Geochemical Study of the Osumi Granodiorite, Southwestern Japan
Abstract
1. Introduction
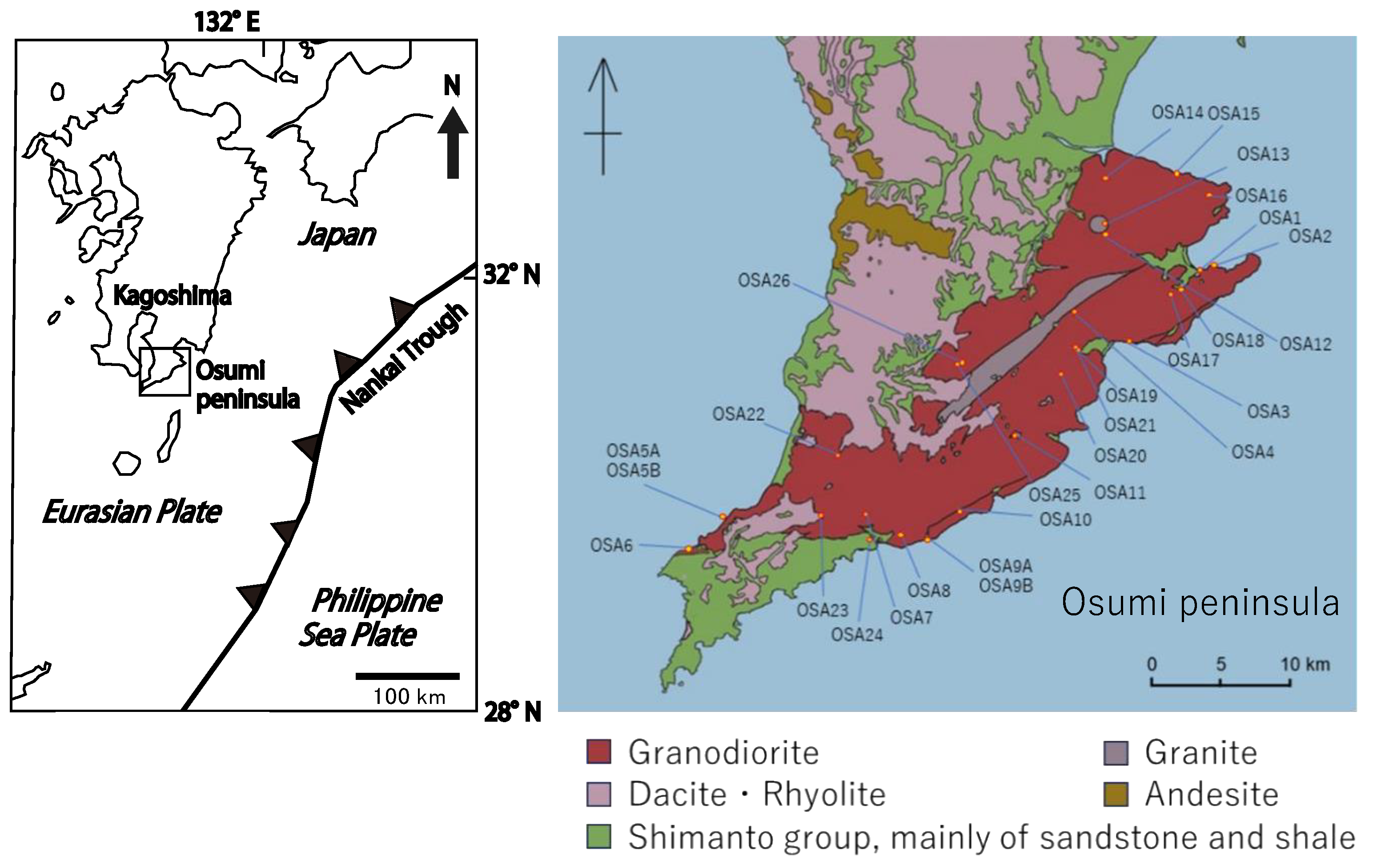
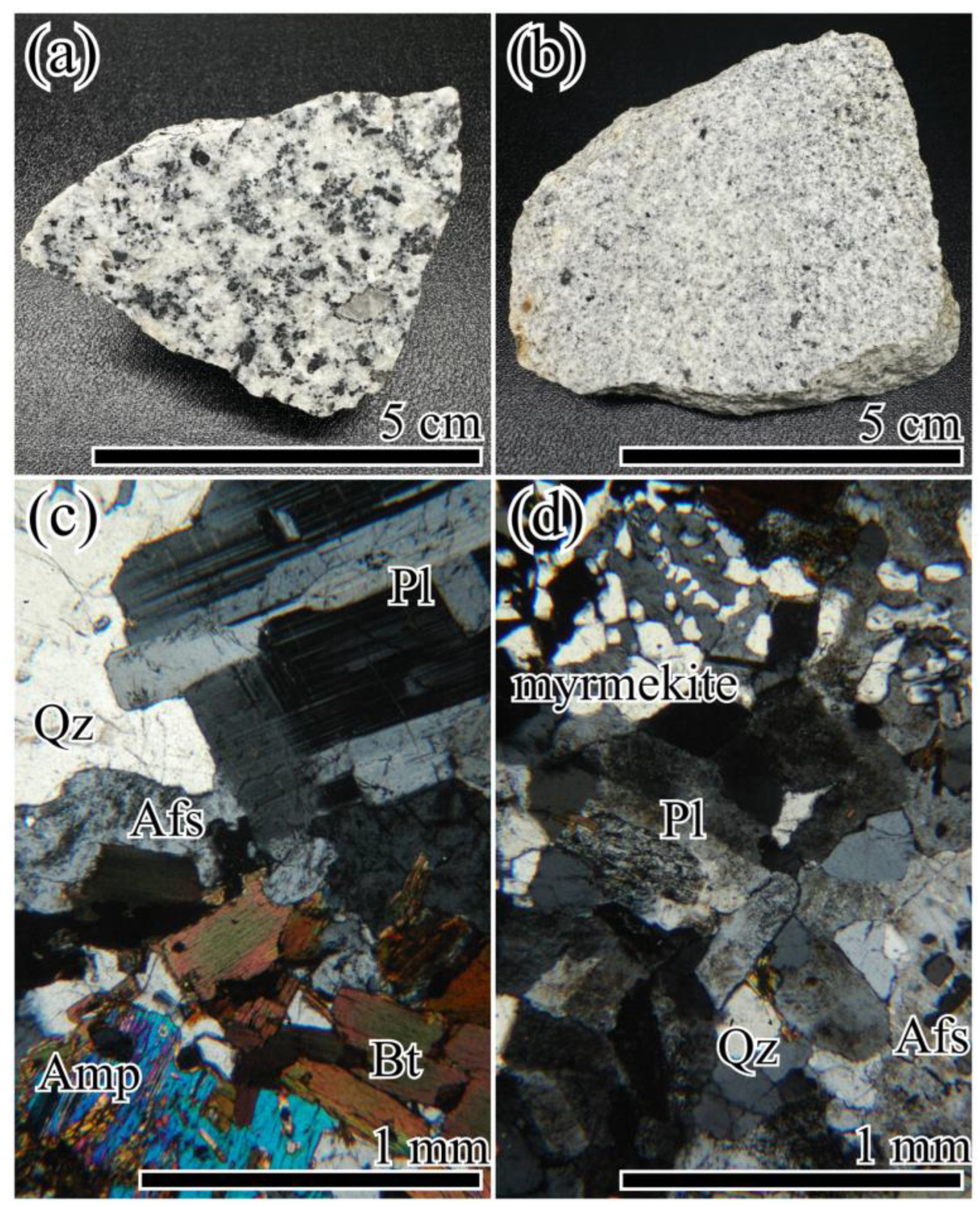
2. Geological Background and Petrography of the Osumi Granodiorite
3. Analytical Methods
3.1. Analysis of Major Elemental Composition and Trace Elemental Composition
3.2. Rare Earth Element Composition (REE) Analysis
3.3. Rb-Sr Isotope Analysis
4. Result
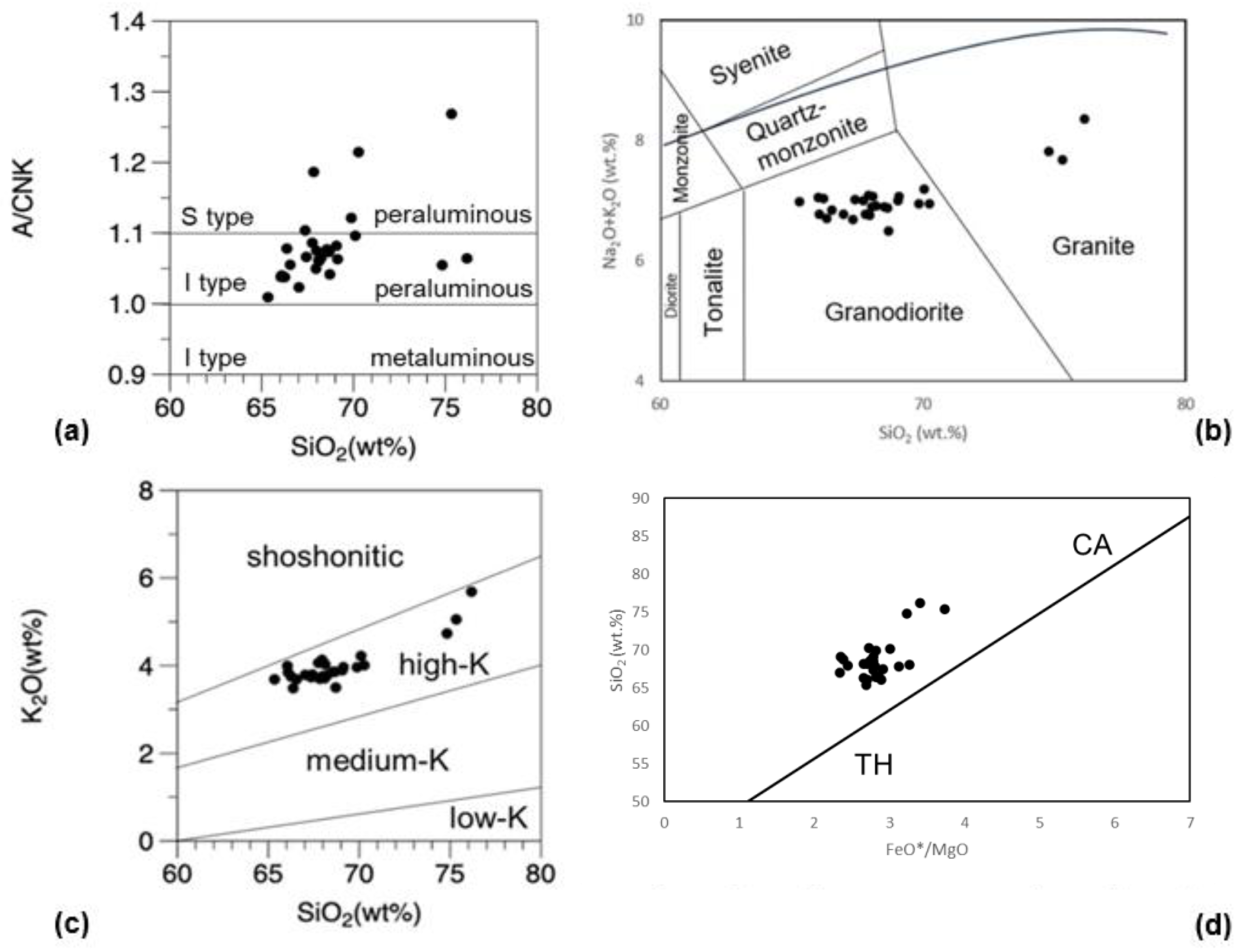
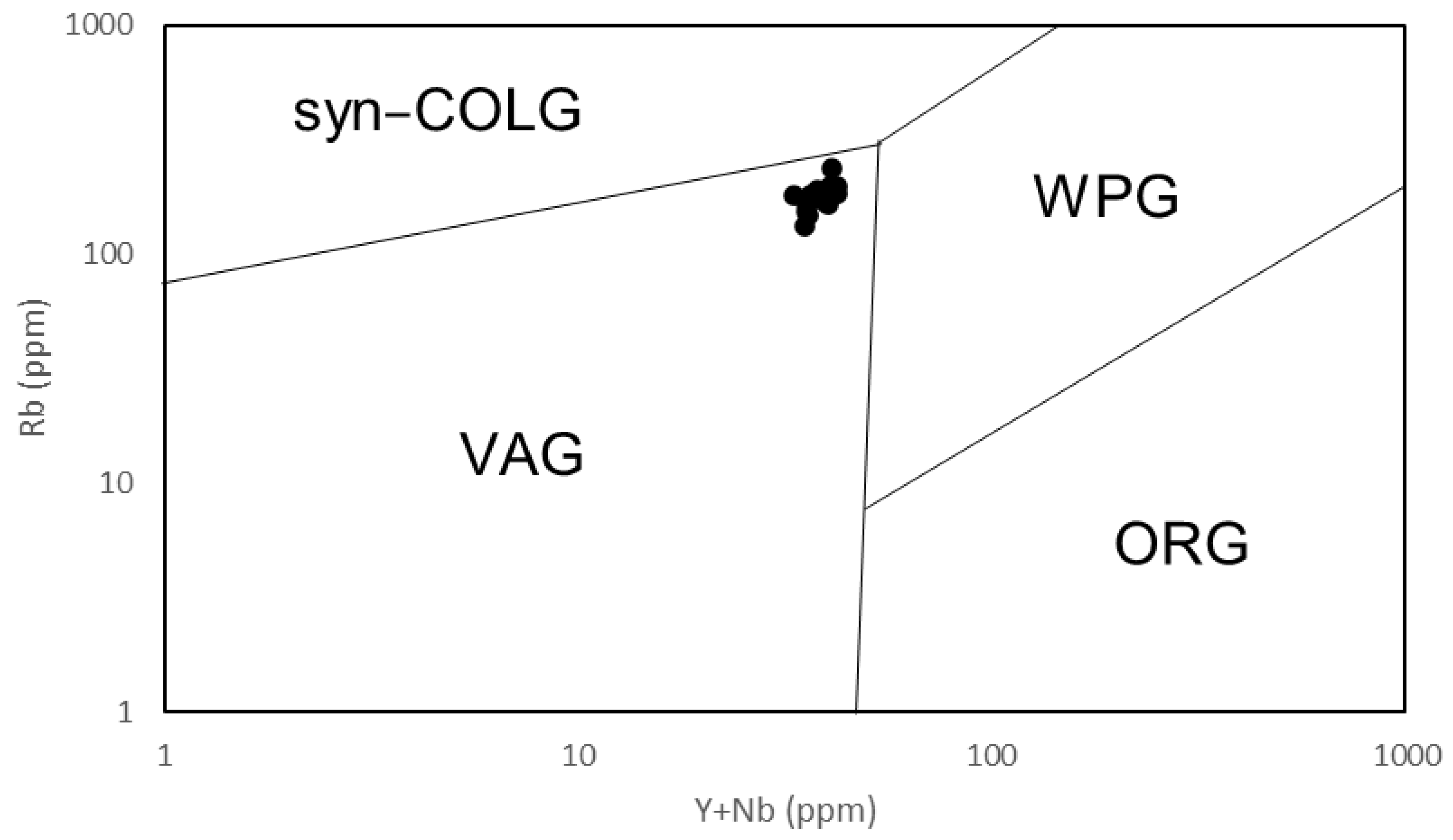
5. Discussion
5.1. Rare Earth Elements
5.2. Spidergram
5.3. Rb–Sr Isotopic System
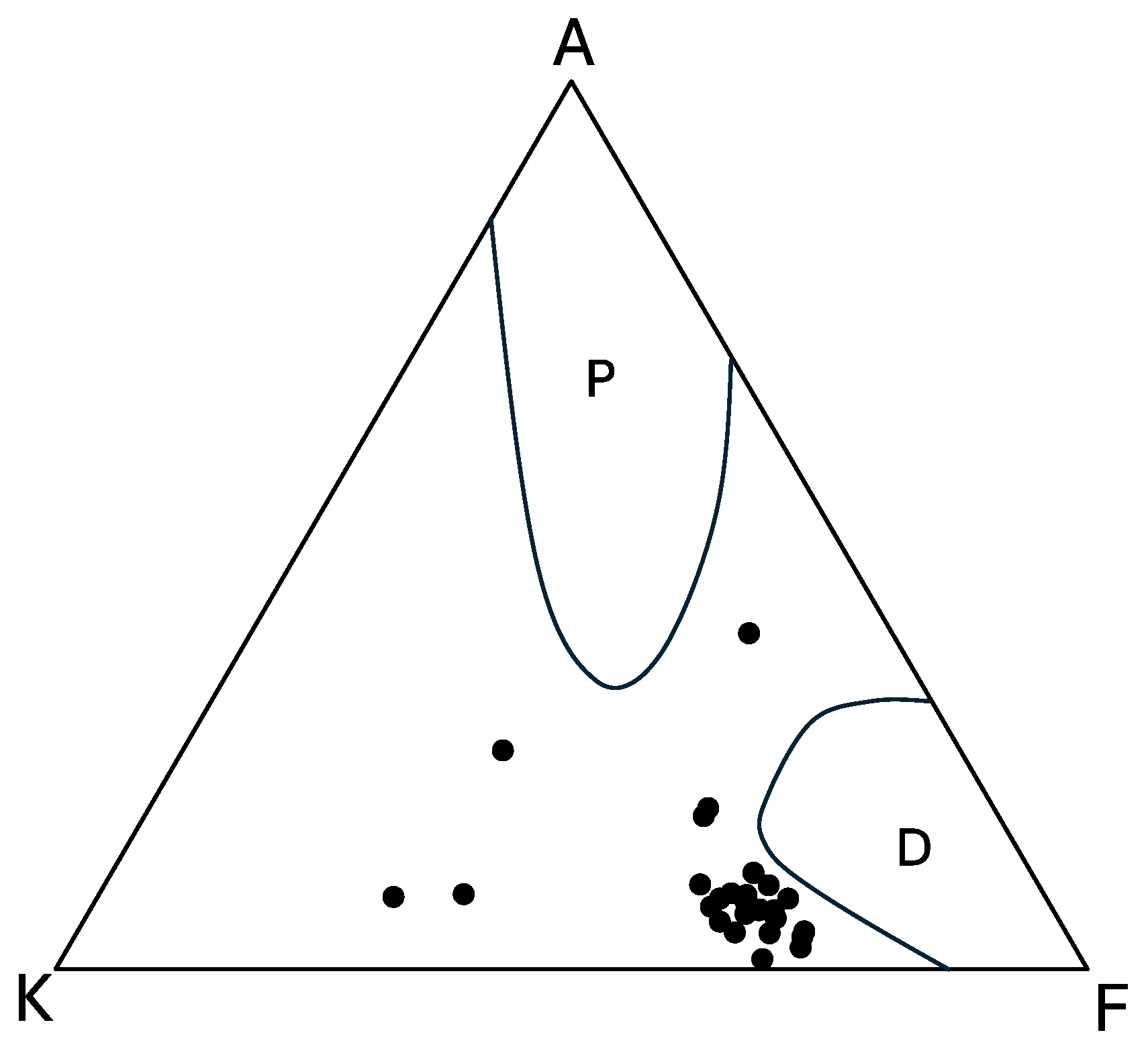
5.4. AKF Diagram
6. Conclusions
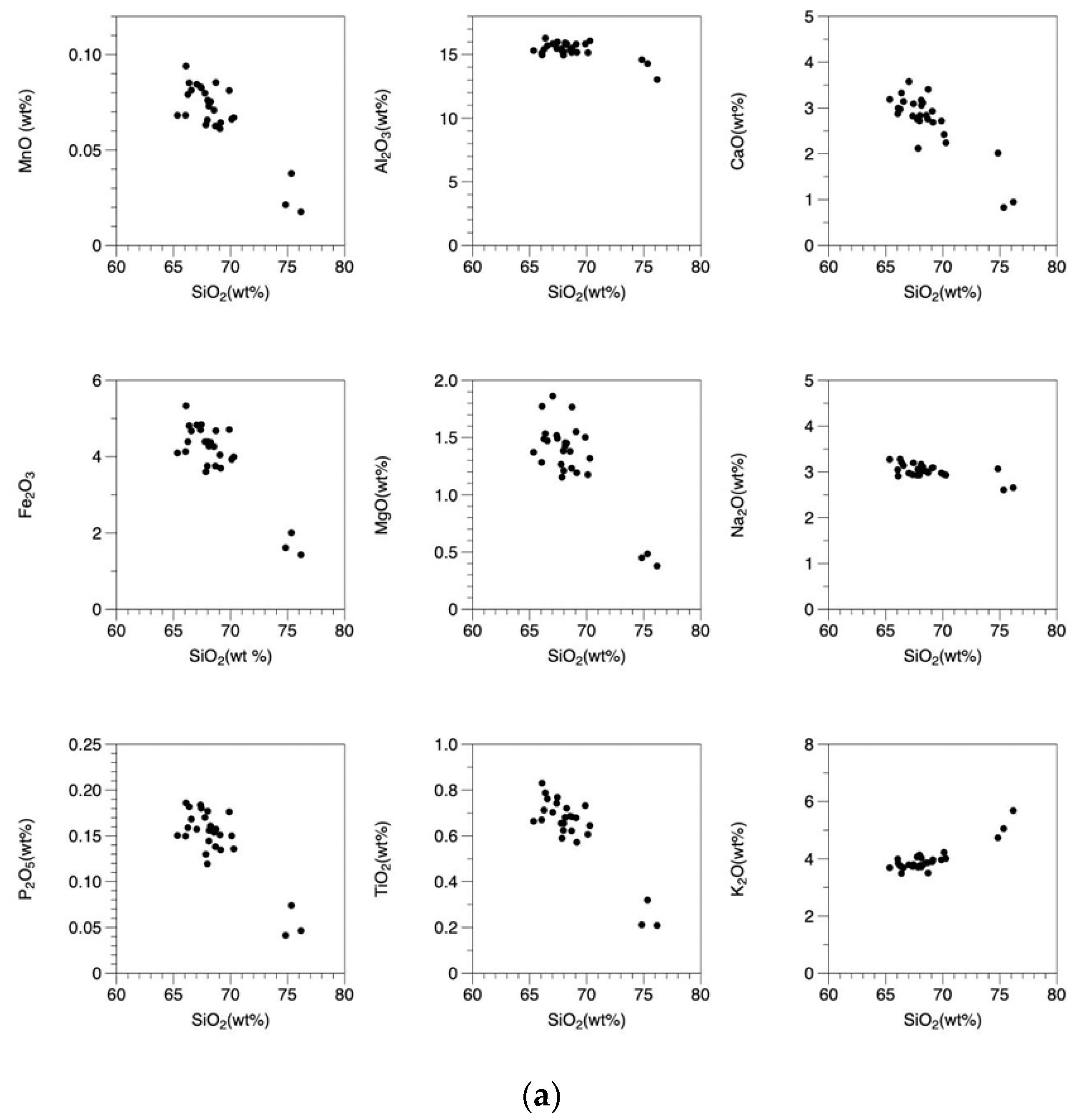
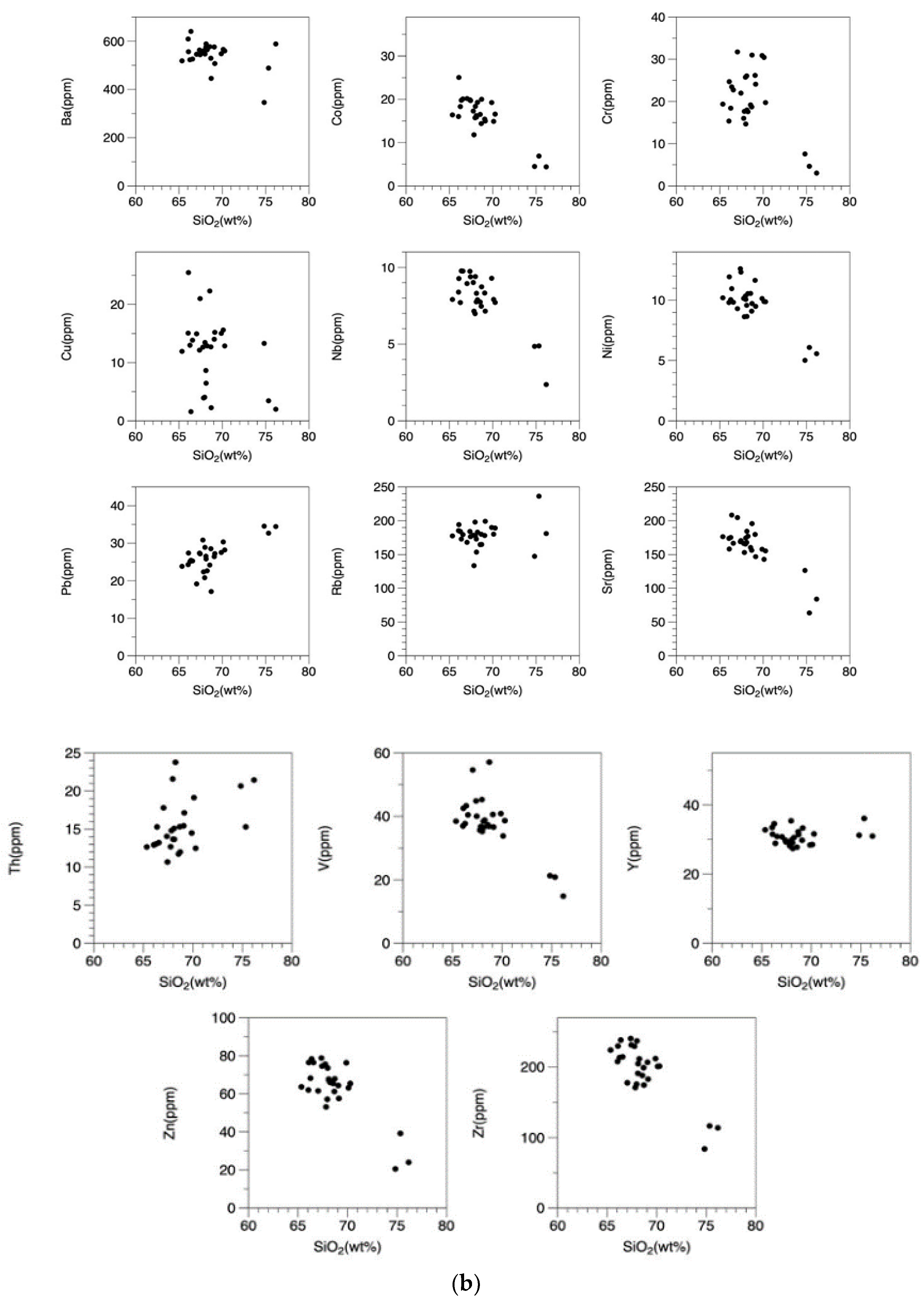
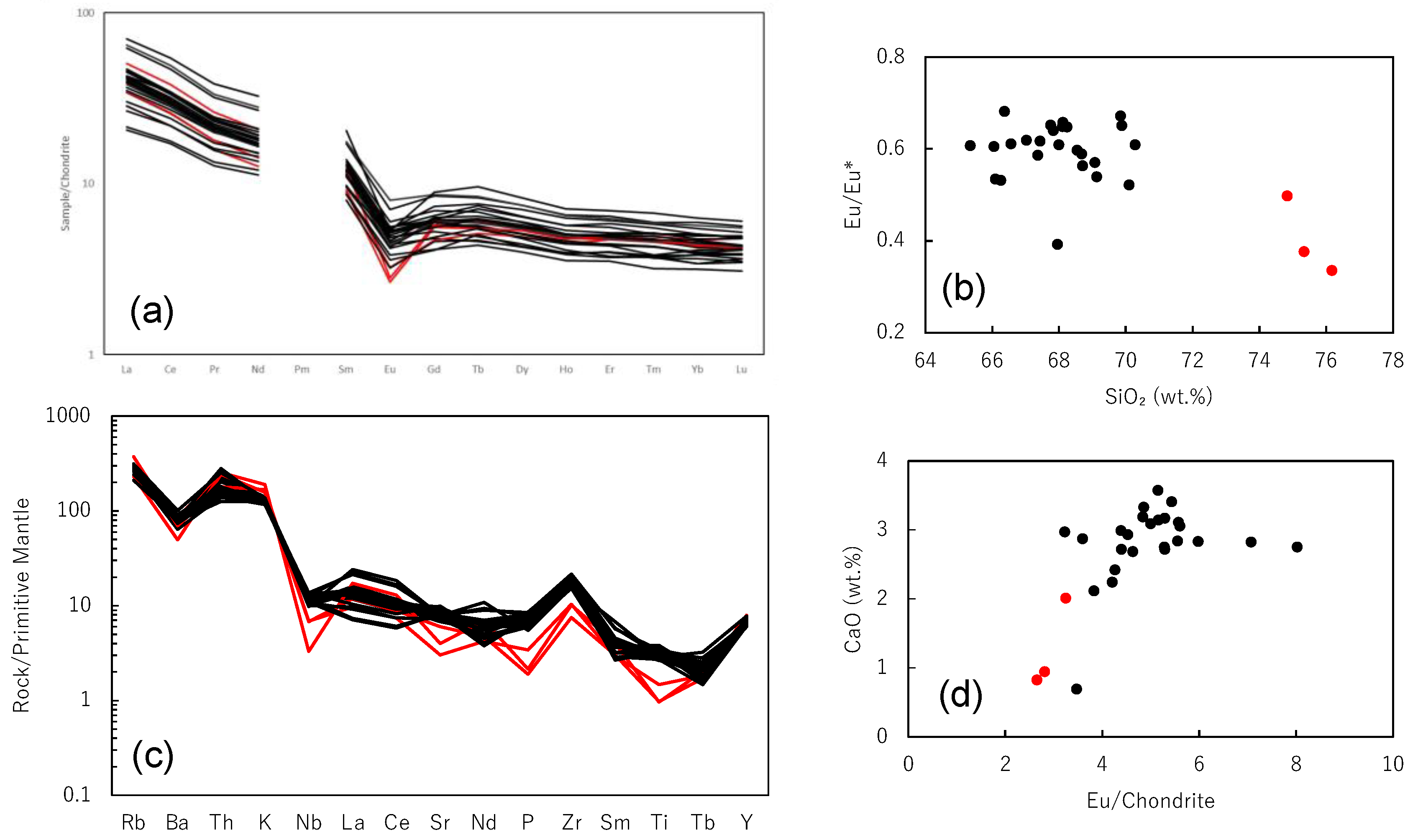
- The Osumi Granodiorite complex is broadly divided into two main rock types: granodiorite with an SiO2 content ranging from 66.1 to 70.1 wt.% and granitic rock with a SiO2 content ranging from 74.8 to 76.2 wt.%. The REE patterns are consistently similar across all samples, characterized by enrichment in light REEs and a somewhat flat, rightward-sloping trend of heavy REEs with approximately 5 times the chondrite values. This rock body exhibits common features with outer zone granitic rocks in southwestern Japan, including higher FeO than CaO and higher K2O than Na2O.
