Paleo-Sedimentary Environment and Formation Mechanism of the Organic-Rich Shale of the Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, China
Abstract
:1. Introduction
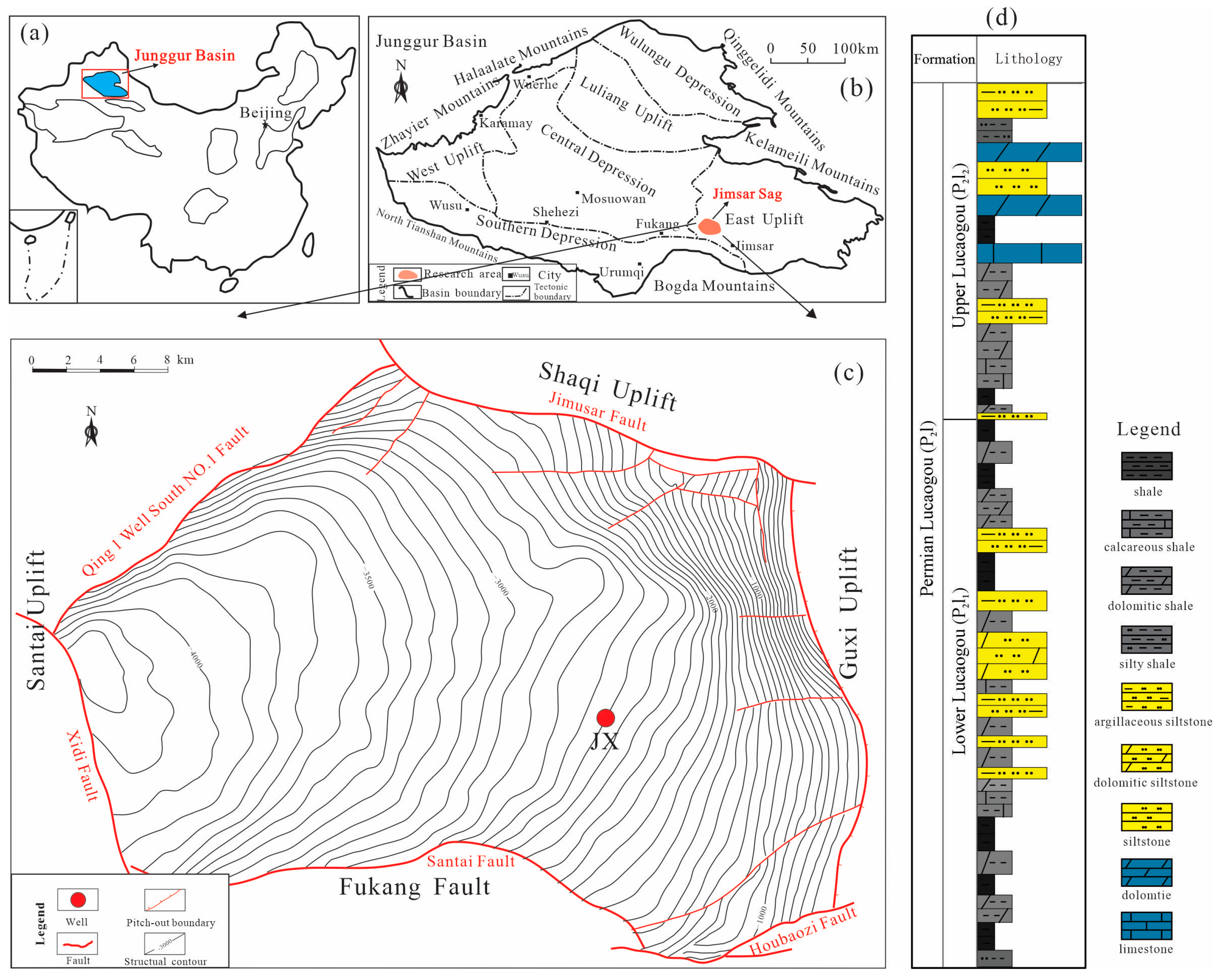
2. Geological Background
3. Samples and Methods
4. Results and Discussion
4.1. Paleo-Environment Analysis
4.1.1. Stratigraphic Division
4.1.2. Paleo-Climate Analysis
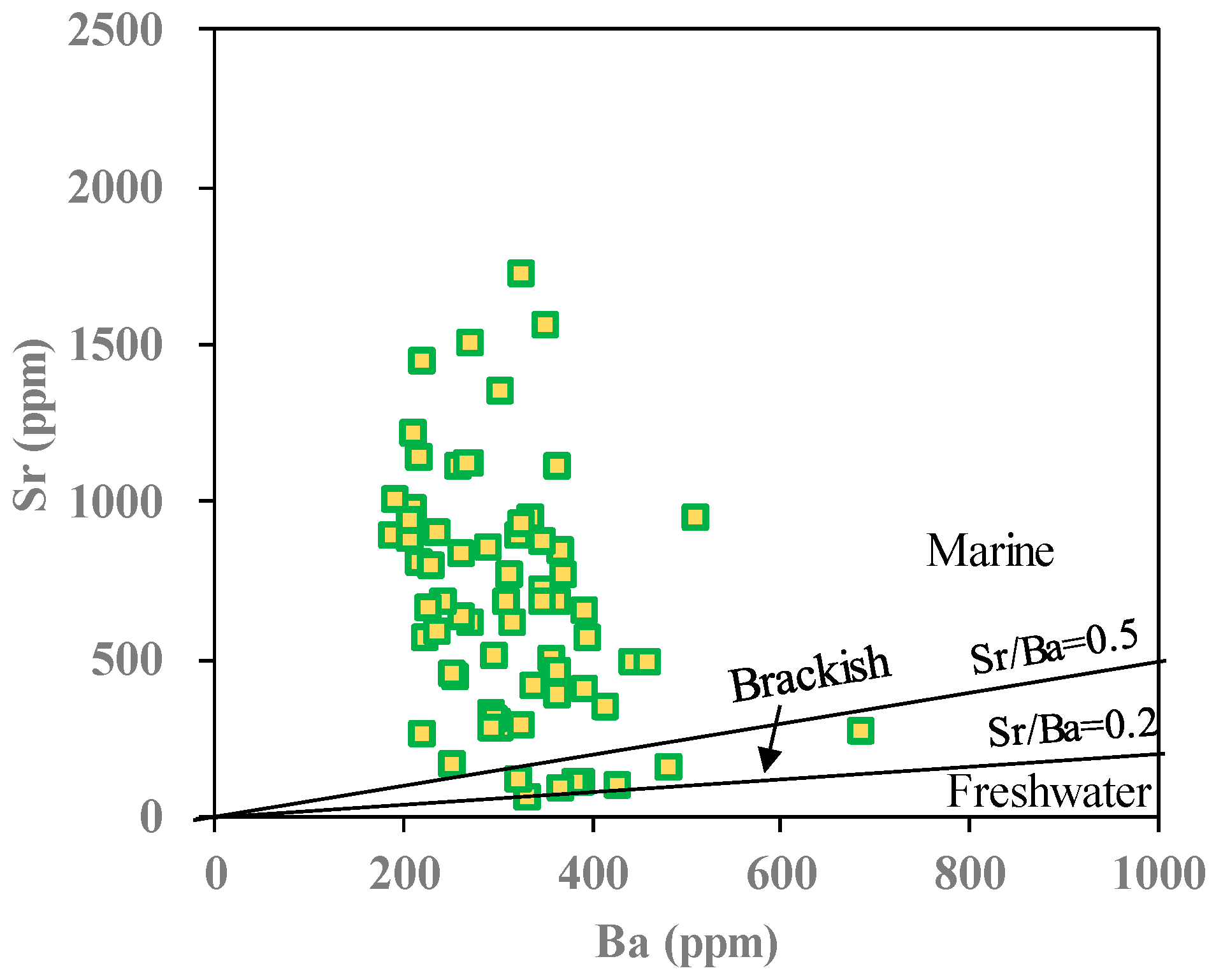
4.1.3. Paleo-Salinity Analysis
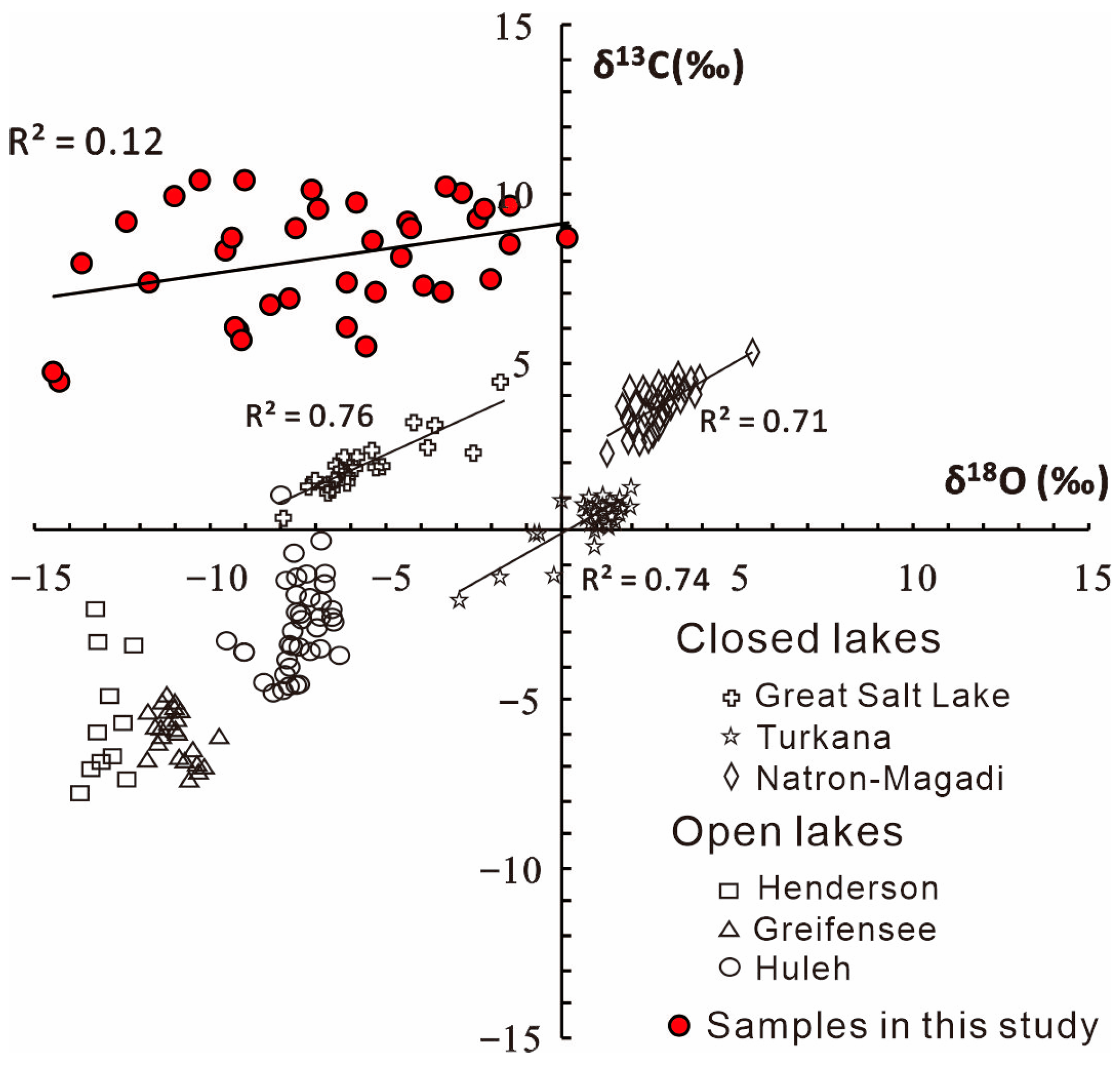
4.1.4. Paleo-Limnological Analysis
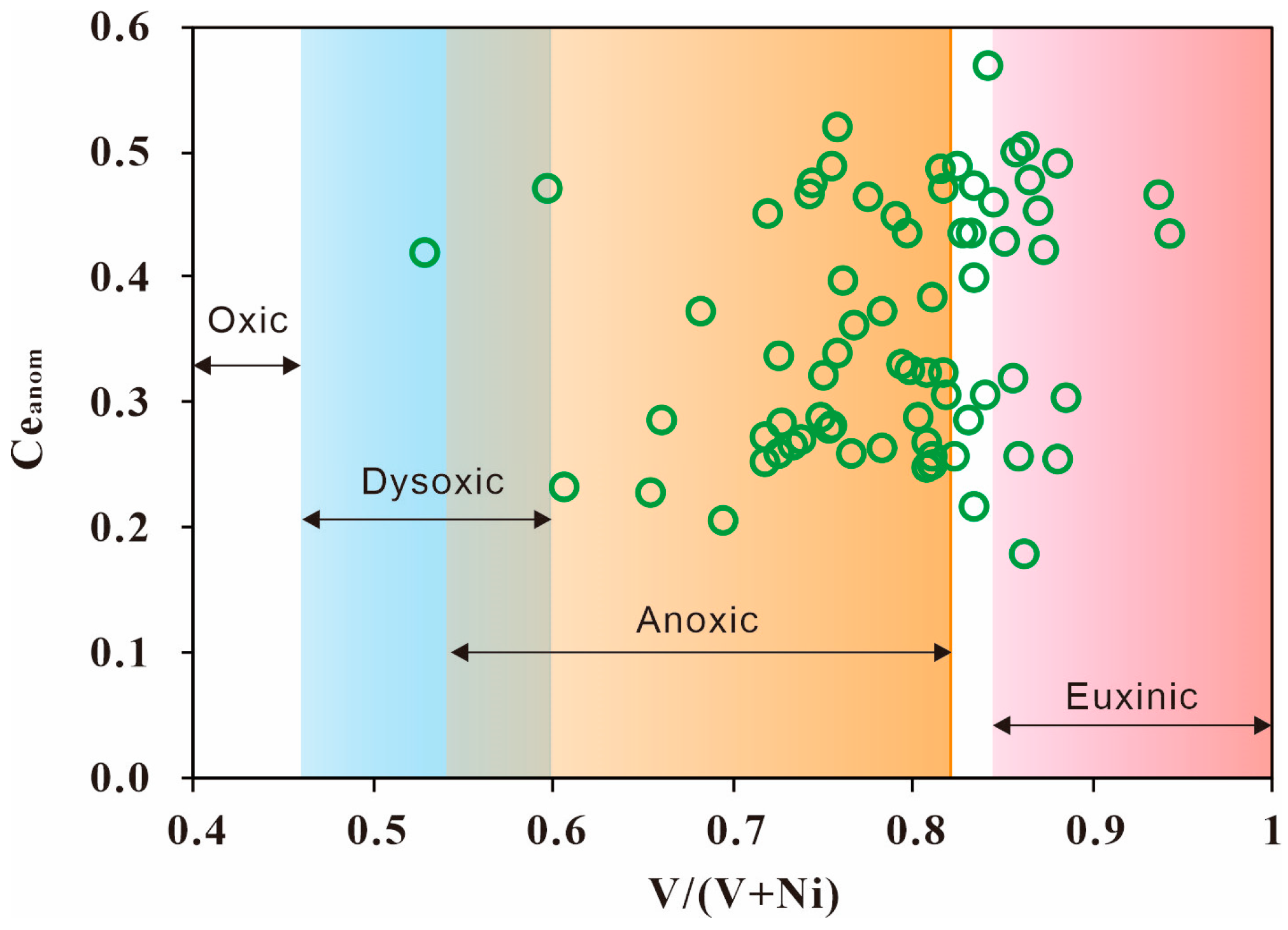
4.1.5. Redox Condition
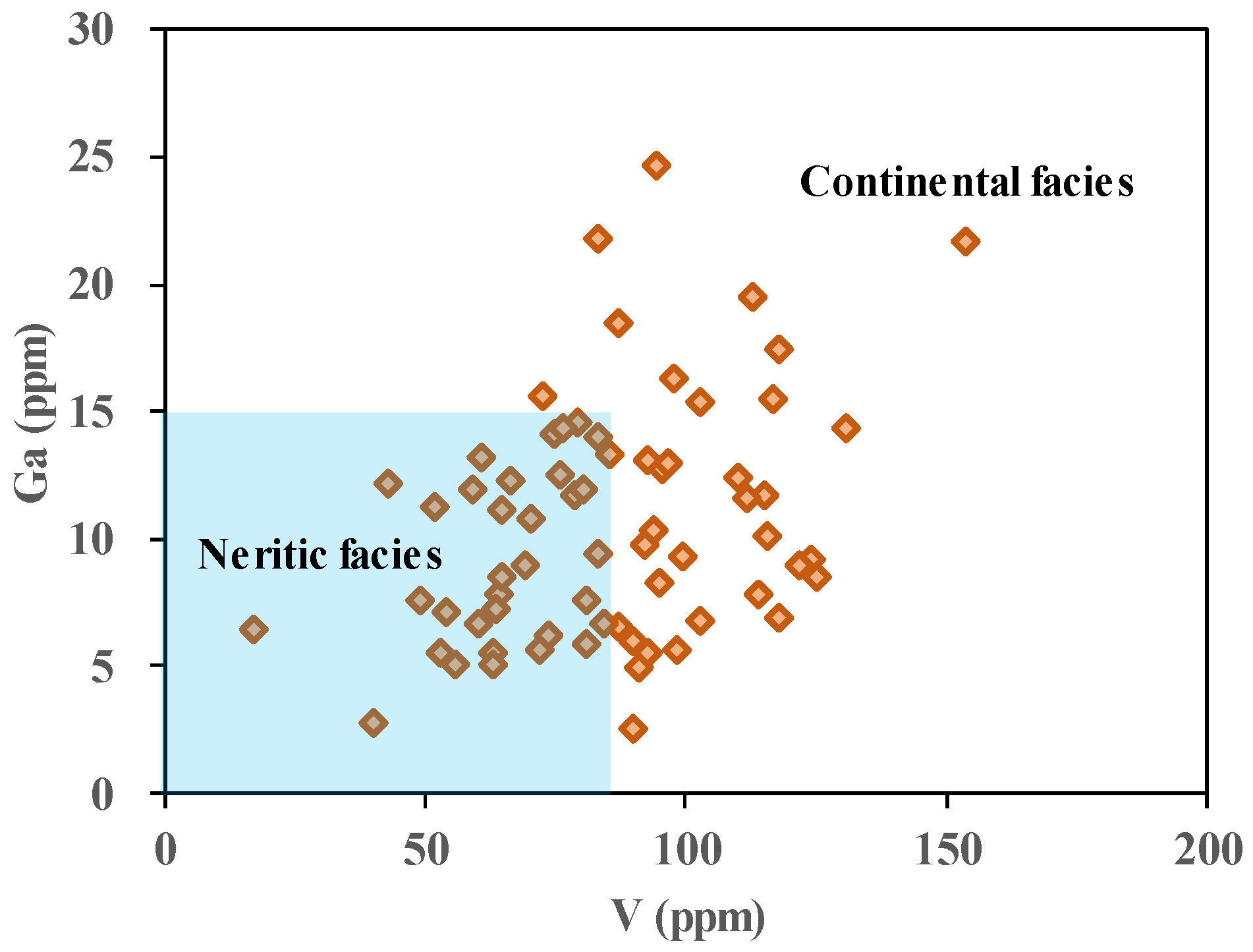
4.1.6. Paleo-Water Depth Analysis
4.1.7. Extreme Environmental Response Analysis
4.2. Formation Mechanism of Organic-Rich Shale
4.2.1. Organic Matter Enrichment Mode
4.2.2. Organic-Rich Shale Sedimentation Model
5. Conclusions
- The Lucaogou Formation, divided into six layers (L6 to L1) from bottom to top, showcases varying lithology, logging response, geochemical attributes, and sedimentary structures. The deposition of this formation primarily occurred within a hot and arid climate, in a saltwater depositional environment marked by salinity fluctuations that corresponded with changes in climate conditions.
