Abstract
Oxidised pellets have become an indispensable high-quality charge for blast furnaces. Nevertheless, high-quality pellet feeds are becoming scarcer and scarcer. To broaden the range of sources of pellet feeds and reduce the production cost of pellets, more steel mills are predicted to use coarse iron ore fines with a relatively low iron grade and low impurities for the preparation of desirable pellet feeds through a typical wet grinding–settling–filtering process. In this work, the grinding, settling and filtering behaviour of Brazilian and Australian iron ore fines are studied and compared, with the aim of discovering the internal relationship between the mineralogical characteristics of different iron ore types and their grinding–settling–filtering performance. Additionally, the effects of ore blending on pellet preparation were investigated. The results show that, usually, the higher the hardness of the iron ore, the more grinding energy is required. Australian and Brazilian ore fines exhibit good grindability, with a Bond work index of about 10–15 kW·h/t. Furthermore, ore blending can reduce grinding energy consumption and improve settling and filtration rates, and the addition of finely ground Australian ores improves the balling performance of pellet mixtures. At the same bentonite content, the ball drop strength of the three blends with added Australian ore is significantly higher than that of the base blend, and the fired pellets obtained from Blend 1, Blend 2 and Blend 3 blends exhibit good metallurgical properties.
1. Introduction
Oxidised pellets have become an essential charge material for blast furnace ironmaking due to their uniform particle size, high iron grade, excellent metallurgical properties and high mechanical strength. Oxidised pellets exhibit significant advantages, notably including low energy consumption, minimal environmental impact and a high iron grade in the resulting products. Among these advantages, the energy consumption during the oxidation pelletisation process is only half that of the sintering process. This highlights the efficiency and environmental benefits of the pelletising method, contributing to high-grade iron-containing products with reduced energy requirements and environmental pollutants. Additionally, the application of oxide pellets in blast furnace smelting can contribute to increased production, coke savings, improved economic indicators of ironmaking technology, reduced pig iron costs and enhanced economic benefits. The raw iron ore concentrate used for pellet production undergoes mineral processing and grinding. However, pellet production requires iron ore concentrate with a suitable particle size distribution and specific surface area, a low crystalline water mass fraction and a reasonable chemical composition [1]. The primary sources of iron ore for pellets are Australia, Brazil and Russia. Nonetheless, due to the rapid development of the global steel industry, high-quality and stable sources of iron ore are increasingly scarce. Magnetite concentrate is the main raw material for producing oxidised pellets, but high-grade magnetite resources are becoming increasingly scarce and expensive. The rich ore fines used for sintering are generally hematite, which has a coarse particle size and is an abundant resource. To expand the availability of pellet feed and reduce production costs, steel mills are utilising cost-effective coarse iron ore fines with a high iron grade and few impurities to prepare ideal pellet feed through the conventional wet grinding–settlement–filtration process. This approach reduces the cost of ore preparation for pelletisation and widens the range of sources of iron ore concentrate for pelletisation.
The primary objective of fine grinding of iron ore fines is to increase the specific surface area to meet the requirements of pellet feed, while avoiding over-grinding. The optimum fineness of concentrates as pellet feed is within the range of 80–90 per cent smaller than 0.074 mm as well as 50–60 per cent smaller than 0.043 mm, and the corresponding specific surface areas (SSAs) are 1500–2000 cm2/g [2]. Fine grinding of iron ore fines can be realised by wet or dry grinding processes, with the former requiring additional settling and filtering steps in production [3]. Wet grinding is generally preferred over dry grinding because it is more energy-efficient [4]. In addition, wet grinding has advantages such as strong fluidity, high efficiency and no agglomeration [5]. The increased energy consumption of dry grinding is attributed to the extended grinding time required to achieve a product with a specific particle size distribution, with a majority of the energy being dissipated as heat [6]. Studies have shown that the energy-consumption differences between dry and wet grinding can be as high as 30–50 per cent. The settling and filtering performance of different iron ore types after fine grinding and filtration can vary considerably. Therefore, to enhance the settling and filtering performance of ore fines, it is crucial to understand the relationship between the material properties of different iron ores and their settling and filtering performance. By blending ores with those that possess excellent settling and filtering performance, a suitable mixture can be achieved that results in optimal grindability, settling and filtration properties. This approach meets the grinding and beneficiation process requirements while also reducing the energy consumption associated with grindability.
In recent years, scholars have conducted research on the use of finely ground ore powder in the preparation of pelletised ore. The Tata Steel Plant in India employed a dry ball-milling method to finely grind coarse hematite powder [7]. With appropriate amounts of bentonite and suitable balling conditions, the finely ground product could be used to prepare high-quality oxide pellets. The fine grinding of hematite coupled with magnetite was suitable for the preparation of acidic pellets. Additionally, research results indicate that incorporating finely ground hematite improves the quality of green pellets and final fired pellets while reducing the reduction swelling index [8]. Combined pre-treatment of hematite fines using ball milling and high-pressure roller milling was used for the preparation of fluxed pellets. The study revealed that milling pre-treatment could enhance the balling performance of hematite, addressing issues such as poor balling properties and high sintering temperatures [9].
There is a lack of research on the settling and filtration performance of wet-ground iron ore fines. This study investigates and compares the grinding, settling and filtration performance of iron ore fines from Brazil and Australia. The aim is to explore the intrinsic relationship between the process mineralogy and settling and filtration performance of different ore fines, optimise the fine grinding and settling and filtration process of Brazilian and Australian ores and improve their settling and filtration performance by rational blending. The goal is to optimise the process flow and improve resource utilisation. The addition of finely ground Australian ore powder improves the performance of pelletisation compared to using a single ore. With the same bentonite content, the drop strength of the three blends with added Australian ore is significantly higher than that of the base blend, and the fired pellets obtained from Blend 1, Blend 2 and Blend 3 blends exhibit good metallurgical properties.
2. Materials and Methods
2.1. Materials
The experimental study utilised six types of iron ore fines, including three from Australia (designated as samples A1–A3) and three from Brazil (designated as samples B1–B3), as shown in Table 1. All six types of ores have a high content of iron and a low content of silicon, with total iron content exceeding 60 per cent and SiO2 content below 5 per cent. They are characterised by low levels of harmful elements such as sulphur, phosphorus and non-ferrous metals. In particular, for the three Australian ore fines with high LOI, the iron grade of the pellets is significantly increased after roasting. These ores only require grinding to achieve particle size and specific surface area requirements for pellet production, without the need for beneficiation. M1 is the raw material for subsequent pellet preparation, characterised by a high iron grade. Therefore, from these perspectives, they are suitable raw materials for pellet production. However, this may result in an increase in the energy consumption required for the hardening of oxidised pellets.

Table 1.
Chemical composition of iron ore fines (wt.%).
The particle size distribution of the iron ores varies among the different countries, as shown in Table 2 for the six types of iron ore fines. Since the −0.074 mm content of B1 is basically more than 90%, no further grinding is required. Thus, the particle size characteristics of the iron ore fines, excluding B1, are more suitable for sintering. However, if these iron ore fines are directly used as pellet feed, fine grinding is necessary.

Table 2.
Size distribution of the iron ore fines (wt%).
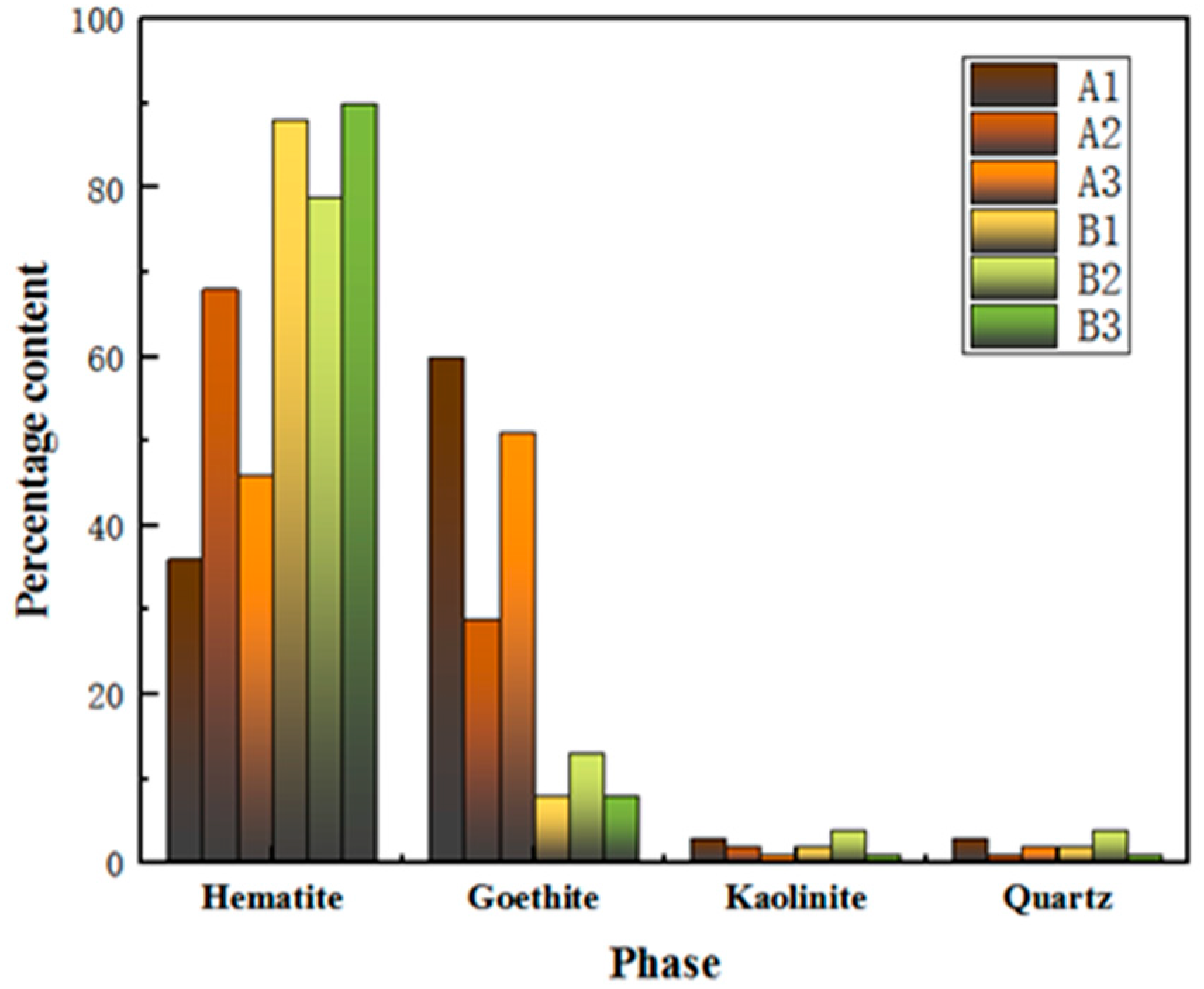
The process mineralogy of three Australian ores and three Brazilian ores is depicted in Figure 1 and Table 3. From the illustration, it is evident that the primary iron-bearing phases in these six iron ores are hematite and goethite. Notably, the content of goethite is significantly higher in Australian ores than in Brazilian ores.
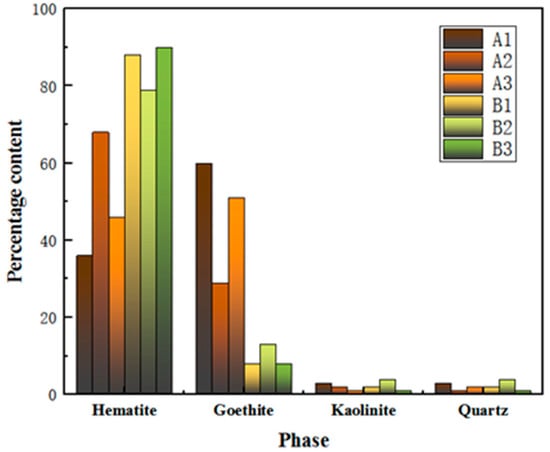
Figure 1.
Phase content of iron ore fines.

Table 3.
Process mineralogical analysis of iron ore fines.
2.2. Methods
2.2.1. Grindability
In this paper, the standard Bond method was used to measure the Bond work index (Wi) by ball milling to estimate the grindability of iron ore fines [10,11,12,13].
In accordance with the particle size requirements of the Bond ball mill and to ensure a closely matched feed particle size, the iron ore was crushed to −3 mm using a jaw crusher. Based on the recommended process parameters of grinding concentration, grinding media filling rate and grinding time in the ball milling process according to the reference grinding process system, ball-milling sample preparation for pelletisation experiments was conducted. The experimental cone ball mill used in the study was a model RK/ZQM(BM), with dimensions of φ160 × 60 mm, and the cylinder rotated at a speed of 112 r/min. The grinding slurry concentration was 7 per cent, and each grinding process used 300 g of ore sample.
