Phosphates on Mars and Their Importance as Igneous, Aqueous, and Astrobiological Indicators
Abstract
1. Introduction
2. Meteorite Summary and Mineral Assemblages
2.1. Merrillite
2.2. Apatite
2.3. Tuite
REE Phosphates
3. Phosphate Detections and Analyses from Spacecraft Missions
3.1. Early Missions
3.2. Spirit
3.3. Opportunity
3.4. Curiosity
3.5. Mars 2020 PIXL
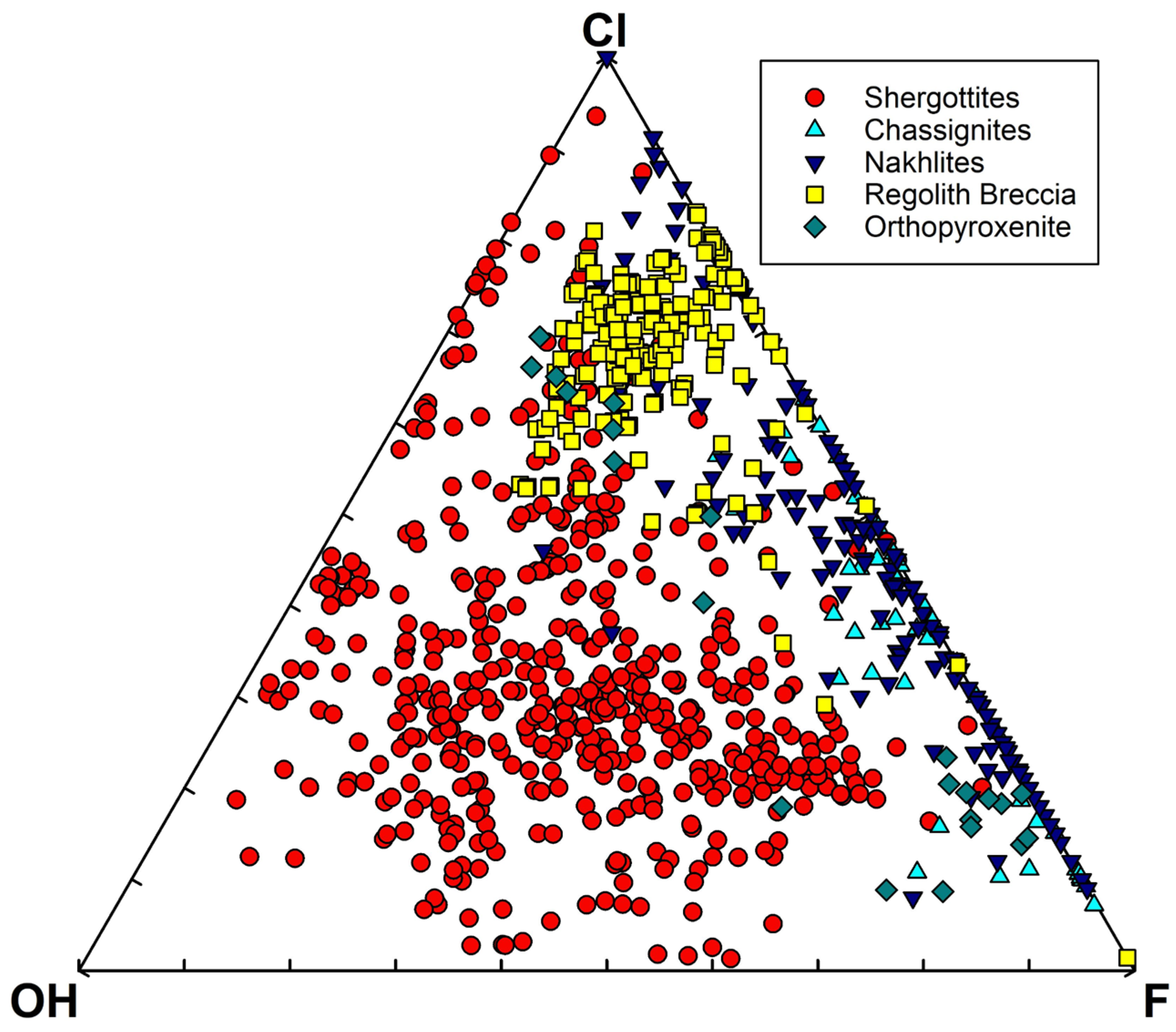
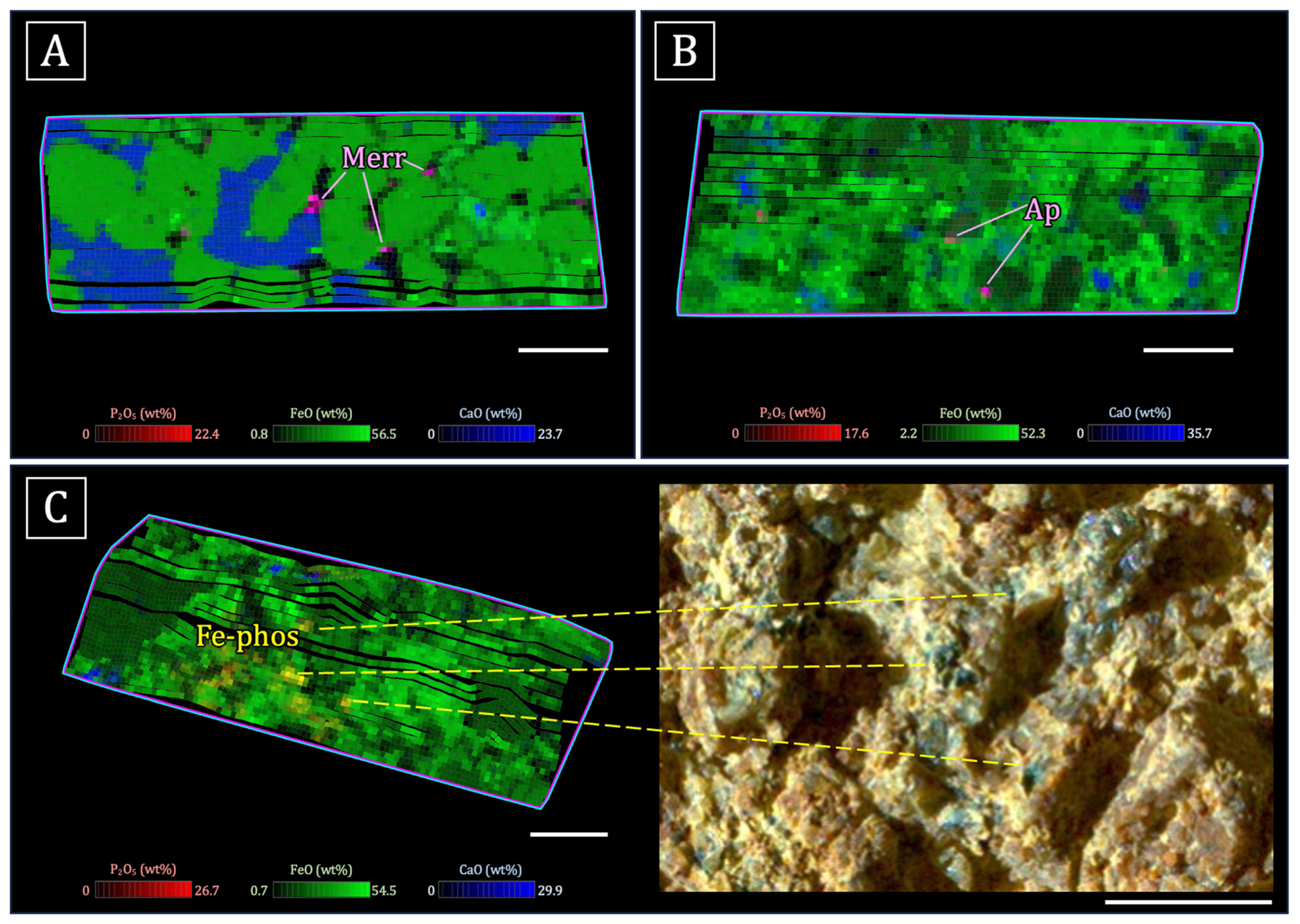
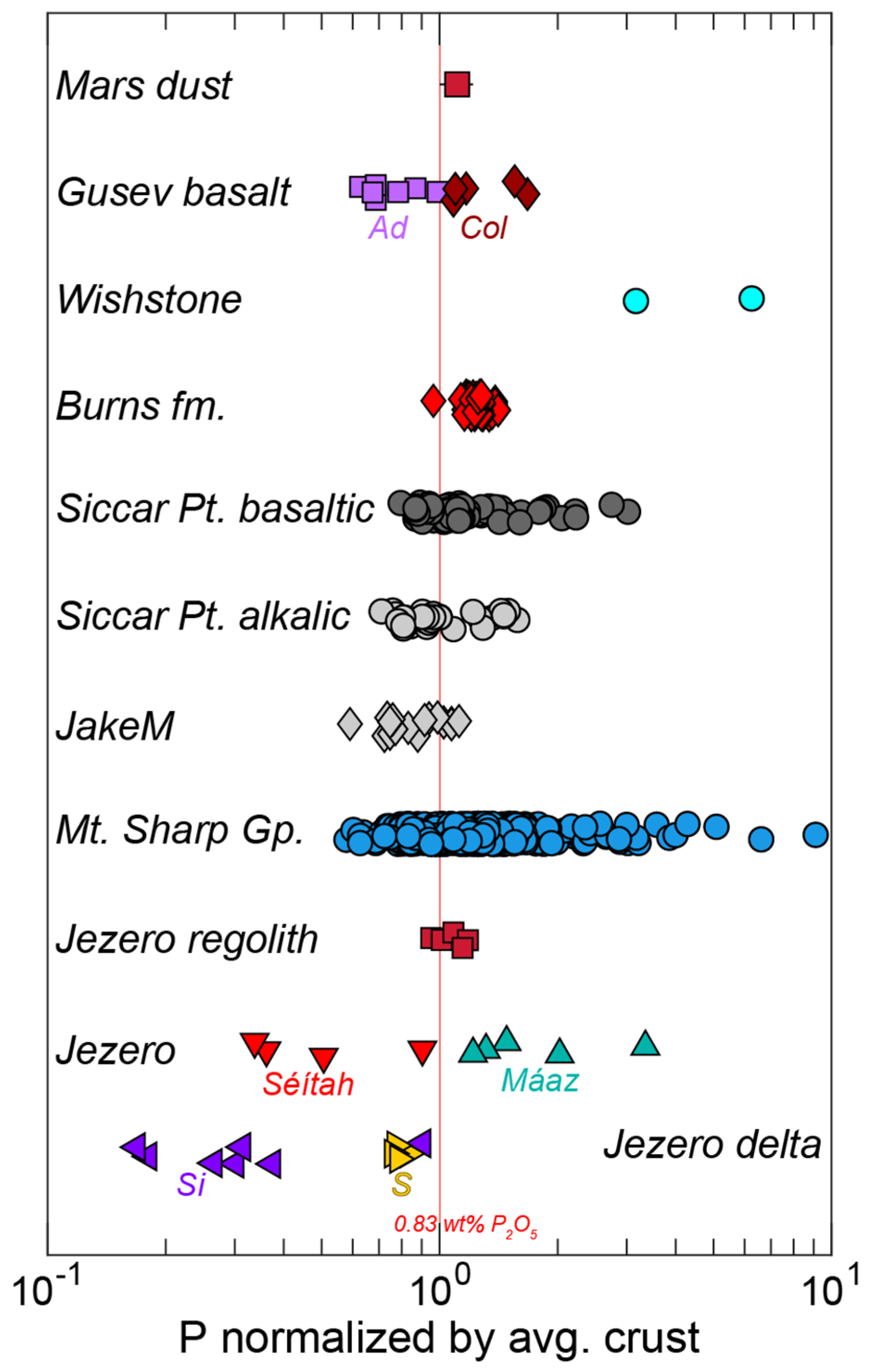
- Igneous phosphate minerals observed within or interstitial to mesostasis/feldspar and adjacent to other late-stage phases such as Fe-Ti oxides [165,167]. The Ca:P (molar) ratios and mixing trends in the igneous rocks on the Jezero crater floor are consistent with apatite and merrillite. Merrillite is dominant in the olivine cumulate rocks of the Séítah formation [167], while the highly evolved basalts of the Maaz formation contain apatite and merrillite [165]. However, it should also be noted that merrillite and apatite are commonly intergrown in igneous martian meteorites at length scales below PIXL’s spot size (i.e., [25,29]; Figure 1).
- A phosphate phase with Ca:P (molar) = ~1 was detected in the igneous rocks of the crater floor, associated with altered olivine, Cl-rich alteration, and mesostasis [165]. Sediments of the Jezero fan also contain rare grains of this mineral. It is likely a secondary phase, possibly brushite [165,166].
- Detrital phosphate minerals in the sedimentary rocks of the Jezero fan. Stoichiometry (e.g., Ca:P ratios) from mixing trends are consistent with apatite and merrillite, suggesting that the grains might be from igneous source rocks [166].
- Blue/green Fe phosphates have been identified in the conglomerate outcrop (Onahu) within the western fan of Jezero crater. These Fe phosphates have an Fe:P ratio and color consistent with those of vivianite (Fe3[PO4]·2H2O; although the exact Fe phosphate phase is not certain at this time) and occur in the matrix surrounding clasts of Fe-Mg silicate minerals [34].
