Phase Transformation of Arsenic, Antimony and Lead in High-Grade Copper Matte Converting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Research Methods
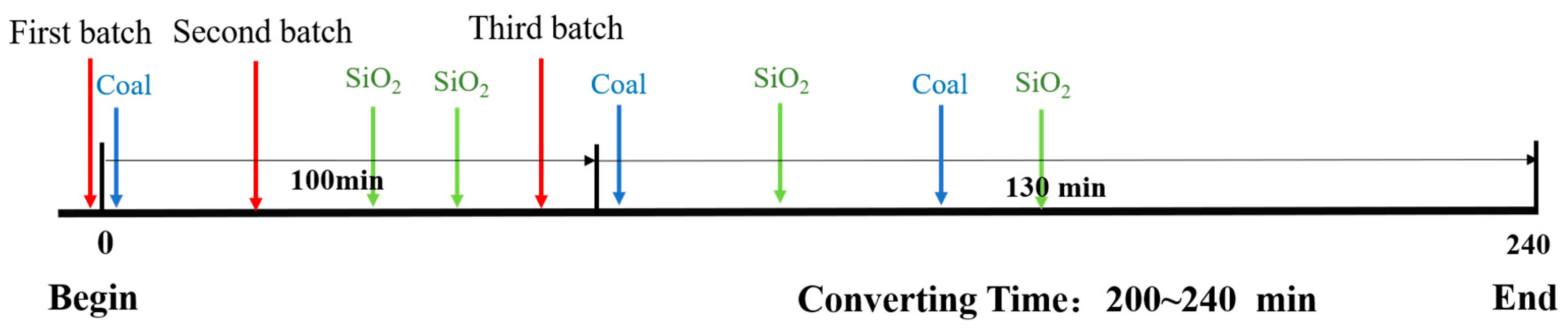
2.2.1. Experimental Methods
2.2.2. Analytical Methods
3. Results and Discussion
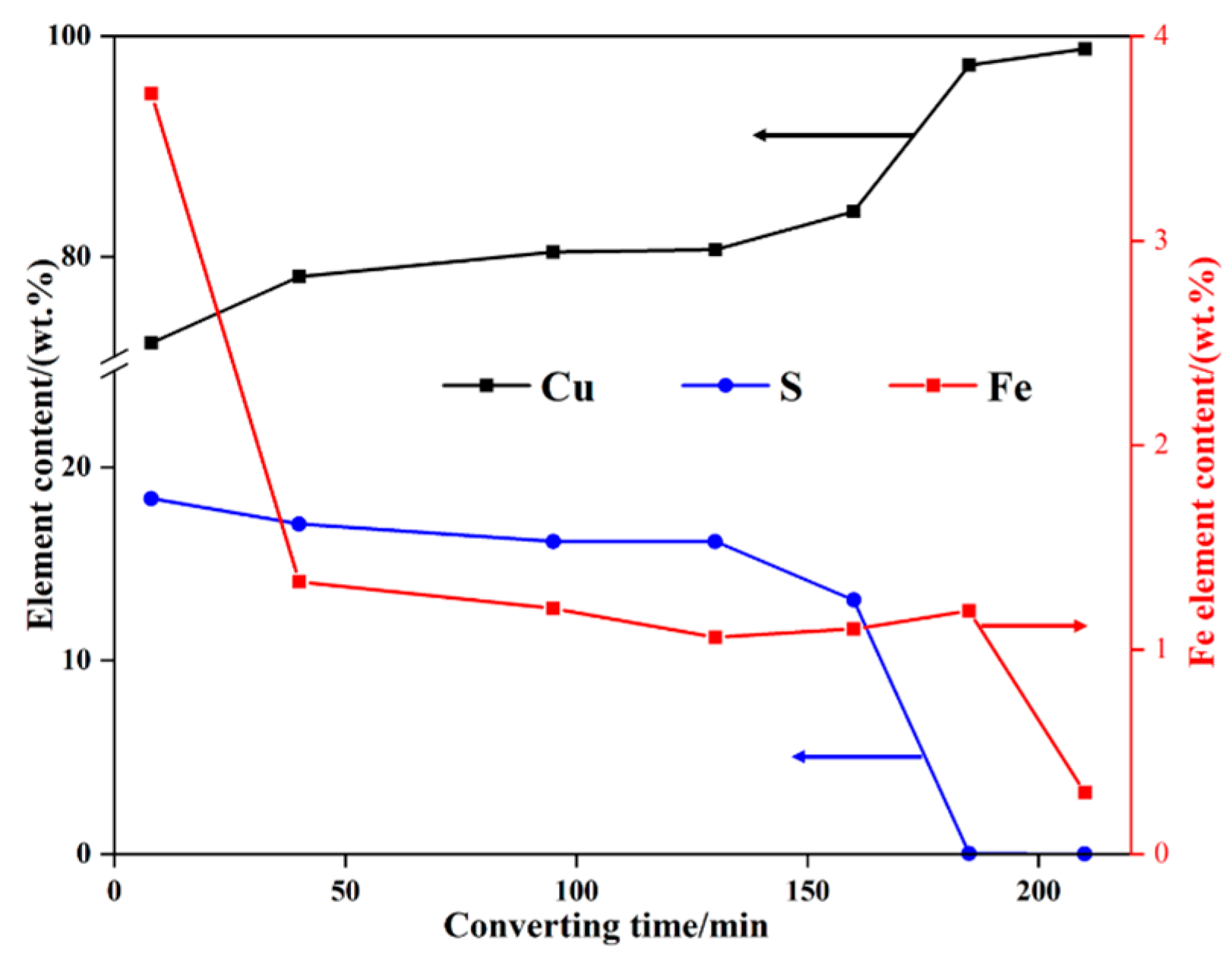
3.1. Transformation of the Main Elements during Converting
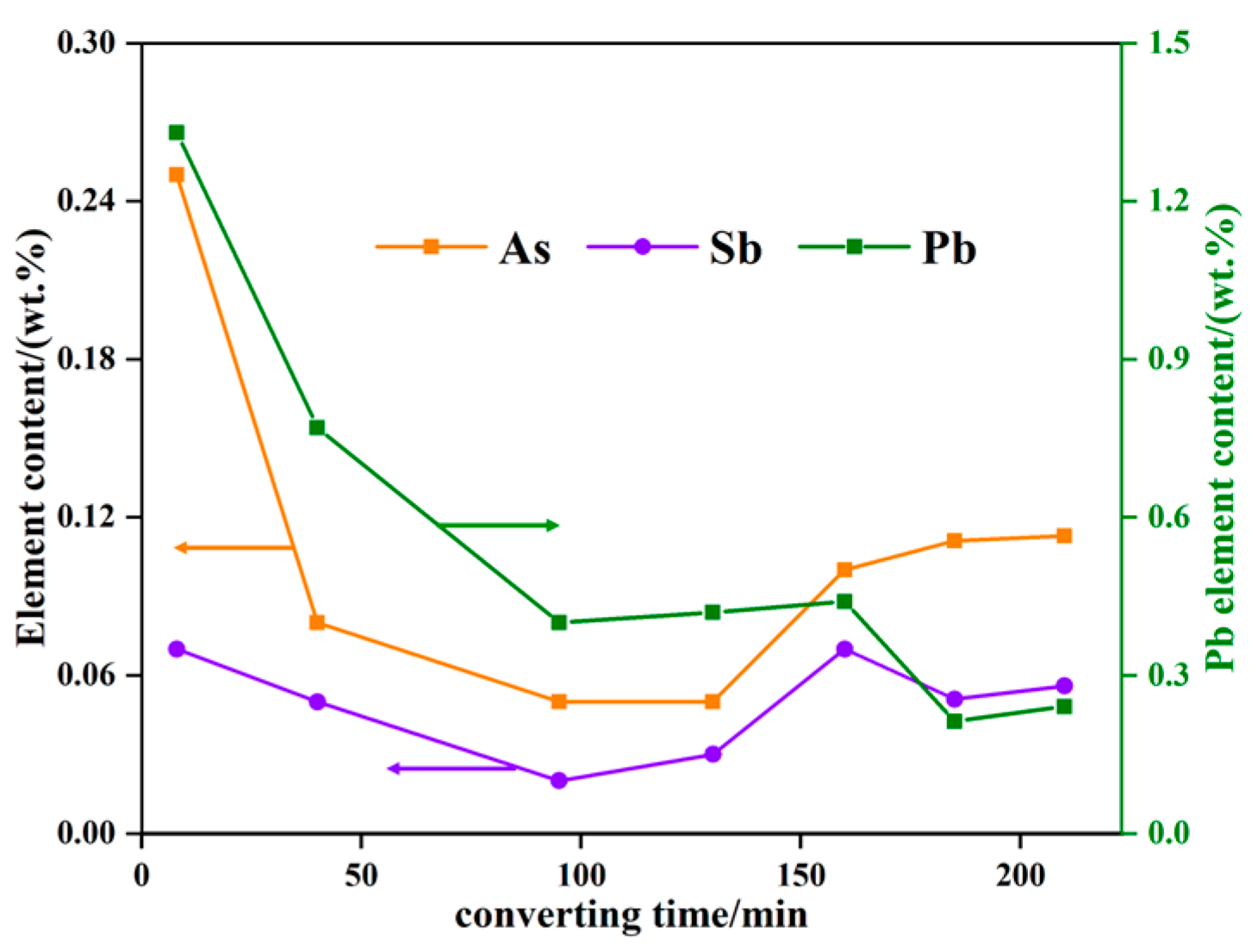
3.2. Transformation of Impurity Elements during Converting
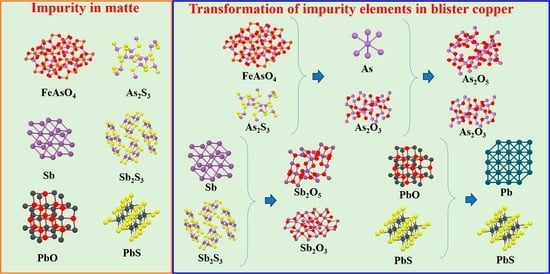
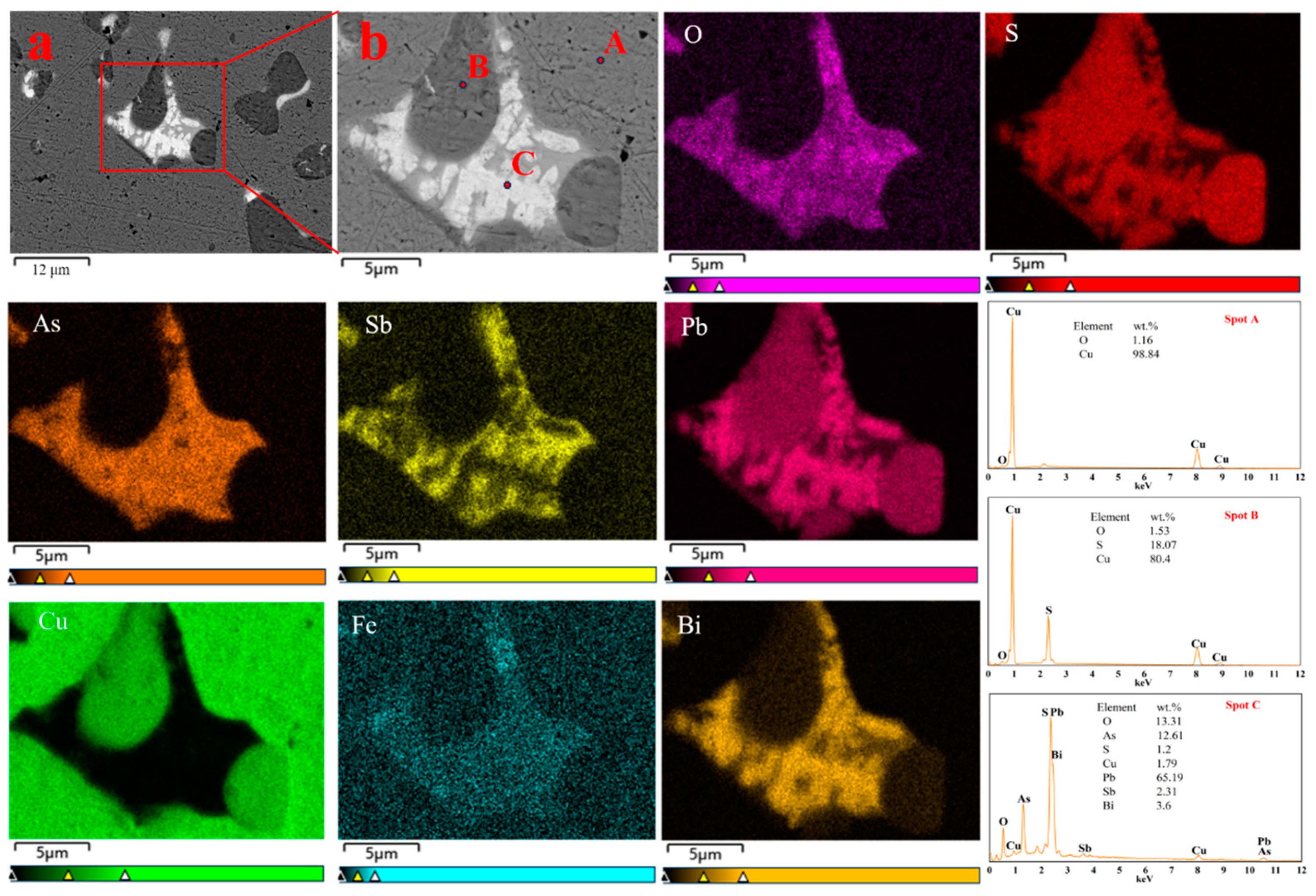
3.2.1. The Form of Impurity Elements in the High-Grade Matte
3.2.2. Impurity Element Content and Distribution in Melt
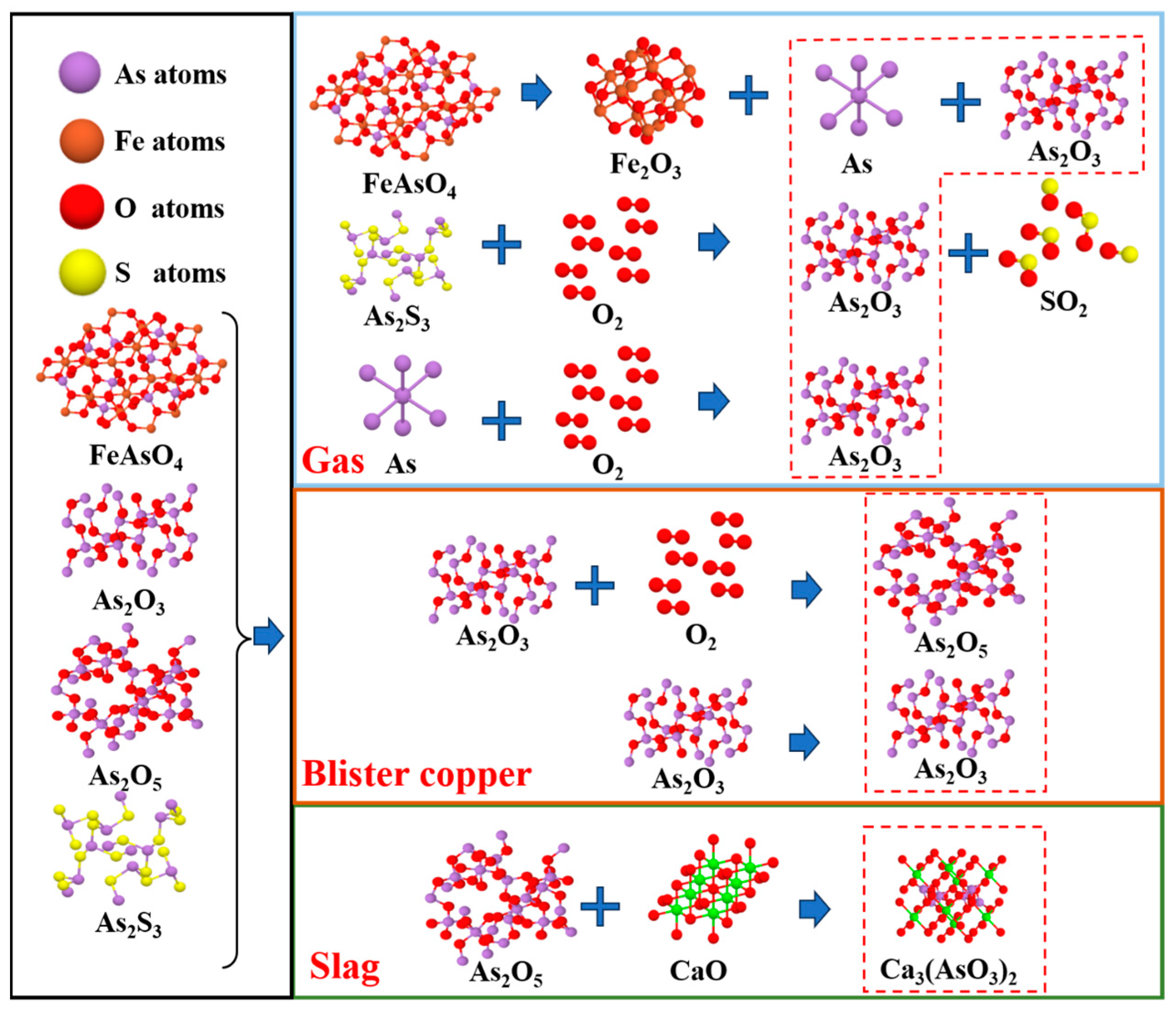
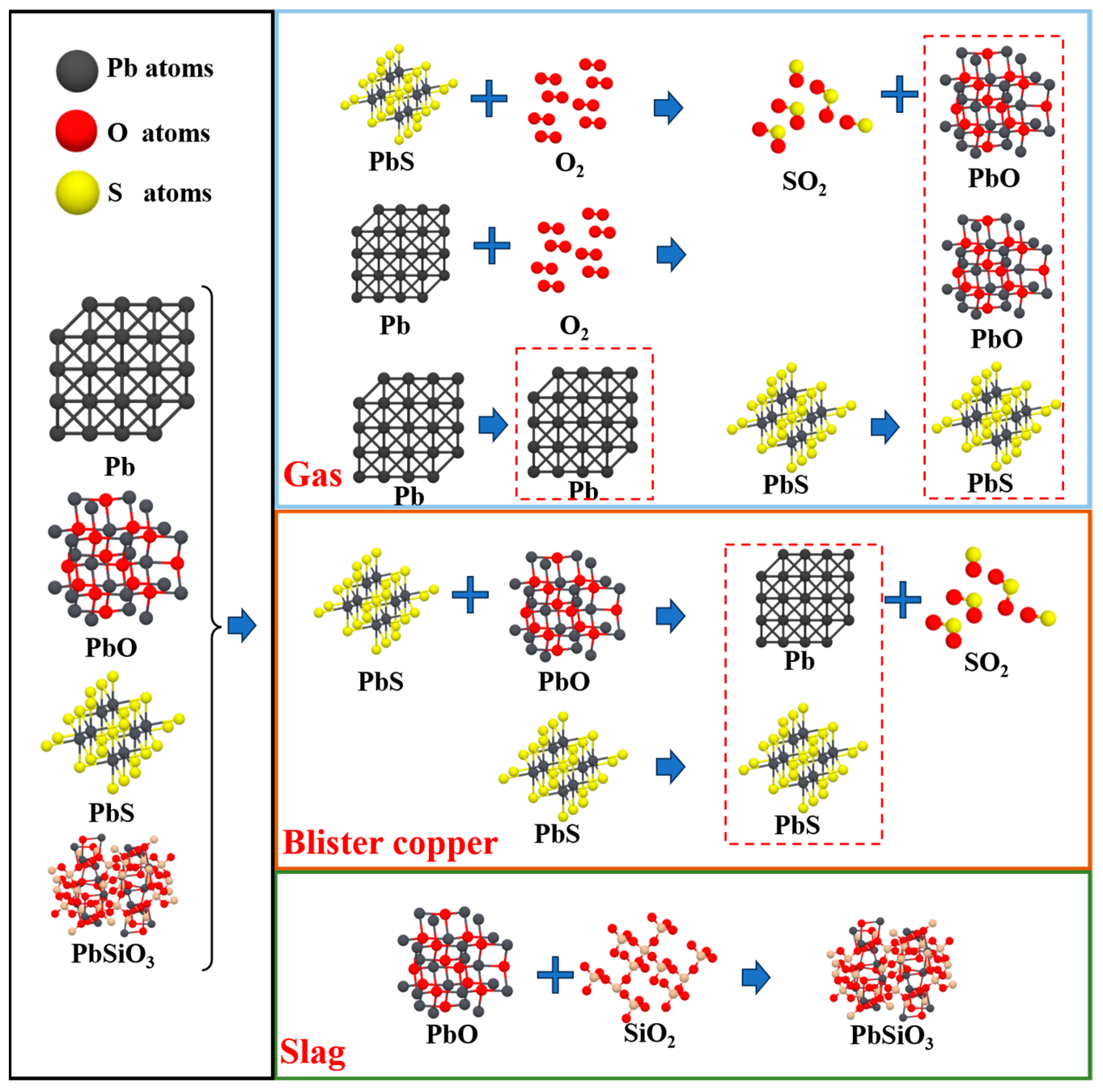
3.2.3. Phase Transformation Mechanism of Impurity Elements
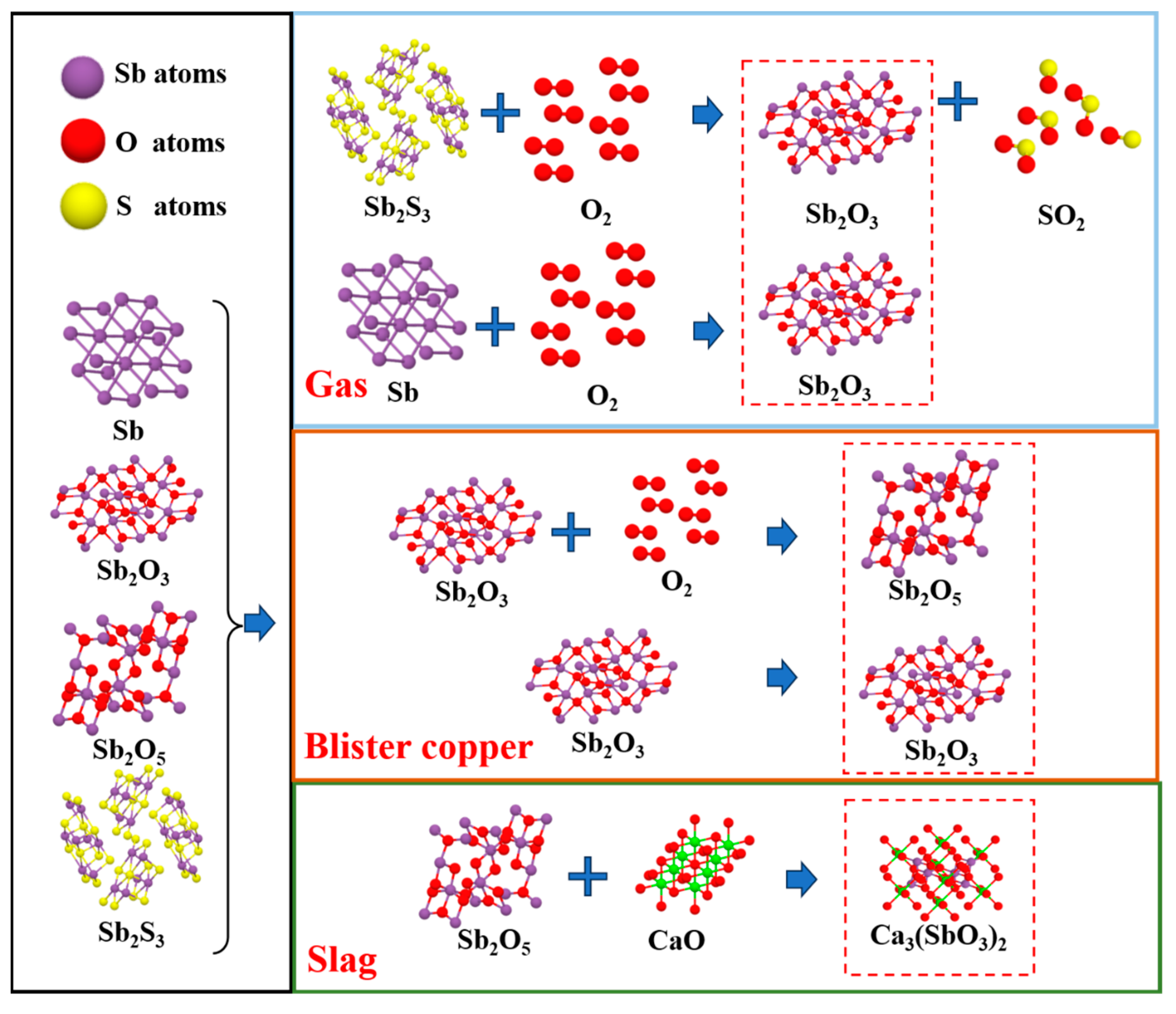
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taskinen, P.; Akdogan, G.; Kojo, I.; Lahtinen, M.; Jokilaakso, A. Matte converting in copper smelting. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2019, 128, 58–73. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, L. The practice of copper matte converting in China. Miner. Process. Extr. Metall. 2018, 128, 117–124. [Google Scholar] [CrossRef]
