In Situ Modification of Activated Carbon Made from Camellia oleifera Shell with Na2EDTA for Enhanced La3+ Recovery
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
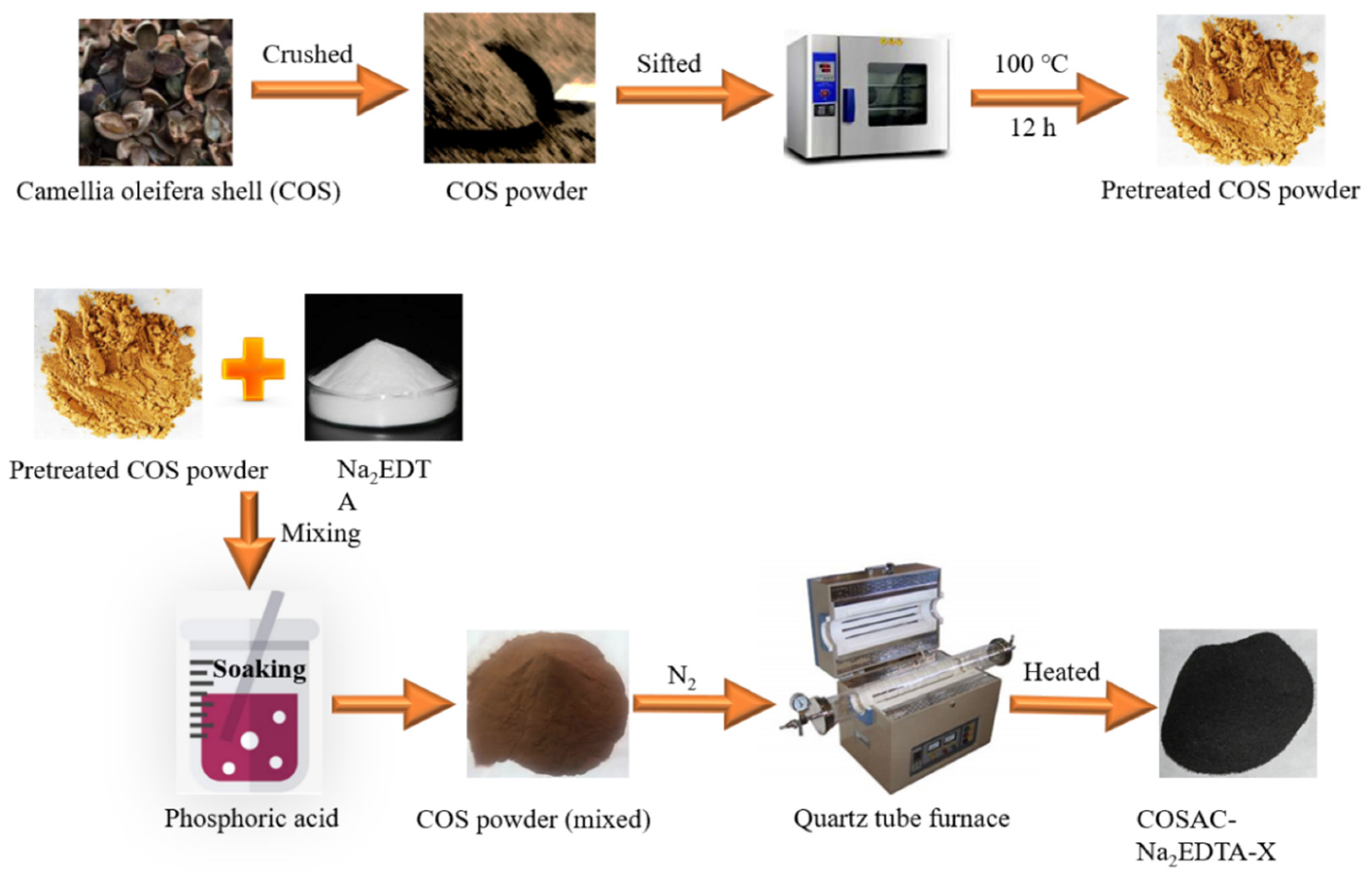
2.2. Synthesis of COSAC-Na2EDTA
2.3. Characterization
2.4. Adsorption and Recovery Experiment
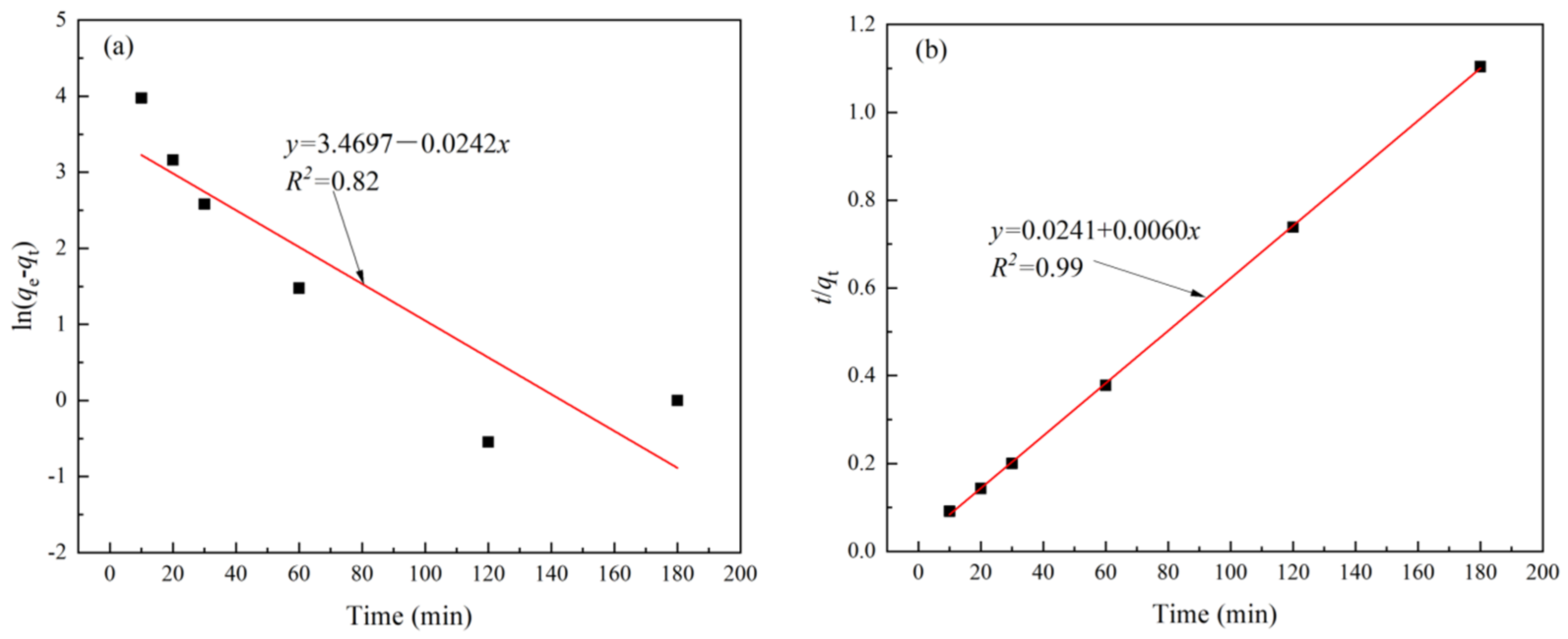
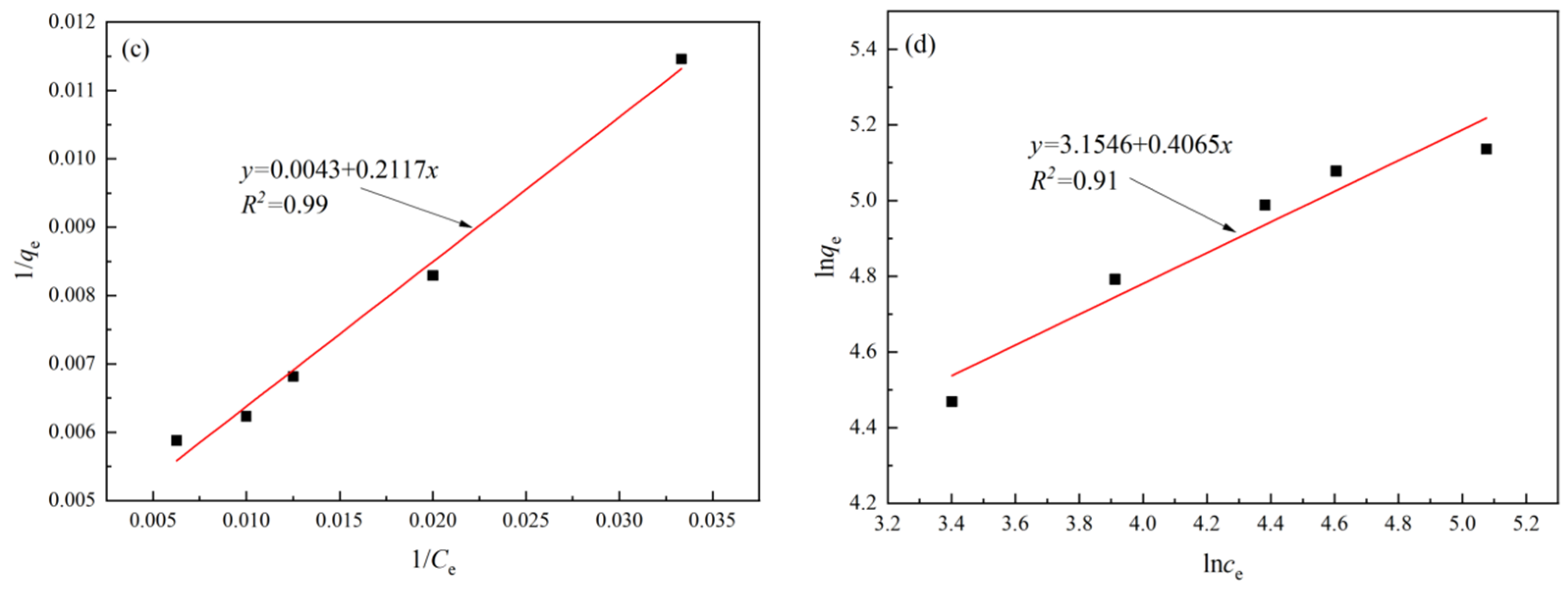
2.5. Kinetic and Isothermal Models
3. Results and Discussion
3.1. Properties of COSAC at Different Preparation Conditions
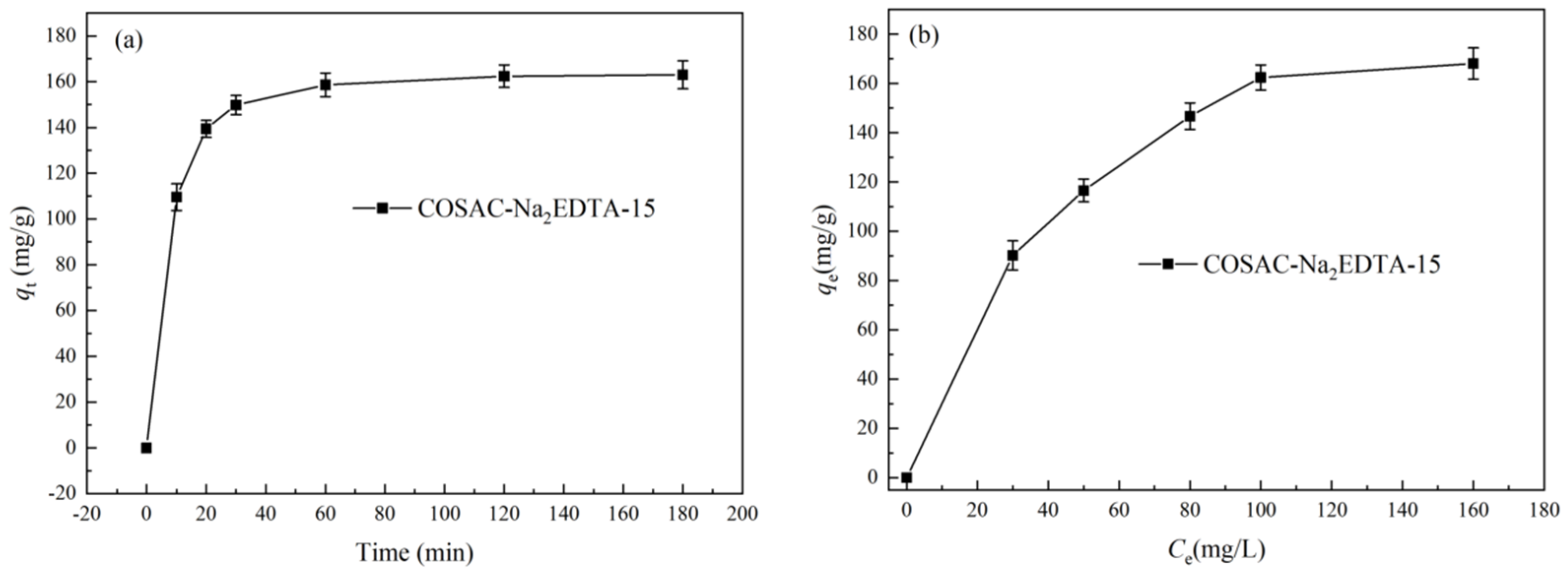
3.2. Kinetics and Isotherms
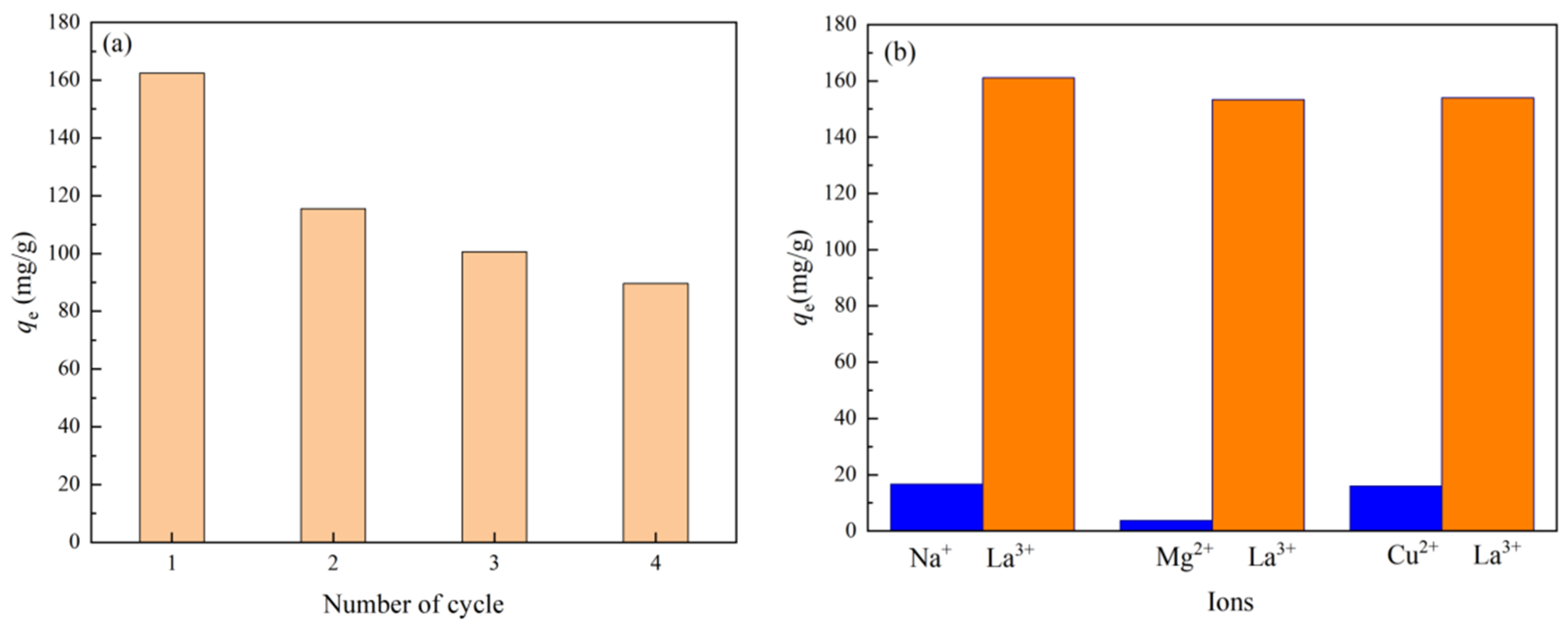
3.3. Adsorption Selectivity and Recycling Characteristics
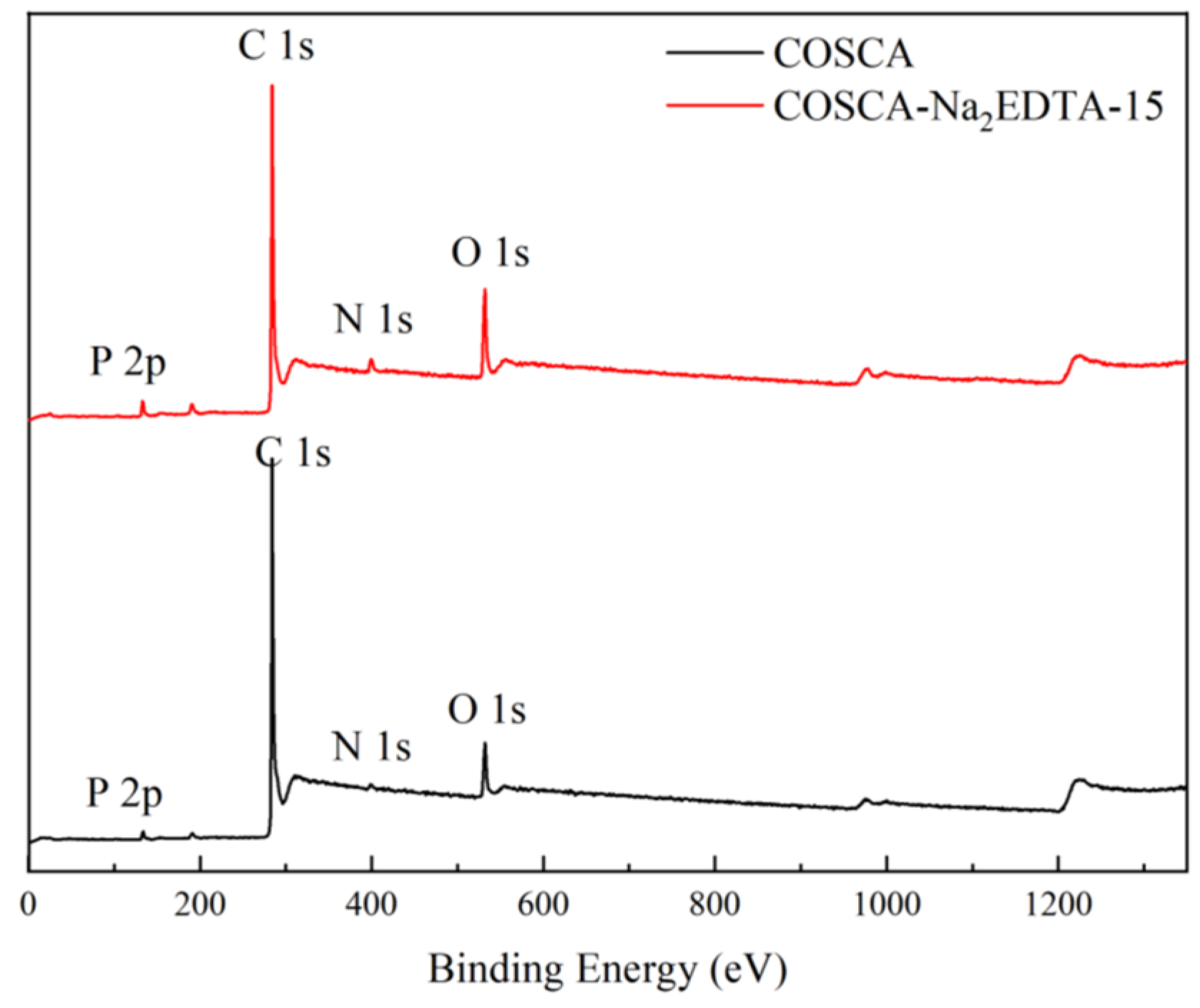
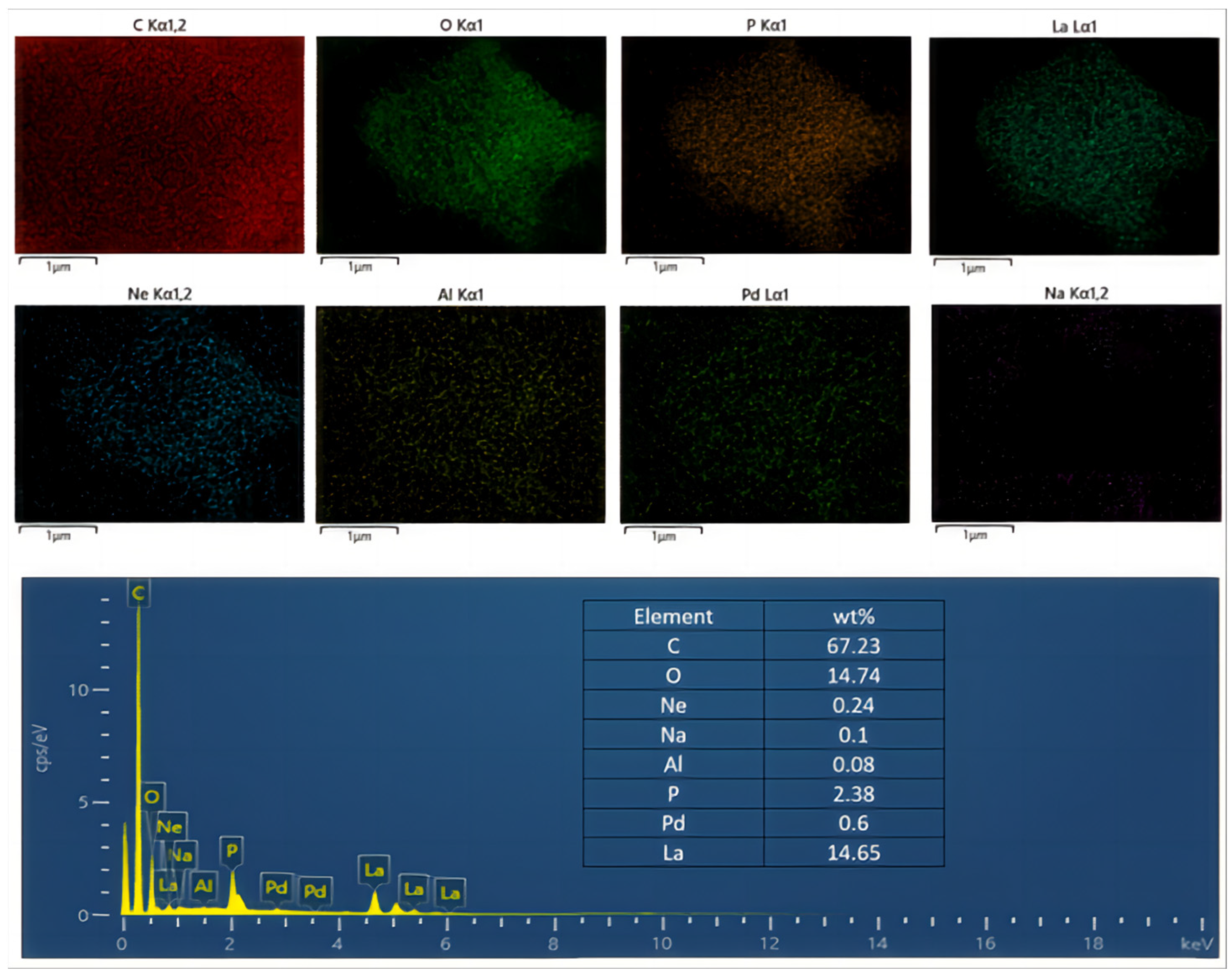
3.4. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.H.; Liu, X.G.; Shi, M.X.; Qin, Y.Y.; Li, X.C. Study on the adsorption of lanthanum ion imprinted on SBA-15/Y. J. Indian Chem. Soc. 2022, 99, 100425. [Google Scholar] [CrossRef]
- Arul Manikandan, N.; Lens, P.N.L. Biorefining of green macroalgal (Ulva sp.) biomass and its application in the adsorptive recovery of rare earth elements (REEs). Sep. Purif. Technol. 2022, 303, 122200. [Google Scholar] [CrossRef]
- Ponou, J.; Dodbiba, G.; Anh, J.-W.; Fujita, T. Selective recovery of rare earth elements from aqueous solution obtained from coal power plant ash. J. Environ. Chem. Eng. 2016, 4, 3761–3766. [Google Scholar] [CrossRef]
- Huang, L.J.H.; Xiao, B.; Liu, L.H.; Li, W.H.; Duan, X.G.; Huang, W.F.; Fan, C.Y.; Dong, Y.; Liu, S.M. Selective recovery of Gd(III) by benzimidazole- and benzoxazole-linked 3D porous polymers. J. Water Process Eng. 2023, 51, 103378. [Google Scholar] [CrossRef]
