Surface-Functionalized Nano-Montmorillonite and Its Application as Crude Oil Flow Improver
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Material Preparation
2.3. Differential Scanning Calorimetry (DSC) Analysis
2.4. Wax Crystal Morphology Analysis
2.5. Zeta-Potential Particle Size Analysis
2.6. Scanning Electron Microscope (SEM) Analysis
2.7. FTIR Analysis
2.8. Decentralized Experiments
2.9. Contact Angle Measurement
3. Results and Discussion
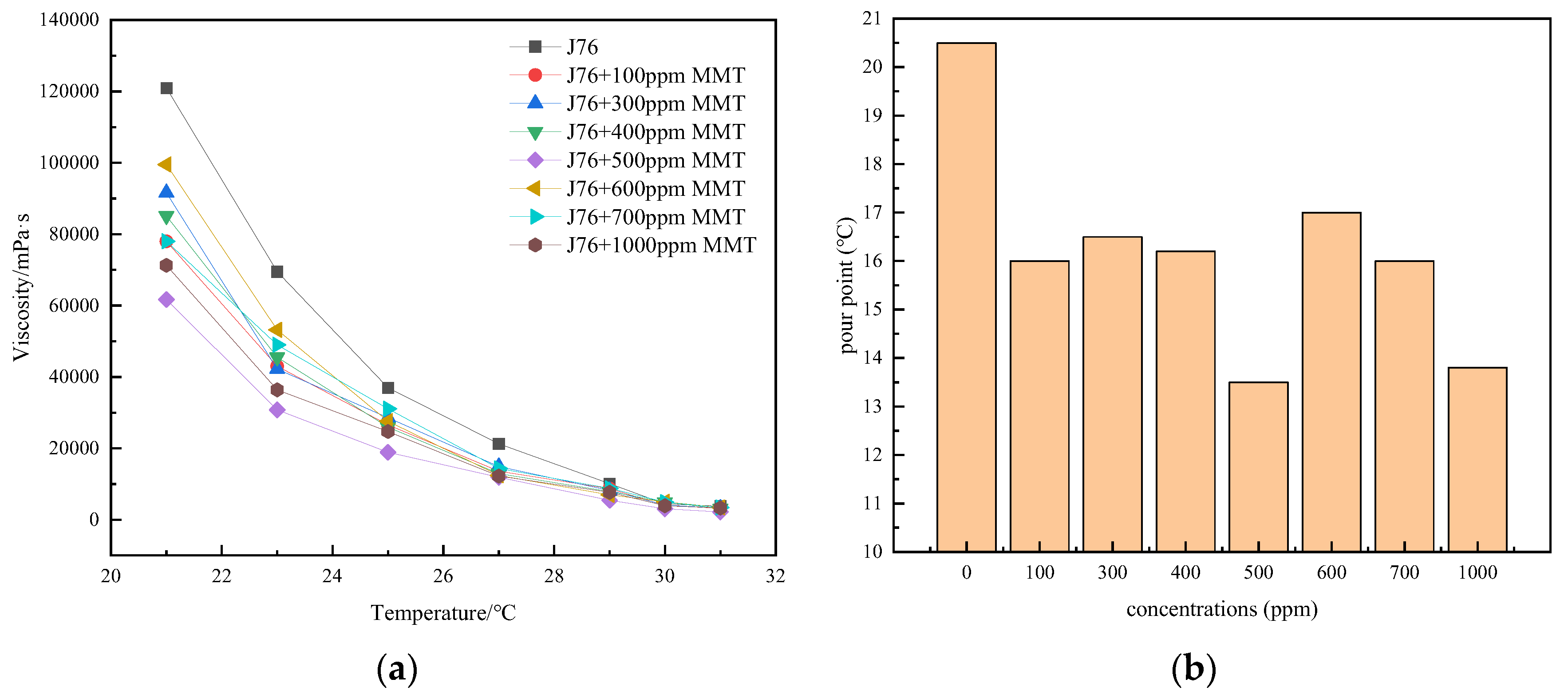
3.1. Effect of Montmorillonite Dosage
3.2. Effect of Particle Size
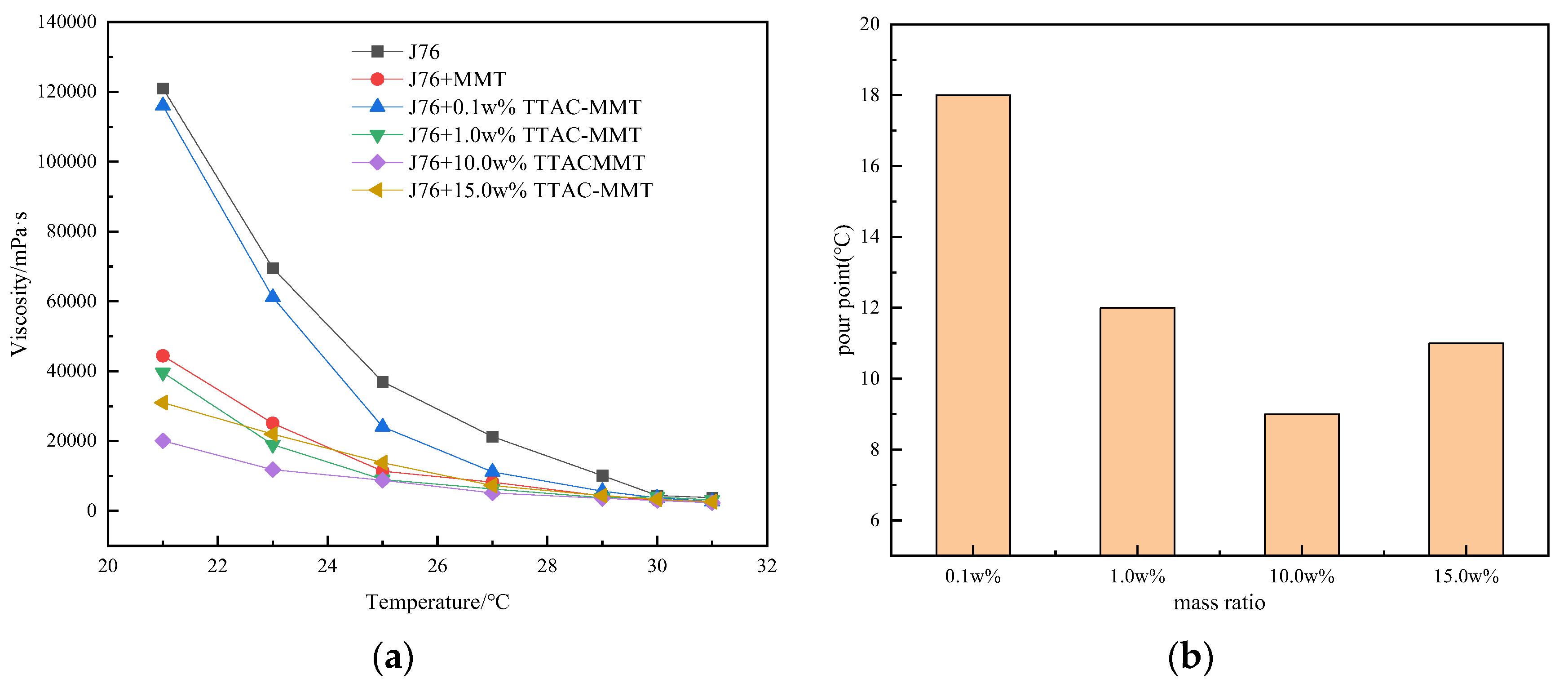
3.3. Effect of Modifier Dosage
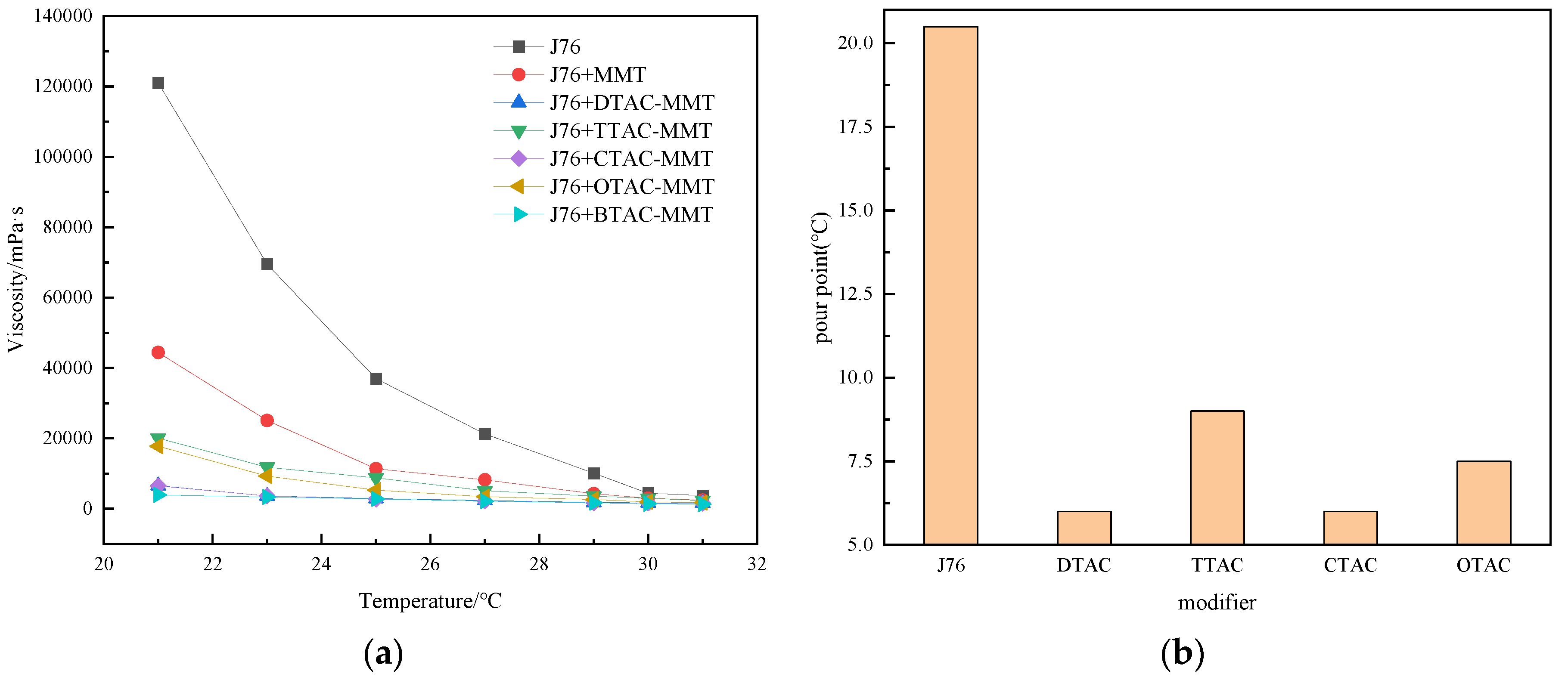
3.4. Effect of Modifier
3.5. B@MMT Dosage Screening
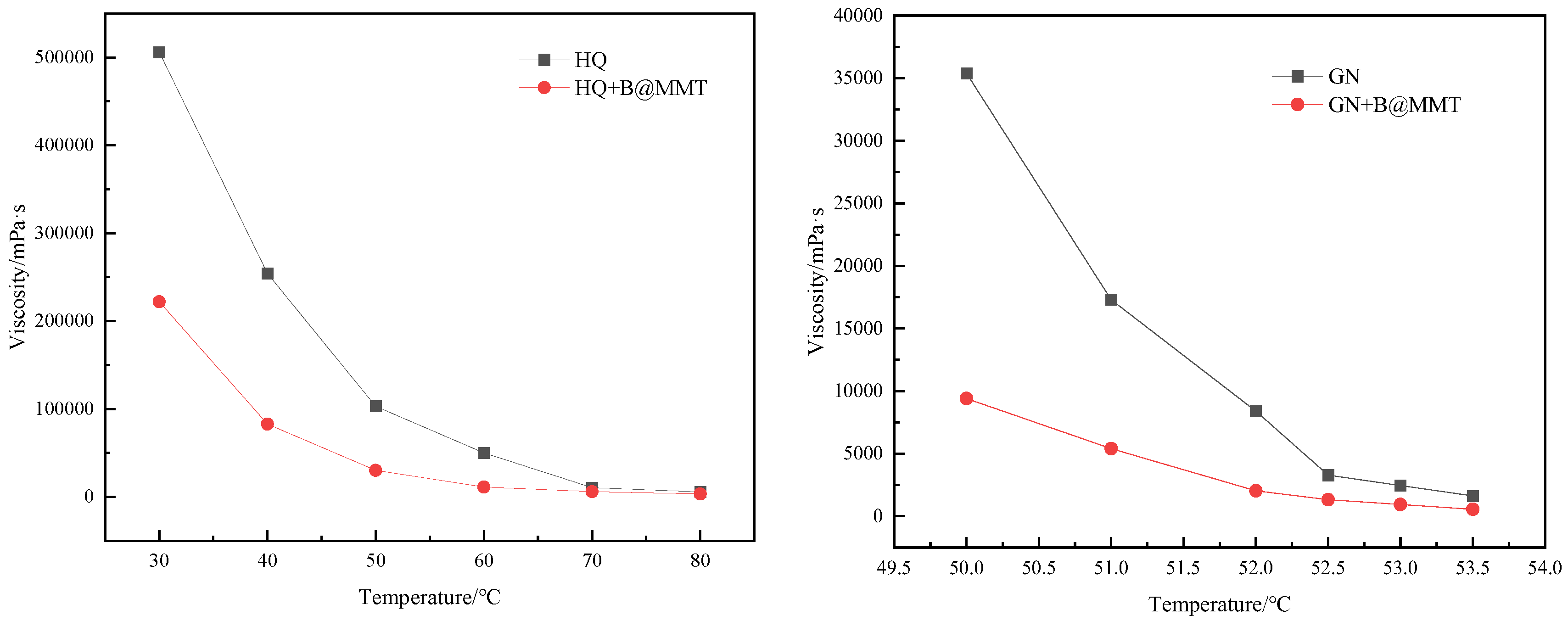
3.6. Universalization Evaluation
3.7. DSC Analysis
3.8. Microscopic Wax Crystal Morphology Analysis
3.9. Particle Size Analysis
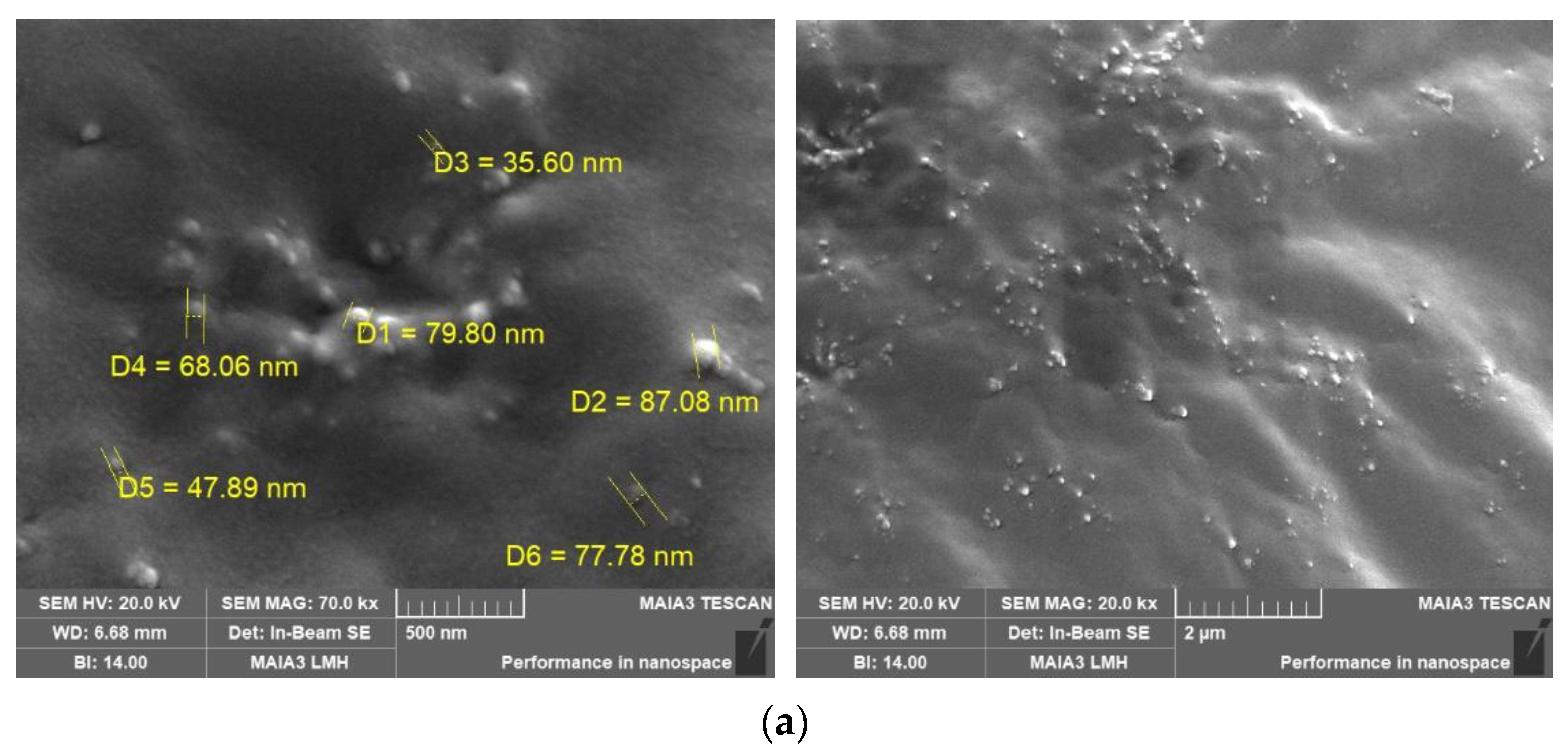
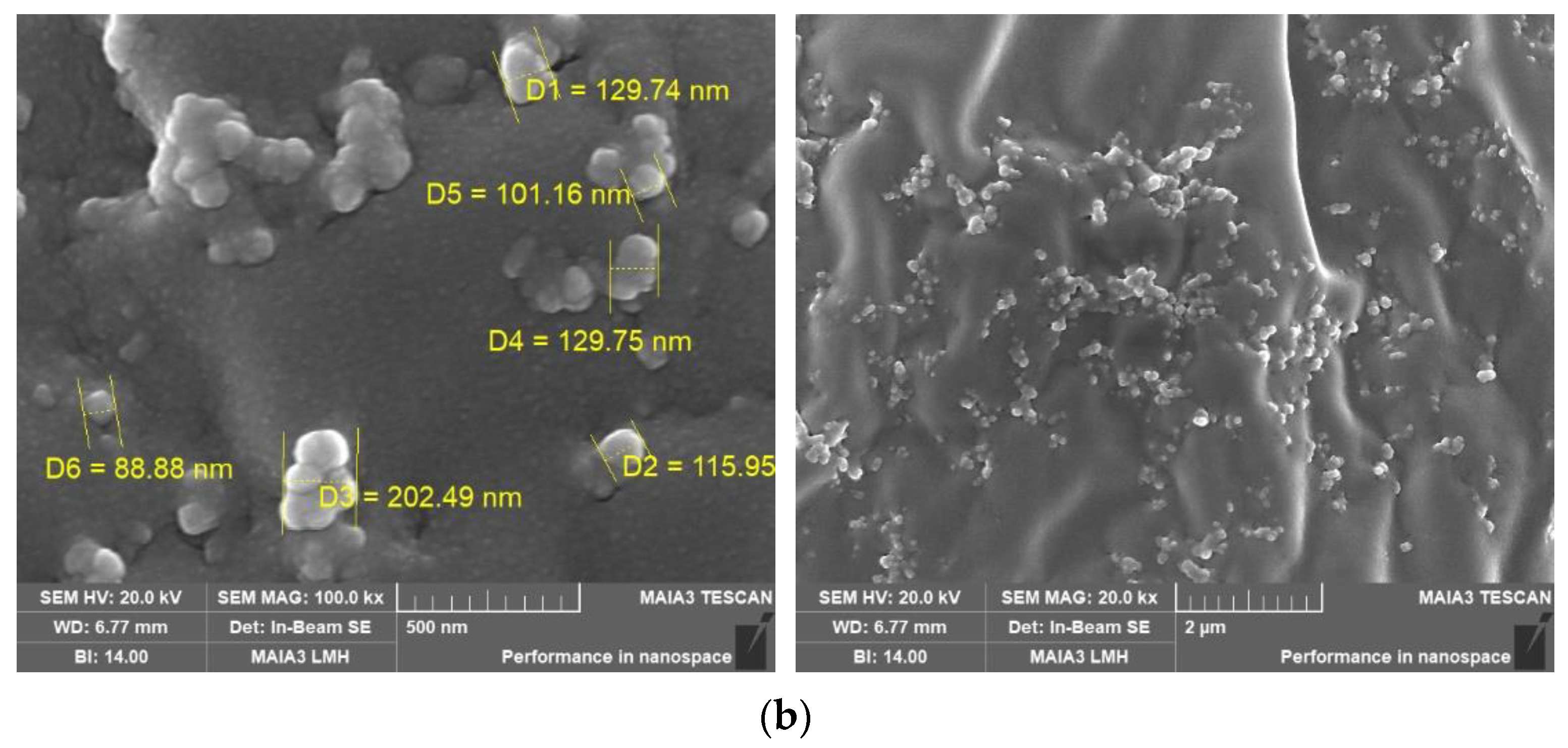
3.10. SEM Analysis
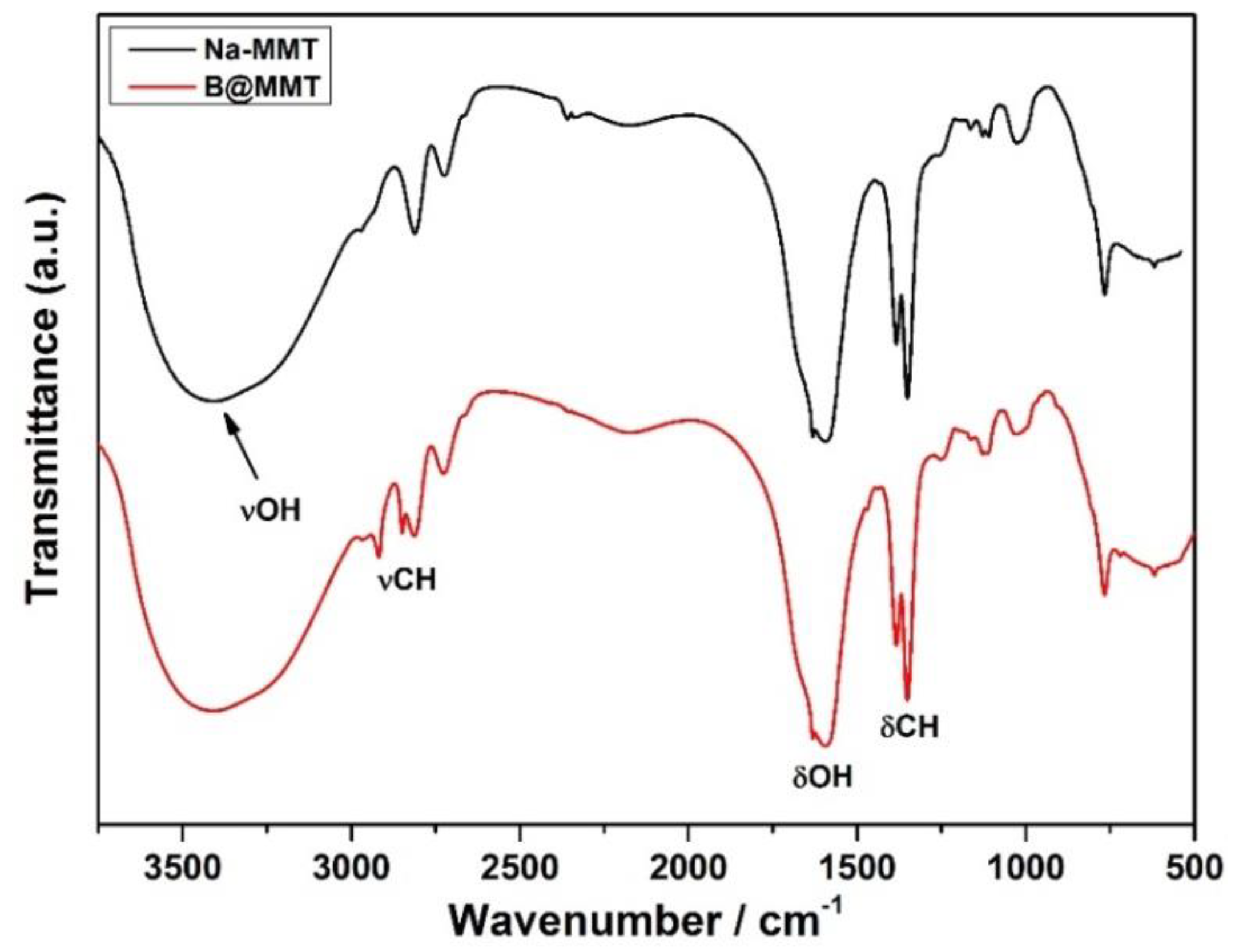
3.11. FTIR Analysis
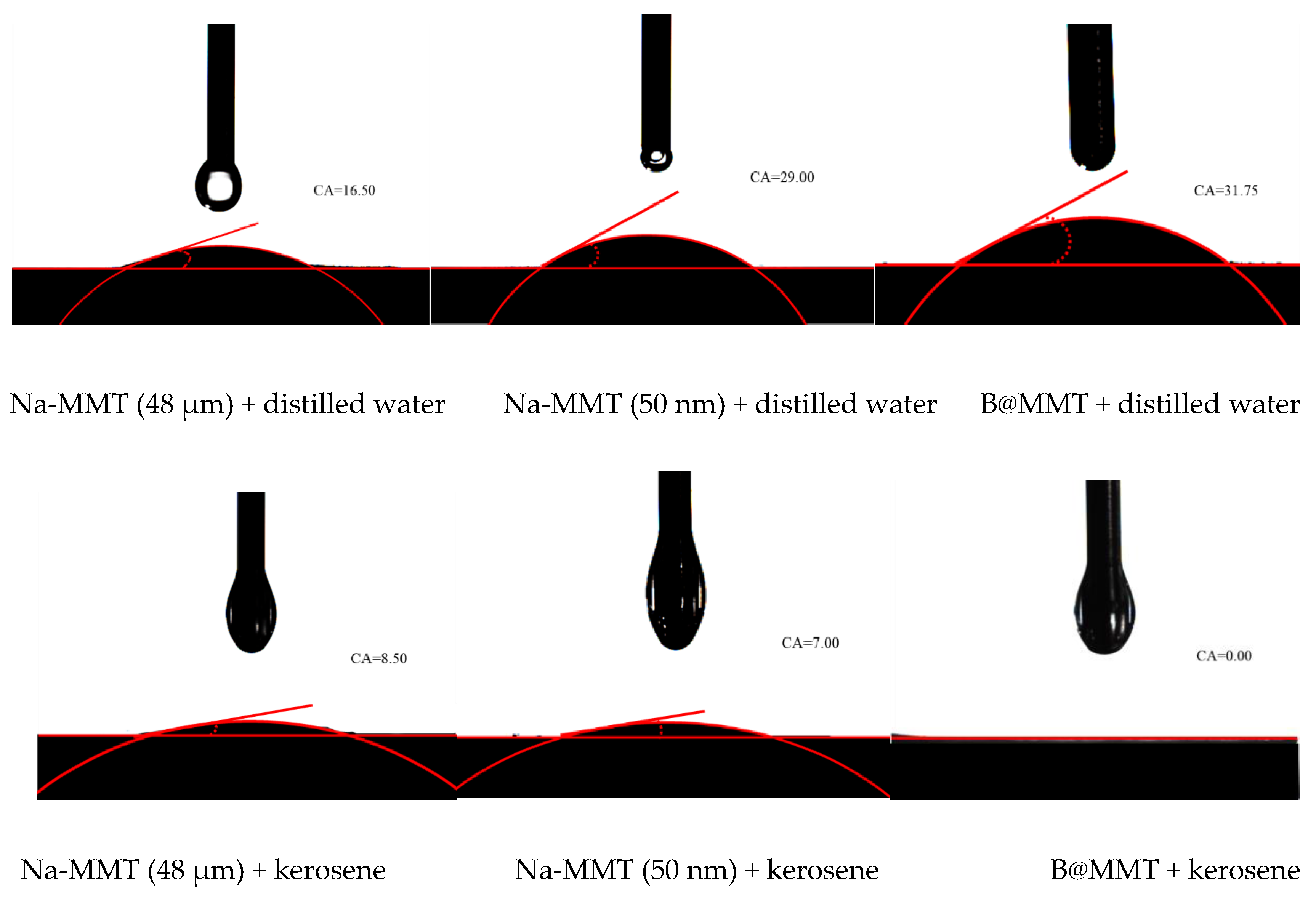
3.12. Contact Angle Analysis
3.13. Dispersion Test
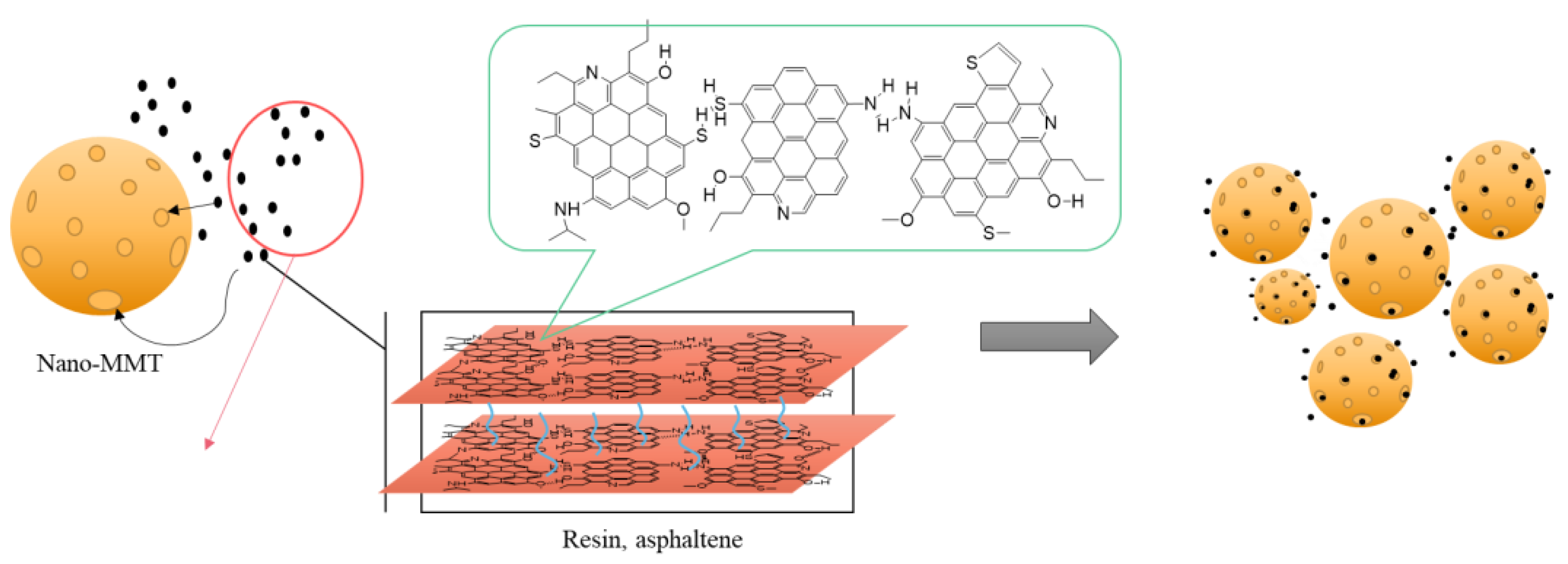
3.14. Mechanism Analysis
3.15. Economic Feasibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, F.; Jiang, T.; Yuan, C.; Varfolomeev, M.A.; Wan, F.; Zheng, Y.; Li, X. An improved viscosity prediction model of extra heavy oil for high temperature and high pressure. Fuel 2022, 319, 123852. [Google Scholar] [CrossRef]