- The Osumi Granodiorite is believed to have originated from a single magma source based on the single trend in the Harker diagrams and similar REE patterns among samples. Geochemical discrimination diagrams and the relationship between FeO*/MgO and SiO2 categorize this rock body as a volcanic arc granite (VAG) belonging to the calc-alkaline series. Therefore, it is inferred that the Osumi Granodiorite formed due to subduction of the Philippine Sea Plate, which triggered felsic magmatic activity.
- Based on the alumina saturation index (ASI), this rock body exhibits a peraluminous composition. Most of the samples are assigned to I-type, but some show characteristics of S-type, indicating a potential influence of chemical composition from sedimentary rocks of the Shimanto Group. The AKF diagram suggests a higher likelihood of contamination by pelitic rocks. It is proposed that the Osumi Granodiorite formed through assimilation during the intrusion process, involving pelitic or psammitic materials from the Shimanto Belt or its lower formations.
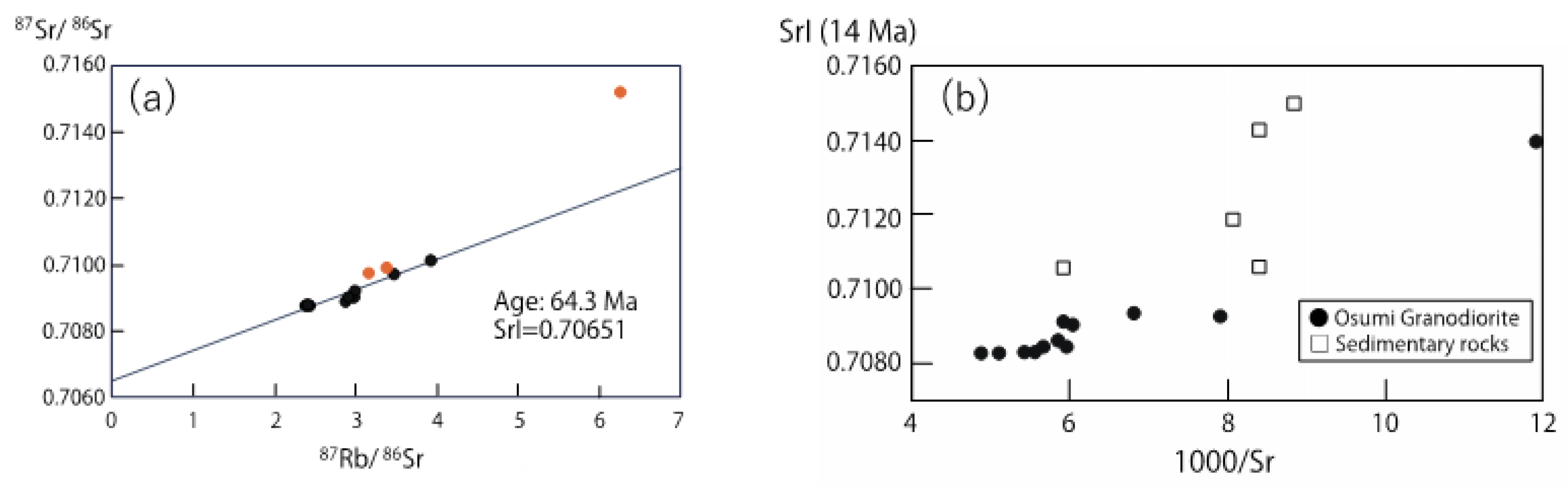
