DIGIT: An In Situ Experiment for Studying the Diffusion of Water and Solutes under Thermal Gradient in the Toarcian Clay Rock at the Tournemire Underground Research Laboratory: Part 1—Goals, Scoping Calculations, Installation and First Results under Unheated Conditions
Abstract
1. Introduction
2. Initial State and Test Dimensioning
2.1. The Tournemire Clay Rock and Site
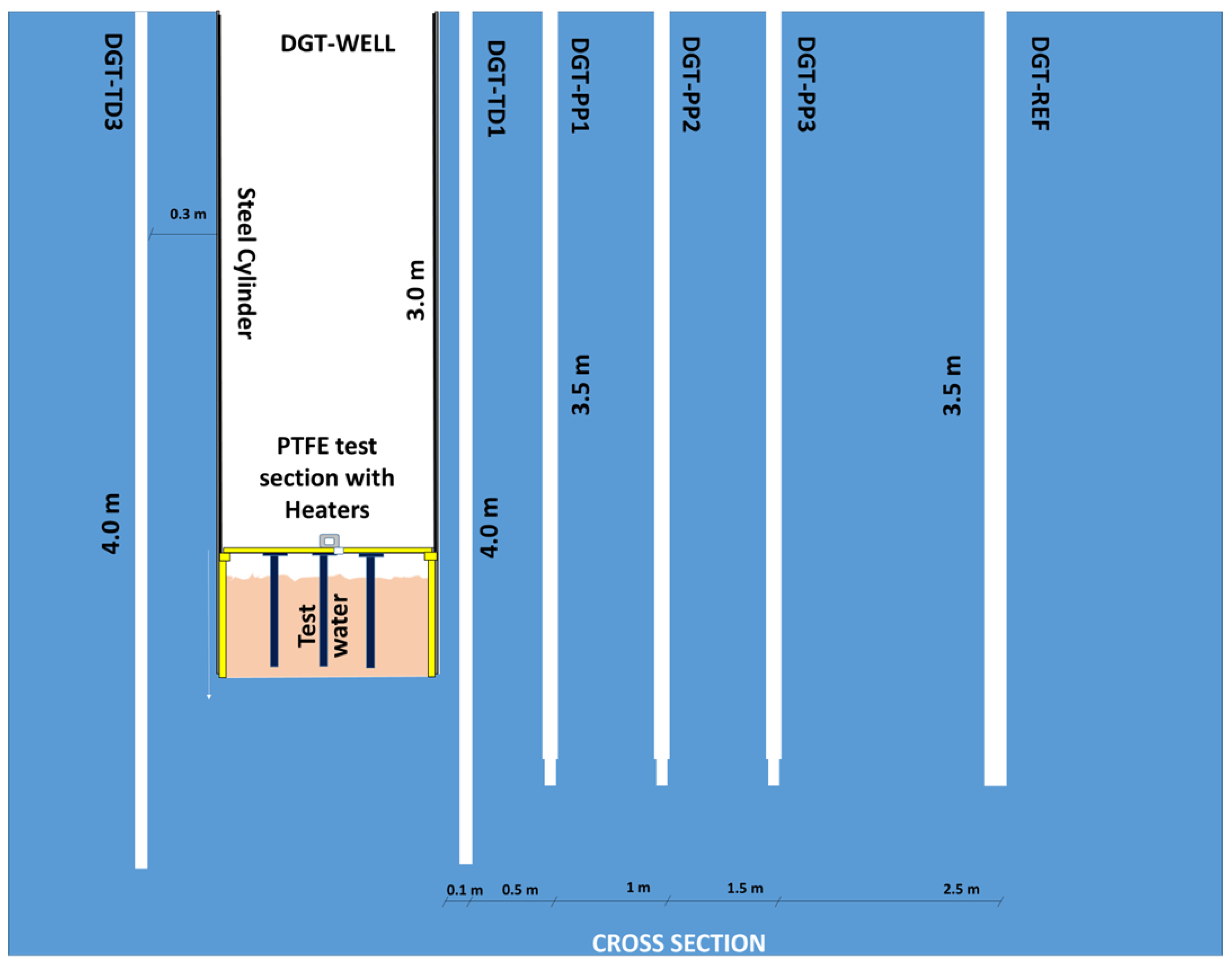
2.2. Test Area Validation
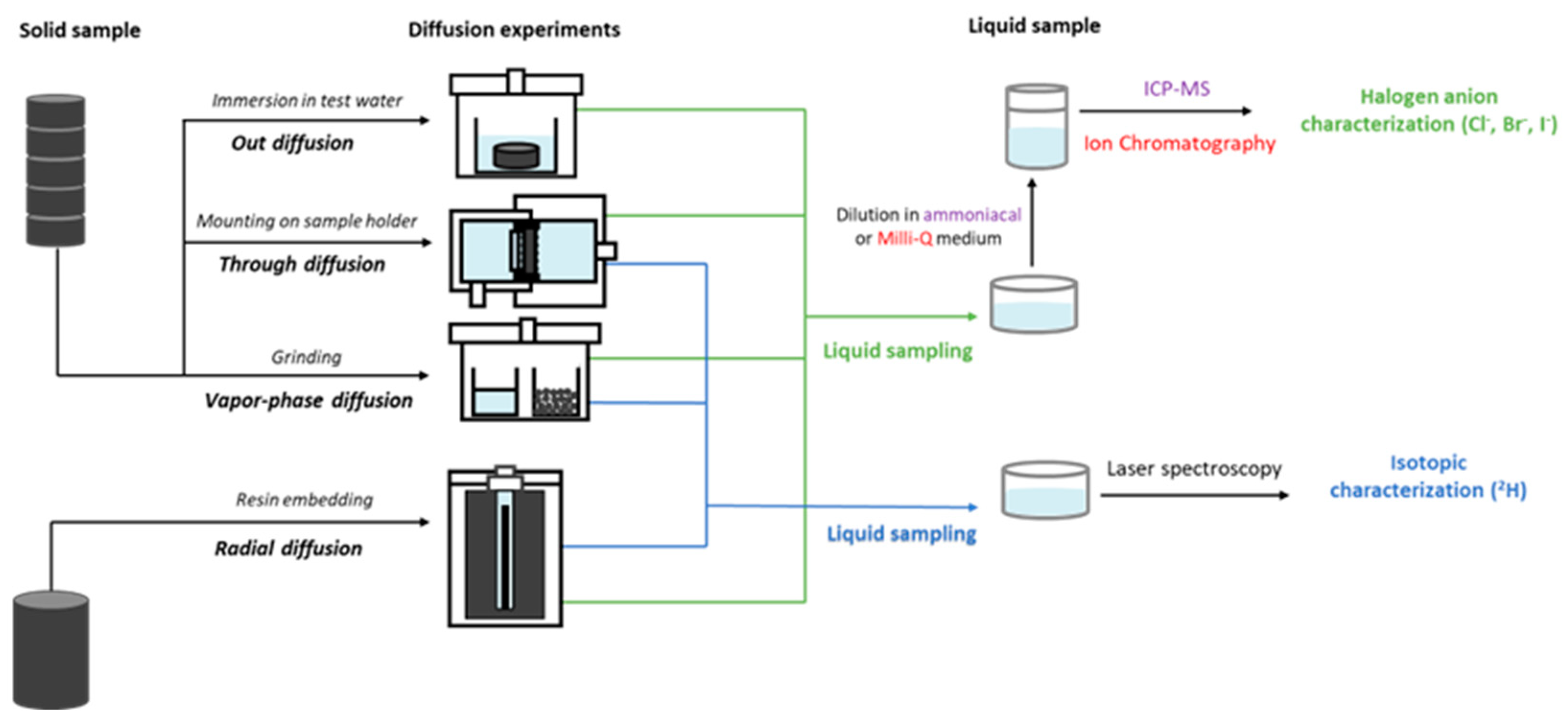
2.3. Tracer Selection and Their Initial Concentrations in Pore Water
2.4. Determination of Heat and Mass Transport Parameters in Their Initial State
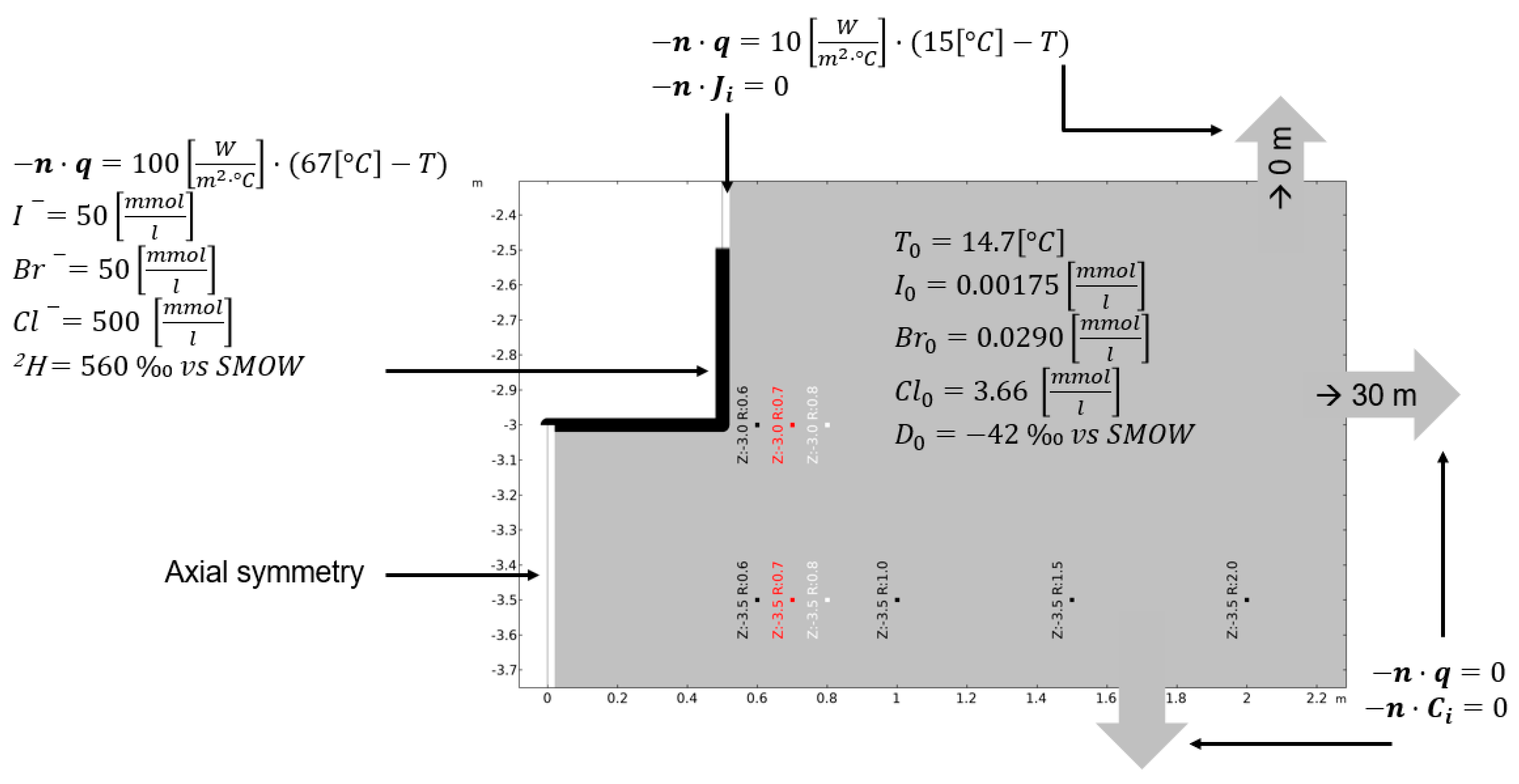
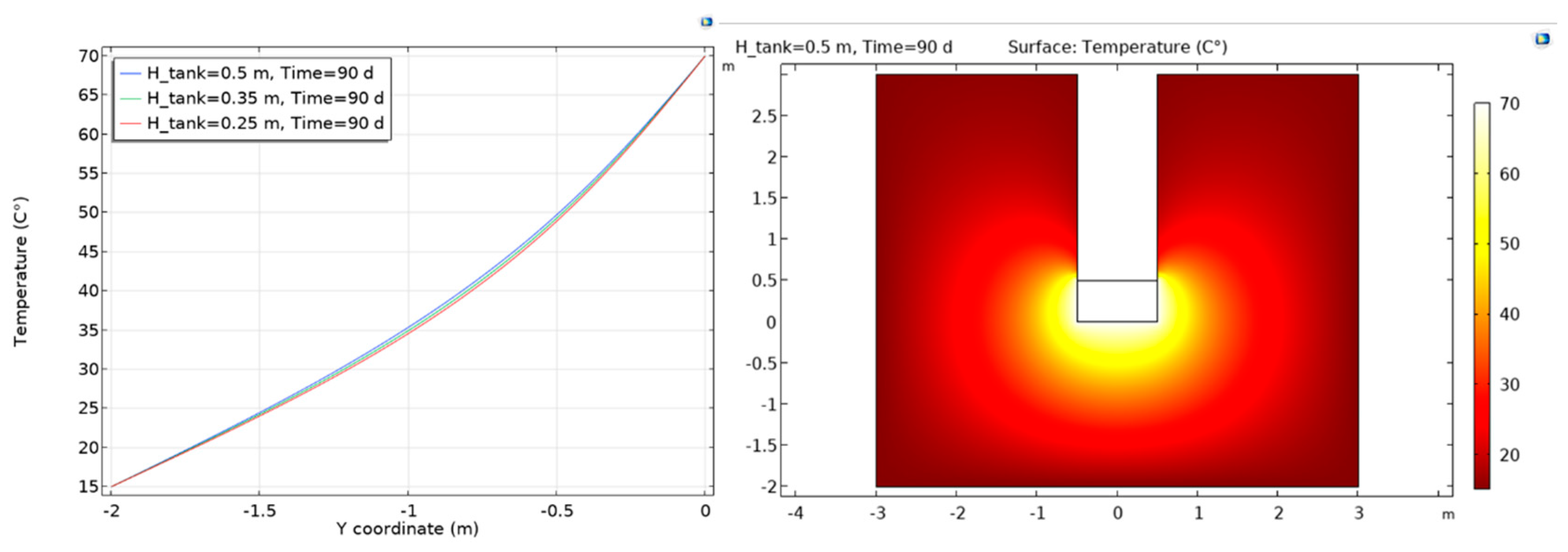
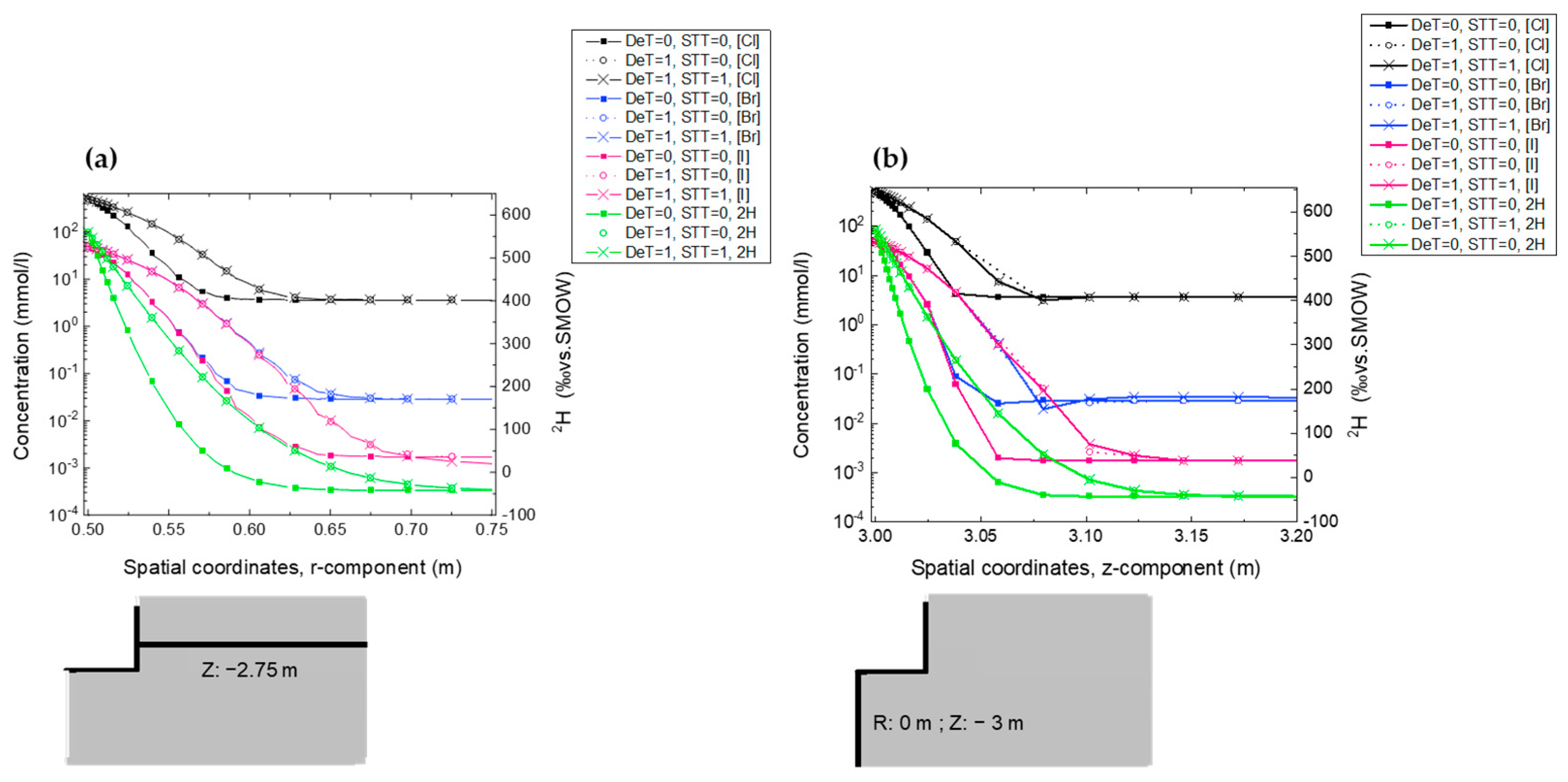
2.5. Scoping Calculations for Experiment Dimensioning and Design
| Parameter | Symbol | Value | Unit | Source |
|---|---|---|---|---|
| ∥ parallel, ⊥ normal to bedding | ||||
| Initial 2H concentration in porewater | −42.0 ± 4 | ‰ vs. SMOW | This study | |
| Initial Cl− concentration in porewater | 3.66 ± 0.02 | mmol L−1 | This study | |
| Initial Br− concentration in porewater | 0.0290 ± 0.0002 | mmol L−1 | This study | |
| Initial I− concentration in porewater | 0.00175 ± 0.00002 | mmol L−1 | This study | |
| Specific heat capacity | Cp | 800 ± 100 | J kg−1 L−1 | [28] |
| Thermal conductivity | λ | 2.2 ± 0.7 ∥ | W m−1 K−1 | [28] |
| Bulk wet density | 2540 ± 2 | Kg m−3 | This study | |
| Total porosity accessible to water | 10 ± 1.0 | vol. % | This study | |
| Total porosity accessible to Cl− | 5.36 ± 0.3 | vol. % | This study | |
| Total porosity accessible to Br− | 5.8 ± 0.5 | vol. % | This study | |
