Experimental Investigation on Gallium and Germanium Migration in Coal Gangue Combustion
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
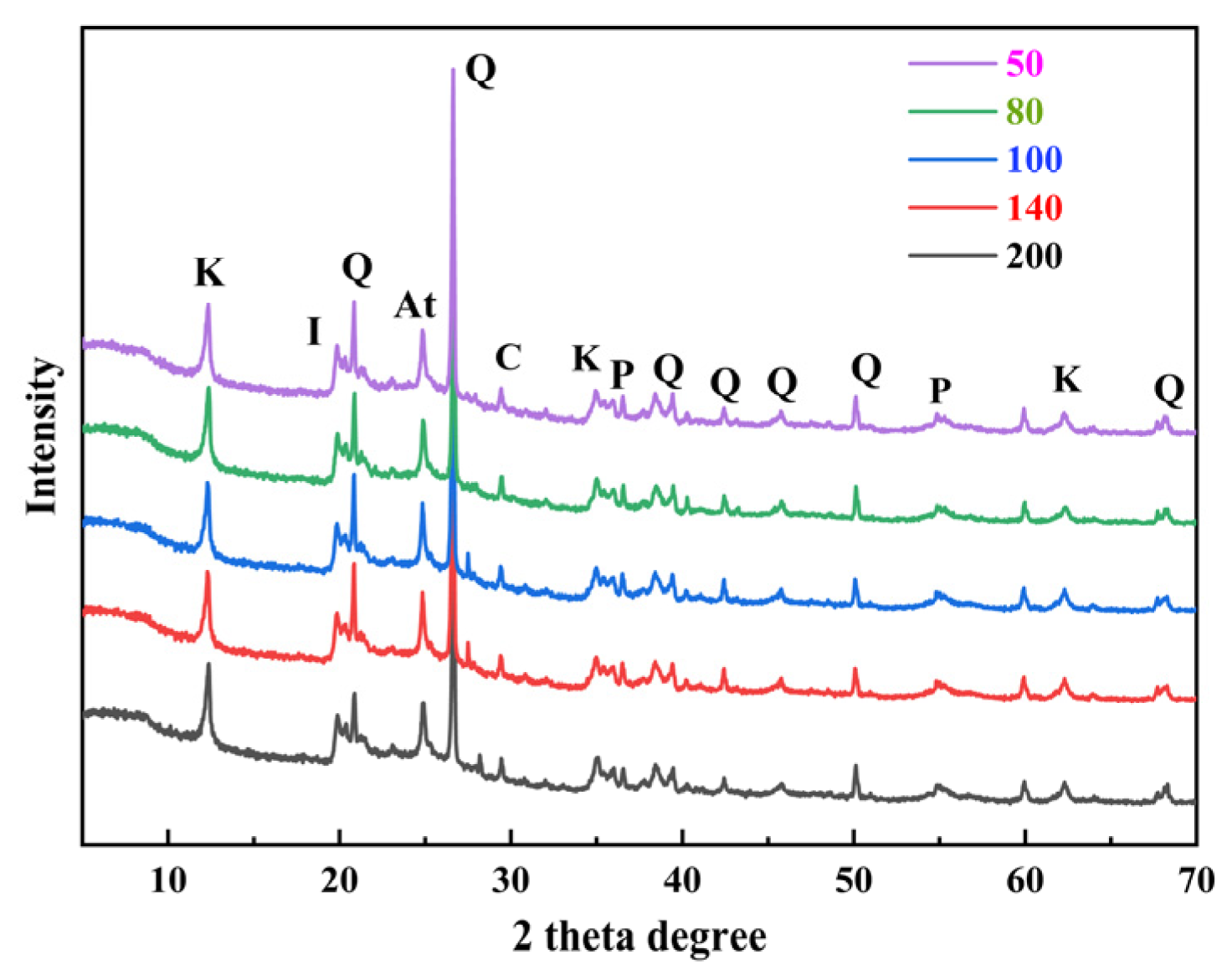
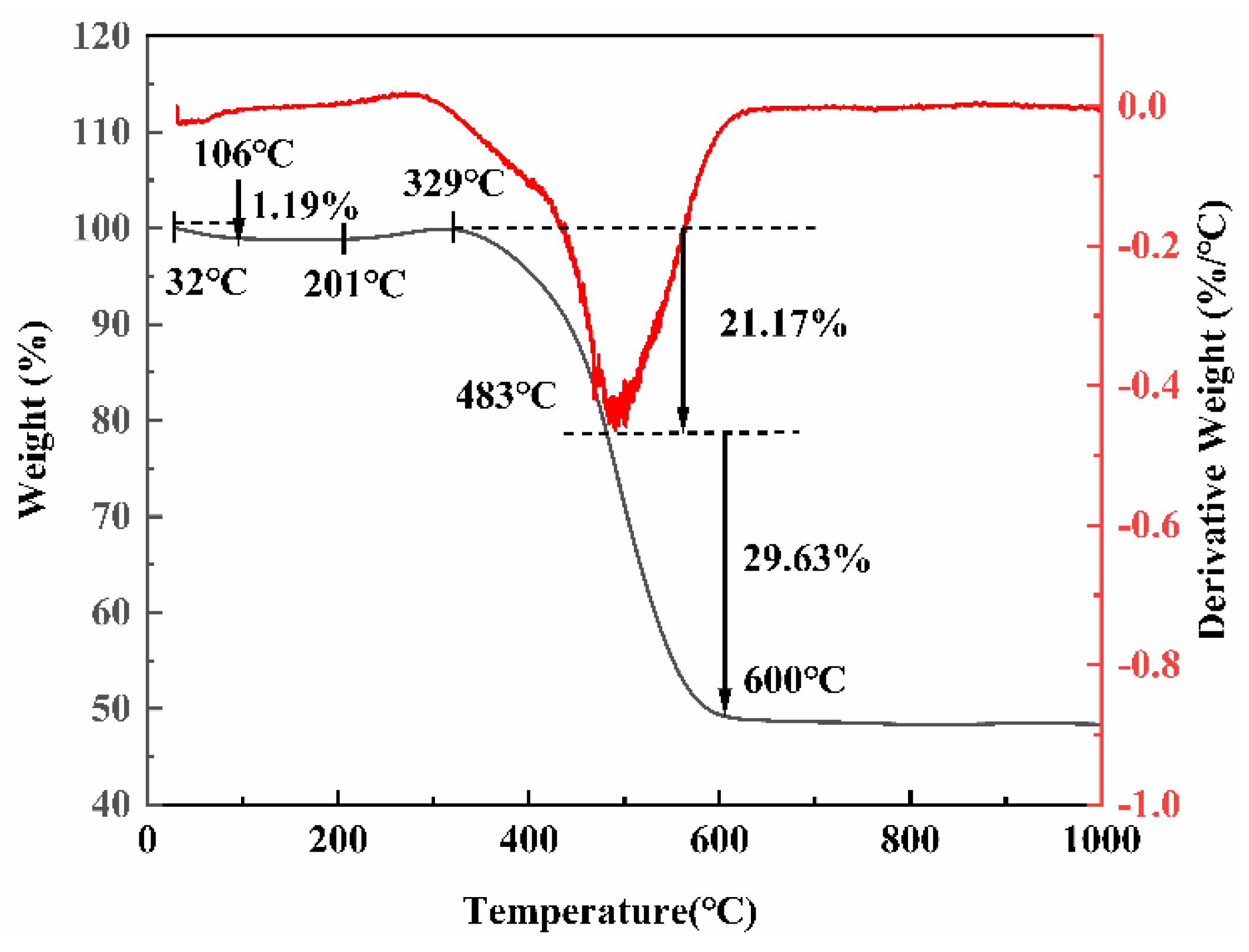
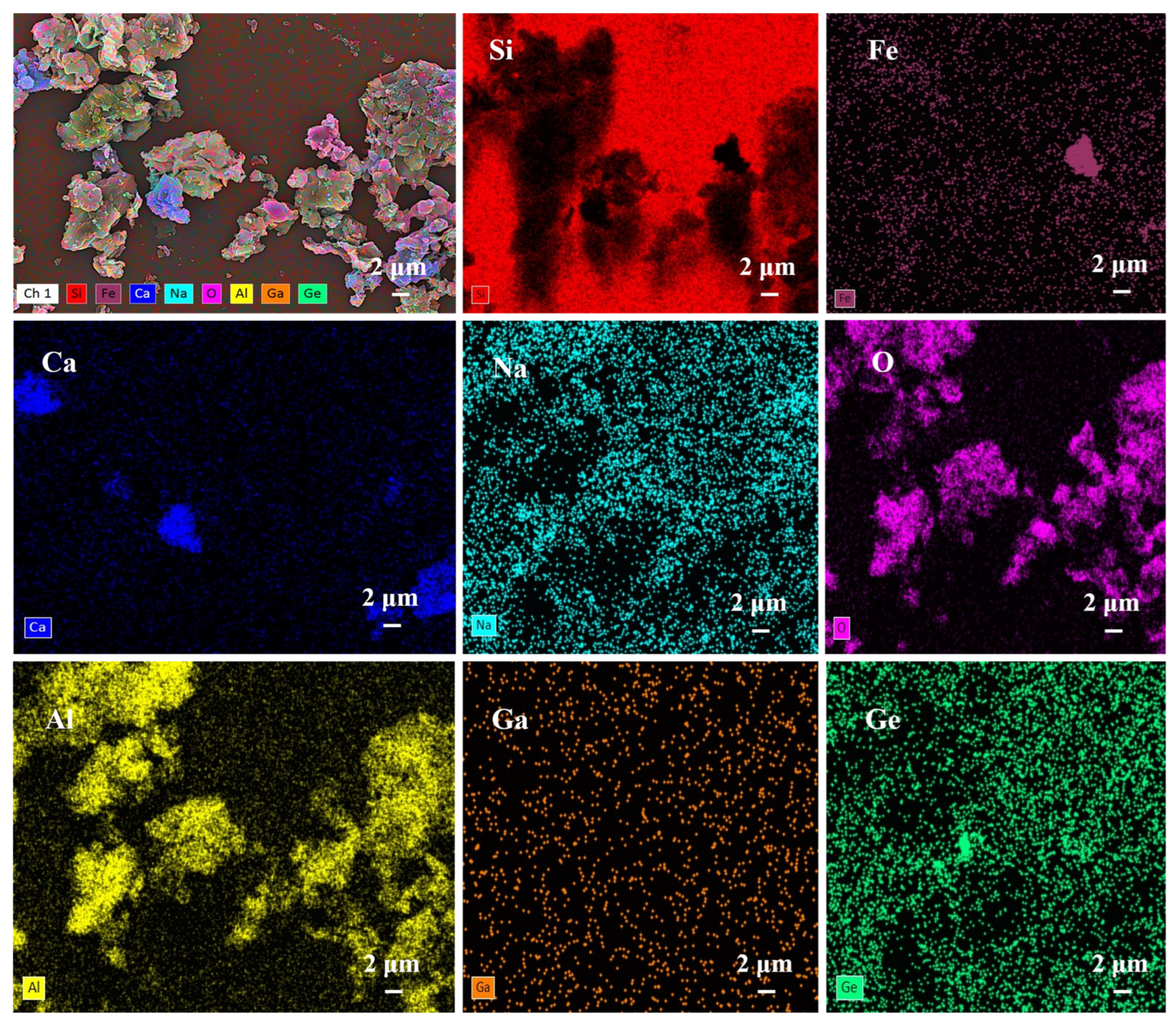
3.1. Geochemical Characteristics of Ga and Ge in Coal Gangue
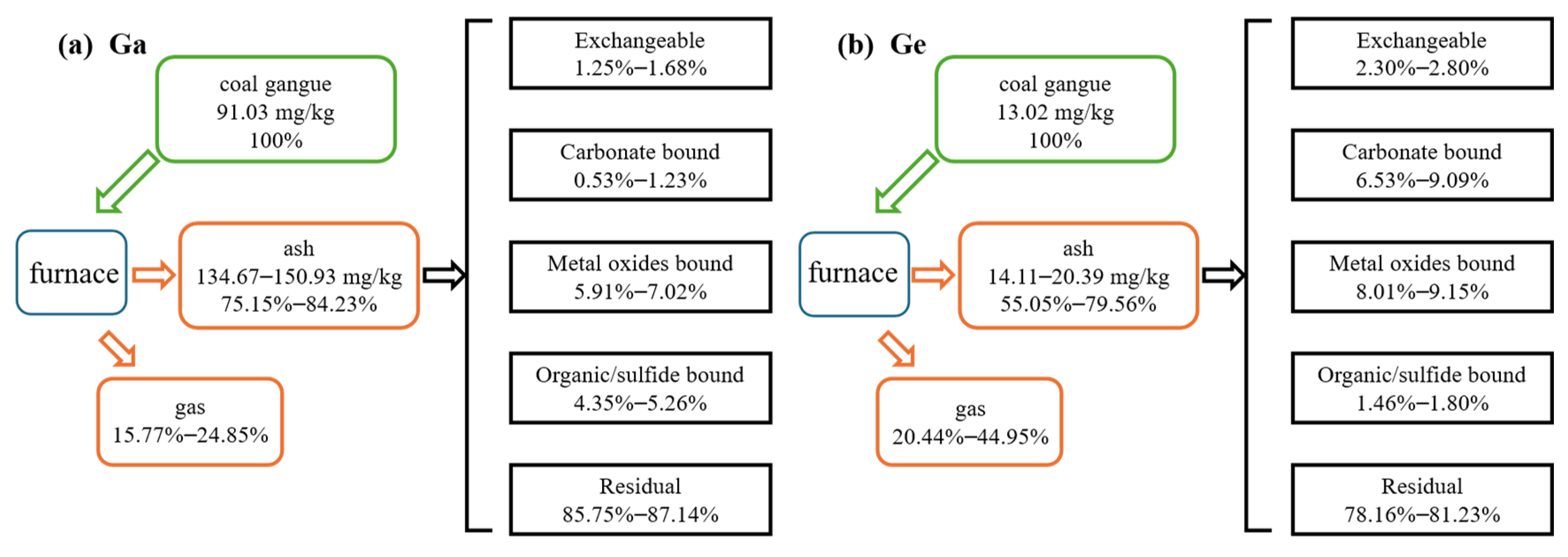
3.2. The Redistribution Characterizations of Ga and Ge during Coal Gangue Combustion
3.3. The Transformation Characterizations of Ga and Ge during Gangue Combustion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Liu, G.; Cheng, S.; Fang, T.; Lam, P.K.S. Thermochemical and Trace Element Behavior of Coal Gangue, Agricultural Biomass and Their Blends during Co-Combustion. Bioresour. Technol. 2014, 166, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Han, Z.; Jia, J.; Wei, C.; Liu, Q.; Wang, X.; Zhou, J.; Li, F.; Miao, S. Occurrence and Distribution of Gallium, Scandium, and Rare Earth Elements in Coal Gangue Collected from Junggar Basin, China. Int. J. Coal Prep. Util. 2019, 39, 389–402. [Google Scholar] [CrossRef]
- Querol, X.; Izquierdo, M.; Monfort, E.; Álvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental Characterization of Burnt Coal Gangue Banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a Promising Source of Critical Elements: Progress and Future Prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Hu, Y.; You, M.; Liu, G.; Dong, Z.; Jiao, F.; Meng, Y. The Potential Utilizing of Critical Element from Coal and Combustion Residues. Energies 2021, 14, 4710. [Google Scholar] [CrossRef]
- Long, J.; Zhang, S.; Luo, K. Discovery of Anomalous Gallium Enriched in Stone Coal: Significance, Provenance and Recommendations. Geosci. Front. 2023, 14, 101538. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability Evaluation of Essential Critical Raw Materials: Cobalt, Niobium, Tungsten and Rare Earth Elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and Recovery of Critical Rare Elements from Contaminated Waters by Living Gracilaria Gracilis. J. Hazard. Mater. 2018, 344, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fan, M.; Tiand, H. Coal and Coal Byproducts: A Large and Developable Unconventional Resource for Critical Materials—Rare Earth Elements. J. Rare Earths 2018, 36, 337–338. [Google Scholar] [CrossRef]