2.2.2. Settling Characteristics
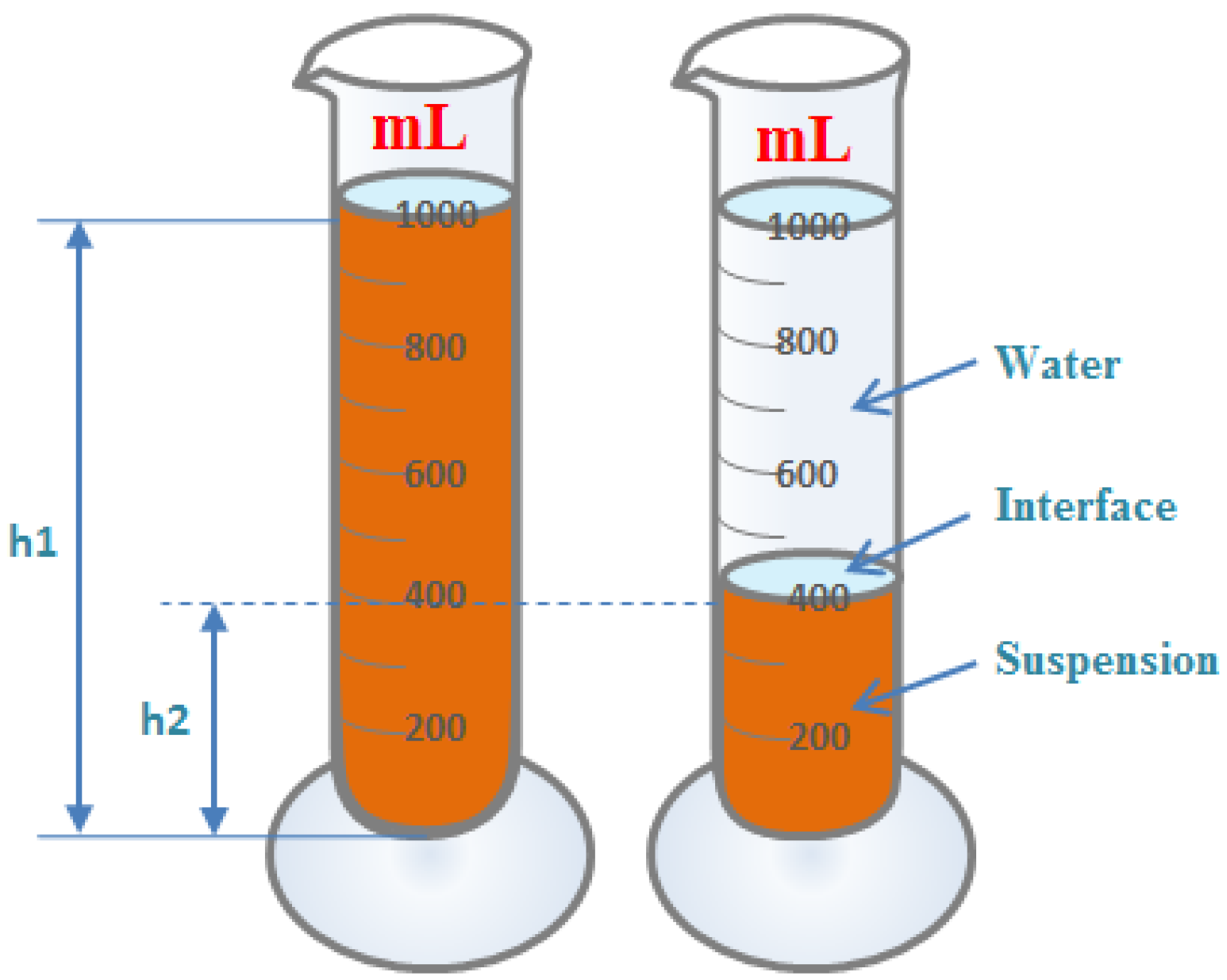
A certain amount of iron ore sample was weighed, and the appropriate amount of water was added. All samples used in the settling experiment were ground to a similar particle size distribution, with 70 per cent of the particles in the slurry smaller than 0.074 mm [7]. The samples used for settling experiments each time had a dry basis weight of 300 g and were pre-mixed to a slurry concentration of about 25% with an initial height of liquid level of about 300 mm. The prepared slurry was poured into a 1000 mL measuring cylinder and shaken evenly. The measuring cylinder was then placed on a horizontal platform, and the real-time settling height (i.e., solid–liquid interface height) of the iron ore vs. settling time was recorded. Recordings were taken every minute for the first 20 min and every 10 min for the subsequent 40 min. A schematic diagram of the experiment on settling characteristics is shown in Figure 2.
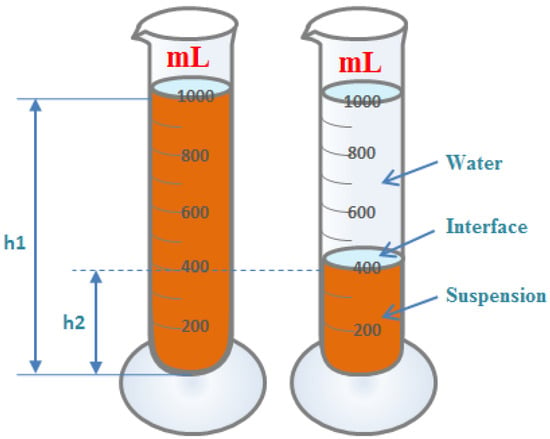
Figure 2.
Experimental diagram of method for determining settling characteristics.
2.2.3. Filtering Characteristics
The bottom suspension obtained after settling of the slurry was further subjected to filtration experiments using the XTLZ-260/200 multi-purpose vacuum filter at the Wuhan Prospecting Machinery factory. First, the iron ore fines underwent wet ball milling, and then the corresponding size of finely ground concentrate was prepared at different concentrations. A 70 per cent concentration of the finely ground iron concentrate slurry was filtered under a vacuum of −0.08 MPa, and the suction filtration time was set from 1 to 6 min. After that, the filtering was stopped, and the moisture content of the filter cake was measured.
2.2.4. Pellet Preparation
According to the predetermined experimental blends, the pellet feeds were mixed uniformly. The ore blends for pellet testing are shown in Table 4. Experimental tests were carried out by adding fine-ground imported powder to the pellet composition. While maintaining the original pellet composition structure, M1 and B3 were used as the main materials, with the proportion of finely ground Australian concentrate controlled at 40%, as shown in Table 4 for the base and three different blends. Various tests on balling, roasting and metallurgical performance of the fine-ground powders were conducted. Subsequently, green pellets were prepared on a disc pelletiser with a diameter (Φ) of 1000 mm, a rotational speed of 32 r/min, a side height (h) of 150 mm and a tilt angle (α) of 47. To achieve specific dimensions and control variables, the moisture content during pellet formation was maintained at approximately 8%. The produced green pellets were manually sieved, and green balls in the size range of 10–12.5 mm were selected for measurement of pellet moisture, drop performance, compressive strength and thermal shock temperature. The pellet-roasting experiment was conducted using a simulated straight-grate process, with a grate pot of 300 mm diameter and 500 mm height. After the roasted pellets cooled to room temperature, pellets in the size range of 10–12.5 mm were sieved for testing of compressive strength and metallurgical performance according to Table 5. Optical microscopy was used for mineral phase analysis of the pellets.

Table 4.
Ore blends for pellet testing.

Table 5.
Metallurgical performance standards.
3. Results and Discussion
3.1. Grindability
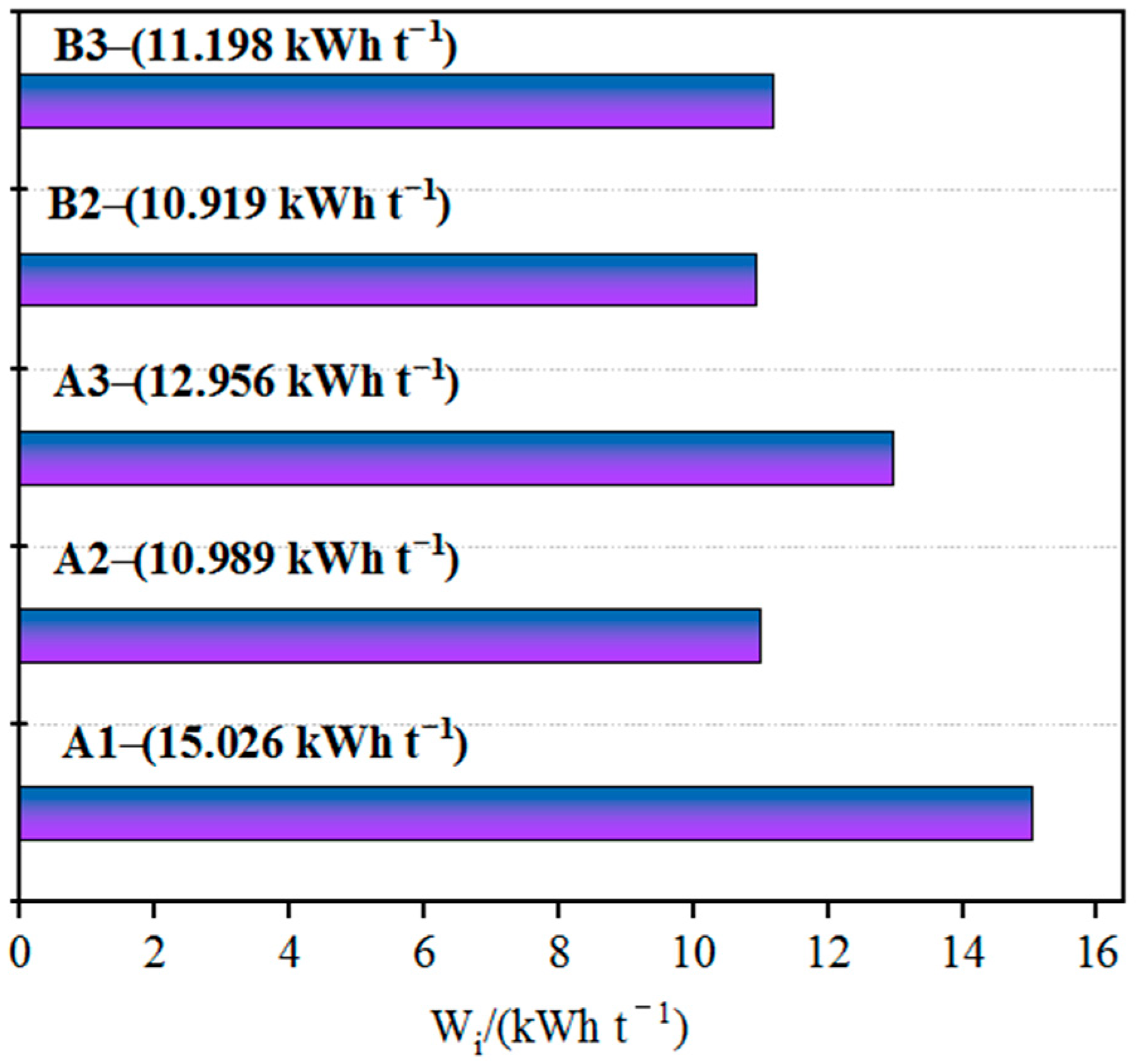
Coarse ores with inappropriate sizes require grinding prior to agglomeration. Grinding can increase the ore’s specific surface area and surface activity, thus improving its agglomeration efficiency [17]. The main factors influencing the fine grinding process include ore properties, equipment performance, construction investment and production cost, but especially ore properties. The Bond work index of imported iron ore concentrates varies greatly among different countries or regions, as shown in Figure 3. According to the Bond theory, the specific energy consumption of ore grinding is proportional to the Bond work index. Strict control of the ore properties is necessary in a specific fine grinding process; otherwise, it will directly lead to one of two serious consequences: either a significant decrease in mill capacity while ensuring the grinding product size or a serious deviation of the grinding product size without adjusting the new feed rate to the mill [18]. Therefore, the Bond work index is the most important factor influencing the selection of the fine grinding process. Although fine grinding can liberate mineral particles that were previously considered to be unprocessable, it may result in high costs in terms of energy consumption and medium usage [19]. For a specific type of iron concentrate purchased globally, its fine grinding performance should be studied in depth. A reliable and practical process flow should be selected both to ensure the stability of grinding, filtering and other operating factors and ensure that production costs such as material consumption, power consumption and reagent consumption are within a reasonable range, achieving the optimal balance between construction investment and production cost.
