Biogenic Calcium Carbonate: Phase Conversion in Aqueous Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Butter Clam Microstructure
2.2. Specimen Preparation
2.3. Aqueous Suspension Experiments
2.4. Characterization
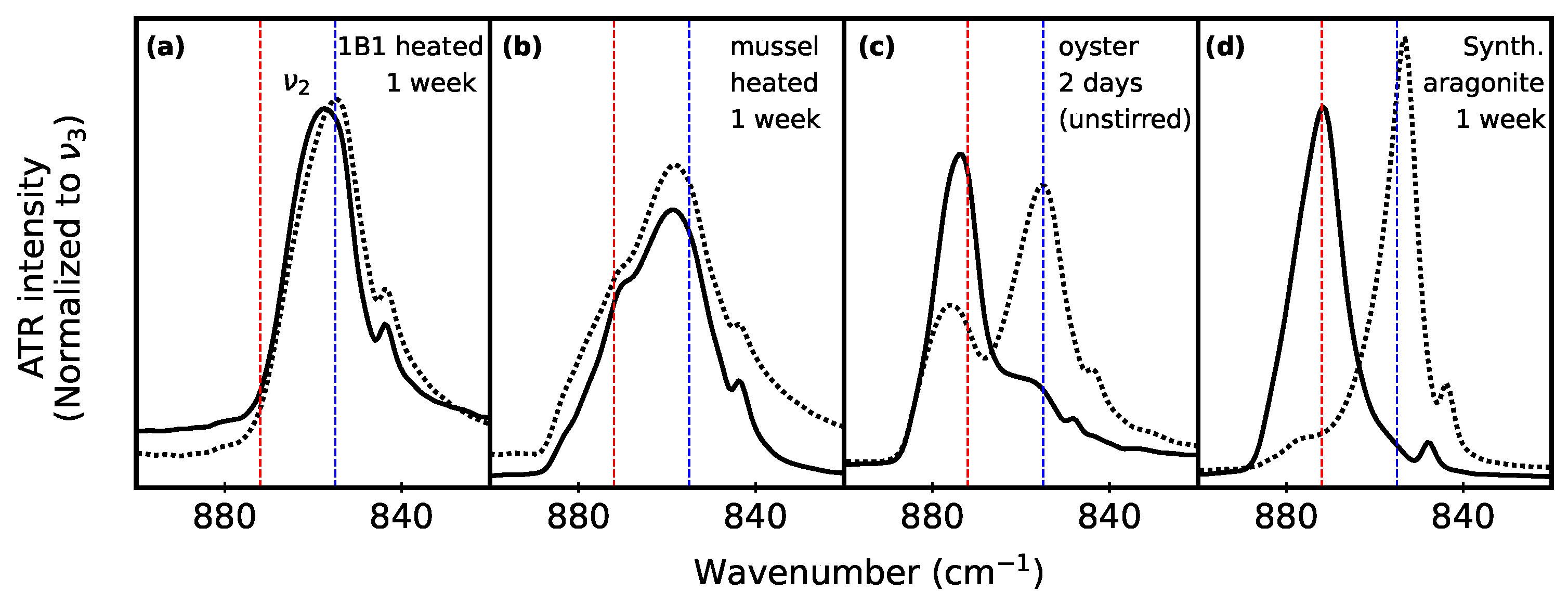
3. Results
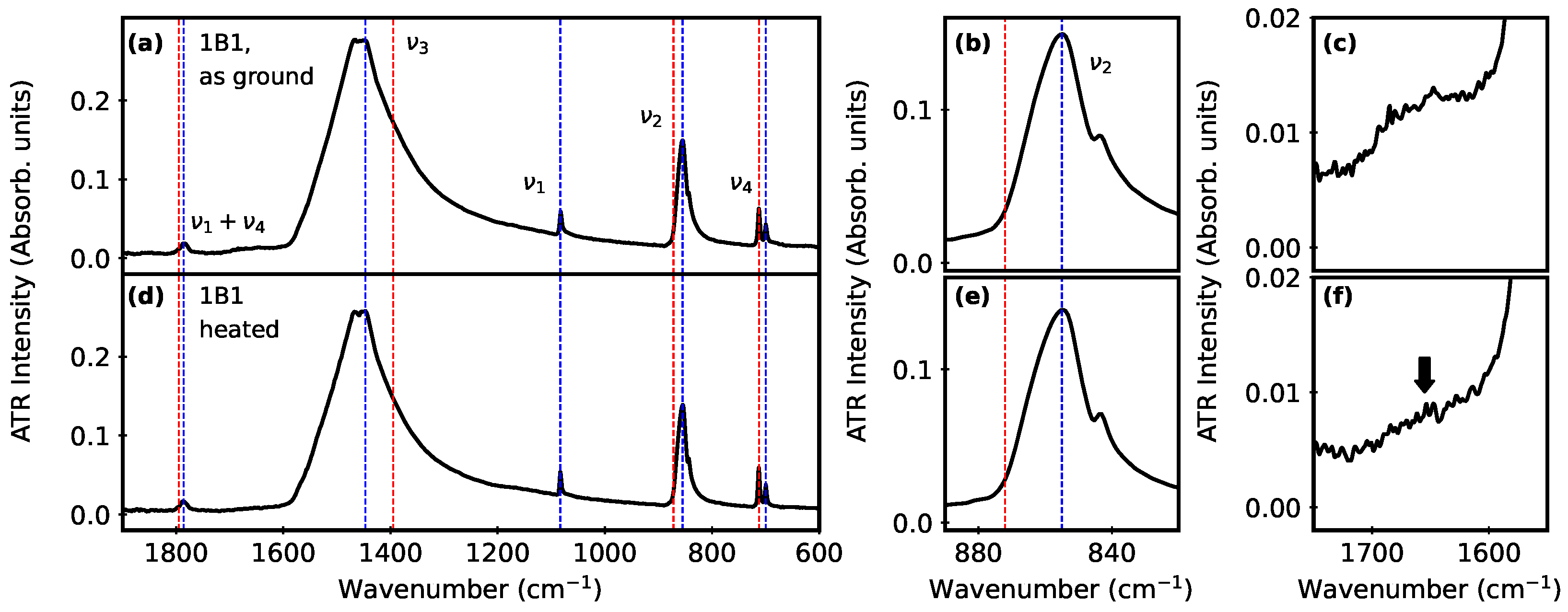
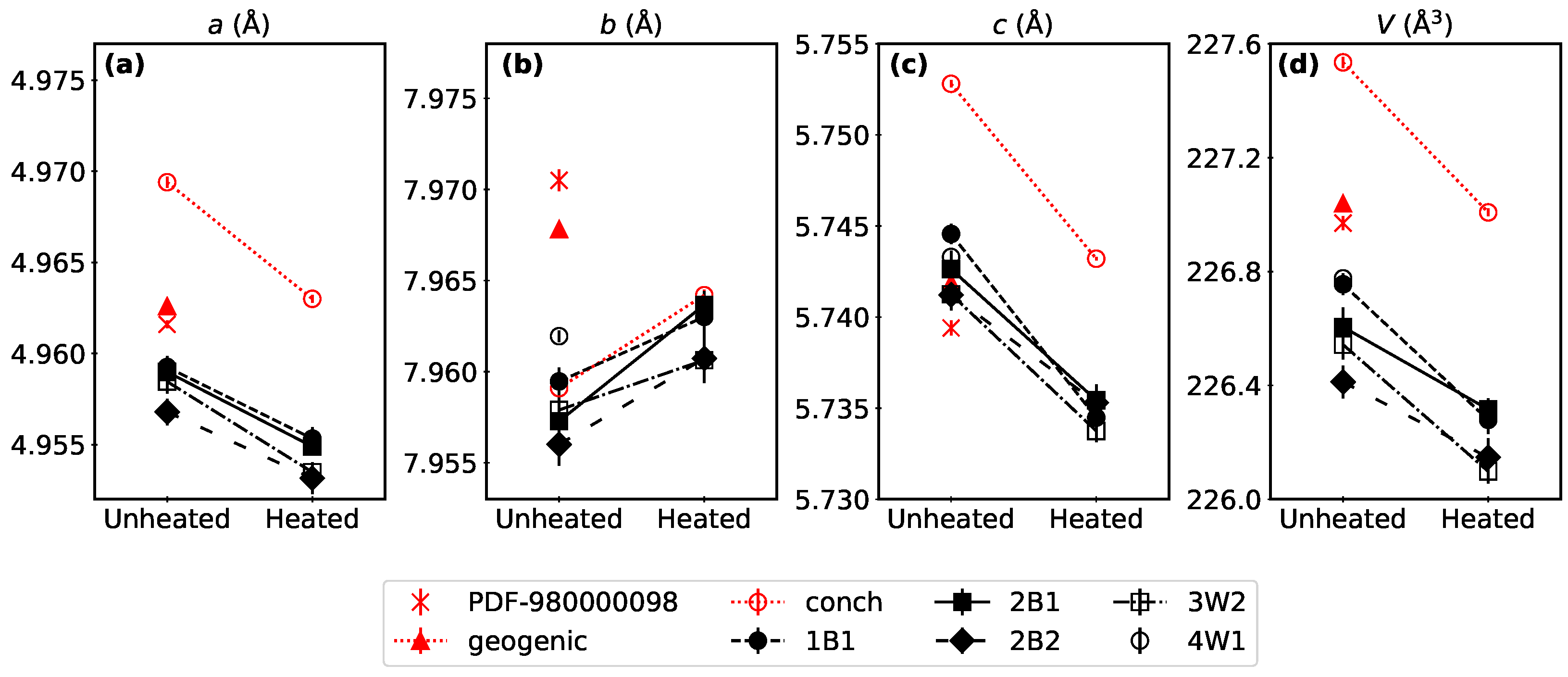
3.1. Clam Mineral Characterization
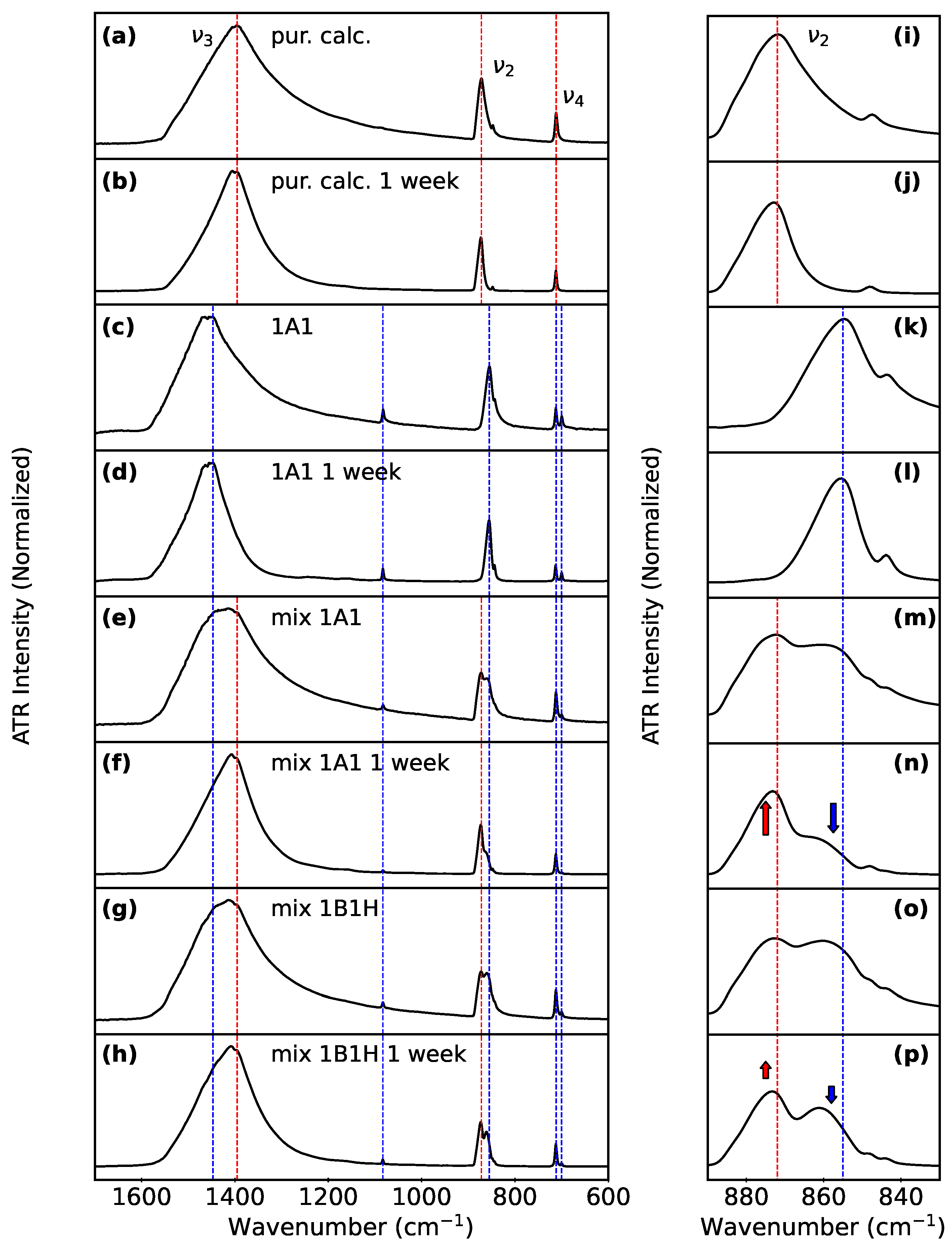
3.2. Monitoring Polymorphs after Aqueous Suspension
3.3. Biogenic vs. Lab-Synthesized
4. Discussion
4.1. Substitutional Impurities
4.2. Polymorphism in Bivalves
4.3. Relevance to Ocean Sediment Studies
5. Conclusions
- (1)
- In ultrapure water with stirring, co-suspensions of powdered biogenic bivalves (butter clams or blue mussels) with powdered purchased calcite showed partial aragonite-to-calcite polymorph conversion within a 1-week time frame.
- (2)
- Even small amounts of synthesized calcite in the lab-synthesized aragonite triggered the aragonite-to-calcite phase conversion in ultrapure water; however, the same was not true for small amounts of biogenic calcite in biogenic aragonite under the same conditions.
- (3)
