.; Hainschwang, T. Shift of CO2-I absorption bands in diamond: A pressure or compositional effect? A FTIR mapping study. Diam. Relat. Mater. 2021, 113, 108280. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Ragozin, A.L.; Shatskii, V.S.; Shebanin, A.P. Variation in the fluid phase composition in the process of natural diamond crystallization. Dokl. Earth Sci. 2001, 379, 571–574. [Google Scholar]
- Smith, E.M.; Kopylova, M.G.; Frezzotti, M.L.; Afanasiev, V.P. Fluid inclusions in Ebelyakh diamonds: Evidence of CO2 liberation in eclogite and the effect of H2O on diamond habit. Lithos 2015, 216, 106–117. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Sobolev, N.V. Experimental study of interaction in the CO2-C system at mantle PT parameters. Dokl. Earth Sci. 2010, 435, 1492–1495. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W. Peridotite, kimberlite, and carbonatite explained in the system CaO-MgO-SiO2-CO2. Geology 1975, 3, 621–624. [Google Scholar] [CrossRef]
- Koziol, A.M.; Newton, R.C. Experimental determination of the reaction: Magnesite + enstatite = forsterite + CO2 in the ranges 6–25 kbar and 700–1100 °C. Am. Mineral. 1998, 83, 213–219. [Google Scholar] [CrossRef]
- Ragozin, A.L.; Shatsky, V.S.; Rylov, G.M.; Goryainov, S.V. Coesite inclusions in rounded diamonds from placers of the Northeastern Siberian Platform. Dokl. Earth Sci. 2002, 384, 385–389. [Google Scholar]
- Ragozin, A.L.; Shatskii, V.S.; Zedgenizov, D.A. New data on the growth environment of diamonds of the variety V from placers of the Northeastern Siberian platform. Dokl. Earth Sci. 2009, 425, 436–440. [Google Scholar] [CrossRef]
- Rohrbach, A.; Schmidt, M.W. Redox freezing and melting in the Earth’s deep mantle resulting from carbon-iron redox coupling. Nature 2011, 472, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Knoche, R.; Sweeney, R.J.; Luth, R.W. Carbonation and decarbonation of eclogites: The role of garnet. Contrib. Mineral. Petrol. 1999, 135, 332–339. [Google Scholar] [CrossRef]
- Luth, R.W. Experimental study of the CaMgSi2O6-CO2 system at 3–8 GPa. Contrib. Mineral. Petrol. 2006, 151, 141–157. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Novoselov, I.D.; Kruk, A.N.; Furman, O.V.; Reutsky, V.N.; Palyanov, Y.N. Experimental modeling of decarbonation reactions resulting in Mg, Fe-garnets and CO2 fluid at the mantle P–T parameters. Russ. Geol. Geophys. 2020, 61, 650–662. [Google Scholar] [CrossRef]
- Shatskiy, A.; Vinogradova, Y.G.; Arefiev, A.V.; Litasov, K.D. Revision of the CaMgSi2O6−CO2 P-T phase diagram at 3–6 GPa. Am. Mineral. 2023, 108, 2338–2347. [Google Scholar] [CrossRef]
- Vinogradova, Y.G.; Shatskiy, A.; Arefiev, A.V.; Litasov, K.D. The equilibrium boundary of the reaction Mg3Al2Si3O12 + 3CO2 = Al2SiO5 + 2SiO2 + 3MgCO3 at 3–6 GPa. Am. Mineral. 2024, 109, 384–391. [Google Scholar] [CrossRef]
- Shatskiy, A.; Vinogradova, Y.G.; Arefiev, A.V.; Litasov, K.D. The system NaAlSi2O6–CaMgSi2O6−CO2 at 3–6.5 GPa: Implications for CO2 stability in the eclogitic suite at depths of 100–200 km. Contrib. Mineral. Petrol. 2023, 178, 22. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Kruk, A.N.; Novoselov, I.D.; Furman, O.V.; Palyanov, Y.N. Decarbonation reactions involving ankerite and dolomite under upper mantle P, T-parameters: Experimental modeling. Minerals 2020, 10, 715. [Google Scholar] [CrossRef]
- Vinogradova, Y.G.; Shatskiy, A.F.; Litasov, K.D. Thermodynamic analysis of the reactions of CO2-fluid with garnets and clinopyroxenes at 3–6 GPa. Geochem. Int. 2021, 59, 851–857. [Google Scholar] [CrossRef]