- Danish, E.Y.; Marwani, H.M.; Almoslehi, K.F.; Bakhsh, E.M. Adsorptive removal of lanthanum based on hydrothermally synthesized iron oxide-titanium oxide nanoparticles. Environ. Sci. Pollut. Res. Int. 2020, 27, 5408–5417. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.H.; Liu, L.H.; Huang, W.F.; Zhao, B.X.; Shen, Z.F.; Bao, Y.Q.; Znad, H. Recovery of lanthanum cations by functionalized magnetic multi-walled carbon nanotube bundles. RSC Adv. 2021, 11, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Kusrini, E.; Wicaksono, W.; Gunawan, C.; Daud, N.Z.A.; Usman, A. Kinetics, mechanism, and thermodynamics of lanthanum adsorption on pectin extracted from durian rind. J. Environ. Chem. Eng. 2018, 6, 6580–6588. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, L.; Bian, X.; Zuo, H.; Dong, H. Adsorption and mineralization of REE-lanthanum onto bacterial cell surface. Environ. Sci. Pollut. Res. Int. 2018, 25, 22334–22339. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Deng, Q.; Wu, Y.; Wu, Y.; Chen, J.; Chen, Y.; Lin, L.; Qiu, Y.; Pan, L.; Zheng, X.; et al. U-Shaped Relationship of Rare Earth Element Lanthanum and Oral Cancer Risk: A Propensity Score-Based Study in the Southeast of China. Fron. Public Health 2022, 10, 905690. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, C.; Zhou, S.; Liang, T.; Wen, X. Preparation of montmorillonite grafted polyacrylic acid composite and study on its adsorption properties of lanthanum ions from aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 9861–9875. [Google Scholar] [CrossRef]