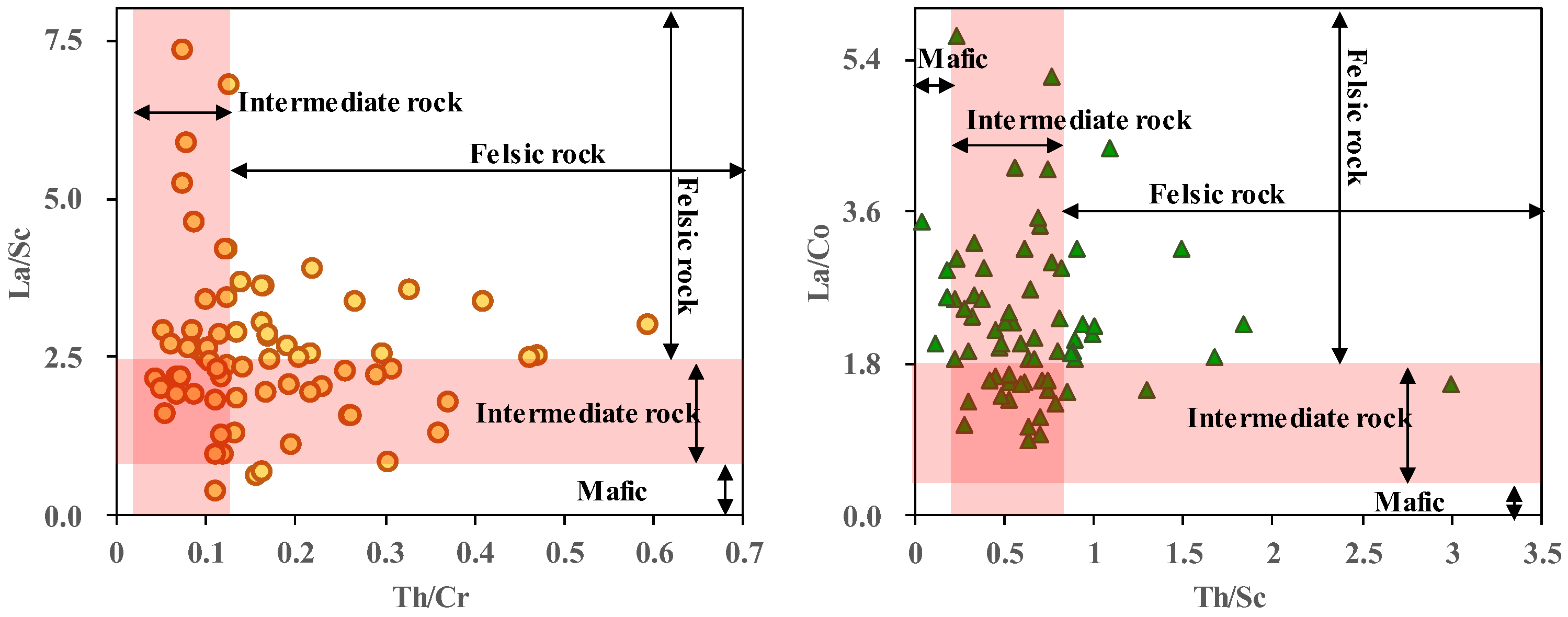
- The Lucaogou Formation formed through semi-closed lake sedimentation, characterized by the lake not being completely closed, and exhibited an anoxic-reducing environment. The paleo-water depth increased gradually from L6 to L1, reflecting concurrent changes in climate and salinity. The predominant source lithology is felsic igneous rock. Small-scale transgression and hydrothermal sedimentation occurred during the deposition.
- The Lucaogou Formation is organically rich due to the stable reducing environment and hot climate. Organic matter is enriched in matrix and laminar modes, formed, respectively, by algal blooming and resuspension transport mechanisms. The matrix organic-rich shale developed in a hot deep-water environment, while the laminar organic-rich shale formed in a shallow water setting, characterized by a warm climate, low salinity, and restricted circulation. The enrichment of organic matter likely contributed to the formation of primary biogenetic dolomite.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.Y.; Hou, L.H.; Luo, X.; Sun, F.F.; Lin, S.H.; Zhang, L.J. Total scanning fluorescence characteristics and implications of shale oil in the Lucaogou Formation, Jimsar Sag, Junggar Basin, NW China. Front. Earth Sci. 2021, 9, 664330. [Google Scholar] [CrossRef]
- Hou, L.H.; Luo, X.; Zhao, Z.Y.; Zhang, L.J. Identification of oil produced from shale and tight reservoirs in the Permian Lucaogou shale sequence, Jimsar Sag, Junggar Basin, NW China. ACS Omega 2021, 6, 2127–2142. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.Q.; Wang, Q.F.; Jiang, S.; Wang, X.L.; Huang, C. Geochemical and geological characteristics of Permian Lucaogou Formation shale of the well Ji174, Jimusar Sag, Junggar Basin, China: Implications for shale oil exploration. Geol. J. 2018, 53, 2371–2385. [Google Scholar] [CrossRef]
- Qi, M.; Han, C.; Ma, C.; Liu, G.; He, X.; Li, G.; Yang, Y.; Sun, R.; Cheng, X. Identification of Diagenetic Facies Logging of Tight Oil Reservoirs Based on Deep Learning—A Case Study in the Permian Lucaogou Formation of the Jimsar Sag, Junggar Basin. Minerals 2022, 12, 913. [Google Scholar] [CrossRef]
- Ding, X.J.; Qian, L.R.; Jiang, W.L.; Yiming, A.; Cao, Z.; Jiang, Z.F.; Zha, M. Volcanic ash content of Permian lucagou shale in the Jimsar sag, Junggar Basin: Evidence from comprehensive analysis of mercury (Hg), major elements, strontium isotope, and thin section. Mar. Pet. Geol. 2023, 157, 106501. [Google Scholar] [CrossRef]
- Hou, L.H.; Ma, W.J.; Luo, X.; Liu, J.Z.; Lin, S.H.; Zhao, Z.Y. Hydrocarbon generation-retention-expulsion mechanism and shale oil producibility of the permian lucaogou shale in the Junggar Basin as simulated by semi-open pyrolysis experiments. Mar. Pet. Geol. 2021, 125, 104880. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, Z.Y.; Hou, L.H.; Lin, S.H.; Sun, F.F.; Zhang, L.J.; Zhang, Y. Experimental methods for the quantitative assessment of the volume fraction of movable shale oil: A case study in the Jimsar Sag, Junggar Basin, China. Front. Earth Sci. 2021, 9, 663574. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.Q.; Wang, X.L. Source rock characteristics of Permian Lucaogou Formation in the Jimusar Sag, Junggar Basin, northwest China, and its significance on tight oil source and occurrence. Geol. J. 2017, 52, 624–645. [Google Scholar] [CrossRef]
- Xie, M.; Yang, W.; Zhao, M.; Li, Y.; Deng, Y.; Gao, Y.; Xu, C.; Hou, H.; Yao, L.; Zhang, Z.; et al. Diagenetic Facies Controls on Differential Reservoir-Forming Patterns of Mixed Shale Oil Sequences in the Saline Lacustrine Basin. Minerals 2023, 13, 143. [Google Scholar] [CrossRef]
- Zeng, W.R.; Zhang, Z.H.; Wang, B.; Chen, X.; Zheng, R.H.; Fu, G.B.; Jin, Y. Formation mechanism of organic-rich mixed sedimentary rocks in saline lacustrine basin, Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, Northwest China. Mar. Pet. Geol. 2023, 156, 106452. [Google Scholar] [CrossRef]