- Shi, W.; Xu, L.; Tao, L.; Zhu, Q.; Bai, J. Flow behaviors and residual oil characteristics of water flooding assisted by multi-effect viscosity reducer in extra heavy oil reservoir. Pet. Sci. Technol. 2023, 41, 1231–1249. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Xia, S. Research progress and development trend of heavy oil emulsifying viscosity reducer: A review. Pet. Sci. Technol. 2021, 39, 550–563. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sircar, A.; Deka, B.; Silviya Anumegalai, A.; Suresh Moorthi, P.; Yasvanthrajan, N. Flow improvers for assured flow of crude oil in midstream pipeline—A review. J. Pet. Sci. Eng. 2018, 164, 24–30. [Google Scholar] [CrossRef]
- Du, Y.; Huang, C.; Jiang, W.; Yan, Q.; Li, Y.; Chen, G. Preparation of surface modified nano-hydrotalcite and its applicaiton as a flow improver for crude oil. Fuel 2024, 357, 130005. [Google Scholar] [CrossRef]
- Zang, Y.; Liu, G.; Ji, W.; Li, Y.; Chen, G. Resource utilization of expired progesterone medicines as flow improver for waxy crude oils. J. Environ. Manag. 2024, 349, 119524. [Google Scholar] [CrossRef]
- Eke, W.I.; Kyei, S.K.; Achugasim, O.; Ajienka, J.A.; Akaranta, O. Pour point depression and flow improvement of waxy crude oil using polyethylene glycol esters of cashew nut shell liquid. Appl. Petrochem. Res. 2021, 11, 199–208. [Google Scholar] [CrossRef]
- Li, R.; Huang, Q.; Huo, F.; Fan, K.; Li, W.; Zhang, D. Effect of shear on the thickness of wax deposit under laminar flow regime. J. Pet. Sci. Eng. 2019, 181, 106212. [Google Scholar] [CrossRef]