- Liu, E.; Chen, L.; Dai, J.; Wang, Y.; Li, C.; Yan, Y. Fabrication of phosphate functionalized chiral nematic mesoporous silica films for the efficient and selective adsorption of lanthanum ions. J. Mol. Liq. 2019, 277, 786–793. [Google Scholar] [CrossRef]
- Abdel-Magied, A.F.; Abdelhamid, H.N.; Ashour, R.M.; Zou, X.; Forsberg, K. Hierarchical porous zeolitic imidazolate frameworks nanoparticles for efficient adsorption of rare-earth elements. Microporous Mesoporous Mater. 2019, 278, 175–184. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Pinto, D.; Lima, É.C.; Knani, S.; Grimm, A.; Silva, L.F.O.; Cadaval, T.R.S.; Dotto, G.L. Lanthanum uptake from water using chitosan with different configurations. React. Funct. Polym. 2022, 180, 105395. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Cui, L. Selective capture of lanthanum and lead cations over biomass-derived ion-imprinted biomacromolecule adsorbents. J. Mol. Liq. 2019, 291, 111290. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Liu, H. In-situ modification of activated carbon with ethylenediaminetetraacetic acid disodium salt during phosphoric acid activation for enhancement of nickel removal. Powder Technol. 2018, 325, 113–120. [Google Scholar] [CrossRef]
- Guo, H.; Bi, C.; Zeng, C.; Ma, W.; Yan, L.; Li, K.; Wei, K. Camellia oleifera seed shell carbon as an efficient renewable bio-adsorbent for the adsorption removal of hexavalent chromium and methylene blue from aqueous solution. J. Mol. Liq. 2018, 249, 629–636. [Google Scholar] [CrossRef]
