ImhoflotTM Flotation Cell Performance in Mini-Pilot and Industrial Scales on the Acacia Copper Ore
Abstract
:1. Introduction
2. Materials and Methods
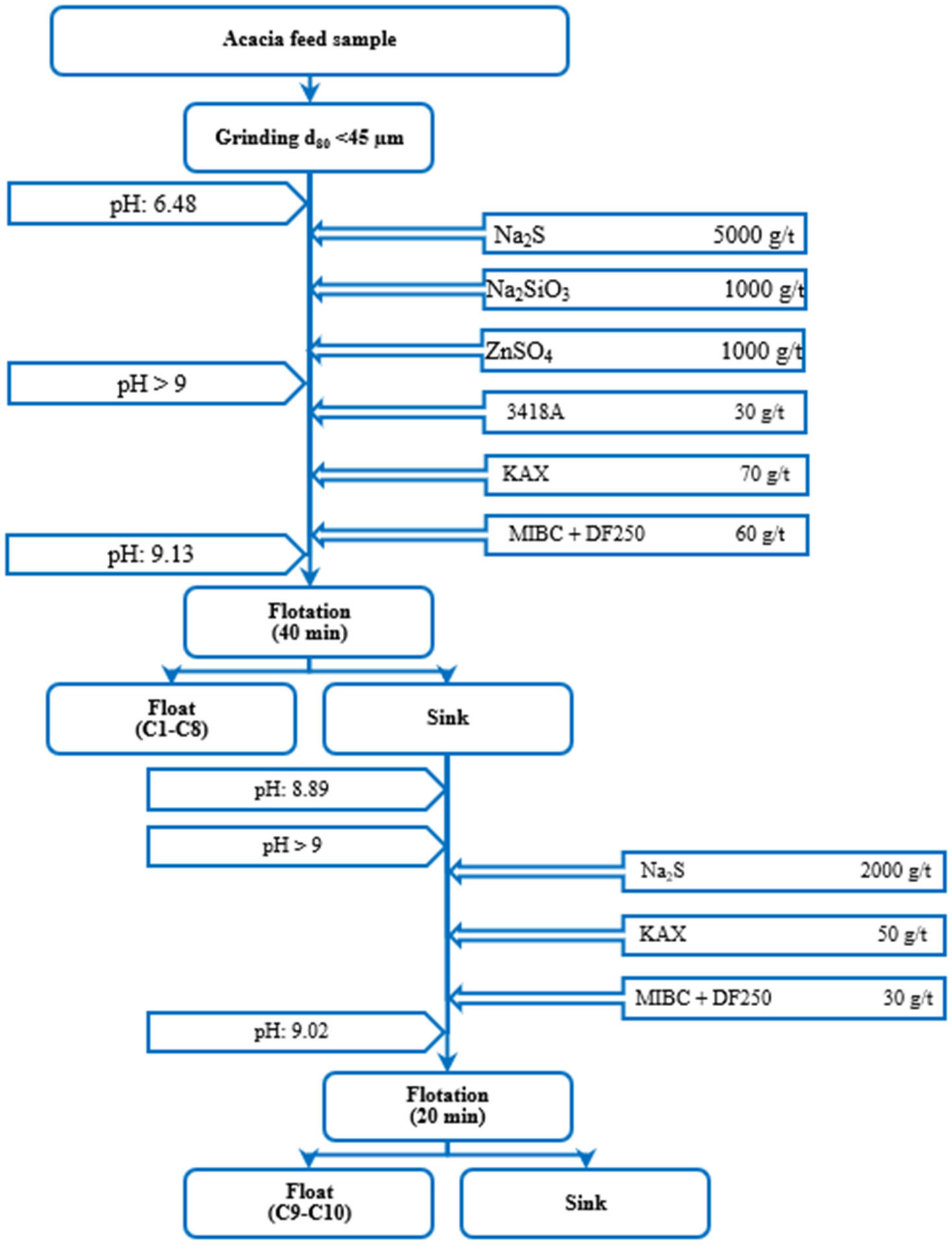
2.1. Material and Sample Preparation
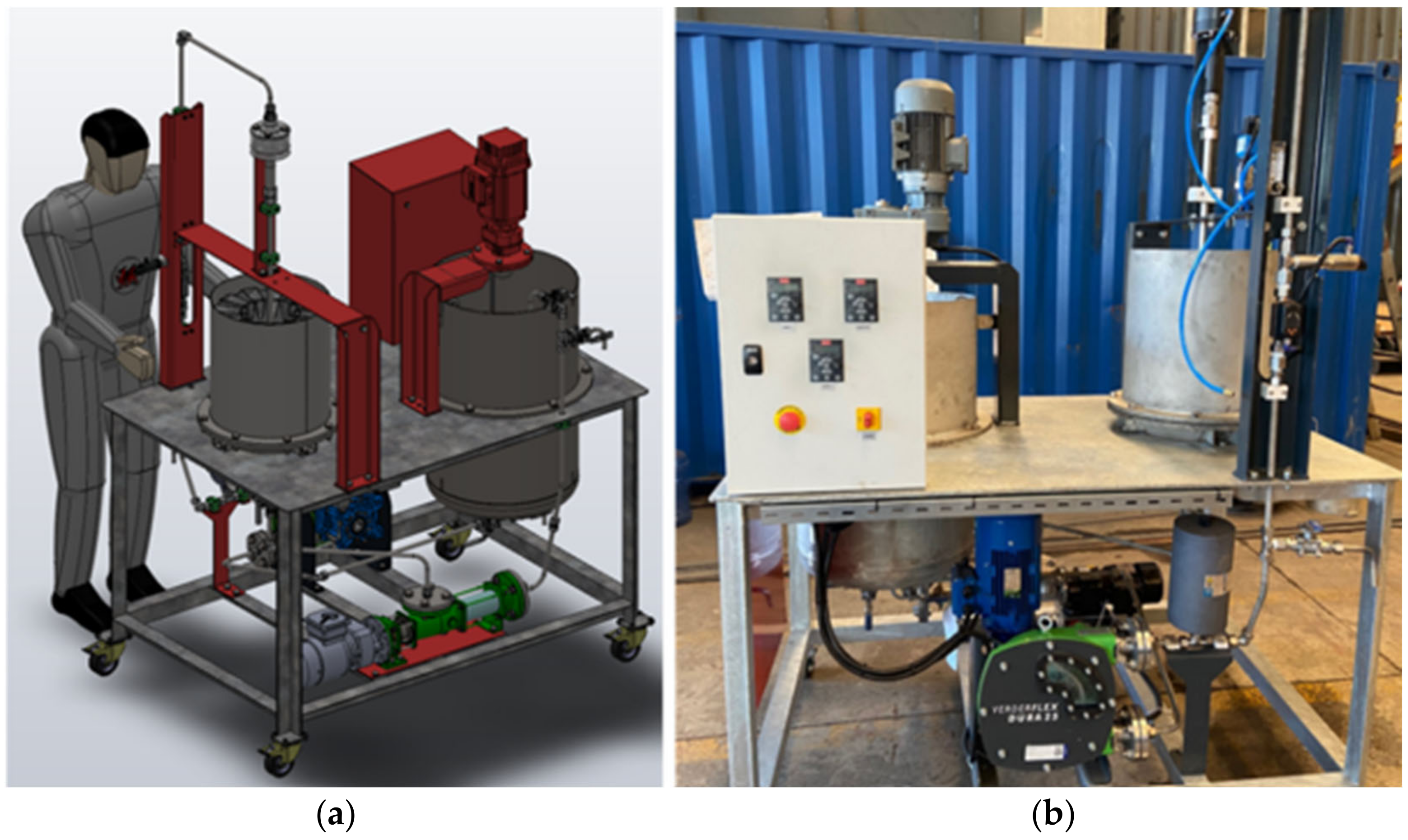
2.2. Design and Fabrication of the VM-04 Cell
2.3. Experimental Procedure
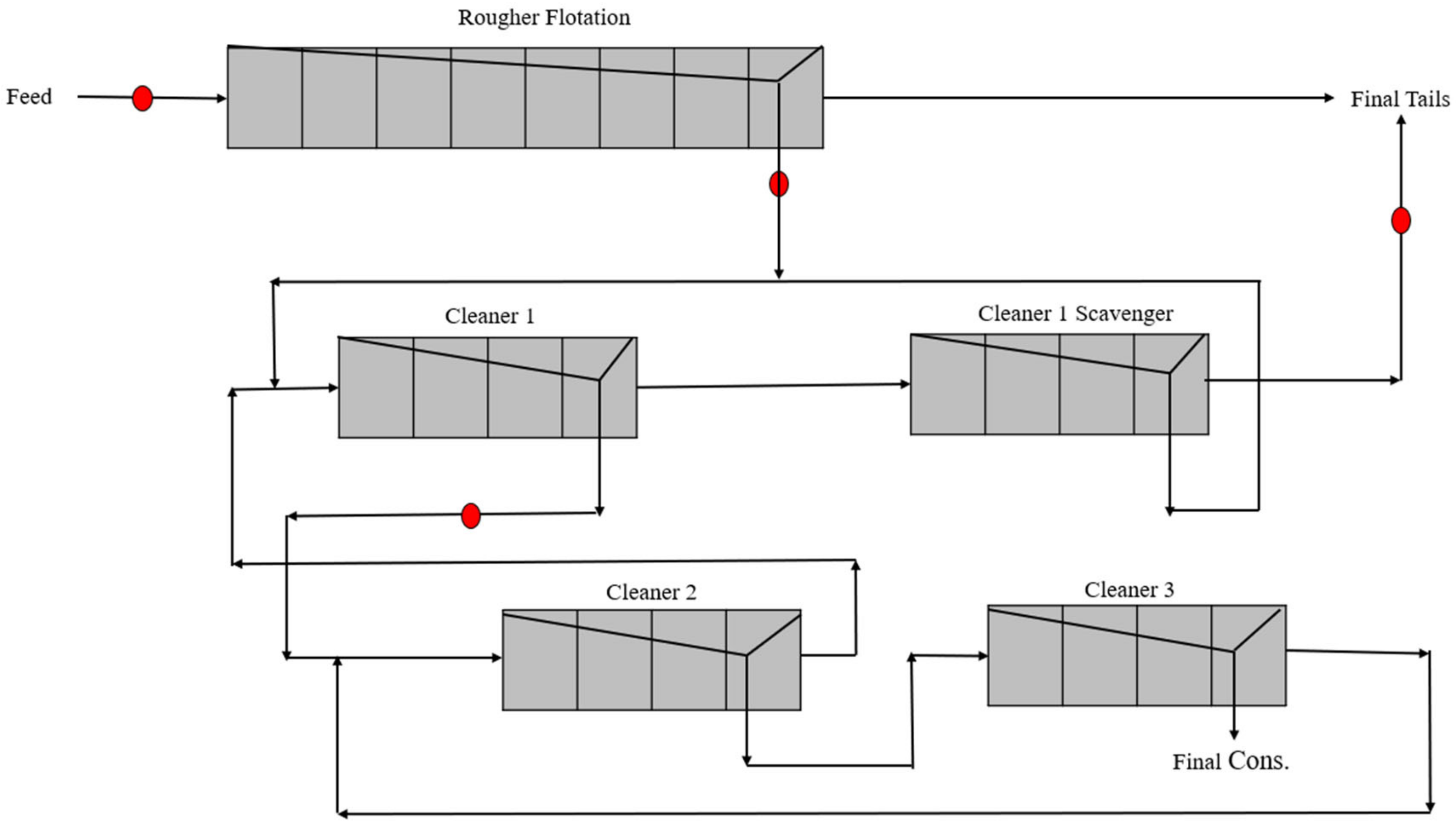
2.4. Acacia Concentration Plant
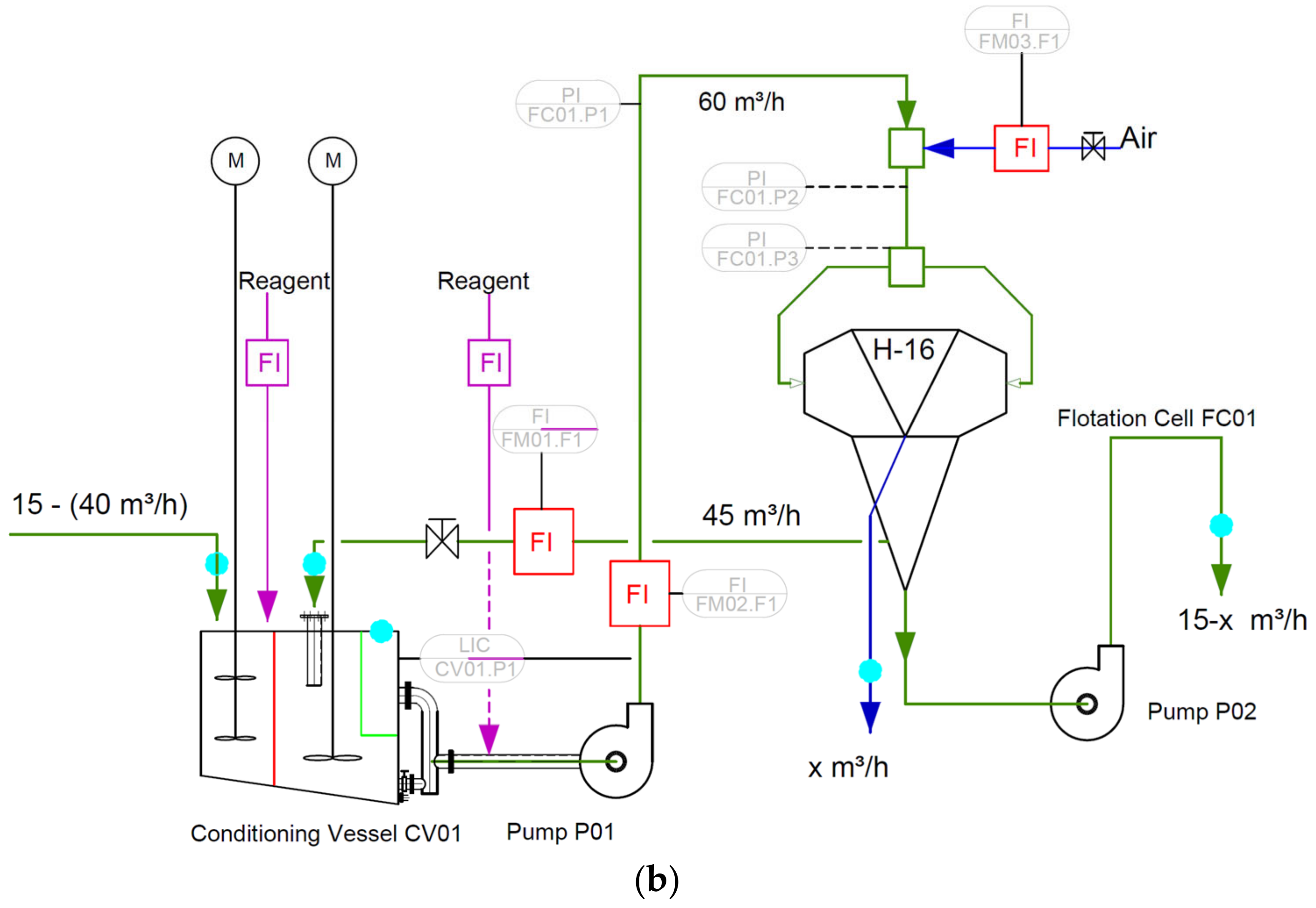
2.5. H-16 Cell and Sampling Campaign
3. Results and Discussions
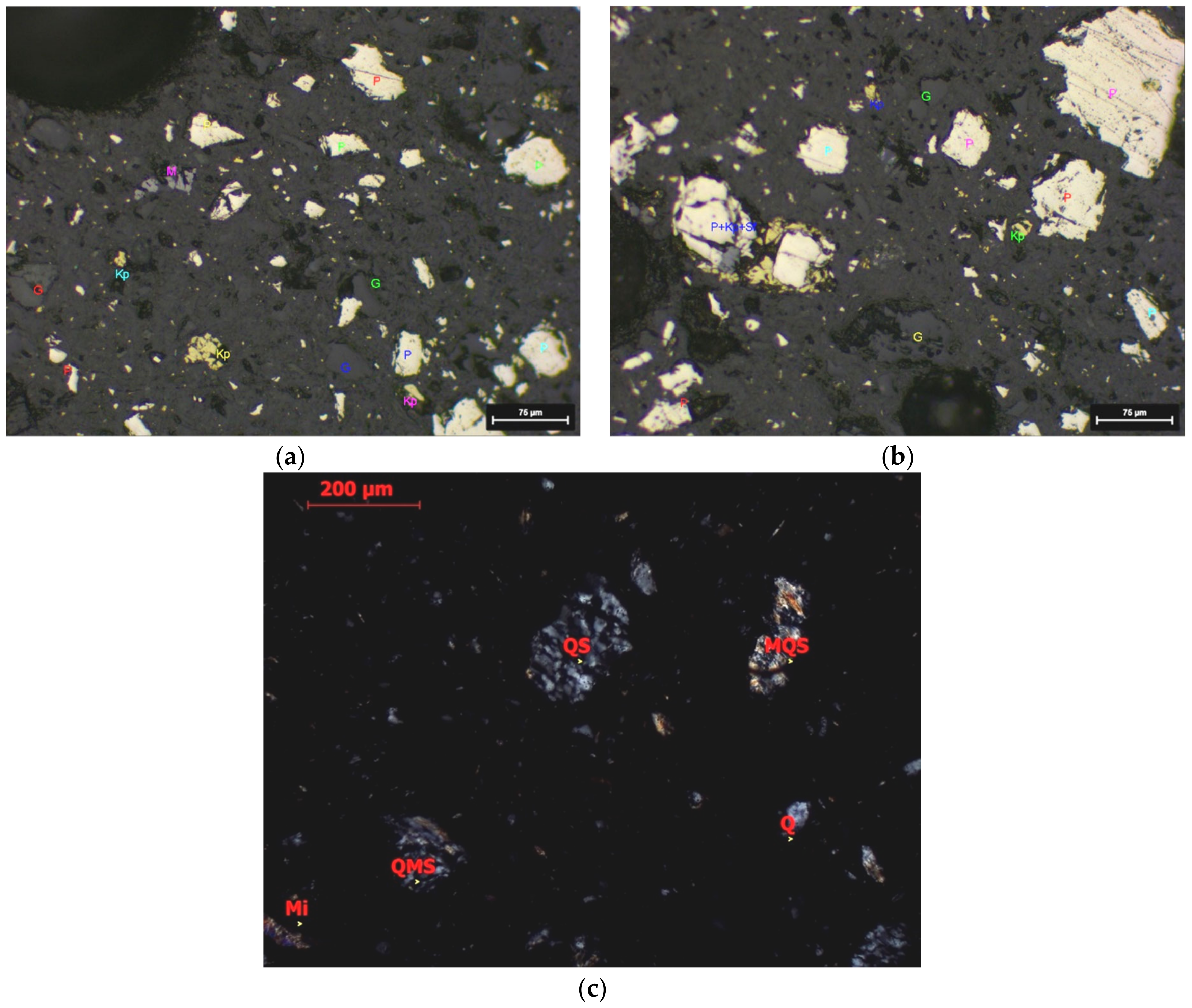
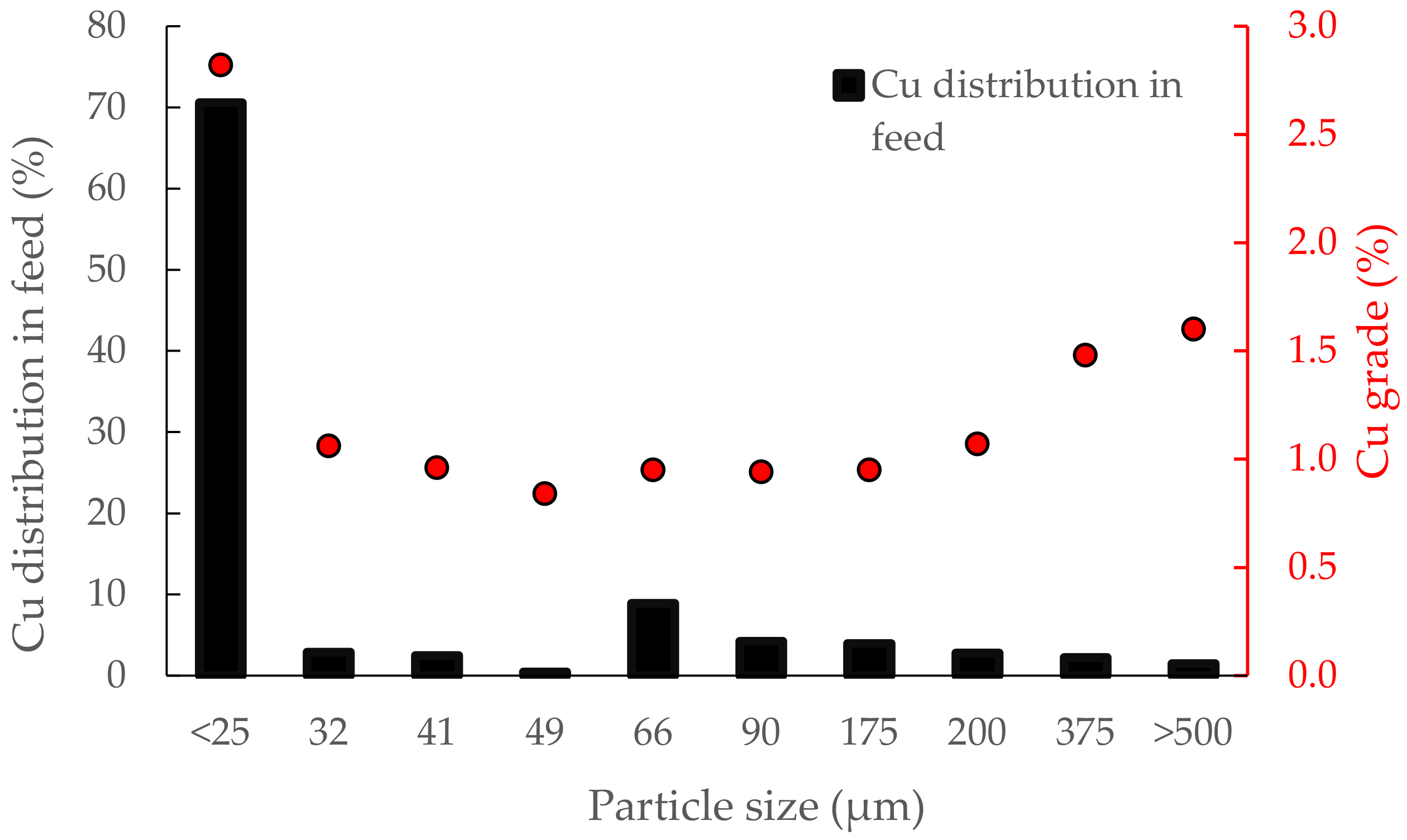
3.1. Sample Properties
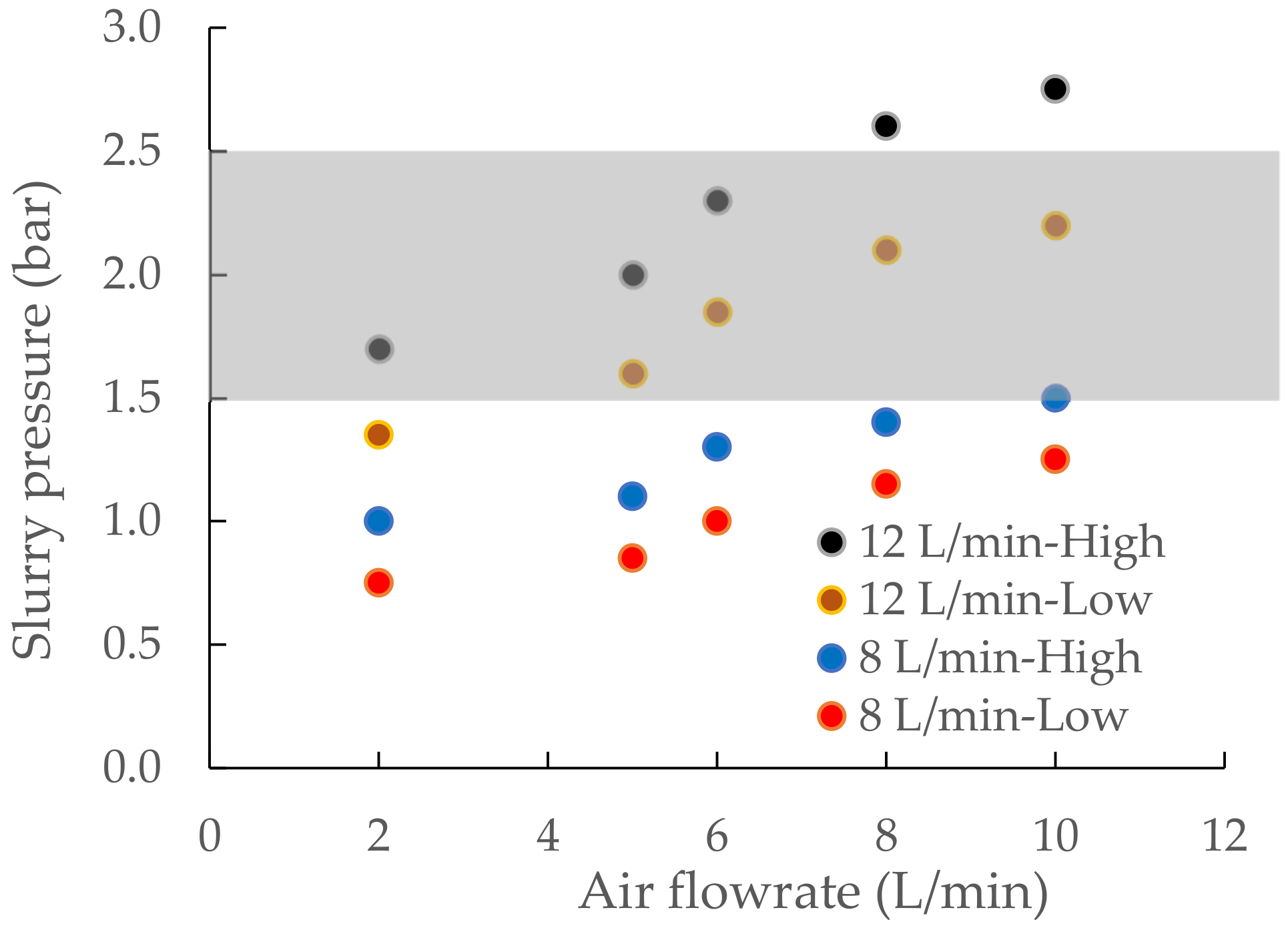
3.2. Operating Parameters of the VM-04 Cell
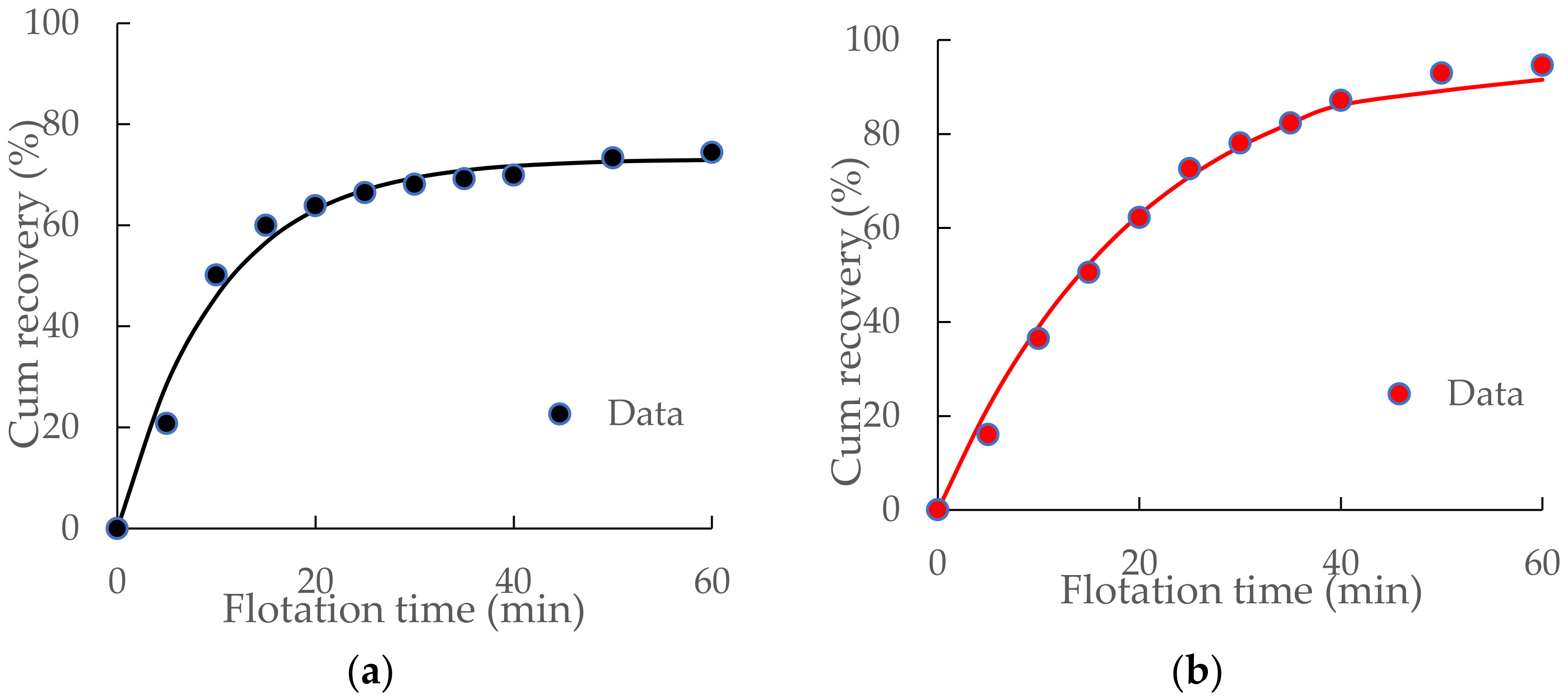
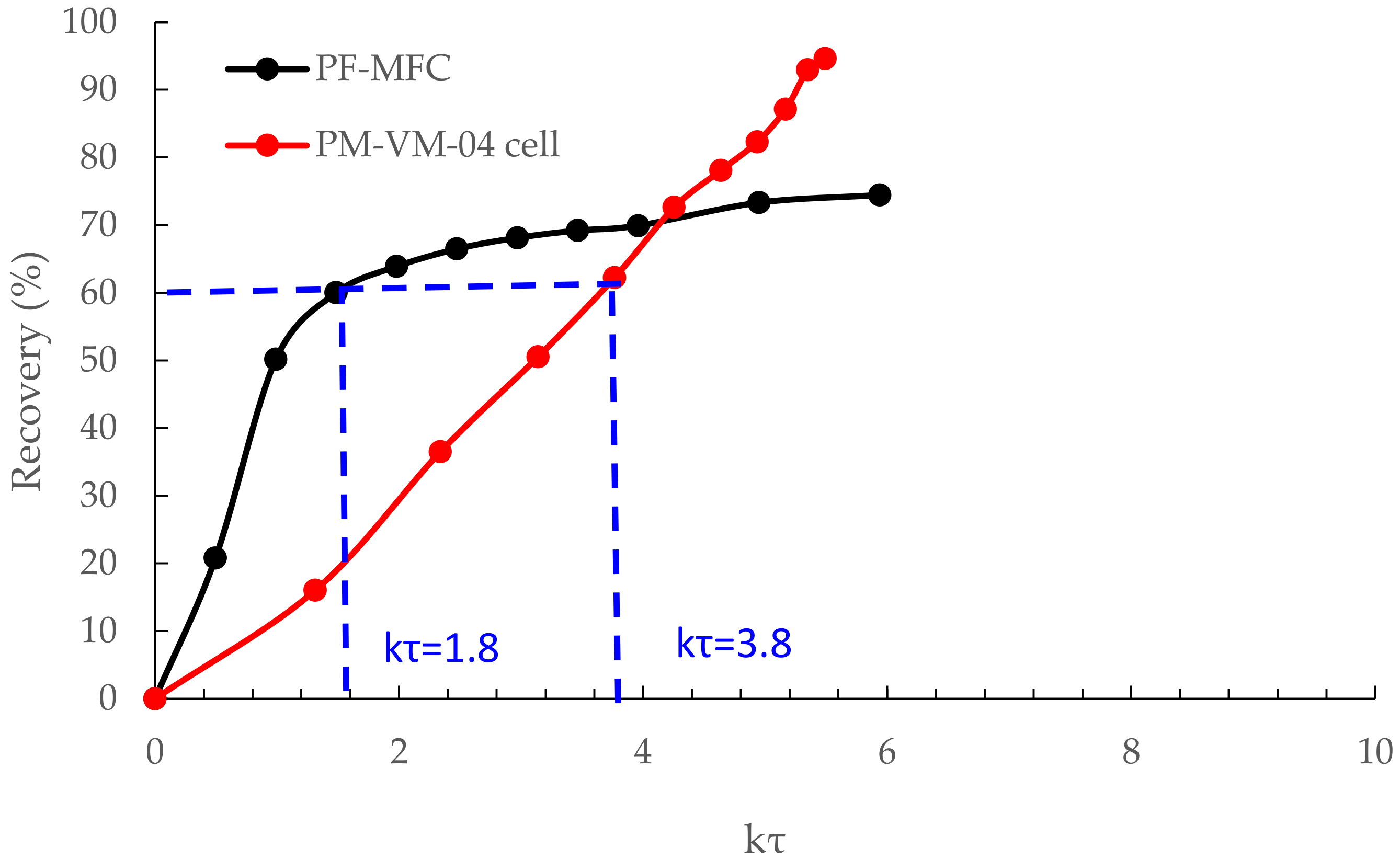
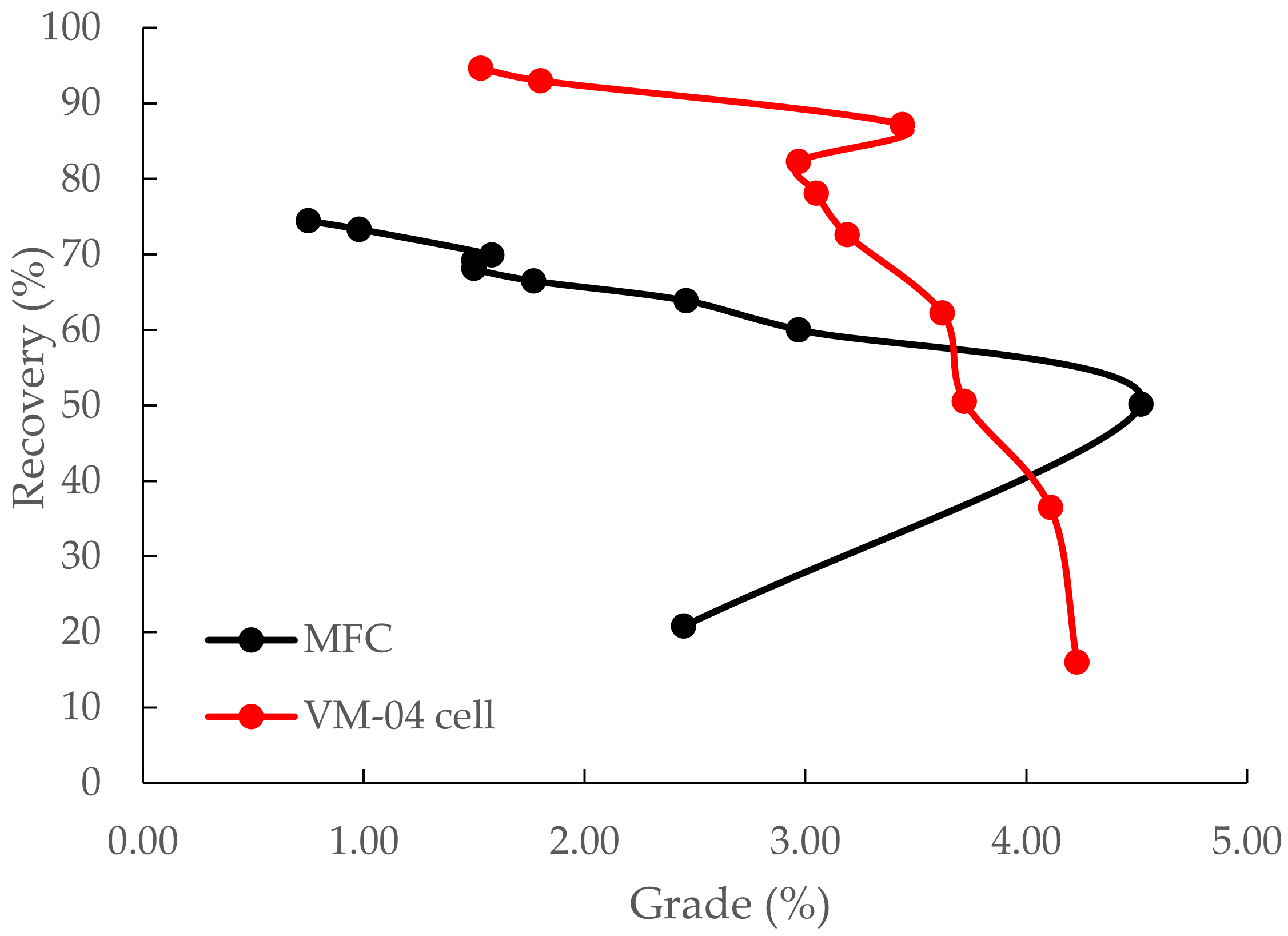
3.3. Flotation Kinetic Results of ImhoflotTM VM-04 and Mechanical Cells
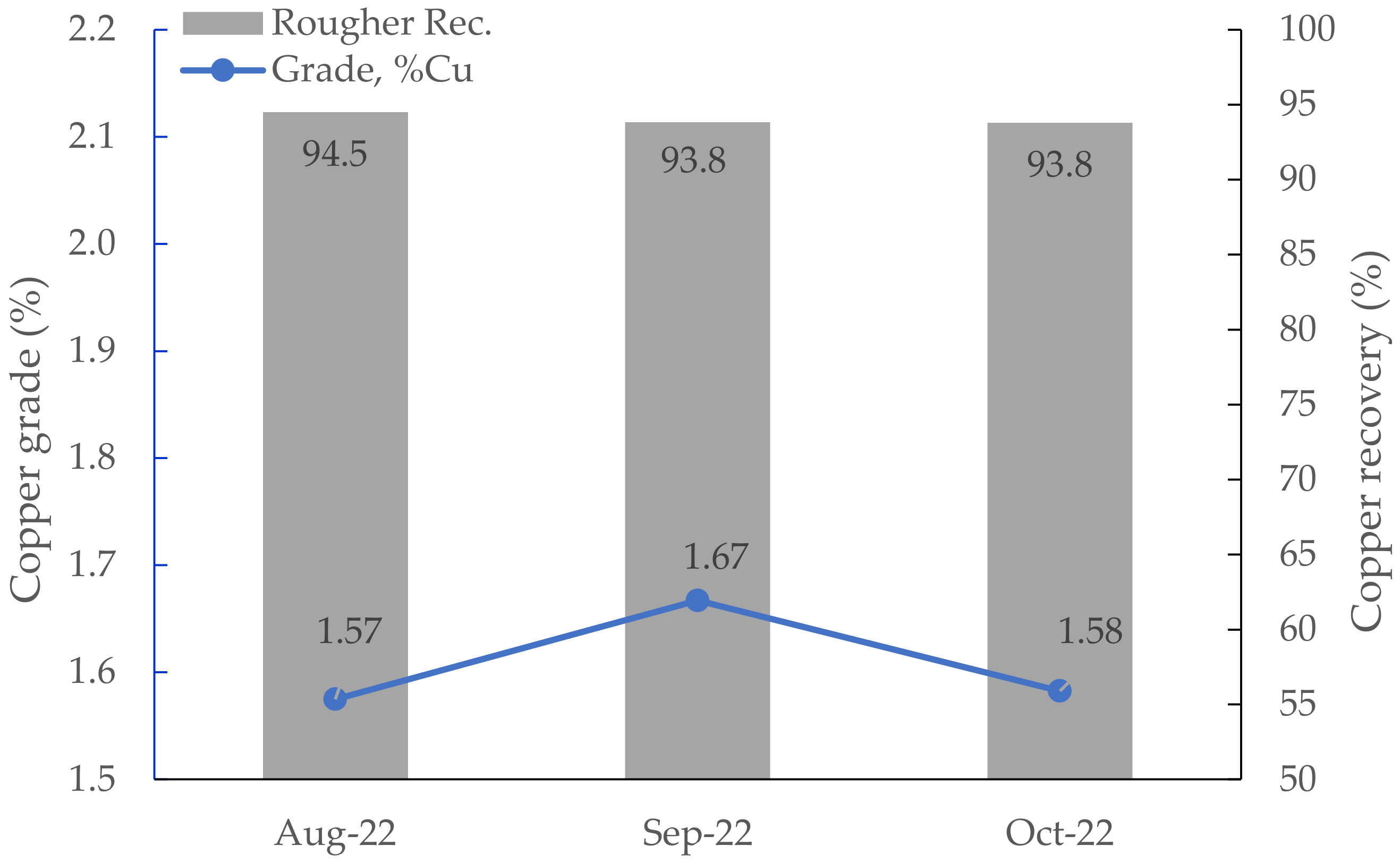
3.4. Results of Industrial Surveys
4. Conclusions and Future Works
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Yang, Y.; Han, D.; Shen, Z.; Shi, S. Hydrodynamic and Metallurgical Evaluation of the 680 m3 Flotation Cell in Industrial Application. In Proceedings of the Flotation ’19, Cape Town, South Africa, 11–14 November 2019. [Google Scholar]
