Assessing the Performance of CO2-Mineralized Underground Backfilling Materials through the Variation Characteristics of Infrared Radiation Temperature Index
Abstract
:1. Introduction
2. Materials and Methods
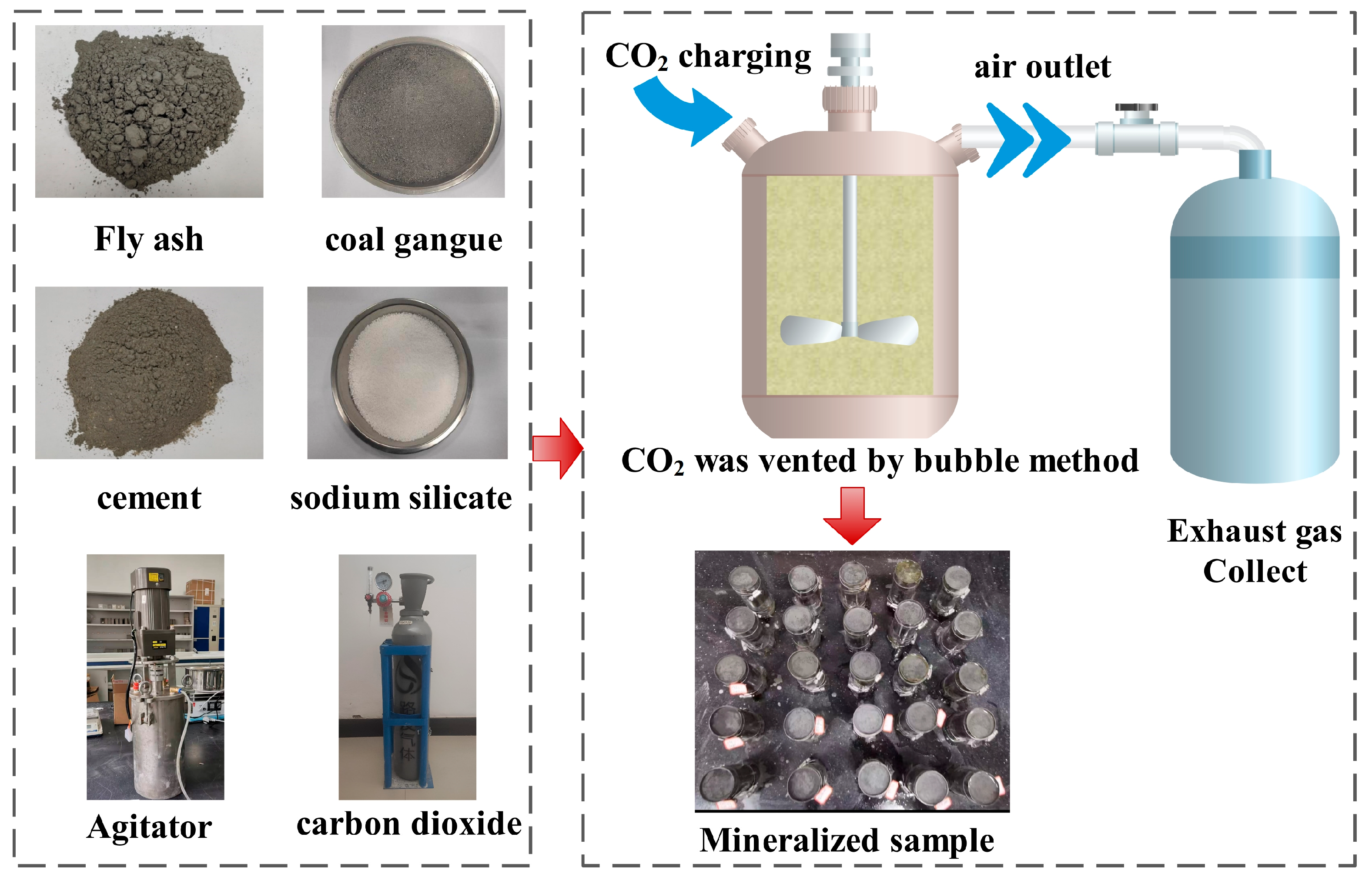
2.1. Sample Making
2.2. Experimental Steps
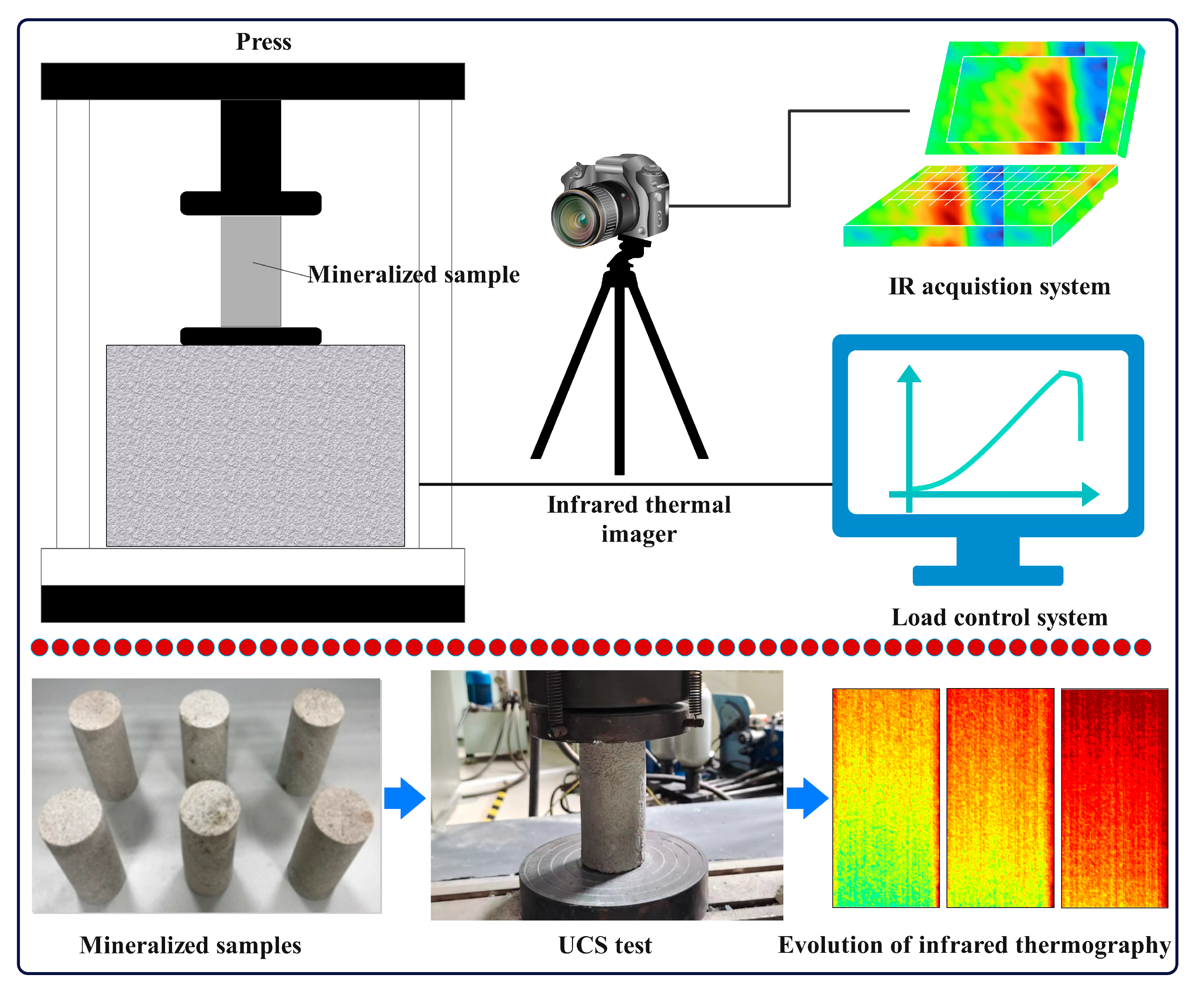
2.3. Test Equipment
2.4. Test Method
3. Experimental Results
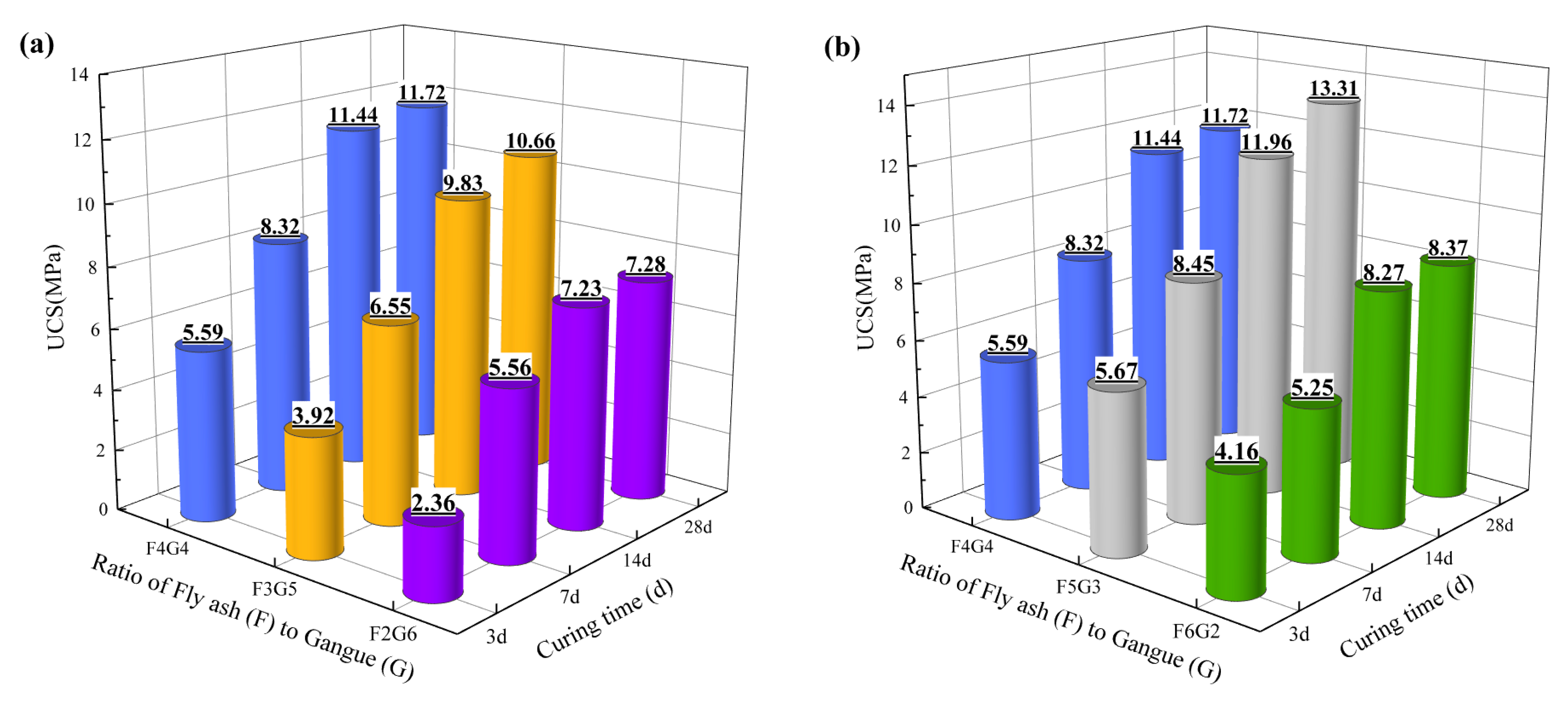
3.1. Unconfined Compressive Strength (UCS)
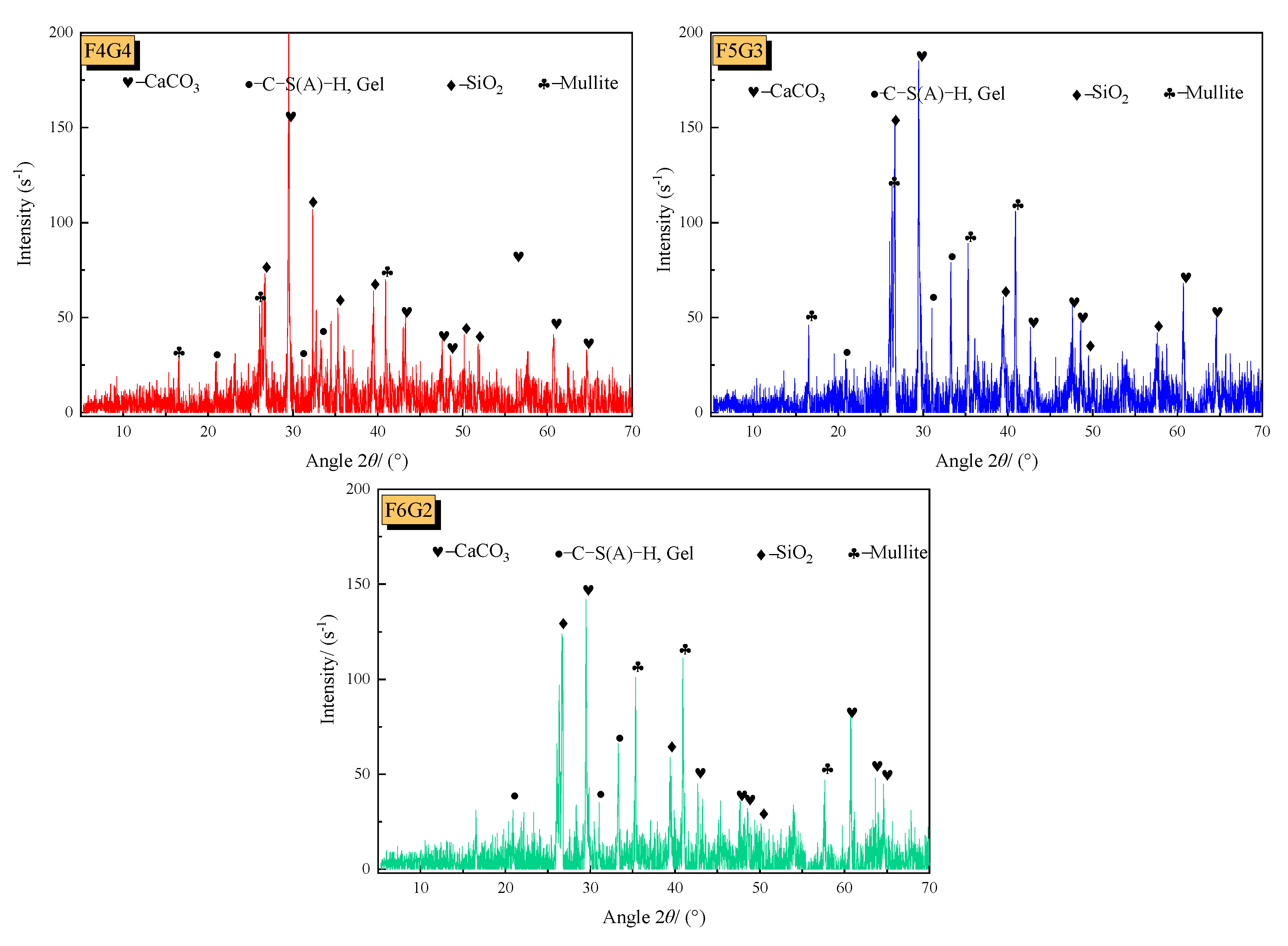