- Zhang, Y.; Nakano, J.; Liu, L.; Wang, X.; Zhang, Z. Trace Element Partitioning Behavior of Coal Gangue-Fired CFB Plant: Experimental and Equilibrium Calculation. Environ. Sci. Pollut. Res. 2015, 22, 15469–15478. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.; Wang, X.; Zhao, L.; Ren, D.; Finkelman, R.B. Valuable Elements in Chinese Coals: A Review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; Huang, W.; Li, T.; Li, X.; Liu, H.; Zhao, L.; et al. Petrology, Mineralogy, and Geochemistry of the Ge-Rich Coal from the Wulantuga Ge Ore Deposit, Inner Mongolia, China: New Data and Genetic Implications. Int. J. Coal Geol. 2012, 90, 72–99. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D.; Ma, Y.; Sun, Y. Mineralogy and Geochemistry of Boehmite-Rich Coals: New Insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive Utilization and Environmental Risks of Coal Gangue: A Review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Potentially Useful Elements (Al, Fe, Ga, Ge, U) in Coal Gangue: A Case Study in Weibei Coal Mining Area, Shaanxi Province, Northwestern China. Environ. Sci. Pollut. Res. 2018, 25, 11893–11904. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, X.; Querol, X.; Font, O.; Moreno, N. A Review on the Applications of Coal Combustion Products in China. Int. Geol. Rev. 2018, 60, 671–716. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, Origin and Significance of Mineral Matter in Coal: An Updated Review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Matjie, R.H.; French, D.; Ward, C.R.; Pistorius, P.C.; Li, Z. Behaviour of Coal Mineral Matter in Sintering and Slagging of Ash during the Gasification Process. Fuel Process. Technol. 2011, 92, 1426–1433. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.; Yuan, W.; Liu, B.; Querol, X.; Font, O.; Moreno, N.; Li, J.; Gang, T.; Liang, G. Mineral Composition and Geochemical Characteristics of the Li-Ga-Rich Coals in the Buertaohai-Tianjiashipan Mining District, Jungar Coalfield, Inner Mongolia. Int. J. Coal Geol. 2016, 167, 157–175. [Google Scholar] [CrossRef]
- Qin, S.; Sun, Y.; Li, Y.; Wang, J.; Zhao, C.; Gao, K. Coal Deposits as Promising Alternative Sources for Gallium. Earth-Sci. Rev. 2015, 150, 95–101. [Google Scholar] [CrossRef]
