Sources and Ore-Forming Environment of the Jinchanghe Pb-Zn Polymetallic Skarn Deposit, Baoshan Block, SW China: Constraints from Cu-S Isotopic and Trace Elemental Compositions of Sulfides
Abstract
:1. Introduction
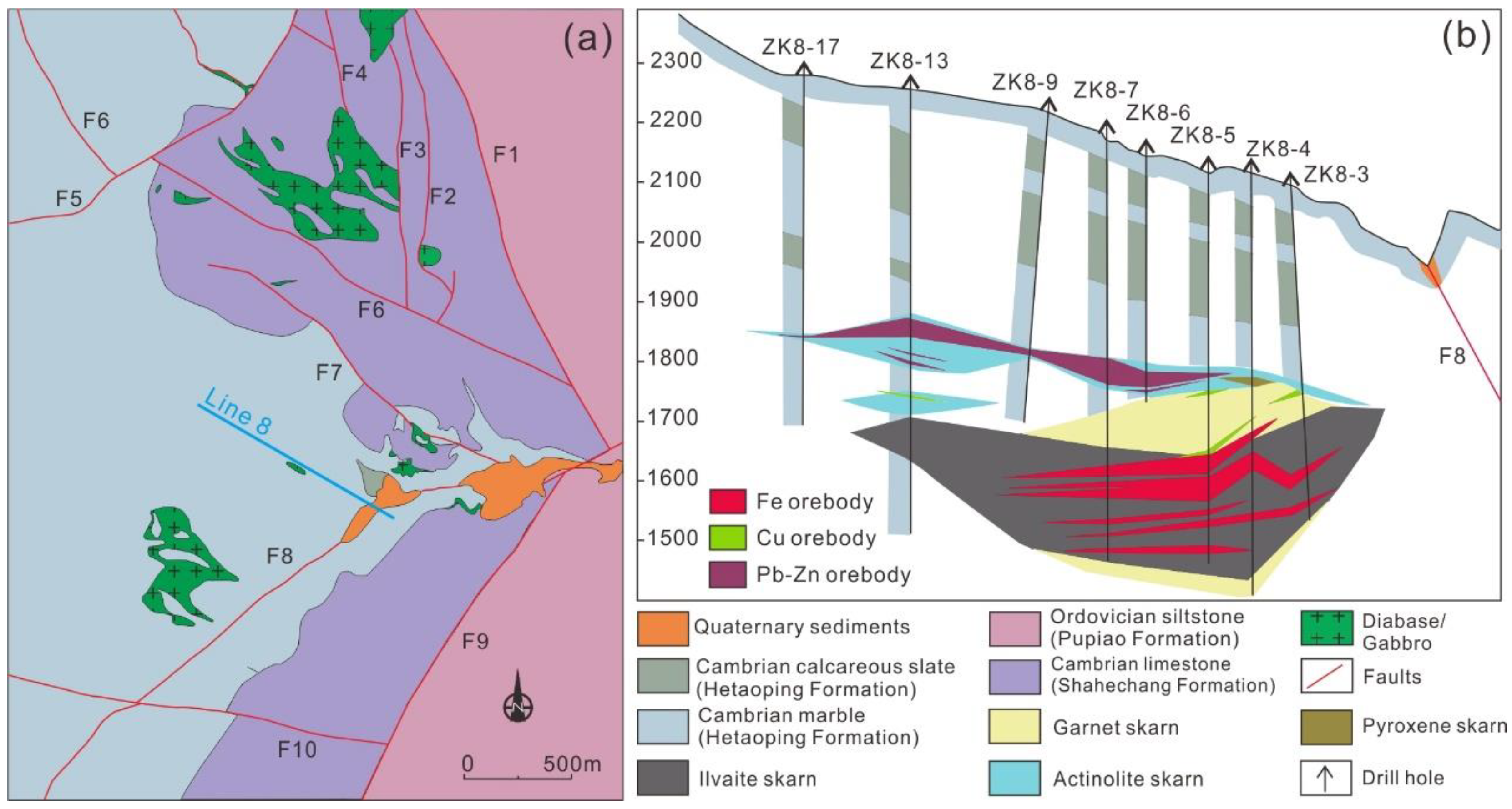
2. Geological Setting
2.1. Regional Geology
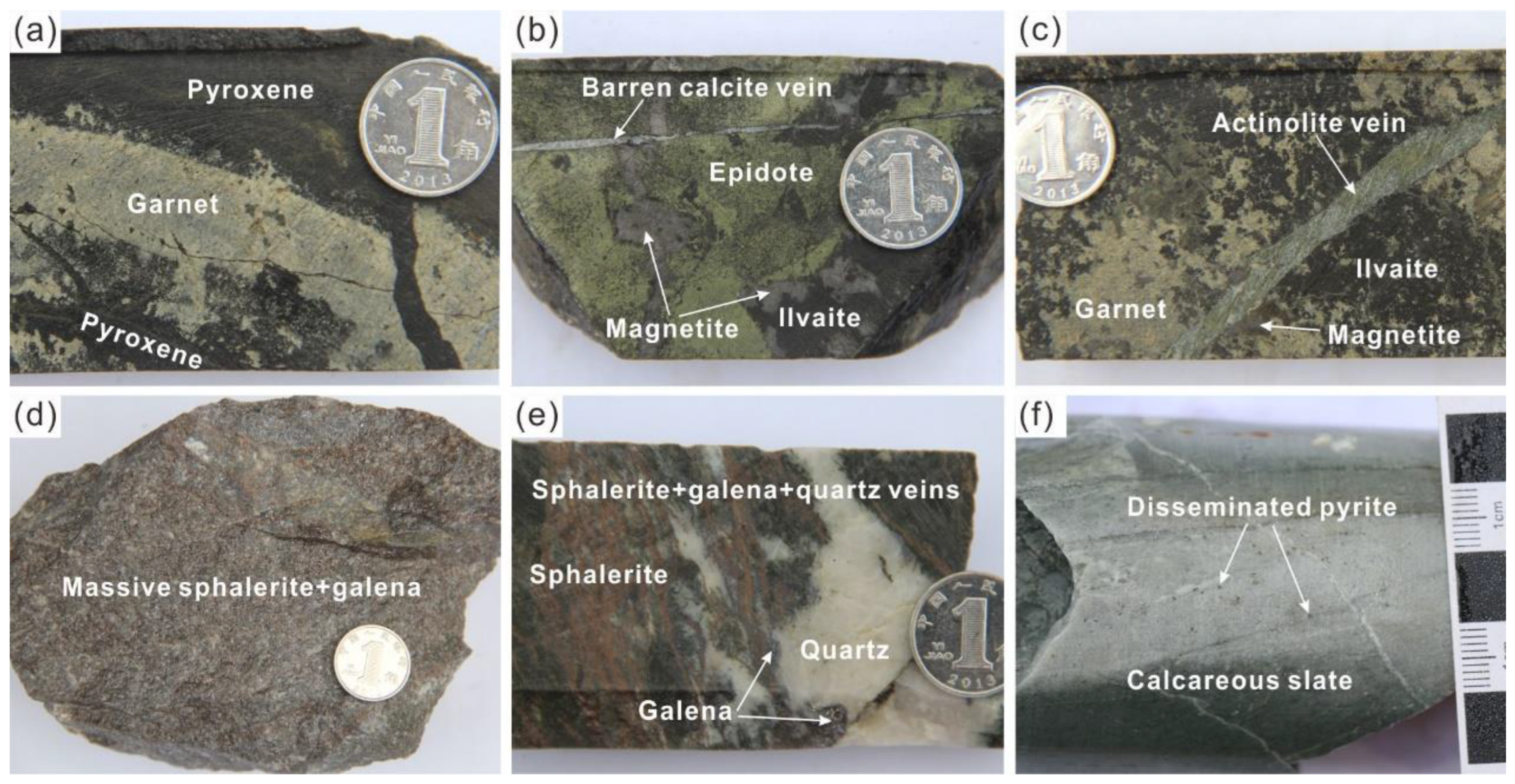
2.2. Deposit Geology
3. Sampling and Analytical Methods
4. Analytical Results
4.1. Sulfur and Copper Isotopes