- Mahto, V.; Verma, D.; Kumar, A.; Sharma, V.P. Wax deposition in flow lines under dynamic conditions. Pet. Sci. Technol. 2014, 32, 1996–2003. [Google Scholar] [CrossRef]
- Ansari, F.; Shinde, S.B.; Paso, K.G.; Sjöblom, J.; Kumar, L. Chemical additives as flow improvers for waxy crude oil and model oil: A critical review analyzing structure–efficacy relationships. Energy Fuels 2022, 36, 3372–3393. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, S.; Zhang, X.; Dong, S.; Yu, T.; Slaný, M.; Chen, G. Enhanced aquathermolysis of heavy oil catalysed by bentonite supported Fe(III) complex in the present of ethanol. J. Chem. Technol. Biotechnol. 2022, 97, 1128–1137. [Google Scholar] [CrossRef]
- Eke, W.I.; Kyei, S.K.; Ajienka, J.; Akaranta, O. Effect of bio-based flow improver on the microscopic and low-temperature flow properties of waxy crude oil. J. Pet. Explor. Prod. Technol. 2021, 11, 711–724. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Y.; Han, X.; Xie, K.; Liu, R.; Zheng, W.; Li, J.; Huang, C.; Wang, X.; Zhang, L. Experimental Study on Modified Lotus Stem Biochar-Based Catalysts for Heavy Oil Aquathermolysis. Mol. Catal. 2024, 554, 113848. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, Y.; Qi, G.; Yu, T.; Xin, X.; Zhao, B.; Chen, G. Oil-Soluble Exogenous Catalysts and Reservoir Minerals Synergistically Catalyze the Aquathermolysis of Heavy Oil. Molecules 2023, 28, 6766. [Google Scholar] [CrossRef]