- Heating to remove organics and/or mechanical periostracum removal did not accelerate polymorph conversion (when powdered in an aqueous suspension) within a 1-week time frame.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium Carbonate Formation and Dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Jacob, D.; Soldati, A.; Wirth, R.; Huth, J.; Wehrmeister, U.; Hofmeister, W. Nanostructure, composition and mechanisms of bivalve shell growth. Geochim. Cosmochim. Acta 2008, 72, 5401–5415. [Google Scholar] [CrossRef]
- de Paula, S.M.; Silveira, M. Studies on molluscan shells: Contributions from microscopic and analytical methods. Micron 2009, 40, 669–690. [Google Scholar] [CrossRef]
- Murphy, J.N.; Schneider, C.M.; Mailänder, L.K.; Lepillet, Q.; Hawboldt, K.; Kerton, F.M. Wealth from waste: Blue mussels (Mylitus edulis) offer up a sustainable source of natural and synthetic nacre. Green Chem. 2019, 21, 3920–3929. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Adkins, J.F.; Naviaux, J.D.; Subhas, A.V.; Dong, S.; Berelson, W.M. The dissolution rate of CaCO3 in the ocean. Annu. Rev. Mar. Sci. 2021, 13, 57–80. [Google Scholar] [CrossRef]
- Sulpis, O.; Agrawal, P.; Wolthers, M.; Munhoven, G.; Walker, M.; Middleburg, J.J. Aragonite dissolution protects calcite at the seafloor. Nat. Commun. 2022, 13, 1104. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Kababya, S.; Poduska, K.M.; Schmidt, A. Surface passivation by embedment of polyphosphate inhibits the aragonite-to-calcite thermodynamic pump. J. Am. Chem. Soc. 2023, 145, 25938–25941. [Google Scholar] [CrossRef]