3.6. Global Observations from Mars Missions
4. Phosphates as Aqueous Indicators
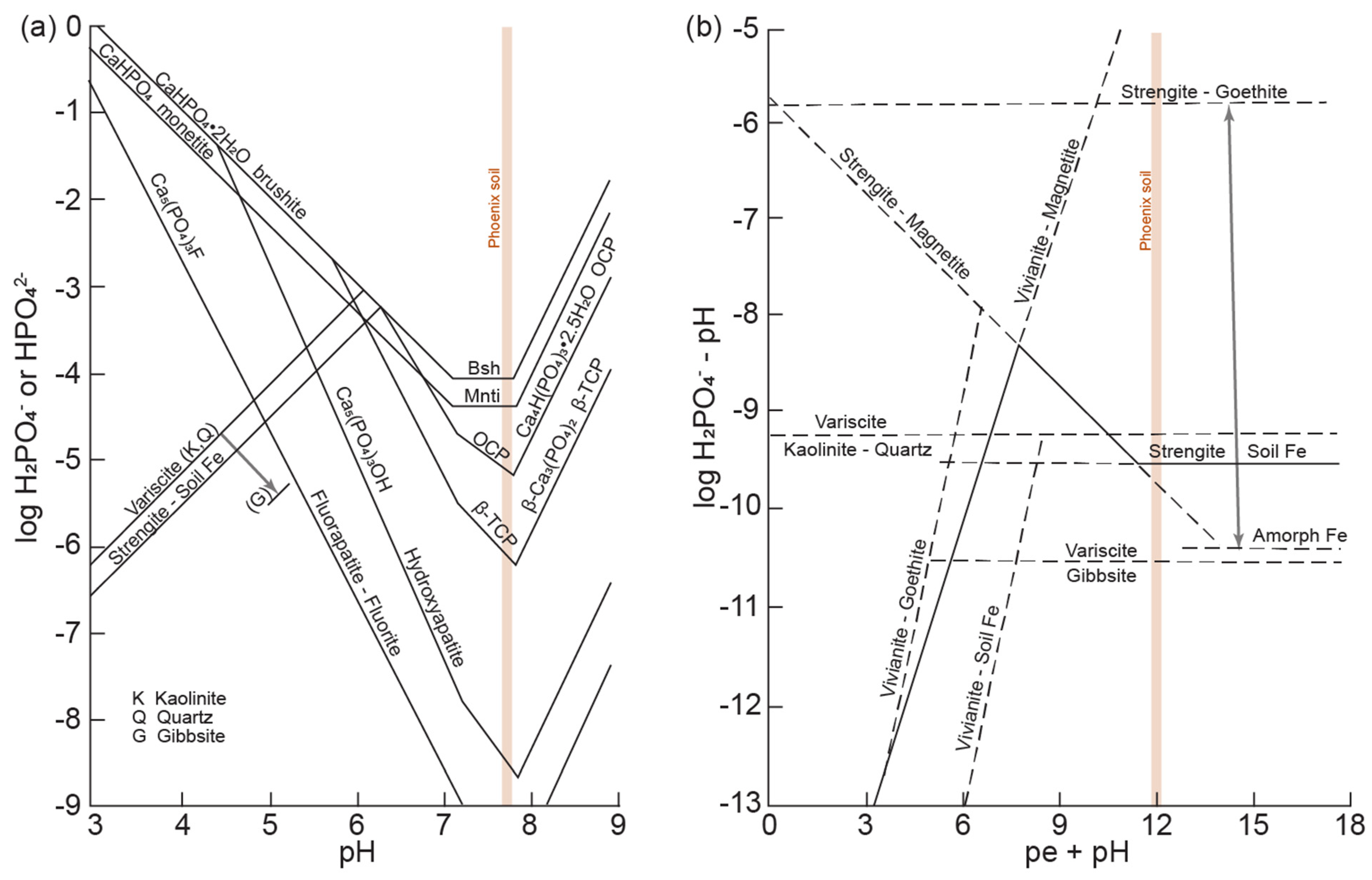
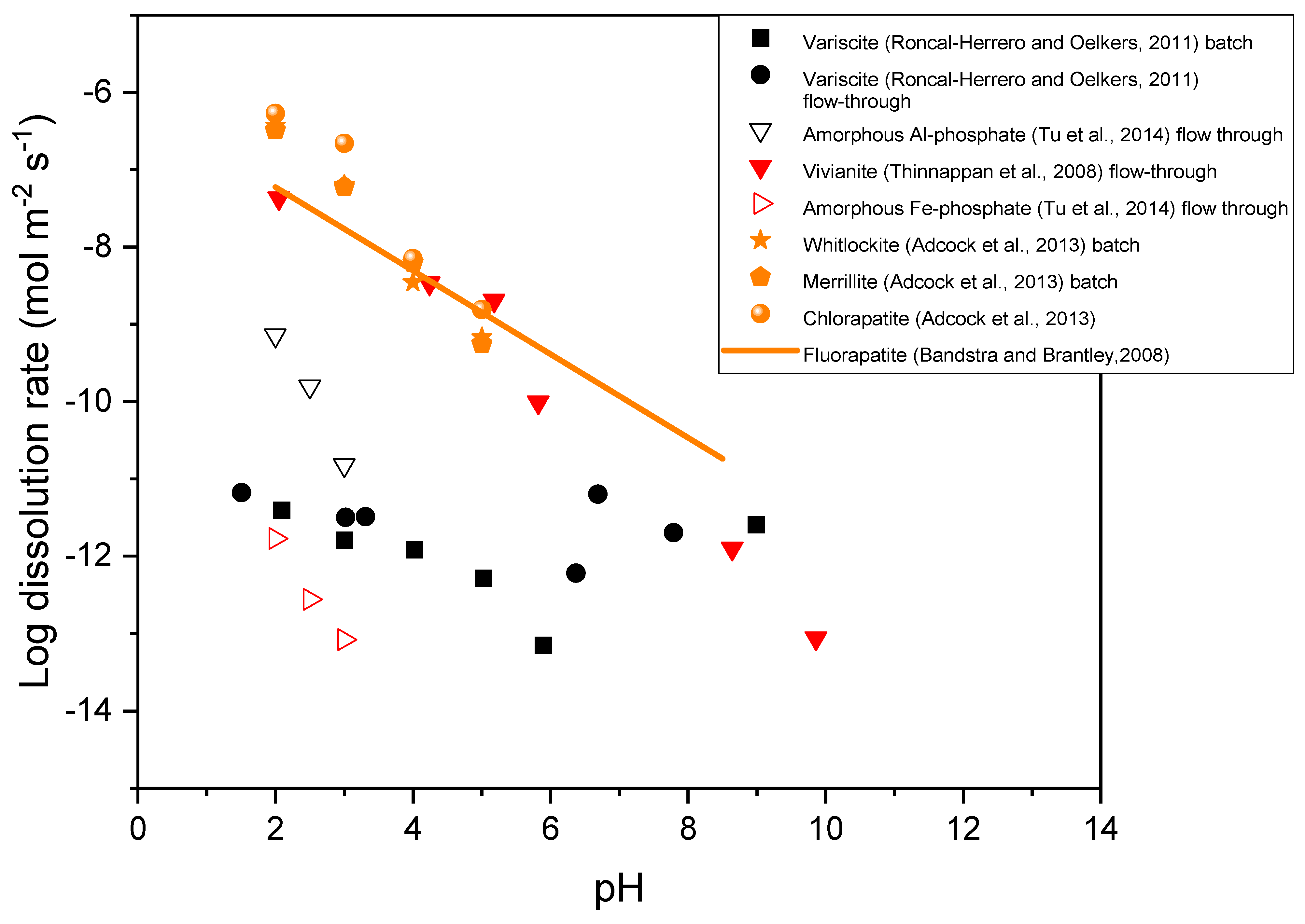
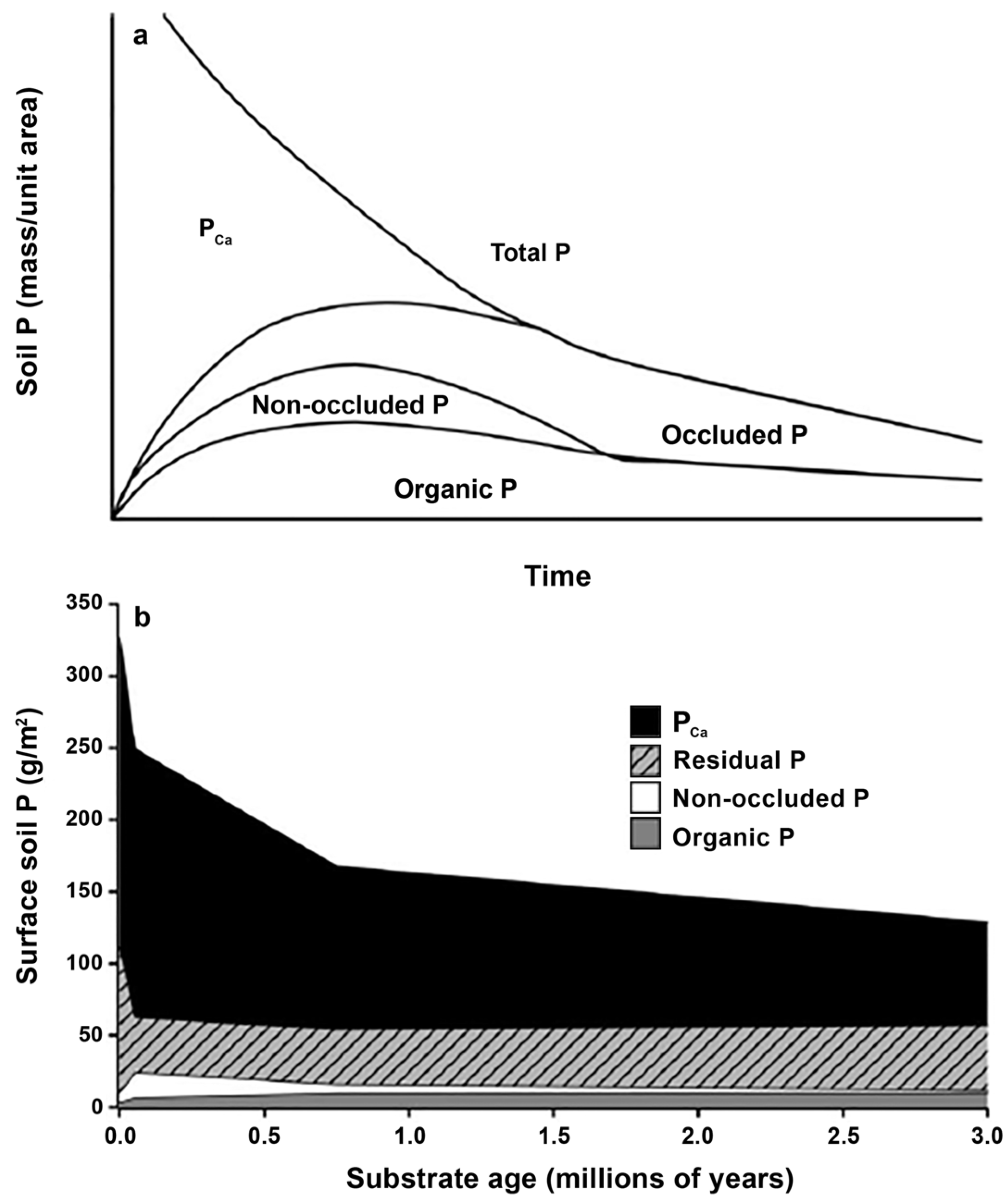
4.1. Dissolution of Phosphate Minerals
4.2. Hydrothermal Phosphates as Temperature Indicators
4.3. Phosphates as Astrobiological Indicators
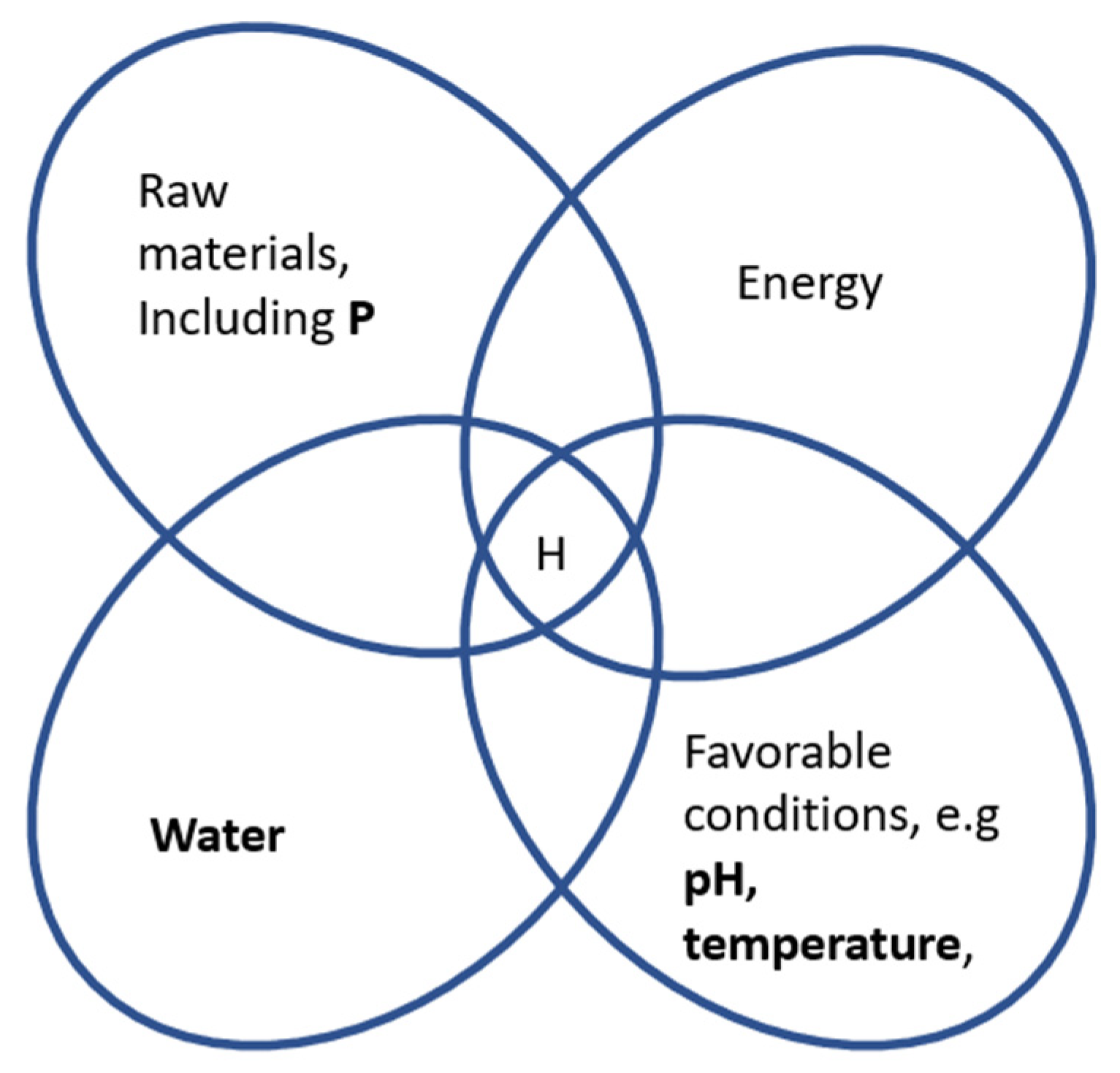
4.3.1. Habitability
4.3.2. Prebiotic Chemistry
4.3.3. Biological Mobilization of Phosphorus (P)
- (i)
- (ii)
- Microorganisms can release the anions of organic acids including citric, malic, malonic, oxalic, succinic, lactic, tartaric, gluconic, 2-ketogluconic, and glycolic acid [234,235]. Organic acids can increase the dissolution of P-bearing minerals by ligand-promoted dissolution and also cause the desorption of P from mineral surfaces by ligand exchange [232,236].
- (iii)
- (iv)
- Microorganisms can physically attach to phosphate-containing minerals. In laboratory cultures, phosphate-limited cyanobacteria have been shown to preferentially attach to fluorapatite surfaces [238].