- Ding, X.J.; Qu, J.X.; Imin, A.; Zha, M.; Su, Y.; Jiang, Z.F.; Jiang, H. Organic matter origin and accumulation in tuffaceous shale of the lower Permian Lucaogou Formation, Jimsar Sag. J. Pet. Sci. Eng. 2019, 179, 696–706. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Qiu, L.W.; Wan, M.; Jia, X.Y.; Cao, Y.C.; Lei, C.W.; Qu, C.S. Depositional model for a salinized lacustrine basin: The Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, NW China. J. Asian Earth Sci. 2019, 178, 81–85. [Google Scholar] [CrossRef]
- Su, Y.; Zha, M.; Ding, X.J.; Qu, J.X.; Gao, C.H.; Jin, J.H.; Iglauer, S. Petrographic, palynologic and geochemical characteristics of source rocks of the Permian Lucaogou formation in Jimsar Sag, Junggar Basin, NW China: Origin of organic matter input and depositional environments. J. Pet. Sci. Eng. 2019, 183, 106364. [Google Scholar] [CrossRef]
- Wang, W.; Cui, H.; Tan, J.; Liu, J.; Song, X.; Wang, J.; Chen, L. Permian Cyanobacterial Blooms Resulted in Enrichment of Organic Matter in the Lucaogou Formation in the Junggar Basin, NW China. Minerals 2023, 13, 537. [Google Scholar] [CrossRef]
- Graham, S.A.; Brassell, S.; Carroll, A.R.; Xiao, X.; Demaison, G.; McKnight, C.L.; Liang, Y.; Hendrix, M.S. Characteristics of selected petroleum source rocks, Xianjiang Uygur autonomous region, Northwest China. AAPG Bull. 1990, 74, 493–512. [Google Scholar]
- Carroll, A.R.; Brassell, S.C.; Graham, S.A. Upper Permian lacustrine oil shales, southern Junggar basin, northwest China. Am. AAPG Bull. 1992, 76, 1874–1902. [Google Scholar]
- Wu, H.G.; Zhou, J.J.; Hu, W.X.; Sun, F.N.; Kang, X.; Zhang, Y.F.; He, W.J.; Feng, C.C. Origin of authigenic albite in a lacustrine mixed-deposition sequence (Lucaogou Formation, Junggar Basin) and its diagenesis implications. Energy Explor. Exploit 2022, 40, 132–154. [Google Scholar] [CrossRef]
- Tao, H.F.; Qiu, Z.; Qu, Y.Q.; Liu, J.; Qin, Z.; Xie, Z.B.; Qiu, J.L.; Liu, B. Geochemistry of middle permian lacustrine shales in the Jimusar sag, Junggar Basin, NW China: Implications for hydrothermal activity and organic matter enrichment. J. Asian Earth Sci. 2022, 232, 105267. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Liu, Y.Q.; Jiao, X.; Ma, L.T.; Zhou, D.W.; Li, H.; Cao, Q.; Zhao, M.R.; Yang, Y.Y. Petrological and organic geochemical characteristics of the Permian Lucaogou Formation in the Jimsar sag, Junggar Basin, NW China: Implications on the relationship between hydrocarbon accumulation and volcanic-hydrothermal activities. J. Pet. Sci. Eng. 2022, 210, 110078. [Google Scholar] [CrossRef]
- Wang, Y.C.; Cao, J.; Tao, K.Y.; Xiao, W.Y.; Xiang, B.L.; Li, E.T.; Pan, C.C. Absence of β-carotane as proxies of hydrothermal activity in brackish lacustrine sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 587, 110801. [Google Scholar] [CrossRef]
- Carroll, A.R. Upper Permian lacustrine organic facies evolution, southern Junggar Basin, NW China. Org. Geochem. 1998, 28, 649–667. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, H.; Tao, H.F.; Carroll, A.R.; Qin, J.M.; Chen, J.Q.; Yuan, X.G.; Wang, C.S. Paleoenvironmental setting, mechanism and consequence of massive organic carbon burial in the Permian Junggar Basin, NW China. J. Asian Earth Sci. 2020, 194, 104222. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.Q.; Jiang, S.; Wang, Q.F.; Zheng, X.W.; Ding, X.G.; Zhao, Y.; Zhu, C.X.; Li, H. Oil content evaluation of lacustrine organic-rich shale with strong heterogeneity: A case study of the Middle Permian Lucaogou Formation in Jimusaer Sag, Junggar Basin, NW China. Fuel 2018, 222, 196–205. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Zhu, R.; Wu, L.; Xu, X. Chemo-sedimentary facies analysis of fine-grained sediment formations: An example from the Lucaogou Fm in the Jimusaer sag, Junggar Basin, NW China. Mar. Pet. Geol. 2019, 110, 388–402. [Google Scholar] [CrossRef]
- Peters, K.E. Guidelines of evaluating petroleum source rock using programmed pyrolysis. AAPG Bull. 1986, 70, 318–329. [Google Scholar]
- Lerman, A. (Ed.) Lakes: Chemistry, Geology, Physics; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Stuiver, M. Climate versus changes in 13C content of the organic component of lake sediments during the Late Quaternary. Quat. Res. 1975, 5, 251–262. [Google Scholar] [CrossRef]