- Huang, L.; Peng, H.; Xiao, Z.; Wu, H.; Fu, G.; Wan, Y.; Bi, H. Production of furfural and 5-hydroxymethyl furfural from Camellia oleifera fruit shell in [Bmim]HSO4/H2O/1,4-dioxane biphasic medium. Ind. Crop. Prod. 2022, 184, 115006. [Google Scholar] [CrossRef]
- Liu, X.; Xie, M.; Hu, Y.; Li, S.; Nie, S.; Zhang, A.; Wu, H.; Li, C.; Xiao, Z.; Hu, C. Facile preparation of lignin nanoparticles from waste Camellia oleifera shell: The solvent effect on the structural characteristic of lignin nanoparticles. Ind. Crop. Prod. 2022, 183, 114943. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zhang, F.; Jiang, J. Xylo-oligosaccharides and lignin production from Camellia oleifera shell by malic acid hydrolysis at mild conditions. Bioresour. Technol. 2021, 341, 125897. [Google Scholar] [CrossRef]
- Liu, X.; Meng, W.; Cheng, S.; Xing, B.; Zheng, Y.; Ren, X.; Xue, M.; Zhang, C.; Xia, H. Utilization of camellia oleifera shell for production of valuable products by pyrolysis. Arab. J. Chem. 2022, 15, 104348. [Google Scholar] [CrossRef]
- Chen, P.; Hu, C.; Gu, J.; Lin, X.; Yang, C.; Leu, S.-Y.; Guan, L. Pyrolysis characteristics of tea oil camellia (Camellia oleifera Abel.) shells and their chemically pre-treated residues: Kinetics, mechanisms, product evaluation and joint optimization. J. Anal. Appl. Pyrolysis 2022, 164, 105526. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, W.; Han, M.; Zhang, F.; Lei, F.; Cheng, X.; Ning, R.; Wang, K.; Ji, L.; Jiang, J. Production of xylooligosaccharides from Camellia oleifera Abel fruit shell using a shell-based solid acid catalyst. Bioresour. Technol. 2022, 365, 128173. [Google Scholar] [CrossRef]