- Wei, Q.; Cui, C.; Dai, S. Organic Association of Ge in the Coal-Hosted Ore Deposits: An Experimental and Theoretical Approach. Ore Geol. Rev. 2020, 117, 103291. [Google Scholar] [CrossRef]
- Besari, D.A.A.; Anggara, F.; Rosita, W.; Petrus, H.T. Characterization and Mode of Occurrence of Rare Earth Elements and Yttrium in Fly and Bottom Ash from Coal-Fired Power Plants in Java, Indonesia. Int. J. Coal Sci. Technol. 2022, 9, 20. [Google Scholar] [CrossRef]
- Fu, B.; Liu, G.; Sun, M.; Hower, J.C.; Mian, M.M.; Wu, D.; Wang, R.; Hu, G. Emission and Transformation Behavior of Minerals and Hazardous Trace Elements (HTEs) during Coal Combustion in a Circulating Fluidized Bed Boiler. Environ. Pollut. 2018, 242, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Sheng, W.; Li, L.; Zheng, L.; Miao, C.; Sun, R. Alteration Behavior of Mineral Structure and Hazardous Elements during Combustion of Coal from a Power Plant at Huainan, Anhui, China. Environ. Pollut. 2018, 239, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; Xie, P.; Jiang, Y.; Hood, M.M.; O’Keefe, J.M.; Song, H. Petrological, Geochemical, and Mineralogical Compositions of the Low-Ge Coals from the Shengli Coalfield, China: A Comparative Study with Ge-Rich Coals and a Formation Model for Coal-Hosted Ge Ore Deposit. Ore Geol. Rev. 2015, 71, 318–349. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Jiang, J.; Hower, J.C.; Song, X.; Jiang, Y.; Wang, X.; Gornostaeva, T.; Li, X.; et al. Composition and Modes of Occurrence of Minerals and Elements in Coal Combustion Products Derived from High-Ge Coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Shao, P.; Wang, W.; Chen, L.; Duan, P.; Qian, F.; Ma, M.; Xiong, W.; Yu, S. Distribution, Occurrence, and Enrichment of Gallium in the Middle Jurassic Coals of the Muli Coalfield, Qinghai, China. J. Geochem. Explor. 2018, 185, 116–129. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Characterization and recovery of rare earth elements and other critical metals (Co, Cr, Li, Mn, Sr, and V) from the calcination products of a coal refuse sample. Fuel 2020, 267, 117236. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Zhou, C.; Du, J.; Zhang, Y.; Sun, J.; Wu, W.; Liu, G. Redistribution and Transformation Mechanisms of Gallium and Germanium during Coal Combustion. Fuel 2021, 305, 121532. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Zhou, G.; Gu, Y. Exploring a Promising Technology for the Extraction of Gallium from Coal Fly Ash. Int. J. Coal Prep. Util. 2022, 42, 1712–1723. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Yan, Z.; Fang, T.; Wang, R. Transformation Behavior of Mineral Composition and Trace Elements during Coal Gangue Combustion. Fuel 2012, 97, 644–650. [Google Scholar] [CrossRef]
- Dai, S.; Jiang, Y.; Ward, C.R.; Gu, L.; Seredin, V.V.; Liu, H.; Zhou, D.; Wang, X.; Sun, Y.; Zou, J.; et al. Mineralogical and Geochemical Compositions of the Coal in the Guanbanwusu Mine, Inner Mongolia, China: Further Evidence for the Existence of an Al (Ga and REE) Ore Deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Hower, J.C.; Eble, C.F.; Wang, N.; Dai, S. Distribution of Rare Earth Elements and Other Critical Elements in Beneficiated Pennsylvania Anthracites. Fuel 2021, 304, 121400. [Google Scholar] [CrossRef]
- Dai, S.; Hower, J.C.; Finkelman, R.B.; Graham, I.T.; French, D.; Ward, C.R.; Eskenazy, G.; Wei, Q.; Zhao, L. Organic Associations of Non-Mineral Elements in Coal: A Review. Int. J. Coal Geol. 2020, 218, 103347. [Google Scholar] [CrossRef]
- Duan, P.; Wang, W.; Ma, M. Distribution Characteristics of Trace Elements in Different Density Fractions of High-Germanium Coal from Wulantuga, Inner Mongolia, China. Coal Geol. Explor. 2022, 50, 14. [Google Scholar]
- Metwally, Y.M.; Chesnokov, E.M. Clay Mineral Transformation as a Major Source for Authigenic Quartz in Thermo-Mature Gas Shale. Appl. Clay Sci. 2012, 55, 138–150. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of Trace Elements in Chinese Coals: A Review of Abundances, Genetic Types, Impacts on Human Health, and Industrial Utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Seredin, V.V.; Finkelman, R.B. Metalliferous Coals: A Review of the Main Genetic and Geochemical Types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Shi, J.; Su, H.; Li, Y.; Huang, Z.; Wang, Y.; Gao, L. Quantitative Analysis of Heat Release during Coal Oxygen-Lean Combustion in a O2/CO2/N2 Atmosphere by TG-DTG-DSC. Sci. Rep. 2022, 12, 6690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Qin, J.; Chen, T.; Wu, J. TG-FTIR Study on Co-Combustion of Bituminous Coal Semicoke and Lignite. J. Therm. Anal. Calorim. 2020, 147, 1849–1858. [Google Scholar] [CrossRef]