- Bowen, R. Isotopes and Climates; Elsevier Applied Science: London, UK; New York, NY, USA, 1991; pp. 128–131. [Google Scholar]
- Wei, W.; Algeo, T.J. Elemental proxies for paleosalinity analysis of ancient shales and mudrocks. Geochem. Cosmochim. Acta 2020, 287, 341–366. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chen, Z.L.; Zhang, W.G. Quaternary stratigraphy and trace element indices of the Yangtze Delta, Eastern China, with special reference to marine transgressions. Quat. Res. 1997, 47, 181–191. [Google Scholar] [CrossRef]
- Keith, M.L.; Weber, J.V. Isotopic composition and environmental classification of selected limestones and fossils. Gcochim. Cosmochim. Acta 1964, 23, 1786–1816. [Google Scholar]
- Talbot, M.R.; Kelts, K. Paleolimnological signatures from carbon and oxygen isotopic ratios in carbonates from organic carbon-rich lacustrine sediments. AAPG Mem. 1990, 50, 99–112. [Google Scholar]
- Talbot, M.R. A review of the paleohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. 1990, 80, 261–279. [Google Scholar]
- Kelts, K.; Talbot, M. Lacustrine carbonates as geochemical archives of environmental change and biotic/abiotic interactions. In Large Lakes: Ecology, Structure and Function; Tilzer, M.M., Serruya, C., Eds.; Science and Technology Publishers: Madison, WI, USA, 1989; pp. 288–315. [Google Scholar]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennaylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A.C. Comparion of geochemical indices used for the interpretation of palaeoredox conditions inancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Wright, J.; Schrader, H.; Holser, W.T. Paleoredox variations in ancient oceans recorded by rare earth elements in fossil apatite. Geochim. Cosmochim. Acta 1987, 51, 631–644. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, F.; Ji, H.; Jiang, S.; Liu, X.; Zhao, Z.; Wu, Y.; Xiong, H.; Li, Y.; Wang, Z. Effects of paleosedimentary environment on organic matter enrichment in a saline lacustrine rift basin—A case study of Paleogene source rock in the Dongpu Depression, Bohai Bay Basin. J. Pet. Sci. Eng. 2020, 195, 107658. [Google Scholar] [CrossRef]
- Wersin, P.; Hohener, P.; Giovanoli, R.; Stumm, W. Early diagenetic influences on iron transformations in a freshwater lake sediment. Chem. Geol. 1991, 90, 233–252. [Google Scholar] [CrossRef]
- Katz, B.; Lin, F. Lacustrine basin unconventional resource plays: Key differences. Mar. Pet. Geol. 2014, 56, 255–265. [Google Scholar] [CrossRef]
- Wang, S.M. Physics and chemistry of saline lakes. J. Lake Sci. 1993, 5, 278–286. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific: Oxford, UK, 1985; p. 312. [Google Scholar]
- Mclennan, S.M.; Taylor, S.R.; Mcculloch, M.T.; Maynard, J.B. Geochemistry and Nd-Sr isotopic composition of deep sea turbidites: Crustal evolution and platetectonic associations. Geochim. Cosmochim. Acta 1990, 54, 2015–2050. [Google Scholar] [CrossRef]
- Huntsman, M.P.; Kampunzu, A.B.; Vink, B.; Ringrose, S. Cryptic indicators of provenance from the geochemistry of the Okavango Delta sediments, Botswana. Sediment. Geol. 2005, 174, 123–148. [Google Scholar] [CrossRef]
- Cullers, R.L. The geochemistry of shales, siltstones and stones of Pennsylvanian-Permian age, Colorado, USA: Implications for provenance and metamorphic studies. Lithos 2000, 51, 181–203. [Google Scholar] [CrossRef]
- Adachi, M.; Yamamoto, K.; Sugisaki, R. Hydrothermal chert and associated siliceous rocks from the northern pacific their geological significance as indication of ocean ridge activity. Sediment. Geol. 1986, 47, 125–148. [Google Scholar] [CrossRef]
- Bostrom, K.; Kraemer, T.; Gartner, S. provenance and accumulation rates of opaline silica, Al, Ti, Fe, Mn, Cu, Ni and Co in pacific pelagic sediments. Chem. Geol. 1973, 11, 123–148. [Google Scholar] [CrossRef]
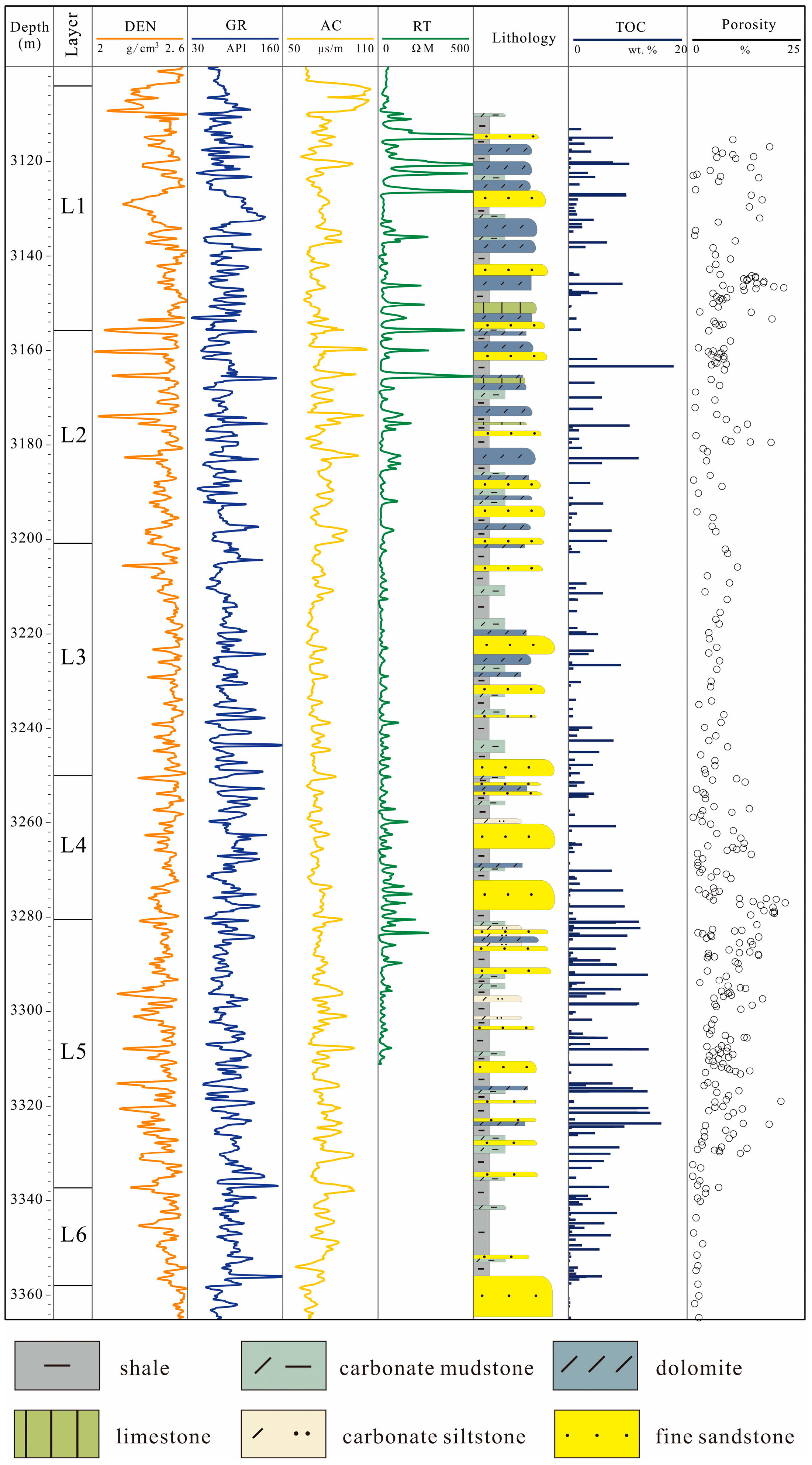
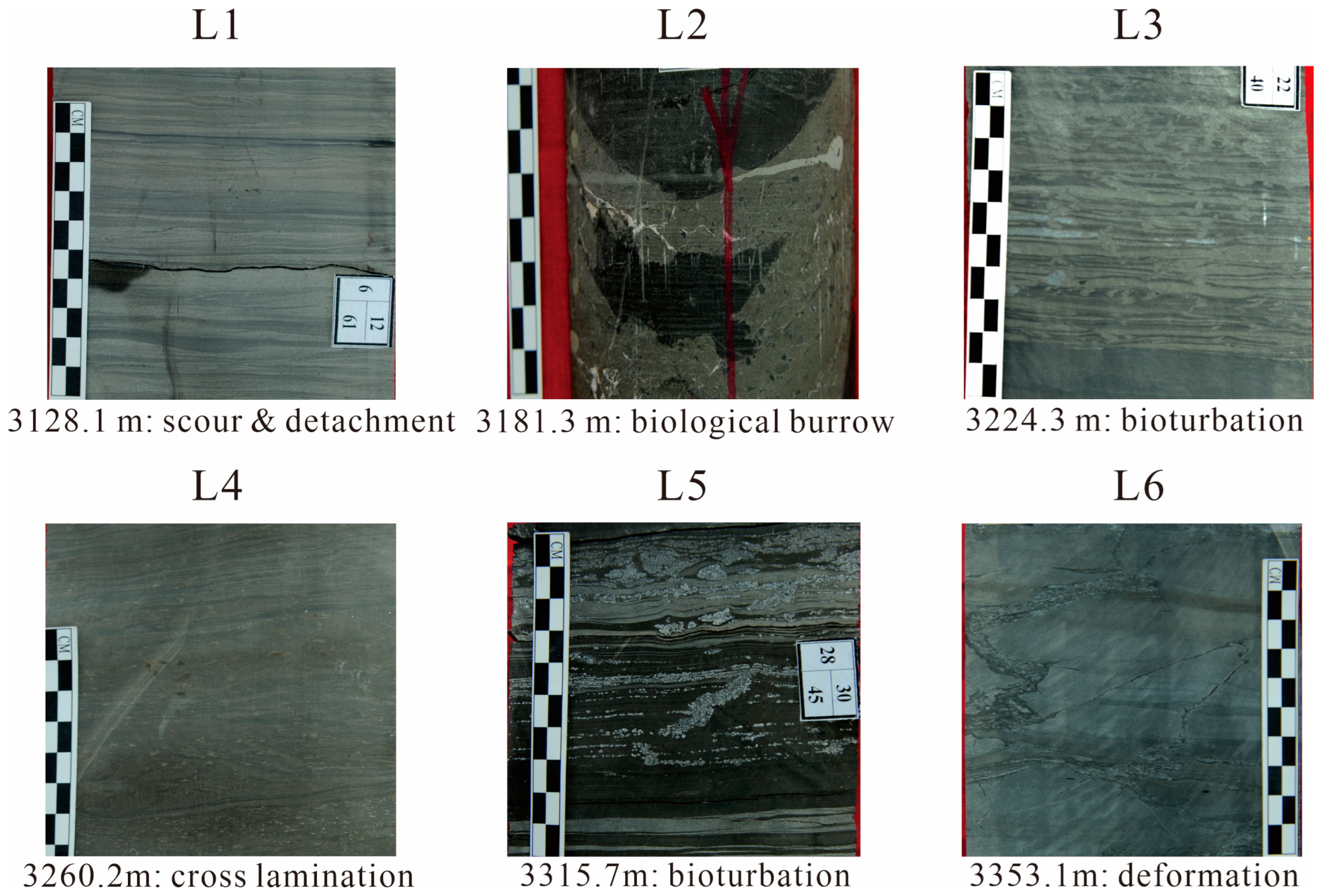



| Layer | Sedimentary Structures | Logging Response Characteristics | TOC (%) | Lithology |
|---|---|---|---|---|