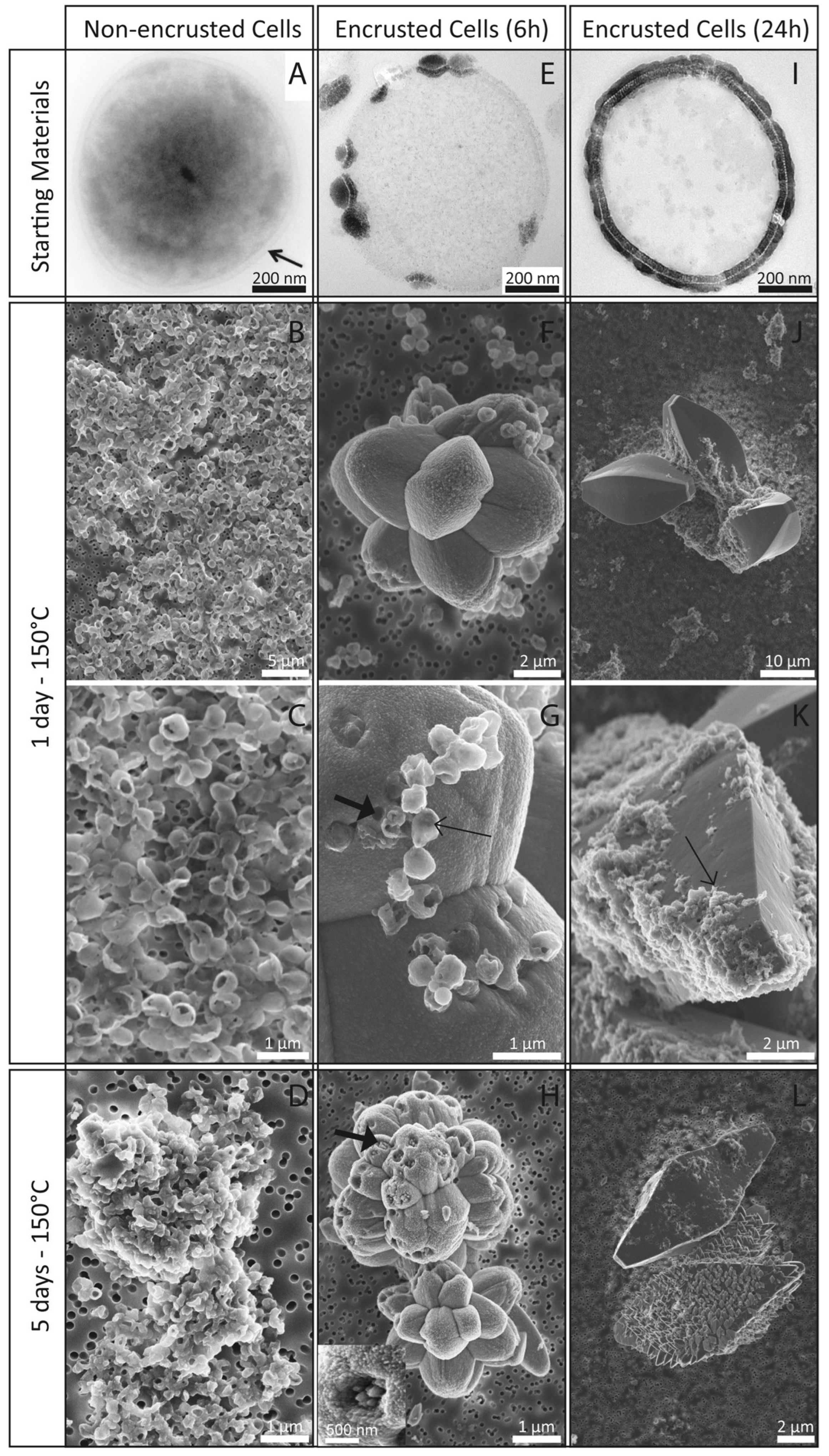
4.3.4. Microbially Mediated Biomineralization
4.3.5. Biologically Induced Mineralization (BIM)
4.3.6. Biologically Controlled Mineralization (BCM)
4.3.7. Oxygen Isotope Composition of Phosphate
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of Activated Pyrimidine Ribonucleotides in Prebiotically Plausible Conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef]
- Clark, F.E. Terrestrial Nitrogen Cycles; Clark, F.E., Rosswell, T., Eds.; Swedish Natural Science Research Council: Stockholm, Sweden, 1981; pp. 363–374. [Google Scholar]
- Chadwick, O.A.; Derry, L.A.; Vitousek, P.M.; Huebert, B.J.; Hedin, L.O. Changing Sources of Nutrients during Four Million Years of Ecosystem Development. Nature 1999, 397, 491–497. [Google Scholar] [CrossRef]
- Filippelli, G.M. The Global Phosphorus Cycle. Rev. Mineral. Geochem. 2002, 48, 391–425. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The Fate of Phosphorus during Pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Neaman, A.; Chorover, J.; Brantley, S.L. Element Mobility Patterns Record Organic Ligands in Soils on Early Earth. Geology 2005, 33, 117–120. [Google Scholar] [CrossRef]
- Horodyskyj, L.B.; White, T.S.; Kump, L.R. Substantial Biologically Mediated Phosphorus Depletion from the Surface of a Middle Cambrian Paleosol. Geology 2012, 40, 503–506. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Meloche, J.D.; Fyfe, W.S.; Murray, R.G.E. Diagenesis of Metals Chemically Complexed to Bacteria: Laboratory Formation of Metal Phosphates, Sulfides, and Organic Condensates in Artificial Sediments. Appl. Environ. Microbiol. 1983, 45, 1094–1108. [Google Scholar] [CrossRef]
- Kish, A.; Miot, J.; Lombard, C.; Guigner, J.-M.; Bernard, S.; Zirah, S.; Guyot, F. Preservation of Archaeal Surface Layer Structure During Mineralization. Sci. Rep. 2016, 6, 26152. [Google Scholar] [CrossRef]
- Miot, J.; Bernard, S.; Bourreau, M.; Guyot, F.; Kish, A. Experimental Maturation of Archaea Encrusted by Fe-Phosphates. Sci. Rep. 2017, 7, 16984. [Google Scholar] [CrossRef]
- Farmer, J.D.; Des Marais, D.J. Exploring for a Record of Ancient Martian Life. J. Geophys. Res. Planets 1999, 104, 26977–26995. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Arrhenius, G. Phosphates and Carbon on Mars: Exobiological Implications and Sample Return Considerations. J. Geophys. Res. Planets 1998, 103, 28495–28511. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-s. The Composition of the Earth. Chem. Evol. Mantle 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Taylor, G.J. The Bulk Composition of Mars. Geochemistry 2013, 73, 401–420. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Nekvasil, H. Maskelynite-Hosted Apatite in the Chassigny Meteorite. Am. Mineral. 2008, 93, 676–684. [Google Scholar] [CrossRef]
- Bridges, J.C.; Grady, M.M. Evaporite Mineral Assemblages in the Nakhlite (Martian) Meteorites. Earth Planet. Sci. Lett. 2000, 176, 267–279. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Blake, R.E.; Coath, C.D. Ion Microprobe Measurements of 18O/16O Ratios of Phosphate Minerals in the Martian Meteorites ALH84001 and Los Angeles. Geochim. Cosmochim. Acta 2003, 67, 2289–2298. [Google Scholar] [CrossRef]
- McSween, H.; Treiman, A.H. Martian Meteorites. In Planetary Materials; Papike, J., Ed.; Mineral Society of America: Washington, DC, USA, 1998; pp. F1–F53. [Google Scholar]
- Barnes, J.J.; McCubbin, F.M.; Santos, A.R.; Day, J.M.D.; Boyce, J.W.; Schwenzer, S.P.; Ott, U.; Franchi, I.A.; Messenger, S.; Anand, M.; et al. Multiple Early-Formed Water Reservoirs in the Interior of Mars. Nat. Geosci. 2020, 13, 260–264. [Google Scholar] [CrossRef]
- Brounce, M.; Boyce, J.W.; McCubbin, F.M. Sulfur in Apatite from the Nakhla Meteorite Record a Late-Stage Oxidation Event. Earth Planet. Sci. Lett. 2022, 595, 117784. [Google Scholar] [CrossRef]
- Filiberto, J.; Gross, J.; McCubbin, F.M. Constraints on the Water, Chlorine, and Fluorine Content of the Martian Mantle. Meteorit. Planet. Sci. 2016, 51, 2023–2035. [Google Scholar] [CrossRef]
- Filiberto, J.; Treiman, A.H. Martian Magmas Contained Abundant Chlorine, but Little Water. Geology 2009, 37, 1087–1090. [Google Scholar] [CrossRef]
- Gross, J.; Filiberto, J.; Bell, A.S. Water in the Martian Interior: Evidence for Terrestrial MORB Mantle-like Volatile Contents from Hydroxyl-Rich Apatite in Olivine–Phyric Shergottite NWA 6234. Earth Planet. Sci. Lett. 2013, 369–370, 120–128. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Boyce, J.W.; Srinivasan, P.; Santos, A.R.; Elardo, S.M.; Filiberto, J.; Steele, A.; Shearer, C.K. Heterogeneous Distribution of H2O in the Martian Interior: Implications for the Abundance of H2O in Depleted and Enriched Mantle Sources. Meteorit. Planet. Sci. 2016, 51, 2036–2060. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Shearer, C.K.; Burger, P.V.; Hauri, E.H.; Wang, J.; Elardo, S.M.; Papike, J.J. Volatile Abundances of Coexisting Merrillite and Apatite in the Martian Meteorite Shergotty: Implications for Merrillite in Hydrous Magmas. Am. Mineral. 2014, 99, 1347–1354. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Elardo, S.M.; Shearer, C.K., Jr.; Smirnov, A.; Hauri, E.H.; Draper, D.S. A Petrogenetic Model for the Comagmatic Origin of Chassignites and Nakhlites: Inferences from Chlorine-Rich Minerals, Petrology, and Geochemistry. Meteorit. Planet. Sci. 2013, 48, 819–853. [Google Scholar] [CrossRef]
- Shearer, C.K.; Messenger, S.; Sharp, Z.D.; Burger, P.V.; Nguyen, A.N.; McCubbin, F.M. Distinct Chlorine Isotopic Reservoirs on Mars. Implications for Character, Extent and Relative Timing of Crustal Interactions with Mantle-Derived Magmas, Evolution of the Martian Atmosphere, and the Building Blocks of an Early Mars. Geochim. Cosmochim. Acta 2018, 234, 24–36. [Google Scholar] [CrossRef]
- Shearer, C.K.; Burger, P.V.; Papike, J.J.; McCubbin, F.M.; Bell, A.S. Crystal Chemistry of Merrillite from Martian Meteorites: Mineralogical Recorders of Magmatic Processes and Planetary Differentiation. Meteorit. Planet. Sci. 2015, 50, 649–673. [Google Scholar] [CrossRef]
- Schmidt, M.E.; McCoy, T.J. The Evolution of a Heterogeneous Martian Mantle: Clues from K, P, Ti, Cr, and Ni Variations in Gusev Basalts and Shergottite Meteorites. Earth Planet. Sci. Lett. 2010, 296, 67–77. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M.; Forster, P.M. Readily Available Phosphate from Minerals in Early Aqueous Environments on Mars. Nat. Geosci. 2013, 6, 824–827. [Google Scholar] [CrossRef]
- Bridges, J.C.; Grady, M.M. A Halite-Siderite-Anhydrite-Chlorapatite Assemblage in Nakhla: Mineralogical Evidence for Evaporites on Mars. Meteorit. Planet. Sci. 1999, 34, 407–415. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Blake, R.E. Evidence for an Acidic Ocean on Mars from Phosphorus Geochemistry of Martian Soils and Rocks. Geology 2006, 34, 953–956. [Google Scholar] [CrossRef]
- Kizovski, T.V.; Schmidt, M.E.; O’Neil, L.; Klevang, D.; Tosca, N.; Tice, M.; Cable, M.; Hausrath, E.; Adcock, C.T.; Hurowitz, J.; et al. Fe-Phosphates in the Jezero Crater Fan: Implications for Habitability and Sample Return; Lunar and Planetary Institute: Houston, TX, USA, 2024; p. Abstract #2615. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter (O): Basel, Switzerland, 2016; pp. 1–30. ISBN 978-3-11-041710-4. [Google Scholar]
- Economou, T. Chemical Analyses of Martian Soil and Rocks Obtained by the Pathfinder Alpha Proton X-ray Spectrometer. Radiat. Phys. Chem. 2001, 61, 191–197. [Google Scholar] [CrossRef]
- Rieder, R.; Economou, T.; Wänke, H.; Turkevich, A.; Crisp, J.; Brückner, J.; Dreibus, G.; McSween, H.Y. The Chemical Composition of Martian Soil and Rocks Returned by the Mobile Alpha Proton X-ray Spectrometer: Preliminary Results from the X-ray Mode. Science 1997, 278, 1771–1774. [Google Scholar] [CrossRef]
- Gellert, R.; Rieder, R.; Brückner, J.; Clark, B.C.; Dreibus, G.; Klingelhöfer, G.; Lugmair, G.; Ming, D.W.; Wänke, H.; Yen, A.; et al. Alpha Particle X-ray Spectrometer (APXS): Results from Gusev Crater and Calibration Report. J. Geophys. Res. Planets 2006, 111, E02S05. [Google Scholar] [CrossRef]
- Usui, T.; McSween, H.Y., Jr.; Clark, B.C., III. Petrogenesis of High-Phosphorous Wishstone Class Rocks in Gusev Crater, Mars. J. Geophys. Res. Planets 2008, 113, E12S44. [Google Scholar] [CrossRef]
- Rieder, R.; Gellert, R.; Anderson, R.C.; Bruckner, J.; Clark, B.C.; Dreibus, G.; Economou, T.; Klingelhofer, G.; Lugmair, G.W.; Ming, D.W.; et al. Chemistry of Rocks and Soils at Meridiani Planum from the Alpha Particle X-ray Spectrometer. Science 2004, 306, 1746–1749. [Google Scholar] [CrossRef]
- Yen, A.S.; Ming, D.W.; Vaniman, D.T.; Gellert, R.; Blake, D.F.; Morris, R.V.; Morrison, S.M.; Bristow, T.F.; Chipera, S.J.; Edgett, K.S. Multiple Stages of Aqueous Alteration along Fractures in Mudstone and Sandstone Strata in Gale Crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Rampe, E.B.; Blake, D.F.; Bristow, T.F.; Ming, D.W.; Vaniman, D.T.; Morris, R.V.; Achilles, C.N.; Chipera, S.J.; Morrison, S.M.; Tu, V.M. Mineralogy and Geochemistry of Sedimentary Rocks and Eolian Sediments in Gale Crater, Mars: A Review after Six Earth Years of Exploration with Curiosity. Geochemistry 2020, 80, 125605. [Google Scholar] [CrossRef]
- Nachon, M.; Mangold, N.; Forni, O.; Kah, L.C.; Cousin, A.; Wiens, R.C.; Anderson, R.; Blaney, D.; Blank, J.G.; Calef, F. Chemistry of Diagenetic Features Analyzed by ChemCam at Pahrump Hills, Gale Crater, Mars. Icarus 2017, 281, 121–136. [Google Scholar] [CrossRef]
- Treiman, A.H.; Lanza, N.L.; VanBommel, S.; Berger, J.; Wiens, R.; Bristow, T.; Johnson, J.; Rice, M.; Hart, R.; McAdam, A. Manganese-Iron Phosphate Nodules at the Groken Site, Gale Crater, Mars. Minerals 2023, 13, 1122. [Google Scholar] [CrossRef]
- VanBommel, S.J.; Berger, J.A.; Gellert, R.; O’Connell-Cooper, C.D.; McCraig, M.A.; Thompson, L.M.; Fedo, C.M.; Des Marais, D.J.; Fey, D.M.; Yen, A.S. Elemental Composition of Manganese-and Phosphorus-Rich Nodules in the Knockfarril Hill Member, Gale Crater, Mars. Icarus 2023, 392, 115372. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Sullivan, R.; Goreva, Y.; Zorzano, M.P.; Cardarelli, E.; Vaughan, A.; Cousin, A.; Siljestrom, S.; Shumway, A.; VanBommel, S.; et al. The First Regolith Samples from Mars; Lunar and Planetary Institute: Houston, TX, USA, 2023; p. Abstract #2379. [Google Scholar]
- Herd, C.D.K.; Bosak, T.; Farley, K.A.; Stack, K.M.; Benison, K.C.; Cohen, B.A.; Czaja, A.D.; Debaille, V.; Goreva, Y.; Hausrath, E.M.; et al. Sampling by the NASA Perseverance Rover for Mars Sample Return; Lunar and Planetary Institute: Houston, TX, USA, 2023; p. Abstract #6140. [Google Scholar]
- Simon, J.I.; Hickman-Lewis, K.; Cohen, B.A.; Mayhew, L.E.; Shuster, D.L.; Debaille, V.; Hausrath, E.M.; Weiss, B.P.; Bosak, T.; Zorzano, M.-P.; et al. Samples Collected From the Floor of Jezero Crater With the Mars 2020 Perseverance Rover. J. Geophys. Res. Planets 2023, 128, e2022JE007474. [Google Scholar] [CrossRef]
- Gooding, J.L.; Wentworth, S.J.; Zolensky, M.E. Calcium Carbonate and Sulfate of Possible Extraterrestrial Origin in the EETA 79001 Meteorite. Geochim. Cosmochim. Acta 1988, 52, 909–915. [Google Scholar] [CrossRef]
- Hurowitz, J.A.; McLennan, S.; Tosca, N.; Arvidson, R.; Michalski, J.R.; Ming, D.W.; Schroder, C.; Squyres, S.W. In Situ and Experimental Evidence for Acidic Weathering of Rocks and Soils on Mars. J. Geophys. Res. 2006, 111, E02S19. [Google Scholar] [CrossRef]
- Ming, D.W.; Mittlefehldt, D.W.; Morris, R.V.; Golden, D.C.; Gellert, R.; Yen, A.; Clark, B.C.; Squyres, S.W.; Farrand, W.H.; Ruff, S.W.; et al. Geochemical and Mineralogical Indicators for Aqueous Processes in the Columbia Hills of Gusev Crater, Mars. J. Geophys. Res. 2006, 111, E02S12. [Google Scholar] [CrossRef]
- Boanini, E.; Pagani, S.; Tschon, M.; Rubini, K.; Fini, M.; Bigi, A. Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization. J. Funct. Biomater. 2022, 13, 65. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Dumitraş, D.-G.; Marincea, Ş.; Fransolet, A.-M. Brushite in the Bat Guano Deposit from the “Dry” Cioclovina Cave (Sureanu Mountains, Romania). Neues Jahrb. Fuer Mineral. Abh. 2004, 180, 45–64. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M. Weathering Profiles in High-P Rocks at Gusev Crater, Mars, Suggest Dissolution of Phosphate Minerals into near-Neutral Waters. Astrobiology 2015, 15, 1060–1075. [Google Scholar] [CrossRef]
- Rubin, A.; Ma, C. Meteorite Mineralogy; Cambridge University Press: Cambridge, UK, 2021; Volume 26. [Google Scholar]
- Mathew, M.; Takagi, S. Structures of Biological Minerals in Dental Research. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1035. [Google Scholar] [CrossRef]
- Lane, M.D.; Bishop, J.L.; Darby Dyar, M.; King, P.L.; Parente, M.; Hyde, B.C. Mineralogy of the Paso Robles Soils on Mars. Am. Mineral. 2008, 93, 728–739. [Google Scholar] [CrossRef]
- Blake, D.F.; Morris, R.V.; Kocurek, G.; Morrison, S.M.; Downs, R.T.; Bish, D.; Ming, D.W.; Edgett, K.S.; Rubin, D.; Goetz, W.; et al. Curiosity at Gale Crater, Mars: Characterization and Analysis of the Rocknest Sand Shadow. Science 2013, 341, 1239505. [Google Scholar] [CrossRef]
- Morris, R.V.; Vaniman, D.T.; Blake, D.F.; Gellert, R.; Chipera, S.J.; Rampe, E.B.; Ming, D.W.; Morrison, S.M.; Downs, R.T.; Treiman, A.H.; et al. Silicic Volcanism on Mars Evidenced by Tridymite in High-SiO2 Sedimentary Rock at Gale Crater. Proc. Natl. Acad. Sci. USA 2016, 113, 7071–7076. [Google Scholar] [CrossRef]
- Achilles, C.N.; Downs, R.T.; Ming, D.W.; Rampe, E.B.; Morris, R.V.; Treiman, A.H.; Morrison, S.M.; Blake, D.F.; Vaniman, D.T.; Ewing, R.C.; et al. Mineralogy of an Active Eolian Sediment from the Namib Dune, Gale Crater, Mars. J. Geophys. Res. Planets 2017, 122, 2344–2361. [Google Scholar] [CrossRef]
- Morrison, S.M.; Downs, R.T.; Blake, D.F.; Vaniman, D.T.; Ming, D.W.; Hazen, R.M.; Treiman, A.H.; Achilles, C.N.; Yen, A.S.; Morris, R.V.; et al. Crystal Chemistry of Martian Minerals from Bradbury Landing through Naukluft Plateau, Gale Crater, Mars. Am. Mineral. 2018, 103, 857–871. [Google Scholar] [CrossRef]