- Yianatos, J.; Vallejos, P. Limiting conditions in large flotation cells: Froth recovery and bubble loading. Miner. Eng. 2022, 185, 107695. [Google Scholar] [CrossRef]
- Finch, J.A.; Tan, Y.H. On limits to flotation cell size. Minerals 2023, 13, 411. [Google Scholar] [CrossRef]
- Harbort, G. Pneumatic Flotation, Chapter 7.3. In SME Mineral Processing and Extractive Metallurgy Handbook, Society for Mining, Metallurgy, and Exploration (SME); ASM International: Cleveland, OH, USA, 2019; pp. 931–957. [Google Scholar]
- Hassanzadeh, A. A short Pragmatic Overview on the Development of Flotation Machines from Historical, Mechanical and Metallurgical Perspectives. In Proceedings of the 9th International Congress of Mining Machinery and Technologies, Izmir, Türkiye, 13–15 September 2023; pp. 10–15. [Google Scholar]
- Hassanzadeh, A.; Safari, M.; Hoang, D.H.; Khoshdast, H.; Albijanic, B.; Kowalczuk, P.B. Technological assessments on recent developments in fine and coarse particle flotation systems. Miner. Eng. 2022, 180, 107509. [Google Scholar] [CrossRef]
- Harris, C.C.; Chakravarti, A.; Degaleesan, S.N. A recycle flow flotation machine model. Int. J. Miner. Process. 1975, 2, 39–58. [Google Scholar] [CrossRef]
- Hoang, D.H.; Imhof, R.; Sambrook, T.; Bakulin, A.E.; Murzabekov, K.M.; Abubakirov, B.A.; Baygunakova, R.K.; Rudolph, M. Recovery of fine gold loss to tailings using advanced reactor pneumatic flotation ImhoflotTM. Miner. Eng. 2022, 184, 107649. [Google Scholar] [CrossRef]
- Huynh, L.; Araya, R.; Seaman, D.R.; Harbort, G.; Munro, P.D. Improved cleaner circuit design for better performance using the Jameson cell. In Proceedings of the 12th AUSIMM Mill Operator’s Conference, Townsville, Australia, 1–3 September 2014; pp. 141–152. [Google Scholar]
- Hassanzadeh, A.; Safari, M.; Khoshdast, H.; Güner, M.K.; Hoang, D.H.; Sambrook, T.; Kowalczuk, P.B. Introducing key advantages of intensified flotation cells over conventionally used mechanical and column cells. Physicochem. Probl. Miner. Process. 2022, 58, 155101. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Froth Flotation. In Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 265–380. [Google Scholar]