- van de Mortel, H.; Delaigue, L.; Humphreys, M.P.; Middelburg, J.J.; Ossebaar, S.; Bakker, K.; Alexandre, J.P.T.; van Leeuwen-Tolboom, A.W.E.; Wolthers, M.; Sulpis, O. Laboratory observation of the buffering effect of aragonite dissolution at the seafloor. J. Geophys. Res. Biogeosci. 2024, 129, e2023JG007581. [Google Scholar] [CrossRef]
- Yoshioka, S.; Ohde, S.; Kitano, Y.; Kanamori, N. Behaviour of Magnesium and Strontium During the Transformation of Coral Aragonite to Calcite in Aquatic Environments. Mar. Chem. 1986, 18, 35–48. [Google Scholar] [CrossRef]
- Krauskopf, K.B.; Bird, D.K. Solution-Mineral equilibria Part 1: Carbonates. Introd. Geochem. 1995, 3, 61–83. [Google Scholar]
- Sulpis, O.; Lix, C.; Mucci, A.; Boudreau, B.P. Calcite dissolution kinetics at the sediment-water interface in natural seawater. Mar. Chem. 2017, 195, 70–83. [Google Scholar] [CrossRef]
- Zhang, G.; Morales, J.; García-Ruiz, J.M. Growth behaviour of silica/carbonate nanocrystalline composites of calcite and aragonite. J. Mater. Chem. B 2017, 5, 1658–1663. [Google Scholar] [CrossRef]
- Triunfo, C.; Gärtner, S.; Marchini, C.; Fermani, S.; Maoloni, G.; Goffredo, S.; Morales, J.G.; Cölfen, H.; Falini, G. Recovering and Exploiting Aragonite and Calcite Single Crystals with Biologically Controlled Shapes from Mussel Shells. ACS Omega 2022, 7, 43992–43999. [Google Scholar] [CrossRef]