- Morris, R.V.; Rampe, E.B.; Vaniman, D.T.; Christoffersen, R.; Yen, A.S.; Morrison, S.M.; Ming, D.W.; Achilles, C.N.; Fraeman, A.A.; Le, L.; et al. Hydrothermal Precipitation of Sanidine (Adularia) Having Full Al,Si Structural Disorder and Specular Hematite at Maunakea Volcano (Hawai’i) and at Gale Crater (Mars). J. Geophys. Res. Planets 2020, 125, e2019JE006324. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Jones, R.H. Extraterrestrial Apatite: Planetary Geochemistry to Astrobiology. Elements 2015, 11, 183–188. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Taylor, J.; Glover, E.; Ball, A.; Nojorka, J. Nanocrystalline Fluorapatite Mineralization in the Calciphile Rock-Boring Bivalve Lithophaga: Functional and Phylogenetic Significance. Biol. J. Linn. Soc. 2023, 138, 229–245. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M.; Rampe, E.B.; Yang, H.; Downs, R.T. The Crystal Structure and Chemistry of Natural Giniite and Implications for Mars. Am. Mineral. 2023, 108, 430–438. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Tschauner, O. Natural Fumarolic Alteration of Fluorapatite, Olivine, and Basaltic Glass, and Implications for Habitable Environments on Mars. Astrobiology 2013, 13, 1049–1064. [Google Scholar] [CrossRef]
- McCollom, T.M.; Donaldson, C.; Moskowitz, B.; Berquó, T.S.; Hynek, B. Phosphorous Immobility During Formation of the Layered Sulfate Deposits of the Burns Formation at Meridiani Planum. J. Geophys. Res. Planets 2018, 123, 1230–1254. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Lou, X.; Wang, Z.; Xing, B. Biochar-Induced Negative Carbon Mineralization Priming Effects in a Coastal Wetland Soil: Roles of Soil Aggregation and Microbial Modulation. Sci. Total Environ. 2018, 610/611, 951–960. [Google Scholar] [CrossRef]
- Clark, B.C.; Van Hart, D.C. The Salts of Mars. Icarus 1981, 45, 370–378. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; LeGeros, J.P. Hydroxyapatite. In Bioceramics and Their Clinical Applications; Woodhead Publishing: Cambridge, UK, 2008; pp. 367–394. ISBN 978-1-84569-204-9. [Google Scholar]
- Hench, L.L.; Thompson, I. Twenty-First Century Challenges for Biomaterials. J. R. Soc. Interface 2010, 7, S379–S391. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M.; Forster, P.M.; Tschauner, O.; Sefein, K.J. Synthesis and Characterization of the Mars-Relevant Phosphate Minerals Fe-and Mg-Whitlockite and Merrillite and a Possible Mechanism That Maintains Charge Balance during Whitlockite to Merrillite Transformation. Am. Mineral. 2014, 99, 1221–1232. [Google Scholar] [CrossRef]
- Thorpe, M.T.; Bristow, T.F.; Rampe, E.B.; Grotzinger, J.P.; Fox, V.K.; Bennett, K.A.; Bryk, A.B.; Yen, A.S.; Vasavada, A.R.; Vaniman, D.T. The Mineralogy and Sedimentary History of the Glen Torridon Region, Gale Crater, Mars. In Proceedings of the LPSC 2021, Houston, TX, USA, 15–19 March 2021. [Google Scholar]
- Lanza, N.L.; Gasda, P.; Ari, E.; Comellas, J.; Caravaca, G.; Rampe, E.B.; Williams, A.J.; Meslin, P.-Y.; Dehouck, E.; Mangold, N. Chemistry of Manganese-Bearing Materials at the Groken Drill Site, Gale Crater, Mars. In Proceedings of the 52nd Lunar and Planetary Science Conference, Woodlands, TX, USA, 15–19 March 2021. [Google Scholar]
- Drouet, C.; Loche, M.; Fabre, S.; Meslin, P.-Y. On the Occurrence of Jahnsite/Whiteite Phases on Mars: A Thermodynamic Study. Am. Mineral. 2022, 107, 1807–1817. [Google Scholar] [CrossRef]
- Britvin, S.N.; Galuskina, I.O.; Vlasenko, N.S.; Vereshchagin, O.S.; Bocharov, V.N.; Krzhizhanovskaya, M.G.; Shilovskikh, V.V.; Galuskin, E.V.; Vapnik, Y.; Obolonskaya, E.V. Keplerite, Ca9(Ca0.5☐0.5)Mg(PO4)7, a New Meteoritic and Terrestrial Phosphate Isomorphous with Merrillite, Ca9NaMg(PO4)7. Am. Mineral. 2021, 106, 1917–1927. [Google Scholar] [CrossRef]
- Slabić, A. Shock-Induced Geochemical Variations in the Keplerite-Bearing Assemblages of Tissint and Intergrown Apatite-Merrillite Assemblages of ALH 84001, 146. PhD Thesis, University of Houston-Clear Lake, Houston, TX, USA, 2022. [Google Scholar]
- Adcock, C.T.; Tschauner, O.; Hausrath, E.M.; Udry, A.; Luo, S.N.; Cai, Y.; Ren, M.; Lanzirotti, A.; Newville, M.; Kunz, M.; et al. Shock-Transformation of Whitlockite to Merrillite and the Implications for Meteoritic Phosphate. Nat. Commun. 2017, 8, 14667. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. THE MATERIAL BONE: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Gu, L.; Hu, S.; Anand, M.; Tang, X.; Ji, J.; Zhang, B.; Wang, N.; Lin, Y. Occurrence of Tuite and Ahrensite in Zagami and Their Significance for Shock-Histories Recorded in Martian Meteorites. Am. Mineral. 2022, 107, 1018–1029. [Google Scholar] [CrossRef]
- Baziotis, I.P.; Liu, Y.; DeCarli, P.S.; Jay Melosh, H.; McSween, H.Y.; Bodnar, R.J.; Taylor, L.A. The Tissint Martian Meteorite as Evidence for the Largest Impact Excavation. Nat. Commun. 2013, 4, 1404. [Google Scholar] [CrossRef]
- Tu, V.M.; Hausrath, E.M.; Tschauner, O.; Iota, V.; Egeland, G.W. Dissolution Rates of Amorphous Al- and Fe-Phosphates and Their Relevance to Phosphate Mobility on Mars†. Am. Mineral. 2014, 99, 1206–1215. [Google Scholar] [CrossRef]
- Ruff, S.W.; Hamilton, V.E. Wishstone to Watchtower: Amorphous Alteration of Plagioclase-Rich Rocks in Gusev Crater, Mars. Am. Mineral. 2017, 102, 235–251. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Microbes as Geologic Agents: Their Role in Mineral Formation. Geomicrobiol. J. 1999, 16, 135–153. [Google Scholar] [CrossRef]
- Hazen, R.M.; Downs, R.T.; Morrison, S.M.; Tutolo, B.M.; Blake, D.F.; Bristow, T.F.; Chipera, S.J.; McSween, H.Y.; Ming, D.; Morris, R.V.; et al. On the Diversity and Formation Modes of Martian Minerals. J. Geophys. Res. Planets 2023, 128, e2023JE007865. [Google Scholar] [CrossRef]
- Brady, M.P.; Tostevin, R.; Tosca, N.J. Marine Phosphate Availability and the Chemical Origins of Life on Earth. Nat. Commun. 2022, 13, 5162. [Google Scholar] [CrossRef]
- Rothe, M.; Kleeberg, A.; Hupfer, M. The Occurrence, Identification and Environmental Relevance of Vivianite in Waterlogged Soils and Aquatic Sediments. Earth-Sci. Rev. 2016, 158, 516–564. [Google Scholar] [CrossRef]
- Jang, H.L.; Jin, K.; Lee, J.; Kim, Y.; Nahm, S.H.; Hong, K.S.; Nam, K.T. Revisiting Whitlockite, the Second Most Abundant Biomineral in Bone: Nanocrystal Synthesis in Physiologically Relevant Conditions and Biocompatibility Evaluation. ACS Nano 2014, 8, 634–641. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Beckett, J.R.; Chen, Y.; Guan, Y. Rare-Earth-Element Minerals in Martian Breccia Meteorites NWA 7034 and 7533: Implications for Fluid–Rock Interaction in the Martian Crust. Earth Planet. Sci. Lett. 2016, 451, 251–262. [Google Scholar] [CrossRef]
- do Nascimento-Dias, B.L. Combination between Ca, P and Y in the Martian Meteorite NWA 6963 Could Be Used as a Strategy to Indicate Liquid Water Reservoirs on Ancient Mars? Int. J. Astrobiol. 2019, 18, 151–156. [Google Scholar] [CrossRef]
- McSween, H.Y., Jr. SNC Meteorites: Are They Martian Rocks? Geology 1984, 12, 3–6. [Google Scholar] [CrossRef]
- McSween, H.Y. What We Have Learned about Mars from SNC Meteorites. Meteoritics 1994, 29, 757–779. [Google Scholar] [CrossRef]
- Warren, P.H. Lunar and Martian Meteorite Delivery Services. Icarus 1994, 111, 338–363. [Google Scholar] [CrossRef]
- Gattacceca, J.; McCubbin, F.M.; Grossman, J.N.; Schrader, D.L.; Chabot, N.L.; D’Orazio, M.; Goodrich, C.; Greshake, A.; Gross, J.; Joy, K.H.; et al. The Meteoritical Bulletin, No. 111. Meteorit. Planet. Sci. 2023, 58, 901–904. [Google Scholar] [CrossRef]
- Bogard, D.D.; Johnson, P. Martian Gases in an Antartic Meteorite? Science 1983, 221, 651–654. [Google Scholar] [CrossRef]
- McSween, H.Y. Petrology on Mars. Am. Mineral. 2015, 100, 2380–2395. [Google Scholar] [CrossRef]
- Udry, A.; Howarth, G.H.; Herd, C.D.K.; Day, J.M.D.; Lapen, T.J.; Filiberto, J. What Martian Meteorites Reveal About the Interior and Surface of Mars. J. Geophys. Res. Planets 2020, 125, 1–34. [Google Scholar] [CrossRef]
- Agee, C.B.; Wilson, N.V.; McCubbin, F.M.; Ziegler, K.; Polyak, V.J.; Sharp, Z.D.; Asmerom, Y.; Nunn, M.H.; Shaheen, R.; Thiemens, M.H.; et al. Unique Meteorite from Early Amazonian Mars: Water-Rich Basaltic Breccia Northwest Africa 7034. Science 2013, 339, 780–785. [Google Scholar] [CrossRef]
- Humayun, M.; Nemchin, A.; Zanda, B.; Hewins, R.H.; Grange, M.; Kennedy, A.; Lorand, J.P.; Göpel, C.; Fieni, C.; Pont, S.; et al. Origin and Age of the Earliest Martian Crust from Meteorite NWA 7533. Nature 2013, 503, 513–516. [Google Scholar] [CrossRef]
- Britvin, S.N.; Krivovichev, S.V.; Armbruster, T. Ferromerrillite, Ca9NaFe2+(PO4)7, a New Mineral from the Martian Meteorites, and Some Insights into Merrillite–Tuite Transformation in Shergottites. Eur. J. Mineral. 2016, 28, 125–136. [Google Scholar] [CrossRef]
- Hughes, J.M.; Jolliff, B.L.; Rakovan, J. The Crystal Chemistry of Whitlockite and Merrillite and the Dehydrogenation of Whitlockite to Merrillite. Am. Mineral. 2008, 93, 1300–1305. [Google Scholar] [CrossRef]
- Hughes, J.M.; Jolliff, B.L.; Gunter, M.E. The Atomic Arrangement of Merrillite from the Fra Mauro Formation, Apollo 14 Lunar Mission: The First Structure of Merrillite from the Moon. Am. Mineral. 2006, 91, 1547–1552. [Google Scholar] [CrossRef]
- Jolliff, B.L.; Hughes, J.M.; Freeman, J.J.; Zeigler, R.A. Crystal Chemistry of Lunar Merrillite and Comparison to Other Meteoritic and Planetary Suites of Whitlockite and Merrillite. Am. Mineral. 2006, 91, 1583–1595. [Google Scholar] [CrossRef]
- Dowty, E. Phosphate in Angra Dos Reis: Structure and Composition of the Ca3 (PO4)2 Minerals. Earth Planet. Sci. Lett. 1977, 35, 347–351. [Google Scholar] [CrossRef]
- Ionov, D.; Hofmann, A.; Merlet, C.; Gurenko, A.; Hellebrand, E.; Montagnac, G.; Gillet, P.; Prikhodko, V. Discovery of Whitlockite in Mantle Xenoliths: Inferences for Water- and Halogen-Poor Fluids and Trace Element Residence in the Terrestrial Upper Mantle. Earth Planet. Sci. Lett. 2006, 244, 201–217. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Zedgenizov, D.A. First Find of Merrillite, Ca3(PO4)2, in a Terrestrial Environment as an Inclusion in Lower-Mantle Diamond. Am. Mineral. 2022, 107, 1652–1655. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Phillips, B.L.; Adcock, C.T.; Tait, K.T.; Steele, A.; Vaughn, J.S.; Fries, M.D.; Atudorei, V.; Vander Kaaden, K.E.; Hausrath, E.M. Discreditation of Bobdownsite and the Establishment of Criteria for the Identification of Minerals with Essential Monofluorophosphate (PO3F2–). Am. Mineral. 2018, 103, 1319–1328. [Google Scholar] [CrossRef]
- Frondel, C. Whitlockite: A New Calcium Phosphate, Ca3(PO4)2. Am. Mineral. 1941, 26, 145–152. [Google Scholar]
- Howarth, G.H.; Liu, Y.; Chen, Y.; Pernet-Fisher, J.F.; Taylor, L.A. Postcrystallization Metasomatism in Shergottites: Evidence from the Paired Meteorites LAR 06319 and LAR 12011. Meteorit. Planet. Sci. 2016, 51, 2061–2072. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Boyce, J.W.; Novák-Szabó, T.; Santos, A.R.; Tartèse, R.; Muttik, N.; Domokos, G.; Vazquez, J.; Keller, L.P.; Moser, D.E.; et al. Geologic History of Martian Regolith Breccia Northwest Africa 7034: Evidence for Hydrothermal Activity and Lithologic Diversity in the Martian Crust: Geologic History of NWA 7034. J. Geophys. Res. Planets 2016, 121, 2120–2149. [Google Scholar] [CrossRef]
- Santos, A.R.; Agee, C.B.; McCubbin, F.M.; Shearer, C.K.; Burger, P.V.; Tartèse, R.; Anand, M. Petrology of Igneous Clasts in Northwest Africa 7034: Implications for the Petrologic Diversity of the Martian Crust. Geochim. Cosmochim. Acta 2015, 157, 56–85. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Zhang, J.; Hao, J.; Xing, W.; Zhang, T.; Yang, W.; Changela, H. Ancient Geologic Events on Mars Revealed by Zircons and Apatites from the Martian Regolith Breccia NWA 7034. Meteorit. Planet. Sci. 2019, 54, 850–879. [Google Scholar] [CrossRef]
- Xie, X.; Zhai, S.; Chen, M.; Yang, H. Tuite, γ-Ca3(PO4)2, Formed by Chlorapatite Decomposition in a Shock Vein of the Suizhou L6 Chondrite. Meteorit. Planet. Sci. 2013, 48, 1515–1523. [Google Scholar] [CrossRef]
- Xie, X.; Minitti, M.E.; Chen, M.; Mao, H.-K.; Wang, D.; Shu, J.; Fei, Y. Tuite, γ-Ca3(PO4)2: A New Mineral from the Suizhou L6 Chondrite. Eur. J. Mineral. 2003, 15, 1001–1005. [Google Scholar] [CrossRef]
- Balta, J.B.; Sanborn, M.E.; McSween, H.Y.; Wadhwa, M. Magmatic History and Parental Melt Composition of Olivine-Phyric Shergottite LAR 06319. Meteorit Planet Sci 2013, 48, 1359–1382. [Google Scholar] [CrossRef]
- Chowdhury, P.; Brounce, M.; Boyce, J.W.; McCubbin, F.M. The Oxidation State of Sulfur in Apatite of Martian Meteorite—Shergotty. J. Geophys. Res. Planets 2023, 128, e2022JE007634. [Google Scholar] [CrossRef]
- Darling, J.R.; White, L.F.; Kizovski, T.; Černok, A.; Moser, D.E.; Tait, K.T.; Dunlop, J.; Langelier, B.; Douglas, J.O.; Zhao, X.; et al. The Shocking State of Apatite and Merrillite in Shergottite Northwest Africa 5298 and Extreme Nanoscale Chlorine Isotope Variability Revealed by Atom Probe Tomography. Geochim. Cosmochim. Acta 2021, 293, 422–437. [Google Scholar] [CrossRef]
- Gross, J.; Filiberto, J.; Herd, C.D.; Daswani, M.M.; Schwenzer, S.P.; Treiman, A.H. Petrography, Mineral Chemistry, and Crystallization History of Olivine-phyric Shergottite NWA 6234: A New Melt Composition. Meteorit. Planet. Sci. 2013, 48, 854–871. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Anand, M.; Franchi, I.A.; Zhao, X.; Zhang, J.; Hao, J.; Zhang, T.; Yang, W.; Changela, H. Deuterium and 37Chlorine Rich Fluids on the Surface of Mars: Evidence From the Enriched Basaltic Shergottite Northwest Africa 8657. J. Geophys. Res. Planets 2020, 125, 1–15. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Zhang, J.; Hao, J.; Feng, L.; Xu, L.; Yang, W.; Yang, J. NanoSIMS Analyses of Apatite and Melt Inclusions in the GRV 020090 Martian Meteorite: Hydrogen Isotope Evidence for Recent Past Underground Hydrothermal Activity on Mars. Geochim. Cosmochim. Acta 2014, 140, 321–333. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Hauri, E.H.; Elardo, S.M.; Vander Kaaden, K.E.; Wang, J.; Shearer, C.K., Jr. Hydrous Melting of the Martian Mantle Produced Both Depleted and Enriched Shergottites. Geology 2012, 40, 683–686. [Google Scholar] [CrossRef]