- Zhou, Z.; Slaný, M.; Kuzielová, E.; Zhang, W.; Ma, L.; Dong, S.; Zhang, J.; Chen, G. Influence of reservoir minerals and ethanol on catalytic aquathermolysis of heavy oil. Fuel 2022, 307, 121871. [Google Scholar] [CrossRef]
- Ridzuan, N.; Subramanie, P.; Uyop, M.F. Effect of pour point depressant (PPD) and the nanoparticles on the wax deposition, viscosity and shear stress for Malaysian crude oil. Pet. Sci. Technol. 2020, 38, 929–935. [Google Scholar] [CrossRef]
- Kiyingi, W.; Guo, J.-X.; Xiong, R.-Y.; Su, L.; Yang, X.-H.; Zhang, S.-L. Crude oil wax: A review on formation, experimentation, prediction, and remediation techniques. Pet. Sci. 2022, 19, 2343–2357. [Google Scholar] [CrossRef]
- Alnaimat, F.; Ziauddin, M. Wax deposition and prediction in petroleum pipelines. J. Pet. Sci. Eng. 2020, 184, 106385. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Yang, L.; Dai, Y.; Zhang, J.; He, L.; Cui, J.; Shen, J.; Wang, Z. Recoverable Magnetic Fe-MOF Immobilized Carrier to Immobilize Two Microorganisms for Reduction of Heavy Oil Viscosity and Oilfield Wastewater COD. J. Water Proc. Eng. 2023, 56, 104459. [Google Scholar] [CrossRef]
- Wu, R.; Yan, Y.; Li, X.; Tan, Y. Preparation and Controllable Heavy Oil Viscosity Reduction Performance of pH-Responsive Star Block Copolymers. J. Mol. Liq. 2023, 389, 122925. [Google Scholar] [CrossRef]
- Vahabzadeh Asbaghi, E.; Assareh, M.; Nazari, F. An effective procedure for wax formation modeling using multi-solid approach and PC-SAFT EOS for petroleum fluids with PNA characterization. J. Pet. Sci. Eng. 2021, 207, 109103. [Google Scholar] [CrossRef]