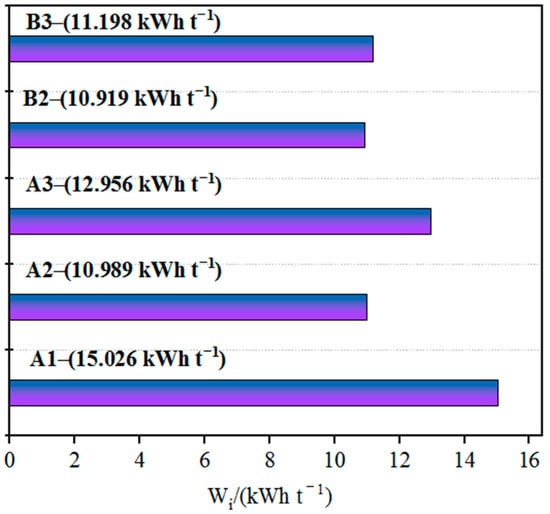
Figure 3.
Bond work indices of the samples of different iron ore fines.
According to the ranking of grindability based on the Bond work index (from high to low, with corresponding energy consumption from low to high), B2 > A2 > B3 > A3 > A1; B2 exhibits good grindability and low grinding energy consumption, while A1 exhibits the poorest grindability and slightly higher grinding energy consumption. All five types of iron ore fines show good grindability, with Bond work indices of about 10–15 kW·h/t; at these values, materials are considered to be relatively easy to grind.
Analysis of the Relationship between Grindability and Ore Mineralogy
Ore hardness is a reflection of the specific mineral composition of the ore itself and its physical and mechanical properties. Ores with a dense structure, very small crystals and high hardness are more difficult to grind. Therefore, such ores require a longer grinding time in the grinding process to ensure that the required grinding fineness is achieved, but generally speaking, ore hardness mainly affects the processing capacity of the mill. On the other hand, ores with low hardness or well-developed decomposition are easy to crush, and the processing capacity per unit volume of the mill is high.
The following relationship, using linear regression, can be represented as in Equation (1) [20]:
where the wear resistance P is inversely proportional to the Vickers hardness H and the hardness dispersion value D (the magnitude of the hardness dispersion value D reflects the degree of adaptability between the uniformity of ore hardness or the complexity of the mineral composition and processing technology), indicating that the higher the hardness, the lower the p value and the more wear-resistant the rock. Conversely, if the rock is less wear-resistant, its hardness is lower.
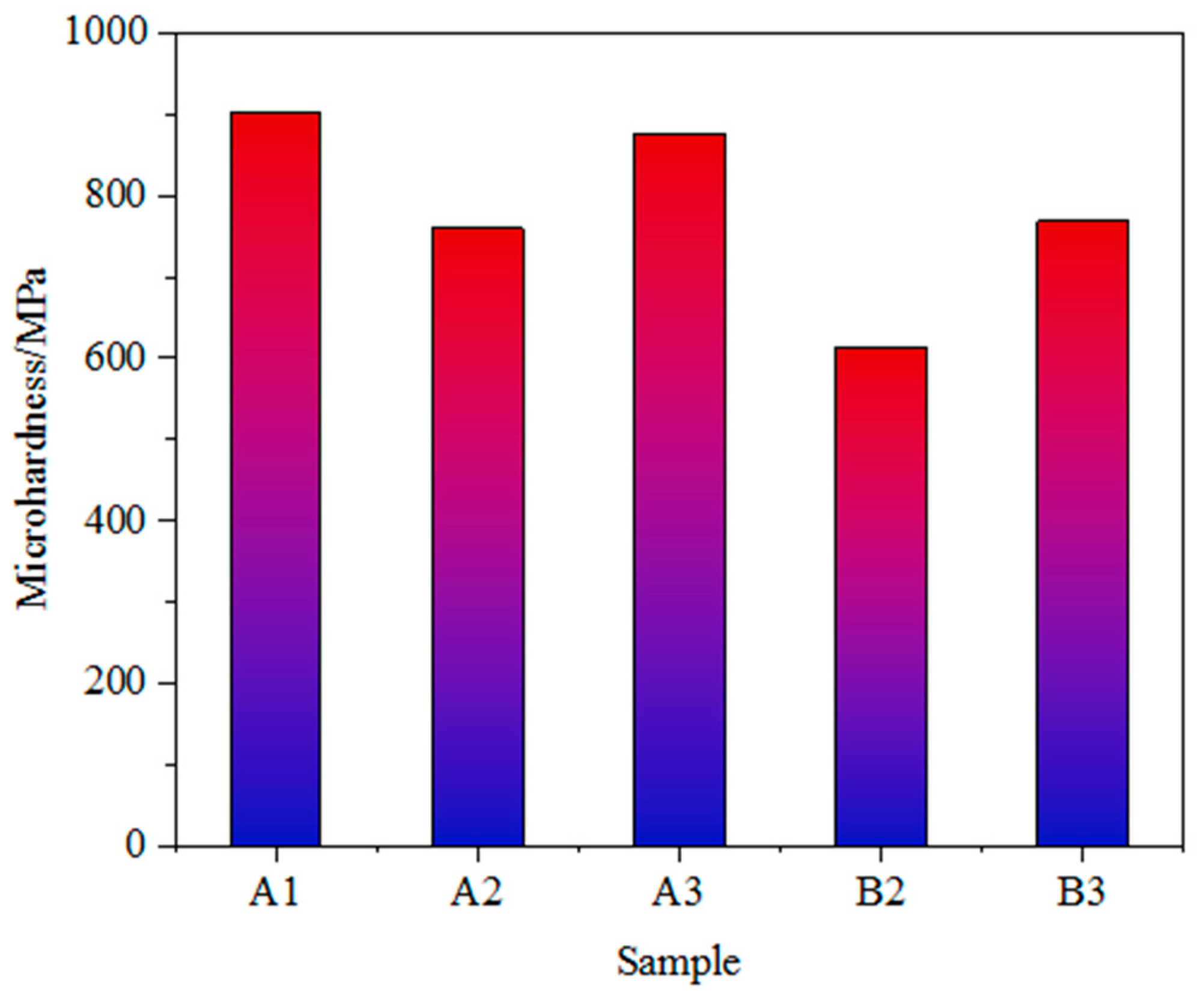
As shown in Figure 3 and Figure 4, the Vickers microhardness of iron ore fines is positively correlated with the Bond work index, and the grindability is largely dependent on the hardness of the iron ore. Sample A1 of iron ore fines has the highest microhardness and the lowest grindability; therefore, a longer grinding time is required to achieve the required grinding fineness. On the other hand, ore sample B1 has the lowest microhardness and the lowest Bond work index among the five samples of iron ore fines, resulting in lower grinding energy consumption.
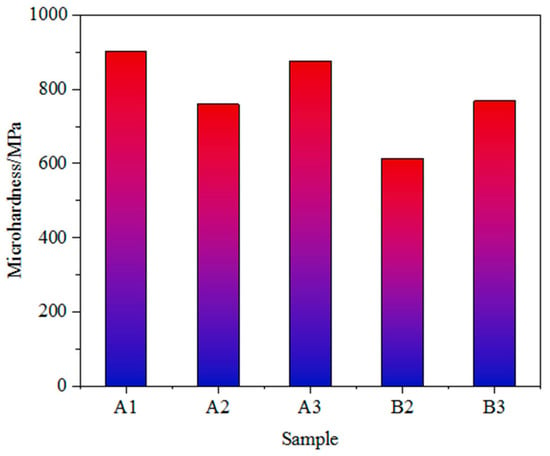
Figure 4.
Vickers microhardness of the samples of iron ore fines.
In addition to microhardness, factors such as ore texture, pore size, goethite content and soft mineral content also affect the grinding effect. The higher the content of goethite in the ore, the more difficult it is to grind. This is due to the physical properties of goethite, which has high hardness and poor toughness and can easily form small particles during the grinding process, leading to increased difficulty in grinding. At the same time, as the content of goethite increases, the frictional force between ore particles during grinding also increases, affecting the grinding effect. The high content of goethite in samples A1 and A3, both exceeding 50 per cent, may be one of the reasons for their poor grindability. The low content of goethite in sample B3 may be one of the reasons for its good grindability.
The mineral texture characteristics, such as mineral particle size, shape, association and spatial distribution, have a certain impact on grinding. If the minerals are interlocked, different types of minerals in the ore are interlocked and distributed, resulting in more interfacial boundaries between minerals and irregular shapes of mineral particles. This can lead to the formation of small mineral particles that are difficult to grind, resulting in poor grindability. If the minerals are enclosed, some minerals in the ore are surrounded by other minerals, and the hardness, toughness and fracture characteristics of these enclosed minerals may be different from those of the external minerals, making them difficult to grind. Sample A3 contains goethite intercalated with adjacent iron ores such as hematite and magnetite, and the magnetite mainly exists inside the large hematite crystals. This may be one of the reasons for its high Bond work index.
3.2. Settling and Filtration Characteristics
After wet ball milling, it is generally necessary to remove water from the ground concentrates to achieve a product containing 8–10 per cent moisture, which is suitable for the pelletising process. This dewatering is traditionally accomplished through a combination of settlement and filtration using thickeners or sedimentation tanks and a filter press or vacuum filters, respectively. Therefore, the settlement and filtration characteristics of iron ore slurry are crucial to the efficiency of the pellet feed preparation and subsequent pelletising process.
3.2.1. Settling Characteristics
Settlement is the process of solid particles suspended in a fluid separating from the fluid under the action of gravity. Settling testing of mineral slurries is one of the fundamental tests in mineral-processing operations for tailings, concentrates, or intermediate materials, aimed at measuring the settling velocity of solid material groups in a slurry of a certain concentration, as a basis for the design of a beneficiation plant or settling area and thickener tank size [21].
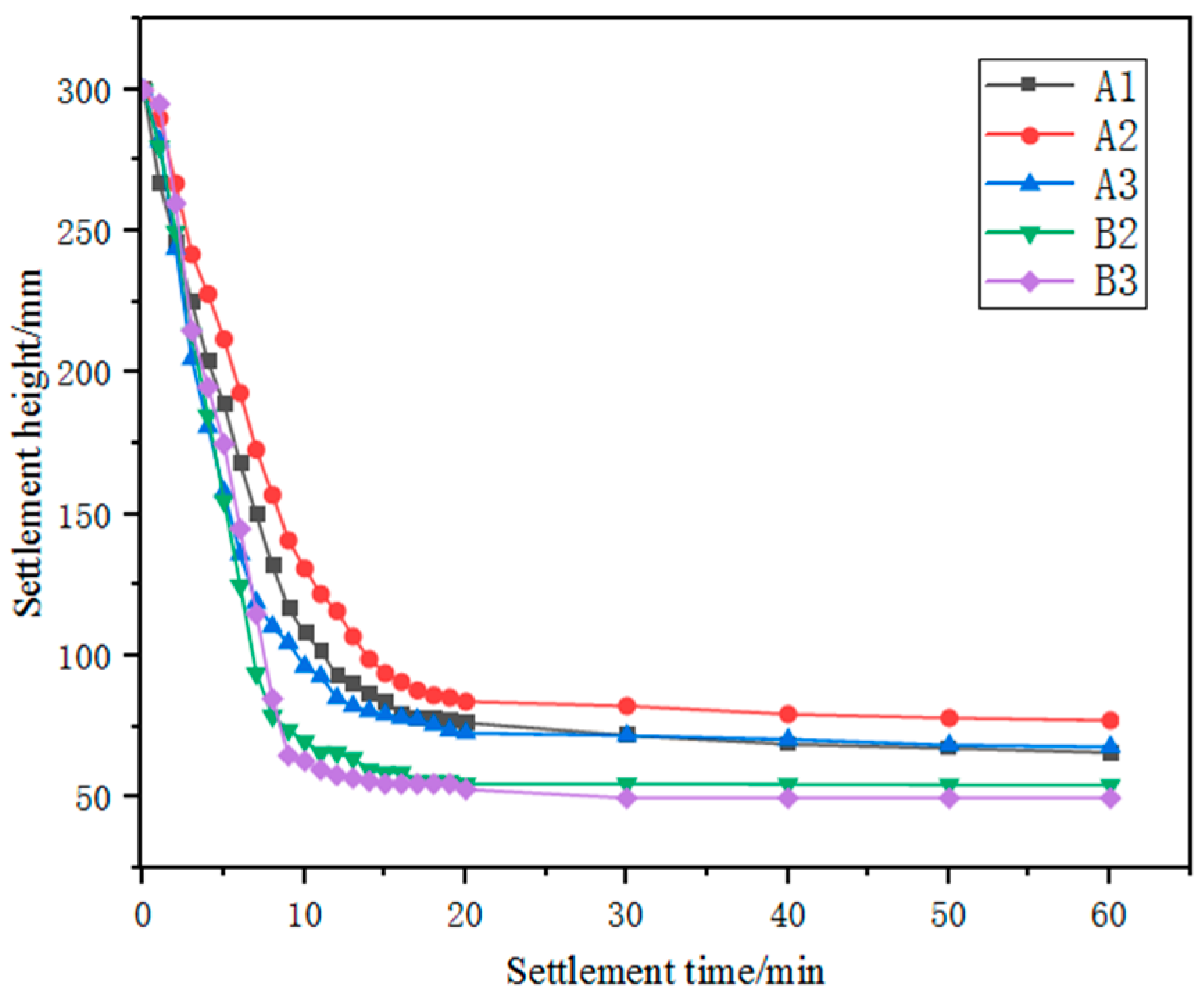
The settling performance of the ground iron ores is shown in Figure 5. The static settling process of the slurry can be roughly divided into three stages: in the initial constant settling stage (within about 10–15 min), the particles in the grinding products of the five types of iron ore fines were less affected by the surrounding particles or the tube wall, and the particle spacing was wide. The fine particles underwent intense Brownian motion and collided and aggregated with each other, resulting in fast settling rates of 14.20, 13.40, 14.62, 15.00 and 16.33 cm/min for samples A1, A2, A3, B2 and B3 respectively. In the subsequent 5–10 min, the settling stage transitioned to the interference settling stage, during which the settling rate of the ore particles decreased significantly. After a settling time of more than 20 min, the process transitioned to the compression stage, during which the settling rate was slowest due to the gradual increase in the concentration and viscosity of the sediment layer [22]. According to the Newtonian viscosity law, the drag force on the particles gradually increases, making the particles more susceptible to severe interference from the surrounding particles during settling. Overall, the static settling performance of the three Australian ore fines was poorer than that of the Brazilian ore fines, and a settling time of more than 20 min was required.