| Total porosity accessible to I− | 4.9 ± 0.5 | vol. % | This study | |
| Effective diffusion coefficient for 2H ∥ | 3.23 ± 0.618 × 10−11 | m2 s−1 | This study | |
| Effective diffusion coefficient for Cl− ∥ | 2.60 ± 0.115 × 10−12 | m2 s−1 | This study | |
| Effective diffusion coefficient for Br− ∥ | 2.58 × 10−12 | m2 s−1 | This study | |
| Effective diffusion coefficient for I− ∥ | 4.03 × 10−12 | m2 s−1 | This study | |
| values | De∥/De⊥ | 3 | [19] | |
| values | De∥/De⊥ | 3.0 ± 0.4 | [17] | |
| values | De∥/De⊥ | 2.6 ± 0.4 | [17] | |
| values | De∥/De⊥ | 3.8 ± 0.4 | [17] | |
| Activation energy for 2H | 22.2 ± 1.78 | kJ mol−1 | [55] | |
| Activation energy for anions | 20.3 ± 1.84 | kJ mol−1 | [55] | |
| Soret coefficient | ST | 1 × 10−2 | K−1 | [22] |
| Reference temperature | Tref | 288.15 (15) | K (°C) | This study |
| Input temperature at the test section wall | Ttest section | 343.15 (70) | K (°C) | This study |
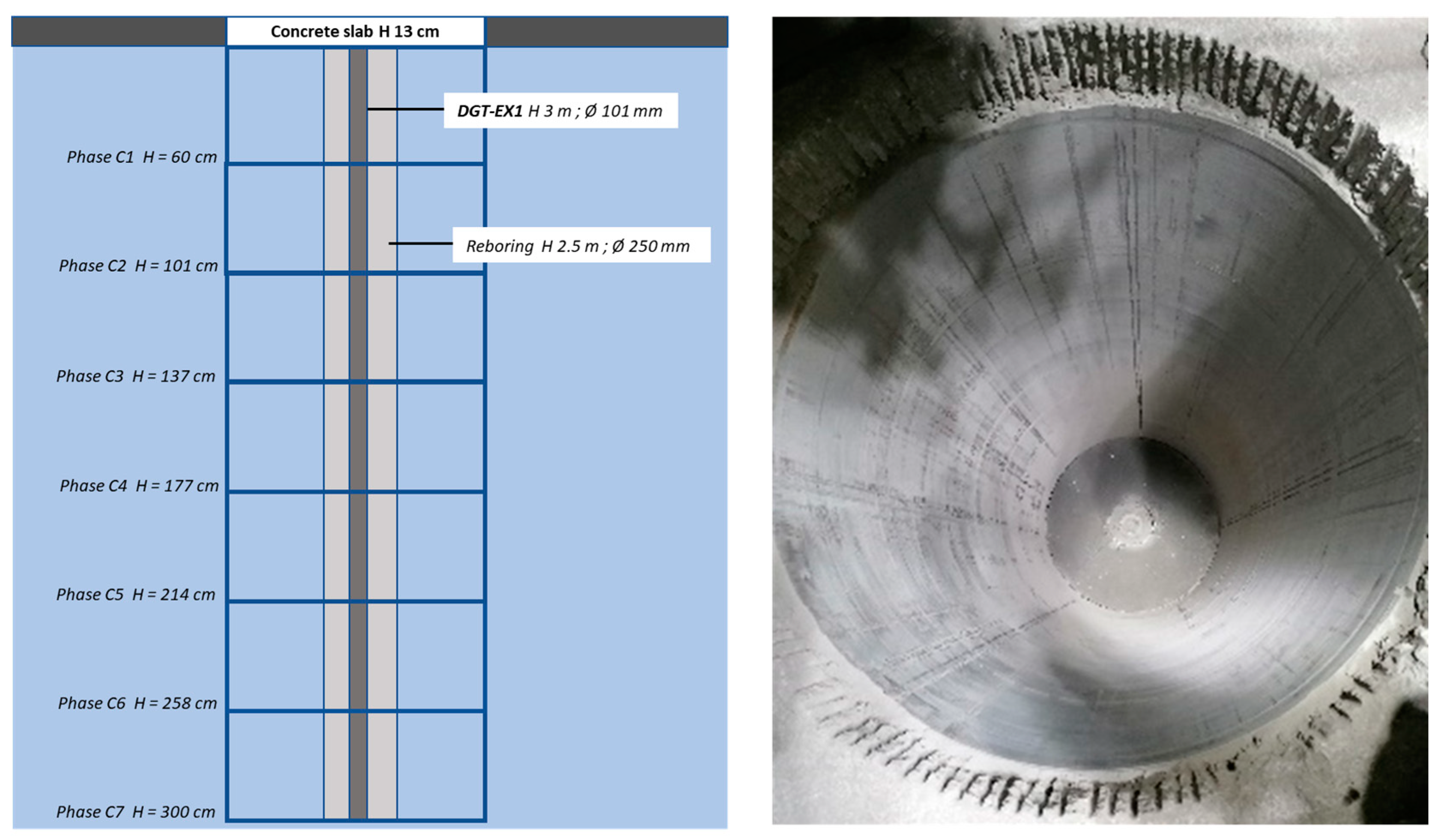
3. Installation of the DIGIT Experiment
4. Discussion
5. Preliminary Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Andra. Argile. Synthesis-Evaluation of the Feasibility of a Geological Repository in an Argillaceous Formation. 2005. Available online: https://www.andra.fr/cigeo/les-documents-de-reference (accessed on 27 March 2024).
- Andra. Authorization File for the Creation of the Cigéo Basic Nuclear Facility (INB). December 2022. Available online: https://www.andra.fr/sites/default/files/2023-04/Pi%C3%A8ce%2007-VPRS-PARTIE%203%20D%C3%A9monstration%20s%C3%BBret%C3%A9-Volume%2008-D%C3%A9monstration%20de%20s%C3%BBret%C3%A9%20apr%C3%A8s-fermeture.pdf (accessed on 27 March 2024).
