Use of Metallic Mercury in Artisanal Gold Mining by Amalgamation: A Review of Temporal and Spatial Trends and Environmental Pollution
Abstract
:1. Introduction
1.1. History of Gold Mining and Use of Mercury in Amalgamation Techniques
1.2. Historic and Present Time Gold Rushes around the World
| Timeline | Remarks | References |
|---|---|---|
| Early 2700 B.C. | The Phoenicians and Carthaginians used Hg to amalgamate precious metals, recovered upon heating. | [15,58,59] |
| Before 4th Century B.C. | The Egyptians, Greeks, and Romans used Hg in the making of cosmetics and medicines and for amalgamation of gold and other precious metals. | [8,74] |
| In the 4th Century B.C. | Hg was used in religious ceremonies and to extract other metals—this was first documented by Aristotle. | [8,56,75] |
| 2nd Century B.C. | Alchemists in China used Hg in processes that tried to convert base metals to gold. | [15,58] |
| From 50 to 68 A.D. | First application of amalgamation technique to mining gold in Bosnia under Emperor Nero (56–68 A.D.) This technology had a widespread application among the Romans in 50 A.D. Its environmental problems led the Romans to prohibit this activity in Italy after less than 100 years. | [8,76] |
| About 1400 A.D. | Gold mining was revived after the fall of the Roman Empire in the 11th century in Central Europe, around the Harz Mountains in Germany. ** Amalgamation and retorting processes were applied in gold extraction. | [8] |
| In 1500s A.D. | The use of Hg was launched in the Americas in the 16th century by the Spanish to amalgamate silver and gold in Mexico, Peru, and Bolivia by applying the “Patio” process. Later, the Patio amalgamation technique was industrialized and used for the recovery of gold and silver. | [10,21,33,38,43] |
| [10,68,77] | ||
| 1400–1600 A.D. | Gold was produced by the amalgamation technique using Cu plates in areas of European explorations. | [78] |
| In 1800s A.D. | Gold mining with Hg began in North America, New Zealand, Australia, and Russia. | [38,43,63,68,69,70,71,72,73,74,75,76,77,78,79,80,81] |
| End of 1800s | The use of the “Patio” process continued through the end of the 19th century. This process, in spite of its convenience, led to the discharge of unprecedented amounts of Hg to the American environment. | [21,33,38,43] |
| During the 1980s | An increase in gold price boosted a new gold mining boom using Hg for amalgamation occurring in South America, Africa, and Southeast Asia. | [21,82] |
| Today | Small-scale artisanal gold mining in gold-rich developing tropical nations continues to use the amalgamation technique intensively and often unrestrictedly, whereas larger companies in developed nations have long replaced Hg amalgamation practice with cyanidation and oxidation methods to extract gold from crude ores. The impact on biodiversity remains poorly studied. | [69,83,84] |
| Gold Rush Events | Remarks | References |
|---|---|---|
| North America from 1799 onwards | Gold mining commenced in USA with gold rushes spreading to several states, including North Carolina, Alabama, California, South Dakota, Colorado, Georgia, and Nevada. In 1896–1901, Klondike, Yukon, Canada experienced a gold rush. In all locations, gold was primarily concentrated using Hg amalgamation techniques. | [67,68,69,77] |
| In 1838 | The discovery of gold in Siberia on the Ulderey River resulted in a gold rush, restricted only to the local population. Significant mining also took place in the Lena Basin from 1846, relying on Hg amalgamation techniques. | [85,86,87,88,89,90] |
| In 1851 | The Australian gold rush started at Bathurst (New South Wales) and Ballarat and Bendigo in Victoria. More gold was discovered in New South Wales, Queensland, Western Australia, and New Zealand subsequently, depending principally on gravity concentration and Hg amalgamation as the main gold extraction technique. | [68,79,81,91,92] |
| Between 1873 and 1886 | South Africa experienced a gold rush following gold discovery in Lyndenburg, Witwatersrand and other localities. Similarly, Hg amalgamation was the method of choice for gold extraction. | [38,68,93] |
| Gold-rich developing nations | Gold mining by Hg amalgamation remains the method of choice used in small-scale mining (SSM) activities. SSM occurs primarily in South and Central America, Africa, and Asia. SSM by amalgamation is still practiced by a few placer miners in Australia, Canada, Russia, and USA. | [21,33,87,88,94,95,96,97,98,99,100] |