- Ma, Z.; Shan, X.; Cheng, F. Distribution Characteristics of Valuable Elements, Al, Li, and Ga, and Rare Earth Elements in Feed Coal, Fly Ash, and Bottom Ash from a 300 MW Circulating Fluidized Bed Boiler. ACS Omega 2019, 4, 6854–6863. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Fu, B.; Liu, Y.; Xue, X. The Transformation and Enrichment of Cd in Fine Particulate Matter during Coal Combustion: The Key Roles of Ti-Bearing Components. Fuel 2021, 292, 120285. [Google Scholar] [CrossRef]
- Yang, W.; Pudasainee, D.; Gupta, R.; Li, W.; Wang, B.; Sun, L. An Overview of Inorganic Particulate Matter Emission from Coal/Biomass/MSW Combustion: Sampling and Measurement, Formation, Distribution, Inorganic Composition and Influencing Factors. Fuel Process. Technol. 2021, 213, 106657. [Google Scholar] [CrossRef]
- Tang, Q.; Du, C.; Liu, J.; Fan, L.; Niu, J.; Miao, C.; Li, W.; Fu, B. The Retention and Speciation Transformation Mechanisms of Chromium during Bituminous Coal Combustion: Effects of Inorganic Minerals and Combustion Temperature. Fuel Process. Technol. 2022, 232, 107273. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Xu, Z.; Sun, H.; Lam, P.K.S. The Retention Mechanism, Transformation Behavior and Environmental Implication of Trace Element during Co-Combustion Coal Gangue with Soybean Stalk. Fuel 2017, 189, 32–38. [Google Scholar] [CrossRef]
- Etschmann, B.; Liu, W.; Li, K.; Dai, S.; Reith, F.; Falconer, D.; Kerr, G.; Paterson, D.; Howard, D.; Kappen, P.; et al. Enrichment of Germanium and Associated Arsenic and Tungsten in Coal and Roll-Front Uranium Deposits. Chem. Geol. 2017, 463, 29–49. [Google Scholar] [CrossRef]
- Bennett, R.; Czechowski, F. Gallium Porphyrins in Bituminous Coal. Nature 1980, 283, 465–467. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, S.H. Detection of Mineralogical Changes in Pyrite Using Measurements of Temperature-Dependence Susceptibilities. Chin. J. Geophys. 2005, 48, 1454–1461. [Google Scholar] [CrossRef]