- Pérez, I.; Moreno-Ventas, I.; Ríos, G. Post-mortem study of magnesia-chromite refractory used in Peirce-Smith Converter for copper-making process, supported by thermochemical calculations. Ceram. Int. 2018, 44, 13476–13486. [Google Scholar] [CrossRef]
- Shishin, D.; Hidayat, T.; Decterov, S.; Jak, E. Thermodynamic modelling of liquid slag-matte-metal equilibria applied to the simulation of the Peirce-Smith converter. In Advances in Molten Slags, Fluxes, and Salts, Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts 2016; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1379–1388. [Google Scholar]
- Ahmed, H.; Ricardez-Sandoval, L.; Vilkko, M. Optimal Scheduling of the Peirce-Smith Converter in the Copper Smelting Process. Processes 2021, 9, 2004. [Google Scholar] [CrossRef]
- Filippou, D.; St-Germain, P.; Grammatikopoulos, T. Recovery of metal values from copper—Arsenic minerals and other related resources. Miner. Process. Extr. Metall. Rev. 2007, 28, 247–298. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Liu, F.; Sohn, H.Y. Experimental Study on Bubble Distribution and Splashing in a Peirce-Smith Copper Converter. Metall. Mater. Trans. B 2021, 52, 440–450. [Google Scholar] [CrossRef]
- Kulczycka, J.; Lelek, Ł.; Lewandowska, A.; Wirth, H.; Bergesen, J.D. Environmental Impacts of Energy-Efficient Pyrometallurgical Copper Smelting Technologies: The Consequences of Technological Changes from 2010 to 2050. J. Ind. Ecol. 2016, 20, 304–316. [Google Scholar] [CrossRef]
- Yao, L.; Min, X.; Xu, H.; Ke, Y.; Wang, Y.; Lin, Z.; Liang, Y.; Liu, D.; Xu, Q.; He, Y. Physicochemical and environmental properties of arsenic sulfide sludge from copper and lead-zinc smelter. Trans. Nonferrous Met. Soc. China 2020, 30, 1943–1955. [Google Scholar] [CrossRef]
- Henao, H.; Moyano, A. Conversion of mattes with high grade of copper. In Proceedings of the 9th International Copper Conference, Kobe, Japan, 13–16 November 2016; pp. 901–908. [Google Scholar]