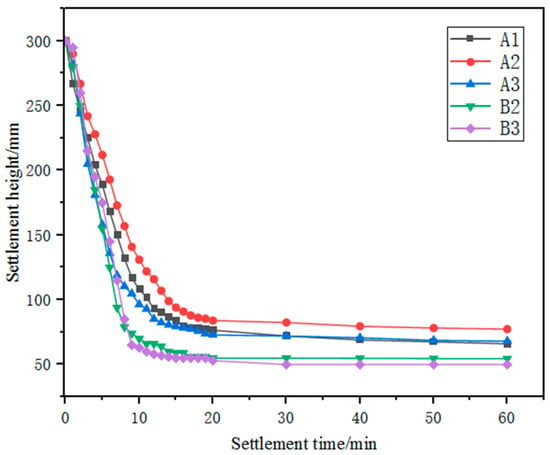
Figure 5.
Relationship between settlement height and settlement time for different iron ore fines.
3.2.2. Analysis of the Relationship between Settling Performance and Ore Mineralogy
Porosity is an important physical property or parameter of ores, reflecting the number and size of pores in the ore and has an important impact on ore processing. Generally, the greater the porosity of the ore, the smaller its density, resulting in slower settling speeds. Australian ore samples A1, A2 and A3 generally have higher porosity, which may be related to their slower settling speeds (with the ore settling time exceeding 20 min).
A higher content of goethite in an ore typically has a negative impact on settlement and filtration performance. Minerals with a high content of goethite have smaller and elongated particles, resulting in slower settling speeds and potentially longer settling times. Therefore, it can be inferred that, under other identical conditions, the higher the content of goethite, the poorer the settlement ability. Australian ore samples A1, A2 and A3 generally have high hematite needle contents, all exceeding 40 per cent, which may be one of the reasons for their longer settling times.
Clay minerals, including kaolinite, typically have smaller particle sizes and are often plate-like or fibrous in shape. Clay minerals with a high content and fine particle size easily form a colloidal suspension, causing slower settling speeds. Therefore, ores with high kaolinite content generally have slower settling speeds.
3.2.3. Filtering Characteristics
Filtration is an operation that separates solids and liquids by passing the liquid in a suspension through the channels of a porous medium under the action of an external force, while retaining the solid particles on the medium surface [23]. The liquid that passes through the filtration medium under pressure is called the filtrate, while the solid particles trapped on the filtration medium’s surface form the filter cake or residue.
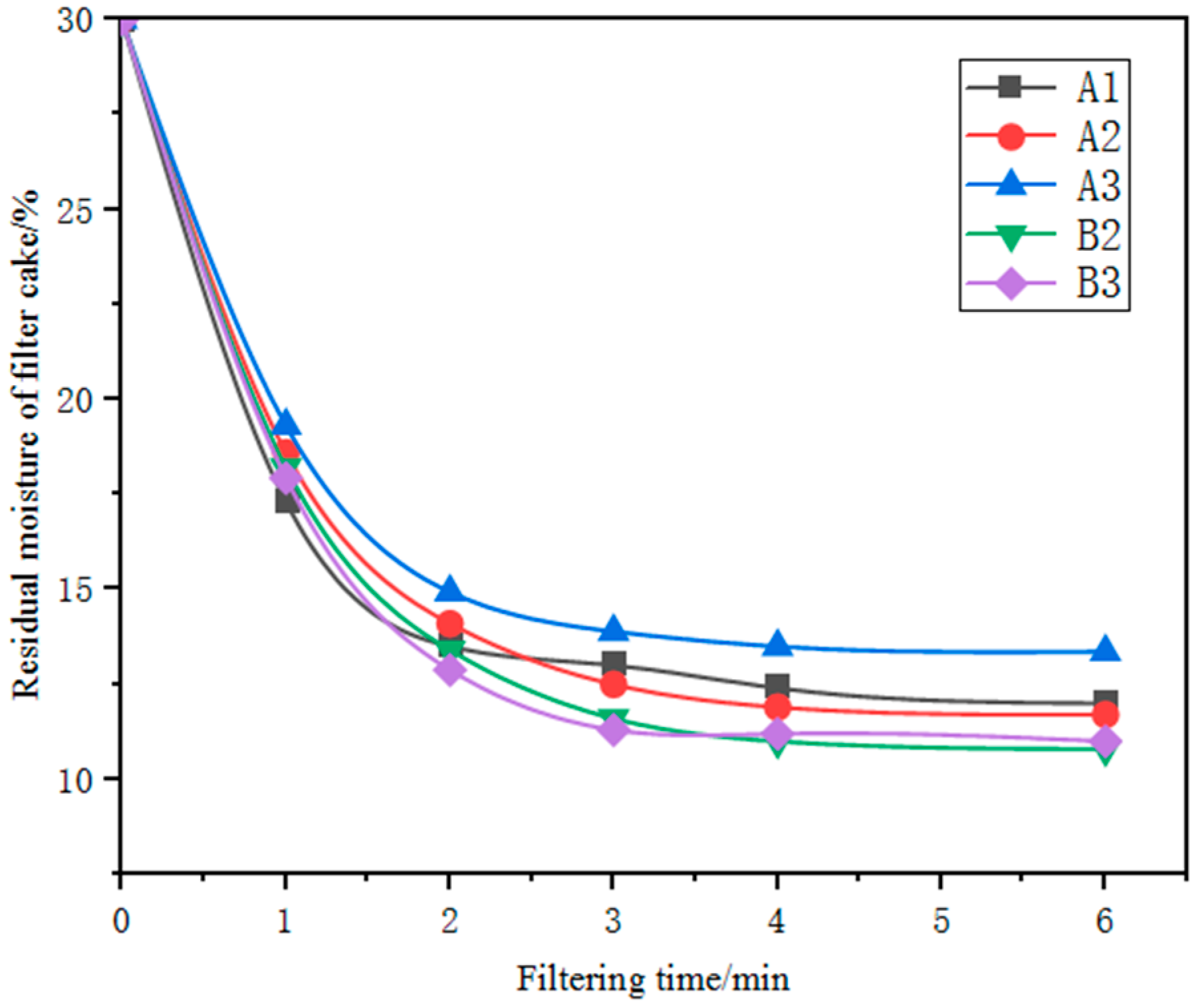
The influence of filtration time on the filtration performance of the five kinds of iron ore fines with a grinding fineness of 70 per cent passing through 0.074 mm was investigated under the conditions of a slurry concentration of 70 per cent and a filtration vacuum of 0.08 MPa. As shown in the Figure 6, with the increase in filtration time, the residual moisture in the filter cake gradually decreased, but the rate of decrease and the filter-cake moisture content varied among the five iron ore fines samples. Moreover, the change in residual moisture in the filter cake was not significant after the filtration time reached 4 min. Even with a filtration time of up to 6 min, the filter-cake moisture content of the five finely ground products remained above 10 per cent, indicating poor filtration performance and difficulty in filtration and dewatering. Due to moisture contents above 10 per cent, further drying of the resultant filter cakes was required before the subsequent balling process.
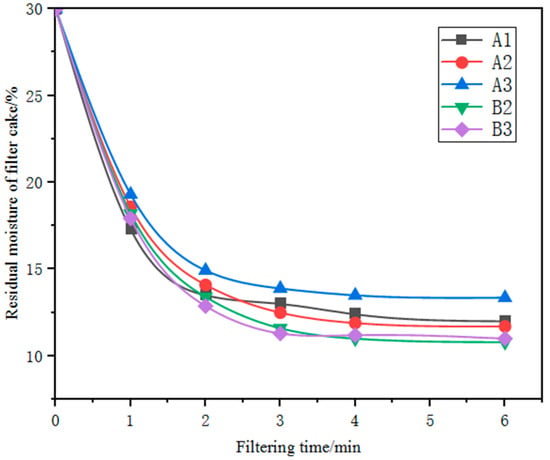
Figure 6.
Relationship between residual moisture of filter cakes and filtering time.
3.2.4. Analysis of the Relationship between Filtering Performance and Ore Mineralogy
The porosity of an ore refers to the proportion of voids in the ore, and a higher porosity indicates more voids in the ore, which generally leads to better filtration performance. If the porosity of an ore is low, water molecules have difficulty penetrating into the interior of the ore, resulting in poor settling and filtration effects. Therefore, in general, a higher porosity leads to better filtration performance. However, the filtration performance of the three Australian ores is also affected by the content of goethite, kaolinite and particle size, despite their relatively large porosity.
Goethite is a fibrous or needle-like mineral that can affect the solid–liquid separation of the ore. When the goethite content in the ore is high, the shape of goethite may cause a staggered stacking phenomenon on the surface of the ore, forming a denser structure, which leads to lower porosity in the ore. As a result, during settling and filtration, the water in the ore has difficulties draining smoothly, leading to poor filtration performance. Therefore, the low content of needle-like iron ore in samples B2 and B3 may be one of the reasons why they have a faster filtration rate and lower residual moisture content in the filter cake.
Kaolinite particles are generally small and tend to fill the voids, making it difficult for water to pass through and leading to poor filtration performance. The three Australian ores all contain varying amounts of kaolinite, which may partially explain why their filtration performance is poor. The kaolinite particles may fill the interstices between other particles, reducing the overall porosity of the ore and hindering the flow of water during filtration.
In summary, while the porosity of an ore is an important factor affecting its filtration performance, other factors such as the content of goethite and kaolinite, particle size and other factors should also be taken into consideration. By understanding and optimising these factors, it is possible to improve the filtration performance of ores and achieve better solid–liquid separation.
3.2.5. The Effect of Combined Ore Blending on Settlement and Filtration
Numerous production practices and experiments have shown that blending hard-to-grind and easy-to-filter materials with easy-to-grind and hard-to-filter materials for fine grinding can improve the filtration and settlement performance of the material, achieving the target for optimising filtration and settlement.