| L1 | Numerous scour structures, fewer horizontal and cross laminations, with a certain amount of bioturbation | Low (<20 ohm) to high (>1000 ohm) electrical resistivity; very low (<2.5 g/cc) density, with slower change than that of other layers; large interval transit time (>80 us/ft) | 2.7/0–10 | Shale and dolomite interbedding, amount of shale increases upward, locally sandwiched dolomitic sandstone |
| L2 | Fewer horizontal laminations, abundant biological disturbance, and numerous scour structures | Large fluctuations in electrical resistivity, saw-toothed curve with spikes and large average value (~1000 ohm); extremely low density of local thin laminae (<2.3 g/cc), with saw-toothed spikes; general interval transit time of >70 us/ft, with local magnification (>90 us/ft) and saw-toothed spikes. | 4.3/0–18 | Dolomitic shale, with locally sandwiched dolomitic sandstone |
| L3 | Several horizontal laminations and bioturbation, with some turbidity structures | Low electrical resistivity (<100 ohm) | 1.9/0–9 | mudstone |
| L4 | More horizontal laminations and cross laminations, with occurrence of thick turbidites | Electrical resistivity increases (high value > 100 ohm), with a dense saw-tooth profile; average density < 2.5 g/cc; large interval transit time (average > 70 us/ft) | 2.0/0–9.5 | Dolomitic silty sandstone |
| L5 | Numerous horizontal laminations, and frequent bioturbation | Electrical resistivity < 40 ohm; density < 2.45 g/cc, saw-toothed curve; saw-toothed interval transit time with an average of >75 us/ft | 4.5/0–16 | Dolomitic mudstone and dolomitic shale, with sandwiched silty sandstone |
| L6 | Numerous thick horizontal laminations with obvious deformation structures | Low (<2.5 g/cc) to high (>2.6 g/cc) density; large (>80 us/ft) to small (<60 us/ft) interval transit times | 1.9/0–8 | Calcareous clay |
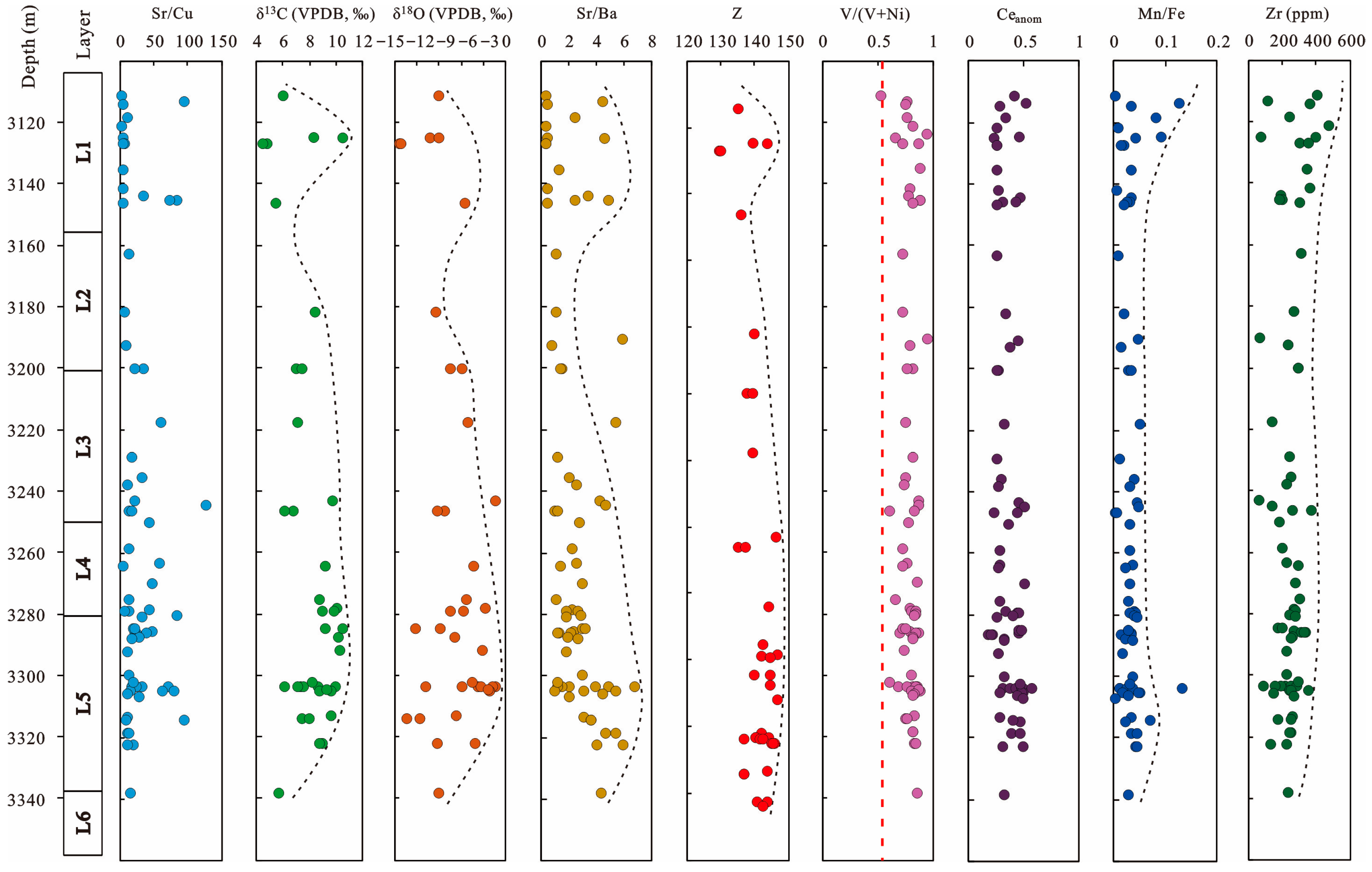
| No. | Depth (m) | Sr/Cu | Sr/Ba | V/(V + Ni) | Mn/Fe | Zr (ppm) | HREE (ppm) | LREE (ppm) | Ga (ppm) | V (ppm) | Th/Cr | Th/Sc | La/Sc | La/Co | Al/(Al + Fe + Mn) | Fe/Ti | δ13C (VPDB, ‰) | δ18O (VPDB, ‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3111.04 | 1.57 | 0.23 | 0.53 | 0.00 | 405.00 | 8.59 | 57.53 | 12.00 | 59.30 | 0.05 | 0.63 | 2.94 | 0.91 | 0.73 | 7.69 | 5.90 | −9.20 |
| 2 | 3113.34 | 93.14 | 4.39 | 0.76 | 0.13 | 109.00 | 14.29 | 69.34 | 6.53 | 17.20 | 0.41 | 0.81 | 3.40 | 2.35 | 0.91 | 10.03 | ||
| 3 | 3114.27 | 3.40 | 0.31 | 0.75 | 0.03 | 362.00 | 18.67 | 117.39 | 17.50 | 118.00 | 0.09 | 0.53 | 2.62 | 1.58 | 0.77 | 6.23 | ||
| 4 | 3121.20 | 1.64 | 0.25 | 0.81 | 0.01 | 471.00 | 24.19 | 148.07 | 18.50 | 87.30 | 0.13 | 1.68 | 6.82 | 1.89 | 0.87 | 3.09 | ||
| 5 | 3124.84 | 3.19 | 0.41 | 0.66 | 0.04 | 397.00 | 33.46 | 181.92 | 13.30 | 61.00 | 0.47 | 1.08 | 2.54 | 4.36 | 0.87 | 2.92 | 10.40 | −10.30 |
| 6 | 3124.80 | 252.98 | 4.50 | 0.95 | 0.09 | 73.80 | 22.25 | 84.91 | 2.26 | 94.60 | 0.30 | 0.33 | 0.86 | 3.24 | 0.43 | 27.25 | 8.30 | −9.20 |
| 7 | 3118.18 | 11.10 | 2.34 | 0.76 | 0.08 | 242.00 | 17.81 | 112.26 | 12.40 | 110.00 | 0.12 | 0.52 | 2.39 | 1.67 | 0.75 | 6.20 | ||
| 8 | 3135.11 | 3.90 | 1.15 | 0.88 | 0.03 | 338.00 | 17.37 | 118.45 | 12.80 | 95.80 | 0.13 | 0.63 | 2.89 | 1.88 | 0.75 | 6.75 | ||
| 9 | 3127.02 | 6.30 | 0.30 | 0.86 | 0.02 | 301.00 | 24.67 | 141.87 | 21.80 | 83.20 | 0.17 | 0.74 | 3.62 | 4.12 | 0.75 | 9.82 | 4.70 | −14.50 |
| 10 | 3127.00 | 4.27 | 0.27 | 0.73 | 0.01 | 351.00 | 27.55 | 152.74 | 24.70 | 94.50 | 0.16 | 0.76 | 3.63 | 5.20 | 0.74 | 9.24 | 4.40 | −14.30 |
| 11 | 3141.55 | 3.90 | 0.40 | 0.78 | 0.00 | 356.00 | 16.57 | 133.05 | 21.70 | 154.00 | 0.12 | 0.90 | 4.20 | 2.10 | 0.72 | 7.86 | ||
| 12 | 3143.82 | 35.02 | 3.27 | 0.78 | 0.03 | 187.00 | 15.76 | 86.11 | 6.67 | 60.30 | 0.12 | 0.27 | 0.96 | 1.07 | 0.74 | 6.07 | ||
| 13 | 3145.19 | 83.24 | 4.81 | 0.87 | 0.03 | 191.00 | 12.79 | 76.44 | 5.61 | 63.20 | 0.13 | 0.32 | 1.32 | 2.37 | 0.64 | 11.33 | ||
| 14 | 3145.29 | 72.37 | 2.35 | 0.89 | 0.02 | 181.00 | 19.51 | 134.52 | 7.82 | 64.10 | 0.11 | 0.23 | 1.83 | 5.70 | 0.58 | 16.50 | ||
| 15 | 3146.10 | 3.33 | 0.34 | 0.81 | 0.02 | 296.00 | 12.68 | 95.34 | 14.40 | 131.00 | 0.09 | 0.53 | 2.91 | 1.38 | 0.80 | 5.48 | 5.40 | −5.60 |
| 16 | 3162.81 | 12.78 | 1.03 | 0.72 | 0.01 | 303.00 | 15.21 | 102.85 | 15.60 | 72.80 | 0.09 | 0.89 | 4.64 | 1.87 | 0.78 | 5.77 | ||