- Slaby, E.; Koch-Mueller, M.; Foerster, H.-J.; Wirth, R.; Rhede, D.; Schreiber, A.; Schade, U. Determination of Volatile Concentrations in Fluorapatite of Martian Shergottite NWA 2975 by Combining Synchrotron FTIR, Raman Spectroscopy, EMPA, and TEM, and Inferences on the Volatile Budget of the Apatite Host-Magma. Meteorit Planet Sci 2016, 51, 390–406. [Google Scholar] [CrossRef]
- Hewins, R.H.; Humayun, M.; Barrat, J.A.; Zanda, B.; Lorand, J.P.; Pont, S.; Assayag, N.; Cartigny, P.; Yang, S.; Sautter, V. Northwest Africa 8694, a Ferroan Chassignite. Geochim. Cosmochim. Acta 2020, 282, 201–226. [Google Scholar] [CrossRef]
- Birski, L.; Slaby, E.; Chatzitheodoridis, E.; Wirth, R.; Majzner, K.; Kozub-Budzyn, G.A.; Slama, J.; Liszewska, K.M.; Kocjan, I.; Zagorska, A. Apatite from NWA 10153 and NWA 10645-the Key to Deciphering Magmatic and Fluid Evolution History in Nakhlites. Minerals 2019, 9, 695. [Google Scholar] [CrossRef]
- Davidson, J.; Wadhwa, M.; Hervig, R.L.; Stephant, A. Water on Mars. Earth Planet Sci. Lett. 2020, 552, 116597. [Google Scholar] [CrossRef]
- Boctor, N.Z.; Alexander, C.M.O.; Wang, J.; Hauri, E. The Sources of Water in Martian Meteorites. Geochim. Cosmochim. Acta 2003, 67, 3971–3989. [Google Scholar] [CrossRef]
- Baird, A.K.; Toulmin, P., 3rd; Clark, B.C.; Rose, H.J.J.; Keil, K.; Christian, R.P.; Gooding, J.L. Mineralogic and Petrologic Implications of Viking Geochemical Results from Mars: Interim Report. Science 1976, 194, 1288–1293. [Google Scholar] [CrossRef]
- Clark, B.C. Geochemical Components in Martian Soil. Geochim. Cosmochim. Acta 1993, 57, 4575–4581. [Google Scholar] [CrossRef]
- Clark, B.C.; Baird, A.K.; Weldon, R.J.; Tsusaki, D.M.; Schnabel, L.; Candelaria, M.P. Chemical Composition of Martian Fines. J. Geophys. Res. Solid Earth 1982, 87, 10059–10067. [Google Scholar] [CrossRef]
- Clark, B.C.; Baird, A.K.; Rose, H.J.; Toulmin, P.; Keil, K.; Castro, A.J.; Kelliher, W.C.; Rowe, C.D.; Evans, P.H. Inorganic Analyses of Martian Surface Samples at the Viking Landing Sites. Science 1976, 194, 1283–1288. [Google Scholar] [CrossRef]
- Brückner, J.; Dreibus, G.; Rieder, R.; Wänke, H. Refined Data of Alpha Proton X-ray Spectrometer Analyses of Soils and Rocks at the Mars Pathfinder Site: Implications for Surface Chemistry. J. Geophys. Res. 2003, 108, 8094. [Google Scholar] [CrossRef]
- Foley, C.N.; Economou, T.; Clayton, R.N. Final Chemical Results from the Mars Pathfinder Alpha Proton X-ray Spectrometer. J. Geophys. Res. Planets 2003, 108, 8096. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Hecht, M.H.; West, S.J.; Morookian, J.M.; Young, S.M.M.; Quinn, R.; Grunthaner, P.; Wen, X.; Weilert, M.; Cable, C.A. The MECA Wet Chemistry Laboratory on the 2007 Phoenix Mars Scout Lander. J. Geophys. Res. 2009, 114, E00A19. [Google Scholar] [CrossRef]
- Hurowitz, J.A.; McLennan, S.M.; McSween, H.Y.; DeSouza, P.A.J.; Klingelhofer, G. Mixing Relationships and the Effects of Secondary Alteration in the Wishstone and Watchtower Classes of Husband Hill, Gusev Crater, Mars. J. Geophys. Res. 2006, 111, E12S14. [Google Scholar] [CrossRef]
- Gellert, R.; Rieder, R.; Anderson, R.C.; Bruckner, J.; Clark, B.C.; Dreibus, G.; Economou, T.; Klingelhofer, G.; Lugmair, G.W.; Ming, D.W.; et al. Chemistry of Rocks and Soils in Gusev Crater from the Alpha Particle X-ray Spectrometer. Science 2004, 305, 829–832. [Google Scholar] [CrossRef]
- Ruff, S.W.; Christensen, P.R.; Blaney, D.L.; Farrand, W.H.; Johnson, J.R.; Michalski, J.R.; Moersch, J.E.; Wright, S.P.; Squyres, S.W. The Rocks of Gusev Crater as Viewed by the Mini-TES Instrument. J. Geophys. Res. 2006, 111, E12S18. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Golden, D.C.; Morris, R.V.; Agresti, D.G.; Ming, D.W. Acid Sulfate Alteration of Fluorapatite, Basaltic Glass and Olivine by Hydrothermal Vapors and Fluids: Implications for Fumarolic Activity and Secondary Phosphate Phases in Sulfate-Rich Paso Robles Soil at Gusev Crater, Mars. J. Geophys. Res. Planets 2013, 118, 1–13. [Google Scholar] [CrossRef]
- Gellert, R.; Yen, A.S. Elemental Analyses of Mars from Rovers Using the Alpha-Particle X-ray Spectrometer. In Remote Compositional Analysis: Techniques for Understanding Spectroscopy, Mineralogy, and Geochemistry of Planetary Surfaces; Cambridge University Press: Cambridge, UK, 2019; pp. 555–572. ISBN 978-1-107-18620-0. [Google Scholar]
- Arvidson, R.E.; Squyres, S.W.; Morris, R.V.; Knoll, A.H.; Gellert, R.; Clark, B.C.; Catalano, J.G.; Jolliff, B.L.; McLennan, S.M.; Herkenhoff, K.E.; et al. High Concentrations of Manganese and Sulfur in Deposits on Murray Ridge, Endeavour Crater, Mars. Am. Mineral. 2016, 101, 1389–1405. [Google Scholar] [CrossRef]
- Rampe, E.B.; Bristow, T.F.; Morris, R.V.; Morrison, S.M.; Achilles, C.N.; Ming, D.W.; Vaniman, D.T.; Blake, D.F.; Tu, V.M.; Chipera, S.J.; et al. Mineralogy of Vera Rubin Ridge From the Mars Science Laboratory CheMin Instrument. J. Geophys. Res. Planets 2020, 125, e2019JE006306. [Google Scholar] [CrossRef]
- Forni, O.; Gaft, M.; Toplis, M.J.; Clegg, S.M.; Maurice, S.; Wiens, R.C.; Mangold, N.; Gasnault, O.; Sautter, V.; Le Mouélic, S.; et al. First Detection of Fluorine on Mars: Implications for Gale Crater’s Geochemistry. Geophys. Res. Lett. 2015, 42, 1020–1028. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Ming, D.W.; Rampe, E.B.; Peretyazhko, T.S. Reactive Transport Modeling of Aqueous Alteration in the Murray Formation, Gale Crater, Mars. ACS Earth Space Chem. 2021, 5, 424–435. [Google Scholar] [CrossRef]
- Rampe, E.B.; Ming, D.W.; Blake, D.F.; Bristow, T.F.; Chipera, S.J.; Grotzinger, J.P.; Morris, R.V.; Morrison, S.M.; Vaniman, D.T.; Yen, A.S.; et al. Mineralogy of an Ancient Lacustrine Mudstone Succession from the Murray Formation, Gale Crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 172–185. [Google Scholar] [CrossRef]
- Dehouck, E.; McLennan, S.M.; Meslin, P.-Y.; Cousin, A. Constraints on Abundance, Composition, and Nature of X-ray Amorphous Components of Soils and Rocks at Gale Crater, Mars. J. Geophys. Res. Planets 2014, 119, 2640–2657. [Google Scholar] [CrossRef]
- Smith, R.J.; McLennan, S.M.; Achilles, C.N.; Dehouck, E.; Horgan, B.H.N.; Mangold, N.; Rampe, E.B.; Salvatore, M.; Siebach, K.L.; Sun, V. X-ray Amorphous Components in Sedimentary Rocks of Gale Crater, Mars: Evidence for Ancient Formation and Long-Lived Aqueous Activity. J. Geophys. Res. Planets 2021, 126, e2020JE006782. [Google Scholar] [CrossRef]
- Achilles, C.N.; Sutter, B.; Lybrand, R.; Ming, D.W.; McAdam, A.C.; Zaharescu, G.D.; Morse, Z.; Honniball, C.; Whelley, P.; Richardson, J.; et al. Investigating Icelandic Soils as an Analog for Pedogenic Processes on Mars; Lunar and Planetary Institute: Houston, TX, USA, 2022; p. Abstract #2472. [Google Scholar]
- Pandey, A.; Rampe, E.; Morris, R.V.; Peretyazhko, T.; Niles, P.B.; Archer, D.A.; Casbeer, P.; Clark, J.; Sutter, B.; Chipera, S.J.; et al. Cryo-Formation of Sulfate Salts: Implications for Ancient Aqueous Environments on Mars; Lunar and Planetary Institute: Houston, TX, USA, 2024; p. Abstract #2301. [Google Scholar]
- Berger, J.A.; Gellert, R.; Boyd, N.I.; King, P.L.; McCraig, M.A.; O’Connell-Cooper, C.D.; Schmidt, M.E.; Spray, J.G.; Thompson, L.M.; VanBommel, S.J.V.; et al. Elemental Composition and Chemical Evolution of Geologic Materials in Gale Crater, Mars: APXS Results from Bradbury Landing to the Vera Rubin Ridge. J. Geophys. Res. Planets 2020, 125, e2020JE006536. [Google Scholar] [CrossRef]
- Achilles, C.N.; Rampe, E.B.; Downs, R.T.; Bristow, T.F.; Ming, D.W.; Morris, R.V.; Vaniman, D.T.; Blake, D.F.; Yen, A.S.; McAdam, A.C.; et al. Evidence for Multiple Diagenetic Episodes in Ancient Fluvial-Lacustrine Sedimentary Rocks in Gale Crater, Mars. J. Geophys. Res. Planets 2020, 125, e2019JE006295. [Google Scholar] [CrossRef]
- Meslin, P.-Y.; Gasda, P.; L’Haridon, J.; Forni, O.; Lanza, N.; Lamm, S.; Johnson, J.R.; Wiens, R.C.; Thompson, L.; Rapin, W.; et al. Detection of Hydrous Manganese and Iron Oxides with Variable Phosphorus and Magnesium Contents in the Lacustrine Sediments of the Murray Formation, Gale, Mars. In Proceedings of the 49th Lunar and Planetary Science Conference, Houston, TX, USA, 19–23 March 2018. [Google Scholar]
- Berger, J.A. Supporting Information Document for Manganese Mobility in Gale Crater, Mars: Leached Bedrock and Localized Enrichments. J. Geophys. Res. Planets 2022, 127, e2021JE007171. [Google Scholar] [CrossRef]
- Berger, J.A.; Schmidt, M.E.; Izawa, M.R.M.; Gellert, R.; Ming, D.W.; Rampe, E.B.; VanBommel, S.J.; McAdam, A.C. Phosphate Stability in Diagenetic Fluids Constrains the Acidic Alteration Model for Lower Mt. Sharp Sedimentary Rocks in Gale Crater, Mars. In Proceedings of the 47th Lunar and Planetary Science Conference, Woodlands, TX, USA, 21–25 March 2016. [Google Scholar]
- Berger, J.A.; Ming, D.W.; Morris, R.V.; Peretyazhko, T.; Rampe, E.B.; Schmidt, M.E.; Tu, V. Importance of Sulfur for Phosphorus Mobility on the Martian Surface. In Proceedings of the AGU Fall Meeting 2022, Chicago, IL, USA, 12–16 December 2022; Volume 2022, p. P12C-01. [Google Scholar]
- Berger, J.A.; Ming, D.W.; Morris, R.V.; King, P.L.; Schmidt, M.E.; Tu, V.M. Aluminum Phosphate-Sulfate Minerals: The Fate of Mobile Phosphorus in Sulfur-Rich, Mars-Relevant Systems. In Proceedings of the 51st Lunar and Planetary Science Conference, Woodlands, TX, USA, 16–20 March 2020. [Google Scholar]
- Kronyak, R.E.; Kah, L.C.; Edgett, K.S.; Van Bommel, S.J.; Thompson, L.M.; Wiens, R.C.; Sun, V.Z.; Nachon, M. Mineral-Filled Fractures as Indicators of Multigenerational Fluid Flow in the Pahrump Hills Member of the Murray Formation, Gale Crater, Mars. Earth Space Sci. 2019, 6, 238–265. [Google Scholar] [CrossRef]
- Yen, A.S.; Morris, R.V.; Ming, D.W.; Schwenzer, S.P.; Sutter, B.; Vaniman, D.T.; Treiman, A.H.; Gellert, R.; Achilles, C.N.; Berger, J.A.; et al. Formation of Tridymite and Evidence for a Hydrothermal History at Gale Crater, Mars. J. Geophys. Res. Planets 2021, 126, e2020JE006569. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Ming, D.W.; Peretyazhko, T.S.; Rampe, E.B. Reactive Transport and Mass Balance Modeling of the Stimson Sedimentary Formation and Altered Fracture Zones Constrain Diagenetic Conditions at Gale Crater, Mars. Earth Planet. Sci. Lett. 2018, 491, 1–10. [Google Scholar] [CrossRef]
- Farley, K.A.; Williford, K.H.; Stack, K.M.; Bhartia, R.; Chen, A.; de la Torre, M.; Hand, K.; Goreva, Y.; Herd, C.D.K.; Hueso, R.; et al. Mars 2020 Mission Overview. Space Sci. Rev. 2020, 216, 142. [Google Scholar] [CrossRef]
- Udry, A.; Ostwald, A.; Sautter, V.; Cousin, A.; Beyssac, O.; Forni, O.; Dromart, G.; Benzerara, K.; Nachon, M.; Horgan, B.; et al. A Mars 2020 Perseverance SuperCam Perspective on the Igneous Nature of the Máaz Formation at Jezero Crater and Link With Séítah, Mars. J. Geophys. Res. Planets 2023, 128, e2022JE007440. [Google Scholar] [CrossRef]
- Beyssac, O.; Forni, O.; Cousin, A.; Udry, A.; Kah, L.C.; Mandon, L.; Clavé, O.E.; Liu, Y.; Poulet, F.; Quantin Nataf, C.; et al. Petrological Traverse of the Olivine Cumulate Séítah Formation at Jezero Crater, Mars: A Perspective From SuperCam Onboard Perseverance. J. Geophys. Res. Planets 2023, 128, e2022JE007638. [Google Scholar] [CrossRef]
- Scheller, E.L.; Razzell Hollis, J.; Cardarelli, E.L.; Steele, A.; Beegle, L.W.; Bhartia, R.; Conrad, P.; Uckert, K.; Sharma, S.; Ehlmann, B.L.; et al. Aqueous Alteration Processes in Jezero Crater, Mars—Implications for Organic Geochemistry. Science 2022, 378, 1105–1110. [Google Scholar] [CrossRef]
- Kizovski, T.V.; Schmidt, M.E.; Liu, Y.; Clark, B.C.; Tice, M.; Herd, C.D.K.; Hurowitz, J.; VanBommel, S.; Henley, T.; Allwood, A. Minor Minerals Analyzed by PIXL—A Major Part of Igneous Rock Petrogenesis at Jezero Crater; Lunar and Planetary Institute: Houston, TX, USA, 2022; p. Abstract #2384. [Google Scholar]
- Kizovski, T.V.; O’Neil, L.; Schmidt, M.; Hurowitz, J.; Treiman, A.; Pedersen, D.; Liu, Y.; Tice, M.; Herd, C.; Labrie, J.; et al. Minor Minerals in the Jezero Crater Delta Analyzed by PIXL—Provenance and Mars Sample Return Implications; Lunar and Planetary Institute: Houston, TX, USA, 2023; p. Abstract #2855. [Google Scholar]
- Liu, Y.; Tice, M.M.; Schmidt, M.E.; Treiman, A.H.; Kizovski, T.V.; Hurowitz, J.A.; Allwood, A.C.; Henneke, J.; Pedersen, D.A.K.; VanBommel, S.J.; et al. An Olivine Cumulate Outcrop on the Floor of Jezero Crater, Mars. Science 2022, 377, 1513–1519. [Google Scholar] [CrossRef]
- Allwood, A.C.; Wade, L.A.; Foote, M.C.; Elam, W.T.; Hurowitz, J.A.; Battel, S.; Dawson, D.E.; Denise, R.W.; Ek, E.M.; Gilbert, M.S.; et al. PIXL: Planetary Instrument for X-ray Lithochemistry. Space Sci. Rev. 2020, 216, 134. [Google Scholar] [CrossRef]
- Farley, K.A.; Stack, K.M.; Shuster, D.L.; Horgan, B.H.N.; Hurowitz, J.A.; Tarnas, J.D.; Simon, J.I.; Sun, V.Z.; Scheller, E.L.; Moore, K.R.; et al. Aqueously Altered Igneous Rocks Sampled on the Floor of Jezero Crater, Mars. Science 2022, 377, eabo2196. [Google Scholar] [CrossRef]
- Tice, M.M.; Hurowitz, J.A.; Allwood, A.C.; Jones, M.W.M.; Orenstein, B.J.; Davidoff, S.; Wright, A.P.; Pedersen, D.A.K.; Henneke, J.; Tosca, N.J.; et al. Alteration History of Séítah Formation Rocks Inferred by PIXL X-ray Fluorescence, X-ray Diffraction, and Multispectral Imaging on Mars. Sci. Adv. 2022, 8, eabp9084. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S. Planetary Crusts: Their Composition, Origin and Evolution, 1st ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN 0-521-14201-6. [Google Scholar]
- Yen, A.S.; Gellert, R.; Schröder, C.; Morris, R.V.; Bell, J.F.; Knudson, A.T.; Clark, B.C.; Ming, D.W.; Crisp, J.A.; Arvidson, R.E.; et al. An Integrated View of the Chemistry and Mineralogy of Martian Soils. Nature 2005, 436, 49–54. [Google Scholar] [CrossRef]
- Ming, D.W.; Gellert, R.; Morris, R.V.; Arvidson, R.E.; Brückner, J.; Clark, B.C.; Cohen, B.A.; d’Uston, C.; Economou, T.; Fleischer, I.; et al. Geochemical Properties of Rocks and Soils in Gusev Crater, Mars: Results of the Alpha Particle X-ray Spectrometer from Cumberland Ridge to Home Plate. J. Geophys. Res. 2008, 113, 28. [Google Scholar] [CrossRef]
- Gellert, R. MER APXS Derived Oxide Data Bundle; NASA: Washington, DC, USA, 2004.