- Guner, M.K.; Hassanzadeh, A.; Vinnett, L.; Yianatos, J.; Kowalczuk, P.B. Effects of operating parameters on residence time distribution in a REFLUX flotation cell. Miner. Eng. 2023, 204, 108439. [Google Scholar] [CrossRef]
- Vinnett, L.; Yianatos, J.; Hassanzadeh, A.; Díaz, F.; Henríquez, F. Residence time distribution measurements and modeling in an industrial-scale Siemens flotation cell. Minerals 2023, 13, 678. [Google Scholar] [CrossRef]
- Yanez, A.; Kupka, N.; Tunç, B.; Suhonen, J.; Rinne, A. Fine and ultrafine flotation with the Concorde CellTM—A journey. Miner. Eng. 2024, 206, 108538. [Google Scholar] [CrossRef]
- Huynh, L.; Kohli, I.; Osborne, D.; De Waal, H.; Walstra, C. Design and performance aspects of coal flotation –experiences with the Jameson cell. Jameson Cell-2020 Compend. Tech. Pap. 2020; 185–196. [Google Scholar]
- Tabosa, E.; Vianna, S.; Valery, W.; Duffy, K.; Holtham, P.; Pyle, L.; Andrade, B. Modeling pneumatic flotation cells for circuit design and optimization. In Proceedings of the XXX International Mineral Processing Congress, Cape Town, South Africa, 18–22 October 2020; pp. 3081–3091. [Google Scholar]
- Parkes, S.; Wang, P.; Galvin, K.P. Investigating the system flotation kinetics of fine chalcopyrite in a REFLUX™ flotation cell: Part II low-grade ores. Miner. Eng. 2024, 207, 108548. [Google Scholar] [CrossRef]