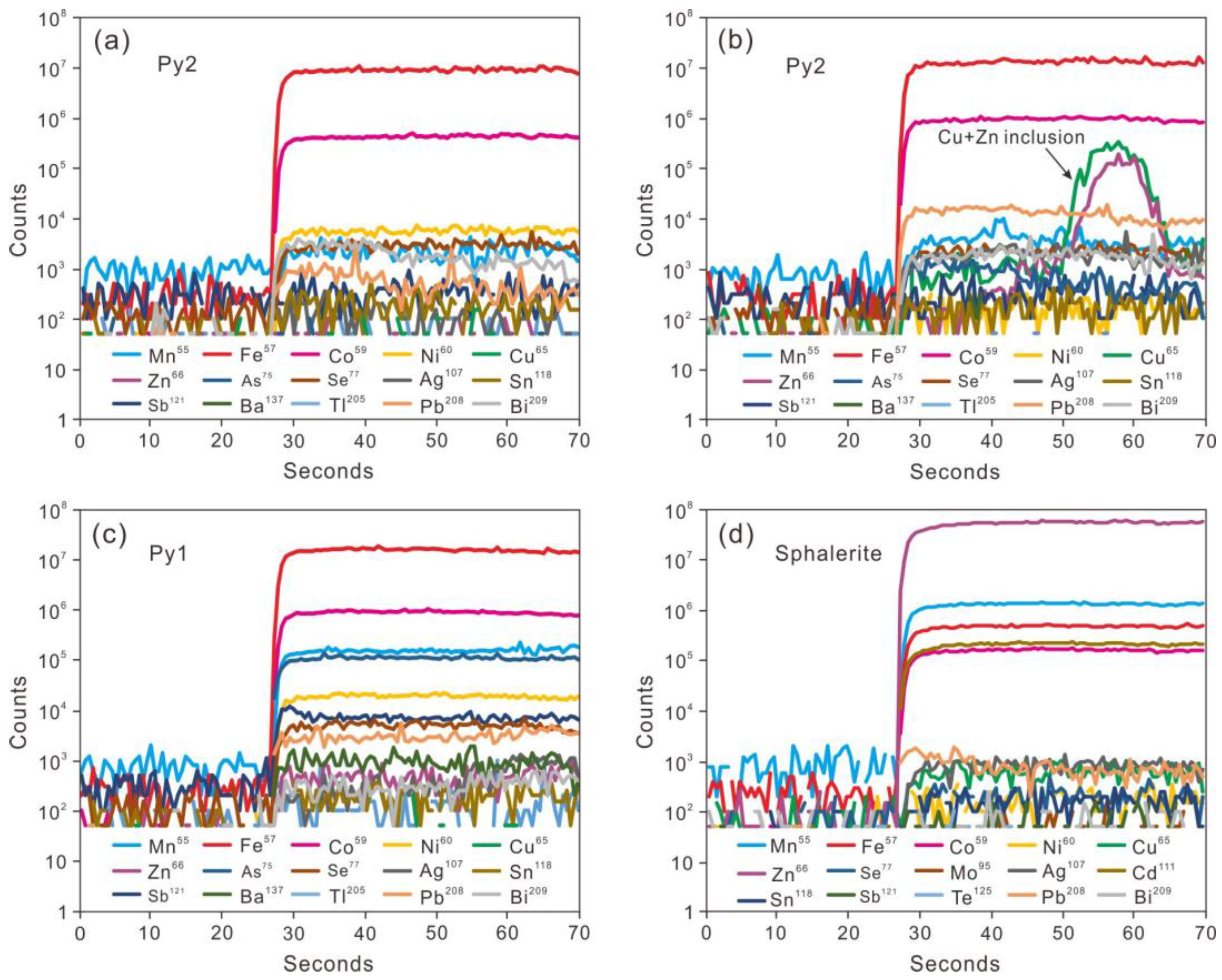
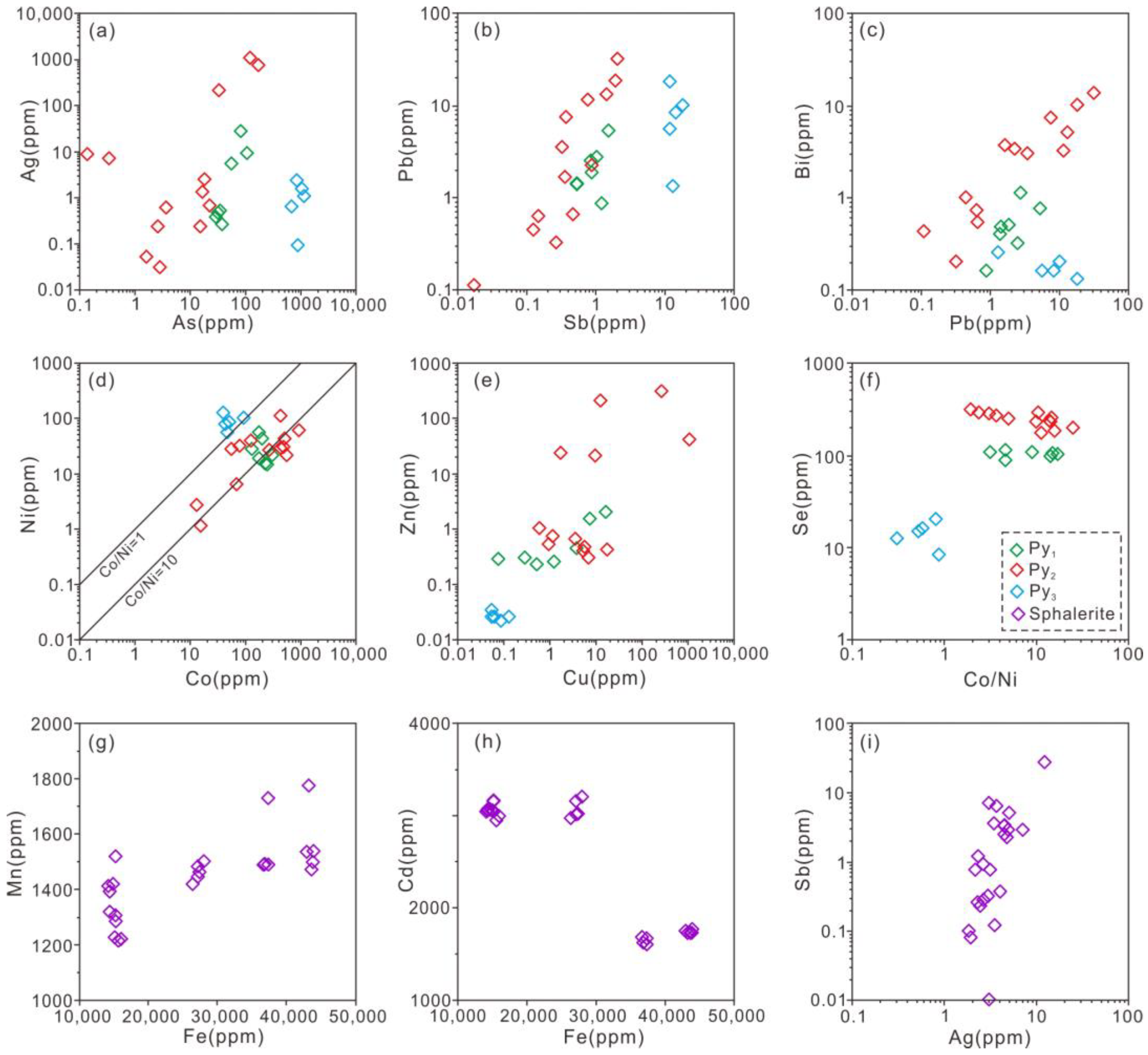
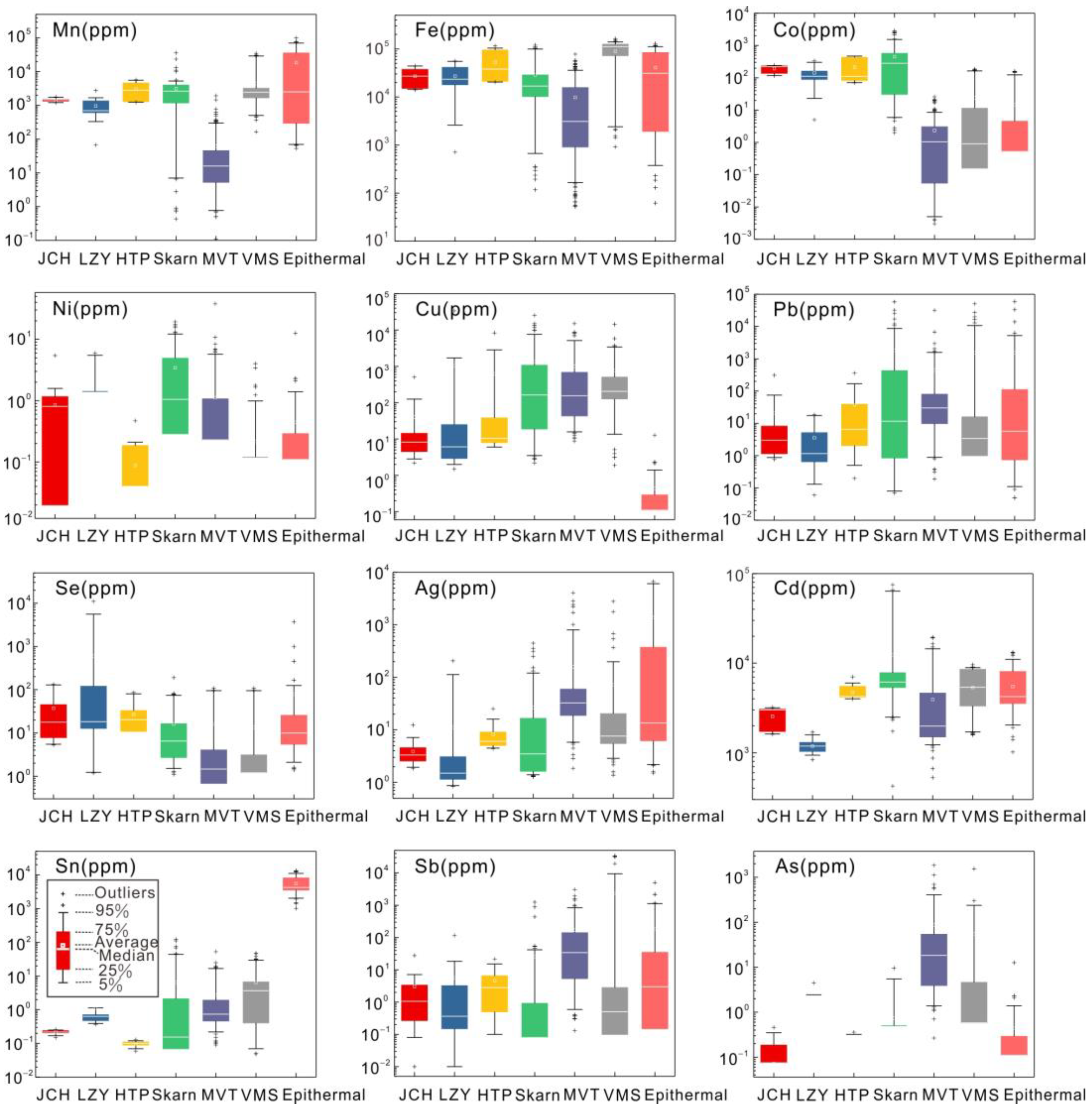
4.2. Trace Elements of Pyrite and Sphalerite
5. Discussion
5.1. Trace Element Distributions in Pyrite and Sphalerite
5.2. Origin of Ore-forming Materials
5.3. Implications for Ore-forming Temperature
6. Conclusions
- (1)
- In the Jinchanghe skarn Pb-Zn polymetallic deposit, trace element Mn, Co, Ni, Cu, Zn, As, Cd, Sn, Sb, Te, Tl, Pb and Bi were detected in pyrite, and Mn, Fe, Co, Ni, Cu, Zn, As, Se, Ag, Cd, Sn, Sb, Te, Au, Pb and Bi were detected in sphalerite. Those elements dominantly enter the pyrite and sphalerite crystal lattice by isomorphic substitution, except for some Zn and Cu occuring as sulfide inclusions in Py1.
- (2)
- Cu-S isotopic and trace elemental compositions jointly demonstrate a magmatic-hydrothermal origin for the Jinchanghe Pb-Zn polymetallic deposit with some sedimentary host-rock contributions.