| 17 | 3181.77 | 6.44 | 0.97 | 0.73 | 0.02 | 260.00 | 11.54 | 83.27 | 13.10 | 92.80 | 0.04 | 0.30 | 2.17 | 1.35 | 0.75 | 6.95 | 8.30 | −9.60 |
| 18 | 3190.64 | 485.53 | 5.61 | 0.96 | 0.04 | 61.70 | 12.16 | 55.50 | 1.33 | 75.50 | 0.11 | 0.04 | 0.40 | 3.48 | 0.32 | 55.59 | ||
| 19 | 3192.50 | 8.59 | 0.71 | 0.78 | 0.01 | 230.00 | 9.77 | 74.20 | 12.30 | 66.40 | 0.06 | 0.45 | 2.72 | 1.65 | 0.73 | 8.13 | ||
| 20 | 3199.99 | 32.97 | 1.44 | 0.81 | 0.03 | 288.00 | 22.27 | 127.66 | 15.40 | 103.00 | 0.10 | 0.76 | 3.41 | 3.02 | 0.81 | 5.45 | 6.90 | −7.70 |
| 21 | 3200.04 | 21.56 | 1.30 | 0.77 | 0.03 | 286.00 | 22.98 | 141.78 | 13.40 | 85.70 | 0.12 | 0.82 | 4.21 | 2.94 | 0.80 | 5.48 | 7.30 | −6.10 |
| 22 | 3217.63 | 60.52 | 5.34 | 0.75 | 0.05 | 134.00 | 30.52 | 133.79 | 5.52 | 53.10 | 0.36 | 0.33 | 1.31 | 2.61 | 0.76 | 5.48 | 7.00 | −5.30 |
| 23 | 3228.90 | 16.91 | 1.09 | 0.81 | 0.01 | 234.00 | 20.87 | 117.89 | 14.20 | 74.70 | 0.22 | 1.48 | 3.91 | 3.17 | 0.75 | 7.61 | ||
| 24 | 3235.38 | 31.70 | 1.91 | 0.75 | 0.04 | 250.00 | 19.03 | 102.88 | 11.20 | 65.00 | 0.14 | 0.47 | 1.85 | 1.99 | 0.71 | 8.12 | ||
| 25 | 3237.77 | 9.57 | 2.49 | 0.73 | 0.03 | 221.00 | 18.48 | 101.66 | 9.49 | 83.20 | 0.23 | 0.85 | 2.05 | 1.47 | 0.72 | 6.67 | ||
| 26 | 3242.98 | 20.36 | 4.21 | 0.87 | 0.04 | 56.40 | 14.72 | 77.46 | 2.55 | 90.00 | 0.16 | 0.11 | 0.64 | 2.04 | 0.40 | 21.56 | 9.60 | −1.50 |
| 27 | 3244.62 | 125.23 | 4.55 | 0.86 | 0.05 | 139.00 | 15.39 | 97.35 | 5.08 | 63.20 | 0.16 | 0.18 | 0.71 | 2.92 | 0.55 | 16.37 | ||
| 28 | 3246.26 | 12.74 | 0.94 | 0.83 | 0.00 | 254.00 | 13.67 | 86.61 | 14.60 | 79.50 | 0.08 | 0.70 | 2.65 | 1.18 | 0.47 | 30.87 | 6.70 | −8.30 |
| 29 | 3246.21 | 16.21 | 1.09 | 0.61 | 0.00 | 366.00 | 23.54 | 135.95 | 19.50 | 113.00 | 0.14 | 0.91 | 3.69 | 3.16 | 0.78 | 6.37 | 6.00 | −9.30 |
| 30 | 3250.08 | 43.28 | 2.64 | 0.77 | 0.03 | 179.00 | 16.34 | 99.58 | 7.59 | 49.30 | 0.12 | 0.37 | 2.19 | 2.59 | 0.70 | 8.37 | ||
| 31 | 3258.69 | 13.10 | 2.11 | 0.73 | 0.03 | 194.00 | 20.44 | 121.82 | 9.38 | 99.40 | 0.46 | 1.84 | 2.49 | 2.27 | 0.68 | 8.05 | ||
| 32 | 3263.36 | 57.84 | 2.51 | 0.76 | 0.03 | 218.00 | 20.73 | 105.84 | 10.80 | 70.50 | 0.17 | 0.61 | 1.95 | 3.18 | 0.73 | 7.79 | ||
| 33 | 3264.16 | 4.58 | 1.27 | 0.72 | 0.02 | 289.00 | 17.91 | 91.74 | 12.60 | 76.10 | 0.16 | 1.29 | 3.06 | 1.51 | 0.80 | 4.81 | 9.10 | −4.40 |
| 34 | 3269.74 | 46.07 | 2.88 | 0.86 | 0.03 | 269.00 | 16.77 | 100.06 | 11.70 | 115.00 | 0.05 | 0.23 | 1.61 | 1.87 | 0.70 | 9.21 | ||
| 35 | 3275.25 | 11.76 | 0.99 | 0.66 | 0.03 | 296.00 | 20.39 | 95.23 | 14.40 | 76.70 | 0.10 | 0.79 | 2.51 | 1.34 | 0.79 | 6.38 | 8.60 | −5.40 |
| 36 | 3278.33 | 42.46 | 2.11 | 0.79 | 0.04 | 266.00 | 20.77 | 98.62 | 10.40 | 93.80 | 0.07 | 0.23 | 2.20 | 2.58 | 0.73 | 7.12 | 10.00 | −2.80 |
| 37 | 3279.11 | 12.95 | 2.57 | 0.80 | 0.04 | 265.00 | 13.09 | 91.94 | 6.85 | 103.00 | 0.22 | 0.66 | 1.95 | 1.86 | 0.71 | 6.96 | 9.70 | −5.80 |
| 38 | 3279.01 | 6.60 | 1.78 | 0.85 | 0.03 | 272.00 | 18.03 | 124.86 | 10.10 | 116.00 | 0.59 | 2.99 | 3.02 | 1.57 | 0.72 | 7.03 | 8.90 | −7.60 |
| 39 | 3280.07 | 83.43 | 2.82 | 0.83 | 0.04 | 241.00 | 14.44 | 101.93 | 6.63 | 87.20 | 0.09 | 0.24 | 1.91 | 3.05 | 0.66 | 10.93 | ||
| 40 | 3280.53 | 30.96 | 1.75 | 0.82 | 0.04 | 272.00 | 20.29 | 122.51 | 9.75 | 92.40 | 0.10 | 0.38 | 2.65 | 2.93 | 0.79 | 5.27 | ||
| 41 | 3285.45 | 47.59 | 2.24 | 0.79 | 0.03 | 266.00 | 17.95 | 110.91 | 11.80 | 79.00 | 0.05 | 0.27 | 2.00 | 2.46 | 0.78 | 5.86 | ||
| 42 | 3286.00 | 38.03 | 2.02 | 0.86 | 0.02 | 332.00 | 20.99 | 125.64 | 12.20 | 42.70 | 0.08 | 0.70 | 5.26 | 3.44 | 0.87 | 3.18 | ||
| 43 | 3286.14 | 24.20 | 1.25 | 0.84 | 0.03 | 304.00 | 20.33 | 160.04 | 12.00 | 80.20 | 0.08 | 0.56 | 5.88 | 4.14 | 0.84 | 4.38 | ||
| 44 | 3286.06 | 21.01 | 1.07 | 0.70 | 0.01 | 320.00 | 21.72 | 161.76 | 16.30 | 97.60 | 0.07 | 0.69 | 7.35 | 3.53 | 0.78 | 6.20 | ||
| 45 | 3287.39 | 27.34 | 1.86 | 0.81 | 0.02 | 251.00 | 12.04 | 70.85 | 7.21 | 54.30 | 0.22 | 0.88 | 2.56 | 1.95 | 0.76 | 6.90 | 10.10 | −7.10 |
| 46 | 3287.98 | 15.75 | 2.58 | 0.82 | 0.03 | 248.00 | 17.17 | 122.11 | 6.76 | 84.20 | 0.27 | 0.93 | 3.38 | 2.27 | 0.65 | 8.96 | ||