- Sellin, P.; Leupin, O. The Use of Clay as an Engineered Barrier in Radioactive-Waste Management—A Review. Clays Clay Miner. 2014, 61, 477–498. [Google Scholar] [CrossRef]
- Swedish Radiation Safety Authority (SRSA). 2021:15 National Plan: Responsible and Safe Management of Spent Fuel and Radioactive Waste in Sweden. Strålsäkerhetsmyndigheten. Available online: https://www.stralsakerhetsmyndigheten.se/publikationer/rapporter/avfall--transport--fysiskt-skydd/2021/202115/ (accessed on 27 March 2024).
- Nagra. Technical Report NTB 21-01. Nagra. 14 December 2021. Available online: https://nagra.ch/downloads/technischer-bericht-ntb-21-01/ (accessed on 27 March 2024).
- Nuclear waste Management Organization (NWMO). Canada’s Deep Geological Repository. Available online: https://www.nwmo.ca/en/canadas-plan/canadas-deep-geological-repository (accessed on 27 March 2024).
- Zou, L.; Cvetkovic, V. Disposal of high-level radioactive waste in crystalline rock: On coupled processes and site development. Rock Mech. Bull. 2023, 2, 100061. [Google Scholar] [CrossRef]
- Vinsot, A.; Lundy, M.; Linard, Y. O2 Consumption and CO2 Production at Callovian-oxfordian Rock Surfaces. Procedia Earth Planet. Sci. 2017, 17, 562–565. [Google Scholar] [CrossRef]
- Guo, R.; Thatcher, K.E.; Seyedi, D.M.; Plúa, C. Calibration of the thermo-hydro-mechanical parameters of the Callovo-Oxfordian claystone and the modelling of the ALC experiment. Int. J. Rock Mech. Min. Sci. 2020, 132, 104351. [Google Scholar] [CrossRef]
- Gens, A.; Vaunat, J.; Garitte, B.; Wileveau, Y. In situ behaviour of a stiff layered clay subject to thermal loading: Observations and interpretation. Géotechnique 2007, 57, 207–228. [Google Scholar] [CrossRef]