2. Methods
3. Results and Discussion
3.1. Gold Mining Processes and Amalgamation Technology
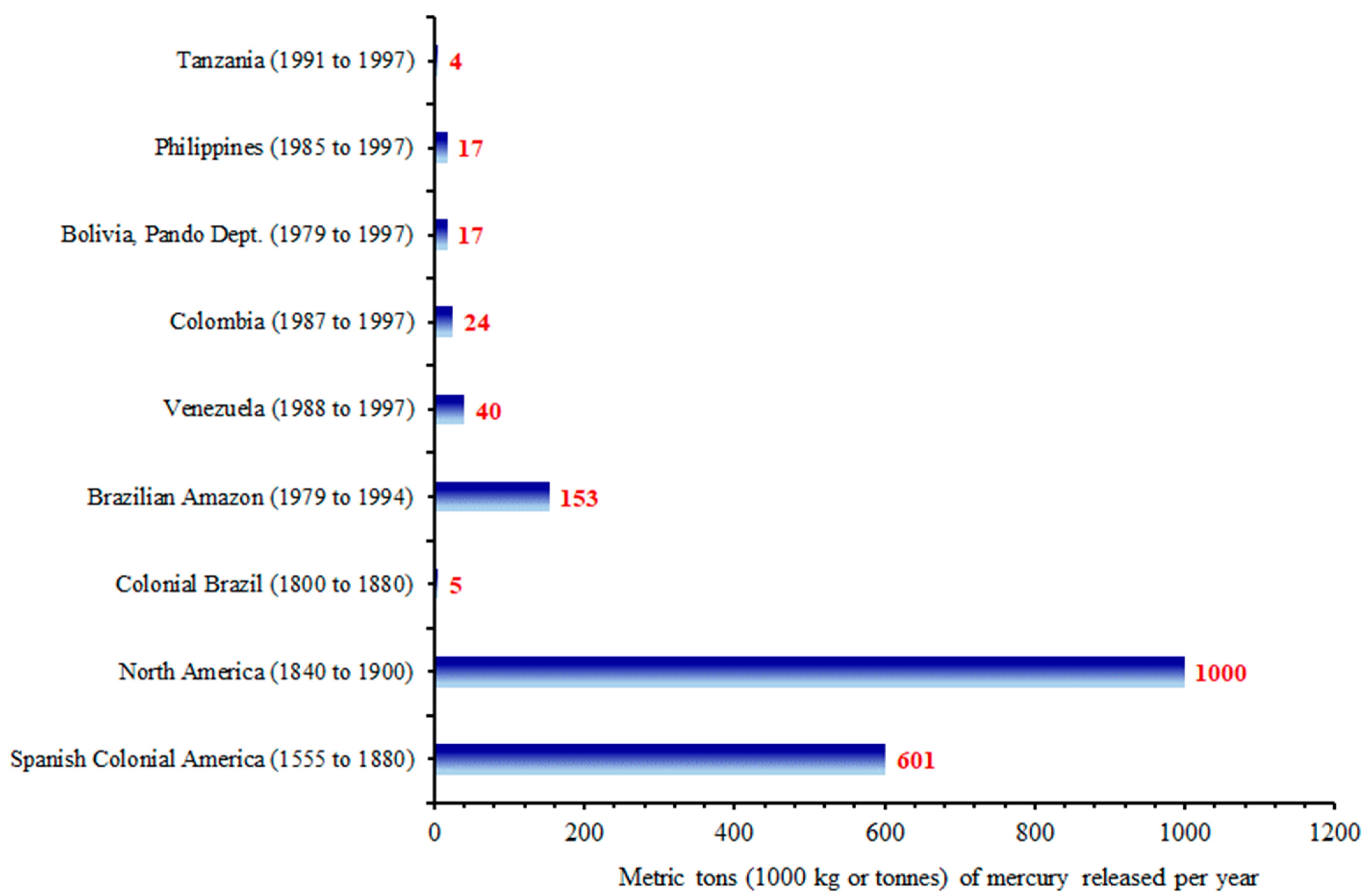
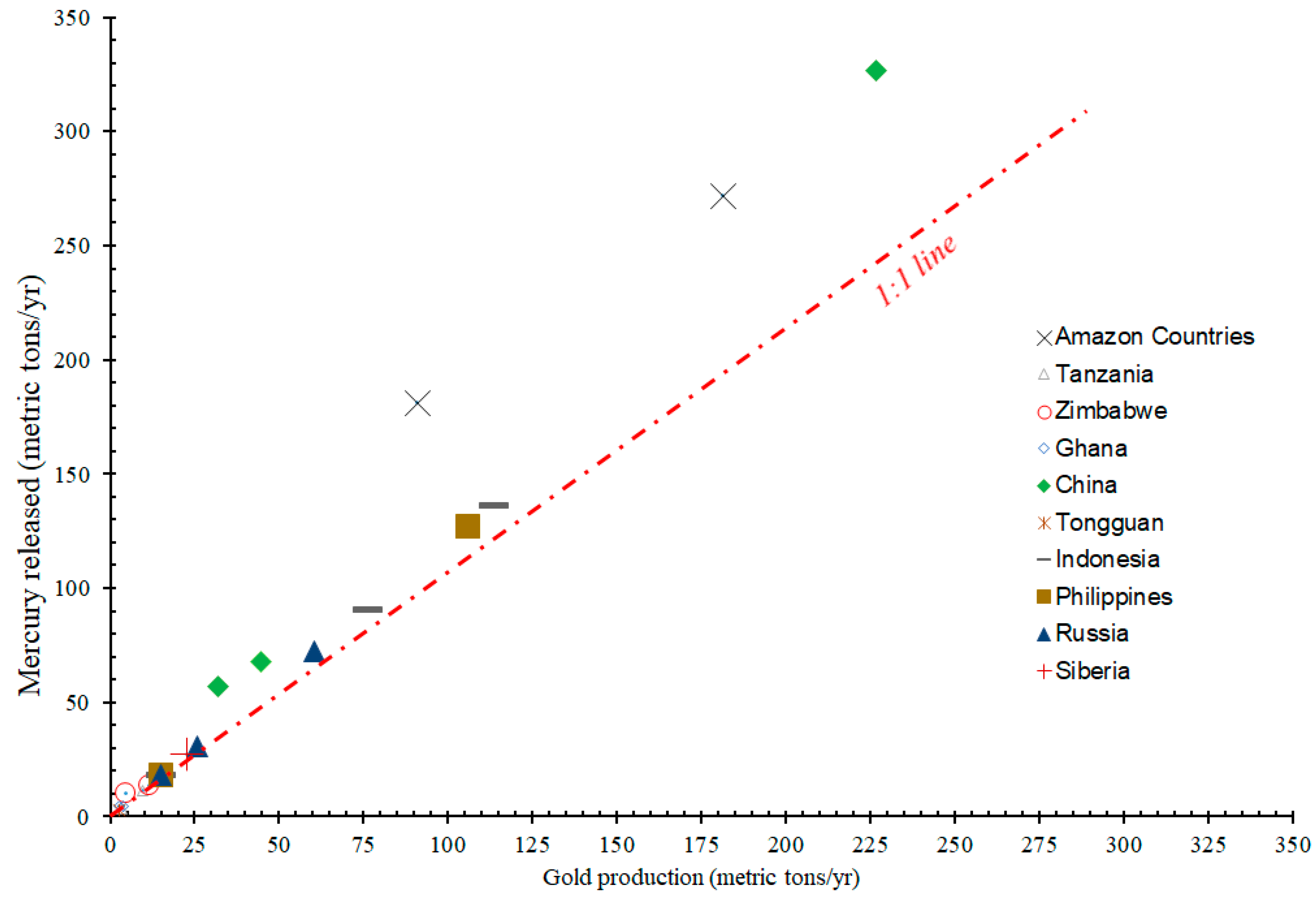
3.2. Mercury Discharges into the Environment Due to Past and Present Gold Mining Activities
| Countries and Mining Sites | Years | Au Production (Metric Tons) | Hg Consumption (Metric Tons) | Hg Release/Loss (Metric Tons) | Receptors | Remarks | References |
|---|---|---|---|---|---|---|---|
| Spanish America | 1550–1880 | 12,7006 * | 34,2916 ** | 17,7808 | Hg trapped in tailings/soils | Mercury is still present in mine tailings, soils, and sediments | [15,26,43,119] |
| USA | 1804–1995 | 11,793 | 31,842 ** | 54,431 | Environment | Hazardous waste Superfund sites. Pollution by mine tailings | [25,43,57,67,77,112,120,121,122] |
| Comstock Lode, Nevada (USA) | 1859–1890 | 4406 * | 11,897 ** | 5761 to 6169 | Carson River Basin. Hg lost to streams in both NV and CA | Contaminated milling tailings: Hg persists in water and sediments; mercurialism among miners | [106,112,123,124] |
| Dahlonega Mining District, North Georgia (USA) | 1820–1900 | 9.7–20.7 * | 26.2–55.9 ** | 13.6–29.0 | Dahlonega Mining District | Hg pollution of both terrestrial and aquatic environments | [25,119,125] |
| Canada | 1800s | 20.1 * | 54.2 ** | 28.1 | Air, water, soil | Anthropogenic release | [28] |
| Goldenville, Nova Scotia (Canada) | 1860–1940 | 4.4 * | 11.9 ** | 6.2 | Air, water, soil | 2.72 × 108 metric ton tailings with 6.2 metric tons of Hg | [69] |
| Alaska and Klondike goldfields | 1800s | 1525 ** | 4119 | 2136 * | Processing sites | 1.4 × 10−3 metric tons of Hg lost for every kg of Au | [38] |
| Wales (Gold belt of Gwynedd) | 1860–1916 | 3.40 | 9.2 ** | 4.8 * | High levels of Hg in Mawddach River | Hg pollution of a river system. | [126] |
| Australia Bendigo, Victoria | 1850–1930 | 583.2 * | 1575 ** | 817 | Hg contamination of waterways and biota | Weight of Hg used was same as weight of Au recovered | [81,91,92,127,128] |
| New Zealand | 1860–1870s | 20–544.3 | 54–1470 ** | 28–762.0 * | Air, water, soil | Legacy of Hg impacted river systems | [79,97,129] |
| Former Soviet Union | 1800s | 6.2–14.5 | 16.7–39.1 ** | 31.0 to 73 | Air, water, soil | High Hg levels in water, soil, and sediments; 0.38 MT Hg used on rugs of sluices; 5.4 MT Hg used on dredges | [85,86,87,88,89,90] |
| Regions | Hg (Metric Tons) | ||
|---|---|---|---|
| 2000 | 2005 | 2010 | |
| Africa | >32.7 to >51.7 | 40 | 200 |
| Asia | >294.8 to >430.9 | 160 | 240 |
| South America | >96.2 to >130.6 | 80 | 160 |
| Former Soviet Union | 18.1 | * NA | NA |
| World Total | >441.8 to >631.4 | NA | 727 |
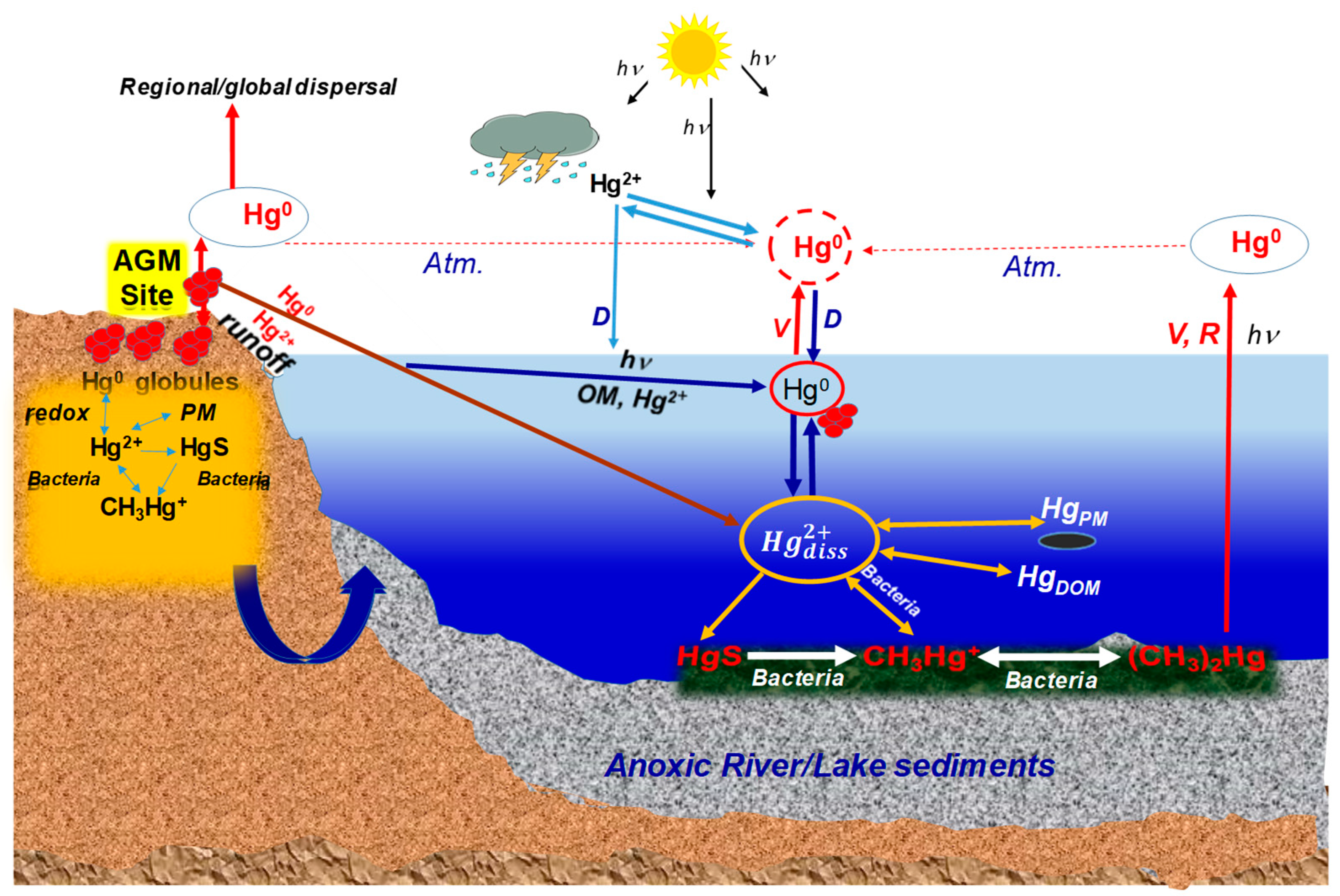
3.3. Fate of Mercury Introduced into the Environment
3.4. Gold Mining and Mercury Pollution of Terrestrial Landscapes
3.5. Mercury and Human Health Impacts
3.6. Ongoing Efforts to Curve Hg Contamination of Natural Systems by AGM
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Itri, F.M. Environmental Mercury Problem; Technical report; Chemical Rubber Company Press: Michigan State University, Lansing, MI, USA, 1972; Volume 12, 124p. [Google Scholar]
- Kazantzis, G. Mercury exposure and early effects: An overview. Med. Lav. 2002, 93, 139–147. [Google Scholar] [PubMed]
- Taux, K.; Kraus, T.; Kaifie, A. Mercury exposure and its health effects in workers in the Artisanal and Small-Scale Gold Mining (ASGM) sector—A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 2081. [Google Scholar] [CrossRef] [PubMed]
- Kobal, A.B.; Grum, D.K. Scopoli’s work in the field of mercurialism in light of today’s knowledge: Past and present perspectives. Am. J. Ind. Med. 2010, 53, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury—Current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Prescott, G.W.; Baird, M.; Geenen, S.; Nkuba, B.; Phelps, J.; Webb, E.L. Formalizing artisanal and small-scale gold mining: A grand challenge of the Minamata Convention. One Earth 2022, 5, 242–251. [Google Scholar] [CrossRef]
- Afrifa, J.; Opoku, Y.K.; Gyamerah, E.O.; Ashiagbor, G.; Sorkpor, R.D. The clinical importance of the mercury problem in artisanal small-scale gold mining. Front. Public Health 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- D’Itri, P.A.; D’Itri, F.M. Mercury Contamination: A Human Tragedy; John Wiley and Sons, Inc.: New York, NY, USA, 1977. [Google Scholar]