| 47 | 3284.63 | 19.31 | 2.89 | 0.72 | 0.03 | 168.00 | 12.42 | 80.94 | 5.98 | 89.90 | 0.20 | 0.44 | 1.11 | 2.21 | 0.65 | 9.93 | 10.40 | −9.00 |
| 48 | 3284.61 | 22.02 | 3.11 | 0.75 | 0.03 | 193.00 | 17.67 | 115.95 | 6.26 | 73.90 | 0.37 | 0.99 | 1.78 | 2.17 | 0.70 | 7.64 | 9.10 | −12.40 |
| 49 | 3292.01 | 11.39 | 1.70 | 0.74 | 0.02 | 217.00 | 23.65 | 115.93 | 7.31 | 63.80 | 0.17 | 0.65 | 2.83 | 2.68 | 0.61 | 11.51 | 10.20 | −3.30 |
| 50 | 3299.63 | 12.14 | 2.93 | 0.80 | 0.04 | 222.00 | 18.86 | 110.82 | 8.31 | 95.20 | 0.17 | 0.58 | 2.46 | 2.05 | 0.72 | 6.94 | ||
| 51 | 3303.39 | 32.84 | 1.89 | 0.82 | 0.01 | 209.00 | 15.19 | 99.17 | 8.98 | 69.10 | 0.10 | 0.48 | 2.44 | 2.04 | 0.62 | 17.56 | 7.20 | −3.90 |
| 52 | 3303.42 | 21.57 | 1.47 | 0.68 | 0.03 | 244.00 | 19.70 | 119.46 | 11.60 | 112.00 | 0.11 | 0.55 | 2.31 | 2.31 | 0.76 | 6.55 | 8.50 | −1.50 |
| 53 | 3303.47 | 17.12 | 1.14 | 0.85 | 0.01 | 283.00 | 15.96 | 100.90 | 15.50 | 117.00 | 0.07 | 0.49 | 2.19 | 1.43 | 0.75 | 7.64 | 6.00 | −6.10 |
| 54 | 3303.48 | 69.57 | 6.67 | 0.83 | 0.13 | 86.20 | 8.55 | 46.66 | 2.84 | 40.10 | 0.26 | 0.63 | 1.57 | 1.06 | 0.64 | 7.03 | 9.90 | −11.00 |
| 55 | 3303.45 | 16.42 | 3.80 | 0.76 | 0.02 | 186.00 | 24.41 | 98.78 | 5.85 | 81.00 | 0.26 | 0.70 | 1.59 | 0.97 | 0.71 | 6.27 | 7.40 | −2.00 |
| 56 | 3303.43 | 22.82 | 4.76 | 0.84 | 0.03 | 154.00 | 18.80 | 118.75 | 5.67 | 72.10 | 0.31 | 0.71 | 2.33 | 1.61 | 0.67 | 7.85 | 7.00 | −3.40 |
| 57 | 3304.91 | 14.51 | 0.86 | 0.80 | 0.01 | 351.00 | 24.44 | 109.26 | 14.00 | 83.50 | 0.14 | 0.67 | 2.36 | 2.12 | 0.74 | 6.03 | 8.70 | 0.20 |
| 58 | 3304.86 | 78.91 | 3.00 | 0.88 | 0.05 | 236.00 | 15.98 | 88.59 | 8.56 | 64.70 | 0.12 | 0.29 | 1.28 | 1.96 | 0.67 | 11.70 | 9.50 | −2.20 |
| 59 | 3304.80 | 62.38 | 5.37 | 0.87 | 0.05 | 243.00 | 15.51 | 92.29 | 7.62 | 80.80 | 0.11 | 0.18 | 0.96 | 2.59 | 0.61 | 12.00 | 9.20 | −2.40 |
| 60 | 3305.88 | 11.32 | 4.33 | 0.83 | 0.03 | 145.00 | 23.67 | 107.43 | 5.52 | 92.70 | 0.29 | 0.87 | 2.24 | 1.93 | 0.63 | 8.98 | ||
| 61 | 3306.69 | 27.16 | 1.97 | 0.82 | 0.00 | 264.00 | 18.77 | 102.42 | 11.30 | 51.60 | 0.12 | 0.74 | 2.85 | 1.49 | 0.24 | 58.52 | ||
| 62 | 3302.13 | 18.25 | 1.10 | 0.60 | 0.03 | 291.00 | 14.93 | 100.99 | 13.00 | 96.70 | 0.07 | 0.42 | 1.92 | 1.61 | 0.79 | 6.12 | 8.10 | −4.60 |
| 63 | 3313.18 | 9.63 | 3.02 | 0.83 | 0.03 | 254.00 | 21.89 | 122.94 | 8.52 | 125.00 | 0.12 | 0.50 | 3.46 | 2.29 | 0.62 | 9.90 | 9.50 | −6.90 |
| 64 | 3314.20 | 9.19 | 3.52 | 0.74 | 0.02 | 247.00 | 21.34 | 111.90 | 9.26 | 124.00 | 0.19 | 0.74 | 2.08 | 1.60 | 0.63 | 9.64 | 7.30 | −11.70 |
| 65 | 3314.18 | 94.72 | 3.52 | 0.76 | 0.07 | 165.00 | 12.79 | 73.27 | 5.06 | 56.00 | 0.19 | 0.79 | 2.68 | 1.95 | 0.70 | 6.69 | 7.90 | −13.60 |
| 66 | 3318.35 | 10.91 | 5.34 | 0.81 | 0.03 | 244.00 | 15.81 | 114.26 | 6.95 | 118.00 | 0.20 | 0.61 | 2.51 | 1.58 | 0.63 | 8.56 | ||
| 67 | 3318.36 | 13.14 | 4.61 | 0.82 | 0.04 | 235.00 | 14.82 | 116.33 | 5.70 | 98.40 | 0.26 | 0.59 | 2.27 | 1.57 | 0.64 | 8.56 | ||
| 68 | 3322.25 | 19.05 | 5.86 | 0.83 | 0.04 | 125.00 | 19.92 | 123.21 | 5.00 | 90.90 | 0.30 | 0.59 | 2.55 | 2.05 | 0.63 | 8.65 | 8.90 | −4.30 |
| 69 | 3322.29 | 10.35 | 3.90 | 0.84 | 0.04 | 219.00 | 17.24 | 108.73 | 8.98 | 122.00 | 0.33 | 1.00 | 3.56 | 2.25 | 0.72 | 7.46 | 8.70 | −9.40 |
| 70 | 3338.05 | 14.27 | 4.26 | 0.86 | 0.03 | 232.00 | 19.39 | 87.07 | 7.91 | 114.00 | 0.17 | 0.52 | 2.86 | 2.41 | 0.73 | 6.28 | 5.60 | −9.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Lin, S.; Luo, X.; Zhang, L. Paleo-Sedimentary Environment and Formation Mechanism of the Organic-Rich Shale of the Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, China. Minerals 2024, 14, 635. https://doi.org/10.3390/min14070635
Zhao Z, Lin S, Luo X, Zhang L. Paleo-Sedimentary Environment and Formation Mechanism of the Organic-Rich Shale of the Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, China. Minerals. 2024; 14(7):635. https://doi.org/10.3390/min14070635
Chicago/Turabian StyleZhao, Zhongying, Senhu Lin, Xia Luo, and Lijun Zhang. 2024. "Paleo-Sedimentary Environment and Formation Mechanism of the Organic-Rich Shale of the Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, China" Minerals 14, no. 7: 635. https://doi.org/10.3390/min14070635