- Tian, C.; Rao, Y.; Su, G.; Huang, T.; Xiang, C. The Thermal Decomposition Behavior of Pyrite-Pyrrhotite Mixtures in Nitrogen Atmosphere. J. Chem. 2022, 2022, 8160007. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Li, J.; Qin, H. Magnetic Properties Related to Thermal Treatment of Pyrite. Sci. China Ser. D-Earth Sci. 2008, 51, 1144–1153. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous Biolithes: World Averages for Trace Element Contents in Black Shales and Coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Fang, T.; Wu, D.; Lam, P.K.S. Partitioning and Transformation Behavior of Toxic Elements during Circulated Fluidized Bed Combustion of Coal Gangue. Fuel 2014, 135, 1–8. [Google Scholar] [CrossRef]
- Frandsen, F.; Dam-Johansen, K.; Rasmussen, P. Trace Elements from Combustion and Gasification of Coal—An Equilibrium Approach. Prog. Energy Combust. Sci. 1994, 20, 115–138. [Google Scholar] [CrossRef]
- Yu, J.; Lucas, J.A.; Wall, T.F. Formation of the Structure of Chars during Devolatilization of Pulverized Coal and Its Thermoproperties: A Review. Prog. Energy Combust. Sci. 2007, 33, 135–170. [Google Scholar] [CrossRef]
- Xu, M.; Yan, R.; Zheng, C.; Qiao, Y.; Han, J.; Sheng, C. Status of Trace Element Emission in a Coal Combustion Process: A Review. Fuel Process. Technol. 2004, 85, 215–237. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Xu, Z.; Sun, H.; Lam, P.K.S. Retention Mechanisms of Ash Compositions on Toxic Elements (Sb, Se and Pb) during Fluidized Bed Combustion. Fuel 2018, 213, 98–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Seetharaman, S.; Liu, L.; Wang, X.; Zhang, Z. Effects of Chemistry and Mineral on Structural Evolution and Chemical Reactivity of Coal Gangue during Calcination: Towards Efficient Utilization. Mater. Struct. 2015, 48, 2779–2793. [Google Scholar] [CrossRef]
- Du, G.; Zhuang, X.; Izquierdo, M.; Alastuey, A.; Moreno, T.; Font, O. Ge Distribution in the Wulantuga High-Germanium Coal Deposit in the Shengli Coalfield, Inner Mongolia, Northeastern China. Int. J. Coal Geol. 2009, 78, 16–26. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Braekman-Danheux, C.; Laurent, P.; Thiemann, T.; Fontana, A. Behaviour, Capture and Inertization of Some Trace Elements during Combustion of Refuse-Derived Char from Municipal Solid Waste. Fuel 1999, 78, 1131–1145. [Google Scholar] [CrossRef]
- Reddy, M.S.; Basha, S.; Joshi, H.V.; Jha, B. Evaluation of the Emission Characteristics of Trace Metals from Coal and Fuel Oil Fired Power Plants and Their Fate during Combustion. J. Hazard. Mater. 2005, 123, 242–249. [Google Scholar] [CrossRef]