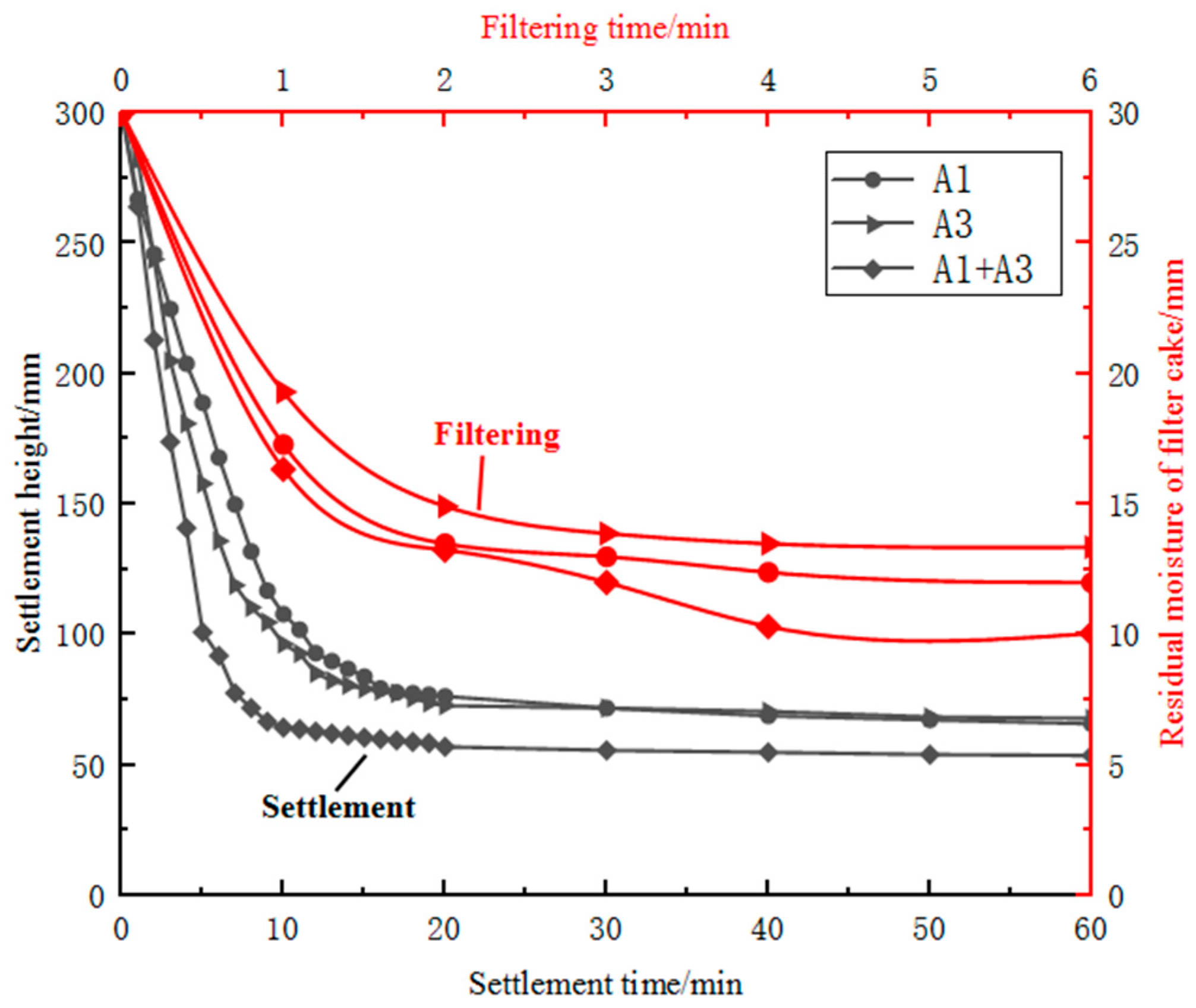
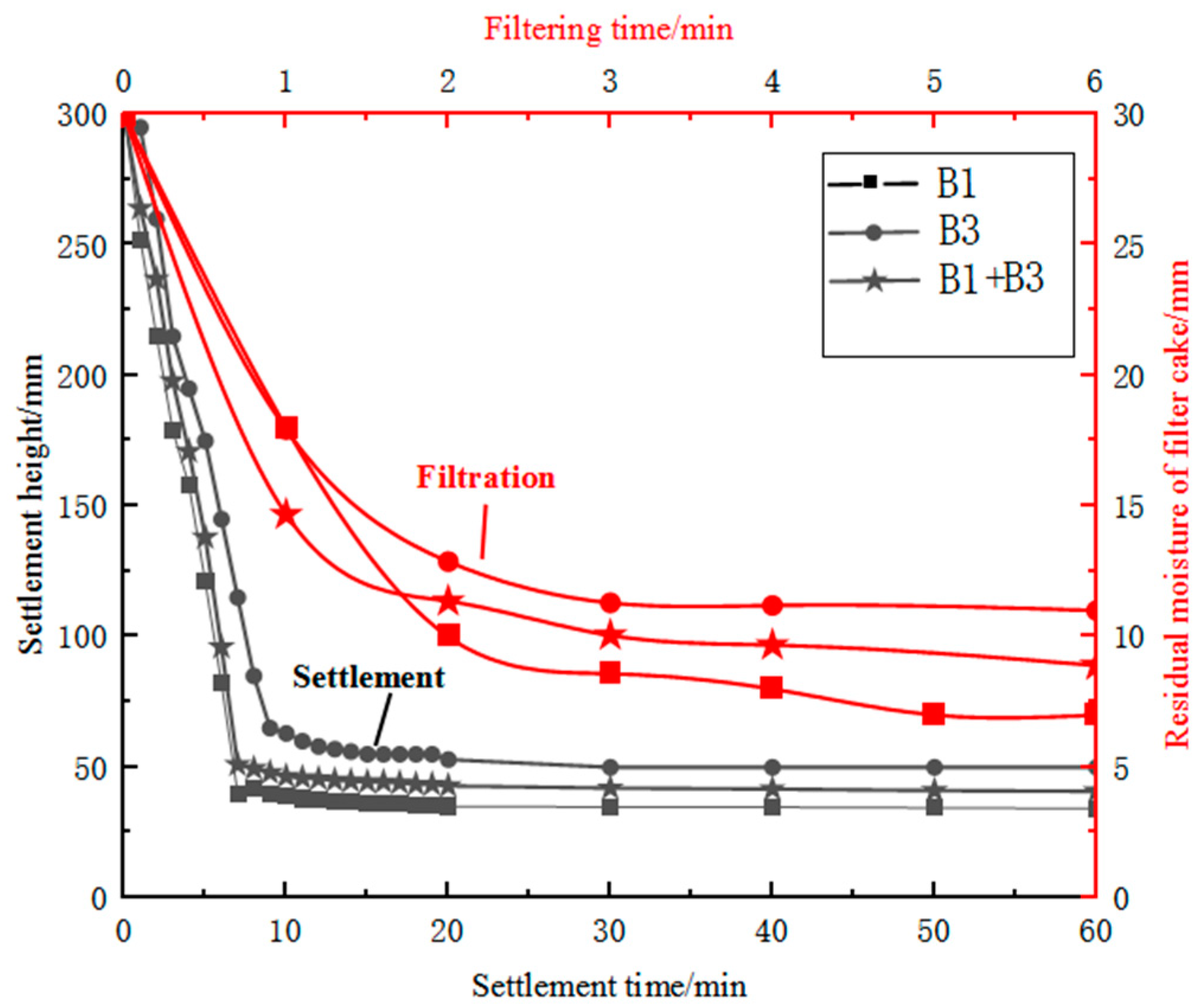
After wet ball milling, the moisture content of sample B3 exceeded 50 per cent and therefore required settlement and filtration treatment. During the wet ball milling of sample B3, the particles underwent lattice deformation and surface defects, resulting in increased surface activity of the particles [24]. The particle size improved after ball milling, and the mass fraction of fine particles increased; the specific surface area also increased, and the hydrophilicity was improved. Additionally, during settlement, highly active kaolin particles produced electrostatic repulsive forces in the aqueous medium, increasing the slurry viscosity and hindering settlement. During filtration, irregularly shaped fine particles in sample B3 filled the pores of the filter cake, reducing its porosity, and the increased hydrophilicity of the finely ground kaolin particles made it difficult to remove moisture from the filter cake. Sample B3 after fine grinding had poor settlement and filtration performance, and it was difficult to obtain a filter cake with a moisture content of less than 10 per cent using existing filtration equipment. Using a large proportion of sample B3 alone was difficult, which affected production efficiency and smooth operation. In contrast, fine specular iron ore concentrate on its own was easy to settle and filter and could achieve a filter cake moisture content of less than 10 per cent in 2 min of filtration time. Therefore, the settlement and filtration performance of finely ground sample B3 was improved by blending it with coarser and regularly shaped sample B1 concentrate. Based on the differences in mineral surface properties, settlement and enhanced filtration technology based on carrier particles with regular particle size, fewer lattice defects and poor surface hydrophilicity could be developed [25]. A comparison of the settlement and filtration performance of single ores and blended ores is represented in Figure 7. The addition of sample B1 concentrate to iron ore slurry was found to reduce both the settlement time and filter-cake moisture content. Increasing the concentration of sample B1 resulted in a reduction of approximately 5 min in the settlement time. By finely grinding sample B3 and blending it with sample B1 concentrate, the filter-cake moisture content could be controlled to below 10 per cent. By intelligently blending materials and controlling the blend of materials, finely ground imported fines suitable for pelletising could be produced. This solved the problem of fine particle pollution of water bodies and the difficulty of achieving the required moisture content of pelletising raw materials, which improved resource utilisation.
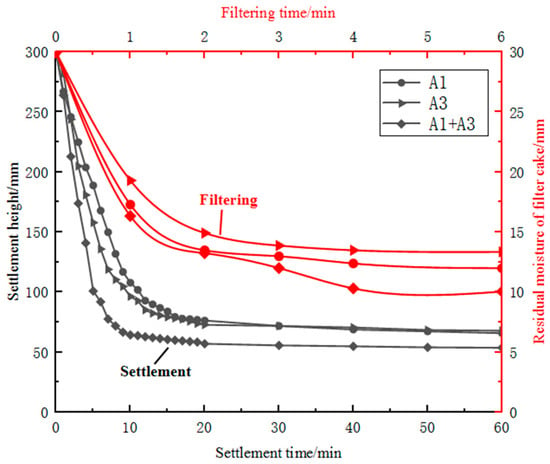
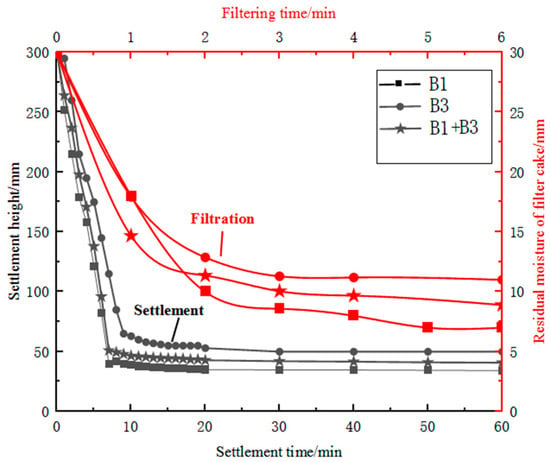
Figure 7.
Comparison of settlement and filtration performance of single ores and blended ores.
By blending sample A1 concentrate with Australian iron ore, the settlement time could be reduced from over 20 min to around 10 min, with a maximum settling rate of 23.55 cm/min during the first 10 min. For all cases, higher filtration pressure and longer filtration time resulted in lower residual moisture content of the filter cake. Filtration of samples A1 and A3 as single ores exhibited poor performance, with over 10 per cent residual moisture content even after 6 min of filtration. However, adding sample A1 improved the filtration performance of sample A3. Blending samples reduced the residual moisture content to around 10 per cent after 6 min of filtration. This suggests that blending of ores and concentrates can be a promising approach to improving the efficiency and quality of mineral processing.
3.3. Effect on Pellet Preparation
According to the material requirements for pellet production, fine grinding or blending of imported coarse ore powder is carried out to produce fine-grained iron concentrate for pellet formulation. The variety and diverse specifications of the finely ground powder results in lower levels of harmful elements such as potassium, sodium and zinc, thereby enhancing the resource security of pellet raw materials. At the same time, it also increases the selectivity of raw materials for technical personnel.
Experimental tests were carried out by adding finely ground imported powder to the pellet composition. Subsequently, various tests on balling, roasting and metallurgical performance of the fine-ground powders were conducted.
3.3.1. Effect on the Quality of Green Balls
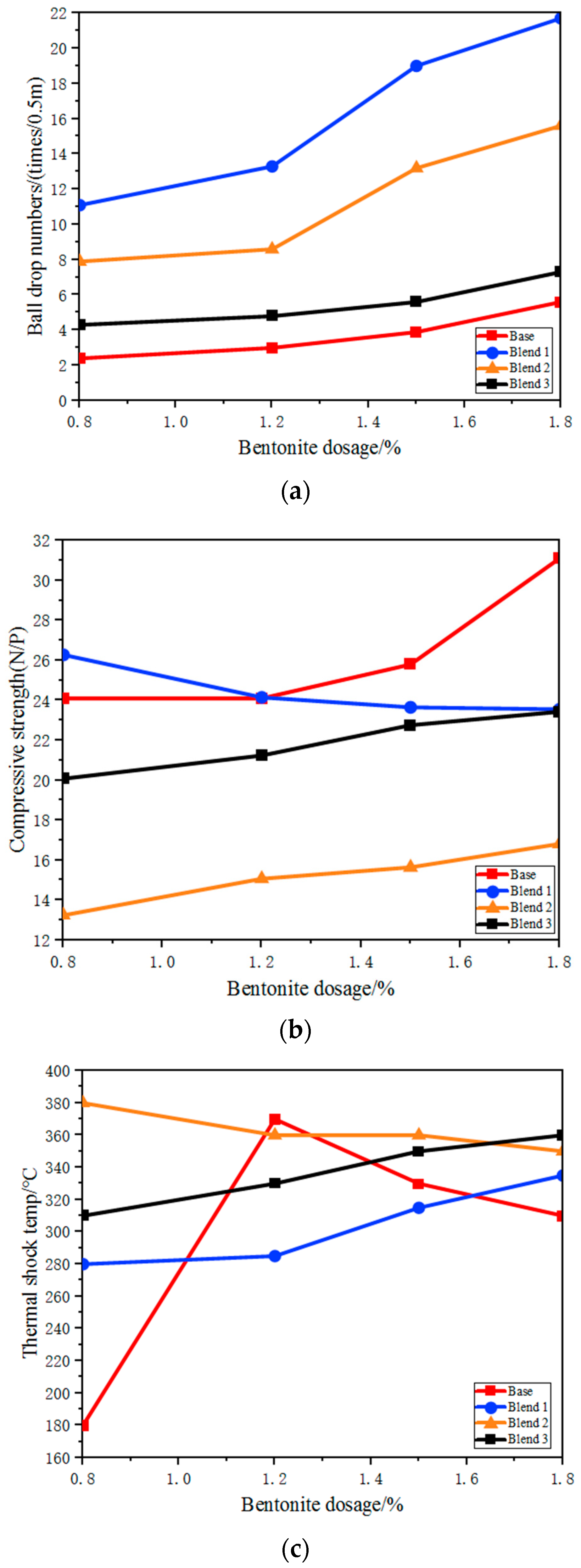
The quality of green pellets has a significant impact on the quality of the fired pellet. The quality of green pellets should be within an appropriate range, neither too high nor too low. For example, if the green pellet’s strength is too low, it may result in fracture during transportation, drying and roasting, leading to increased powder content, hindering material layer permeability and affecting the roasting process. Therefore, green pellets have a drop number of 5–8 times per 0.5 m, not exceeding 10 times per 0.5 m [8].