- Klein, D. Mercury in marine environment. In Applied Spectroscopy; Society for Applied Spectroscopy: Frederick, MD, USA, 1969; Volume 23, 647p. [Google Scholar]
- Wise, E. Gold and Gold Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology 10; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Bakir, F.; Damluji, S.F.; Amin-Zaki, L.; Murtadha, M.; Khalidi, A.; al-Rawi, N.Y.; Tikriti, S.; Dahahir, H.I.; Clarkson, T.W.; Smith, J.C.; et al. Methylmercury poisoning in Iraq. Science 1973, 181, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Geomicrobiology, 4th ed.; Marcel Dekker Inc.: New York, USA, 2002. [Google Scholar]
- Derban, L.K. Outbreak of food poisoning due to alkyl-mercury fungicide. Arch. Environ. Health Int. J. 1974, 28, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Zolnikov, T.R.; Ortiz, D.R. A systematic review on the management and treatment of mercury in artisanal gold mining. Sci. Total Environ. 2018, 633, 816–824. [Google Scholar] [CrossRef]
- Nandiyanto, A.; Ragadhita, R.; Al Husaeni, D.; Nugraha, W. Research trend on the use of mercury in gold mining: Literature review and bibliometric analysis. Moroc. J. Chem. 2023, 11, 11. [Google Scholar]
- Tsang, V.W.; Lockhart, K.; Spiegel, S.J.; Yassi, A. Occupational health programs for artisanal and small-scale gold mining: A systematic review for the WHO global plan of action for workers’ health. Ann. Glob. Health 2019, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Diringer, S.E.; Feingold, B.J.; Ortiz, E.J.; Gallis, J.A.; Araujo-Flores, J.M.; Berky, A.; Pan, W.K.; Hsu-Kim, H. River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru. Environ. Sci. Process Impacts 2015, 17, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Wade, L. Mercury pollution. Gold’s dark side. Science 2013, 341, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, N.; Keeler, G.J.; Nriagu, J.O. Regional differences in worldwide emissions of mercury to the atmosphere. Atmos. Environ. 1996, 30, 2981–2987. [Google Scholar] [CrossRef]
- Lacerda, L.D. Global mercury emissions from gold and silver mining. Water Air Soil. Pollut. 1997, 97, 209–221. [Google Scholar] [CrossRef]
- Villas Bôas, R.C.; Beinhoff, C.; Silva, A.R.B.d. Mercury in the Tapajós Basin; CETEM/CYTED/IMAAC/UNIDO. 2001; 198p. Available online: http://mineralis.cetem.gov.br/handle/cetem/688 (accessed on 7 April 2024).
- Cohn, J.; Stern, E.W. Gold and Gold Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Drace, K.; Kiefer, A.M.; Veiga, M.M.; Williams, M.K.; Ascari, B.; Knapper, K.A.; Logan, K.M.; Breslin, V.M.; Skidmore, A.; Bolt, D.A.; et al. Mercury-free, small-scale artisanal gold mining in Mozambique: Utilization of magnets to isolate gold at clean tech mine. J. Clean. Prod. 2012, 32, 88–95. [Google Scholar] [CrossRef]
- Saim, A.K. Mercury (Hg) use and pollution assessment of ASGM in Ghana: Challenges and strategies towards Hg reduction. Environ. Sci. Pollut. Res. 2021, 28, 61919–61928. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, W.C.; de Lacerda, L.D.; Malm, O.; Souza, C.M.; da Silveira, E.G.; Bastos, W.R. Mercury concentrations in inland waters of gold-mining areas in Rondonia, Brazil. Sci. Total Environ. 1989, 87–88, 233–240. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The mercury problem in artisanal and small-scale gold mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef]
- Korte, F.; Coulston, F. Some considerations on the impact on ecological chemical principles in practice with emphasis on gold mining and cyanide. Ecotoxicol. Environ. Saf. 1998, 41, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Shamley, D.J.; Sack, J.S. Mercury poisoning. A case report and comment on 6 other cases. S. Afr. Med. J. 1989, 76, 114–116. [Google Scholar] [PubMed]
- Spiegel, S.J.; Veiga, M.M. Building capacity in small-scale mining communities: Health, ecosystem sustainability, and the Global Mercury Project. EcoHealth 2005, 2, 361–369. [Google Scholar] [CrossRef]
- Hangi, A. Environmental Impacts of Small Scale Mining: A Case Study of Merelani, Kahama, Nzega, Geita, and Musoma. (CEEST) Centre for Energy, Environment, Science, and Technology (CEEST), Research Report series No 1. 1996. Available online: https://searchworks.stanford.edu/view/4436190 (accessed on 7 April 2024).