3.2. X-ray Diffraction (XRD)
3.3. Average Infrared Radiation Temperature (AIRT)
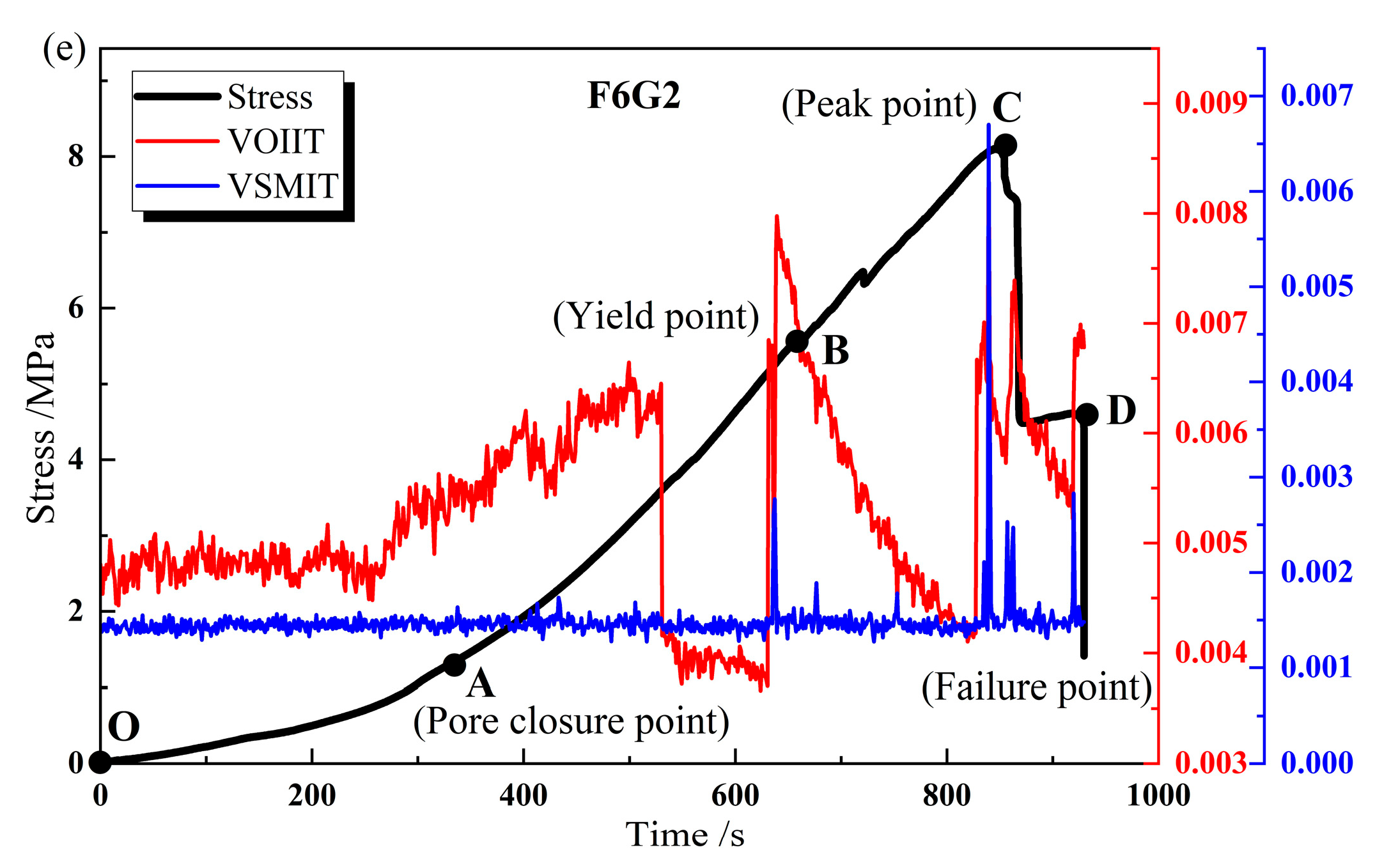
3.4. Variance of Original Infrared Image Temperature (VOIIT) and Variance of Successive Minus Infrared Image Temperature (VSMIT)
4. Mineralized Material Underground Backfilling Method
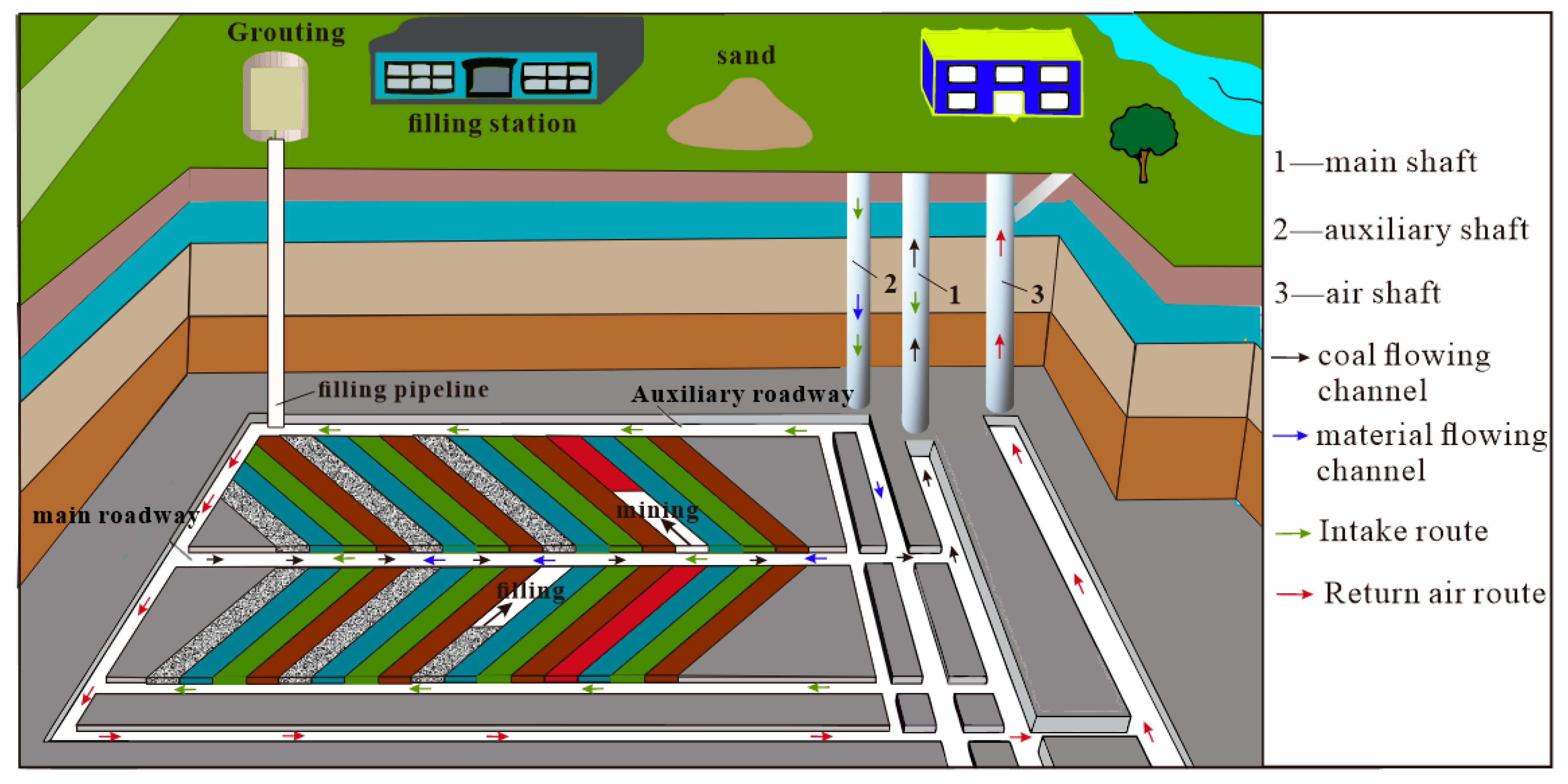
4.1. Working Face Layout
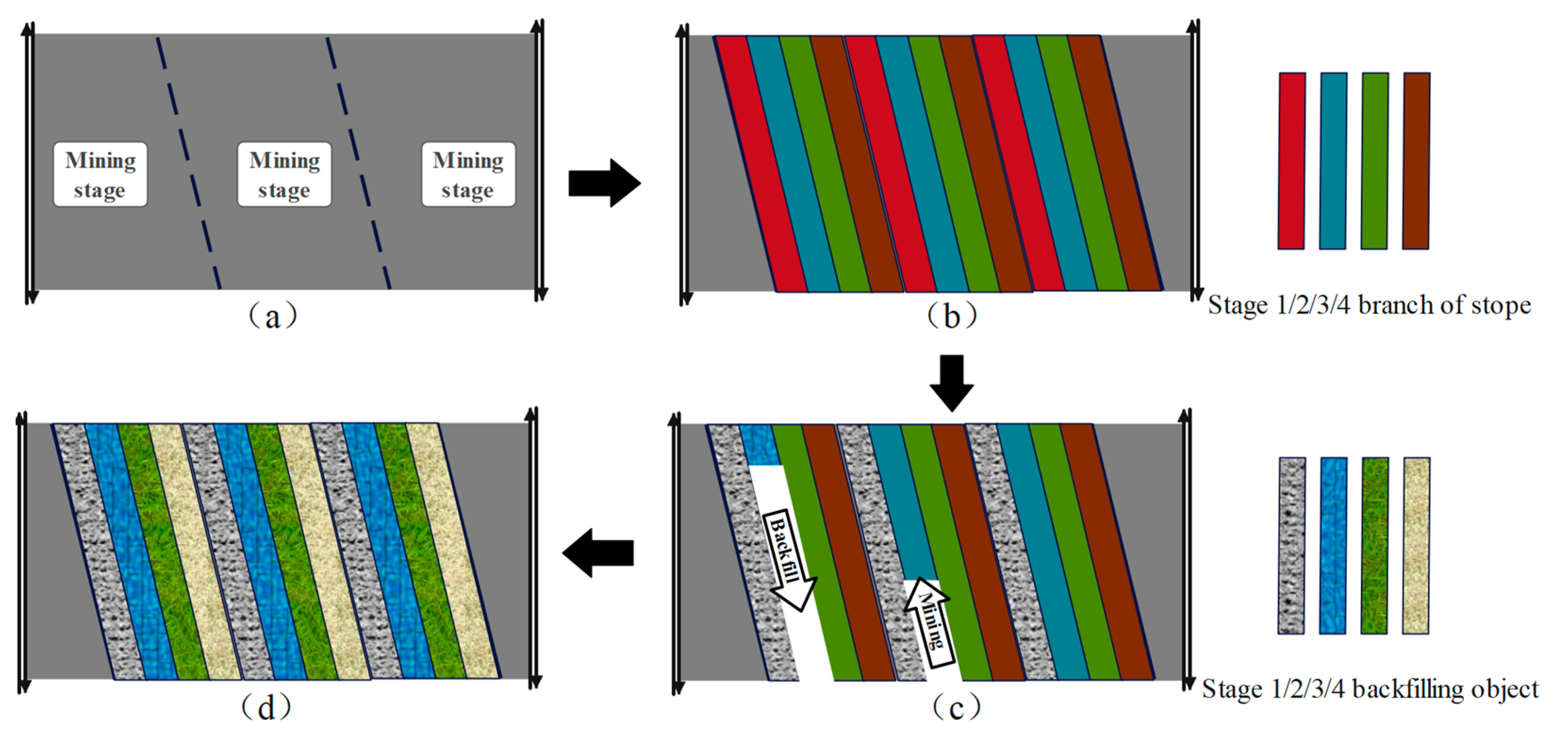
4.2. Mining Sequence in the Branch Roadway of the Stope