- Novoselov, I.D.; Palyanov, Y.N.; Bataleva, Y.V. Experimental modeling of the interaction between garnets of mantle parageneses and CO2 fluid at 6.3 GPa and 950–1550 °C. Russ. Geol. Geophys. 2023, 64, 379–393. [Google Scholar] [CrossRef]
- Connolly, J.A.D. The geodynamic equation of state: What and how. Geochem. Geophys. Geosystems 2009, 10, Q10014. [Google Scholar] [CrossRef]
- Connolly, J. Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet. Sci. Lett. 2005, 236, 524–541. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 2011, 29, 333–383. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Green, E.C.R.; Powell, R. Melting of peridotites through to granites: A simple thermodynamic model in the system KNCFMASHTOCr. J. Petrol. 2018, 59, 881–900. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R.T.J.B. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 1998, 16, 309–343. [Google Scholar] [CrossRef]
- Franzolin, E.; Schmidt, M.W.; Poli, S. Ternary Ca–Fe–Mg carbonates: Subsolidus phase relations at 3.5 GPa and a thermodynamic solid solution model including order/disorder. Contrib. Mineral. Petrol. 2011, 161, 213–227. [Google Scholar] [CrossRef]
- Holland, T.; Powell, R. A Compensated-Redlich-Kwong (CORK) equation for volumes and fugacities of CO2 and H2O in the range 1 bar to 50 kbar and 100–1600 °C. Contrib. Mineral. Petrol. 1991, 109, 265–273. [Google Scholar] [CrossRef]
- Boyd, F.R. Garnet peridotites and the system CaSiO3-MgSiO3-Al2O3. Mineral. Soc. Amer. Spec. Pap. 1970, 3, 63–75. [Google Scholar]
- Maaloe, S.; Wyllie, P.J. Join grossularite-pyrope at 30 kb and its petrological significance. Am. J. Sci. 1979, 279, 288–301. [Google Scholar] [CrossRef]
- Sekine, T.; Wyllie, P.J. Phase relationships in the join grossularite-pyrope 7.5 persent H2O at 30 kb. Am. J. Sci. 1983, 283, 435–453. [Google Scholar] [CrossRef]
- Surkov, N.V.; Gartvich, Y.G. Experimental study of phase equilibria in the pyrope-grossular join at a pressure of 30 kbar. Petrology 2000, 8, 84–95. [Google Scholar]
- Orlando, A.; Borrini, D. Synthesis of pyrope-grossular garnets: An experimental study at P = 2.5 GPa. Mineral. Petrol. 2003, 78, 37–51. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasov, K.D.; Kupriyanov, I.N.; Ohtani, E.; Palyanov, Y.N. Phase relations in the system FeCO3-CaCO3 at 6 GPa and 900–1700 °C and its relation to the system CaCO3-FeCO3-MgCO3. Am. Mineral. 2014, 99, 773–785. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Minin, D.A.; Chanyshev, A.D.; Litasov, K.D. Revision of the CaCO3–MgCO3 phase diagram at 3 and 6 GPa. Am. Mineral. 2018, 103, 441–452. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Ohtani, E.; Borzdov, Y.M.; Khmelnikov, A.I.; Palyanov, Y.N. Phase relations in the K2CO3-FeCO3 and MgCO3-FeCO3 systems at 6 GPa and 900–1700 °C. Eur. J. Mineral. 2015, 27, 487–499. [Google Scholar] [CrossRef]
- Taylor, L.A.; Snyder, G.A.; Keller, R.; Remley, D.A.; Anand, M.; Wiesli, R.; Valley, J.; Sobolev, N.V. Petrogenesis of group A eclogites and websterites: Evidence from the Obnazhennaya kimberlite, Yakutia. Contrib. Mineral. Petrol. 2003, 145, 424–443. [Google Scholar] [CrossRef]
- Taylor, L.A.; Neal, C.R. Eclogites with oceanic crustal and mantle signatures from the Bellsbank kimberlite, South Africa, Part I: Mineralogy, petrography, and whole rock chemistry. J. Geol. 1989, 97, 551–567. [Google Scholar] [CrossRef]
- Coleman, R.G.; Lee, D.E.; Beatty, L.B.; Brannock, W.W. Eclogites and eclogites: Their differences and similarities. Geol. Soc. Am. Bull. 1965, 76, 483–508. [Google Scholar] [CrossRef]