- Bensenouci, F.; Michelot, J.L.; Matray, J.M.; Savoye, S.; Massault, M.; Vinsot, A. Coupled study of water-stable isotopes and anions in porewater for characterizing aqueous transport through the Mesozoic sedimentary series in the eastern Paris Basin. Mar. Pet. Geol. 2014, 53, 88–101. [Google Scholar] [CrossRef]
- Rebeix, R.; La Salle, C.L.G.; Jean-Baptiste, P.; Lavastre, V.; Fourré, E.; Bensenouci, F.; Matray, J.M.; Landrein, P.; Shouakar-Stash, O.; Frape, S.K.; et al. Chlorine transport processes through a 2000 m aquifer/aquitard system. Mar. Pet. Geol. 2014, 53, 102–116. [Google Scholar] [CrossRef]
- Trémosa, J. Influence of Osmotic Processes on the Excess-Hydraulic Head Measured in the Toarcian/Domerian Argillaceous Formation of Tournemire. Ph.D. Thesis, Universite de Paris VI, Paris, France, 2010. Available online: https://theses.fr/2010PA066670 (accessed on 9 April 2024).
- Gonçalvès, J.; Matray, J.M.; Yu, C.J. Assessing relevant transport processes in Opalinus Clay at the Mont Terri rock laboratory using excess-pressure, concentration and temperature profiles. Appl. Clay Sci. 2023, 242, 107016. [Google Scholar] [CrossRef]
- Yu, C.J. Comparative Study of Convective and Diffusive Transport Phenomena within the Opalinus Clay of Mont Terri. Ph.D. Thesis, Aix-Marseille, Marseille, France, 2017. Available online: http://www.theses.fr/2017AIXM0409/document (accessed on 9 April 2024).
- Bensenouci, F. Apport Des Traceurs Naturels à La Compréhension Des Transferts Au Sein Des Formations Argileuses Compactées. Ph.D. Thesis, Université Paris-Sud 11, Bures-sur-Yvette, France, 2010. Available online: https://theses.fr/2010PA112156 (accessed on 9 April 2024).