4.2.1. Mining Sequence of Stope Branches
4.2.2. Coal Mining Operation in the Branch of the Stope
4.2.3. Backfilling Operation in the Branch of the Stope
4.3. Production System
4.3.1. Transportation System
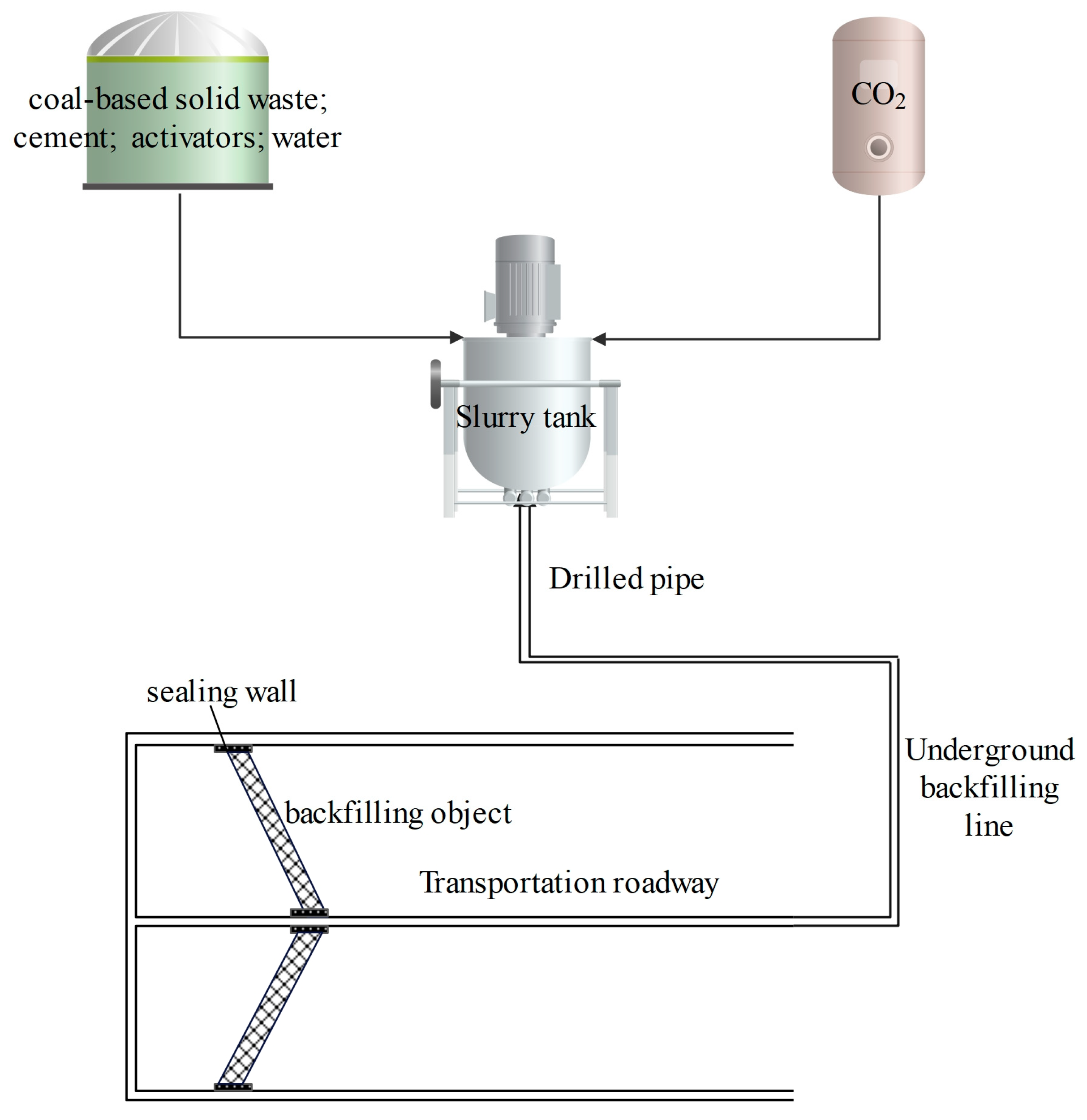
4.3.2. Backfill System
5. Discussion
5.1. Further Prospects
5.1.1. Optimize the CO2-Mineralized Coal-Based Solid Waste Program
5.1.2. Temperature Change in the Reaction Stage of the CO2-Mineralization of Coal-Based Solid Waste
5.2. Advantages and Disadvantages of Different Infrared Indicators
5.3. Temperature Change Mechanism of the Backfill Rupture Process
5.3.1. Thermal Effect during Pore Compaction Stage
5.3.2. Thermal Effect during Elastic Deformation
5.3.3. Thermal Effect in Plastic Deformation and Failure Stage
6. Conclusions
- (1)
- The UCS gradually increases with curing time, reaching basic stability at 14 days, with minimal changes in strength at 28 days compared to 14 days. Under the same curing time, the UCS of the F2G6 scheme is lower, while the mineralization effects of the F3G5, F4G4, and F5G3 schemes are relatively better; in particular, the UCS of the F5G3 scheme can reach up to 13.31 MPa, indicating that an increase in the ratio of fly ash (F) to coal gangue (G) can improve the strength of mineralized specimens to a certain extent. The XRD results indicate that hydration reactions ultimately produce gel structure (C-S-H) and calcium carbonate (CaCO3), both of which can effectively improve the strength of mineralized specimens.