- Van Bommel, S.J. MER APXS Derived Oxide Data Bundle; NASA: Washington, DC, USA, 2020.
- Gellert, R. MSL MARS Alpha Particle X-ray Spectrometer 4/5 RDR V1.0. NASA Planetary Data System; NASA: Washington, DC, USA, 2013. [CrossRef]
- Gellert, R. MSL APXS Calibrated Data Collection; NASA: Washington, DC, USA, 2012.
- Thompson, L.M.; Spray, J.G.; O’Connell-Cooper, C.; Berger, J.A.; Yen, A.; Gellert, R.; Boyd, N.; McCraig, M.A.; VanBommel, S.J. Alteration at the Base of the Siccar Point Unconformity and Further Evidence for an Alkaline Provenance at Gale Crater: Exploration of the Mount Sharp Group, Greenheugh Pediment Cap Rock Contact With APXS. J. Geophys. Res. Planets 2022, 127, e2021JE007178. [Google Scholar] [CrossRef]
- Goetz, W.; Bertelsen, P.; Binau, C.S.; Gunnlaugsson, H.P.; Hviid, S.F.; Kinch, K.M.; Madsen, D.E.; Madsen, M.B.; Olsen, M.; Gellert, R.; et al. Indication of Drier Periods on Mars from the Chemistry and Mineralogy of Atmospheric Dust. Nature 2005, 436, 62–65. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Perrett, G.M.; Bray, S.L.; Bradley, N.J.; Lee, R.E.; Berger, J.A.; Campbell, J.L.; Ly, C.; Squyres, S.W.; Tesselaar, D. Dusty Rocks in Gale Crater: Assessing Areal Coverage and Separating Dust and Rock Contributions in APXS Analyses. J. Geophys. Res. Planets 2018, 123, 1649–1673. [Google Scholar] [CrossRef]
- VanBommel, S.J.; Knight, A.L.; Schmidt, M.E.; Yingst, R.A.; Henneke, J.; Klevang, D.; Allwood, A.C.; Hurowitz, J.A.; Tice, M.M.; Cable, M.L.; et al. Compositional and Volumetric Analyses of Dust on the PIXL Calibration Target; Lunar and Planetary Institute: Houston, TX, USA, 2024; p. Abstract #1598. [Google Scholar]
- Piccoli, P.M.; Candela, P.A. Apatite in Igneous Systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Adcock, C.T.; Udry, A.; Hauausrath, E.M.; Tschauauner, O. Craters of the Moon National Monument Basalts as Unshocked Compositional and Weathering Analogs for Martian Rocks and Meteorites. Am. Mineral. 2018, 103, 502–516. [Google Scholar] [CrossRef]
- Dymek, R.F.; Owens, B.E. Petrogenesis of Apatite-Rich Rocks (Nelsonites and Oxide-Apatite Gabbronorites) Associated with Massif Anorthosites. Econ. Geol. 2001, 96, 797–815. [Google Scholar] [CrossRef]
- Ihlen, P.M.; Schiellerup, H.; Gautneb, H.; Skår, Ø. Characterization of Apatite Resources in Norway and Their REE Potential—A Review. Ore Geol. Rev. 2014, 58, 126–147. [Google Scholar] [CrossRef]
- Tollari, N.; Toplis, M.J.; Barnes, S.-J. Predicting Phosphate Saturation in Silicate Magmas: An Experimental Study of the Effects of Melt Composition and Temperature. Geochim. Cosmochim. Acta 2006, 70, 1518–1536. [Google Scholar] [CrossRef]
- Krasnova, N.I.; Petrov, T.G.; Balaganskaya, E.G.; Garcia, D.; Moutte, J.; Zaitsev, A.N.; Wall, F. Introduction to Phoscorites: Occurrence, Composition, Nomenclature and Petrogenesis. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Wall, F., Zaitsev, A.N., Eds.; Mineralogical Society of Great Britain and Ireland: Middlesex, UK, 2004; ISBN 978-0-903056-22-9. [Google Scholar]
- Crews, T.E.; Kitayama, K.; Fownes, J.H.; Riley, R.H.; Herbert, D.A.; Mueller-Dombois, D.; Vitousek, P.M. Changes in Soil Phosphorus Fractions and Ecosystem Dynamics across a Long Chronosequence in Hawaii. Ecology 1995, 76, 1407–1424. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Farrington, H. Nutrient Limitation and Soil Development: Experimental Test of a Biogeochemical Theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem Properties and Forest Decline in Contrasting Long-Term Chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef]
- Brucker, E.; Spohn, M. Formation of Soil Phosphorus Fractions along a Climate and Vegetation Gradient in the Coastal Cordillera of Chile. Catena 2019, 180, 203–211. [Google Scholar] [CrossRef]
- Roncal-Herrero, T.; Oelkers, E.H. Does Variscite Control Phosphate Availability in Acidic Natural Waters? An Experimental Study of Variscite Dissolution Rates. Geochim. Cosmochim. Acta 2011, 75, 416–426. [Google Scholar] [CrossRef]
- Wilson, S.G.; Dahlgren, R.A.; Margenot, A.J.; Rasmussen, C.; O’Geen, A.T. Expanding the Paradigm: The Influence of Climate and Lithology on Soil Phosphorus. Geoderma 2022, 421, 115809. [Google Scholar] [CrossRef]
- Selmants, P.C.; Hart, S.C. Phosphorus and Soil Development: Does the Walker and Syers Model Apply to Semiarid Ecosystems? Ecology 2010, 91, 474–484. [Google Scholar] [CrossRef]
- Lindsay, W.L. Phosphates. In Chemical Equilibria in Soils; Wiley: Hoboken, NJ, USA, 1979; p. 478. ISBN 978-0-471-02704-1. [Google Scholar]
- Quinn, R.C.; Chittenden, J.D.; Kounaves, S.P.; Hecht, M.H. The Oxidation-Reduction Potential of Aqueous Soil Solutions at the Mars Phoenix Landing Site. Geophys. Res. Lett. 2011, 38, L14202. [Google Scholar] [CrossRef]
- Bandstra, J.Z.; Brantley, S.L. Data Fitting Techniques with Applications to Mineral Dissolution Kinetics. In Kinetics of Water-Rock Interaction; Brantley, S.L., Kubicki, J.D., White, A.F., Eds.; Springer: New York, NY, USA, 2008; pp. 211–257. ISBN 978-0-387-73563-4. [Google Scholar]
- Huffman, E.O.; Cate, W.E.; Deming, M.E. Rates and mechanisms of dissolution of some ferric phosphates. Soil Sci. 1960, 90, 8–15. [Google Scholar] [CrossRef]
- Thinnappan, V.; Merrifield, C.M.; Islam, F.S.; Polya, D.A.; Wincott, P.; Wogelius, R.A. A Combined Experimental Study of Vivianite and As (V) Reactivity in the pH Range 2–11. Arsen. Groundw. South-East Asia Emphas. Cambodia Vietnam 2008, 23, 3187–3204. [Google Scholar] [CrossRef]
- Vitarella, D.; Moss, O.; Dorman, D.C. Pulmonary Clearance of Manganese Phosphate, Manganese Sulfate, and Manganese Tetraoxide by CD Rats Following Intratracheal Instillation. Inhal. Toxicol. 2000, 12, 941–957. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Vlek, P.L.G.; Chien, S.H. Phosphate Minerals. In Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; p. 1244. [Google Scholar]
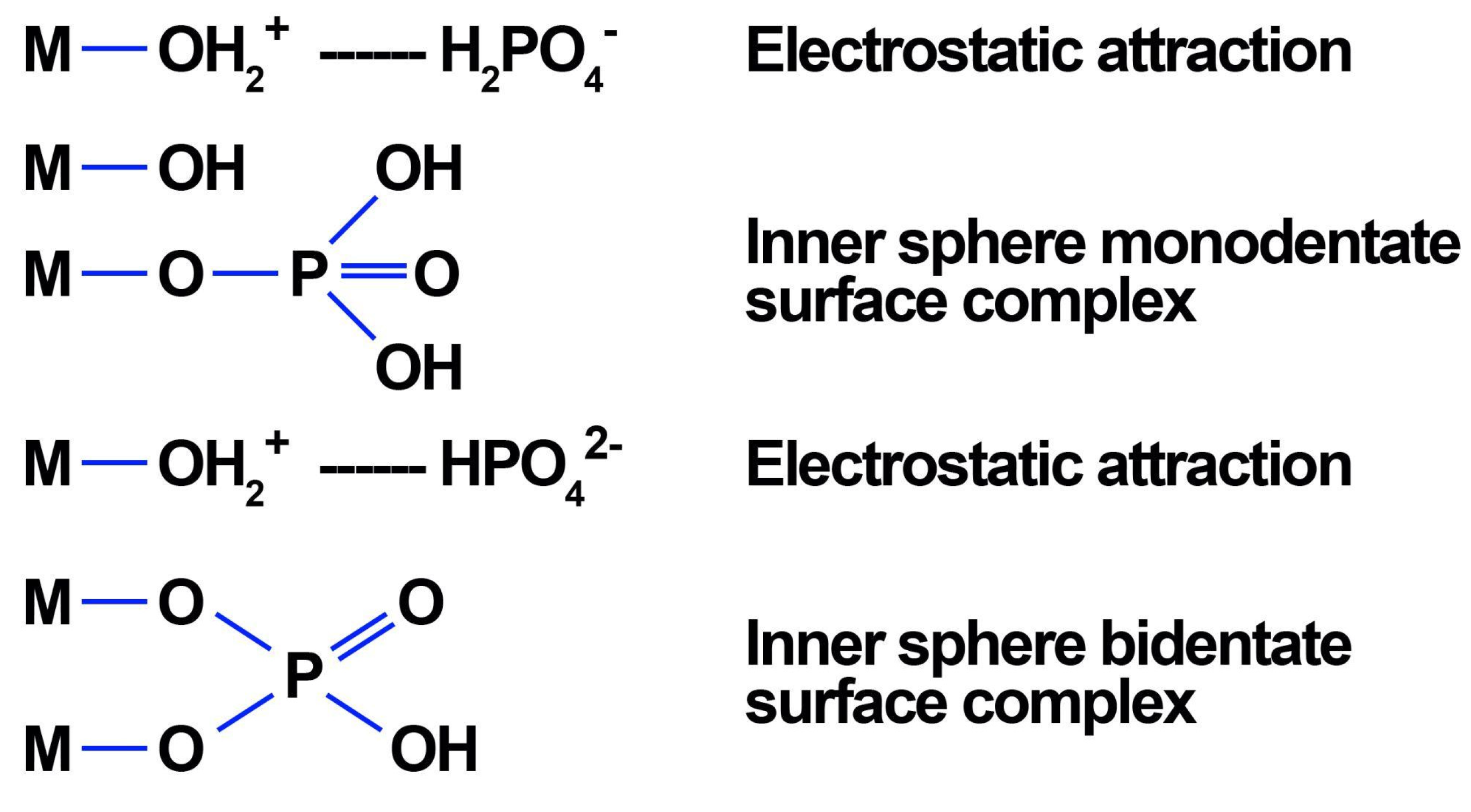
- Kwon, K.D.; Kubicki, J.D. Molecular Orbital Theory Study on Surface Complex Structures of Phosphates to Iron Hydroxides: Calculation of Vibrational Frequencies and Adsorption Energies. Langmuir 2004, 20, 9249–9254. [Google Scholar] [CrossRef]
- Rampe, E.B.; Morris, R.V.; Archer, P.D.; Agresti, D.G.; Ming, D.W. Recognizing Sulfate and Phosphate Complexes Chemisorbed onto Nanophase Weathering Products on Mars Using In-Situ and Remote Observationsk. Am. Mineral. 2016, 101, 678–689. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing Phosphate Adsorption by Mg/Al Layered Double Hydroxide Functionalized Biochar with Different Mg/Al Ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Roncal-Herrero, T.; Rodríguez-Blanco, J.D.; Benning, L.G.; Oelkers, E.H. Precipitation of Iron and Aluminum Phosphates Directly from Aqueous Solution as a Function of Temperature from 50 to 200 °C. Cryst. Growth Des. 2009, 9, 5197–5205. [Google Scholar] [CrossRef]
- Hsu, P.H. Crystallization of Iron(III) Phosphate at Room-Temperature. Soil Sci. Soc. Am. J. 1982, 46, 928–932. [Google Scholar] [CrossRef]
- Hsu, P.H. Crystallization of Variscite At Room Temperature. Soil Sci. 1982, 133, 305–313. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Dell, C.I. Diagenetic Formation of Iron Phosphates in Recent Lake Sediments. Am. Mineral. 1974, 59, 13. [Google Scholar]
- Tosca, N.J.; McLennan, S.M.; Lindsley, D.H.; Schoonen, M.A.A. Acid-Sulfate Weathering of Synthetic Martian Basalt: The Acid Fog Model Revisited. J. Geophys. Res. 2004, 109, E05003. [Google Scholar] [CrossRef]
- Golden, D.C.; Stewart, R.B.; Tillman, R.W.; White, R.E. Partially Acidulated Reactive Phosphate Rock (PAPR) Fertilizer and Its Reactions in Soil. Fertil. Res. 1991, 28, 295–304. [Google Scholar] [CrossRef]
- Tang, R.; Hass, M.; Wu, W.; Gulde, S.; Nancollas, G.H. Constant Composition Dissolution of Mixed Phases. II. Selective Dissolution of Calcium Phosphates. J. Colloid Interface Sci. 2003, 260, 379–384. [Google Scholar] [CrossRef]
- Borg, L.; Drake, M.J. A Review of Meteorite Evidence for the Timing of Magmatism and of Surface or Near-Surface Liquid Water on Mars. J Geophys Res 2005, 110, E12S03. [Google Scholar] [CrossRef]
- Boyle, F.W.; Lindsay, W.L. Manganese Phosphate Equilibrium Relationships in Soils1. Soil Sci. Soc. Am. J. 1986, 50, 588–593. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. Planetary Crusts: Their Composition, Origin, and Evolution; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. GSA Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
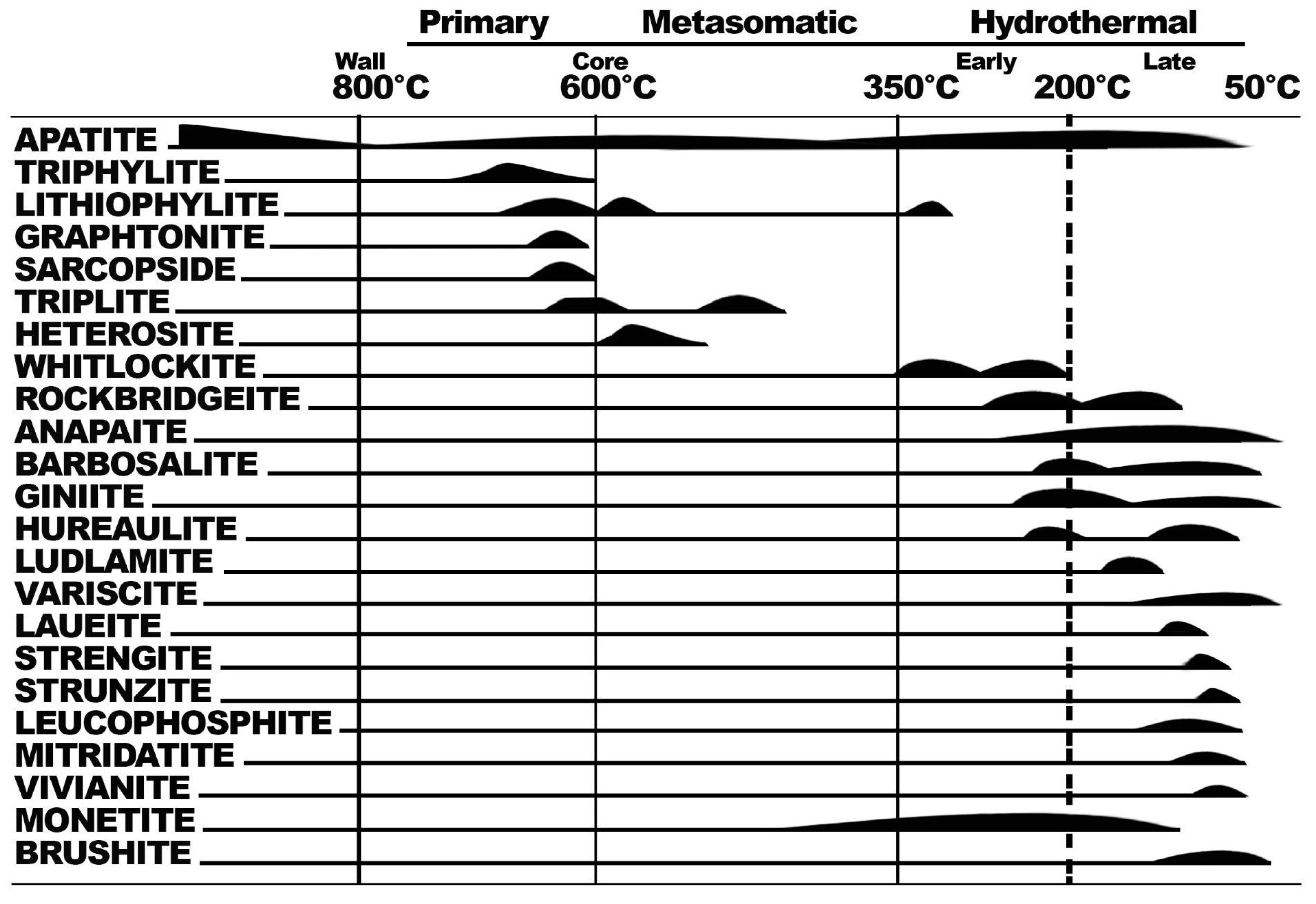
- Fisher, D.J. Pegmatite Phosphates and Their Problems. Am. Mineral. 1958, 43, 27. [Google Scholar]
- Dill, H.G.; Melcher, F.; Gerdes, A.; Weber, B. The Origin and Zoning of Hypogene and Supergene Fe-Mn-Mg-Sc-U-REE Phosphate Mineralization from the Newly Discovered Trutzhofmuhle Aplite, Hagendorf Pegmatite Province, Germany. Can Miner. 2008, 46, 1131–1157. [Google Scholar] [CrossRef]
- Hawthorne, F.C. Structure and Chemistry of Phosphate Minerals. Miner. Mag 1998, 62, 141–164. [Google Scholar] [CrossRef]
- Moore, P.B. Pegmatite Phosphates: Descriptive Mineralogy and Crystal Chemistry. Mineral. Rec. 1973, 4, 28. [Google Scholar]
- Hays, L.E.; Graham, H.V.; Des Marais, D.J.; Hausrath, E.M.; Horgan, B.; McCollom, T.M.; Parenteau, M.N.; Potter-McIntyre, S.L.; Williams, A.J.; Lynch, K.L. Biosignature Preservation and Detection in Mars Analog Environments. Astrobiology 2017, 17, 363–400. [Google Scholar] [CrossRef]
- Hoehler, T.M. An Energy Balance Concept for Habitability. Astrobiology 2007, 7, 824–838. [Google Scholar] [CrossRef]
- Reeves, J.P.; Dowben, R.M. Formation and Properties of Thin-walled Phospholipid Vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef]
- Deamer, D.W.; Oro, J. Role of Lipids in Prebiotic Structures. Biosystems 1980, 12, 167–175. [Google Scholar] [CrossRef]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of Phospholipids and Membranes in Prebiotic Conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef]
- Gulick, A. Phosphorus as a Factor in the Origin of Life. Am. Sci. 1955, 43, 479–489. [Google Scholar]