- Louis, V.; Besseau, L.; Lartaud, F. Step in Time: Biomineralisation of Bivalve’s Shell. Front. Mar. Sci. 2022, 9, 906085. [Google Scholar] [CrossRef]
- Gao, B.; Poduska, K.M. Comparing Polyphosphate and Orthophosphate Treatments of Solution-Precipitated Aragonite Powders. Solids 2022, 3, 684–696. [Google Scholar] [CrossRef]
- Schöne, B.R.; Dunca, E.; Fiebig, J.; Pfeiffer, M. Mutvei’s solution: An ideal agent for resolving microgrowth structures of biogenic carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 228, 149–166. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Lorrain, A.; Navez, J.; Taylor, J.W.; André, L.; Keppens, E.; Baeyens, W.; Dehairs, F. Strong biological controls on Sr/Ca ratios in aragonitic marine bivalve shells. Geochem. Geophys. Geosystems 2005, 6, Q05009. [Google Scholar] [CrossRef]
- Ulens, H. The Potentials of Saxidomus Giganteus as a Paleoclimate Proxy. Master’s Thesis, Gent University, Flanders, Belgium, 2003. [Google Scholar]
- Huang, Q.; Wu, H.; Schöne, B.R. A novel trophic archive: Practical considerations of compound-specific amino acid δ15N analysis of carbonate-bound organic matter in bivalve shells (Arctica islandica). Chem. Geol. 2023, 615, 121220. [Google Scholar] [CrossRef]