- International Labour Organization. Social and Labour Issues in Small-Scale Mines. Report for Discussion at the Tripartite Meeting on Social and Labour Issues in Small-Scale Mines; ILO: Geneva, Switzerland, 1999; 58p. [Google Scholar]
- De Lacerda, L.D. Mercury from Gold and Silver Mining: A Chemical Time Bomb? with 29 Tables; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- United Nations. Recent Developments in Small-Scale Mining: A Report of the Secretary-General of the United Nations. Nat. Resour. Forum 1996, 20, 215–225. [Google Scholar] [CrossRef]
- Veiga, M.M. Mercury in artisanal gold mining in Latin America: Facts, fantasies and solutions. In Proceedings of the UNIDO-Expert Group Meeting—Introducing New Technologies for Abatement of Global Mercury Pollution Deriving from Artisanal Gold Mining, Vienna, Austria, 1–3 July 1997. [Google Scholar]
- Compeau, G.; Bartha, R. Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol. 1985, 50, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Reuther, R. Mercury accumulation in sediment and fish from rivers affected by alluvial gold mining in the brazilian Madeira river basin, Amazon. Environ. Monit. Assess. 1994, 32, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O.; Wong, H.K.T. Gold rushes and mercury pollution. Met. Ions Biol. Syst. 1997, 34, 131–160. [Google Scholar]
- Seccatore, J.; Veiga, M.; Origliasso, C.; Marin, T.; De Tomi, G. An estimation of the artisanal small-scale production of gold in the world. Sci. Total Environ. 2014, 496, 662–667. [Google Scholar] [CrossRef]
- UNEP. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; United Nations Environment Programme Chemicals Branch, Division of Technology, Industry and Economics (DTIE): Geneva, Switzerland, 2013. [Google Scholar]
- Velásquez-López, P.C.; Veiga, M.M.; Hall, K. Mercury balance in amalgamation in artisanal and small-scale gold mining: Identifying strategies for reducing environmental pollution in Portovelo-Zaruma, Ecuador. J. Clean. Prod. 2010, 18, 226–232. [Google Scholar] [CrossRef]
- Porcella, D.; Ramel, C.; Jernelov, A. Global mercury pollution and the role of gold mining: An overview. Water Air Soil. Pollut. 1997, 97, 205–207. [Google Scholar] [CrossRef]
- Nriagu, J.O. Mercury pollution from the past mining of gold and silver in the Americas. Sci. Total Environ. 1994, 149, 167–181. [Google Scholar] [CrossRef]
- Lacerda, L.; Marins, R.; Barcellos, C.; Molisani, M. Sepetiba Bay: A case study of the environmental geochemistry of heavy metals in a subtropical coastal lagoon. In Environmental Geochemistry in Tropical and Subtropical Environments; Springer: Berlin/Heidelberg, Germany, 2004; pp. 293–318. [Google Scholar]
- Malm, O.; Pfeiffer, W.C.; Souza, C.M.; Reuther, R. Mercury pollution due to gold mining in the Madeira River basin, Brazil. ambio 1990, 19, 11–15. [Google Scholar]
- Martinelli, L.A.; Ferreira, J.R.; Forsberg, B.R.; Victoria, R.L. Mercury contamination in the Amazon: A gold rush consequence. Ambio 1988, 252–254. [Google Scholar]
- Pfeiffer, W.C.; Malm, O.; Souza, C.; de Lacerda, L.D.; Silveira, E.; Bastos, W. Mercury in the Madeira river ecosystem, Rondonia, Brazil. For. Ecol. Manag. 1991, 38, 239–245. [Google Scholar] [CrossRef]
- Babut, M.; Sekyi, R.; Rambaud, A.; Potin-Gautier, A.; Tellier, S.; Bannerman, W.; Beinhoff, C. Improving the environmental management of small-scale gold mining in Ghana: A case study of Dumasi. J. Clean. Prod. 2003, 11, 215–221. [Google Scholar] [CrossRef]
- Bonzongo, J.-C.; Donkor, A.; Nartey, V.; Lacerda, L. Mercury pollution in Ghana: A case study of environmental impacts of artisanal gold mining in Sub-Saharan Africa. In Environmental Geochemistry in Tropical and Subtropical Environments; Springer: Berlin/Heidelberg, Germany, 2004; pp. 135–156. [Google Scholar]
- Campbell, L.; Dixon, D.; Hecky, R. A review of mercury in Lake Victoria, East Africa: Implications for human and ecosystem health. J. Toxicol. Environ. Health Part B 2003, 6, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Feng, X.; Qiu, G.; Jiang, H. Mercury contaminations from gold mining using amalgamation technique in Xiaoqinling Region, Shanxi Province, PR China. J. Phys. IV Proc. 2003, 107, 345–348. [Google Scholar] [CrossRef]
- Ikingura, J.R.; Mutakyahwa, M.; Kahatano, J. Mercury and mining in Africa with special reference to Tanzania. Water Air Soil. Pollut. 1997, 97, 223–232. [Google Scholar] [CrossRef]
- Kambey, J.L.; Farrell, A.; Bendell-Young, L. Influence of illegal gold mining on mercury levels in fish of North Sulawesi’s Minahasa Peninsula, (Indonesia). Environ. Pollut. 2001, 114, 299–302. [Google Scholar] [CrossRef]
- Limbong, D.; Kumampung, J.; Rimper, J.; Arai, T.; Miyazaki, N. Emissions and environmental implications of mercury from artisanal gold mining in north Sulawesi, Indonesia. Sci. Total Environ. 2003, 302, 227–236. [Google Scholar] [CrossRef]
- Merchant, B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals 1998, 26, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Müezzinoğlu, A. A review of environmental considerations on gold mining and production. Crit. Rev. Environ. Sci. Tech. 2003, 33, 45–71. [Google Scholar] [CrossRef]
- Craig, J.R.; Rimstidt, J.D. Gold production history of the United States. Ore Geol. Rev. 1998, 13, 407–464. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Sci. Total Environ. 2003, 304, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Hylander, L.D.; Meili, M. The rise and fall of mercury: Converting a resource to refuse after 500 years of mining and pollution. Crit. Rev. Environ. Sci. Technol. 2005, 35, 1–36. [Google Scholar] [CrossRef]
- Kirk, R.E.; Othmer, D.F. Encyclopedia of Chemical Technology; John Wiley and Sons Inc: New York, USA, 1981; Volume 53, p. 143. [Google Scholar]
- Chang, L.W. Mercury-related neurological syndromes and disorders. Miner. Met. Neurotoxicology 1997, 17, 169–176. [Google Scholar]
- Clarkson, T.W. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997, 34, 369–403. [Google Scholar] [CrossRef]
- Churchill, C. The Age of Gold: The California Gold Rush and the New American Dream; J West Inc.: Manhattan, KS, USA, 2004; p. 82. [Google Scholar]
- Pomeroy, E.; Starr, K. Americans and the California Dream, 1850–1915; Oxford University Press: New York, NY, USA, 1973; pp. 18–494. [Google Scholar] [CrossRef]
- Starr, K. Golden Dreams: California in an Age of Abundance, 1950–1963; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Brands, H. The Age of Gold: The California Gold Rush and the New American Dream; Doubleday Publishers-Random House, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Da Rosa, C.D.; Lyon, J.S.; Hocker, P.M. Golden Dreams, Poisoned Streams: How Reckless Mining Pollutes America’s Waters, and How We Can Stop It; Mineral Policy Center: Washington, DC, USA, 1997. [Google Scholar]
- Marsden, J.; House, I. The Chemistry of Gold Extraction; E. Horwood: New York, NY, USA, 1992. [Google Scholar]
- Wong, H.K.T.; Gauthier, A.; Nriagu, J.O. Dispersion and toxicity of metals from abandoned gold mine tailings at Goldenville, Nova Scotia, Canada. Sci. Total Environ. 1999, 228, 35–47. [Google Scholar] [CrossRef]
- Andrist, R.K. The Gold Rush, 1st ed.; New World City, Inc.: Boston, MA, USA, 2015; 145p. [Google Scholar]
- Koschmann, A.; Bergendahl, M. Principal Gold-Producing Districts. United States US Geol. Surv. Prof. Pap. 1968, 610, 283. [Google Scholar]
- Lewis, R. The North Carolina Gold Rush. North Carolina Museum of History. 2006. Available online: https://www.ncpedia.org/industry/gold-rush (accessed on 25 May 2024).