- (3)
- The trace elemental compositions of pyrite and sphalerite indicate that the ore-forming temperature of the sulfide-ore stage in the Jinchanghe distal skarn Pb-Zn polymetallic deposit is medium (ca. 260 ℃).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Wang, Q.; Li, G.; Li, C.; Wang, C. Tethys tectonic evolution and its bearing on the distribution of important mineral deposits in the Sanjiang region, SW China. Gondwana Res. 2014, 26, 419–437. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Q. Gold mineralization in China: Metallogenic provinces, deposit types and tectonic framework. Gondwana Res. 2016, 36, 219–274. [Google Scholar] [CrossRef]
- Deng, J.; Chen, F.; Shu, Q.; Wang, Q.; Li, G.; Cui, X.; Huizenga, J.M.; Hu, X. Mineralogy, fluid inclusion and stable isotope study of the Jinchanghe Zn-Pb-Fe-Cu skarn deposit in southwestern China. Miner. Deposita 2024, 59, 795–813. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.; Zhou, Y.; Liu, B.; Xie, Y.; Dong, Y.; Yang, C.; Dong, W. Skarn mineralogy and zoning model of the Jinchanghe copper-zinc-iron polymetallic deposit in Yunnan Province. Acta Petrol. Mineral. 2014, 33, 127–148. (In Chinese) [Google Scholar]
- Huang, H.; Zhang, C.; Zhou, Y.; Xie, H.; Liu, B.; Xie, Y.; Dong, Y.; Yang, C.; Dong, W. Rb-Sr isochron age of Jinchanghe Fe-Cu-Pb-Zn polymetallic deposit in Yunnan Province and its geological significance. Miner. Depos. 2014, 33, 123–136. (In Chinese) [Google Scholar]
- Li, F.; Liu, X.; Zhu, J.; Zhou, Y.; Zhao, C.; Wang, J.; Li, S.; Lu, B.; Cao, Z.; Zhou, J. The geochemistry and geochronology of garnet from the Jinchanghe Fe-Cu-Pb-Zn deposit, Baoshan Block, western Yunnan. Ore Geol. Rev. 2023, 162, 105669. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Liu, X.; Zhang, N.; Zhu, J.; Chen, J.; Zhang, C.; Luo, Y. S-Pb isotope characteristics and source tracing of ore-forming materials in the Jinchanghe Fe–Cu–Pb–Zn polymetallic deposit Baoshan block western Yunnan Province. Geol. Bull. China 2020, 39, 552–562. (In Chinese) [Google Scholar]
- Chen, F.C.; Cheng, X.L.; Han, R.S.; Li, G.J.; Liu, J.Y.; Chang, H.; Jia, Z.; Cheng, Y. The fractionation of iron isotope and its constraints on the sources of ore-forming materials in the Jinchanghe skarn polymetallic deposit in Sanjiang region, Southwest China. Acta Petrol. Sin. 2022, 38, 157–171. (In Chinese) [Google Scholar]
- Chen, F.; Deng, J.; Wang, Q.; Huizenga, J.M.; Li, G.; Gu, Y. LA-ICP-MS trace element analysis of magnetite and pyrite from the Hetaoping Fe-Zn-Pb skarn deposit in Baoshan block, SW China: Implications for ore-forming processes. Ore Geol. Rev. 2020, 117, 103309. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Danyushevsky, L.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.; McGoldrick, P.; Shelley, M. Routine quantitative multi-element analysis of sulphide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Env. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Chen, F.; Deng, J.; Wang, Q.; Li, G.; Shu, Q.; Yang, C.; Liu, J.; Xu, R. The source and evolution of ore fluids in the Heiniuwa gold deposit, Baoshan block, Sanjiang region: Constraints from sulfide trace element, fluid inclusion and stable isotope studies. Ore Geol. Rev. 2018, 95, 725–745. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, C.; Deng, M.; Bai, F.; Chen, F. Genesis of Caoziwa Pb-Zn Deposit in Tengchong Block, SW China: Constraints from Sulfur Isotopic and Trace Elemental Compositions of Sulfides. Minerals 2024, 14, 82. [Google Scholar] [CrossRef]
- Burchfiel, B.C.; Chen, Z. Tectonics of the Southeastern Tibetan Plateau and Its Adjacent Foreland; Geological Society of America: Boulder, CO, USA, 2013; Volume 210, pp. 1–164. [Google Scholar]
- Yang, X.; Jia, X.; Xiong, C.; Bai, X.; Huang, B.; Luo, G.; Yang, C. LA-ICP-MS zircon U-Pb age of metamorphic basic volcanic rock in Gongyanghe Group of southern Gaoligong Mountain, western Yunnan Province, and its geological significance. Geol. Bull. China 2012, 31, 264–276. (In Chinese) [Google Scholar]
- Dong, M.; Dong, G.; Mo, X.; Santosh, M.; Zhu, D.; Yu, J.; Nie, F.; Hu, Z. Geochemistry, zircon U-Pb geochronology and Hf isotopes of granites in the Baoshan Block, Western Yunnan: Implications for Early Paleozoic evolution along the Gondwana margin. Lithos 2013, 179, 36–47. [Google Scholar] [CrossRef]
- Li, G.-J.; Wang, Q.-F.; Huang, Y.-H.; Gao, L.; Yu, L. Petrogenesis of middle Ordovician peraluminous granites in the Baoshan block: Implications for the early Paleozoic tectonic evolution along East Gondwana. Lithos 2016, 245, 76–92. [Google Scholar] [CrossRef]
- Ye, L.; Gao, W.; Cheng, Z.; Yang, Y.; Tao, Y. LA-ICP-MS Zircon U-Pb Geochronology and Petrology of the Muchang Alkali Granite, Zhenkang County, Western Yunnan Province, China. Acta Geol. Sin. Engl. 2010, 84, 1488–1499. [Google Scholar]
- Tao, Y.; Hu, R.Z.; Zhu, F.L.; Ma, Y.S.; Ye, L.; Cheng, Z.T. Ore-forming age and the geodynamic background of the Hetaoping lead-zinc deposit in Baoshan, Yunnan. Acta Petrol. Sin. 2010, 26, 1760–1772. (In Chinese) [Google Scholar]