- Hallmann, N.; Burchell, M.; Schöne, B.R.; Irvine, G.V.; Maxwell, D. High-resolution sclerochronological analysis of the bivalve mollusk Saxidomus gigantea from Alaska and British Columbia: Techniques for revealing environmental archives and archaeological seasonality. J. Archaeol. Sci. 2009, 36, 2353–2364. [Google Scholar] [CrossRef]
- Available online: https://commons.wikimedia.org/wiki/File:Clam_(PSF).jpg (accessed on 21 March 2024).
- Gao, B.; Poduska, K.M. Tracking Amorphous Calcium Carbonate Crystallization Products with Far-Infrared Spectroscopy. Minerals 2023, 13, 110. [Google Scholar] [CrossRef]
- Christ, C.L.; Hostetler, P.B.; Siebert, R.M. Stabilities of calcite and aragonite. J. Geophys. Res. 1974, 2, 175–184. [Google Scholar]
- JADE 10; Materials Data: Livermore, CA, USA, 2019.
- Gates-Rector, S.; Blanton, T. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N.; Armbruster, T.; Danisi, R.M. The power of databases: The RRUFF project. Highlights Mineral. Crystallogr. 2015, 1, 25. [Google Scholar]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Kimmel Center for Archaeological Science (Weizmann Institute of Science). Infrared Standards Library. 2021. Available online: https://centers.weizmann.ac.il/kimmel-arch/infrared-spectra-library (accessed on 24 June 2024).