- Rb–Sr whole-rock isochron and SrI–1000/Sr diagrams suggest that the Osumi Granodiorite formed through heterogeneous assimilation of magma derived from a single source with the sedimentary materials from the Shimanto Group. The original magma might be derived from mantle, which is indicated by the relatively lower Sr isotopic ratios compared with sedimentary rocks.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakajima, T. Granites of Japan: A review at 2017. J. Geol. Soc. Jpn. 2018, 124, 603–625. [Google Scholar] [CrossRef]
- Shinjoe, H. Origin of the granodiorite in the forearc region of southwest Japan: Melting of the Shimanto accretionary prism. Chem. Geol. 1997, 134, 237–255. [Google Scholar] [CrossRef]
- Nishimura, K.; Yanagi, T. In situ crystallization observed in the Osumi granodiorite batholith. Earth Planet. Sci. Lett. 2000, 180, 185–199. [Google Scholar] [CrossRef]
- Takahashi, M. Anatomy of a middle Miocene Valles-type caldera cluster: Geology of the Okueyama volcano-plutonic complex, Southwest Japan. J. Volcanol. Geotherm. Res. 1986, 29, 33–70. [Google Scholar] [CrossRef]
- Miura, D. Arcuate pyroclastic conduits, ring faults, and coherent floor at Kumano caldera, southwest Honshu, Japan. J. Volcanol. Geotherm. Res. 1999, 92, 271–294. [Google Scholar] [CrossRef]