| Step | Extraction Procedure | Speciation |
|---|---|---|
| 1 | One gram sample was extracted with 10 mL 1.0 M MgCl2 (pH = 7.0) under room temperature for 1 h, and the suspension was achieved by centrifugation at 3500 rpm for 20 min. | (F1) Ion exchange |
| 2 | The residual solid from step 1 was extracted with 10 mL 1 M sodium acetate (pH = 5.0) under room temperature and agitated continuously for 5 h. The suspension was achieved by centrifugation at 3500 rpm for 20 min. | (F2) Carbonate-bound |
| 3 | The residual solid from step 2 was treated with 20 mL 0.04 M NH2OH·HCl in 25% (v/v) under room temperature and agitated continuously for 6 h. The suspension was achieved by centrifugation at 3500 rpm for 20 min. | (F3) Metal-oxide-bound |
| 4 | The residual solid from step 3 was treated with 3 mL 0.02 M HNO3 and 5 mL 30% H2O2 (pH = 2.0) at 85 °C for 2 h. Another 3 mL aliquot of 30% H2O2 (pH = 2.0 with HNO3) was then added at 85 °C and agitated for 3 h. After cooling, 5 mL of 3.2 M NH4OAc in 20% (v/v) HNO3 was added, and the sample was diluted to 100 mL and agitated continuously for 30 min. | (F4) Organic/ Sulfide-bound |
| 5 | The residual solid from step 4 was digested with an HCl–HNO3–HF mixture according to the procedure used for bulk samples. | (F5) Residual |
| The Proximate and Ultimate Analysis of Gangue (wt.%) | |||||
|---|---|---|---|---|---|
| Proximate analysis, dry basis | Moisture | Ash | Volatile matter | Fixed carbon | / |
| Coal gangue | 1.19 | 50.80 | 18.38 | 29.63 | / |
| Ultimate analysis, daf | C | H | N | O | S |
| Coal gangue | 23.44 | 3.91 | 0.72 | 8.74 | 0.53 |
| Chemical Composition | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | LOI a |
|---|---|---|---|---|---|---|---|---|---|
| Coal gangue | 55.07 | 2.60 | 26.44 | 9.07 | 0.21 | 1.88 | 0.17 | 3.08 | 15.37 |
| Particle Sizes (Mesh) | 50 | 80 | 100 | 140 | 200 |
|---|---|---|---|---|---|
| Ga | 85.09 | 88.40 | 91.03 | 92.53 | 94.80 |
| Ge | 10.60 | 11.59 | 13.02 | 14.87 | 15.66 |
| Particle Sizes (Mesh) | 50 | 80 | 100 | 140 | 200 | |
|---|---|---|---|---|---|---|
| Ga (%) | F1 | 0.03 | 0.09 | 0.16 | 0.07 | 0.15 |
| F2 | 0.91 | 1.43 | 2.15 | 2.01 | 1.80 | |
| F3 | 6.23 | 6.47 | 4.07 | 5.89 | 5.31 | |
| F4 | 8.95 | 9.06 | 5.74 | 7.97 | 6.21 | |
| F5 | 83.88 | 82.95 | 87.88 | 84.06 | 86.53 | |