- Gibb, H.; O’Leary, K.G. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: A comprehensive review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef]
- Nriagu, J.O. The Biogeochemistry of Mercury in the Environment; Elsevier, North-Holland Biomedical Press: Amsterdam, The Netherlands, 1979; p. 696. [Google Scholar]
- Lutz Ehrlich, H. Methods in Geomicrobiology. In Geomicrobiology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 153–181. [Google Scholar] [CrossRef]
- Hentschel, T.; Priester, M. Mercury contamination in developing countries through gold amalgamation in small-scale mining: Some processing alternatives. Nat. Resour. Dev. 1992, 35, 67–77. [Google Scholar]
- Nriagu, J.O. Legacy of Mercury Pollution. Nature 1993, 363, 589. [Google Scholar] [CrossRef]
- Marsden, J.; House, I. The Chemistry of Gold Extraction; SME: New York, NY, USA, 2006. [Google Scholar]
- Manten, A. Geo-scientific aspects of the discovery exploration and development of New Zealand. Atlas-News Suppl. Earth-Sci. Rev. 1968, 4, A229–A252. [Google Scholar] [CrossRef]
- Pfeiffer, W.C.; Delacerda, L.D. Mercury Inputs into the Amazon Region, Brazil. Environ. Technol. Lett. 1988, 9, 325–330. [Google Scholar] [CrossRef]
- Bycroft, B.; Coller, B.; Deacon, G.; Coleman, D.; Lake, P. Mercury contamination of the Lerderberg River, Victoria, Australia, from an abandoned gold field. Environ. Pollut. Ser. Ecol. Biol. 1982, 28, 135–147. [Google Scholar] [CrossRef]
- Kehrig, H.A.; Malm, O.; Akagi, H. Methylmercury in hair samples from different riverine groups, Amazon, Brazil. Water Air Soil. Pollut. 1997, 97, 17–29. [Google Scholar] [CrossRef]
- Pirrone, N.; Ivo, A.; Keeler, G.J.; Nriagu, J.O.; Rossmann, R.; Robbins, J.A. Historical atmospheric mercury emissions and depositions in North America compared to mercury accumulations in sedimentary records. Atmos. Environ. 1998, 32, 929–940. [Google Scholar] [CrossRef]
- Omotehinse, A.O.; Samson, O. A systematic review of artisanal and small-scale mining: Impacts in alleviating poverty in Africa. SN Soc. Sci. 2022, 2, 197. [Google Scholar] [CrossRef]
- Laperdina, T.; Melnikova, M.; Khvostova, T. Mercury contamination of the environment due to gold mining in Zabaikalye. In Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances; Springer: Berlin/Heidelberg, Germany, 1996; pp. 415–427. [Google Scholar]
- Laperdina, T.; Melnikova, M.; Khvostova, T. Gold mining in Siberia as a source of mercury contamination of the environment. In Mercury Contaminated Sites: Characterization, Risk Assessment and Remediation; Ebinghaus, R., Turner, R., Larceda, L., Vasiliev, O., Salomons, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 357–374. [Google Scholar]
- Laperdina, T.; Tupyakov, A.; Yegorov, A.; Melnikova, M.; Askarova, O.; Banshchikov, V.; Khvostova, T.; Tsybikdorzhiev, Z.; Bochko, O. Environment pollution by mercury in the zone of influence of transbaikalian gold mining plants. Chem. Sustain. Dev. 1995, 3, 53–62. [Google Scholar]
- Laperdina, T.G. Estimation of mercury and other heavy metal contamination in traditional gold-mining areas of Transbaikalia. Geochem. Explor. Environ. Anal. 2002, 2, 219–223. [Google Scholar] [CrossRef]
- Vasiliev, O.F.; Obolenskiy, A.A.; Yagolnitser, M.A. Mercury as a pollutant in Siberia: Sources, fluxes and a regional budget. Sci. Total Environ. 1998, 213, 73–84. [Google Scholar] [CrossRef]
- Yagolnitser, M.; Sokolov, V.; Rabtsev, A.; Obolenskii, A.; Ozerova, N.; Dvurechenskaya, S.Y.; Sukhenko, S. Industrial mercury sources in Siberia. In Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1996; Volume 21, pp. 429–440. [Google Scholar] [CrossRef]
- Churchill, R.C.; Meathrel, C.E.; Suter, P.J. A retrospective assessment of gold mining in the Reedy Creek sub-catchment, northeast Victoria, Australia: Residual mercury contamination 100 years later. Environ. Pollut. 2004, 132, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Lawrence, S.; Turnbull, J. Mercury use and loss from gold mining in nineteenth-century Victoria. Proc. R. Soc. Vic. 2015, 127, 44–54. [Google Scholar] [CrossRef]
- Rendall, R.E.G.; Vansittert, G.C.H. Improvement in Conditions at an Amalgamation Plant after an Industrial-Hygiene Survey. S. Afr. Med. J. 1984, 66, 413–414. [Google Scholar] [PubMed]
- Greer, J. The price of gold. Environmental costs of the new gold rush. Ecologist 1993, 23, 91–96. [Google Scholar]
- Lin, Y.H.; Guo, M.X.; Gan, W.M. Mercury pollution from small gold mines in China. Water Air Soil. Pollut. 1997, 97, 233–239. [Google Scholar] [CrossRef]
- Maponga, O.; Ngorima, C.F. Overcoming environmental problems in the gold panning sector through legislation and education: The Zimbabwean experience. J. Clean. Prod. 2003, 11, 147–157. [Google Scholar] [CrossRef]
- Leigh, D.S. Mercury-tainted overbank sediment from past gold mining in north Georgia, USA. Environ. Geol. 1997, 30, 244–251. [Google Scholar] [CrossRef]
- James, L.P. The Mercury Tromol Mill—An Innovative Gold Recovery Technique, and a Possible Environmental Concern. J. Geochem. Explor. 1994, 50, 493–500. [Google Scholar] [CrossRef]
- Donoghue, A.M. Mercury toxicity due to the smelting of placer gold recovered by mercury amalgam. Occup. Med.-Oxf. 1998, 48, 413–415. [Google Scholar] [CrossRef]
- Pfeiffer, W.; Lacerda, L.; Salomons, W.; Malm, O. Environmental fate of mercury from gold mining in the Brazilian Amazon. Environ. Rev. 1993, 1, 26–37. [Google Scholar] [CrossRef]
- Miller, J.R.; Lechler, P.J. Mercury partitioning within alluvial sediments of the Carson River valley, Nevada: Implications for sampling strategies in tropical environments. In Environmental Geochemistry in the Tropics; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 1998; Volume 72, pp. 211–233. [Google Scholar] [CrossRef]
- De Lacerda, L.; Salomons, W.; Pfeiffer, W.; Bastos, W. Mercury distribution in sediment profiles from lakes of the high Pantanal, Mato Grosso State, Brazil. Biogeochemistry 1991, 14, 91–97. [Google Scholar] [CrossRef]
- Salomons, W. Environmental-Impact of Metals Derived from Mining Activities—Processes, Predictions, Prevention. J. Geochem. Explor. 1995, 52, 5–23. [Google Scholar] [CrossRef]
- Mallas, J.; Benedicto, N. Mercury and Goldmining in the Brazilian Amazon. Ambio 1986, 15, 248–249. [Google Scholar]
- Ikingura, J.R.; Akagi, H. Monitoring of fish and human exposure to mercury due to gold mining in the Lake Victoria goldfields, Tanzania. Sci. Total Environ. 1996, 191, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.H. The History of the Comstock Lode, 1850–1997; University of Nevada Press: Reno, NV, USA, 1998; Volume 24. [Google Scholar]
- Henderson, E.H. Stamp-milling and amalgamation practices at Goldenville, Nova Scotia. Can. Inst. Min. Metall. Bull. 1935, 278, 222–240. [Google Scholar]
- Amegbey, N.A.; Dankwa, J.; Al-Hassan, S. Small scale mining in Ghana-Techniques and environmental considerations. Int. J. O/Surf. Min. Reclam. Environ. 1997, 11, 135–138. [Google Scholar] [CrossRef]
- Hypolito, R.; Sumi, E.M. Recovery of mercury of amalgams in digging gold–Retorta RHYP. Geociências 2019, 38, 567–573. [Google Scholar] [CrossRef]
- Odumo, B.O.; Carbonell, G.; Angeyo, H.K.; Patel, J.P.; Torrijos, M. and Rodríguez Martín, J.A. Impact of gold mining associated with mercury contamination in soil, biota sediments and tailings in Kenya. Environ. Sci. Pollut. Res. 2014, 21, 12426–12435. [Google Scholar] [CrossRef]
- Miller, J.R.; Lechler, P.J. Importance of temporal and spatial scale in the analysis of mercury transport and fate: An example from the Carson River system, Nevada. Environ. Geol. 2003, 43, 315–325. [Google Scholar] [CrossRef]