- Chaydarreh, K.C.; Lin, X.; Dandan, L.; Zhang, W.; Guan, L.; Hu, C. Developing 3-layer tea oil camellia (Camellia oleifera Abel.) shells-based particleboard with systematic study on particle geometry and distribution. Ind. Crop. Prod. 2022, 179, 114682. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Y.; Liu, Y.; Chang, S. Anatomical structure of Camellia oleifera shell. Protoplasma 2018, 255, 1777–1784. [Google Scholar] [CrossRef]
- Long, H.; Gu, J.; Jiang, J.; Guan, L.; Lin, X.; Zhang, W.; Hu, C. Mechanically strong and biodegradable holocellulose films prepared from Camellia oleifera shells. Carbohydr. Polym. 2023, 299, 120189. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zhang, X.; Li, P.; Lei, F.; Jiang, J. Co-production of xylooligosaccharides and activated carbons from Camellia oleifera shell treated by the catalysis and activation of zinc chloride. Bioresour. Technol. 2020, 306, 123131. [Google Scholar] [CrossRef]
- Mei, L.; Qiao, H.; Ke, F.; Peng, C.; Hou, R.; Wan, X.; Cai, H. One-step synthesis of zirconium dioxide-biochar derived from Camellia oleifera seed shell with enhanced removal capacity for fluoride from water. Appl. Surf. Sci. 2020, 509, 144685. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Cheng, H.; Cheng, J.; Du, K.; Hu, Y.; Chen, Y. High mesoporosity phosphorus-containing biochar fabricated from Camellia oleifera shells: Impressive tetracycline adsorption performance and promotion of pyrophosphate-like surface functional groups (C-O-P bond). Bioresour. Technol. 2021, 329, 124922. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, B.; Luo, L.; Deng, B.; Shad, N.; Hu, D.; Aly, H.M.; Zhang, L. Effects of hydroxyapatite and modified biochar derived from Camellia oleifera fruit shell on soil Cd contamination and N2O emissions. Ind. Crop. Prod. 2022, 177, 114476. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, X.; Gao, Y.; Zhang, W.; Zhou, L.; Zeng, X.; Liu, W.; Jiang, Q.; Jiang, C.; Wang, S. Iron oxide loaded biochar/attapulgite composites derived camellia oleifera shells as a novel bio-adsorbent for highly efficient removal of Cr(VI). J. Clean. Prod. 2021, 317, 128412. [Google Scholar] [CrossRef]
- Zeng, B.; Zeng, X.; Hu, L.; Huang, L.; Huang, Y.; Zhou, Y.; Liu, G.; Huang, W. Activated carbon from Camellia oleifera shells for adsorption of Y(iii): Experimental and DFT studies. RSC Adv. 2024, 14, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hou, L.; Wang, Z.; Gu, P.; Chen, G.; Jiang, R. A new function of spent activated carbon in BAC process: Removing heavy metals by ion exchange mechanism. J. Hazard. Mater. 2018, 359, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bud, J.; Byambajav, E.; Tsubouchi, N. Influence of ammonia treatment on the CO2 adsorption of activated carbon. J. Environ. Chem. Eng. 2022, 10, 107273. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, L.; Huang, W.; Zhang, D.; Zeng, X.; Yao, X. Glycine functionalized activated carbon derived from navel orange peel for enhancement recovery of Gd(Ⅲ). J. Rare Earths 2022, 40, 1794–1802. [Google Scholar] [CrossRef]