- Forjanes, P.; Roda, M.S.; Greiner, M.; Griesshaber, E.; Lagos, N.A.; Veintemillas-Verdaguer, S.; Astilleros, J.M.; Fernández-Díaz, L.; Schmahl, W.W. Long-term experimental diagenesis of aragonitic biocarbonates: From organic matter loss to abiogenic calcite formation. Biogeosci. Discuss 2021, 2021, 1–53. [Google Scholar] [CrossRef]
- Pokroy, B.; Fitch, A.; Zolotoyabko, E. Structure of biogenic aragonite (CaCO3). Cryst. Growth Des. 2007, 7, 1580–1583. [Google Scholar] [CrossRef]
- Pokroy, B.; Fieramosca, J.; Von Dreele, R.; Fitch, A.; Caspi, E.; Zolotoyabko, E. Atomic structure of biogenic aragonite. Chem. Mater. 2007, 19, 3244–3251. [Google Scholar] [CrossRef]
- Caspi, E.N.; Pokroy, B.; Lee, P.; Quintana, J.; Zolotoyabko, E. On the structure of aragonite. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 129–132. [Google Scholar] [CrossRef]
- Sun, W.; Jayaraman, S.; Chen, W.; Persson, K.A.; Ceder, G. Nucleation of metastable aragonite CaCO3 in seawater. Proc. Natl. Acad. Sci. USA 2015, 112, 3199–3204. [Google Scholar] [CrossRef]
- Chave, K.E. A Solid Solution between Calcite and Dolomite. J. Geol. 1952, 60, 190–192. [Google Scholar] [CrossRef]
- Wasylenki, L.E.; Dove, P.M.; Wilson, D.S.; De Yoreo, J.J. Nanoscale effects of strontium on calcite growth: An in situ AFM study in the absence of vital effects. Geochim. Cosmochim. Acta 2005, 69, 3017–3027. [Google Scholar] [CrossRef]
- Astilleros, J.; Fernández-Díaz, L.; Putnis, A. The role of magnesium in the growth of calcite: An AFM study. Chem. Geol. 2010, 271, 52–58. [Google Scholar] [CrossRef]
- Hashim, M.S.; Kaczmarek, S.E. The transformation of aragonite to calcite in the presence of magnesium: Implications for marine diagenesis. Earth Planet. Sci. Lett. 2021, 574, 117166. [Google Scholar] [CrossRef]
- Mills, J.V.; Barnhart, H.A.; DePaolo, D.J.; Lammers, L.N. New insights into Mn2+ and Mg2+ inhibition of calcite growth. Geochim. Cosmochim. Acta 2022, 334, 338–367. [Google Scholar] [CrossRef]
- Walter, L.M.; Morse, J.W. The dissolution kinetics of shallow marine carbonates in seawater: A laboratory study. Geochim. Cosmochim. Acta 1985, 49, 1503–1513. [Google Scholar] [CrossRef]