The impact of different blends on the quality of green pellets is shown in Figure 8. It is evident that, with the same bentonite content, the drop strength of the three blends with the addition of Australian ore was significantly increased compared to that of the base blend, with the A2 blend showing the most noticeable improvement in drop strength. Therefore, it is possible to reduce the amount of bentonite (from 1.8% to 0.9–1.2%), thereby enhancing the grade of the pellet, while also reducing the cost of the pellets.
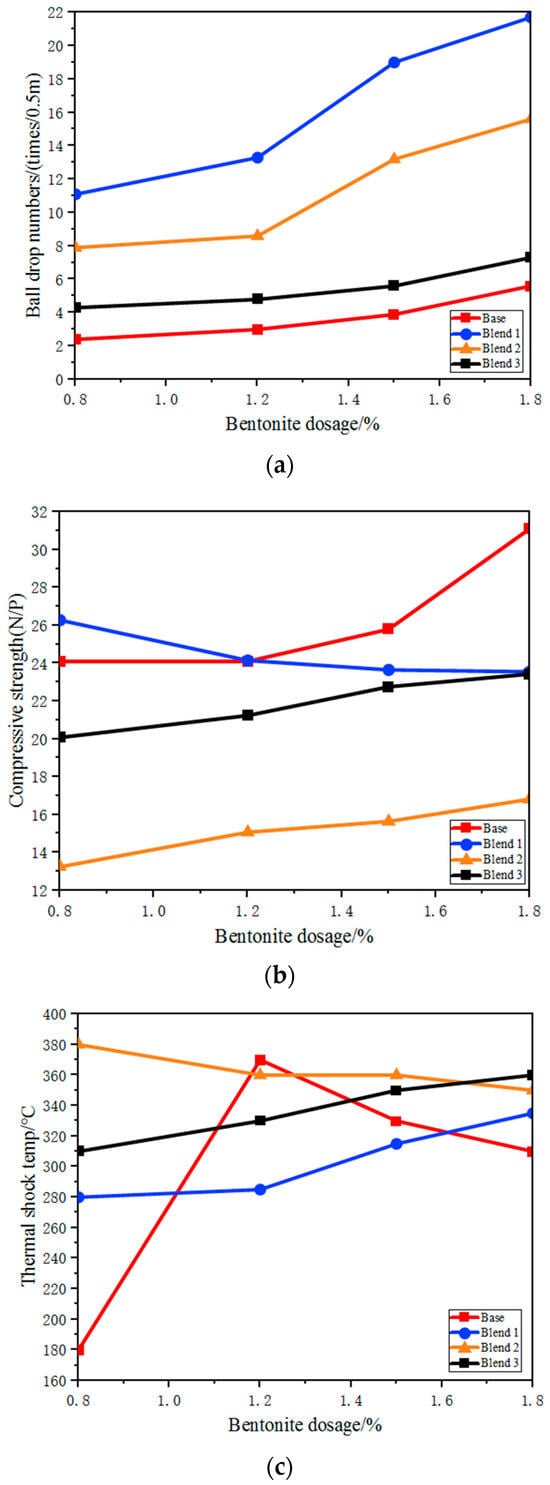
Figure 8.
The effect of different bentonite on the performance of green balls. (a) The effect of bentonite dosage on ball drop numbers of green balls with different proportions; (b) The effect of bentonite dosage on compressive strength of green balls with different proportions; (c) The effect of bentonite dosage on thermal shock temperature of green Balls with different proportions.
The thermal shock temperature refers to the temperature at which the structure of the green pellets is disrupted. If green-pellet bursting occurs during the drying process, it can significantly deteriorate the permeability of the material layer, further affecting the preheating and roasting process, leading to reduced productivity, decreased quality of the fired pellets and an increased return fines rate. There are many factors influencing the bursting temperature of green pellets, such as the physicochemical properties of the fine ore powder, bentonite blend and green-pellet moisture content. The addition of Australian ore blends can generally improve the bursting temperature, with the B2 blend having a higher bursting temperature and greater stability of green pellets when the A3 blend is added.
3.3.2. Effect on the Quality of the Fired Pellets
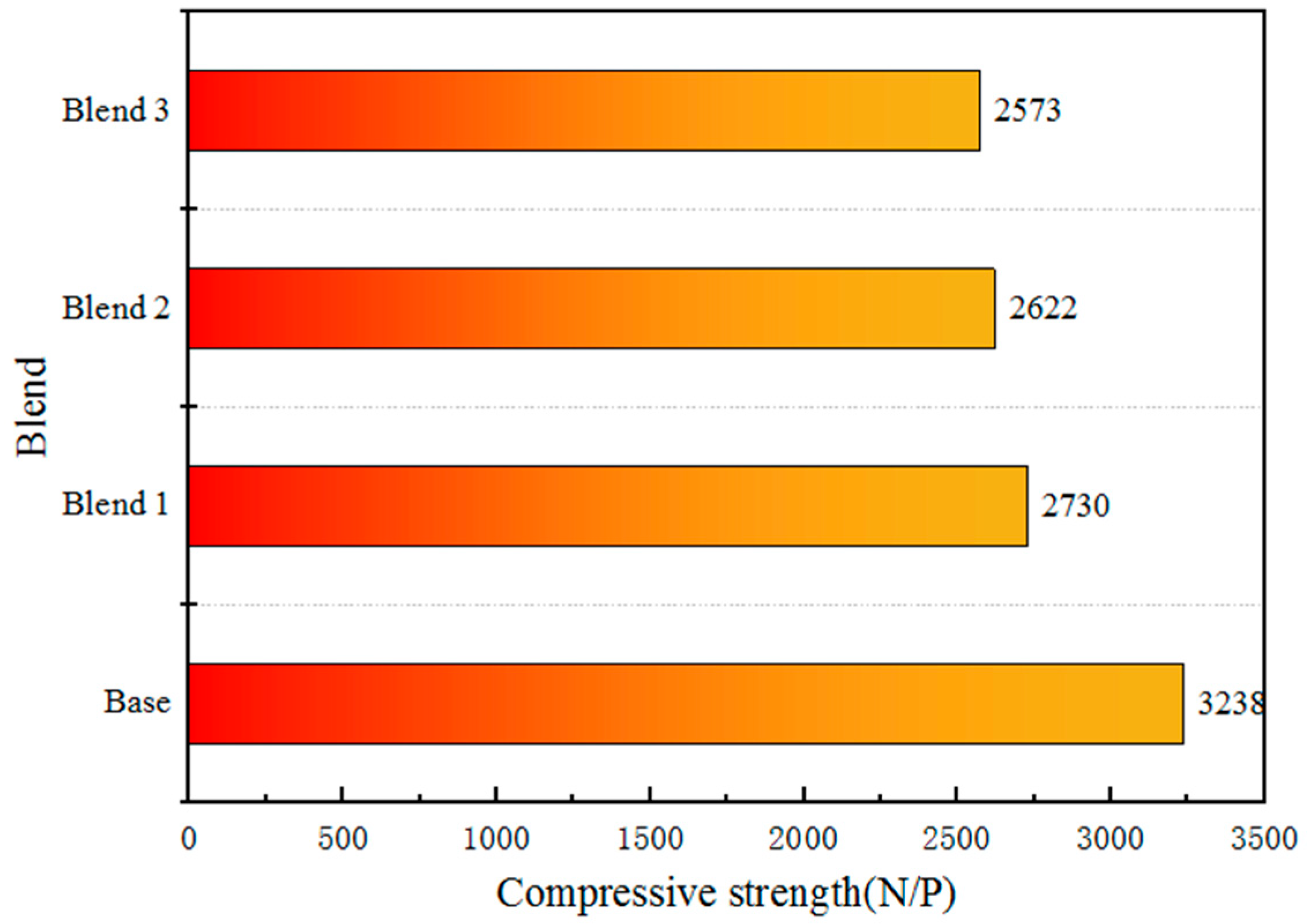
The optimal roasting system after the optimisation of four blends are illustrated in Table 6. The impact of different blends of added Australian ore on the compressive strength of fired pellets is illustrated in Figure 9. From the figure, it can be observed that the Base without added Australian ore, under the condition of roasting temperature at 1200 °C and roasting time of 12 min, still exhibits a compressive strength of over 3200 N per pellet. However, due to its excessively dense structure, this may reduce its reducibility and affect its metallurgical performance. In contrast, for the three blends with added Australian ore, the strength of the pellets can reach 2500 N per pellet, which essentially meets the requirements.

Table 6.
Optical operation parameters of straight grate pelletisation process for different ore blends.
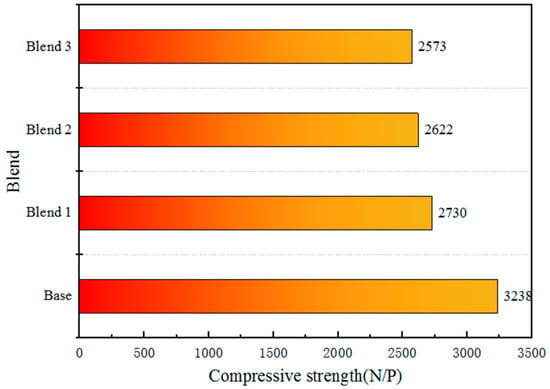
Figure 9.
Variation of compressive strength of fired ore with blends.
Based on the Figure 1, the goethite content in Australian ores is higher than that of Brazilian ores. Generally, goethite in ores tends to have higher crystalline water content. Australian ores, in particular, exhibit higher crystalline water content compared to Brazilian ores, necessitating an endothermic decomposition during the roasting process and requiring higher roasting temperatures. This results in increased energy consumption during the iron smelting process, leading to elevated production costs. Moreover, as the crystalline water content in Australian ores increases, the removal of crystalline water leads to an increase in pores in the pellets as shown in the Figure 9. Therefore, the Base structure is dense, while the blends with higher crystalline water content, such as Blend 1–3, exhibit numerous pores. The removal of crystalline water results in a porous microstructure with improved reactivity and reduction performance but leads to a decrease in compressive strength.
In general, higher crystalline water content is associated with greater loss on ignition. From the data in Table 1, it is evident that the loss on ignition in Australian ores is higher than that in Brazilian ores. Controlling loss on ignition is crucial in pellet preparation, as excessively high loss on ignition can lead to the excessive combustion of combustible substances in the raw materials. This results in insufficient binder content in the pellets, leading to a decline in pellet quality. The reduction in pellet quality can adversely affect the smelting efficiency and product quality of the iron blast furnace. Additionally, elevated loss on ignition releases more particulate matter, emissions and harmful substances, intensifying atmospheric pollution. High loss on ignition results in reduced raw material utilisation, increasing production costs. Raw materials are a significant cost factor in the iron smelting process; thus, excessive loss on ignition necessitates a higher volume of raw materials to meet production demands, further escalating costs.
Blend 2, which adds A2, is highlighted in Table 1 for exhibiting lower LOI than A1 and A3. Additionally, process mineralogy indicates that A2 has lower goethite content, implying the lowest crystalline water content among the three Australian ores. This suggests that, during the roasting process, blend 2 with A2 requires a lower temperature to achieve the desired roasting pellet quality.