- (2)
- The average infrared radiation temperature (AIRT) of mineralized samples exhibits a continuous rise during the loading process, and some samples demonstrate a sudden decrease in the AIRT during the severe damage stage, indicating a strong positive correlation between the AIRT and loading stress. In addition, the increase in the AIRT amplitude is mostly observed to rise with the increase in the ratio of F and G, with the highest temperature rise of up to 0.83 °C observed in the F5G3 sample, indicating that F exhibits larger temperature variations compared to G during the loading process.
- (3)
- The variance of original infrared image temperature (VOIIT) and the variance of successive minus infrared image temperature (VSMIT) can be used as sensitive response indicators for sample rupture. When the indicators fluctuate violently or rise rapidly, they reflect the expansion of internal cracks in the sample, proving that they can serve as precursors to severe sample failure.
- (4)
- The construction of parallel backfilling space for mining and backfilling can ensure that the branch roadways of the same mining stage are spaced apart from each other, and the coal mining and backfilling are always in an independent and stable working space, achieving efficient mining of coal resources, rapid backfilling of mineralized materials, and controlling the movement of overlying strata.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xi, X.; Sang, S.; Liu, S. Progress in research of CO2 fixation and sequestration by coal mine solid waste mineralization and co-disposal of pollution and carbon reduction. J. China Coal Soc. 2023, 1075, 1–16. (In Chinese) [Google Scholar]
- Seifritz, W. CO2 Disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Mazzella, A.; Errico, M.; Spiga, D. CO2 uptake capacity of coal fly ash: Influence of pressure and temperature on direct gas-solid carbonation. J. Environ. Chem. Eng. 2016, 4, 4120–4128. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R.; Prigiobbe, V. Comparison of different reaction routes for carbonation of APC residues. Energy Procedia 2009, 1, 4851–4858. [Google Scholar] [CrossRef]
- Mayoral, M.C.; Andrés, J.M.; Gimeno, M.P. Optimization of mineral carbonation process for CO2 sequestration by lime-rich coal ashes. Fuel 2013, 106, 448–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Liu, Y.; Li, H.; Wang, Z.; Lang, Z. Comparison of three typical industrial solid wastes on the performance of CO2 mineralization and sequestration. Chin. J. Environ. Eng. 2021, 15, 2344–2355. (In Chinese) [Google Scholar]
- Ren, G.; Liao, H.; Wu, H.; Gao, H.; Yan, Z.; Cheng, F. Carbonation characteristics of fly ash, carbide slag and their mixtures. Chin. J. Environ. Eng. 2018, 12, 2295–2300. (In Chinese) [Google Scholar]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Chu, H.W.; Wang, C.F.; Chiang, P.C. CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor. J. Hazard. Mater. 2011, 195, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhai, J.; Ngo, I. Experimental study on preparation of negative carbon filling material for water protection mining by CO2 mineralization of coal-based solid waste. J. China Coal Soc. 2022, 47, 4228–4236. (In Chinese) [Google Scholar]
- Devaraj, V.; Mangottiri, V.; Balu, S. Prospects of sustainable geotechnical applications of manufactured sand slurry as controlled low strength material. Constr. Build. Mater. 2023, 400, 132747. [Google Scholar] [CrossRef]
- Devaraj, V.; Mangottiri, V.; Balu, S. Sustainable Approach for Using M-Sand Slurry as a Controlled Low Strength Material. J. Solid Waste Technol. Manag. 2023, 49, 333–339. [Google Scholar] [CrossRef]
- Do, T.M.; Kang, G.O.; Go, G.H.; Kim, Y.S. Evaluation of Coal Ash–Based CLSM Made with Cementless Binder as a Thermal Grout for Borehole Heat Exchangers. J. Mater. Civ. Eng. 2019, 31, 04019027. [Google Scholar] [CrossRef]
- Devaraj, V.; Mangottiri, V.; Balu, S. Sustainable utilization of industrial wastes in controlled low-strength materials: A review. Environ. Sci. Pollut. Res. 2023, 30, 14008–14028. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yuan, N.; Wang, S.; Hao, X.; Zhang, X.; Wang, D.; Yang, X. The Effect of Bottom Ash Ball-Milling Time on Properties of Controlled Low-Strength Material Using Multi-Component Coal-Based Solid Wastes. Sustainability 2022, 14, 9949. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, N.; Wang, S.; Zhang, X.; Lin, C.; Wu, X.; Wang, Q.; Wang, D. Optimization of Controlled Low-Strength Material from Multi-Component Coal-Based Solid Waste. Sustainability 2024, 16, 1513. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Elsworth, D. Morphological complexity and azimuthal disorder of evolving pore space in low-maturity oil shale during in-situ thermal upgrading and impacts on permeability. Pet. Sci. 2024, 03.020, 1–13. [Google Scholar] [CrossRef]
- Das, S.K.; Mahamaya, M.; Reddy, K.R. Coal mine overburden soft shale as a controlled low strength Material. Int. J. Min. Reclam. Environ. 2020, 34, 725–747. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Z.; Guan, W.; Wang, W.; Xu, X.; Song, Y.; Liu, H.; Su, H.; Zhao, B.; Hou, D. Review of the Backfill Materials in Chinese Underground Coal Mining. Minerals 2023, 13, 473. [Google Scholar] [CrossRef]
- Lai, X.; Sun, H.; Shan, P.; Wang, C.; Cui, N.; Yang, Y. Acoustic emission and temperature variation in failure process of hard rock pillars sandwiched between thick coal seams of extremely steep. Chin. J. Rock Mech. Eng. 2015, 34, 2285–2292. (In Chinese) [Google Scholar]