- Leupin, O.X.; Van Loon, L.R.; Gimmi, T.; Wersin, P.; Soler, J.M. Exploring diffusion and sorption processes at the Mont Terri rock laboratory (Switzerland): Lessons learned from 20 years of field research. Swiss J. Geosci. 2017, 110, 391–403. [Google Scholar] [CrossRef]
- Van Loon, L.R.; Soler, J.M.; Müller, W.; Bradbury, M.H. Anisotropic Diffusion in Layered Argillaceous Rocks: A Case Study with Opalinus Clay. Environ. Sci. Technol. 2004, 38, 5721–5728. [Google Scholar] [CrossRef]
- Motellier, S.; Devol-Brown, I.; Savoye, S.; Thoby, D.; Alberto, J.C. Evaluation of tritiated water diffusion through the Toarcian clayey formation of the Tournemire experimental site (France). J. Contam. Hydrol. 2007, 94, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Davarzani, H.; Marcoux, M.; Costeseque, P.; Quintard, M. Experimental measurement of the effective diffusion and thermodiffusion coefficients for binary gas mixture in porous media. Chem. Eng. Sci. 2010, 65, 5092–5104. [Google Scholar] [CrossRef]
- Furry, W.H.; Jones, R.C.; Onsager, L. On the Theory of Isotope Separation by Thermal Diffusion. Phys. Rev. 1939, 55, 1083–1095. [Google Scholar] [CrossRef]
- Rosanne, R.; Paszkuta, M.; Tevissen, E.; Adler, P.M. Thermodiffusion in compact clays. J. Colloid Interface Sci. 2003, 267, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Tremosa, J.; Arcos, D.; Matray, J.M.; Bensenouci, F.; Gaucher, E.C.; Tournassat, C.; Hadi, J. Geochemical characterization and modelling of the Toarcian/Domerian porewater at the Tournemire underground research laboratory. Appl. Geochem. 2012, 27, 1417–1431. [Google Scholar] [CrossRef]
- Matray, J.M.; Savoye, S.; Cabrera, J. Desaturation and structure relationships around drifts excavated in the well-compacted Tournemire’s argillite (Aveyron, France). Eng. Geol. 2007, 90, 1–16. [Google Scholar] [CrossRef]
- Massmann, J. Modeling of Excavation Induced Coupled Hydraulic-Mechanical Processes in Claystone. 2009. Available online: https://www.osti.gov/etdeweb/biblio/21269578 (accessed on 9 April 2024).
- Cornet, F.H. 15—The HTPF and the Integrated Stress Determination Methods. In Rock Testing and Site Characterization; Hudson, J.A., Ed.; Pergamon Press Ltd.: Oxford, UK, 1993; pp. 413–432. [Google Scholar] [CrossRef]
- Savoye, S.; Michelot J luc Wittebroodt, C.; Altinier, M.V. Contribution of the diffusive exchange method to the characterization of pore-water in consolidated argillaceous rocks. J. Contam. Hydrol. 2006, 86, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Bonin, B. Deep geological disposal in argillaceous formations: Studies at the Tournemire test site. J. Contam. Hydrol. 1998, 35, 315–330. [Google Scholar] [CrossRef]
- Okay, G.O.N.C.A.; Cosenza, P.; Ghorbani, A.; Camerlynck, C.; Cabrera, J.; Florsch, N.; Revil, A. Localization and characterization of cracks in clay-rocks using frequency and time-domain induced polarization. Geophys. Prospect. 2013, 61, 134–152. [Google Scholar] [CrossRef]
- Pearson, F.J. What is the porosity of a mudrock? Geol. Soc. Lond. Spec. Publ. 1999, 158, 9–21. [Google Scholar] [CrossRef]
- Pearson, F.J.; Arcos, D.; Bath, A.; Boisson, J.Y.; Fernandez, A.M.; Gäbler, H.E.; Gaucher, E.; Gautschi, A.; Griffault, L.; Hernán, P.; et al. Mont Terri Project—Geochemistry of Water in the Opalinus Clay Formation at the Mont Terri Rock Laboratory. Bundesamt für Wasser und Geologie. Bern. 2003. Available online: https://www.admin.ch/gov/de/start/dokumentation/medienmitteilungen.msg-id-1375.html (accessed on 27 March 2024).
- Zhang, J. Oxygen Isotope Fractionation between Carbonate Minerals and Carbonic Acid Systems and Constraints for Environmental Science and Geological Processes. Molecules 2024, 29, 698. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.R.; Soler, J.M.; Jakob, A.; Bradbury, M.H. Effect of confining pressure on the diffusion of HTO, 36Cl− and 125I− in a layered argillaceous rock (Opalinus Clay): Diffusion perpendicular to the fabric. Appl. Geochem. 2003, 18, 1653–1662. [Google Scholar] [CrossRef]
- Van Loon, L.R.; Glaus, M.A.; Müller, W. Anion exclusion effects in compacted bentonites: Towards a better understanding of anion diffusion. Appl. Geochem. 2007, 22, 2536–2552. [Google Scholar] [CrossRef]
- Wigger, C.; Van Loon, L.R. Importance of Interlayer Equivalent Pores for Anion Diffusion in Clay-Rich Sedimentary Rocks. Environ. Sci. Technol. 2017, 51, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Savoye, S.; Michelot, J.L.; Wittebroodt, C. Evaluation of the reversibility of iodide uptake by argillaceous rocks by the radial diffusion method. Radiochim. Acta 2006, 94, 699–704. [Google Scholar] [CrossRef]
- van der Kamp, G.; Van Stempvoort, D.R.; Wassenaar, L.I. The Radial Diffusion Method: 1. Using intact cores to determine isotopic composition, chemistry, and effective porosities for groundwater in aquitards. Water Resour. Res. 1996, 32, 1815–1822. [Google Scholar] [CrossRef]
- De Hoog, F.R.; Knight, J.H.; Stokes, A.N. An Improved Method for Numerical Inversion of Laplace Transforms. SIAM J. Sci. Stat. Comput. 1982, 3, 357–366. [Google Scholar] [CrossRef]
- Patriarche, D.; Michelot, J.L.; Ledoux, E.; Savoye, S. Diffusion as the main process for mass transport in very low water content argillites: 1. Chloride as a natural tracer for mass transport—Diffusion coefficient and concentration measurements in interstitial water. Water Resour. Res. 2004, 40, W01516. [Google Scholar] [CrossRef]
- Bachir-Bey, N.; Matray, J.M. Origin of Halides (Cl− and Br−) and of Their Stable Isotopes (d37Cl and d81Br) at the Tournemire URL (France)—Experimental and Numerical Approach. Published online 1 May 2014. p. 2245. Available online: https://ui.adsabs.harvard.edu/abs/2014EGUGA..16.2245B/abstract (accessed on 27 March 2024).
- Wittebroodt, C. Transfert de l’iode dans l’argilite de Tournemire. 2009. Available online: https://www.irsn.fr/sites/default/files/documents/larecherche/formation_recherche/theses/theses-soutenues/dei/2009-these-wittebroodt.pdf (accessed on 27 March 2024).
- Savoye, S.; Frasca, B.; Grenut, B.; Fayette, A. How mobile is iodide in the Callovo–Oxfordian claystones under experimental conditions close to the in situ ones? J. Contam. Hydrol. 2012, 142–143, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Altinier, M.V. Etude de La Composition Isotopique Des Eaux Porales de l’argilite de Tournemire: Inter-Comparaison Des Méthodes de Mesure et Relations Avec Les Paramètres Pétrophysiques. Université Paris-Sud 11, Bures-sur-Yvette, France. 2006. Available online: https://theses.fr/2006PA112065 (accessed on 27 March 2024).
- LGR-ICOS Enhanced Performance Benchtop Analyzers GLA431 Series. Measurement Products. Available online: https://new.abb.com/products/measurement-products/analytical/laser-gas-analyzers/laser-analyzers/lgr-icos-enhanced-performance-benchtop-analyzers/lgr-icos-enhanced-performance-benchtop-analyzers-gla431-series (accessed on 27 March 2024).