- De Lacerda, L. Updating global Hg emissions from small-scale gold mining and assessing its environmental impacts. Environ. Geol. 2003, 43, 308–314. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Strode, S.; Jaegle, L.; Selin, N.E. Impact of mercury emissions from historic gold and silver mining: Global modeling. Atmos. Environ. 2009, 43, 2012–2017. [Google Scholar] [CrossRef]
- Malm, O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ. Res. 1998, 77, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Suemasu, K.; Veiga, M.M. Estimation of mercury losses and gold production by artisanal and small-scale gold mining (ASGM). J. Sustain. Metall. 2021, 7, 1045–1059. [Google Scholar] [CrossRef]
- Camargo, J.A. Contribution of Spanish-American silver mines (1570-1820) to the present high mercury concentrations in the global environment: A review. Chemosphere 2002, 48, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.M.; Baker, R.F.; Fried, M.B.; Withers, D. Protocols for Environmental and Health Assessment of Mercury Released by Artisanal and Small-Scale Gold Miners; Global Mercury Project, UNIDO(02)/G563: Vienna, Austria, 2004. [Google Scholar]
- Mastrine, J.A.; Bonzongo, J.-C.J.; Lyons, W.B. Mercury concentrations in surface waters from fluvial systems draining historical precious metals mining areas ink southeastern USA. Appl. Geochem. 1999, 14, 147–158. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jaegle, L.; Thompson, L.; Streets, D.G. Six centuries of changing oceanic mercury. Glob. Biogeochem. Cycles 2014, 28, 1251–1261. [Google Scholar] [CrossRef]
- Engstrom, D.R.; Fitzgerald, W.F.; Cooke, C.A.; Lamborg, C.H.; Drevnick, P.E.; Swain, E.B.; Balogh, S.J.; Balcom, P.H. Atmospheric Hg emissions from preindustrial gold and silver extraction in the Americas: A reevaluation from lake-sediment archives. Environ. Sci. Technol. 2014, 48, 6533–6543. [Google Scholar] [CrossRef]
- Streets, D.G.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. [Google Scholar] [CrossRef]
- Akagi, H.; Castillo, E.S.; Cortes-Maramba, N.; Francisco-Rivera, A.T.; Timbang, T.D. Health assessment for mercury exposure among schoolchildren residing near a gold processing and refining plant in Apokon, Tagum, Davao del Norte, Philippines. Sci. Total Environ. 2000, 259, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, J.; Gonzales, G.F.; Steenland, K. Mercury exposures in informal gold miners and relatives in southern Peru. Int. J. Occup. Environ. Health 2013, 12, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Leigh, D.S. Mercury Contamination and Floodplain Sedimentation from Former Gold Mines in North Georgia. J. Am. Water Resour. Assoc. 1994, 30, 739–748. [Google Scholar] [CrossRef]
- Fuge, R.; Pearce, N.J.G.; Perkins, W.T. Mercury and Gold Pollution. Nature 1992, 357, 369. [Google Scholar] [CrossRef]
- Craw, D.; Chappell, D.A. Metal redistribution in historic mine wastes, Coromandel Peninsula, New Zealand. N. Z. J. Geol. Geophys. 2000, 43, 187–198. [Google Scholar] [CrossRef]
- Stephens, C.; Ahern, M. Worker and Community Health Impacts Related to Mining Operations Internationally: A Rapid Review of the Literature; International Institute for Environment and Development (IIED), World Business Council for Sustainable Development, London School of Hygiene & Tropical Medicine: London, UK, 2001. [Google Scholar]
- Newcombe, V.C. Mercury Use in the Goldmining Industry: A Retrospective Examination of Elemental Mercury Use in the Gold Mining Industry of the West Coast of New Zealand in the Period 1984–1988. Master’s Thesis, Massey University, Wellington, New Zealand, 2008. [Google Scholar]
- van Straaten, P. Human exposure to mercury due to small scale gold mining in northern Tanzania. Sci. Total Environ. 2000, 259, 45–53. [Google Scholar] [CrossRef] [PubMed]
- van Straaten, P. Mercury contamination associated with small-scale gold mining in Tanzania and Zimbabwe. Sci. Total Environ. 2000, 259, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.D.; Williams, T.M.; Breward, N.; Apostol, A.; Miguel, J.; Miranda, C. Mercury contamination associated with artisanal gold mining on the island of Mindanao, the Philippines. Sci. Total Environ. 1999, 228, 95–109. [Google Scholar] [CrossRef]
- Cramer, S. Problems facing the Philippines. Int. Mining. 1990, 29–30. [Google Scholar]
- Dórea, J.G.; Marques, R.C. Mercury levels and human health in the Amazon Basin. Ann. Hum. Biol. 2016, 43, 349–359. [Google Scholar] [CrossRef]
- Koekkoek, B. Measuring Global Progress towards a Transition away from Mercury Use in Artisanal and Small-Scale Gold Mining; Royal Roads University: Victoria, BC, Canada, 2013; 74p. [Google Scholar]
- McCredie, A.; Ealey, E.H.M. Mercury and Mining Pollution in the Upper Goulburn River; Report No 9; Graduate School of Environmental Science, Monash University: Clayton, VIC, Australia, 1982. [Google Scholar]
- Tiller, D. Mercury in the Freshwater Environment: The Contamination of Waterbodies in Victoria as a Result of Past Gold Mining Activities; Environment Protection Authority SRS 90/005, Ministry for Planning and Environment: Melbourne, Australia, 1990. [Google Scholar]
- Fabris, G. Lake Wellington Mercury Pilot Study; Fisheries Victoria Research Report Series, 51; Department of Primary Industries: Victoria, Australia, 2012; 13p. [Google Scholar]
- Alpers, C.N.; Hunerlach, M.P.; May, J.T.; Hothem, R.L. Mercury Contamination from Historical Gold Mining in California; US Department of the Interior, US Geological Survey: Washington, DC, USA, 2000. [Google Scholar]
- Isenberg, A.C. Mining California: An Ecological History; Macmillan: New York, NY, USA, 2005. [Google Scholar]
- Singer, M.B.; Aalto, R.; James, L.A.; Kilham, N.E.; Higson, J.L.; Ghoshal, S. Enduring legacy of a toxic fan via episodic redistribution of California gold mining debris. Proc. Natl. Acad. Sci. USA 2013, 110, 18436–18441. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.; Smith, M. The rush started here II: Hard rock gold mining in North Carolina, 1825 to 1864. Earth Sci. Hist. 2006, 25, 69–106. [Google Scholar] [CrossRef]
- Lecce, S.; Pavlowsky, R.; Schlomer, G. Mercury contamination of active channel sediment and floodplain deposits from historic gold mining at Gold Hill, North Carolina, USA. Environ. Geol. 2008, 55, 113–121. [Google Scholar] [CrossRef]
- Wayne, D.M.; Warwick, J.J.; Lechler, P.J.; Gill, G.A.; Lyons, W.B. Mercury contamination in the Carson River, Nevada: A preliminary study of the impact of mining wastes. Water Air Soil. Pollut. 1996, 92, 391–408. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.; Stewart, R.B.; Robinson, B.H. Phytoremediation of mercury-contaminated mine tailings by induced plant-mercury accumulation. Environ. Pract. 2004, 6, 165–175. [Google Scholar] [CrossRef]
- Nichols, F.H.; Cloern, J.E.; Luoma, S.N.; Peterson, D.H. The modification of an estuary. Science 1986, 231, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, H.M.; Jacob, D.J.; Amos, H.M.; Streets, D.G.; Sunderland, E.M. Historical Mercury releases from commercial products: Global environmental implications. Environ. Sci. Technol. 2014, 48, 10242–10250. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O. Global metal pollution: Poisoning the biosphere? Environ. Sci. Policy Sustain. Dev. 1990, 32, 7–33. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Tesha, A.; Drasch, K.; Roider, G.; Taylor, H.; Appleton, D.; Siebert, U. Health assessment of artisanal gold miners in Tanzania. Sci. Total Environ. 2010, 408, 796–805. [Google Scholar] [CrossRef]