3.3.3. Effect on the Metallurgical Properties of Pellets
The metallurgical performance of the fired pellets is presented in Table 7. As shown in Table 7, the RI of the fired pellets of blend 1–3 is close, ranging between 67% and 68%, obviously higher than that of the base. The lower RI of the base is probably due to the denser structure of the fired pellets (compressive strength of approximately 3200 N per pellet). The impact of other blends on the reducibility performance of the fired oxide pellets is not significant.

Table 7.
Fired pellet metallurgical performance test results.
The low-temperature reduction disintegration index (RDI+3.15) of the fired pellets is mostly greater than 95%. The blend exhibits higher low-temperature reduction disintegration RDI+3.15 due to denser structure of the fired pellets. Additionally, compared to A1 and A3, the addition of A3 in blend 2 shows better resistance to low-temperature reduction disintegration. Typically, poorer low-temperature reduction disintegration performance results in higher fines generation in the upper part of the blast furnace, leading to decreased productivity and increased energy consumption.
Furthermore, different blends exhibit varying reduction swelling performance of the fired pellets. However, the reducibility of the reference scheme is relatively low, which results in a protracted reduction process, sluggish crystal transformation and minimal lattice expansion. Consequently, this leads to a swelling rate of merely 0.62%. Additionally, the reduction swelling of fired pellets made from A1 and A2 is better than those made from A3. However, the reduction swelling rates of all blends are below 15%, indicating excellent reduction swelling performance.
3.3.4. Effect on the Microstructure of Fired Pellets
During the production process of pelletised ore, the internal microstructure of the fired pellets is one of the important factors affecting their compressive strength and reducibility. Therefore, studying the microstructure inside the hematite pellets using microscopy and scanning electron microscopy can help reveal the mechanisms behind the high mechanical strength and good reducibility of the pellets during the roasting process.
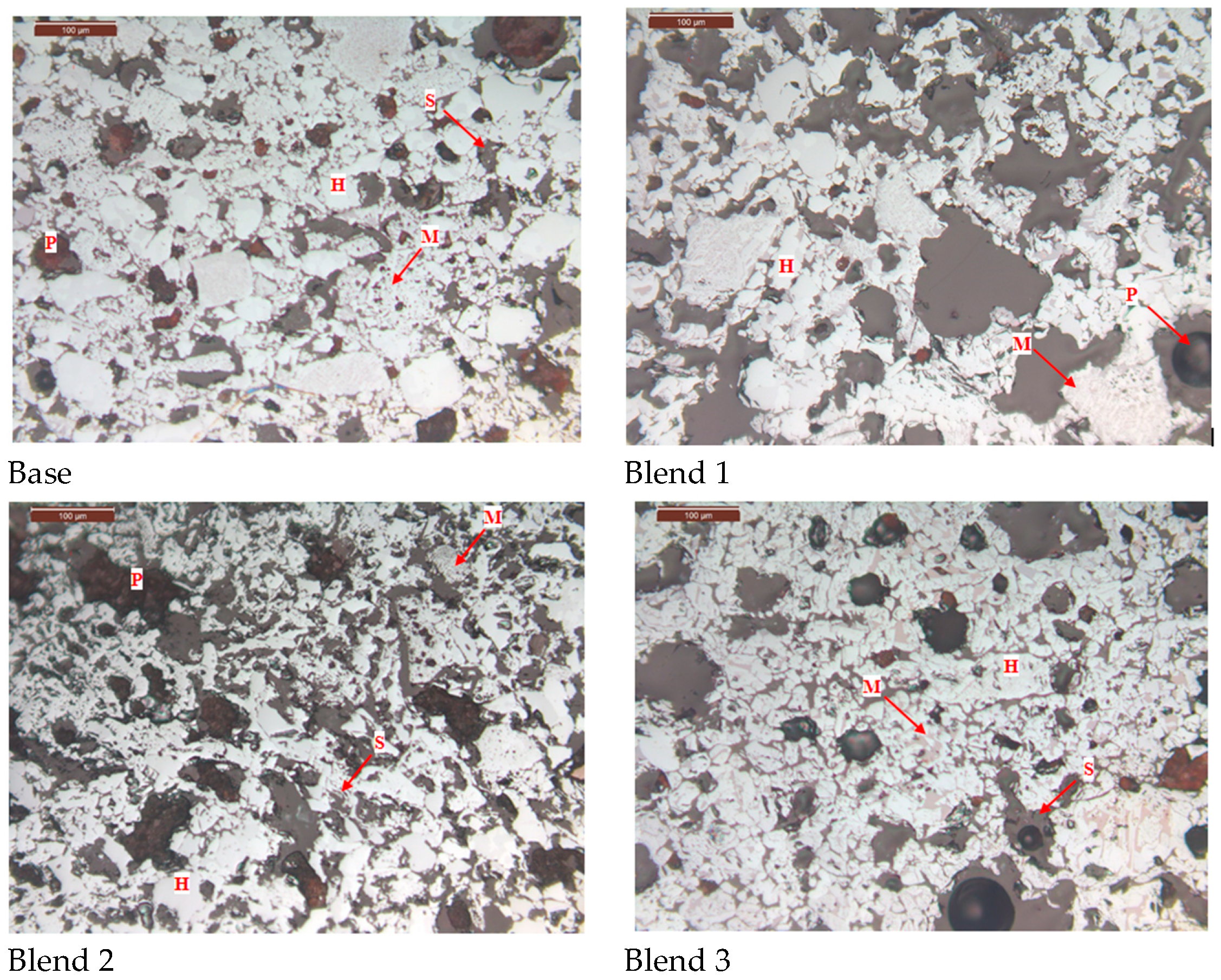
The microstructure of the fired pellets is shown in Figure 10. It is evident that the addition of Australian ore leads to the generation of more pores during the roasting process, resulting in an increased porosity of the pellets and a decrease in the degree of particle connectivity. Furthermore, there is a deterioration in the recrystallisation degree of Fe2O3, leading to a decrease in the compressive strength of the fired pellets. However, there is a slight improvement in reducibility.
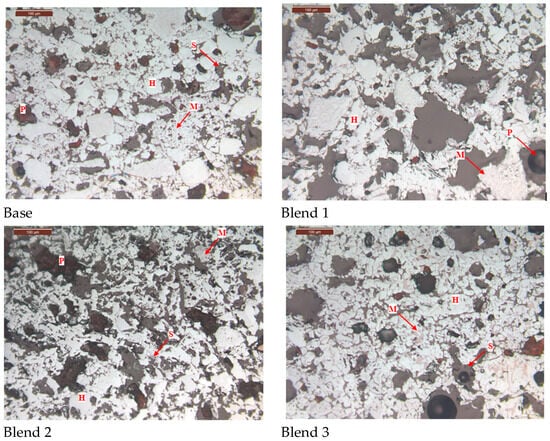
Figure 10.
Comparison of microstructures of fired ores. (H—Hematite, M—Magnetite, S—Silicate, P—Pore).
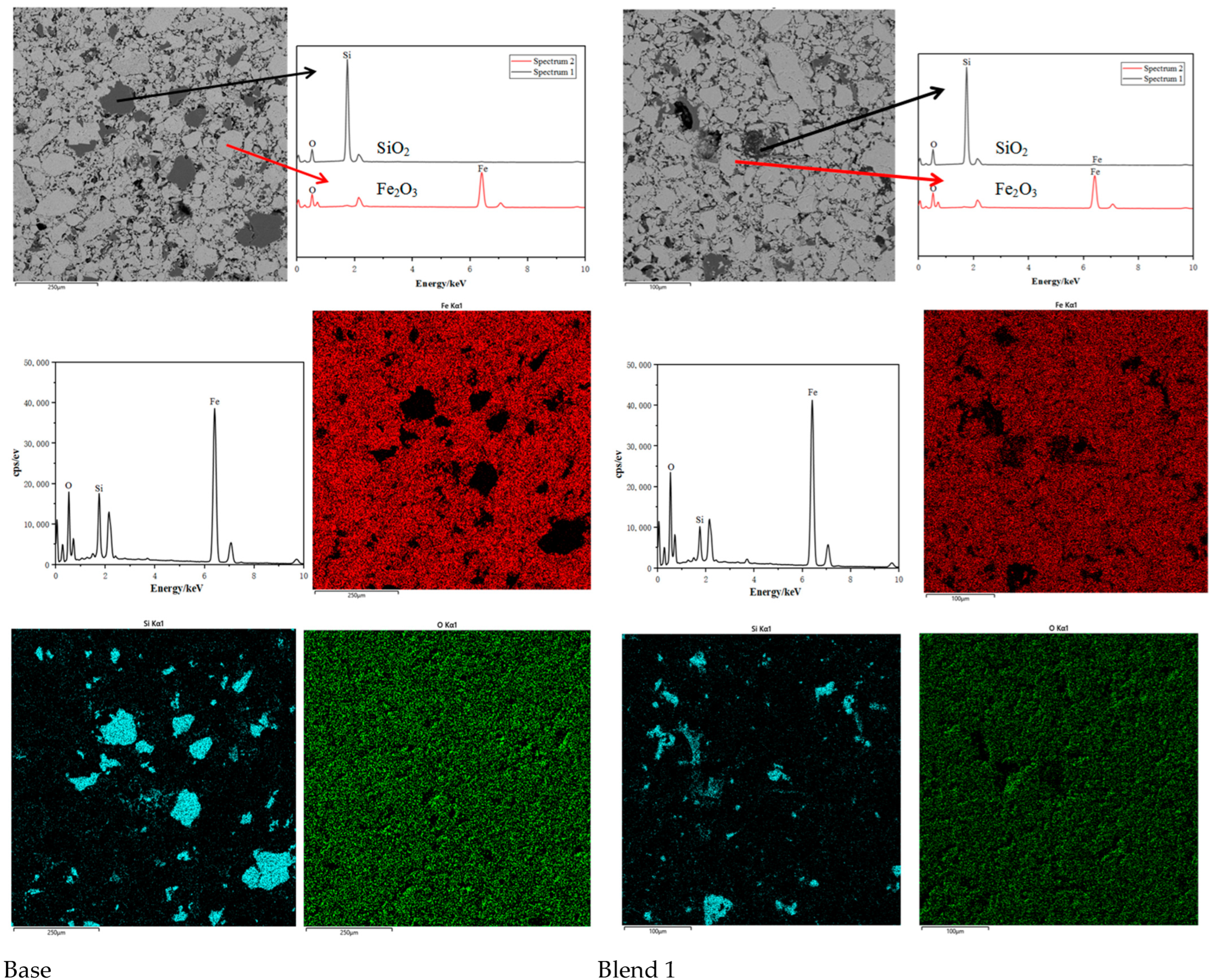
Comparing the microstructures of the four blends, it is evident that the Base blend is significantly denser with lower porosity compared to Blend 1–3. This observation is further supported by Figure 11 and Figure 12. Additionally, a silicate slag phase is commonly present in the interstices of oxidised iron particles. It often reshapes the particles by dissolving their sharp edges into the liquid phase, eliminating voids and consolidating the particles into a denser structure [26]. From this perspective, the presence of the liquid phase promotes pellet consolidation. However, on the other hand, the dense structure of the Base blend is likely to increase the internal diffusion resistance of reducing gases during the reduction process, leading to inferior reducibility.
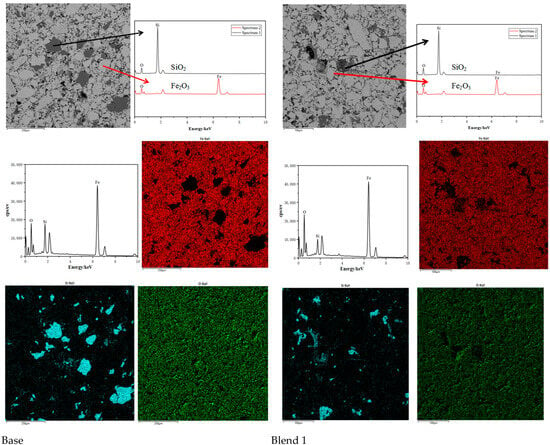
Figure 11.