- Gorny, J.; Jardin, C.; Diez, O.; Galceran, J.; Gourgiotis, A.; Happel, S.; Coppin, F.; Février, L.; Simonucci, C.; Cazala, C. Dissolved iodide in marine waters determined with Diffusive Gradients in Thin-films technique. Anal. Chim. Acta 2021, 1177, 338790. [Google Scholar] [CrossRef]
- Kerstel, E.T.; Gagliardi, G.; Gianfrani, L.; Meijer, H.A.J.; Van Trigt, R.; Ramaker, R. Simultaneous Determination of the 2H/1H, 17O/16O, and 18O/16O Isotope Abundance Ratios in Water by Means of Laser Spectrometry. Anal. Chem. 1999, 71, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Gianfrani, L.; Gagliardi, G.; Van Burgel, M.; Kerstel, E.T. Isotope analysis of water by means of near-infrared dual-wavelength diode laser spectroscopy. Opt. Express. OE 2003, 11, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Aminot, A.; Kerouel, R. Hydrologie des écosystèmes marins. In Paramètres et analyses. Ifremer MA0401; Ifremer: Plouzané, France, 2004. [Google Scholar]
- Mügler, C.; Filippi, M.; Montarnal, P.; Martinez, J.M.; Wileveau, Y. Determination of the thermal conductivity of opalinus clay via simulations of experiments performed at the Mont Terri underground laboratory. J. Appl. Geophys. 2006, 58, 112–129. [Google Scholar] [CrossRef]
- Fuchs, S.; Norden, B.; Peksa, R. GFZ Thermal Petrophysics Lab—Data Report 2024-01: Thermal Properties of the Shaly Jurassic (Upper Toarcian) Formation at the Tournemire Underground Research Lab (Tournemire, France). GFZ Data Services. 2024. Available online: https://dataservices.gfz-potsdam.de/portal/ (accessed on 27 March 2024).
- van Brakel, J.; Heertjes, P.M. Analysis of diffusion in macroporous media in terms of a porosity, a tortuosity and a constrictivity factor. Int. J. Heat Mass Transf. 1974, 17, 1093–1103. [Google Scholar] [CrossRef]
- Dullien, F.A.L. Porous Media: Fluid Transport and Pore Structure; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Appelo, C.A.J.; Van Loon, L.R.; Wersin, P. Multicomponent diffusion of a suite of tracers (HTO, Cl, Br, I, Na, Sr, Cs) in a single sample of Opalinus Clay. Geochim. Cosmochim. Acta 2010, 74, 1201–1219. [Google Scholar] [CrossRef]
- Savoye, S.; Goutelard, F.; Beaucaire, C.; Charles, Y.; Fayette, A.; Herbette, M.; Larabi, Y.; Coelho, D. Effect of temperature on the containment properties of argillaceous rocks: The case study of Callovo–Oxfordian claystones. J. Contam. Hydrol. 2011, 125, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.R.; Müller, W.; Iijima, K. Activation energies of the self-diffusion of HTO, 22Na+ and 36Cl− in a highly compacted argillaceous rock (Opalinus Clay). Appl. Geochem. 2005, 20, 961–972. [Google Scholar] [CrossRef]
- Bailly, D.; Matray, J.M.; Ababou, R. Temporal behavior of a ventilated claystone at the Tournemire URL: Cross-spectral analyses focused on daily harmonics. Eng. Geol. 2014, 183, 137–158. [Google Scholar] [CrossRef]
- Boudreau, B. Diagenetic Models and Their Implementation: Modelling Transport and Reactions in Aquatic Sediments. 2 January 2013. Available online: https://www.researchgate.net/profile/Bernard-Boudreau/publication/235335761_Diagenetic_models_and_their_implementation_modelling_transport_and_reactions_in_aquatic_sediments/links/0fcfd510fd916cfdde000000/Diagenetic-models-and-their-implementation-modelling-transport-and-reactions-in-aquatic-sediments.pdf (accessed on 27 March 2024).




| Upper Toarcian (Reference Level) | |
|---|---|
| Parameter | Range |
| Mineralogy [23] | |
| Clay fraction (illite, I/S R1, chlorite, kaolinite) [wt %] | 44–87 |
| Mica, Illite | 7–22 |
| Chlorite [wt %] | 1–7 |
| Kaolinite [wt %] | 8–15 |
| Illite/Smectite mixed layers–R1 type [wt %] | 28–43 |
| Quartz + Opal [wt %] | 17–22 |
| Calcite + Dolomite/Ankerite [wt %] | 11–19 |
| Pyrite, Siderite, Fe Oxy-hydroxides [wt %] | 2–4 |
| Total Organic Carbon [wt %] | 0.9 |
| Petrophysical properties [24] | |
| Total (Physical) & water loss porosity [vol %] | 9–10 |
| Water content [wt %] | 3–4 |
| Density (humid) [g/cm3] | 2.50–2.60 |
| Grain density [g/cm3] | 2.70–2.71 |
| Specific surface area (N2 BET + BJH) [m2/g] | 22–26 |
| Mechanical properties [13,25,26] | |
| Elastic module E-module (P) [GPa] | 20–28 |
| Elastic module E-module (N) [GPa] | 5–13 |
| Uniaxial compressive strength (P) [MPa] | 57 |
| Uniaxial compressive strength (N) [MPa] | 20 |
| Free swelling (P) [%] | <1 |
| Free swelling (N) [%] | 2% |
| Stess field σh [MPa] | 1.1–3.1 |
| Stress field σv [MPa] | 3.4–4.2 |
| Stress field σH [Mpa] | 2–6 |
| Intrinsic permeability (k) [m2] | 10−21–10−22 |
| Elastic module E-module (P) [GPa] | 20–28 |
| Elastic module E-module (N) [GPa] | 5–13 |
| Uniaxial compressive strength (P) [MPa] | 57 |
| Uniaxial compressive strength (N) [MPa] | 20 |