- Metso Outotec Corporation. Section 4—Separations, Metso Minerals. In Basics in Minerals Processing, 5th ed.; Metso: Montreal, QC, Canada, 2006. [Google Scholar]
- Parkes, S.; Wang, P.; Galvin, K.P. Revisiting a flotation cell benchmark. Miner. Eng. 2023, 200, 108134. [Google Scholar] [CrossRef]
- Pyle, L.; Tabosa, E.; Vianna, S.; Sinclair, S.; Valery, W. Future (and Present) Trends in Circuit Design. In Proceedings of the IMPC Asia-Pacific 2022, Melbourne, Australia, 22–24 August 2022; pp. 1068–1083. [Google Scholar]
- Seaman, D.R.; Burns, F.; Adamson, B.; Seaman, B.A.; Manton, P. Telfer Processing Plant Upgrade-the Implementation of Additional Cleaning Capacity and the Regrinding of Copper and Pyrite Concentrates. In Proceedings of the 11th AusIMM Mill Operators’ Conference, Tasmania, Australia, 23–31 October 2012; The Australian Institute of Mining and Metallurgy: Melbourne, Australia, 2012; pp. 373–381. [Google Scholar]
- Dickinson, J.; Dabrowski, B.; Lelinski, D.; Christodoulou, L.; Galvin, K. Pilot Trial of a New High-Rate Flotation Machine; Procemin-Geomet: Santiago, Chile, 2019. [Google Scholar]