- Yu, L.; Li, G.J.; Wang, Q.F.; Liu, X.F. Petrogenesis and tectonic significance of the Late Cretaceous magmatism in the northern part of the Baoshan block: Constraints from bulk geochemistry, zircon U-Pb geochronology and Hf isotopic compositions. Acta Petrol. Sin. 2014, 30, 2709–2724. (In Chinese) [Google Scholar]
- Yu, L.; Wang, Q.F.; Li, G.J.; Gao, L. Geochemistry, zircon U-Pb geochronology of granitic pegmatites from Caojian area in the northern Baoshan block, and their geological significance. Acta Petrol. Sin. 2015, 31, 3281–3296. (In Chinese) [Google Scholar]
- Liao, S.; Wang, D.; Tang, Y.; Yin, F.; Sun, Z.; Sun, J. LA-ICP-MS U-Pb age of two-mica granite in the Yunlong tin-tungsten metallogenic belt in Three River region and its geological implications. Acta Petrol. Mineral. 2013, 32, 450–462. [Google Scholar]
- Deng, J.; Wang, Q.; Li, G.; Santosh, M. Cenozoic tectono-magmatic and metallogenic processes in the Sanjiang region, southwestern China. Earth-Sci. Rev. 2014, 138, 268–299. [Google Scholar] [CrossRef]
- Wang, Q.; Deng, J.; Li, G.; Liu, J.; Li, C.; Ripley, E.M. Geochronological, Petrological, and Geochemical Studies of the Daxueshan Magmatic Ni-Cu Sulfide Deposit in the Tethyan Orogenic Belt, Southwest China. Econ. Geol. 2018, 113, 1307–1332. [Google Scholar] [CrossRef]
- Chen, F.; Deng, J.; Shu, Q.; Li, G.; Cui, X.; Zhao, F.; Wang, Q. Geology, fluid inclusion and stable isotopes (O, S) of the Hetaoping distal skarn Zn-Pb deposit, northern Baoshan block, SW China. Ore Geol. Rev. 2017, 90, 913–927. [Google Scholar] [CrossRef]
- Xu, R.; Deng, M.-G.; Li, W.C.; Lai, C.K.; Zaw, K.; Gao, Z.W.; Chen, Y.H.; Niu, C.H.; Liang, G. Origin of the giant Luziyuan Zn-Pb-Fe(-Cu) distal skarn deposit, Baoshan block, SE Tibet: Constraints from Pb-Sr isotopes, calcite C-O isotopes, trace elements and Sm-Nd dating. J. Asian Earth Sci. 2021, 205, 104587. [Google Scholar] [CrossRef]
- Xiao, C.H.; Li, G.J. Geological, sulfur isotopic, and mineral trace element constraints on the genesis of the Xiyi Pb-Zn deposit, Baoshan Block, SW China. J. Asian Earth Sci. 2019, 186, 104056. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Deng, J.; Li, C.; Li, G.; Ripley, E.M. 280–310 Ma rift-related basaltic magmatism in northern Baoshan, SW China: Implications for Gondwana reconstruction and mineral exploration. Gondwana Res. 2020, 77, 1–18. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yang, C.H.; Fu, J.; Zhang, W.W.; Xie, J.; Li, J.B. Metallogenic Models and Prospecting Criteria for the Jinchanghe Au–Cu Polymetallic Ore–Concentration Area in Western Yunnan. Geol. Explor. 2021, 57, 254–268. [Google Scholar]
- Maréchal, C.N.; Télouk, P.; Albarède, F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 1999, 156, 251–273. [Google Scholar] [CrossRef]
- Liu, S.-A.; Li, D.; Li, S.; Teng, F.-Z.; Ke, S.; He, Y.; Lu, Y. High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 122–133. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and Trace Element Zonation in Pyrite Using a Laser Imaging Technique: Implications for the Timing of Gold in Orogenic and Carlin-Style Sediment-Hosted Deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Sung, Y.H.; Brugger, J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Nugus, M. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields Province, Western Australia. Miner. Depos. 2009, 44, 765–791. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Green, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Mineral. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Huston David, L.; Sie Soey, H.; Suter Gary, F.; Cooke David, R.; Both Ross, A. Trace elements in sulfide minerals from eastern Australian volcanic-hosted massive sulfide deposits; Part I, Proton microprobe analyses of pyrite, chalcopyrite, and sphalerite, and Part II, Selenium levels in pyrite; comparison with delta 34 S values and implications for the source of sulfur in volcanogenic hydrothermal systems. Econ. Geol. 1995, 90, 1167–1196. [Google Scholar]
- Grant, H.L.; Hannington, M.D.; Petersen, S.; Frische, M.; Fuchs, S.H. Constraints on the behavior of trace elements in the actively-forming TAG deposit, Mid-Atlantic Ridge, based on LA-ICP-MS analyses of pyrite. Chem. Geol. 2018, 498, 45–71. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.J.; Ulrich, T.; Zhang, J.; Wang, J. Trace element compositions of sulfides from Pb-Zn deposits in the Northeast Yunnan and northwest Guizhou Provinces, SW China: Insights from LA-ICP-MS analyses of sphalerite and pyrite. Ore Geol. Rev. 2022, 141, 104639. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.; Chryssoulis, S.; Li, J.W.; Ma, C.Q.; Parada, M.A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochim. Cosmochim. 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Xie, J.; Ge, L.; Fang, D.; Li, Q.; Qian, L.; Li, Z.; Yan, J.; Sun, W. Geochemistry of pyrite from stratabound massive sulfide deposits, Tongling region, China: Implication for their genesis. Ore Geol. Rev. 2020, 120, 103430. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.M.; Walshe, J.L. Trace-metal nanoparticles in pyrite. Geochim. Cosmochim. 2010, 74, A216. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Sun, H.; Koua, K.A.; Lyu, C. LA-ICP-MS trace element analysis of sphalerite and pyrite from the Beishan Pb-Zn ore district, south China: Implications for ore genesis. Ore Geol. Rev. 2022, 150, 105128. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Hu, Y.; Danyushevskiy, L.; Li, Z.; Huang, Z. Distribution and occurrence of Ge and related trace elements in sphalerite from the Lehong carbonate-hosted Zn-Pb deposit, northeastern Yunnan, China: Insights from SEM and LA-ICP-MS studies. Ore Geol. Rev. 2019, 115, 103175. [Google Scholar] [CrossRef]
- Mathur, R.; Titley, S.; Barra, F.; Brantley, S.; Wilson, M.; Phillips, A.; Munizaga, F.; Maksaev, V.; Vervoort, J.; Hart, G. Exploration potential of Cu isotope fractionation in porphyry copper deposits. J. Geochem. Explor. 2009, 102, 1–6. [Google Scholar] [CrossRef]
- Wang, C.; Bagas, L.; Chen, J.; Yang, L.; Zhang, D.; Du, B.; Shi, K. The genesis of the Liancheng Cu-Mo deposit in the Lanping Basin of SW China: Constraints from geology, fluid inclusions, and Cu-S-H-O isotopes. Ore Geol. Rev. 2018, 92, 113–128. [Google Scholar] [CrossRef]
- Ohmoto, H.; Kaiser, C.J.; Geer, K.A. Systematics of sulphur isotopes in recent marine sediments and ancient sediment-hosted base metal deposits. In Stable Isotopes and Fluid Processes in Mineralization; Herbert, H.K., Ho, S.E., Eds.; University of Western Australia: Perth, Australia, 1990; pp. 70–120. [Google Scholar]
- Clark, C.; Grguric, B.; Mumm, A.S. Genetic implications of pyrite chemistry from the Palaeoproterozoic Olary Domain and overlying Neoproterozoic Adelaidean sequences, northeastern South Australia. Ore Geol. Rev. 2004, 25, 237–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Cheng, J.; Tian, J.; Zhang, L.; Olin, P. Pyrite geochemistry and its implications on Au-Cu skarn metallogeny: An example from the Jiguanzui deposit, Eastern China. Am. Mineral. 2022, 107, 1910–1925. [Google Scholar] [CrossRef]
- Andreas, A. The Metal Content of Magmatic-Hydrothermal Fluids and Its Relationship to Mineralization Potential. Econ. Geol. 2019, 114, 1033–1056. [Google Scholar]
- Shu, Q.; Lai, Y.; Sun, Y.; Wang, C.; Meng, S. Ore Genesis and Hydrothermal Evolution of the Baiyinnuo’er Zinc-Lead Skarn Deposit, Northeast China: Evidence from Isotopes (S, Pb) and Fluid Inclusions. Econ. Geol. 2013, 108, 835–860. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Hu, Y.; Huang, Z.; Danyushevsky, L.; Wang, H. LA-ICP-MS analyses of trace elements in base metal sulfides from carbonate-hosted Zn-Pb deposits, South China: A case study of the Maoping deposit. Ore Geol. Rev. 2021, 130, 130945. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, C.; Yu, H.; Yang, Y.; Zhao, Y.; Zhu, C.; Ding, Q.; Zhou, Y.; Yang, J.; Xu, Y. Element enrichment characteristics: Insights from element geochemistry of sphalerite in Daliangzi Pb–Zn deposit, Sichuan, Southwest China. J. Geochem. Explor. 2018, 186, 187–201. [Google Scholar] [CrossRef]
- Xu, R. Genesis of the Zn-Pb polymetallic deposit in Luziyuan, Baoshan Block, Sanjiang, Southwest China: Constraints of mineral chemistry and isotope geochemistry. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2021. (In Chinese). [Google Scholar]
- Keith, M.; Smith, D.J.; Jenkin, G.R.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Li, R.; Chen, H.; Xia, X.; Yan, Q.; Danyushevsky Leonid, V.; Lai, C. Using integrated in-situ sulfide trace element geochemistry and sulfur isotopes to trace ore-forming fluids: Example from the Mina Justa IOCG deposit (southern Peru). Ore Geol. Rev. 2018, 101, 165–179. [Google Scholar] [CrossRef]
- Rowins, S.M.; Groves, D.I.; McNaughton, N.J.; Palmer, M.R.; Eldridge, C.S. A reinterpretation of the role of granitoids in the genesis of Neoproterozoic gold mineralization in the Telfer Dome, Western Australia. Econ. Geol. 1997, 92, 133–160. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, L.; Wei, C.; Li, Z.; Huang, Z.; Wang, H. Trace Elements in Sphalerite from the Dadongla Zn-Pb Deposit, Western Hunan-Eastern Guizhou Zn-Pb Metallogenic Belt, South China. Acta Geol. Sin-Engl. 2020, 94, 2152–2164. [Google Scholar] [CrossRef]
- Tian, J.; Yang, G. Trace Element Characteristics of Sphalerite from Dulong Tin-polymetallic Deposit in Southeast Yunnan and Their Geological Significances. Geotecton. Metallog. 2022, 46, 1148–1166. (In Chinese) [Google Scholar]
- Ye, L.; Li, Z.L.; Hu, Y.S.; Huang, Z.L.; Zhou, J.X.; Fan, H.F.; Danyushevskiy, L. Trace elements in sulfide from the Tianbaoshan Pb-Zn deposit, Sichuan Province, China: A LA-ICPMS study. Acta. Petrol. Sin. 2016, 32, 3377–3393. (In Chinese) [Google Scholar]
- Keith, M.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R.; Petersen, S.; Bach, W. Effects of temperature, sulfur, and oxygen fugacity on the composition of sphalerite from submarine hydrothermal vents. Geology 2014, 42, 699–702. [Google Scholar] [CrossRef]