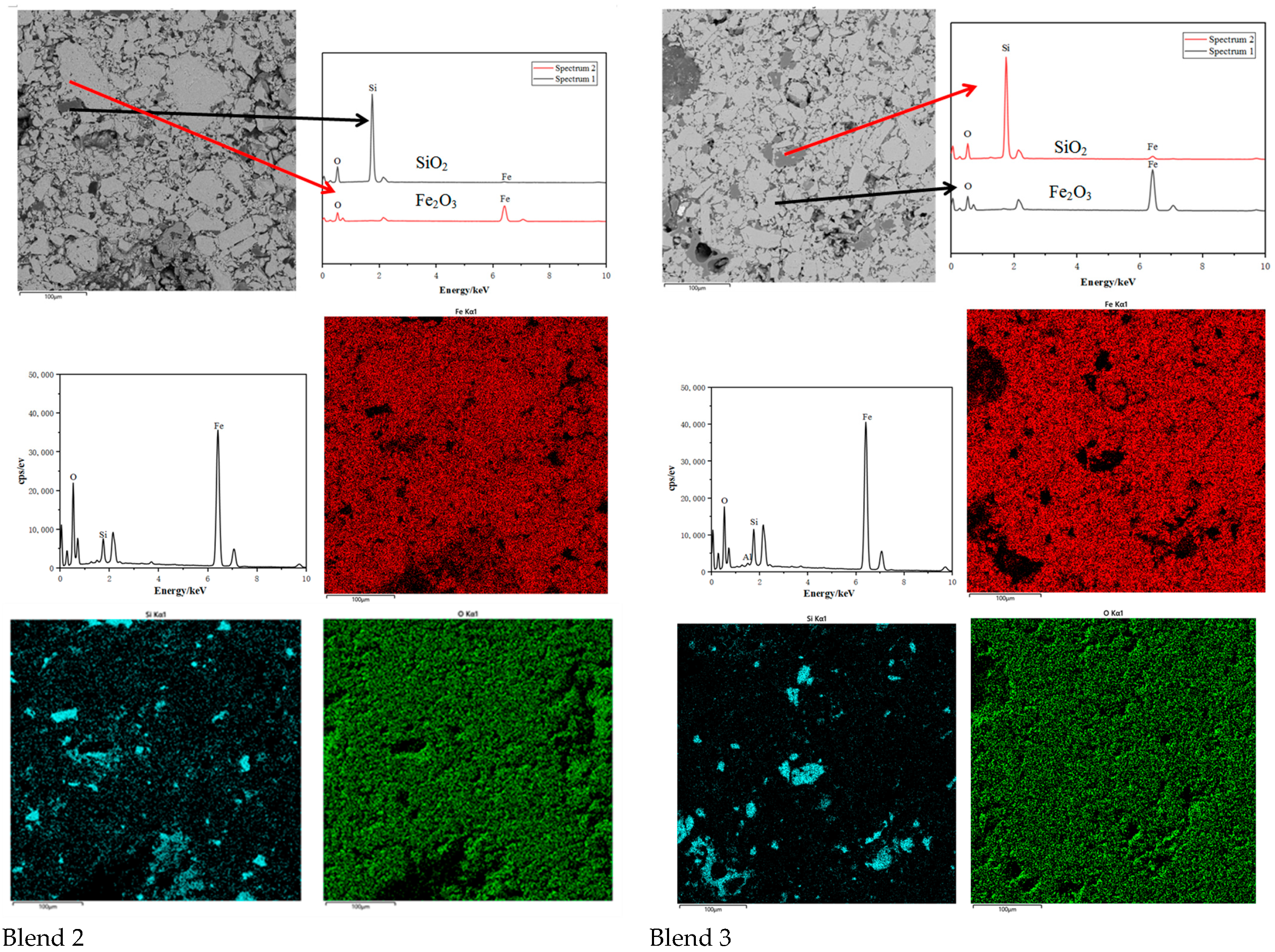
Scanning electron micrographs and Energy Dispersive Spectroscopy of Base and Blend 1. (Si = blue, Fe = red, O = Green).
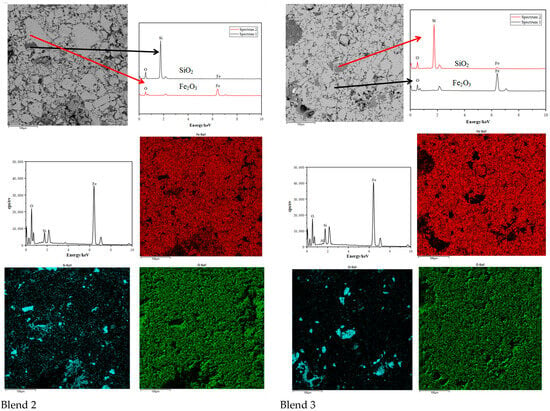
Figure 12.
Scanning electron micrographs and Energy Dispersive Spectroscopy of Blend 2 and Blend 3. (Si = blue, Fe = red, O = Green).
4. Conclusions
- By improving the technology of fine grinding, various imported ore resources can be well adapted and matched, providing more resource options for pellet feed blending. The Bond work index of the five iron ore fines samples investigated ranged from 10 to 15 kW·h/t, indicating good grindability and they can all be considered as materials that are relatively easy to grind. The static settling performance of the three Australian iron ore fines studied in this article was poorer than that of the Brazilian iron ore fines.
- There was reasonably clear relationship between the inherent mineralogical characteristics of the ores and their grinding, settling and filtration performance. Grindability was largely determined by the hardness of the iron ore and in general, the harder the ore, the more grinding energy required. Generally, iron ore with fewer goethite minerals, fewer soft minerals (such as kaolinite) and coarse particle size has good settling and filtration performance.
- By smart ore blending, it is possible to obtain pellet feeds with excellent settling and filtration performance, which can meet the requirements of the grinding and selection process, maximise the production rate of equipment, reduce operating costs and produce high-quality finely ground iron ore concentrate.
- The addition of finely ground Australian ores improve the balling performance of pellet mixtures. The addition of 40% finely-ground Australian iron ores is capable of improving the metallurgical performance of pellet products. And the fired pellets obtained from blend 1, blend 2 and blend 3 exhibit good metallurgical properties, with reduction index (RI) of 67–68%, low-temperature reduction disintegration index (RDI+3.15) generally greater than 95% and reduction swelling index (RSI) less than 15%.
Author Contributions
Conceptualisation, Q.Z. and W.Z.; methodology, Q.Z. and W.Z.; software, Q.Z.; validation, D.Z., J.P. and C.Y.; formal analysis, Q.Z. and W.Z.; investigation, Q.Z. and W.Z.; resources, Q.Z. and W.Z.; data curation, Q.Z. and W.Z.; writing—original draft preparation, Q.Z. and W.Z.; writing—review and editing, C.Y.; visualisation, J.P.; supervision, D.Z.; project administration, J.P. and D.Z.; funding acquisition, J.P. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully thank the Natural Science Foundation of China (Grant No. 52004339) and the China Baowu Low-Carbon Metallurgy Innovation Foundation (BWLCF202216) for providing financial support for the project.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to some information that could compromise the privacy of research participants.
Acknowledgments
The authors would like to acknowledge the Analytical and Testing Center of Central South University, which supplied the facilities for the measurements.
Conflicts of Interest
The authors declare no conflicts of interest. And author W.Z was employed by the company Huai Hai New Material. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yie, K.W. The present state and the forecasting of our country pelletizing industry. Sinter. Pelletizing 2003, 1, 14. [Google Scholar]
- Mohamed, O.A.; Shalabi, M.E.H.; El-Hussiny, N.A.; Khedr, M.H.; Khedr, F.M. Effect of some additives on flowability and compressibility of lactose powders. Powder Technol. 2003, 130, 277–282. [Google Scholar] [CrossRef]
- Fu, J.Y.; Jiang, T.; Zhu, D.Q. Sintering and Pelletizing; Central South University of Technology Press: Changsha, China, 1996; pp. 313–315. [Google Scholar]
- Peltoniemi, M.; Kallio, R.; Tanhua, A.; Luukkanen, S.; Perämäki, P. Mineralogical and surface chemical characterization of flotation feed and products after wet and dry grinding. Miner. Eng. 2020, 156, 106500. [Google Scholar] [CrossRef]
- Gao, E.X.; Zhang, C.; Li, Y.P.; Luo, J.H.; Gao, R.Z.; Rang, J.C. Effects of Grinding Methods on the Flotation Kinetics of Sphalerite and Pyrite. Metal. Mine 2022, 9, 100–106. [Google Scholar]
- Wills, B.A.; Napier-Munn, T.J. Will’s Mineral Processing Technology, 7th ed.; Elsevier Inc.: Oxford, UK, 2006; pp. 108–117, 267–352. [Google Scholar]
- Zhu, D.Q.; Guo, Z.Q.; Pan, J.; Wang, Z.Y. Insights on pretreatment of Indian hematite fines in grate–kiln pelletizing process: The choice of grinding processes. J. Iron Steel Res. Int. 2018, 25, 506–514. [Google Scholar] [CrossRef]
- Ma, L.; Qing, G.L. Experimental study on pelletizing proportioned with grinded MAC fines. Sinter. Pelletizing 2019, 44, 39–41. [Google Scholar]
- Yang, Y.B.; Zhang, J.; Zhong, Q. Effect of pretreatment on the ballability of hematite in preparation of a fluxed pellet. Min. Metall. Eng. 2019, 39, 83–88. [Google Scholar]
- Ahmadi, R.; Shahsavari, S. Investigation of laboratory conditions effect on prediction accuracy of size distribution of industrial ball mill discharge by using a perfect mixing model. Miner. Eng. 2009, 22, 104–106. [Google Scholar] [CrossRef]
- Gent, M.; Menendez, M.; Torano, J.; Torno, S. Effect of grinding aids on the grinding energy consumed during grinding of calcite in a stirred ball mill. Powder Technol. 2012, 224, 217–222. [Google Scholar] [CrossRef]
- Magdalinovic, N.; Trumic, M.; Trumic, G.; Magdalinovic, S.; Trumic, M. Grinding of hematite ore with a high specific surface area using a planetary ball mill. Int. J. Miner. Process. 2012, 114–117, 48–50. [Google Scholar] [CrossRef]
- Ipek, H.; Ucbas, Y.; Hosten, C. Effect of particle size distribution on grinding kinetics in dry and wet ball milling operations. Miner. Eng. 2005, 18, 981–983. [Google Scholar] [CrossRef]
- GB/T 13241-2017; Iron Ore–Determination of Reducibility. China National Standardization Administration: Beijing, China, 2017. Available online: https://www.chinesestandard.net/PDF.aspx/GBT13241-2017 (accessed on 14 October 2017).
- GB/T 13240-2018; Iron Ore Pellets for Blast Furnace Feedstocks—Determination of the Free-Swelling Index. China National Standardization Administration: Beijing, China, 2018. Available online: https://www.codeofchina.com/standard/GBT13240-2018.html (accessed on 1 February 2019).
- GB/T 13242-2017; Iron Ores—Low-Temperature Disintegration Test—Method Using Cold Tumbling after Static Reduction. China National Standardization Administration: Beijing, China, 2017. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT13242-2017 (accessed on 14 October 2017).
- Long, H.M.; Chun, T.J.; Wang, P.; Meng, Q.M.; Di, Z.X.; Li, J.X. Grinding kinetics of vanadium-titanium magnetite concentrate in a damp mill and its properties. Metall. Mater. Trans. B 2017, 48, 815–822. [Google Scholar] [CrossRef]
- Xing, W.; Yang, H.L.; Xi, Z.W. Study on Fine Grinding Technology of Imported Iron Concentrate. Min. Eng. 2020, 18, 43–46. [Google Scholar]
- De Bakker, J. Energy Use of Fine Grinding in Mineral Processing. Metall. Mater. Trans. E 2013, 1, 8–19. [Google Scholar] [CrossRef]
- Zhou, C.M.; Shao, G.Y. Weighted Vickers Hardness and Grindability of Granite. Stone 1997, 1, 18–20; 30. [Google Scholar]
- Su, G.H.; Wang, Z.Q.; Li, H.Y. Application of modifier to improve sedimentation and filtration performance of superfine slurry of laterite nickel ore. World Nonferrous Met. 2017, 16, 247–248. [Google Scholar]
- Cai, F.W.; Zhou, X.T.; Xia, J.P.; Luo, Z.Q.; Man, L.; Zhu, L. Study on Settling Character of Bauxite Tailings Slurry. Bull. Chin. Ceram. Soc. 2014, 33, 1544–1549. [Google Scholar]
- He, J.W.; An, G.; Wang, K.; Kang, H.J.; Zhao, W.C.; Liu, C.J. Shougang Jingtang imported fine grinding technology. Hebei Metall. 2023, 2, 33–36. [Google Scholar]
- Peng, D.S.; Pan, J.; Li, J.; Tian, H.Y.; Yang, C.C.; Shi, X.J.; Sheng, W.J. Experimental study on the preparation of fluxed pellets from fine grinding carajas powder. Sinter. Pelletizing 2021, 4, 50–57. [Google Scholar]
- Yi, L.J.; Jiang, L.H.; Li, J.; Niu, C.S.; Chen, J.H.; Zhu, D.Q.; Yang, C.C.; Tian, H.Y. Technology development and application of diversified low cost clean production of high quality pellet products. Sinter. Pelletizing 2022, 47, 95–103. [Google Scholar]
- Yang, C.C.; Zhu, D.Q.; Pan, J. Oxidation and Induration Characteristics of Pellets Made from Western Australian Ultrafine Magnetite Concentrates and Its Utilization Strategy. J. Iron Steel Res. (Int.) 2016, 23, 924–932. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).