- Cardona, N.; MacKey, P.J.; Coursol, P.; Parada, R.; Parra, R.A. Optimizing Peirce-Smith converters using thermodynamic modeling and plant sampling. JOM 2012, 64, 546–550. [Google Scholar] [CrossRef]
- Wang, Z. Effect of Impurities Distribution on Matte Grade Change by Using Oxygen-riched Side Blowing Furnace. Non-Ferr. Min. Metall. 2018, 34, 2. [Google Scholar]
- Chen, C.; Zhang, J.; Bai, M.; Wei, S. Investigation on the Copper Content of Matte Smelting Slag in Peirce-Smith Converter. J. Univ. Sci. Technol. Beijing 2001, 8, 177–181. [Google Scholar]
- Wilkomirsky, I.; Parra, R.; Parada, F.; Balladares, E. Continuous Converting of Copper Matte to Blister Copper in a High-Intensity Molten-Layer Reactor. JOM 2014, 66, 1687–1693. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Tian, Q.; Chen, M.; Zhao, B. Reaction Mechanism and Distribution Behavior of Arsenic in the Bottom Blown Copper Smelting Process. Metals 2017, 7, 302. [Google Scholar] [CrossRef]
- Sohn, H.S.; Fukunaka, Y.; Oishi, T.; Sohn, H.Y.; Asaki, Z. Kinetics of As, Sb, Bi and Pb volatilization from industrial copper matte during Ar + O2 bubbling. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2005, 35, 651–661. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Huang, L.; Xu, Z. Trend and recovery of arsenic, antimony and bismuth in copper smelting. Nonferrous Met. Sci. Eng. 2019, 10, 13–19. [Google Scholar]
- Li, Q.; Li, B.; Yan, X.; Wang, Q.; Li, S.; Liu, H.; Liang, Y. A review of arsenic reaction behavior in copper smelting process and its disposal techniques. J. Cent. South Univ. 2023, 30, 2510–2541. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Wang, Q.; Wang, S.; Tian, Q. Copper and arsenic substance flow analysis of pyrometallurgical process for copper production. Trans. Nonferrous Met. Soc. China 2022, 32, 364–376. [Google Scholar] [CrossRef]
- Wang, S. Arsenic Distribution Surveying in Copper Smelting Process of Jinlong Copper Co. Ltd. Nonferrous Met. Eng. 2016, 6, 3. [Google Scholar]
- Wang, E.; Shu, B.; Zhang, X.; Sheng, Q. Lead Occurrence State Change and Lead Removal Technical Discussion of High Lead Matte by P-S Converter in Blowing Process. Yunnan Metall. 2023, 52, 5. [Google Scholar]
- Hidayat, T.; Henao, H.M.; Hayes, P.C.; Jak, E. Phase equilibria studies of the Cu-Fe-O-Si system in equilibrium with air and with metallic copper. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 1034–1045. [Google Scholar] [CrossRef]
- Henao, H.; Kohnenkamp, E.; Rojas, L.; Moyano, A. Experimental Determination of the Effect of CaO and Al2O3 in Slag Systems Related to the Conversion Process of High Copper Matte Grade. Minerals 2019, 9, 716. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, F.; Gao, L.; Zhou, X.; Shi, Z.; Li, N. Numerical simulation study on the effects of co-injection of pulverized coal and SPL (Spent Pot-Lining) into the blast furnace. Fuel 2023, 354, 129368. [Google Scholar] [CrossRef]
- Pérez, I.; Moreno-Ventas, I.; Ríos, G.; Bravo, T. Study of Industrial Copper Matte Converting Using Micrography and Thermochemical Calculations. Metall. Mater. Trans. B 2020, 51, 1432–1445. [Google Scholar] [CrossRef]

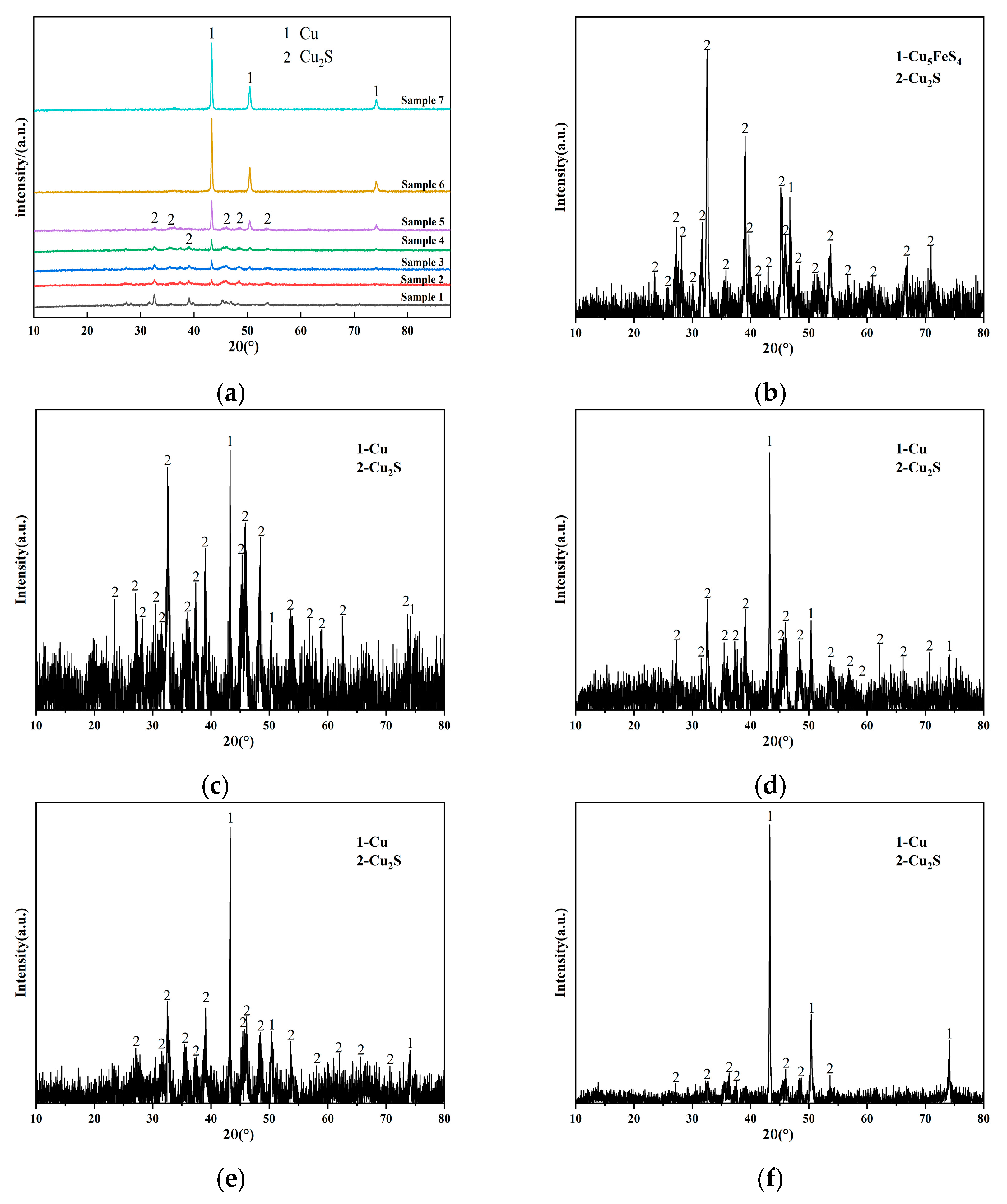


| Element | Cu | Fe | S | Pb | Zn | Ca | As | Si | Ni | Sb |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 72.22 | 3.72 | 18.38 | 1.38 | 0.39 | 0.28 | 0.25 | 0.2 | 0.18 | 0.07 |
| Species | Mad | Aad | Vad | Fcad |