- Day, H.W. A revised diamond-graphite transition curve. Am. Mineral. 2012, 97, 52–62. [Google Scholar] [CrossRef]
- Hasterok, D.; Chapman, D.S. Heat production and geotherms for the continental lithosphere. Earth Planet. Sci. Lett. 2011, 307, 59–70. [Google Scholar] [CrossRef]
- Katsura, T. A revised adiabatic temperature profile for the mantle. J. Geophys. Res. Solid Earth 2022, 127, e2021JB023562. [Google Scholar] [CrossRef]
- Berman, R.G. Thermobarometry using multi-equilibrium calculations: A new technique with petrological application. Can. Mineral. 1991, 29, 833–855. [Google Scholar]
- Ogasawara, Y.; Liou, J.G.; Zhang, R.Y. Thermochemical calculation of log fO2–T–P stability relations of diamond-bearing assemblages in the model system CaO–MgO–SiO2–CO2–H2O. Russ. Geol. Geophys. 1997, 2, 587–598. [Google Scholar]
- Huang, W.L.; Wyllie, P.J. Melting relationships in the systems CaO-CO2 and MgO-CO2 to 33 kilobars. Geochim. Cosmochim. Acta 1976, 40, 129–132. [Google Scholar]
- Dalton, J.A.; Presnall, D.C. Carbonatitic melts along the solidus of model lherzolite in the system CaO-MgO-Al2O3-SiO2-CO2 from 3 to 7 GPa. Contrib. Mineral. Petrol. 1998, 131, 123–135. [Google Scholar] [CrossRef]
- Sokol, A.G.; Palyanova, G.A.; Palyanov, Y.N.; Tomilenko, A.A.; Melenevsky, V.N. Fluid regime and diamond formation in the reduced mantle: Experimental constraints. Geochim. Et Cosmochim. Acta 2009, 73, 5820–5834. [Google Scholar] [CrossRef]
- Sokol, A.G.; Pal’yanov, Y.N.; Pal’yanova, G.A.; Tomilenko, A.A. Diamond crystallization in fluid and carbonate-fluid systems under mantle P-T conditions: 1. Fluid composition. Geochem. Int. 2004, 42, 830–838. [Google Scholar]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Liquid immiscibility and phase relations in the join KAlSi3O8–CaMg(CO3)2 ± NaAlSi2O6 ± Na2CO3 at 6 GPa: Implications for diamond-forming melts. Chem. Geol. 2020, 550, 119701. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Effect of water on carbonate-silicate liquid immiscibility in the system KAlSi3O8–CaMgSi2O6–NaAlSi2O6–CaMg(CO3)2 at 6 GPa: Implications for diamond-forming melts. Am. Mineral. 2021, 106, 165–173. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Pal’yanova, G.A.; Borzdov, Y.M.; Sobolev, N.V. Diamond and graphite crystallization in COH fluid at PT parameters of the natural diamond formation. Dokl. Earth Sci. 2000, 375, 1395–1398. [Google Scholar]
- Sokol, A.G.; Pal’yanov, Y.N.; Pal’yanova, G.A.; Khokhryakov, A.F.; Borzdov, Y.M. Diamond and graphite crystallization from C-O-H fluids under high pressure and high temperature conditions. Diam. Relat. Mater. 2001, 10, 2131–2136. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N. Diamond formation via carbonate or CO2 reduction under pressures and temperatures of the lithospheric mantle: Review of experimental data. Minerals 2023, 13, 940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradova, Y.G.; Shatskiy, A. Stability of CO2 Fluid in Eclogitic Mantle Lithosphere: Thermodynamic Calculations. Minerals 2024, 14, 403. https://doi.org/10.3390/min14040403
Vinogradova YG, Shatskiy A. Stability of CO2 Fluid in Eclogitic Mantle Lithosphere: Thermodynamic Calculations. Minerals. 2024; 14(4):403. https://doi.org/10.3390/min14040403
Chicago/Turabian StyleVinogradova, Yulia G., and Anton Shatskiy. 2024. "Stability of CO2 Fluid in Eclogitic Mantle Lithosphere: Thermodynamic Calculations" Minerals 14, no. 4: 403. https://doi.org/10.3390/min14040403