- Pasek, M.; Lauretta, D. Extraterrestrial Flux of Potentially Prebiotic C, N, and P to the Early Earth. Orig. Life Evol. Biosph. 2008, 38, 5–21. [Google Scholar] [CrossRef]
- Pasek, M.; Block, K. Lightning-Induced Reduction of Phosphorus Oxidation State. Nat. Geosci 2009, 2, 553–556. [Google Scholar] [CrossRef]
- Ritson, D.J.; Mojzsis, S.J.; Sutherland, J.D. Supply of Phosphate to Early Earth by Photogeochemistry after Meteoritic Weathering. Nat. Geosci. 2020, 13, 344–348. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C. A Carbonate-Rich Lake Solution to the Phosphate Problem of the Origin of Life. Proc. Natl. Acad. Sci. USA 2020, 117, 883–888. [Google Scholar] [CrossRef]
- McGill, W.B.; Cole, C.V. Comparative Aspects of Cycling of Organic C, N, S and P through Soil Organic Matter. Geoderma 1981, 26, 267–286. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Solubilization of Inorganic Phosphates by Microorganisms Isolated from Forest Soils. Soil Biol. Biochem. 1992, 24, 389–395. [Google Scholar] [CrossRef]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–198. ISBN 978-3-642-15271-9. [Google Scholar]
- Rogers, J.R.; Bennett, P.C. Mineral Stimulation of Subsurface Microorganisms: Release of Limiting Nutrients from Silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh Kumar, G.; Parekh, L.J.; Poole, P.S. Role of Soil Microorganisms in Improving P Nutrition of Plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Kucey, R.M.N.; Janzen, H.H.; Leggett, M.E. Microbially Mediated Increases in Plant-Available Phosphorus. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1989; Volume 42, pp. 199–228. [Google Scholar]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus Saturation and pH Differentially Regulate the Efficiency of Organic Acid Anion-Mediated P Solubilization Mechanisms in Soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Banfield, J.F.; Barker, W.W.; Welch, S.A.; Taunton, A. Biological Impact on Mineral Dissolution: Application of the Lichen Model to Understanding Mineral Weathering in the Rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar] [CrossRef]
- Schaperdoth, I.; Liermann, L.J.; Brantley, S.L. The Effect of Polymeric Substances on Apatite Reactivity in the Presence of a Freshwater Cyanobacterium. Geomicrobiol. J. 2007, 24, 79–91. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Neaman, A.; Brantley, S.L. Elemental Release Rates from Dissolving Basalt and Granite with and without Organic Ligands. Am. J. Sci. 2009, 309, 633–660. [Google Scholar] [CrossRef]
- Neaman, A.; Chorover, J.; Brantley, S.L. Effects of Organic Ligands on Granite Dissolution in Batch Experiments at pH 6. Am. J. Sci. 2006, 306, 451–473. [Google Scholar] [CrossRef]
- Neaman, A.; Chorover, J.; Brantley, S.L. Implications of the Evolution of Organic Acid Moieties for Basalt Weathering over Geological Time. Am. J. Sci. 2005, 305, 147–185. [Google Scholar] [CrossRef]
- Goyne, K.W.; Brantley, S.L.; Chorover, J. Rare Earth Element Release from Phosphate Minerals in the Presence of Organic Acids. Chem. Geol. 2010, 278, 1–14. [Google Scholar] [CrossRef]
- Goyne, K.W.; Brantley, S.L.; Chorover, J. Effects of Organic Acids and Dissolved Oxygen on Apatite and Chalcopyrite Dissolution: Implications for Using Elements as Organomarkers and Oxymarkers. Chem. Geol. 2006, 234, 28–45. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyajima, K.; Nakagaki, M.; Shimabayashi, S. Interactions of Aspartic Acid, Alanie, and Lysine with Hydroxyapatite. Chem. Pharm. Bull. 1989, 37, 2897–2901. [Google Scholar] [CrossRef]
- Hsu, J.; Fox, J.L.; Powell, G.L.; Otsuka, M.; Higuchi, W.I.; Yu, D.; Wong, J.; LeGeros, R.Z. Quantitative Relationship between Carbonated Apatite Metastable Equilibrium Solubility and Dissolution Kinetics. J. Colloid Interface Sci. 1994, 168, 356–372. [Google Scholar] [CrossRef]
- Welch, S.A.; Taunton, A.E.; Banfield, J.F. Effect of Microorganisms and Microbial Metabolites on Apatite Dissolution. Geomicrobiol. J. 2002, 19, 343–367. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Cui, J.; Wei, Z. Interaction between Low Molecular Weight Organic Acids and Hydroxyapatite with Different Degrees of Crystallinity. Colloids Surf. Physicochem. Eng. Asp. 2011, 392, 67–75. [Google Scholar] [CrossRef]
- Bartlett, C.L.; Hausrath, E.M.; Adcock, C.T.; Huang, S.; Harrold, Z.R.; Udry, A. Effects of Organic Compounds on Dissolution of the Phosphate Minerals Chlorapatite, Whitlockite, Merrillite, and Fluorapatite: Implications for Interpreting Past Signatures of Organic Compounds in Rocks, Soils and Sediments. Astrobiology 2018, 18, 1543–1558. [Google Scholar] [CrossRef]
- Driese, S.G.; Jirsa, M.A.; Ren, M.; Brantley, S.L.; Sheldon, N.D.; Parker, D.; Schmitz, M. Neoarchean Paleoweathering of Tonalite and Metabasalt: Implications for Reconstructions of 2.69Ga Early Terrestrial Ecosystems and Paleoatmospheric Chemistry. Precambrian Res. 2011, 189, 1–17. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Navarre-Sitchler, A.K.; Sak, P.B.; Williams, J.Z.; Brantley, S.L. Soil Profiles as Indicators of Mineral Weathering Rates and Organic Interactions for a Pennsylvania Diabase. Chem. Geol. 2011, 290, 89–100. [Google Scholar] [CrossRef]
- Medaris, L.G.; Driese, S.G.; Stinchcomb, G.E. The Paleoproterozoic Baraboo Paleosol Revisited: Quantifying Mass Fluxes of Weathering and Metasomatism, Chemical Climofunctions, and Atmospheric pCO2 in a Chemically Heterogeneous Protolith. Precambrian Res. 2017, 301, 179–194. [Google Scholar] [CrossRef]
- Brantley, S.L.; Lebedeva, M.; Hausrath, E.M. A Geobiological View of Weathering and Erosion. In Fundamentals of Geobiology; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 205–227. ISBN 978-1-118-28087-4. [Google Scholar]
- Banfield, J.F.; Moreau, J.W.; Chan, C.S.; Welch, S.A.; Little, B. Mineralogical Biosignatures and the Search for Life on Mars. Astrobiology 2001, 1, 447–465. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N. Looking for Traces of Life in Minerals. Comptes Rendus Palevol 2009, 8, 617–628. [Google Scholar] [CrossRef]
- Beveridge, T.J. Role of Cellular Design in Bacterial Metal Accumulation and Mineralization. Annu. Rev. Microbiol. 1989, 43, 147–171. [Google Scholar] [CrossRef]
- Brock, J.; Schulz-Vogt, H.N. Sulfide Induces Phosphate Release from Polyphosphate in Cultures of a Marine Beggiatoa Strain. ISME J. 2011, 5, 497–506. [Google Scholar] [CrossRef]
- Goldhammer, T.; Brüchert, V.; Ferdelman, T.G.; Zabel, M. Microbial Sequestration of Phosphorus in Anoxic Upwelling Sediments. Nat. Geosci. 2010, 3, 557–561. [Google Scholar] [CrossRef]
- Schulz, H.N.; Schulz, H.D. Large Sulfur Bacteria and the Formation of Phosphorite. Science 2005, 307, 416–418. [Google Scholar] [CrossRef]
- Cosmidis, J.; Benzerara, K.; Morin, G.; Busigny, V.; Lebeau, O.; Jézéquel, D.; Noël, V.; Dublet, G.; Othmane, G. Biomineralization of Iron-Phosphates in the Water Column of Lake Pavin (Massif Central, France). Geochim. Cosmochim. Acta 2014, 126, 78–96. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Fyfe, W.S.; Schultze-Lam, S.; Ferris, F.G.; Beveridge, T.J. Iron Phosphate Precipitation by Epilithic Microbial Biofilms in Arctic Canada. Can. J. Earth Sci. 1994, 31, 1320–1324. [Google Scholar] [CrossRef]
- Bailey, J.V.; Corsetti, F.A.; Greene, S.E.; Crosby, C.H.; Liu, P.; Orphan, V.J. Filamentous Sulfur Bacteria Preserved in Modern and Ancient Phosphatic Sediments: Implications for the Role of Oxygen and Bacteria in Phosphogenesis. Geobiology 2013, 11, 397–405. [Google Scholar] [CrossRef]
- Xiao, S.; Knoll, A.H. Fossil Preservation in the Neoproterozoic Doushantuo Phosphorite Lagerstatte, South China. Lethaia 1999, 32, 219–240. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Arrhenius, G.; McKeegan, K.D.; Harrison, T.M.; Nutman, A.P.; Friend, C.R.L. Evidence for Life on Earth before 3800 Million Years Ago. Nature 1996, 384, 55–59. [Google Scholar] [CrossRef]
- Wacey, D.; Saunders, M.; Roberts, M.; Menon, S.; Green, L.; Kong, C.; Culwick, T.; Strother, P.; Brasier, M.D. Enhanced Cellular Preservation by Clay Minerals in 1 Billion-Year-Old Lakes. Sci. Rep. 2014, 4, 5841. [Google Scholar] [CrossRef]
- Steiner, M.H.; Hausrath, E.H.; Sun, H.J. Synthesis of Potential Phosphate Mineral Biosignatures Under Mars Relevant Conditions. In Proceedings of the 44th Annual Lunar and Planetary Science Conference, Woodlands, TX, USA, 18–22 March 2013; Volume 44, p. 2761. [Google Scholar]
- Yang, H.; Sun, H.J.; Downs, R.T. Hazenite, KNaMg2(PO4)2.14H2O, a New Biologically Related Phosphate Mineral, from Mono Lake, California, U.S.A. Am. Mineral. 2011, 96, 675–681. [Google Scholar] [CrossRef]
- Yang, H.; Sun, H.J. Crystal Structure of a New Phosphate Compound, Mg2KNa(PO4)2·14H2O. J. Solid State Chem. 2004, 177, 2991–2997. [Google Scholar] [CrossRef]
- Boskey, A.; Spevak, L.; Tan, M.; Doty, S.B.; Butler, W.T. Dentin Sialoprotein (DSP) Has Limited Effects on In Vitro Apatite Formation and Growth. Calcif. Tissue Int. 2000, 67, 472–478. [Google Scholar] [CrossRef]
- Boskey, A.L.; Spevak, L.; Doty, S.B.; Rosenberg, L. Effects of Bone CS-Proteoglycans, DS-Decorin, and DS-Biglycan on Hydroxyapatite Formation in a Gelatin Gel. Calcif. Tissue Int. 1997, 61, 298–305. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-Hydroxyapatite Interactions in Vitro: Inhibition of Hydroxyapatite Formation and Growth in a Gelatin-Gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Doty, S.; Sabsay, B.; Veis, A. Concentration-Dependent Effects of Dentin Phosphophoryn in the Regulation of in Vitro Hydroxyapatite Formation and Growth. Bone Miner. 1990, 11, 55–65. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Appel, J. The Effects of Noncollagenous Matrix Proteins on Hydroxyapatite Formation and Proliferation in a Collagen Gel System. Connect. Tissue Res. 1989, 21, 171–178. [Google Scholar] [CrossRef]
- Boskey, A.L. Osteopontin and Related Phosphorylated Sialoproteins: Effects on Mineralization. Ann. N. Y. Acad. Sci. 1995, 760, 249–256. [Google Scholar] [CrossRef]
- Boskey, A.L. Hydroxyapatite Formation in a Dynamic Collagen Gel System: Effects of Type I Collagen, Lipids, and Proteoglycans. J. Phys. Chem. 1989, 93, 1628–1633. [Google Scholar] [CrossRef]