- Astilleros, J.; Pina, C.; Fernández-Díaz, L.; Putnis, A. Metastable phenomena on calcite 101-4 surfaces growing from Sr2+–Ca2+– aqueous solutions. Chem. Geol. 2003, 193, 93–107. [Google Scholar] [CrossRef]
- Weiss, I.M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zool. 2002, 293, 478–491. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Xie, L.; Zhang, R. Amorphous calcium carbonate: A precursor phase for aragonite in shell disease of the pearl oyster. Biochem. Biophys. Res. Commun. 2018, 497, 102–107. [Google Scholar] [CrossRef]
- Grünewald, T.A.; Checchia, S.; Dicko, H.; Le Moullac, G.; Sham Koua, M.; Vidal-Dupiol, J.; Duboisset, J.; Nouet, J.; Grauby, O.; Di Michiel, M.; et al. Structure of an amorphous calcium carbonate phase involved in the formation of Pinctada margaritifera shells. Proc. Natl. Acad. Sci. USA 2022, 119, e2212616119. [Google Scholar] [CrossRef]
- Fitzer, S.C.; Chung, P.; Maccherozzi, F.; Dhesi, S.S.; Kamenos, N.A.; Phoenix, V.R.; Cusack, M. Biomineral shell formation under ocean acidification: A shift from order to chaos. Sci. Rep. 2016, 6, 21076. [Google Scholar] [CrossRef]
- Ramesh, K.; Hu, M.Y.; Thomsen, J.; Bleich, M.; Melzner, F. Mussel larvae modify calcifying fluid carbonate chemistry to promote calcification. Nat. Commun. 2017, 8, 1709. [Google Scholar] [CrossRef] [PubMed]
- Simmer, R.A.; Jansen, E.J.; Patterson, K.J.; Schnoor, J.L. Climate Change and the Sea: A Major Disruption in Steady State and the Master Variables. ACS Environ. Au 2023, 3, 195–208. [Google Scholar] [CrossRef]
- Castellan, G.; Angeletti, L.; Canese, S.; Mazzoli, C.; Montagna, P.; Schiaparelli, S.; Taviani, M. Visual Imaging of Benthic Carbonate-Mixed Factories in the Ross Sea Region Marine Protected Area, Antarctica. Minerals 2021, 11, 833. [Google Scholar] [CrossRef]
- Cubillas, P.; Köhler, S.; Prieto, M.; Chaïrat, C.; Oelkers, E.H. Experimental determination of the dissolution rates of calcite, aragonite, and bivalves. Chem. Geol. 2005, 216, 59–77. [Google Scholar] [CrossRef]