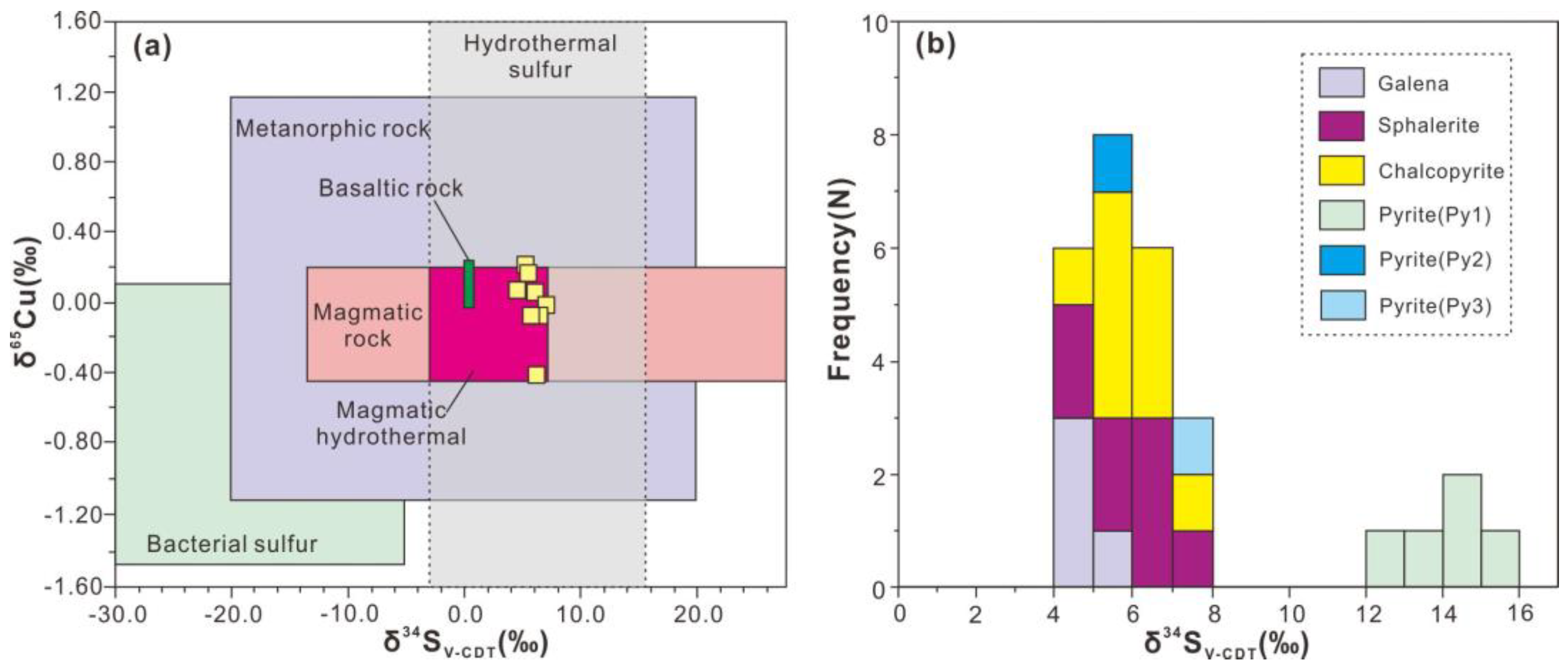

| Sample No. | Mineral | Sample Description | δ34S(CDT)‰ | δ65Cu (‰) | 2SD |
|---|---|---|---|---|---|
| JCH-16-02 | Sphalerite | Sphalerite massive ore | 4.5 | ND | ND |
| JCH-16-04 | Chalcopyrite | Chalcopyrite-quartz-calcite vein | 7.1 | 0.00 | 0.03 |
| JCH-16-05 | Chalcopyrite | Chalcopyrite-sphalerite-galena massive ore | 5.1 | ND | ND |
| Galena | 4.6 | ND | ND | ||
| Sphalerite | 6.2 | ND | ND | ||
| JCH-16-08 | Chalcopyrite | Chalcopyrite-quartz vein | 5.3 | 0.22 | 0.03 |
| JCH-16-09 | Sphalerite | Sphalerite stockwork ore | 4.5 | ND | ND |
| JCH-16-10 | Chalcopyrite | Chalcopyrite-galena-sphalerite vein | 6.2 | −0.40 | 0.02 |
| Galena | 4.7 | ND | ND | ||
| Sphalerite | 5.8 | ND | ND | ||
| JCH-16-11 | Chalcopyrite | Sphalerite-chalcopyrite massive ore | 5.5 | 0.17 | 0.05 |
| Sphalerite | 7.3 | ND | ND | ||
| JCH-16-12 | Pyrite | Pyrite-chalcopyrite-sphalerite vein | 5.9 | ND | ND |
| Chalcopyrite | 6.5 | −0.06 | 0.02 | ||
| Sphalerite | 6.7 | ND | ND | ||
| JCH-16-13 | Chalcopyrite | Chalcopyrite-quartz vein | 4.6 | 0.08 | 0.02 |
| JCH-16-15 | Sphalerite | Pyrite-sphalerite massive ore | 5.2 | ND | ND |
| Pyrite | 7.1 | ND | ND | ||
| JCH-16-16 | Chalcopyrite | Chalcopyrite-quartz vein | 5.8 | −0.06 | 0.04 |
| JCH-16-19 | Galena | Chalcopyrite-sphalerite-galena massive ore | 4.3 | ND | ND |
| Sphalerite | 6.6 | ND | ND | ||
| JCH-16-22 | Chalcopyrite | Galena-chalcopyrite-quartz vein | 6.1 | 0.07 | 0.02 |
| Galena | 5.9 | ND | ND | ||
| JCH-16-25 | Pyrite | Disseminated pyrite in calcareous slate | 14.5 | ND | ND |
| JCH-16-26 | Pyrite | Disseminated pyrite in calcareous slate | 12.3 | ND | ND |
| JCH-16-27 | Pyrite | Disseminated pyrite in marble | 15.1 | ND | ND |
| JCH-16-28 | Pyrite | Disseminated pyrite in marble | 14.2 | ND | ND |
| JCH-16-29 | Pyrite | Disseminated pyrite in marble | 13.6 | ND | ND |
| Pyrite Generation | Sample No. | Analysis Spot | Mn | Co | Ni | Cu | Zn | As | Se | Ag | Cd | Sn | Sb | Te | Tl | Pb | Bi | Co/Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | ||||
| Py1 | JCH-16-26 | 1 | 163.22 | 39.37 | 132.26 | B.D.L | 0.33 | 1102.55 | 12.56 | 1.08 | B.D.L | 0.09 | 127.83 | 0.28 | 0.97 | 1.32 | 0.25 | 0.3 |
| 2 | 98.15 | 45.55 | 57.25 | 0.12 | 0.25 | 645.84 | 20.38 | 0.62 | 0.16 | 0.13 | 116.97 | 0.16 | 0.51 | 5.63 | 0.16 | 0.8 | ||
| 3 | 117.64 | 90.28 | 105.41 | 0.08 | 0.22 | 806.37 | 8.41 | 2.37 | 0.28 | 0.11 | 116.01 | B.D.L | 0.36 | 18.26 | 0.13 | 0.86 | ||
| JCH-16-29 | 1 | 145.05 | 42.65 | 82.35 | 0.05 | 0.25 | 1008.25 | 15.22 | 1.53 | B.D.L | 0.15 | 176.2 | 0.25 | 0.58 | 10.05 | 0.2 | 0.52 | |
| 2 | 148.73 | 50.19 | 89.09 | 0.06 | 0.26 | 857.38 | 16.35 | 0.09 | 0.12 | 0.11 | 140.41 | 0.18 | 0.65 | 8.47 | 0.16 | 0.56 | ||
| Py2 | JCH-16-01 | 1 | B.D.L | 416.12 | 115.6 | 1057.71 | 409.29 | 16.72 | 274.39 | 2.52 | 9.05 | 0.76 | 19.16 | 0.19 | 0.51 | 18.42 | 10.07 | 3.6 |
| 2 | B.D.L | 77.26 | 33.27 | 12.15 | 2131.41 | 21.52 | 298.29 | 0.67 | 32.86 | 0.55 | 7.61 | 0.35 | 0.24 | 11.53 | 3.22 | 2.32 | ||
| JCH-16-03 | 1 | 0.43 | 123.87 | 40.9 | 254.36 | 3112.93 | 15.72 | 293.38 | 1.3 | 37.36 | 0.65 | 14.06 | 0.23 | 0.41 | 13.2 | 5.08 | 3.03 | |
| 2 | B.D.L | 54.91 | 29.12 | 3.48 | 6.59 | 14.35 | 319.4 | 0.23 | 8.6 | 0.19 | 1.23 | 0.35 | 0.16 | 0.44 | 1 | 1.89 | ||
| JCH-16-06 | 1 | 0.22 | 926.75 | 63.92 | 1.6 | 235.36 | 0.13 | 259.43 | 8.6 | 2.32 | 0.14 | 3.69 | 0.13 | 0.12 | 7.55 | 7.33 | 14.5 | |
| 2 | 0.23 | 420.54 | 30.21 | 9.08 | 208.91 | 0.32 | 234.69 | 7.01 | 2 | 0.07 | 3.15 | B.D.L | 0.39 | 3.51 | 2.99 | 13.92 | ||
| 3 | 10.65 | 493.75 | 44.44 | 16.35 | 4.18 | 114.09 | 176.78 | 1103.81 | 6.64 | 0.14 | 8.44 | B.D.L | 0.29 | 2.26 | 3.34 | 11.11 | ||
| JCH-16-08 | 1 | 8.55 | 536.54 | 21.8 | 5.49 | 4.69 | 159.82 | 202.81 | 783.23 | 4.22 | 0.15 | 4.62 | B.D.L | 0.12 | 0.65 | 0.54 | 24.61 | |
| 2 | 4.45 | 483.38 | 31.32 | 6.76 | 2.99 | 30.9 | 188.98 | 216.69 | 15.1 | 0.24 | 3.55 | B.D.L | 0.14 | 1.65 | 3.69 | 15.43 | ||
| JCH-16-14 | 1 | B.D.L | 270.55 | 27.5 | 0.57 | 10.25 | 2.42 | 234.87 | 0.23 | 0.52 | 0.11 | 0.17 | 0.27 | 0.07 | 0.11 | 0.43 | 9.84 | |
| 2 | B.D.L | 13.04 | 2.67 | 5.04 | 3.98 | 3.5 | 257.35 | 0.6 | B.D.L | 0.14 | 19.94 | 0.27 | 0.28 | 32.14 | 13.6 | 4.88 | ||
| 3 | B.D.L | 15.33 | 1.12 | 0.87 | 5.15 | 1.5 | 241.84 | 0.05 | B.D.L | 0.1 | 2.58 | 0.12 | 0.25 | 0.32 | 0.2 | 13.69 | ||
| 4 | B.D.L | 68.13 | 6.61 | 1.08 | 7.36 | 2.67 | 294.74 | 0.03 | 0.13 | 0.11 | 1.43 | 0.23 | 0.13 | 0.63 | 0.72 | 10.31 | ||
| Py3 | JCH-16-04 | 1 | 71.94 | 198.9 | 43.57 | 0.27 | 3.03 | 33.33 | 115.49 | 0.52 | B.D.L | 0.15 | 10.13 | B.D.L | 0.08 | 2.73 | 1.13 | 4.57 |
| 2 | 86.61 | 170.12 | 19.33 | 0.49 | 2.26 | 29.44 | 111.51 | 0.45 | B.D.L | 0.13 | 8.65 | B.D.L | 0.16 | 1.87 | 0.5 | 8.8 | ||
| 3 | 103.96 | 248.26 | 14.67 | 1.19 | 2.54 | 76.24 | 104.77 | 27.51 | B.D.L | 0.15 | 5.14 | B.D.L | 0.25 | 1.4 | 0.4 | 16.92 | ||