- Wang, S.; Sun, Q.; Qiao, J.; Wang, S. Geological guarantee of coal green mining. J. China Coal Soc. 2020, 45, 8–15. (In Chinese) [Google Scholar]
- Wu, Q.; Tu, K.; Zeng, Y.; Liu, S. Discussion on the main problems and countermeasures for building an upgrade version of main energy (coal) industry in China. J. China Coal Soc. 2019, 44, 1625–1636. (In Chinese) [Google Scholar]
- Zhang, Q.; Zhang, J.; Wu, Z.; Chen, Y.; Xia, K.; Liu, H. Function relational expression of backfilled body’s density and its influencing factors. Energy Sources 2019, 43, 2218–2234. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.; Cho, W.; Choi, H.; Cho, G. A Comparative Evaluation of Stress–Strain and Acoustic Emission Methods for Quantitative Damage Assessments of Brittle Rock. Rock Mech. Rock Eng. 2015, 48, 495–508. [Google Scholar] [CrossRef]
- Wu, X.; Gao, X.; Liu, X.; Zhao, K. Abnormality of infrared temperature mutation in the process of saturated siltstone failure. J. China Coal Soc. 2015, 40, 328–336. (In Chinese) [Google Scholar]
- Zhang, D.; Fan, G.; Liu, Y.; Ma, L. Field trials of aquifer protection in longwall mining of shallow coal seams in China. Int. J. Rock Mech. Min. Sci. 2010, 47, 908–914. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.; He, M.; Zhang, F. Experimental investigation of the occurrence of rockburst in a rock specimen through infrared thermography and acoustic emission. Int. J. Rock Mech. Min. Sci. 2017, 93, 250–259. [Google Scholar] [CrossRef]
- Cao, K.; Dong, F.; Liu, W.; Khan, N.; Cui, R.; Li, X.; Hussain, S.; Alarifi, S.; Niu, D. Infrared radiation denoising model of “sub-region-Gaussian kernel function” in the process of sandstone loading and fracture. Infrared Phys. Technol. 2023, 129, 104583. [Google Scholar] [CrossRef]
- Blokhin, D.I.; Sheinin, V.I. Thermomechancal effects in different geomaterials in limiting behavior of cyclic loading. IOP Conf. Ser. Earth Environ. Sci. 2021, 773, 012055. [Google Scholar] [CrossRef]
- Ngo, I.; Ma, L.; Zhai, J.; Wang, Y.; Wei, T.; Ni, Y. Experimental Investigation of CO2-Induced Silica Gel as the Water Blocking Grout Effect of Aquifer Ions. ACS Omega 2022, 7, 27090–27101. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Zhou, C.; Song, L.; Ma, K.; Yue, H. Gas and slurry flow behaviors in a CO2-mineralization spray tower reactor. ALCHE J. 2023, 70, e18329. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, W.; Cheng, W.; Qi, G.; Hu, X.; Su, H.; Wang, M.; Zhang, M.; Liang, Y. Method for rapid mineralization of CO2 with carbide slag in the constant-pressure and continuous-feed way and its reaction heat. Powder Technol. 2022, 398, 117148. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Tian, Y.; Meng, Y.; Chen, H.; Shi, W. The Effect of Alkali Activator on the Properties of Super Sulfate Cement and Concrete. Fly Ash Compr. Util. 2020, 34, 1–6. (In Chinese) [Google Scholar]
- Luo, X.; Wei, C.; Li, X.; Deng, Z.; Li, M.; Fan, G. A green approach to prepare polymorph CaCO3 for clean utilization of salt gypsum residue and CO2 mineralization. Fuel 2023, 333, 126305. [Google Scholar] [CrossRef]
- Hao, T.; Li, F.; Tang, Y.; Wang, Z.; Zhao, L. Infrared radiation characterization of damaged coal rupture based on stress distribution and energy. ACS Omega 2022, 7, 28545–28555. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, W.D.; Ekaputri, J.J.; Fansuri, H. Combination of precipitated-calcium carbonate substitution and dilute-alkali fly ash treatment in a very high-volume fly ash cement paste. Constr. Build. Mater. 2020, 234, 117273. [Google Scholar] [CrossRef]
- Ling, Q.; Mao, X.; Cui, Y.; Bao, J.; Sant, G.; Chen, T.; Zhang, P.; Gao, X.; Bauchy, M. New insights into the early stage nucleation of calcium carbonate gels by reactive molecular dynamics simulations. J. Chem. Phys. 2022, 157, 234501. [Google Scholar]
- Ho, H.J.; Lizuka, A.; Shibata, E. Utilization of low-calcium fly ash via direct aqueous carbonation with a low-energy input: Determination of carbonation reaction and evaluation of the potential for CO2 sequestration and utilization. J. Environ. Manag. 2021, 288, 112411. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Qiao, L.; Li, Q.; Hao, J.; Wu, S. A Method to Predict Rock Fracture with Infrared Thermography Based on Heat Diffusion Analysis. Geofluids 2021, 2021, 6669016. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Li, X. Coupling Study of Acoustic Emission and Infrared Radiation in Sandstone under Water-Rock Interaction. J. Yangtze River Sci. Res. Inst. 2022, 39, 127–133. (In Chinese) [Google Scholar]
- Li, F.; Hao, T.; Wang, G.; Tang, Y.; Yuan, M.; Zhao, L. Mechanical Properties and Fractal Characteristics of Fractured Coal Under Different Gas Pressures. Nat. Resour. Res. 2024, 33, 793–812. [Google Scholar] [CrossRef]
- Ma, L.; Sun, H.; Zhang, Y.; Hu, H.; Zhang, C. The Role of Stress in Controlling Infrared Radiation during Coal and Rock Failures; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 54, pp. 1–12. [Google Scholar]
- Nie, X.; Xiao, B.; Shen, D.; Guo, W. Research on thermal-mechanical stamping forming considering deformation heat and friction heat effect. China Mech. Eng. 2020, 31, 2005–2015. (In Chinese) [Google Scholar]
- Prendes, G.; Suárez, D.; González, C.; Alvarez, F. Infrared thermography methodology applied to detect localized rockfalls in self-supporting underground mines. In Rock Mechanics for Resources, Energy and Environment; CRC Press: Boca Raton, FL, USA, 2013; pp. 825–829. [Google Scholar]