- Iverfeldt, A.; Lindqvist, O. Atmospheric Oxidation of Elemental Mercury by Ozone in the Aqueous Phase. Atmos. Environ. 1986, 20, 1567–1573. [Google Scholar] [CrossRef]
- Lindqvist, O.; Rodhe, H. Atmospheric Mercury—A Review. Tellus Ser. B-Chem. Phys. Meteorol. 1985, 37, 136–159. [Google Scholar] [CrossRef]
- De Lacerda, L.D. Amazon mercury emissions. Nature 1995, 374, 20–21. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Marins, R.V. Anthropogenic mercury emissions to the atmosphere in Brazil: The impact of gold mining. J. Geochem. Explor. 1997, 58, 223–229. [Google Scholar] [CrossRef]
- Marins, R.V.; de Andrade, J.B.; Pereira, P.A.; Paiva, E.C.; Paraquetti, H.H. Sampling techniques for the assessment of anthropogenic vapour and particulate mercury in the Brazilian Amazon atmosphere. J. Environ. Monit. 2000, 2, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.W. Sources of methyl mercury to freshwater ecosystems: A review. Water Air Soil. Pollut. 1995, 80, 697–713. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Southworth, G.; Prestbo, E.M.; Wallschlager, D.; Bogle, M.A.; Price, J. Gaseous methyl- and inorganic mercury in landfill gas from landfills in Florida, Minnesota, Delaware, and California. Atmos. Environ. 2005, 39, 249–258. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Wallschlager, D.; Prestbo, E.M.; Bloom, N.S.; Price, J.; Reinhart, D. Methylated mercury species in municipal waste landfill gas sampled in Florida, USA. Atmos. Environ. 2001, 35, 4011–4015. [Google Scholar] [CrossRef]
- Bonzongo, J.; Lyons, W.; Hines, M.; Warwick, J.; Faganeli, J.; Horvat, M.; Lechler, P.; Miller, J. Mercury in surface waters of three mine-dominated river systems: Idrija River, Slovenia; Carson River, Nevada; and Madeira River, Brazilian Amazon. Geochem. Explor. Environ. Anal. 2002, 2, 111–119. [Google Scholar] [CrossRef]
- Lalonde, J.D.; Amyot, M.; Kraepiel, A.M.; Morel, F.M. Photooxidation of Hg(0) in artificial and natural waters. Environ. Sci. Technol. 2001, 35, 1367–1372. [Google Scholar] [CrossRef]
- Kudo, A. Natural and Artificial Mercury Decontamination—Ottawa River and Minamata Bay (Yatsushiro Sea). Water Sci. Technol. 1992, 26, 217–226. [Google Scholar] [CrossRef]
- Yamamoto, M. Possible Mechanism of Elemental Mercury Oxidation in the Presence of Sh Compounds in Aqueous-Solution. Chemosphere 1995, 31, 2791–2798. [Google Scholar] [CrossRef]
- Yamamoto, M. Stimulation of elemental mercury oxidation in the presence of chloride ion in aquatic environments. Chemosphere 1996, 32, 1217–1224. [Google Scholar] [CrossRef]
- de Magalhaes, M.E.; Tubino, M. A possible path for mercury in biological systems: The oxidation of metallic mercury by molecular oxygen in aqueous solutions. Sci. Total Environ. 1995, 170, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Amyot, M.; Morel, F.M.; Ariya, P.A. Dark oxidation of dissolved and liquid elemental mercury in aquatic environments. Environ. Sci. Technol. 2005, 39, 110–114. [Google Scholar] [CrossRef] [PubMed]
- O’Concubhair, R.; O’Sullivan, D.; Sodeau, J.R. Dark oxidation of dissolved gaseous mercury in polar ice mimics. Environ. Sci. Technol. 2012, 46, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, H.; Mann, B.F.; Liang, L.; Gu, B. Oxidation of dissolved elemental mercury by thiol compounds under anoxic conditions. Environ. Sci. Technol. 2013, 47, 12827–12834. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lin, H.; Zheng, W.; Tomanicek, S.J.; Johs, A.; Feng, X.; Elias, D.A.; Liang, L.; Gu, B. Oxidation and methylation of dissolved elemental mercury by anaerobic bacteria. Nat. Geosci. 2013, 6, 751–754. [Google Scholar] [CrossRef]
- Colombo, M.J.; Ha, J.; Reinfelder, J.R.; Barkay, T.; Yee, N. Oxidation of Hg (0) to Hg (II) by diverse anaerobic bacteria. Chem. Geol. 2014, 363, 334–340. [Google Scholar] [CrossRef]
- Bidone, E.; Castilhos, Z.; Cid de Souza, T.; Lacerda, L. Fish contamination and human exposure to mercury in the Tapajós River Basin, Pará State, Amazon, Brazil: A screening approach. Bull. Environ. Contam. Toxicol. 1997, 59, 194–201. [Google Scholar] [CrossRef]
- Grieb, T.M.; Driscoll, C.T.; Gloss, S.P.; Schofield, C.L.; Bowie, G.L.; Porcella, D.B. Factors Affecting Mercury Accumulation in Fish in the Upper Michigan Peninsula. Environ. Toxicol. Chem. 1990, 9, 919–930. [Google Scholar] [CrossRef]
- Kim, J.P. Methylmercury in Rainbow-Trout (Oncorhynchus-Mykiss) from Lakes Okareka, Okaro, Rotomahana, Rotorua and Tarawera, North-Island, New-Zealand. Sci. Total Environ. 1995, 164, 209–219. [Google Scholar] [CrossRef]
- Gilmour, C.C.; Henry, E.A. Mercury methylation in aquatic systems affected by acid deposition. Environ. Pollut. 1991, 71, 131–169. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.J.; Veiga, M.M.; Veiga, A.T.C. Clean artisanal gold mining: A utopian approach? J. Clean. Prod. 2003, 11, 99–115. [Google Scholar] [CrossRef]
- Valenzuela, A.; Fytas, K. Mercury Management in Small-Scale Mining. Int. J. Surf. Min. Reclam. Environ. 2002, 16, 2–23. [Google Scholar] [CrossRef]
- Yasui, M.; Verity, M.A. Mineral and Metal Neurotoxicology; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Yokoo, E.M.; Valente, J.G.; Grattan, L.; Schmidt, S.L.; Platt, I.; Silbergeld, E.K. Low level methylmercury exposure affects neuropsychological function in adults. Environ. Health 2003, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nakachi, S.; Cheu, T.; Hamada, H.; Ono, Y.; Tsuda, T.; Yanagida, K.; Kizaki, T.; Ohno, H. Monitoring of mercury pollution in Tanzania: Relation between head hair mercury and health. Sci. Total Environ. 1999, 227, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Eisler, R. Health risks of gold miners: A synoptic review. Environ. Geochem. Health 2003, 25, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Fields, S. Tarnishing the earth: Gold mining’s dirty secret. Environ. Health Perspect. 2001, 109, A474–A481. [Google Scholar] [CrossRef]
- Kippen, S. The social and political meaning of the silent epidemic of miners’ phthisis, Bendigo 1860–1960. Soc. Sci. Med. 1995, 41, 491–499. [Google Scholar] [CrossRef]
- Cleary, D.; Thornton, I. The Environmental Impact of Gold Mining in the Brazilian Amazon; Royal Society of Chemistry: London, UK, 1994. [Google Scholar] [CrossRef]
- Castilhos, Z.; Rodrigues-Filho, S.; Cesar, R.; Rodrigues, A.P.; Villas-Bôas, R.; de Jesus, I.; Lima, M.; Faial, K.; Miranda, A.; Brabo, E. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. 2015, 22, 11255–11264. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Maydl, S.; Vosko, M.R.; Roider, G.; Dzaja, D. The Mt. Diwata study on the Philippines 2000-treatment of mercury intoxicated inhabitants of a gold mining area with DMPS (2,3-dimercapto-1-propane-sulfonic acid, Dimaval). Sci. Total Environ. 2003, 307, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Bose-O’Reilly, S.; Lettmeier, B.; Gothe, R.M.; Beinhoff, C.; Siebert, U.; Drasch, G. Mercury as a serious health hazard for children in gold mining areas. Environ. Res. 2008, 107, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Drasch, G.; Bose-O’Reilly, S.; Beinhoff, C.; Roider, G.; Maydl, S. The Mt. Diwata study on the Philippines 1999--assessing mercury intoxication of the population by small scale gold mining. Sci. Total Environ. 2001, 267, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, D.W.; Beamish, F.W.H. Dynamics of Dietary Methylmercury in Rainbow-Trout, Salmo-Gairdneri. Aquat. Toxicol. 1982, 2, 271–290. [Google Scholar] [CrossRef]
- Olson, G. Microbial intervention in trace element-containing industrial process streams and waste products. In The Importance of Chemical “Speciation” in Environmental Processes; Bernhard, M., Brinckman, F.E., Sadler, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 493–512. [Google Scholar]