| Free swelling (P) [%] | <1 |
| Free swelling (N) [%] | 2 |
| Geochemical properties [23] | |
| Total Exchange Capacity (CsCl) [meq/100 g rock] | 8.6–9.0 |
| Exchangeable cations [meq/100 g rock] | Na+: 1.7; K+: 1.2; Ca2+: 3.8; Mg2+: 1.9; CEC: 8.6 |
| Porewater composition [mmol L−1] | Alkalinity: 4.43; SO42−: 9.5; Cl−: 4.5; Ca2+: 1.5; Mg2+: 0.75; Na+: 22.6; K+: 0.77 |
| Porewater composition—other properties | pH: 7.74; I: 0.035; pCO2: 10−2.6 atm; TDS: 2 g/L |
| Water and mass transport properties [19,24,27] | |
| Pore pressure [MPa] | 0.6 |
| Hydraulic conductivity (K) [m/s] | 10−14–10−15 |
| Specific storativity (Ss) [1/m] | 5 × 10−7–10−6 |
| Anions accessible porosity [vol. %] | 2.8–4.7 |
| Cl− and Br− effective diffusion coefficient (DeCl,Br) (P) [m2/s] | 1.2 × 10−12–2 × 10−12 |
| I− effective diffusion coefficient (DeI) (P) [m2/s] | 7 × 10−13–15 × 10−12 |
| Water tracer effective diffusion coefficient (DeW) (P) [m2/s] | 1.7 × 10−11–3 × 10−11 |
| Anion exclusion (DeCl,Br/DeW) | 0.12–0.15 |
| Thermal properties [28] | |
| Thermal conductivity (N) [W/(mK)] | 1.6–1.8 |
| Heat capacity [J/(m3K)] | 1.8–1.9 × 106 |
| ID | Distance from the Well Wall | Drilling Date | Depth/Ground Level | Drilling Method | Borehole out Diameter | Core Diameter | Equipment (Supplier) |
|---|---|---|---|---|---|---|---|
| cm | m | mm | mm | ||||
| DGT-EX1 | 0 | 24 February 2022 | 3 | air-cored | 101 | 96 | None |
| DGT-REF | 250 | 24 February 2022 | 3.5 | 101 | 96 | PT100 (Socotec) + Conductivity probe (WTW) | |
| DGT-TD1 | 10 | 3 May 2022 | 46 | 35 | PT100 at 3 m and at 3.5 m + Sensolux optic fiber | ||
| DGT-TD2 | 20 | 4 May 2022 | 46 | 35 | PT100 at 3 m and at 3.5 m + Sensolux optic fiber | ||
| DGT-TD3 | 30 | 5 May 2022 | 46 | 35 | PT100 at 3 m and at 3.5 m + Sensolux optic fiber | ||
| DGT-PP1 | 50 | 9 May 2022 | 66 (down to 3.38 m) + 45 (down to 3.5 m) | 60 + 35 | Absolute pressure probe (2 MPa max, Sisgeo) + PT1000 (Sisgeo) at 3.5 m | ||
| DGT-PP2 | 100 | 10 May 2022 | 66 (down to 3.38 m) + 45 (down to 3.5 m) | 61 + 35 | Absolute pressure probe (2 MPa max, Sisgeo) + PT1000 (Sisgeo) at 3.5 m | ||
| DGT-PP3 | 150 | 11 May 2022 | 66 (down to 3.38 m) + 45 (down to 3.5 m) | 62 + 35 | Absolute pressure probe (2 MPa max, Sisgeo) + PT1000 (Sisgeo) at 3.5 m | ||
| DGT-INS | 100 | 7 July 2022 | 3+ | 101 | 96 | 2 PT1000 (Sisgeo) + 2 PT100 (Socotec) + 1 Tetracon probe (WTW) + 1 Sensolux sensor (Socotec) |
| Mean Distance from the Borehole Mouth | Bulk Wet Density | Dry Density | Grain Density by Helium Pycnometry | Degree of Saturation | Gravimetric Water Content | Total Porosity | Volumetric Moisture Content | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +/− | rh | σrh | rd | σrd | rs | σrs | S | σS | WCdry,105 °C | σWCdry,105 °C | ntot | σntot | q | σq | |
| cm | cm | g cm−3 | g cm−3 | g cm−3 | g cm−3 | g cm−3 | g cm−3 | % DS | % DS | % DS | % DS | % DS | % DS | % DS | % DS |
| 291.5 | 0.5 | 2.540 | 0.002 | 2.439 | 0.002 | 2.702 | 0.001 | 95.91 | 1.22 | 3.85 | 0.02 | 9.71 | 0.12 | 9.31 | 0.16 |
| 291.5 | 0.5 | 2.541 | 0.002 | 2.441 | 0.002 | 2.706 | 0.003 | 94.56 | 0.99 | 3.81 | 0.02 | 9.77 | 0.08 | 9.24 | 0.13 |
| 292.5 | 0.5 | 2.535 | 0.002 | 2.434 | 0.003 | 2.716 | 0.001 | 90.38 | 1.35 | 3.89 | 0.04 | 10.40 | 0.10 | 9.40 | 0.17 |
| 293.5 | 0.5 | 2.533 | 0.002 | 2.429 | 0.002 | 2.697 | 0.001 | 95.47 | 1.00 | 3.94 | 0.02 | 9.96 | 0.09 | 9.50 | 0.13 |
| 294.5 | 0.5 | 2.536 | 0.002 | 2.437 | 0.003 | 2.715 | 0.004 | 88.65 | 1.80 | 3.76 | 0.04 | 10.24 | 0.17 | 9.08 | 0.24 |
| 295.5 | 0.5 | 2.536 | 0.002 | 2.433 | 0.002 | 2.704 | 0.001 | 97.02 | 0.81 | 4.04 | 0.01 | 10.04 | 0.08 | 9.74 | 0.11 |
| 295.5 | 0.5 | 2.541 | 0.002 | 2.440 | 0.002 | 2.696 | 0.000 | 97.85 | 0.96 | 3.84 | 0.02 | 9.49 | 0.08 | 9.29 | 0.12 |
| 296.5 | 0.5 | 2.546 | 0.002 | 2.450 | 0.002 | 2.696 | 0.004 | 98.68 | 1.69 | 3.72 | 0.01 | 9.15 | 0.15 | 9.03 | 0.22 |
| 296.5 | 0.5 | 2.537 | 0.002 | 2.435 | 0.002 | 2.727 | 0.001 | 88.19 | 0.73 | 3.90 | 0.02 | 10.70 | 0.08 | 9.43 | 0.10 |
| 297.5 | 0.5 | 2.537 | 0.002 | 2.435 | 0.002 | 2.701 | 0.001 | 95.11 | 0.79 | 3.86 | 0.01 | 9.82 | 0.07 | 9.34 | 0.10 |
| 298.5 | 0.5 | 2.540 | 0.002 | 2.443 | 0.002 | 2.683 | 0.001 | 94.29 | 1.05 | 3.47 | 0.02 | 8.93 | 0.09 | 8.42 | 0.12 |
| 298.5 | 0.5 | 2.534 | 0.002 | 2.440 | 0.002 | 2.689 | 0.002 | 91.52 | 1.02 | 3.51 | 0.02 | 9.27 | 0.09 | 8.49 | 0.13 |
| mean values | |||||||||||||||
| 295.2 | 0.5 | 2.538 | 0.002 | 2.438 | 0.002 | 2.703 | 0.002 | 93.97 | 1.12 | 3.80 | 0.02 | 9.79 | 0.10 | 9.19 | 0.14 |
| Analytical Method: IC | ||||
|---|---|---|---|---|
| Cell | Depth | Core | [Cl−]pw | σCl−pw |
| cm | orientation | mmol L−1 | mmol L−1 | |
| DS_1 | 294 | ┴ strat | 3.72 | 0.0225 |