- Vinnett, L.; Yianatos, J.; Díaz, F.; Hassanzadeh, A. Estimating effective volumes in industrial forced-air flotation cells. Miner. Eng. 2024, 211, 108678. [Google Scholar] [CrossRef]
- Hoang, D.H.; Kurzydło, P.; Kwiatkowski, P.; Imhof, R.; Hassanzadeh, A.; Pereira, L.; Rudolph, M. Application of Pneumatic ImhoflotTM G-Cell in Recovering Fine Particles: A Case Study of KGHM Copper Ore. In Proceedings of the MEI Conferences, Cape Town, South Africa, 4–7 November 2023. [Google Scholar]
- Bahr, A.; Ludke, H.; Mehrhoff, F. The development and introduction of a new coal flotation cell. Proc. CIM Bull. 1970, 75, 84. [Google Scholar]
- Alizadeh, A.; Simonis, W. Flotation of finest and ultra-finest size coal particles. Aufbereit.-Tech. 1985, 6, 363–366. [Google Scholar]
- Imhof, R.M.; Battersby, M.J.G.; Brown, J.V.; Lotzien, R.M.; Kleefeld, J. Development of Pneumatic Flotation Incorporating Centrifugal Separation. In Proceedings of the XXII International Mineral Processing Congress, Cape Town, South Africa, 28 October 2003. [Google Scholar]
- Hassanzadeh, A.; Cinar, Y.A.; Gungor, E.; Hoang, D.H. Application of a Self-Aspirated ImhoflotTM H-16-Cell in a Copper Flotation Circuit. In Proceedings of the 2023 IMCET, Antalya, Türkiye, 28 October–1 December 2023; Volume 1133, p. 1141. [Google Scholar]