- Mehrani, Z.; Ebrahimzadeh, H.; Moradi, E.; Yamini, Y. Using three-dimensional poly (vinyl alcohol)/sodium hexametaphosphate nanofiber as a non-toxic and efficient nanosorbent for extraction and recovery of Lanthanide ions from aqueous solutions. J. Mol. Liq. 2020, 307, 112925. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Araucz, K. New titanium oxide sorbent for As(V) and Cr(VI) removal as well as La(III) and Nd(III) recovery. J. Mol. Liq. 2020, 315, 113720. [Google Scholar] [CrossRef]
- Ni, C.; Liu, Q.; Ren, Z.; Hu, H.; Sun, B.; Liu, C.; Shao, P.; Yang, L.; Pavlostathis, S.G.; Luo, X. Selective removal and recovery of La(III) using a phosphonic-based ion imprinted polymer: Adsorption performance, regeneration, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106701. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, D.; Su, T.; Bao, S.; Liao, C.; Wang, Q. Magnetic nanocomposite hydrogel prepared by ZnO-initiated photopolymerization for La (III) adsorption. ACS Appl. Mater. Interfaces 2014, 6, 19840–19849. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, D.L.; Porada, S.; Sillanpää, M. Marine algae: A promising resource for the selective recovery of scandium and rare earth elements from aqueous systems. Chem. Eng. J. 2019, 371, 759–768. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Ogden, M.D.; Moon, E.M.; Soldenhoff, K.L. REE behavior and sorption on weak acid resins from buffered media. J. Ind. Eng. Chem. 2018, 59, 440–455. [Google Scholar] [CrossRef]




| Absorbent | Max. Adsorption Capacity (mg/g) | Equilibrium Time (h) | Refs. |
|---|---|---|---|
| Amine modified activated carbon | 107 | 1.5 | [15] |
| Iron oxide-titanium oxide nanoparticles | 89.63 | 1 | [5] |
| MSFs-1 | 77.74 | 3 | [11] |
| PVA/SHMP HENF | 181.82 | 2 | [39] |
| ADSORBSIA™ As500 | 24.77 | 1 | [40] |