- Kawakami, Y.; Hoshi, H.; Yamaguchi, Y. Mechanism of caldera collapse and resurgence: Observations from the northern part of the Kumano acidic rocks, Kii peninsula, southwest Japan. J. Volcanol. Geotherm. Res. 2007, 167, 263–281. [Google Scholar] [CrossRef]
- Orihashi, Y.; Shinjoe, H.; Anma, R. Elucidation of whole Mid-Miocene granitic magmatism of the outer zone of southwestern Japan, Kyushu province: Constraint from LA-ICPMS U–Pb age determination. Abstr. Annu. Meet. Geochem. Soc. Jpn. 2015, 62, 33. [Google Scholar]
- Miller, J.A.; Shibata, K.; Kawachi, Y. Potassium-argon ages of granitic rocks from the Outer Zone of Kyushu, Japan. Bull. Geol. Surv. Jpn. 1962, 13, 712–714. [Google Scholar]
- Kawano, Y.; Ueda, Y. KA dating on the igneous rocks in Japan (I). J. Jpn. Assoc. Mineral. Petrol. Econ. Geol. 1964, 51, 127–148. [Google Scholar] [CrossRef][Green Version]
- Shibata, K. Contemporaneity of Tertiary granites in the Outer Zone of Southwest Japan. Bull. Geol. Surv. Jpn. 1978, 29, 551–554. [Google Scholar]
- Fabbri, O.; Monié, P.; Fournier, M. Transtensional deformation at the junction between the Okinawa trough back-arc basin and the SW Japan island arc. Geol. Soc. London Spec. Publ. 2004, 227, 297–312. [Google Scholar] [CrossRef]
- Geological Survey of Japan, AIST Seamless Digital Geological Map of Japan. Available online: https://gbank.gsj.jp/seamless/ (accessed on 24 June 2024).