| DS_2 | 295 | 3.42 | 0.0211 | |
| DS_3 | 296 | 3.92 | 0.0245 | |
| DS_4 | 298 | 3.42 | 0.0216 | |
| DS_5 | 299 | 3.84 | 0.0244 | |
| mean | 296 | 3.66 | 0.0228 | |
| Analytical Method: ICP-MS | ||||
| Cell | Depth | Core | [Br−]pw | σBr−pw |
| cm | orientation | mmol L−1 | mmol L−1 | |
| DS_1 | 294 | ┴ strat | 0.029 | 0.000183 |
| DS_2 | 295 | 0.031 | 0.000188 | |
| DS_3 | 296 | 0.026 | 0.000161 | |
| DS_4 | 298 | 0.029 | 0.000185 | |
| DS_5 | 299 | 0.029 | 0.000177 | |
| mean | 296 | 0.029 | 0.000179 | |
| Analytical Method: ICP-MS | ||||
| Cell | Depth | Core | [I−]pw | σI−pw |
| cm | orientation | mmol L−1 | mmol L−1 | |
| DS_1 | 294 | ┴ strat | 0.00154 | 0.0000148 |
| DS_2 | 295 | 0.00177 | 0.0000165 | |
| DS_3 | 296 | 0.00168 | 0.0000166 | |
| DS_4 | 298 | 0.00199 | 0.0000184 | |
| DS_5 | 299 | 0.00178 | 0.0000166 | |
| mean | 296 | 0.00175 | 0.0000166 | |
| Cell | Depth cm | δ²Hpw ‰ vs. V-SMOW | Water Content wt % |
|---|---|---|---|
| PV1 | 245 | −42.1 | 5.20 |
| PV2 | 249 | −42.5 | 4.88 |
| PV3 | 255 | −42.9 | 4.61 |
| PV4 | 260 | −41.2 | 4.59 |
| PV5 | 265 | −42.2 | 4.91 |
| PV6 | 270 | −42.5 | 4.75 |
| PV7 | 274 | −42.9 | 4.73 |
| PV8 | 321 | −42.9 | 5.25 |
| PV9 | 337 | −43.1 | 5.16 |
| PV10 | 338.5 | −43.3 | 5.05 |
| PV11 | 340 | −42.9 | 5.07 |
| PV12 | 341.5 | −42.5 | 4.76 |
| PV13 | 342 | −41.7 | 5.14 |
| Mean | 295.2 | −42.5 | 4.9 |
| Std. Dev. | 41.0 | 0.588 | 0.229 |
| Analytical Method: IC | ||||
|---|---|---|---|---|
| Cell | Depth | Core | [Cl−]pw | σCl−pw |
| cm | orientation | mmol L−1 | mmol L−1 | |
| E1 | 301 | ┴ strat | 435.48 | 2.53 |
| E2 | 302 | 216.21 | 1.25 | |
| E3 | 303 | 114.16 | 0.65 | |
| E4 | 304 | 50.34 | 0.28 | |
| E5 | 305 | 26.71 | 0.15 | |
| E6 | 306 | 6.24 | 0.04 | |
| E7 | 307 | 4.18 | 0.02 | |
| E9 | 309 | 4.37 | 0.03 | |
| mean | 303 | 168.58 | 1.1458 | |
| Cell | Depth | Core | [Br−]pw | σBr−pw |
| cm | orientation | mmol L−1 | mmol L−1 | |
| E1 | 301 | ┴ strat | 41.514 | 0.238 |
| E2 | 302 | 19.815 | 0.114 | |
| E3 | 303 | 10.206 | 0.059 | |
| E4 | 304 | 3.596 | ||
| E5 | 305 | 0.978 | 0.006 | |
| E6 | 306 | 0.110 | 0.001 | |
| E7 | 307 | 0.023 | 0.0001 | |
| E9 | 309 | 0.045 | 0.0003 | |
| mean | 303 | 15.22 | 0.1042 | |
| Cell | Depth | Core | [I−]pw | σI−pw |
| cm | orientation | mmol L−1 | mmol L−1 | |
| E1 | 301 | ┴ strat | 40.95 | 0.239 |
| E2 | 302 | 18.65 | 0.108 | |
| E3 | 303 | 7.72 | 0.044 | |
| E4 | 304 | 1.50 | 0.02 | |
| E5 | 305 | 0.21 | 0.001 | |
| E6 | 306 | 0.1034 | 0.000030 | |
| E7 | 307 | 0.0012 | 0.000012 | |
| E9 | 309 | 0.0041 | 0.000030 | |
| mean | 303 | 13.81 | 0.0981 | |
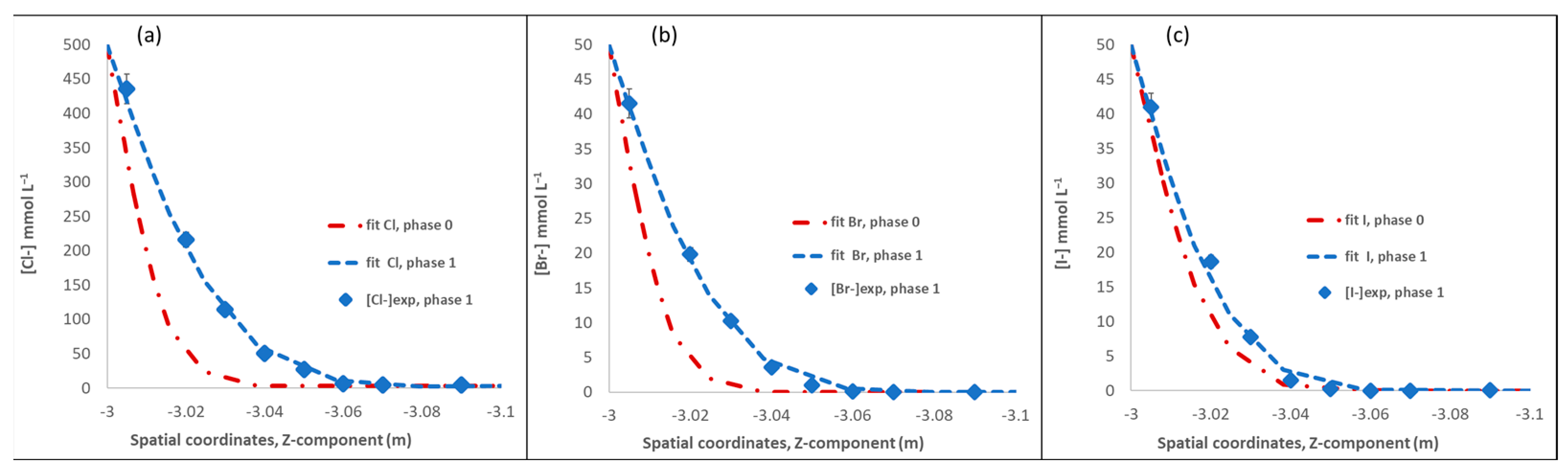
| Title | Phase 0 | Phase 1 | ||
|---|---|---|---|---|
| De | ωacc | De | ωacc | |
| (m2·s−1) × 10−12 | % | (m2 s−1) × 10−12 | % | |
| Cl− | 2.50 | 5.5 | 10.5 | 23 |
| Br− | 2.58 | 5.8 | 9.76 | 21 |
| I− | 4.03 | 4.9 | 6.43 | 17 |
| 2H | 24.8 | 11.5 | currently being acquired | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humbezi Desfeux, M.; Marcoux, M.; Matray, J.-M.; Gorny, J.; Schädle, P.; Pochet, G. DIGIT: An In Situ Experiment for Studying the Diffusion of Water and Solutes under Thermal Gradient in the Toarcian Clay Rock at the Tournemire Underground Research Laboratory: Part 1—Goals, Scoping Calculations, Installation and First Results under Unheated Conditions. Minerals 2024, 14, 563. https://doi.org/10.3390/min14060563
Humbezi Desfeux M, Marcoux M, Matray J-M, Gorny J, Schädle P, Pochet G. DIGIT: An In Situ Experiment for Studying the Diffusion of Water and Solutes under Thermal Gradient in the Toarcian Clay Rock at the Tournemire Underground Research Laboratory: Part 1—Goals, Scoping Calculations, Installation and First Results under Unheated Conditions. Minerals. 2024; 14(6):563. https://doi.org/10.3390/min14060563
Chicago/Turabian StyleHumbezi Desfeux, Maïwenn, Manuel Marcoux, Jean-Michel Matray, Josselin Gorny, Philipp Schädle, and Guillaume Pochet. 2024. "DIGIT: An In Situ Experiment for Studying the Diffusion of Water and Solutes under Thermal Gradient in the Toarcian Clay Rock at the Tournemire Underground Research Laboratory: Part 1—Goals, Scoping Calculations, Installation and First Results under Unheated Conditions" Minerals 14, no. 6: 563. https://doi.org/10.3390/min14060563
APA StyleHumbezi Desfeux, M., Marcoux, M., Matray, J.-M., Gorny, J., Schädle, P., & Pochet, G. (2024). DIGIT: An In Situ Experiment for Studying the Diffusion of Water and Solutes under Thermal Gradient in the Toarcian Clay Rock at the Tournemire Underground Research Laboratory: Part 1—Goals, Scoping Calculations, Installation and First Results under Unheated Conditions. Minerals, 14(6), 563. https://doi.org/10.3390/min14060563