- Wood, J.M. Selected Biochemical Reactions of Environmental Significance. Chem. Scr. 1983, 21, 155–160. [Google Scholar]
- Campbell, L.; Verburg, P.; Dixon, D.G.; Hecky, R.E. Mercury biomagnification in the food web of Lake Tanganyika (Tanzania, East Africa). Sci. Total Environ. 2008, 402, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.M.; Balirwa, J.; Dixon, D.; Hecky, R. Biomagnification of mercury in fish from Thruston Bay, Napoleon Gulf, Lake Victoria (East Africa). Afr. J. Aquat. Sci. 2004, 29, 91–96. [Google Scholar] [CrossRef]
- Choi, S.C.; Bartha, R. Cobalamin-mediated mercury methylation by Desulfovibrio desulfuricans LS. Appl. Environ. Microbiol. 1993, 59, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T. Health effects of metals: A role for evolution? Environ. Health Perspect. 1995, 103 (Suppl. 1), 9–12. [Google Scholar] [CrossRef]
- Clarkson, T.W. The toxicology of mercury and its chemical compounds. In Mercury Pollution: Integration and Synthesis; Watras, C., Huckabee, J., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; p. 621. [Google Scholar]
- Keating, M.H.; Mahaffey, K.; Schoeny, R.; Rice, G.; Bullock, O. Mercury Study Report to Congress. Volume 1. Executive Summary; Environmental Protection Agency: Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- Nierenberg, D.W.; Nordgren, R.E.; Chang, M.B.; Siegler, R.W.; Blayney, M.B.; Hochberg, F.; Toribara, T.Y.; Cernichiari, E.; Clarkson, T. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N. Engl. J. Med. 1998, 338, 1672–1676. [Google Scholar] [CrossRef]
- Menkes, J.H. Man, Metals, and Minerals. In Mineral and Metal Neurotoxicology, 1st ed.; Yasui, M., Verity, M.A., Eds.; CRC Press, Inc.: Boca Raton, FL, USA, 1996; p. 5. [Google Scholar]
- Clarkson, T.W. Mercury: Major issues in environmental health. Environ. Health Perspect. 1993, 100, 31–38. [Google Scholar] [CrossRef]
- Wheatley, B.; Paradis, S. Exposure of Canadian Aboriginal Peoples to Methylmercury. Water Air Soil. Pollut. 1995, 80, 3–11. [Google Scholar] [CrossRef]
- Campbell, L.M.; Hecky, R.E.; Muggide, R.; Dixon, D.G.; Ramlal, P.S. Variation and distribution of total mercury in water, sediment and soil from northern Lake Victoria, East Africa. Biogeochemistry 2003, 65, 195–211. [Google Scholar] [CrossRef]
- Castilhos, Z.C.; Rodrigues-Filho, S.; Rodrigues, A.P.; Villas-Boas, R.C.; Siegel, S.; Veiga, M.M.; Beinhoff, C. Mercury contamination in fish from gold mining areas in Indonesia and human health risk assessment. Sci. Total Environ. 2006, 368, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.S.; Blum, J.D.; Basu, N.; Rajaee, M.; Evers, D.C.; Buck, D.G.; Petrlik, J.; DiGangi, J. Assessment of mercury exposure among small-scale gold miners using mercury stable isotopes. Environ. Res. 2015, 137, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Adler Miserendino, R.; Guimarães, J.R.D.; Schudel, G.; Ghosh, S.; Godoy, J.M.; Silbergeld, E.K.; Lees, P.S.; Bergquist, B.A. Mercury pollution in Amapá, Brazil: Mercury amalgamation in artisanal and small-scale gold mining or land-cover and land-use changes? ACS Earth Space Chem. 2017, 2, 441–450. [Google Scholar] [CrossRef]
- Schudel, G.; Miserendino, R.A.; Veiga, M.M.; Velasquez-López, P.C.; Lees, P.S.; Winland-Gaetz, S.; Guimarães, J.R.D.; Bergquist, B.A. An investigation of mercury sources in the Puyango-Tumbes River: Using stable Hg isotopes to characterize transboundary Hg pollution. Chemosphere 2018, 202, 777–787. [Google Scholar] [CrossRef]
- Schudel, G.; Kaplan, R.; Miserendino, R.A.; Veiga, M.M.; Velasquez-López, P.C.; Guimarães, J.R.D.; Bergquist, B.A. Mercury isotopic signatures of tailings from artisanal and small-scale gold mining (ASGM) in southwestern Ecuador. Sci. Total Environ. 2019, 686, 301–310. [Google Scholar] [CrossRef]
- Goix, S.; Maurice, L.; Laffont, L.; Rinaldo, R.; Lagane, C.; Chmeleff, J.; Menges, J.; Heimbürger, L.-E.; Maury-Brachet, R.; Sonke, J.E. Quantifying the impacts of artisanal gold mining on a tropical river system using mercury isotopes. Chemosphere 2019, 219, 684–694. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Blum, J.D.; Yin, R.; Tsui, M.T.-K.; Yang, Y.H.; Choi, J.W. Mercury stable isotopes for monitoring the effectiveness of the Minamata Convention on Mercury. Earth-Sci. Rev. 2020, 203, 103111. [Google Scholar] [CrossRef]
- Laffont, L.; Menges, J.; Goix, S.; Gentès, S.; Maury-Brachet, R.; Sonke, J.E.; Legeay, A.; Gonzalez, P.; Rinaldo, R.; Maurice, L. Hg concentrations and stable isotope variations in tropical fish species of a gold-mining-impacted watershed in French Guiana. Environ. Sci. Pollut. Res. 2021, 28, 60609–60621. [Google Scholar] [CrossRef] [PubMed]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef]
- Clifford, M.J. Future strategies for tackling mercury pollution in the artisanal gold mining sector: Making the Minamata Convention work. Futures 2014, 62, 106–112. [Google Scholar] [CrossRef]
- Selin, H.; Keane, S.E.; Wang, S.; Selin, N.E.; Davis, K.; Bally, D. Linking science and policy to support the implementation of the Minamata Convention on Mercury. Ambio 2018, 47, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Nakajima, K.; Nansai, K.; Seccatore, J.; Veiga, M.M.; Takaoka, M. Examining the inconsistency of mercury flow in post-Minamata Convention global trade concerning artisanal and small-scale gold mining activity. Resour. Conserv. Recycl. 2022, 185, 106461. [Google Scholar] [CrossRef]
- Spiegel, S.; Keane, S.; Metcalf, S.; Veiga, M. Implications of the Minamata Convention on Mercury for informal gold mining in Sub-Saharan Africa: From global policy debates to grassroots implementation? Environ. Dev. Sustain. 2015, 17, 765–785. [Google Scholar] [CrossRef]
- Keane, S.; Bernaudat, L.; Davis, K.J.; Stylo, M.; Mutemeri, N.; Singo, P.; Twala, P.; Mutemeri, I.; Nakafeero, A.; Etui, I.D. Mercury and artisanal and small-scale gold mining: Review of global use estimates and considerations for promoting mercury-free alternatives. Ambio 2023, 52, 833–852. [Google Scholar] [CrossRef]
- Veiga, M.M.; Angeloci-Santos, G.; Meech, J.A. Review of barriers to reduce mercury use in artisanal gold mining. Extr. Ind. Soc. 2014, 1, 351–361. [Google Scholar] [CrossRef]
- Vieira, R. Mercury-free gold mining technologies: Possibilities for adoption in the Guianas. J. Clean. Prod. 2006, 14, 448–454. [Google Scholar] [CrossRef]
- Martinez, G.; Restrepo-Baena, O.; Veiga, M. The myth of gravity concentration to eliminate mercury use in artisanal gold mining. Extr. Ind. Soc. 2021, 8, 477–485. [Google Scholar] [CrossRef]
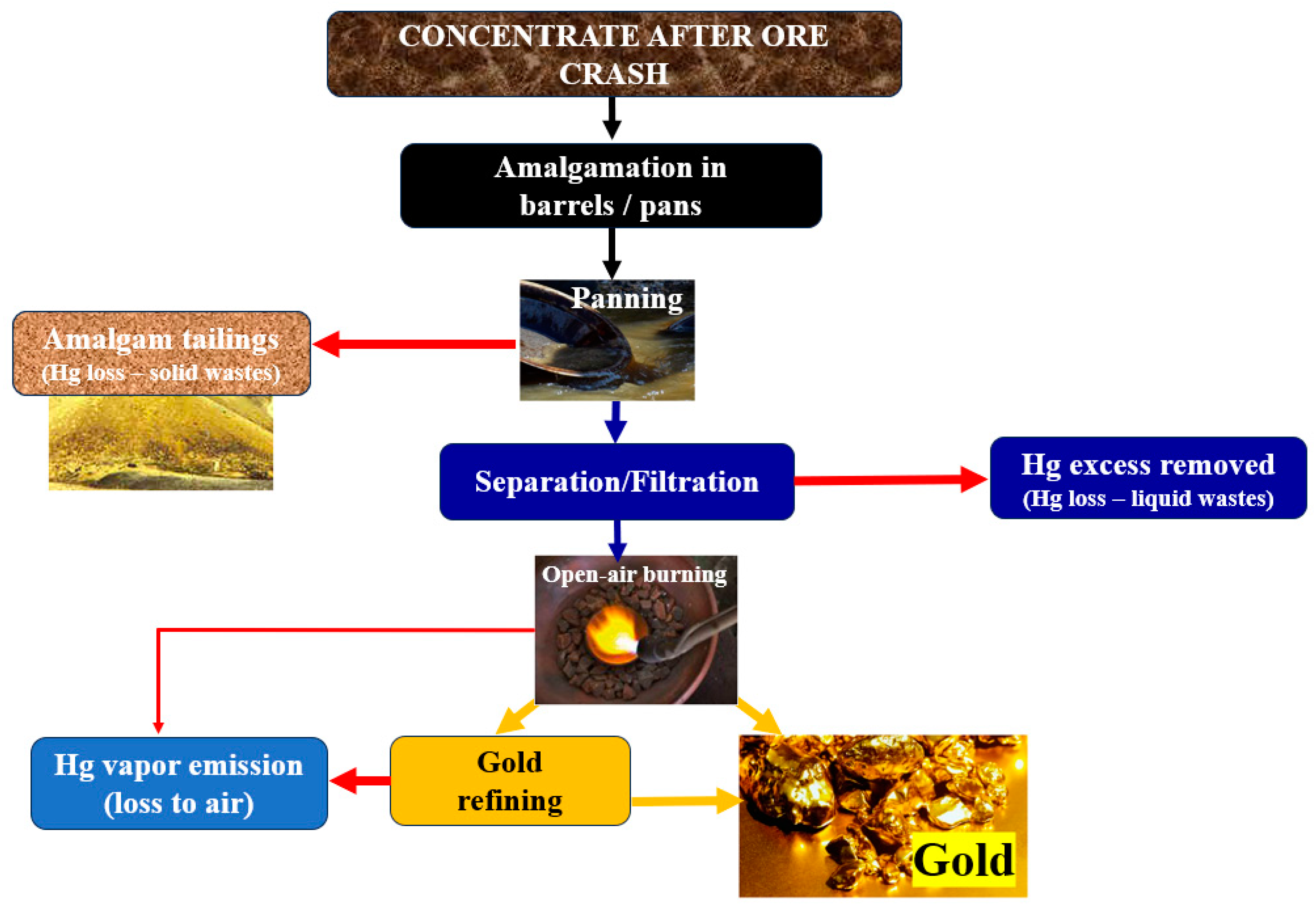
| Developing Nations | Developed Nations |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donkor, A.K.; Ghoveisi, H.; Bonzongo, J.-C.J. Use of Metallic Mercury in Artisanal Gold Mining by Amalgamation: A Review of Temporal and Spatial Trends and Environmental Pollution. Minerals 2024, 14, 555. https://doi.org/10.3390/min14060555
Donkor AK, Ghoveisi H, Bonzongo J-CJ. Use of Metallic Mercury in Artisanal Gold Mining by Amalgamation: A Review of Temporal and Spatial Trends and Environmental Pollution. Minerals. 2024; 14(6):555. https://doi.org/10.3390/min14060555
Chicago/Turabian StyleDonkor, Augustine K., Hossein Ghoveisi, and Jean-Claude J. Bonzongo. 2024. "Use of Metallic Mercury in Artisanal Gold Mining by Amalgamation: A Review of Temporal and Spatial Trends and Environmental Pollution" Minerals 14, no. 6: 555. https://doi.org/10.3390/min14060555