- Shi, H.; Mao, Z.; Ran, L.; Ru, C.; Guo, S.; Dong, H. Heavy Oil Viscosity Reduction through Aquathermolysis Catalyzed by Ni20(NiO)80 Nanocatalyst. Fuel Process. Technol. 2023, 250, 107911. [Google Scholar] [CrossRef]
- Marchesini, F.H.; Alicke, A.A.; de Souza Mendes, P.R.; Ziglio, C.M. Rheological characterization of waxy crude oils: Sample preparation. Energy Fuels 2012, 26, 2566–2577. [Google Scholar] [CrossRef]
- Chi, M.; Cui, J.; Hu, J.; Fan, J.; Du, S.; Xiao, P.; Hu, S.; Sun, S. Silica Janus Nanosheets Anchored with Ni Nanoparticles for In-Situ Upgrading and Stimulus-Responsive Demulsification to Enhance Heavy Oil Recovery. J. Anal. Appl. Pyrolysis 2024, 181, 106603. [Google Scholar] [CrossRef]
- Ridzuan, N.; Adam, F.; Yaacob, Z. Evaluation of the inhibitor selection on wax deposition for Malaysian crude oil. Pet. Sci. Technol. 2016, 34, 366–371. [Google Scholar] [CrossRef]
- Kurniawan, M.; Subramanian, S.; Norrman, J.; Paso, K. Influence of microcrystalline wax on the properties of model wax-oil gels. Energy Fuels 2018, 32, 5857–5867. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery—A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Zhang, H.; Cao, J.; Xiao, C.; Ding, M.; Chen, W.; Chen, M.; Zhang, Z. Preparation and Performance Evaluation of a Branched Functional Polymer for Heavy Oil Recovery. J. Mol. Liq. 2022, 363, 119808. [Google Scholar] [CrossRef]