| JCH-16-11 | 1 | 86.57 | 173.23 | 56.39 | 3.69 | 4.54 | 53.7 | 111.22 | 5.47 | 2.7 | 0.15 | 8.32 | B.D.L | 0.14 | 2.52 | 0.32 | 3.07 | |
| 2 | 94.62 | 299.2 | 21.63 | 0.07 | 2.87 | 102.08 | 100.73 | 9.18 | B.D.L | 0.13 | 12.08 | B.D.L | 0.12 | 0.87 | 0.16 | 13.83 | ||
| JCH-16-15 | 1 | 81.22 | 126.38 | 28.22 | 6.9 | 15.36 | 35.36 | 89.16 | 0.25 | 0.56 | 0.22 | 5.26 | 0.15 | 0.12 | 1.42 | 0.48 | 4.48 | |
| 2 | 72.23 | 225.65 | 15.26 | 15.42 | 20.13 | 27.28 | 108.22 | 0.38 | 0.25 | 0.17 | 15.15 | B.D.L | 0.29 | 5.31 | 0.75 | 14.79 |
| Sample No. | Analysis No. | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Ag | Cd | Sn | Sb | Te | Au | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (wt.%) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | ||
| JCH-16-02 | 1 | 1773 | 43,243 | 138.59 | B.D.L | 13.95 | 62.52% | B.D.L | 49.79 | 2.31 | 1727 | 0.21 | 0.26 | 0.10 | B.D.L | 0.87 | 0.52 |
| 2 | 1534 | 42,949 | 139.64 | B.D.L | 14.55 | 62.55% | B.D.L | 41.15 | 2.66 | 1749 | 0.19 | 0.92 | 0.09 | B.D.L | 2.21 | 1.68 | |
| 3 | 1537 | 43,927 | 140.87 | B.D.L | 72.21 | 62.45% | B.D.L | 41.63 | 4.53 | 1768 | 0.19 | 2.49 | 0.06 | 0.01 | 39.39 | 4.89 | |
| JCH-16-05 | 1 | 1470 | 43,631 | 138.92 | B.D.L | 506.15 | 62.48% | B.D.L | 36.02 | 4.82 | 1725 | 0.21 | 2.26 | 0.13 | 0.02 | 9.80 | 6.29 |
| 2 | 1497 | 43,853 | 136.79 | B.D.L | 12.31 | 62.46% | B.D.L | 33.56 | 3.16 | 1729 | 0.21 | 0.76 | 0.23 | B.D.L | 2.99 | 1.99 | |
| JCH-16-09 | 1 | 1392 | 14,340 | 240.59 | 1.58 | 11.73 | 65.58% | B.D.L | 29.08 | 3.00 | 3070 | 0.21 | 0.32 | 0.05 | B.D.L | 1.22 | 0.01 |
| 2 | 1418 | 14,803 | 238.75 | 1.44 | 2.19 | 65.53% | 0.13 | 21.10 | 2.18 | 3061 | 0.22 | 0.76 | 0.13 | B.D.L | 1.00 | 0.01 | |
| 3 | 1319 | 14,333 | 239.48 | 1.55 | 2.82 | 65.58% | 0.35 | 8.43 | 2.67 | 3052 | 0.24 | 0.28 | 0.05 | B.D.L | 0.77 | 0.01 | |
| 4 | 1411 | 14,119 | 244.47 | 1.45 | 3.32 | 65.60% | 0.16 | 5.50 | 3.54 | 3044 | 0.25 | 0.12 | 0.05 | 0.01 | 0.88 | 0.01 | |
| JCH-16-10 | 1 | 1487 | 36,659 | 117.16 | B.D.L | 92.85 | 63.22% | 0.20 | 132.41 | 4.95 | 1681 | 0.23 | 2.91 | 0.11 | 0.01 | 310.74 | 3.79 |
| 2 | 1728 | 37,325 | 118.78 | 5.43 | 7.43 | 63.15% | 0.24 | 119.63 | 4.55 | 1603 | 0.21 | 3.37 | 0.11 | 0.02 | 5.30 | 1.45 | |
| 3 | 1488 | 37,349 | 117.98 | B.D.L | 4.67 | 63.15% | 0.14 | 129.09 | 2.47 | 1667 | 0.21 | 0.23 | 0.04 | B.D.L | 0.90 | 0.32 | |
| 4 | 1490 | 36,847 | 116.59 | B.D.L | 125.43 | 63.20% | 0.02 | 107.45 | 4.51 | 1621 | 0.23 | 3.33 | 0.08 | 0.02 | 15.18 | 2.32 | |
| JCH-16-12 | 1 | 1461 | 27,367 | 220.10 | 0.84 | 5.83 | 64.20% | 0.26 | 7.56 | 2.34 | 3019 | 0.22 | 1.20 | 0.13 | 0.01 | 2.98 | B.D.L |
| 2 | 1444 | 27,137 | 220.80 | 0.97 | 3.14 | 64.22% | 0.15 | 6.82 | 1.94 | 3012 | 0.25 | 0.08 | 0.03 | B.D.L | 1.19 | 0.01 | |
| 3 | 1418 | 26,361 | 221.80 | 0.84 | 3.32 | 64.31% | 0.46 | 7.59 | 1.85 | 2974 | 0.26 | 0.10 | 0.10 | 0.01 | 1.13 | B.D.L | |
| 4 | 1482 | 27,115 | 224.38 | 0.60 | 4.49 | 64.23% | B.D.L | 5.27 | 3.06 | 3158 | 0.17 | 7.13 | 0.10 | 0.02 | 5.39 | B.D.L | |
| 5 | 1500 | 27,987 | 225.24 | 0.65 | 4.62 | 64.13% | B.D.L | 6.33 | 3.49 | 3205 | 0.19 | 3.59 | 0.02 | B.D.L | 5.41 | B.D.L | |
| JCH-16-15 | 1 | 1306 | 15,178 | 235.33 | 0.18 | 24.64 | 65.49% | 0.19 | 8.07 | 12.36 | 3156 | 0.25 | 27.79 | 0.11 | 0.10 | 74.96 | 0.01 |
| 2 | 1518 | 15,166 | 231.56 | 1.06 | 7.49 | 65.49% | B.D.L | 14.60 | 3.70 | 3166 | 0.23 | 6.46 | 0.01 | 0.01 | 8.12 | B.D.L | |
| JCH-16-19 | 1 | 1285 | 15,213 | 227.96 | 1.26 | 8.45 | 65.48% | 0.18 | 48.24 | 3.08 | 3045 | 0.22 | B.D.L | 0.12 | 0.01 | 1.68 | B.D.L |
| 2 | 1227 | 15,043 | 230.83 | 0.81 | 11.82 | 65.50% | 0.17 | 11.23 | 5.10 | 3054 | 0.24 | 5.10 | 0.04 | 0.01 | 8.27 | B.D.L | |
| 3 | 1215 | 15,595 | 231.20 | 0.80 | 8.02 | 65.44% | B.D.L | 12.07 | 4.06 | 2948 | 0.15 | 0.37 | B.D.L | B.D.L | 1.34 | 0.01 | |
| 4 | 1221 | 15,986 | 231.48 | 0.82 | 13.26 | 65.40% | 0.02 | 11.77 | 7.15 | 2995 | 0.25 | 2.91 | 0.03 | 0.01 | 4.40 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Zhou, Y.; Wang, J.; Zhao, C.; Huang, J.; Li, P.; Wang, H.; Chen, F. Sources and Ore-Forming Environment of the Jinchanghe Pb-Zn Polymetallic Skarn Deposit, Baoshan Block, SW China: Constraints from Cu-S Isotopic and Trace Elemental Compositions of Sulfides. Minerals 2024, 14, 644. https://doi.org/10.3390/min14070644
Cheng X, Zhou Y, Wang J, Zhao C, Huang J, Li P, Wang H, Chen F. Sources and Ore-Forming Environment of the Jinchanghe Pb-Zn Polymetallic Skarn Deposit, Baoshan Block, SW China: Constraints from Cu-S Isotopic and Trace Elemental Compositions of Sulfides. Minerals. 2024; 14(7):644. https://doi.org/10.3390/min14070644
Chicago/Turabian StyleCheng, Xiaolin, Yunman Zhou, Jiyuan Wang, Chengfeng Zhao, Jing Huang, Pengju Li, Hai Wang, and Fuchuan Chen. 2024. "Sources and Ore-Forming Environment of the Jinchanghe Pb-Zn Polymetallic Skarn Deposit, Baoshan Block, SW China: Constraints from Cu-S Isotopic and Trace Elemental Compositions of Sulfides" Minerals 14, no. 7: 644. https://doi.org/10.3390/min14070644