- Ma, L.; Sun, H. Spatial-temporal infrared radiation precursors of coal failure under uniaxial compressive loading. Infrared Phys. Technol. 2018, 93, 144–153. [Google Scholar] [CrossRef]
- Sun, H.; Ma, L.; Liu, W.; Spearing, A.J.S.; Han, J.; Fu, Y. The response mechanism of acoustic and thermal effect when stress causes rock damage. Appl. Acoust. 2021, 180, 108093. [Google Scholar] [CrossRef]
- Cui, R.; Cao, K.; Li, X.; Khan, R.M.A.; Khan, N.M.; Liu, W.; Gao, Q.; Wang, F.; Yang, Y.; Quan, J.; et al. The Infrared Radiation Characteristics of Sandstone Fracture Seepage under Coupled Stress-Hydro Effect. Sustainability 2022, 14, 16454. [Google Scholar] [CrossRef]
- Ma, L.; Jin, Z.; Liu, W.; Zhang, D.; Zhang, Y. Wongawilli roadway backfilling coal mining method—A case study in Wangtaipu coal mine. Int. J. Oil Gas Coal Technol. 2019, 20, 342–359. [Google Scholar] [CrossRef]
- Peng, S.; Bi, Y. Strategic consideration and core technology about environmental ecological restoration in coal mine areas in the Yellow River basin of China. J. China Coal Soc. 2020, 45, 1211–1221. (In Chinese) [Google Scholar]
- Zong, T.; Zhu, G.; Zhang, Q.; Yang, K.; Wang, Y.; Han, Y.; Lv, H.; Gao, J. Solid Backfilling Efficiency Optimization in Coal Mining: Spatiotemporal Linkage Analysis and Case Study. Appl. Sci. 2023, 13, 12298. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, D.; Wang, S.; Xie, Y.; Yu, Y. Water—Preserved mining with the method named “backfilling while mining”. J. China Coal Soc. 2018, 43, 62–69. (In Chinese) [Google Scholar]
- Lu, B.; Li, Y.; Fang, S.; Lin, H.; Zhu, Y. Cemented Backfilling Mining Technology for Gently Inclined Coal Seams Using a Continuous Mining and Continuous Backfilling Method. Shock Vib. 2021, 2021, 6652309. [Google Scholar] [CrossRef]
- Cui, R. Infrared Radiation Monitoring and Early Warning of Coal and Rock Fracture and Water Penetration or Inrush under Coupled Stress-Hydro Effect. Master′s Thesis, China University of Mining and Technology, Xuzhou, China, 2022. (In Chinese). [Google Scholar]
- Wang, D.; Zhou, F.; Dong, Y.; Sun, D.; Yu, B. Experimental Investigation of Thermal Effect on Fracability Index of Geothermal Reservoirs. Nat. Resour. Res. 2021, 30, 273–288. [Google Scholar] [CrossRef]
- Lin, H.; Deng, J.; Zhang, X.; Gao, J. Porothermoelastic Response of a Borehole in Fluid-Saturated Medium Subjected to Thermal Osmosis Effect. Geofluids 2023, 2023, 4030804. [Google Scholar] [CrossRef]
- Yuan, M.; Ouyang, J.; Zheng, S.; Tian, Y.; Sun, R.; Bao, R.; Li, T.; Yu, T.; Li, S.; Wu, D. Research on Ecological Effect Assessment Method of Ecological Restoration of Open-Pit Coal Mines in Alpine Regions. Int. J. Environ. Res. Public Health 2022, 19, 7682. [Google Scholar] [CrossRef]




| Component Name | CaO | SiO2 | Al2O3 | Fe2O3 | K2O | TiO2 | MgO | SO3 | P2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 22.3% | 31.0% | 30.3% | 5.2% | 1.3% | 1.2% | 0.5% | 0.5% | 0.2% | 5.5% |
| G | 1.6% | 43.5% | 18.2% | 3.6% | 1.1% | 0.5% | 0.7% | 2.1% | 0.2% | 29.2% |
| cement | 49.5% | 23.2% | 8.1% | 3.2% | 0.9% | 0.5% | 4.6% | 4.1% | 0.1% | 5.4% |
| Scheme Group | Specimen Number | Mass Fraction of Solids | Mass Fraction of Liquid | Solid–Liquid Mass Fraction Ratio | Curing Time /Day | |||
|---|---|---|---|---|---|---|---|---|
| F | G | Cement | Alkaline Activator | Water | ||||
| 1-1 | 20% | 60% | 20% | 10% | 90% | 70%:30% | 3 | |
| F2G6 | 1-2 | 20% | 60% | 20% | 10% | 90% | 70%:30% | 7 |
| 1-3 | 20% | 60% | 20% | 10% | 90% | 70%:30% | 14 | |
| 1-4 | 20% | 60% | 20% | 10% | 90% | 70%:30% | 28 | |
| 2-1 | 30% | 50% | 20% | 10% | 90% | 70%:30% | 3 | |
| F3G5 | 2-2 | 30% | 50% | 20% | 10% | 90% | 70%:30% | 7 |
| 2-3 | 30% | 50% | 20% | 10% | 90% | 70%:30% | 14 | |
| 2-4 | 30% | 50% | 20% | 10% | 90% | 70%:30% | 28 | |
| 3-1 | 40% | 40% | 20% | 10% | 90% | 70%:30% | 3 | |
| F4G4 | 3-2 | 40% | 40% | 20% | 10% | 90% | 70%:30% | 7 |
| 3-3 | 40% | 40% | 20% | 10% | 90% | 70%:30% | 14 | |
| 3-4 | 40% | 40% | 20% | 10% | 90% | 70%:30% | 28 | |
| 4-1 | 50% | 30% | 20% | 10% | 90% | 70%:30% | 3 | |
| F5G3 | 4-2 | 50% | 30% | 20% | 10% | 90% | 70%:30% | 7 |
| 4-3 | 50% | 30% | 20% | 10% | 90% | 70%:30% | 14 | |
| 4-4 | 50% | 30% | 20% | 10% | 90% | 70%:30% | 28 | |
| 5-1 | 60% | 20% | 20% | 10% | 90% | 70%:30% | 3 | |
| F6G2 | 5-2 | 60% | 20% | 20% | 10% | 90% | 70%:30% | 7 |
| 5-3 | 60% | 20% | 20% | 10% | 90% | 70%:30% | 14 | |
| 5-4 | 60% | 20% | 20% | 10% | 90% | 70%:30% | 28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, G.; Ma, L.; Osemudiamhen, A.E.; Ngo, I.; Gao, Q.; Yu, K.; Guo, Z. Assessing the Performance of CO2-Mineralized Underground Backfilling Materials through the Variation Characteristics of Infrared Radiation Temperature Index. Minerals 2024, 14, 566. https://doi.org/10.3390/min14060566
Cao G, Ma L, Osemudiamhen AE, Ngo I, Gao Q, Yu K, Guo Z. Assessing the Performance of CO2-Mineralized Underground Backfilling Materials through the Variation Characteristics of Infrared Radiation Temperature Index. Minerals. 2024; 14(6):566. https://doi.org/10.3390/min14060566
Chicago/Turabian StyleCao, Guanghui, Liqiang Ma, Arienkhe Endurance Osemudiamhen, Ichhuy Ngo, Qiangqiang Gao, Kunpeng Yu, and Zezhou Guo. 2024. "Assessing the Performance of CO2-Mineralized Underground Backfilling Materials through the Variation Characteristics of Infrared Radiation Temperature Index" Minerals 14, no. 6: 566. https://doi.org/10.3390/min14060566