- Chen, G.; Lin, J.; Hu, W.; Cheng, C.; Gu, X.; Du, W.; Zhang, J.; Qu, C. Characteristics of a crude oil composition and its in situ waxing inhibition behavior. Fuel 2018, 218, 213–217. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.; Zhang, F.; Gu, X.; Du, W.; Zhang, J.; Li, J.; Qu, C. Application of polymethacrylate from waste organic glass as a pour point depressor in heavy crude oil. J. Pet. Sci. Eng. 2018, 165, 1049–1053. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.; Bai, Y.; Zhao, W.; Zhang, J.; Wu, Y.; Gu, X.; Chen, S.; Yu, H. Synthesis of pour point depressant for heavy oil from waste organic glass. Pet. Chem. 2018, 58, 85–88. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Dong, S.; Zhang, J.; Chen, G. Synthesis of aluminium alkylbenzene sulfonate and its behavior as a flow improver for crude oil. Tenside Surfactants Deterg. 2022, 59, 353–361. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Z.; Shi, X.; Zhang, X.; Dong, S.; Zhang, J. Synthesis of alkylbenzenesulfonate and its behavior as flow improver in crude oil. Fuel 2021, 288, 119644. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Zhang, F.; Zhang, X.; Dong, S.; Zhang, J.; Gang, C. Synthesis of barium alkylbenzene sulfonate and its behavior as a flow improver for crude oil. Comptes Rendus Chim. 2021, 24, 83–89. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Yu, T.; Li, Y.; Struhárová, A.; Matejdes, M.; Slaný, M.; Chen, G. The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils. Molecules 2023, 28, 2651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Q.; Li, Y.; Wu, Y.; Li, Q.; Chen, G. Study on the Viscosity Reduction Effect and Mechanism of Aquathermolysis of Heavy Oil Catalyzed by Clay-Supported Mn(II) Complex. React. Kinet. Mech. Catal. 2023, 136, 823–835. [Google Scholar] [CrossRef]
- Wang, P.; Gu, X.; Xue, M.; Li, Y.; Dong, S.; Chen, G.; Zhang, J. Resource utilization of medical waste under COVID-19: Waste mask used as crude oil fluidity improver. J. Clean. Prod. 2022, 358, 131903. [Google Scholar] [CrossRef]
- Mao, J.; Kang, Z.; Yang, X.; Lin, C.; Zheng, L.; Zuo, M.; Mao, J.; Dai, S.; Xue, J.; Ouyang, D. Synthesis and performance evaluation of a nanocomposite pour-point depressant and viscosity reducer for high-pour-point heavy oil. Energy Fuels 2020, 34, 7965–7973. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, J.; Lv, X.; Yu, P.; Ding, X. Preparation and properties of modified nano-montmorillonite viscosity reducers. J. Dispers. Sci. Technol. 2023, 45, 151–160. [Google Scholar] [CrossRef]
- Liang, L.; Wang, L.; Nguyen, A.V.; Xie, G. Heterocoagulation of alumina and quartz studied by zeta potential distribution and particle size distribution measurements. Powder Technol. 2017, 309, 1–12. [Google Scholar] [CrossRef]
- Slaný, M.; Kuzielová, E.; Žemlička, M.; Matejdes, M.; Struhárová, A.; Palou, M.T. Metabentonite and metakaolin-based geopolymers/zeolites: Relation between kind of clay, calcination temperature and concentration of alkaline activator. J. Therm. Anal. Calorim. 