- Shinjo, R.; Woodhead, J.D.; Hergt, J.M. Geochemical variation within the northern Ryuku Arc: Magma source compositions and geodynamic implications. Contrib. Mineral. Petrol. 2000, 140, 263–282. [Google Scholar] [CrossRef]
- Nozawa, T.; Ota, R. Geology of the Uchinoura District. Quadrangle Series, 1:50,000. Geol. Surv. Jpn. 1967, 1–37. [Google Scholar]
- Aridome, C.; Yamamoto, H. Joint system in the Osumi granodiorite batholith, southern Kyushu, Southwest Japan. J. Geol. Soc. Jpn. 2021, 127, 489–495. [Google Scholar] [CrossRef]
- Karakida, Y.; Hayasaka, S.; Hase, Y. (Eds.) Regional Geology of Japan Part 9 Kyushu; Kyoritsu: Shuppan, Tokyo, 1992; p. 371. [Google Scholar]
- Oba, N. Petrochemical Study of Osumi Granite, especially its Heterogeneity and Schistose Structure. Misc. Rep. Res. Inst. Nat. Resour. 1961, 54–55, 191–201. [Google Scholar]
- Yamamoto, M.; Oba, N. Geology and Rocks of the Takakumayama Granite and Osumi Granodiorite. 90th Annu. Meet. Geol. Soc. Japan Field excursion guide 1983. 61–79.
- Hayase, I.; Ishizaka, K. Rb-Sr dating on the rocks in Japan (I), South Western Japan. J. Jpn. Assoc. Mineral. Petrol. Econ. 1967, 58, 201–212. [Google Scholar]
- Yanagi, T.; Yamaguchi, M.; Nozawa, T. Sr whole rock ages of the granites of Minami-osumi and Amami-oshima, South-west Japan. Mem. Fac. Sci. Kyushu Univ. Ser. D Geol. 1971, 21, 163–175. [Google Scholar]
- Nakazaki, M.; Tsuboi, M.; Kanagawa, K.; Kato, T.; Suzuki, K. Quantitative chemical analysis of rocks with X-ray fluorescence analyzer XRF-1800. Bull. Nagoya Univ. Museum 2004, 20, 79–91. [Google Scholar]
- Koga, K.; Tsuboi, M. Petrogenesis of Granitic Rocks in the Hisakajima Island, Goto Archipelago, Southwestern Japan: A Geochemical Study. Minerals 2021, 11, 248. [Google Scholar] [CrossRef]
- Kitanaka, R.; Tsuboi, M.; Ozaki, Y. Biogenic apatitein carbonate concretions with and without fossils investigated in situ by micro-Raman spectroscopy. Sci. Rep. 2023, 13, 9714. [Google Scholar] [CrossRef] [PubMed]
- Vermeesch, P. A free and open toolbox for geochronology. Geosci. Front. 2018, 9, 1479–1493. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene Calc Alkaline Volcanic Rocks from Kastamonu Area, Northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace Element Discrimination Diagrams for the Tectonic Interpretation of Granitic Rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Miyashiro, A. Volcanic rock series in island arcs and active continental margins. Am. J. Sci. 1974, 274, 321–355. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Terakado, Y.; Shimizu, H.; Masuda, A. Nd and Sr isotopic variations in acidic rocks formed under a peculiar tectonic environment in Miocene Southwest Japan. Contrib. Mineral. Petrol. 1988, 99, 1–10. [Google Scholar] [CrossRef]
- Kawano, M.; Takahashi, K.; Nozawa, T. Petrochemistry of Minami-osumi granite in the Uchinoura area, Kyushu, Japan. Bull. Geol. Surv. Jpn. 1966, 17, 533–543. [Google Scholar]
- Chapell, B.W.; White, A.J.R. Two contrasting granite types: 25 years later. Aust. J. Earth Sci. 2001, 48, 489–499. [Google Scholar] [CrossRef]
- Chappell, B.W. Aluminium saturation in I- and S-type granites and the characterization of fractionated haplogranites. Lithos 1999, 46, 535–551. [Google Scholar] [CrossRef]
- Ishihara, S. P2O5 contents vs. S, I, A-type granites in Slovakia and Japan. Chishitsu News 2009, 663, 47–55. [Google Scholar]
- Nakada, S.; Takahashi, M. Regional variation in chemistry of the Miocene intermediate to felsic magmas in the Outer Zone and the Setouchi Province of Southwest Japan. J. Geol. Soc. Jpn. 1979, 85, 571–582. [Google Scholar] [CrossRef]
- Gorai, M. Some problems in the mantle origin hypothesis of calc alkaline magmas. J. Geol. Soc. Jpn. 1970, 76, 529–536. [Google Scholar] [CrossRef]
- Rollinson, H. Using Geochemical Data; Longman: Harlow, UK, 1993; p. 352. [Google Scholar]
- Ewart, A.; Griffin, W.L. Application of proton-microprobe data to trace element partitioning in volcanic rocks. Chem. Geol. 1994, 117, 251–284. [Google Scholar] [CrossRef]
- Ishihara, S.; Chappell, B.W. Chemical compositions of Miocene granitoids of the Okueyama, Hoei mine and Takakumayama granitoids, Outer Zone of SW Japan. Bull. Geol. Surv. Jpn. 2010, 61, 17–38. [Google Scholar] [CrossRef]
- Shinjoe, H.; Sumii, T. Whole rock composition of the Miocene granitic rocks in the Satsuma Peninsula, Kagoshima prefecture. J. Humanit. Nat. Sci. 2006, 121, 13–21. [Google Scholar]
- Phlipotts, J.A. Redox estimation from a calculation of Eu2+ and Eu3+ concentrations in natural phases. Earth Planet. Sci. Lett. 1970, 9, 257–268. [Google Scholar] [CrossRef]
- Moller, P.; Muecke, G.K. Significance of Europium anomalies in silicate melts and crystal-melt equilibria: A re-evaluation. Contrib. Mineral. Petrol. 1984, 87, 242–250. [Google Scholar] [CrossRef]
- Horikoshi, E. Tectonics of Granitic Magma and the Related Mineral Deposits. Soc. Min. Geol. Jpn. 1976, 7, 1–14. [Google Scholar]
- Kobayashi, Y. Early and Middle Miocene Dike Swarms and Regional Tectonic Stress Field in the Southwest Japan. Volcanol. Soc. Jpn. 1979, 2, 203–212. [Google Scholar]