|---|---|---|---|---|
| Content (wt.%) | 0.48 | 15.53 | 8.68 | 75.31 |
| Element | Cu | S | Fe |
|---|---|---|---|
| Sample 1 | 72.22 | 18.38 | 3.72 |
| Sample 2 | 78.21 | 17.06 | 1.33 |
| Sample 3 | 80.44 | 16.16 | 1.20 |
| Sample 4 | 80.68 | 16.15 | 1.06 |
| Sample 5 | 84.12 | 13.13 | 1.10 |
| Sample 6 | 97.38 | 0.02 | 1.19 |
| Sample 7 | 98.86 | 0 | 0.30 |
| Elements | Phase | Content/(wt.%) | Proportion |
|---|---|---|---|
| Arsenic | As2O3 | 0.003 | 1.28% |
| As2S3 | 0.160 | 64.00% | |
| FeAsO4 | 0.086 | 34.40% | |
| others | 0.001 | 0.40% | |
| Total | 0.250 | 100% | |
| Antimony | Sb2O3 | 0.008 | 11.11% |
| Sb | 0.044 | 61.11% | |
| Sb2S3 | 0.003 | 4.17% | |
| Sb2O5 | 0.017 | 23.61% | |
| Total | 0.072 | 100% | |
| Lead | Pb | 0.240 | 17.60% |
| PbS | 0.660 | 47.82% | |
| PbO | 0.370 | 26.85% | |
| PbSiO3 | 0.100 | 7.40% | |
| Total | 1.380 | 100% |
| Element | As | Sb | Pb |
|---|---|---|---|
| Sample 1 | 0.250 | 0.072 | 1.380 |
| Sample 2 | 0.080 | 0.050 | 0.770 |
| Sample 3 | 0.050 | 0.020 | 0.400 |
| Sample 4 | 0.050 | 0.030 | 0.420 |
| Sample 5 | 0.100 | 0.070 | 0.440 |