| Ge (%) | F1 | 0.18 | 0.15 | 0.35 | 0.33 | 0.16 |
| F2 | 1.34 | 1.43 | 1.71 | 1.17 | 1.15 | |
| F3 | 6.78 | 7.96 | 7.93 | 12.13 | 10.26 | |
| F4 | 22.23 | 23.79 | 27.49 | 26.51 | 25.22 | |
| F5 | 69.47 | 66.67 | 62.52 | 59.86 | 63.21 | |
| Particle Size (Mesh) | 50 | 80 | 100 | 140 | 200 | |
|---|---|---|---|---|---|---|
| Ga | 600 °C | 143.07 | 147.55 | 150.93 | 154.51 | 157.40 |
| 700 °C | 140.26 | 145.77 | 149.80 | 152.10 | 155.91 | |
| 800 °C | 130.21 | 142.02 | 147.88 | 149.90 | 154.18 | |
| 900 °C | 128.58 | 139.53 | 145.22 | 147.99 | 151.59 | |
| 1000 °C | 126.36 | 129.84 | 134.67 | 136.92 | 143.11 | |
| Ge | 600 °C | 17.21 | 18.78 | 20.39 | 22.85 | 25.29 |
| 700 °C | 16.59 | 17.54 | 19.24 | 20.60 | 24.53 | |
| 800 °C | 14.78 | 15.13 | 17.34 | 19.50 | 23.93 | |
| 900 °C | 13.81 | 14.05 | 15.23 | 18.33 | 21.86 | |
| 1000 °C | 12.00 | 13.20 | 14.11 | 16.15 | 20.43 | |
| Particle Size (Mesh) | 50 | 80 | 100 | 140 | 200 | |
|---|---|---|---|---|---|---|
| REI (Ga) | 600 °C | 0.854 | 0.848 | 0.842 | 0.848 | 0.843 |
| 700 °C | 0.837 | 0.838 | 0.836 | 0.835 | 0.835 | |
| 800 °C | 0.777 | 0.816 | 0.825 | 0.823 | 0.826 | |
| 900 °C | 0.768 | 0.802 | 0.810 | 0.812 | 0.812 | |
| 1000 °C | 0.754 | 0.746 | 0.752 | 0.752 | 0.767 | |
| REI (Ge) | 600 °C | 0.825 | 0.823 | 0.796 | 0.781 | 0.820 |
| 700 °C | 0.795 | 0.769 | 0.751 | 0.704 | 0.796 | |
| 800 °C | 0.708 | 0.663 | 0.677 | 0.666 | 0.776 | |
| 900 °C | 0.662 | 0.616 | 0.594 | 0.626 | 0.709 | |
| 1000 °C | 0.575 | 0.579 | 0.551 | 0.552 | 0.663 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Zhou, B.; Zhou, C. Experimental Investigation on Gallium and Germanium Migration in Coal Gangue Combustion. Minerals 2024, 14, 476. https://doi.org/10.3390/min14050476
Wu F, Zhou B, Zhou C. Experimental Investigation on Gallium and Germanium Migration in Coal Gangue Combustion. Minerals. 2024; 14(5):476. https://doi.org/10.3390/min14050476
Chicago/Turabian StyleWu, Feitan, Benjun Zhou, and Chuncai Zhou. 2024. "Experimental Investigation on Gallium and Germanium Migration in Coal Gangue Combustion" Minerals 14, no. 5: 476. https://doi.org/10.3390/min14050476
APA StyleWu, F., Zhou, B., & Zhou, C. (2024). Experimental Investigation on Gallium and Germanium Migration in Coal Gangue Combustion. Minerals, 14(5), 476. https://doi.org/10.3390/min14050476