- Nakamura, K.; Uyeda, S. Stress gradient in arc-back arc regions and plate subduction. J. Geophys. Res. 1980, 85, 6419–6428. [Google Scholar] [CrossRef]
| Sample | OSA1 | OSA2 | OSA3 | OSA4 | OSA5A | OSA5B | OSA6 | OSA7 | OSA8 | OSA9A | OSA9B | OSA10 | OSA11 | OSA12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 (wt.%) | 3.93 | 4.71 | 4.05 | 4.68 | 4.83 | 1.61 | 4.68 | 4.27 | 3.70 | 1.43 | 4.30 | 4.38 | 5.33 | 4.84 |
| MnO | 0.07 | 0.08 | 0.06 | 0.08 | 0.08 | 0.02 | 0.09 | 0.07 | 0.06 | 0.02 | 0.07 | 0.08 | 0.09 | 0.08 |
| TiO2 | 0.61 | 0.73 | 0.68 | 0.76 | 0.70 | 0.21 | 0.68 | 0.68 | 0.57 | 0.21 | 0.68 | 0.72 | 0.83 | 0.77 |
| CaO | 2.42 | 2.72 | 2.93 | 3.14 | 3.58 | 2.01 | 3.41 | 3.17 | 2.69 | 0.95 | 3.06 | 3.11 | 3.00 | 3.09 |
| K2O | 4.22 | 3.96 | 3.89 | 3.69 | 3.79 | 4.73 | 3.50 | 3.71 | 3.97 | 5.69 | 4.03 | 3.78 | 3.85 | 3.80 |
| P2O5 | 0.15 | 0.18 | 0.15 | 0.17 | 0.16 | 0.04 | 0.16 | 0.14 | 0.13 | 0.05 | 0.16 | 0.16 | 0.19 | 0.18 |
| SiO2 | 70.10 | 69.87 | 69.07 | 66.55 | 67.03 | 74.82 | 68.71 | 68.10 | 69.13 | 76.17 | 68.12 | 68.24 | 66.09 | 67.43 |
| Al2O3 | 15.15 | 15.84 | 15.82 | 15.70 | 15.86 | 14.59 | 15.51 | 15.91 | 15.17 | 13.03 | 15.79 | 15.85 | 14.97 | 15.98 |
| MgO | 1.18 | 1.50 | 1.55 | 1.47 | 1.86 | 0.45 | 1.77 | 1.43 | 1.19 | 0.38 | 1.45 | 1.45 | 1.77 | 1.49 |
| Na2O | 2.95 | 2.98 | 3.09 | 3.14 | 2.97 | 3.07 | 2.98 | 3.17 | 3.10 | 2.66 | 3.02 | 3.12 | 2.91 | 3.20 |
| Total | 100.76 | 102.58 | 101.29 | 99.38 | 100.86 | 101.56 | 101.48 | 100.66 | 99.71 | 100.56 | 100.68 | 100.89 | 99.02 | 100.86 |
| ASI | 1.10 | 1.12 | 1.08 | 1.06 | 1.02 | 1.05 | 1.04 | 1.06 | 1.06 | 1.06 | 1.06 | 1.07 | 1.04 | 1.07 |
| FeO*/MgO | 3.00 | 2.82 | 2.35 | 2.86 | 2.33 | 3.23 | 2.38 | 2.70 | 2.79 | 3.40 | 2.66 | 2.71 | 2.71 | 2.92 |
| Na2O+K2O | 7.17 | 6.94 | 6.98 | 6.83 | 6.76 | 7.80 | 6.48 | 6.88 | 7.06 | 8.34 | 7.05 | 6.90 | 6.75 | 6.99 |
| Th (ppm) | 19.1 | 14.5 | 15.4 | 13.2 | 17.8 | 20.7 | 12.0 | 15.1 | 17.1 | 21.5 | 13.7 | 23.8 | 13.0 | 10.7 |
| Pb | 30.4 | 27.5 | 26.4 | 25.3 | 19.2 | 34.5 | 17.1 | 26.5 | 27.2 | 34.4 | 25.8 | 22.7 | 27.4 | 27.2 |
| Ba | 565.8 | 547.9 | 576.0 | 526.1 | 545.8 | 345.8 | 445.6 | 575.1 | 507.2 | 588.5 | 588.7 | 564.2 | 556.3 | 543.9 |
| Nb | 7.9 | 9.3 | 8.3 | 9.8 | 8.9 | 4.9 | 8.7 | 7.8 | 7.1 | 2.4 | 8.3 | 7.9 | 9.3 | 9.4 |
| Zr | 200.9 | 211.9 | 206.7 | 214.5 | 177.6 | 83.7 | 174.5 | 191.1 | 182.8 | 113.7 | 204.7 | 211.6 | 229.8 | 231.4 |
| Y | 28.5 | 28.4 | 29.8 | 30.9 | 30.8 | 31.2 | 31.4 | 29.1 | 33.3 | 31.0 | 27.4 | 30.5 | 31.5 | 29.3 |
| Sr | 142.8 | 157.8 | 179.7 | 166.7 | 204.7 | 126.4 | 195.7 | 167.8 | 146.8 | 83.9 | 184.4 | 177.1 | 158.1 | 170.6 |
| Rb | 180.1 | 190.0 | 178.1 | 179.4 | 168.0 | 147.3 | 164.8 | 172.9 | 199.2 | 181.0 | 153.5 | 183.1 | 194.2 | 176.3 |
| Zn | 63.2 | 76.3 | 64.4 | 76.5 | 61.6 | 20.5 | 67.9 | 67.7 | 57.6 | 24.0 | 67.0 | 66.0 | 76.5 | 74.5 |
| Cu | 15.6 | 15.0 | 14.0 | 13.8 | 14.9 | 13.3 | 2.3 | 8.6 | 15.2 | 2.0 | 6.5 | 12.9 | 25.5 | 21.0 |
| Ni | 9.9 | 10.1 | 11.6 | 9.8 | 9.3 | 5.0 | 9.7 | 9.6 | 9.5 | 5.6 | 8.7 | 10.6 | 11.9 | 12.3 |
| Co | 14.9 | 19.2 | 15.4 | 20.1 | 20.2 | 4.5 | 20.0 | 16.2 | 15.0 | 4.4 | 15.9 | 19.3 | 25.0 | 19.7 |
| Cr | 30.4 | 30.9 | 26.2 | 22.7 | 31.7 | 7.6 | 31.0 | 26.1 | 24.1 | 3.0 | 18.0 | 17.6 | 24.7 | 22.0 |
| V | 33.8 | 40.9 | 40.6 | 40.5 | 54.6 | 21.3 | 57.1 | 38.4 | 36.6 | 14.8 | 36.5 | 38.9 | 42.6 | 40.1 |
| Y+Nb | 36.4 | 37.7 | 38.1 | 40.6 | 39.7 | 36.1 | 40.1 | 36.8 | 40.5 | 33.3 | 35.7 | 38.4 | 40.8 | 38.7 |
| Sample | OSA13 | OSA14 | OSA15 | OSA16 | OSA17 | OSA18 | OSA19 | OSA20 | OSA21 | OSA22 | OSA23 | OSA24 | OSA25 | OSA26 |
| Fe2O3 (wt.%) | 2.01 | 4.70 | 4.39 | 4.39 | 4.26 | 4.39 | 3.76 | 4.00 | 4.81 | 3.76 | 3.60 | 4.92 | 4.13 | 4.10 |
| MnO | 0.04 | 0.08 | 0.08 | 0.08 | 0.07 | 0.08 | 0.06 | 0.07 | 0.09 | 0.07 | 0.06 | 0.06 | 0.07 | 0.07 |
| TiO2 | 0.32 | 0.74 | 0.66 | 0.66 | 0.69 | 0.71 | 0.62 | 0.64 | 0.79 | 0.62 | 0.59 | 0.75 | 0.67 | 0.66 |
| CaO | 0.83 | 2.83 | 2.75 | 2.84 | 2.84 | 2.97 | 2.75 | 2.24 | 3.33 | 2.72 | 2.12 | 0.70 | 2.87 | 3.19 |
| K2O | 5.06 | 3.74 | 4.06 | 3.76 | 3.86 | 3.75 | 3.86 | 4.01 | 3.49 | 4.13 | 3.70 | 2.92 | 3.99 | 3.69 |
| P2O5 | 0.07 | 0.18 | 0.17 | 0.18 | 0.15 | 0.16 | 0.14 | 0.14 | 0.18 | 0.12 | 0.13 | 0.10 | 0.15 | 0.15 |
| SiO2 | 75.33 | 67.36 | 67.75 | 67.99 | 68.55 | 66.25 | 68.67 | 70.27 | 66.37 | 67.95 | 67.83 | 69.83 | 66.05 | 65.34 |
| Al2O3 | 14.29 | 15.48 | 15.45 | 15.20 | 15.41 | 15.42 | 15.17 | 16.08 | 16.29 | 14.95 | 15.30 | 16.01 | 15.11 | 15.31 |
| MgO | 0.48 | 1.52 | 1.27 | 1.21 | 1.38 | 1.49 | 1.23 | 1.32 | 1.53 | 1.38 | 1.15 | 1.86 | 1.28 | 1.37 |
| Na2O | 2.61 | 2.94 | 2.93 | 2.99 | 3.02 | 3.28 | 3.00 | 2.93 | 3.21 | 2.93 | 3.06 | 1.78 | 3.05 | 3.27 |
| Total | 101.03 | 99.58 | 99.52 | 99.29 | 100.23 | 98.51 | 99.27 | 101.69 | 100.08 | 98.64 | 97.55 | 98.93 | 97.38 | 97.16 |
| ASI | 1.27 | 1.10 | 1.09 | 1.07 | 1.08 | 1.04 | 1.07 | 1.21 | 1.08 | 1.05 | 1.19 | 2.17 | 1.04 | 1.01 |
| FeO*/MgO | 3.73 | 2.79 | 3.12 | 3.26 | 2.78 | 2.66 | 2.74 | 2.73 | 2.82 | 2.44 | 2.81 | 2.39 | 2.90 | 2.69 |