| Sample 6 | 0.111 | 0.051 | 0.210 |
| Sample 7 | 0.113 | 0.056 | 0.240 |
| Element | Cu | Fe | S | Pb | Zn | As | SiO2 | Ni |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 16.16 | 38.22 | 0.21 | 1.09 | 1.06 | 0.03 | 18.65 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, W.; Yang, Y.; Zhou, S.; Wei, Y.; Li, B. Phase Transformation of Arsenic, Antimony and Lead in High-Grade Copper Matte Converting. Minerals 2024, 14, 499. https://doi.org/10.3390/min14050499
Qu W, Yang Y, Zhou S, Wei Y, Li B. Phase Transformation of Arsenic, Antimony and Lead in High-Grade Copper Matte Converting. Minerals. 2024; 14(5):499. https://doi.org/10.3390/min14050499
Chicago/Turabian StyleQu, Wenkai, Yingbao Yang, Shiwei Zhou, Yonggang Wei, and Bo Li. 2024. "Phase Transformation of Arsenic, Antimony and Lead in High-Grade Copper Matte Converting" Minerals 14, no. 5: 499. https://doi.org/10.3390/min14050499
APA StyleQu, W., Yang, Y., Zhou, S., Wei, Y., & Li, B. (2024). Phase Transformation of Arsenic, Antimony and Lead in High-Grade Copper Matte Converting. Minerals, 14(5), 499. https://doi.org/10.3390/min14050499