- Bouropoulos, N.; Moradian-Oldak, J. Induction of Apatite by the Cooperative Effect of Amelogenin and the 32-kDa Enamelin. J. Dent. Res. 2004, 83, 278–282. [Google Scholar] [CrossRef]
- Hunter, G.; Hauschka, P.; Poole, A.; Rosenberg, L.; Goldberg, H. Nucleation and Inhibition of Hydroxyapatite Formation by Mineralized Tissue Proteins. Biochem. J. 1996, 317, 59–64. [Google Scholar] [CrossRef]
- Hunter, G.K.; Curtis, H.A.; Grynpas, M.D.; Simmer, J.P.; Fincham, A.G. Effects of Recombinant Amelogenin on Hydroxyapatite Formation In Vitro. Calcif. Tissue Int. 1999, 65, 226–231. [Google Scholar] [CrossRef]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of Crystal Formation by Bone Phosphoproteins: Structural Specificity of the Osteopontin-Mediated Inhibition of Hydroxyapatite Formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef]
- Hunter, G.K.; Goldberg, H.A. Nucleation of Hydroxyapatite by Bone Sialoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 8562–8565. [Google Scholar] [CrossRef]
- Iijima, M.; Moriwaki, Y.; Wen, H.B.; Fincham, A.G.; Moradian-Oldak, J. Elongated Growth of Octacalcium Phosphate Crystals in Recombinant Amelogenin Gels under Controlled Ionic Flow. J. Dent. Res. 2002, 81, 69–73. [Google Scholar] [CrossRef]
- Taira, T.; Iijima, M.; Moriwaki, Y.; Kuboki, Y. A New Method for in Vitro Calcification Using Acrylamide Gel and Bovine Serum. Connect. Tissue Res. 1995, 33, 185–192. [Google Scholar] [CrossRef]
- Tartaix, P.H.; Doulaverakis, M.; George, A.; Fisher, L.W.; Butler, W.T.; Qin, C.; Salih, E.; Tan, M.; Fujimoto, Y.; Spevak, L.; et al. In Vitro Effects of Dentin Matrix Protein-1 on Hydroxyapatite Formation Provide Insights into in Vivo Functions. J. Biol. Chem. 2004, 279, 18115–18120. [Google Scholar] [CrossRef]
- Tye, C.E.; Rattray, K.R.; Warner, K.J.; Gordon, J.A.R.; Sodek, J.; Hunter, G.K.; Goldberg, H.A. Delineation of the Hydroxyapatite-Nucleating Domains of Bone Sialoprotein. J. Biol. Chem. 2003, 278, 7949–7955. [Google Scholar] [CrossRef]
- Wen, H.B.; Moradian-Oldak, J.; Fincham, A.G. Dose-Dependent Modulation of Octacalcium Phosphate Crystal Habit by Amelogenins. J. Dent. Res. 2000, 79, 1902–1906. [Google Scholar] [CrossRef]
- Freche, M.; Lacout, J. LEffect of Humic Compounds and Some Organic Acids Added during Dicalcium Phosphate Dihydrate Crystal Growth Process. J. Alloys Compd. 1992, 188, 65–68. [Google Scholar] [CrossRef]
- Grossl, P.R.; Inskeep, W.P. Kinetics of Octacalcium Crystal Growth in the Presence of Organic Acids. Geochim. Cosmochim. Acta 1992, 56, 1955–1961. [Google Scholar] [CrossRef]
- Van Der Houwen, J.A.M.; Valsami-Jones, E. The Application of Calcium Phosphate Precipitation Chemistry to Phosphorus Recovery: The Influence of Organic Ligands. Environ. Technol. 2001, 22, 1325–1335. [Google Scholar] [CrossRef]
- Blake, R.E. Experimental Investigations of Organic and Microbially Mediated Reactions of Aluminosilicates and Phosphates. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 1997. [Google Scholar]
- Blake, R.E.; O’Neil, J.R.; Surkov, A.V. Biogeochemical Cycling of Phosphorus: Insights from Oxygen Isotope Effects of Phosphoenzymes. Am. J. Sci. 2005, 305, 596–620. [Google Scholar] [CrossRef]
- Chang, S.J.; Blake, R.E. Oxygen Isotope Studies of Phosphite Oxidation: Purification and Analysis of Reactants and Products by High-temperature Conversion Elemental Analyzer/Isotope Ratio Mass Spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2039–2044. [Google Scholar] [CrossRef]
- Colman, A.S.; Blake, R.E.; Karl, D.M.; Fogel, M.L.; Turekian, K.K. Marine Phosphate Oxygen Isotopes and Organic Matter Remineralization in the Oceans. Proc. Natl. Acad. Sci. USA 2005, 102, 13023–13028. [Google Scholar] [CrossRef]
- Colman, A.S.; Blake, R.E.; Anna, M.; FOGEL, M.L. 18O of PO4 as a Hydrogeologic Tracer for Nutrient Contamination and Subsurface Microbial Acticity.; 2002.
- Jaisi, D.P.; Blake, R.E.; Liang, Y.; Chang, S.J. Investigation of Compound-Specific Organic-Inorganic Phosphorus Transformation Using Stable Isotope Ratios in Phosphate. In Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 267–292. [Google Scholar]
- Jaisi, D.P.; Blake, R.E. Advances in Using Oxygen Isotope Ratios of Phosphate to Understand Phosphorus Cycling in the Environment. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 125, pp. 1–53. ISBN 0065-2113. [Google Scholar]
- Li, H.; Yu, C.; Wang, F.; Chang, S.J.; Yao, J.; Blake, R.E. Probing the Metabolic Water Contribution to Intracellular Water Using Oxygen Isotope Ratios of PO4. Proc. Natl. Acad. Sci. USA 2016, 113, 5862–5867. [Google Scholar] [CrossRef]
- Liang, Y.; Blake, R.E. Oxygen Isotope Fractionation between Apatite and Aqueous-Phase Phosphate: 20–45 C. Chem. Geol. 2007, 238, 121–133. [Google Scholar] [CrossRef]
- Liang, Y.; Blake, R.E. Oxygen Isotope Composition of Phosphate in Organic Compounds: Isotope Effects of Extraction Methods. Org. Geochem. 2006, 37, 1263–1277. [Google Scholar] [CrossRef]
- Liang, Y.; Blake, R.E. Oxygen Isotope Signature of Pi Regeneration from Organic Compounds by Phosphomonoesterases and Photooxidation. Geochim. Cosmochim. Acta 2006, 70, 3957–3969. [Google Scholar] [CrossRef]
- O’Neil, J.R.; Roe, L.J.; Reinhard, E.; Blake, R.E. A Rapid and Precise Method of Oxygen Isotope Analysis of Biogenic Phosphate. Isr. J. Earth Sci. 1994, 43, 203–212. [Google Scholar]
- Sandy, E.H.; Blake, R.E.; Chang, S.J.; Jun, Y.; Yu, C. Oxygen Isotope Signature of UV Degradation of Glyphosate and Phosphonoacetate: Tracing Sources and Cycling of Phosphonates. J. Hazard. Mater. 2013, 260, 947–954. [Google Scholar] [CrossRef]
- Blake, R.E.; Alt, J.C.; Martini, A.M. Oxygen Isotope Ratios of PO4: An Inorganic Indicator of Enzymatic Activity and P Metabolism and a New Biomarker in the Search for Life. Proc. Natl. Acad. Sci. USA 2001, 98, 2148–2153. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Kukkadapu, R.K.; Stout, L.M.; Varga, T.; Blake, R.E. Biotic and Abiotic Pathways of Phosphorus Cycling in Minerals and Sediments: Insights from Oxygen Isotope Ratios in Phosphate. Environ. Sci. Technol. 2011, 45, 6254–6261. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Blake, R.E.; Kukkadapu, R.K. Fractionation of Oxygen Isotopes in Phosphate during Its Interactions with Iron Oxides. Geochim. Cosmochim. Acta 2010, 74, 1309–1319. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Blake, R.E. Tracing Sources and Cycling of Phosphorus in Peru Margin Sediments Using Oxygen Isotopes in Authigenic and Detrital Phosphates. Geochim. Cosmochim. Acta 2010, 74, 3199–3212. [Google Scholar] [CrossRef]
- Siebers, N.; Bauke, S.L.; Tamburini, F.; Amelung, W. Short-Term Impacts of Forest Clear-Cut on P Accessibility in Soil Microaggregates: An Oxygen Isotope Study. Geoderma 2018, 315, 59–64. [Google Scholar] [CrossRef]
- Tamburini, F.; Pfahler, V.; von Sperber, C.; Frossard, E.; Bernasconi, S.M. Oxygen Isotopes for Unraveling Phosphorus Transformations in the Soil–Plant System: A Review. Soil Sci. Soc. Am. J. 2014, 78, 38–46. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Martinez, E.; Blake, R.E.; Udry, A.; McCubbin, F.M.; Wostbrock, J.; Hayles, J. Examining the Oxygen Isotope Composition of Phosphate as a Potential Biosignature in Returned Martian Samples. In Proceedings of the 2024 Astrobiology Science Conference, Providence, RI, USA, 5–10 May 2024; p. Abstract 211-04. [Google Scholar]

| Name | Formula | Type a | Basis b | Ref. | Bio Ref. |
|---|---|---|---|---|---|
| Anapaite | Ca2Fe(PO4)2·4H2O | S | Met | [49] | |
| Brushite | CaPO3(OH)·2H2O | S | Inf | [50,51] | [52,53,54] |
| Chlorapatite | Ca5(PO4)3Cl | I | Met | [19,50,55,56] | [57] |
| Collinsite | Ca2(Mg,Fe)(PO4)2·2H2O | S | Met | [49] | |
| Ferristrunzite | Fe3+Fe3+2(PO4)2(OH)3·5H2O | S | Inf | [58] | |
| Fluorapatite | Ca5(PO4)3F | I,M,S | Met/ Mars | [41,42,59,60,61,62,63,64] | [65,66] |
| Giniite | Fe2+Fe3+4(PO4)3(OH)5·2H2O | S | Inf | [58,67,68,69] | [70] |
| hydroxyapatite | Ca10(PO4)6(OH)2 | I,M,S | Met | [64,71] | [72,73] |
| Holtedahlite | Mg2(PO4)(OH) | S | Met | [49,74] | |
| Jahnsite group | (Ca,Mn,Na)(Fe,Mn,Mg) (Fe,Mn,Mg)2 (Fe3+)2(PO4)4(OH)3·8H2O | S | Inf | [75,76,77] | |
| Keplerite | Ca9(Ca0.5□ 0.5)Mg(PO4)7 | I,M-Sh | Met | [78,79] | |
| Laueite | Mn2+Fe3+2(PO4)2(OH)2·8H2O | S | Inf | [44] | |
| Merrillite | Ca9(Na,Mg)(PO4)7 | I,M-Sh | Met | [19,39,50,55,80] | [31,74,81] |
| Metavivianite | Fe2+Fe3+2(PO4)2(OH)2·6H2O | S | Inf | [34] | |
| Monetite | Ca(HPO4) | S | Inf | [50] | [81,82] |
| Monazite | REE(PO4) | I | Met | [56] | |
| Strengite | FePO4·2H2O | S | Inf | [58] | |
| Strunzite | Mn2+Fe3+2(PO4)2(OH)2·6H2O | S | Inf | [44] | |
| Tuite | γ-Ca3(PO4)2 | M-Sh | Met | [83,84] | |
| Variscite | AlPO4·2(H2O) | S | Inf | [85,86] | [87] |
| Vivianite | Fe3(PO4)2·8H2O | S | Inf | [34,88,89] | [90] |
| Whitlockite | Ca9(Fe,Mg)HPO4(PO4)6 | S, I | Inf | [50,80] | [91] |
| Xenotime | REE(PO4) | I | Met | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hausrath, E.M.; Adcock, C.T.; Berger, J.A.; Cycil, L.M.; Kizovski, T.V.; McCubbin, F.M.; Schmidt, M.E.; Tu, V.M.; VanBommel, S.J.; Treiman, A.H.; et al. Phosphates on Mars and Their Importance as Igneous, Aqueous, and Astrobiological Indicators. Minerals 2024, 14, 591. https://doi.org/10.3390/min14060591
Hausrath EM, Adcock CT, Berger JA, Cycil LM, Kizovski TV, McCubbin FM, Schmidt ME, Tu VM, VanBommel SJ, Treiman AH, et al. Phosphates on Mars and Their Importance as Igneous, Aqueous, and Astrobiological Indicators. Minerals. 2024; 14(6):591. https://doi.org/10.3390/min14060591
Chicago/Turabian StyleHausrath, E. M., C. T. Adcock, J. A. Berger, L. M. Cycil, T. V. Kizovski, F. M. McCubbin, M. E. Schmidt, V. M. Tu, S. J. VanBommel, A. H. Treiman, and et al. 2024. "Phosphates on Mars and Their Importance as Igneous, Aqueous, and Astrobiological Indicators" Minerals 14, no. 6: 591. https://doi.org/10.3390/min14060591
APA StyleHausrath, E. M., Adcock, C. T., Berger, J. A., Cycil, L. M., Kizovski, T. V., McCubbin, F. M., Schmidt, M. E., Tu, V. M., VanBommel, S. J., Treiman, A. H., & Clark, B. C. (2024). Phosphates on Mars and Their Importance as Igneous, Aqueous, and Astrobiological Indicators. Minerals, 14(6), 591. https://doi.org/10.3390/min14060591