| La-IIP | 62.8 | 0.5 | [41] |
| Valorised Ulva Sp. biomass | 119.297 | 2 | [2] |
| ZnO clay nanocomposite hydrogel | 58.8 | 2 | [42] |
| Sargassum fluitans | 73.62 | 24 | [43] |
| Amberlite IRC86 | 40.60 | -- | [44] |
| COSAC | 50 | 2 | This study |
| COSAC-Na2EDTA-15 | 162.43 | 2 | This study |
| Sample | C (%) | N (%) | P (%) | O (%) |
|---|---|---|---|---|
| COSAC | 89.51 | 1.48 | 1.7 | 7.31 |
| COSAC-Na2EDTA-15 | 80.98 | 4.15 | 2.23 | 12.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zeng, X.; Fan, C.; Liu, L.; Alam, S.; Zeng, B.; Liu, S.; Huang, W.; Shu, R. In Situ Modification of Activated Carbon Made from Camellia oleifera Shell with Na2EDTA for Enhanced La3+ Recovery. Minerals 2024, 14, 560. https://doi.org/10.3390/min14060560
Huang L, Zeng X, Fan C, Liu L, Alam S, Zeng B, Liu S, Huang W, Shu R. In Situ Modification of Activated Carbon Made from Camellia oleifera Shell with Na2EDTA for Enhanced La3+ Recovery. Minerals. 2024; 14(6):560. https://doi.org/10.3390/min14060560
Chicago/Turabian StyleHuang, Lijinhong, Xiangrong Zeng, Chunyan Fan, Lihong Liu, Shafiq Alam, Bin Zeng, Shaomin Liu, Wanfu Huang, and Ronghua Shu. 2024. "In Situ Modification of Activated Carbon Made from Camellia oleifera Shell with Na2EDTA for Enhanced La3+ Recovery" Minerals 14, no. 6: 560. https://doi.org/10.3390/min14060560
APA StyleHuang, L., Zeng, X., Fan, C., Liu, L., Alam, S., Zeng, B., Liu, S., Huang, W., & Shu, R. (2024). In Situ Modification of Activated Carbon Made from Camellia oleifera Shell with Na2EDTA for Enhanced La3+ Recovery. Minerals, 14(6), 560. https://doi.org/10.3390/min14060560