| Na2O+K2O | 7.66 | 6.68 | 6.99 | 6.75 | 6.88 | 7.03 | 6.87 | 6.94 | 6.70 | 7.07 | 6.77 | 4.71 | 7.04 | 6.96 |
| Th (ppm) | 15.3 | 14.0 | 12.7 | 13.7 | 11.7 | 13.0 | 15.3 | 12.5 | 15.3 | 21.6 | 14.8 | 12.0 | 12.9 | 12.7 |
| Pb | 32.7 | 27.4 | 30.8 | 28.9 | 24.2 | 25.1 | 28.5 | 28.2 | 25.4 | 20.8 | 22.4 | 28.6 | 24.3 | 23.8 |
| Ba | 488.4 | 563.0 | 555.8 | 546.8 | 577.1 | 523.1 | 529.2 | 559.7 | 640.4 | 707.0 | 560.1 | 591.7 | 609.1 | 518.5 |
| Nb | 4.9 | 9.7 | 9.0 | 9.4 | 7.7 | 7.7 | 7.5 | 7.7 | 9.8 | 7.0 | 7.1 | 10.1 | 8.4 | 7.9 |
| Zr | 116.3 | 240.4 | 229.5 | 237.1 | 188.1 | 213.6 | 199.1 | 201.2 | 238.3 | 175.7 | 170.9 | 184.4 | 207.8 | 224.1 |
| Y | 36.1 | 29.8 | 28.9 | 29.8 | 27.8 | 34.6 | 32.3 | 31.6 | 28.9 | 35.4 | 28.1 | 25.5 | 33.5 | 32.8 |
| Sr | 63.5 | 169.0 | 166.4 | 174.3 | 160.4 | 175.2 | 156.2 | 155.3 | 208.3 | 165.5 | 152.9 | 134.8 | 173.8 | 176.3 |
| Rb | 236.3 | 184.1 | 178.6 | 177.8 | 164.6 | 184.1 | 179.9 | 189.1 | 172.9 | 198.0 | 133.4 | 145.6 | 185.3 | 177.5 |
| Zn | 39.2 | 78.8 | 75.6 | 73.6 | 65.5 | 68.2 | 61.2 | 65.4 | 78.3 | 57.1 | 53.1 | 97.5 | 62.0 | 63.6 |
| Cu | 3.4 | 12.1 | 12.6 | 13.5 | 22.3 | 13.0 | 12.7 | 12.9 | 1.6 | 4.0 | 3.9 | 29.7 | 15.1 | 11.9 |
| Ni | 6.1 | 12.6 | 10.2 | 10.1 | 10.6 | 10.0 | 9.1 | 9.9 | 11.0 | 10.3 | 8.6 | 17.9 | 9.8 | 10.2 |
| Co | 6.9 | 19.9 | 17.3 | 18.4 | 16.5 | 18.3 | 14.4 | 16.6 | 19.8 | 15.7 | 11.8 | 18.7 | 16.0 | 16.4 |
| Cr | 4.6 | 45.6 | 16.0 | 14.7 | 19.2 | 18.4 | 18.7 | 19.7 | 23.5 | 25.7 | 17.6 | 76.5 | 15.4 | 19.4 |
| V | 20.9 | 44.9 | 35.7 | 35.2 | 37.4 | 37.7 | 36.9 | 38.7 | 43.3 | 45.3 | 36.8 | 89.9 | 37.0 | 38.5 |
| Y+Nb | 41.0 | 39.6 | 37.9 | 39.2 | 35.5 | 42.3 | 39.7 | 39.3 | 38.6 | 42.4 | 35.3 | 35.6 | 41.9 | 40.7 |
| Sample | OSA1 | OSA2 | OSA3 | OSA4 | OSA5A | OSA5B | OSA6 | OSA7 | OSA8 | OSA9A | OSA9B | OSA10 | OSA11 | OSA12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La (ppm) | 8.56 | 8.01 | 9.23 | 9.64 | 9.80 | 8.09 | 8.25 | 10.99 | 9.37 | 11.82 | 10.53 | 9.93 | 8.90 | 9.15 |
| Ce | 17.06 | 15.50 | 17.99 | 18.67 | 18.79 | 15.72 | 16.33 | 20.84 | 18.22 | 22.97 | 20.09 | 19.27 | 17.30 | 17.59 |
| Pr | 1.78 | 1.60 | 1.87 | 1.95 | 1.90 | 1.60 | 1.77 | 2.11 | 1.89 | 2.33 | 2.06 | 1.99 | 1.81 | 1.82 |
| Nd | 7.44 | 6.77 | 7.76 | 8.12 | 7.84 | 6.41 | 7.75 | 8.69 | 7.84 | 9.39 | 8.68 | 8.39 | 7.52 | 7.55 |
| Pm | ||||||||||||||
| Sm | 1.65 | 1.40 | 1.64 | 1.74 | 1.65 | 1.34 | 1.85 | 1.73 | 1.71 | 1.80 | 1.76 | 1.78 | 1.64 | 1.61 |
| Eu | 0.24 | 0.25 | 0.25 | 0.29 | 0.29 | 0.18 | 0.30 | 0.30 | 0.26 | 0.16 | 0.31 | 0.31 | 0.25 | 0.28 |
| Gd | 1.17 | 0.94 | 1.12 | 1.18 | 1.22 | 0.92 | 1.45 | 1.12 | 1.25 | 1.13 | 1.19 | 1.21 | 1.19 | 1.18 |
| Tb | 0.20 | 0.17 | 0.21 | 0.23 | 0.22 | 0.19 | 0.28 | 0.20 | 0.23 | 0.22 | 0.22 | 0.22 | 0.21 | 0.20 |
| Dy | 1.17 | 1.04 | 1.23 | 1.39 | 1.35 | 1.19 | 1.71 | 1.24 | 1.46 | 1.33 | 1.33 | 1.36 | 1.32 | 1.23 |
| Ho | 0.23 | 0.21 | 0.25 | 0.28 | 0.28 | 0.25 | 0.35 | 0.26 | 0.30 | 0.27 | 0.27 | 0.28 | 0.27 | 0.25 |
| Er | 0.60 | 0.59 | 0.69 | 0.77 | 0.80 | 0.74 | 0.97 | 0.70 | 0.82 | 0.75 | 0.75 | 0.78 | 0.75 | 0.71 |
| Tm | 0.09 | 0.09 | 0.10 | 0.11 | 0.12 | 0.12 | 0.14 | 0.10 | 0.12 | 0.11 | 0.11 | 0.11 | 0.11 | 0.10 |
| Yb | 0.55 | 0.56 | 0.65 | 0.71 | 0.81 | 0.76 | 0.89 | 0.64 | 0.77 | 0.70 | 0.69 | 0.73 | 0.72 | 0.63 |
| Lu | 0.08 | 0.09 | 0.09 | 0.11 | 0.12 | 0.12 | 0.13 | 0.10 | 0.12 | 0.10 | 0.10 | 0.10 | 0.10 | 0.09 |
| LaCN | 36.49 | 34.15 | 39.32 | 41.07 | 41.74 | 34.45 | 35.15 | 46.81 | 39.92 | 50.35 | 44.86 | 42.29 | 37.91 | 38.97 |
| Ce | 28.29 | 25.70 | 29.82 | 30.96 | 31.15 | 26.07 | 27.08 | 34.54 | 30.21 | 38.08 | 33.31 | 31.94 | 28.67 | 29.16 |
| Pr | 20.02 | 17.91 | 21.03 | 21.84 | 21.33 | 17.96 | 19.86 | 23.72 | 21.16 | 26.18 | 23.15 | 22.33 | 20.26 | 20.48 |
| Nd | 16.44 | 14.97 | 17.15 | 17.96 | 17.34 | 14.17 | 17.14 | 19.21 | 17.32 | 20.75 | 19.18 | 18.54 | 16.63 | 16.70 |
| Pm | ||||||||||||||
| Sm | 11.23 | 9.54 | 11.13 | 11.82 | 11.19 | 9.11 | 12.59 | 11.74 | 11.63 | 12.23 | 11.97 | 12.09 | 11.16 | 10.93 |
| Eu | 4.26 | 4.39 | 4.53 | 5.16 | 5.15 | 3.25 | 5.43 | 5.29 | 4.63 | 2.81 | 5.60 | 5.57 | 4.38 | 4.99 |
| Gd | 5.96 | 4.77 | 5.68 | 6.03 | 6.20 | 4.68 | 7.38 | 5.68 | 6.34 | 5.75 | 6.07 | 6.14 | 6.03 | 6.01 |
| Tb | 5.44 | 4.60 | 5.67 | 6.22 | 6.03 | 5.10 | 7.60 | 5.60 | 6.43 | 6.02 | 5.96 | 6.13 | 5.87 | 5.54 |
| Dy | 4.80 | 4.28 | 5.05 | 5.72 | 5.57 | 4.90 | 7.06 | 5.13 | 6.01 | 5.49 | 5.48 | 5.61 | 5.44 | 5.09 |
| Ho | 4.09 | 3.85 | 4.50 | 5.04 | 5.04 | 4.44 | 6.28 | 4.63 | 5.32 | 4.80 | 4.83 | 5.03 | 4.90 | 4.49 |
| Er | 3.77 | 3.71 | 4.36 | 4.84 | 5.02 | 4.69 | 6.09 | 4.40 | 5.17 | 4.71 | 4.75 | 4.89 | 4.73 | 4.44 |
| Tm | 3.70 | 3.74 | 4.27 | 4.74 | 5.07 | 4.93 | 5.81 | 4.30 | 5.14 | 4.54 | 4.61 | 4.69 | 4.61 | 4.30 |
| Yb | 3.40 | 3.42 | 4.00 | 4.40 | 4.96 | 4.68 | 5.45 | 3.95 | 4.74 | 4.31 | 4.23 | 4.51 | 4.41 | 3.90 |
| Lu | 3.47 | 3.65 | 3.85 | 4.35 | 4.94 | 4.80 | 5.19 | 4.07 | 4.80 | 4.24 | 4.19 | 4.30 | 4.26 | 3.90 |
| Eu/Eu* | 0.52 | 0.65 | 0.57 | 0.61 | 0.62 | 0.50 | 0.57 | 0.65 | 0.54 | 0.34 | 0.66 | 0.65 | 0.54 | 0.62 |