- Imhof, R.; Battersby, M.; Parra, F.; Sanchez-Pino, S. The Successful Application of Pneumatic Flotation Technology for the Removal of Silica by Reverse Flotation at the Iron Ore Pellet Plant of Compañía Minera Huasco, Chile. In Proceedings of the Centenary of Flotation Symposium, Brisbane, Australia, 6–9 June 2005; pp. 1–8. [Google Scholar]
- Lima, P.N.; Peres, A.E.C.; Goncalves, T.A.R. Comparative evaluation between mechanical and pneumatic cells for quartz flotation in the iron ore industry, REM. Int. Eng. J. Ouro Pereto 2018, 71, 437–442. [Google Scholar] [CrossRef]
- Imhof, R.; Fletcher, M.; Vathavooran, A.; Singh, A. Application of Imhoflot G-Cell centrifugal flotation technology. J. South. Afr. Inst. Min. Metall. 2007, 107, 623–631. [Google Scholar]
- Battersby, M.; Battersby, R.M.; Flatman, S.; Imhof, R.; Sprenger, H.; Bragado, T. Recovery of Ultrafine Using Imhoflot Pneumatic Flotation—Two Pilot Plant Case Studies Recovering Nickel and Zinc From Tailings Streams. 2011. Available online: https://www.maelgwyn.com/wp-content/uploads/2016/04/Recovery_of_Ultra_fines.pdf (accessed on 30 January 2024).