- Hadjittofis, E.; Vargas, S.M.; Litster, J.D.; Sedransk Campbell, K.L. The role of surface energy in the apparent solubility of two different calcite crystal habits. Proc. R. Soc. A 2021, 477, 20210200. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.Y.; Bénézeth, P.; Pokrovsky, O.; Shvarov, Y.V.; Castillo, A.; Schott, J. Experimental determination of calcite solubility and the stability of aqueous Ca–and Na–carbonate and–bicarbonate complexes at 100–160 °C and 1–50 bar pCO2 using in situ pH measurements. Geochim. Cosmochim. Acta 2020, 290, 352–365. [Google Scholar] [CrossRef]

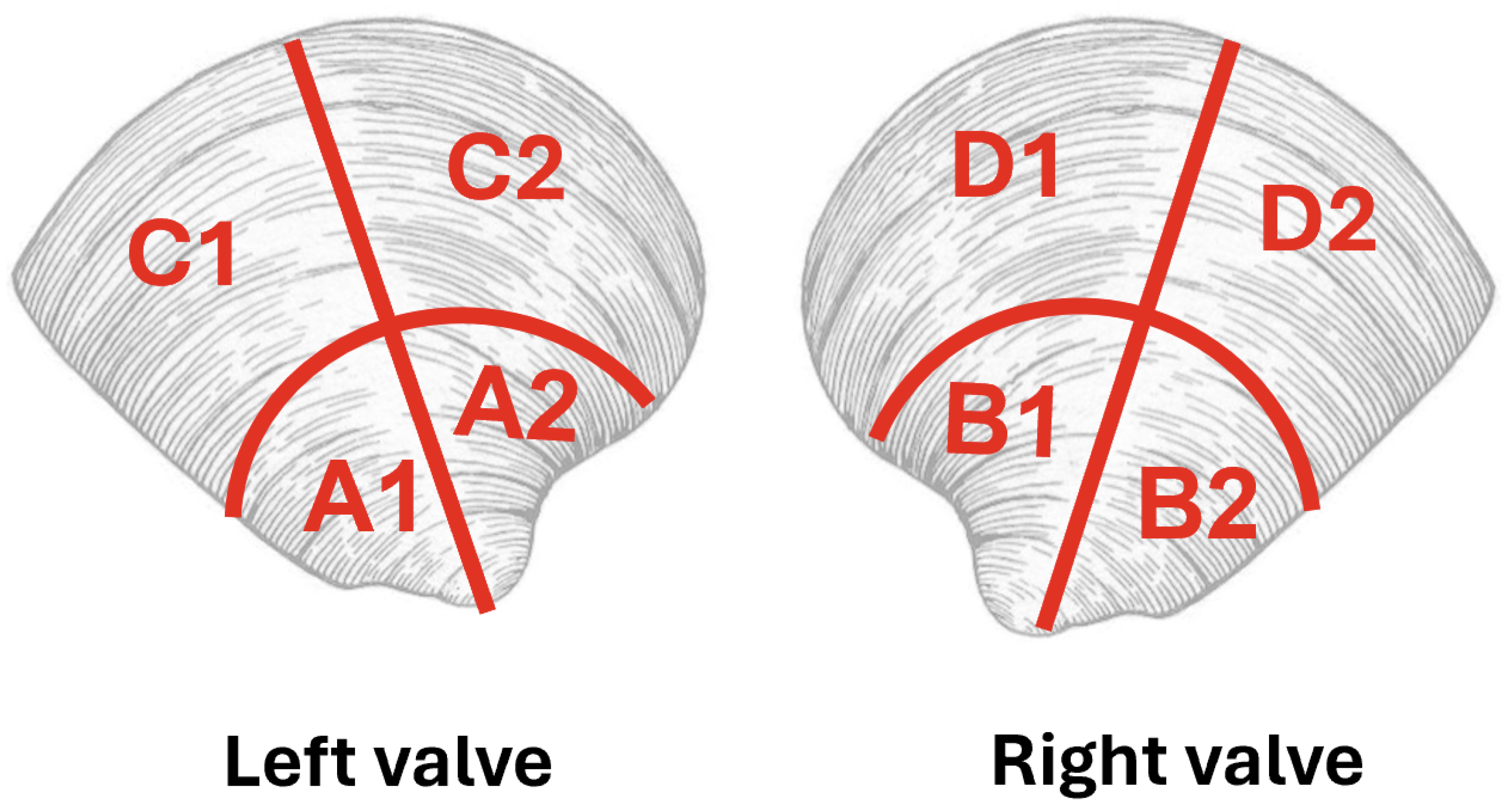

| Label | Valve | Hinge? | Periostracum? | Mass % | Alone (mg:mL) | Mix ((mg):mL) | Stir? |
|---|---|---|---|---|---|---|---|
| Clam 1 | |||||||
| 1A1 | left | yes | yes | – | 50:5 | (50 + 50):10 | yes |
| 1A2 | left | yes | no | – | 50:5 | (50 + 50):10 | yes |
| 1C1 | left | no | yes | – | 100:10 | (50 + 50):10 | yes |
| 1B1 | right | yes | no | – | 10:1 | – | yes |
| no | −2.9 | 50:5 | (50 + 50):10 | yes | |||
| 1B2 | right | yes | yes | – | – | – | yes |
| yes | −2.4 | 50:5 | (50 + 50):10 | yes | |||
| Clam 2 | |||||||
| 2B1 | right | yes | no | −3.1 | – | – | – |
| 2B2 | right | yes | yes | −2.6 | – | – | – |
| Clam 3 | |||||||
| 3W1 | – | – | yes | – | 100:10 | (50 + 50):10 | yes |
| 3W2 | – | – | yes | −2.1 | 100:10 | (50 + 50):10 | yes |
| Clam 4 | |||||||
| 4W1 | – | – | yes | – | – | (50 + 50):10 | no |
| yes | – | 100:10 | – | yes | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Acosta, B.; Breen, J.J.; Burchell, M.; Poduska, K.M. Biogenic Calcium Carbonate: Phase Conversion in Aqueous Suspensions. Minerals 2024, 14, 682. https://doi.org/10.3390/min14070682
Espinosa-Acosta B, Breen JJ, Burchell M, Poduska KM. Biogenic Calcium Carbonate: Phase Conversion in Aqueous Suspensions. Minerals. 2024; 14(7):682. https://doi.org/10.3390/min14070682
Chicago/Turabian StyleEspinosa-Acosta, Brian, Jake J. Breen, Meghan Burchell, and Kristin M. Poduska. 2024. "Biogenic Calcium Carbonate: Phase Conversion in Aqueous Suspensions" Minerals 14, no. 7: 682. https://doi.org/10.3390/min14070682
APA StyleEspinosa-Acosta, B., Breen, J. J., Burchell, M., & Poduska, K. M. (2024). Biogenic Calcium Carbonate: Phase Conversion in Aqueous Suspensions. Minerals, 14(7), 682. https://doi.org/10.3390/min14070682