| sample | OSA13 | OSA14 | OSA15 | OSA16 | OSA17 | OSA18 | OSA19 | OSA20 | OSA21 | OSA22 | OSA23 | OSA24 | OSA25 | OSA26 |
| La (ppm) | 6.67 | 14.58 | 15.25 | 10.07 | 10.73 | 4.85 | 10.64 | 6.26 | 7.10 | 16.52 | 6.66 | 6.99 | 5.05 | 9.79 |
| Ce | 13.20 | 28.19 | 29.58 | 20.56 | 20.71 | 10.37 | 20.71 | 13.20 | 14.51 | 32.75 | 13.20 | 14.04 | 10.74 | 18.78 |
| Pr | 1.40 | 2.85 | 2.98 | 2.16 | 2.18 | 1.13 | 2.12 | 1.43 | 1.54 | 3.41 | 1.40 | 1.43 | 1.19 | 1.92 |
| Nd | 5.73 | 12.19 | 12.72 | 9.50 | 9.49 | 5.09 | 9.07 | 6.52 | 6.85 | 14.69 | 6.10 | 5.87 | 5.44 | 8.29 |
| Pm | ||||||||||||||
| Sm | 1.32 | 2.53 | 2.59 | 2.03 | 1.97 | 1.18 | 1.90 | 1.44 | 1.43 | 3.01 | 1.28 | 1.11 | 1.27 | 1.67 |
| Eu | 0.15 | 0.40 | 0.45 | 0.33 | 0.31 | 0.18 | 0.30 | 0.24 | 0.27 | 0.30 | 0.21 | 0.19 | 0.20 | 0.27 |
| Gd | 1.09 | 1.67 | 1.70 | 1.37 | 1.27 | 0.90 | 1.22 | 0.96 | 1.03 | 1.76 | 0.81 | 0.69 | 0.80 | 1.10 |
| Tb | 0.20 | 0.30 | 0.31 | 0.25 | 0.27 | 0.17 | 0.26 | 0.20 | 0.18 | 0.35 | 0.16 | 0.13 | 0.18 | 0.22 |
| Dy | 1.29 | 1.81 | 1.81 | 1.45 | 1.57 | 1.03 | 1.56 | 1.18 | 1.08 | 2.01 | 0.96 | 0.85 | 1.04 | 1.35 |
| Ho | 0.26 | 0.37 | 0.36 | 0.28 | 0.32 | 0.22 | 0.32 | 0.25 | 0.22 | 0.40 | 0.20 | 0.18 | 0.22 | 0.28 |
| Er | 0.77 | 1.02 | 0.99 | 0.77 | 0.93 | 0.63 | 0.88 | 0.69 | 0.63 | 1.11 | 0.56 | 0.52 | 0.63 | 0.79 |
| Tm | 0.11 | 0.14 | 0.14 | 0.11 | 0.13 | 0.09 | 0.12 | 0.09 | 0.09 | 0.16 | 0.08 | 0.08 | 0.09 | 0.12 |
| Yb | 0.71 | 0.96 | 0.93 | 0.76 | 0.83 | 0.62 | 0.83 | 0.66 | 0.59 | 1.02 | 0.51 | 0.55 | 0.62 | 0.75 |
| Lu | 0.10 | 0.14 | 0.13 | 0.11 | 0.12 | 0.09 | 0.12 | 0.10 | 0.09 | 0.15 | 0.07 | 0.08 | 0.09 | 0.10 |
| LaCN | 28.41 | 62.11 | 64.96 | 42.92 | 45.70 | 20.68 | 45.35 | 26.69 | 30.24 | 70.40 | 28.36 | 29.79 | 21.52 | 41.72 |
| Ce | 21.88 | 46.73 | 49.04 | 34.09 | 34.34 | 17.19 | 34.34 | 21.88 | 24.05 | 54.29 | 21.89 | 23.27 | 17.81 | 31.13 |
| Pr | 15.75 | 31.97 | 33.46 | 24.26 | 24.48 | 12.74 | 23.81 | 16.02 | 17.29 | 38.28 | 15.72 | 16.02 | 13.34 | 21.51 |
| Nd | 12.66 | 26.94 | 28.12 | 21.00 | 20.99 | 11.25 | 20.04 | 14.41 | 15.15 | 32.47 | 13.48 | 12.98 | 12.02 | 18.32 |
| Pm | ||||||||||||||
| Sm | 8.96 | 17.17 | 17.60 | 13.79 | 13.36 | 8.03 | 12.95 | 9.78 | 9.69 | 20.43 | 8.69 | 7.57 | 8.61 | 11.38 |
| Eu | 2.65 | 7.07 | 8.02 | 5.97 | 5.56 | 3.22 | 5.28 | 4.21 | 4.85 | 5.29 | 3.83 | 3.47 | 3.59 | 4.83 |
| Gd | 5.55 | 8.49 | 8.62 | 6.97 | 6.48 | 4.60 | 6.21 | 4.88 | 5.23 | 8.93 | 4.12 | 3.53 | 4.09 | 5.58 |
| Tb | 5.45 | 8.29 | 8.44 | 6.85 | 7.42 | 4.66 | 7.11 | 5.45 | 4.90 | 9.64 | 4.38 | 3.64 | 5.03 | 6.17 |
| Dy | 5.31 | 7.46 | 7.46 | 5.96 | 6.47 | 4.26 | 6.42 | 4.86 | 4.45 | 8.27 | 3.97 | 3.51 | 4.30 | 5.56 |
| Ho | 4.73 | 6.58 | 6.53 | 5.07 | 5.69 | 3.89 | 5.68 | 4.44 | 3.93 | 7.15 | 3.54 | 3.21 | 3.96 | 5.08 |
| Er | 4.85 | 6.45 | 6.24 | 4.86 | 5.83 | 3.97 | 5.54 | 4.33 | 3.99 | 6.97 | 3.52 | 3.26 | 3.96 | 4.96 |
| Tm | 4.68 | 5.96 | 5.91 | 4.67 | 5.49 | 3.80 | 5.09 | 3.81 | 3.67 | 6.73 | 3.19 | 3.23 | 3.69 | 4.89 |
| Yb | 4.37 | 5.94 | 5.71 | 4.67 | 5.08 | 3.82 | 5.08 | 4.08 | 3.65 | 6.29 | 3.17 | 3.36 | 3.82 | 4.63 |
| Lu | 4.18 | 5.67 | 5.52 | 4.36 | 4.86 | 3.81 | 4.80 | 4.14 | 3.51 | 6.04 | 3.07 | 3.20 | 3.64 | 4.28 |
| Eu/Eu* | 0.38 | 0.59 | 0.65 | 0.61 | 0.60 | 0.53 | 0.59 | 0.61 | 0.68 | 0.39 | 0.64 | 0.67 | 0.61 | 0.61 |
| Sample | Rb (ppm) | Sr (ppm) | 87Rb/86Sr | 87Sr/86Sr | SrI (14 Ma) |
|---|---|---|---|---|---|
| OSA3 | 178.1 | 179.7 | 2.87 | 0.70888 | 0.7083 |
| OSA5A | 168.0 | 204.7 | 2.37 | 0.70875 | 0.7083 |
| OSA5B | 147.3 | 126.4 | 3.37 | 0.70992 | 0.7092 |
| OSA6 | 164.8 | 195.7 | 2.44 | 0.70875 | 0.7083 |
| OSA7 | 172.9 | 167.8 | 2.98 | 0.70903 | 0.7084 |
| OSA8 | 199.2 | 146.8 | 3.93 | 0.71013 | 0.7093 |
| OSA9A | 181.0 | 83.9 | 6.25 | 0.71521 | 0.7140 |
| OSA9B | 153.5 | 184.4 | 2.41 | 0.70879 | 0.7083 |
| OSA12 | 176.3 | 170.6 | 2.99 | 0.70921 | 0.7086 |
| OSA14 | 184.1 | 169.0 | 3.15 | 0.70975 | 0.7091 |
| OSA22 | 198.0 | 165.5 | 3.46 | 0.70971 | 0.7090 |
| OSA26 | 177.5 | 176.3 | 2.91 | 0.70901 | 0.7084 |
| Sedimentary rocks * | |||||
| Omine-7 | 153.0 | 124.0 | 3.57 | 0.71258 | 0.7119 |
| Omine-11 | 76.4 | 169.0 | 1.31 | 0.71081 | 0.7105 |
| Wada-2 | 113.0 | 113.0 | 2.90 | 0.71557 | 0.7150 |
| Wada-4 | 53.0 | 119.0 | 1.29 | 0.71085 | 0.7106 |
| Miyazaki-5 | 165.0 | 119.0 | 4.01 | 0.71508 | 0.7143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Shimooka, K.; Tsuboi, M. Geochemical Study of the Osumi Granodiorite, Southwestern Japan. Minerals 2024, 14, 680. https://doi.org/10.3390/min14070680
Xue H, Shimooka K, Tsuboi M. Geochemical Study of the Osumi Granodiorite, Southwestern Japan. Minerals. 2024; 14(7):680. https://doi.org/10.3390/min14070680
Chicago/Turabian StyleXue, Haozhen, Kazuya Shimooka, and Motohiro Tsuboi. 2024. "Geochemical Study of the Osumi Granodiorite, Southwestern Japan" Minerals 14, no. 7: 680. https://doi.org/10.3390/min14070680
APA StyleXue, H., Shimooka, K., & Tsuboi, M. (2024). Geochemical Study of the Osumi Granodiorite, Southwestern Japan. Minerals, 14(7), 680. https://doi.org/10.3390/min14070680