- Imhof, R.M.; Lotzien, R.M.; Sobek, S. Pneumatic Flotation: A Reliable Procedure for a Correct Plant Layout. In Proceedings of the XVIII International Mineral Processing Congress, Sydney, Australia, 23–28 May 1993; pp. 971–978. [Google Scholar]
- Hassanzadeh, A.; Gungor, E.; Samet, E.; Durunesil, D.; Hoang, D.H.; Köse, B. A Comparative Study between ImhoflotTM and Mechanical Flotation Cells. In Proceedings of the MEI Conferences, Cape Town, South Africa, 4–7 November 2023. Flotation 23. [Google Scholar]
- Bayraktar, Z.; Ayhan, R.; Baharlı, A. Improving the fine flotation recovery of fine particles with magnetic conditioning technology. 2022. [Google Scholar]
- Duan, J.; Fornasiero, D.; Ralston, R. Calculation of the flotation rate constant of chalcopyrite particles in an ore. Int. J. Miner. Process. 2003, 72, 227–237. [Google Scholar] [CrossRef]
- Asghari, M.; Salmani Nuri, O.; Allahkarami, E. Analysis of kinetic models for chalcopyrite flotation: Effect of operating parameters. Geosystem Eng. 2019, 22, 263–270. [Google Scholar] [CrossRef]
- Guner, M.K.; Hassanzadeh, A.; Vinnett, L.; Yianatos, J.; Kowalczuk, P.B. Residence Time Distribution Measurements and Modeling of Lab-Scale Imhoflot™ Pneumatic Flotation Cell and REFLUX™ Flotation Cell. In Proceedings of the 17th IMPS 2022, Istanbul, Türkiye, 15–17 December 2022; pp. 393–401. [Google Scholar]
- Lotter, N.O.; Kormos, L.J.; Oliveira, J.; Fragomeni, D.; Whiteman, E. Modern process mineralogy: Two case studies. Miner. Eng. 2011, 24, 638–650. [Google Scholar] [CrossRef]
- Lee, R.; Chen, X.; Peng, Y. Flotation performance of chalcopyrite in the presence of an elevated pyrite proportion. Miner. Eng. 2022, 177, 107387. [Google Scholar] [CrossRef]
- Agheli, S.; Hassanzadeh, A.; Vaziri Hassas, B.; Hasanzadeheh, M. Effect of pyrite content of feed and configuration of locked particles on rougher flotation of copper in low and high pyritic ore types. Int. J. Min. Sci. Technol. 2018, 28, 167–176. [Google Scholar] [CrossRef]
- Karakashev, S.; Grozev, B.; Ozdemir, O.; Guven, O.; Ata, S.; Bournival, G.; Batjargal, K.; Boylu, F.; Hristova, S.; Çelik, M.S. Physical restrictions of the flotation of fine particles and ways to overcome them. Physicochem. Probl. Miner. Process. 2022, 58, 153944. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of Fine Particles: A Review. Miner. Process. Extr. Metall. Rev. 2021, 42, 473–483. [Google Scholar] [CrossRef]


| Parameter | Content |
|---|---|
| Solid content (%, w/w) | 25 |
| Agitator (Hz) | 26 |
| Feed flow rate (L/min) | 10 |
| Feed pump (Hz) | 26.8 |
| Tailing pump (Hz) | 49.9 |
| Nozzle diameter (mm) | 3.5 |
| Venturi diameter (mm) | 5.5 |
| Air flow rate (L/min) | 10 |
| Pulp pressure (bar) | 1.60 |
| Location | Solid Content (%w/w) | Fresh Feed Flowrate (m3/h) | Feed Flowrate to Aerator (m3/h) | pH | Air Flowrate (m3/h) | P80 (µm) | Reagent, Dosage (g/t) |
|---|---|---|---|---|---|---|---|
| Pre-rougher | 30 | 15 | 41 | 7.5 * | 17–21 | 63–70 | PAX, 90–130 |
| Rougher concentrate | 20–30 | 14–17 | 35 | 7.5 | 15–18 | 63–70 | PAX, 140–155 ** |
| Cleaner-scavenger tailings *** | 20 | 16–18 | 34 | 7.5–8 | 18–20 | 20–25 | Aero 3894, 10–20 |
| Cleaner-1 Concentrate *** | 20 | 16–17 | 41 | 7.5–8 | 18–19 | 20–25 | No additional reagent |
| Component | Mg | Cu | Fe | Zn | Pb | S | Al | Si | Ca | Zn | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.37 | 1.87 | 26.69 | 0.18 | 0.01 | 25.91 | 0.87 | 31.13 | 0.44 | 0.18 | 10.1 |
| Component | Co | Ni | Mo | Ba | Sc | Be | Ag | Au | V | Mn | As |
| Content (ppm) | 366.1 | 39.55 | 33.94 | 28.81 | <1.000 | <1.000 | 9.304 | 0.1 | 27.15 | 1208 | 47.19 |
| Position in the Circuit | H-16 ImhoflotTM Cell | Conventional Plant Flotation Cells | |||
|---|---|---|---|---|---|
| ER | Recovery (%) | ER | Recovery (%) | No. × Cell Size (m3) | |
| Pre-rougher | 4.84 | 89 | 1.89 | 96 | - |
| Rougher concentrate | 2.97 | 61 | - | - | 8 × 50 |
| Cleaner-scavenger tailing | 2.67 | 38 | - | - | 4 × 50 |
| Cleaner-1 concentrate | 1.76 | 64 | 1.45 | 60 | 4 × 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanzadeh, A.; Gungor, E.; Samet, E.; Durunesil, D.; Hoang, D.H.; Vinnett, L. ImhoflotTM Flotation Cell Performance in Mini-Pilot and Industrial Scales on the Acacia Copper Ore. Minerals 2024, 14, 590. https://doi.org/10.3390/min14060590
Hassanzadeh A, Gungor E, Samet E, Durunesil D, Hoang DH, Vinnett L. ImhoflotTM Flotation Cell Performance in Mini-Pilot and Industrial Scales on the Acacia Copper Ore. Minerals. 2024; 14(6):590. https://doi.org/10.3390/min14060590
Chicago/Turabian StyleHassanzadeh, Ahmad, Ekin Gungor, Ehsan Samet, Doruk Durunesil, Duong H. Hoang, and Luis Vinnett. 2024. "ImhoflotTM Flotation Cell Performance in Mini-Pilot and Industrial Scales on the Acacia Copper Ore" Minerals 14, no. 6: 590. https://doi.org/10.3390/min14060590