2023, 148, 10531–10547. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, S.; Zhou, H.; Li, Q.; Yang, J. Cis-9-Octadecenylamine Modified Ferric Oxide and Ferric Hydroxide for Catalytic Viscosity Reduction of Heavy Crude Oil. Fuel 2022, 322, 124159. [Google Scholar] [CrossRef]
- Saulick, Y.; Lourenço, S.D.N.; Baudet, B.A. A semi-automated technique for repeatable and reproducible contact angle measurements in granular materials using the sessile drop method. Soil Sci. Soc. Am. J. 2017, 81, 241–249. [Google Scholar] [CrossRef]
- Marmur, A. Soft contact: Measurement and interpretation of contact angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Xie, Q.; Zhu, H.; Xu, W.; Liu, Z. Mechanism analysis of heavy oil viscosity reduction by ultrasound and viscosity reducers based on molecular dynamics simulation. ACS Omega 2022, 7, 36137–36149. [Google Scholar] [CrossRef]
- He, L.; Dai, Y.; Hou, J.; Gao, Y.; Zhang, D.; Cui, J.; Zhang, J.; Zhu, H.; Shen, J. MXene Based Immobilized Microorganism for Chemical Oxygen Demand Reduction of Oilfield Wastewater and Heavy Oil Viscosity Reduction to Enhance Recovery. J. Environ. Chem. Eng. 2023, 11, 109376. [Google Scholar] [CrossRef]
- Li, N.; Ma, H.; Wang, T.; Sun, C.; Xia, S. Effect of Molecular Weight on the Properties of Water-Soluble Terpolymers for Heavy Oil Viscosity Reduction. J. Taiwan Inst. Chem. Eng. 2023, 144, 104738. [Google Scholar] [CrossRef]



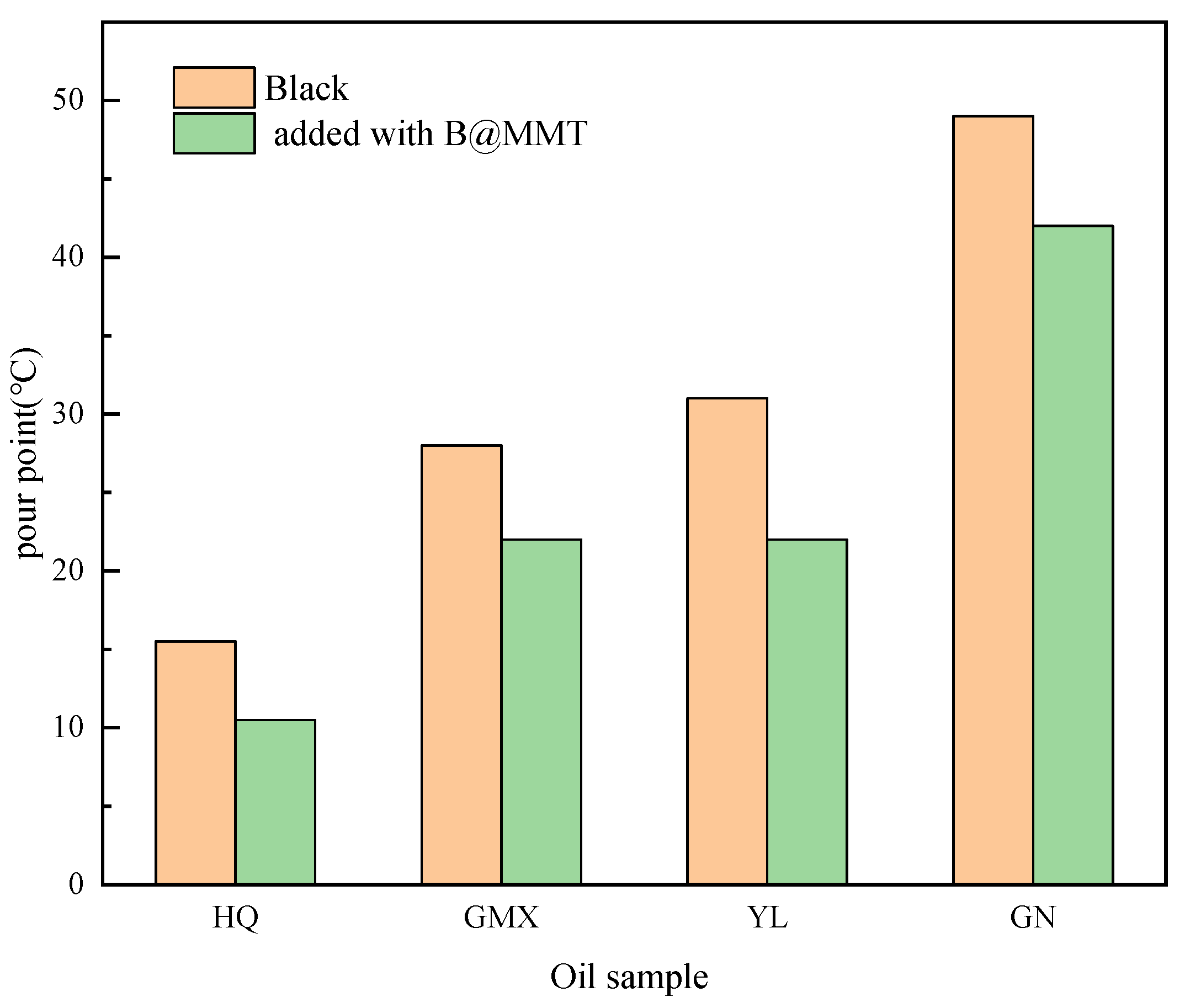
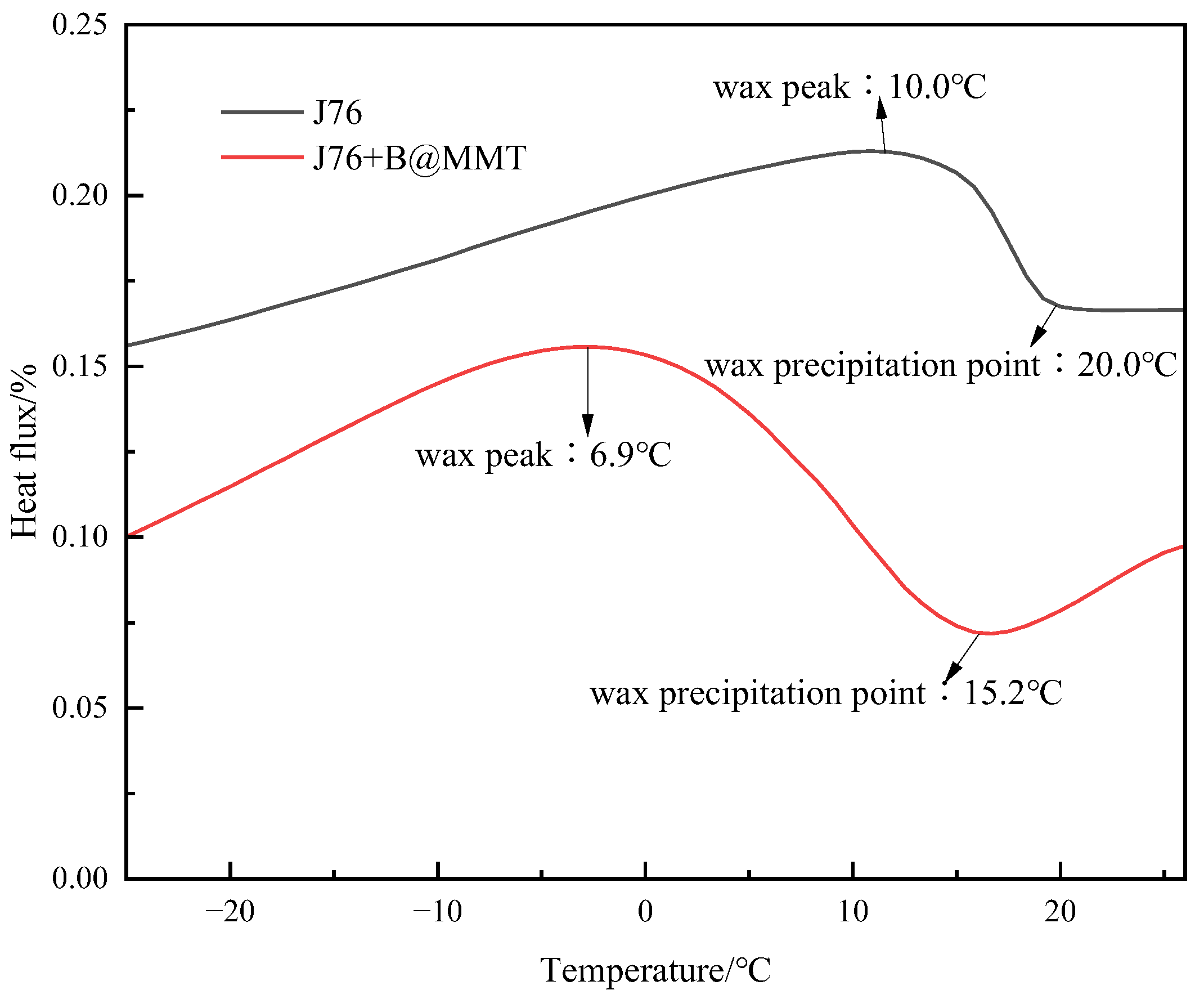
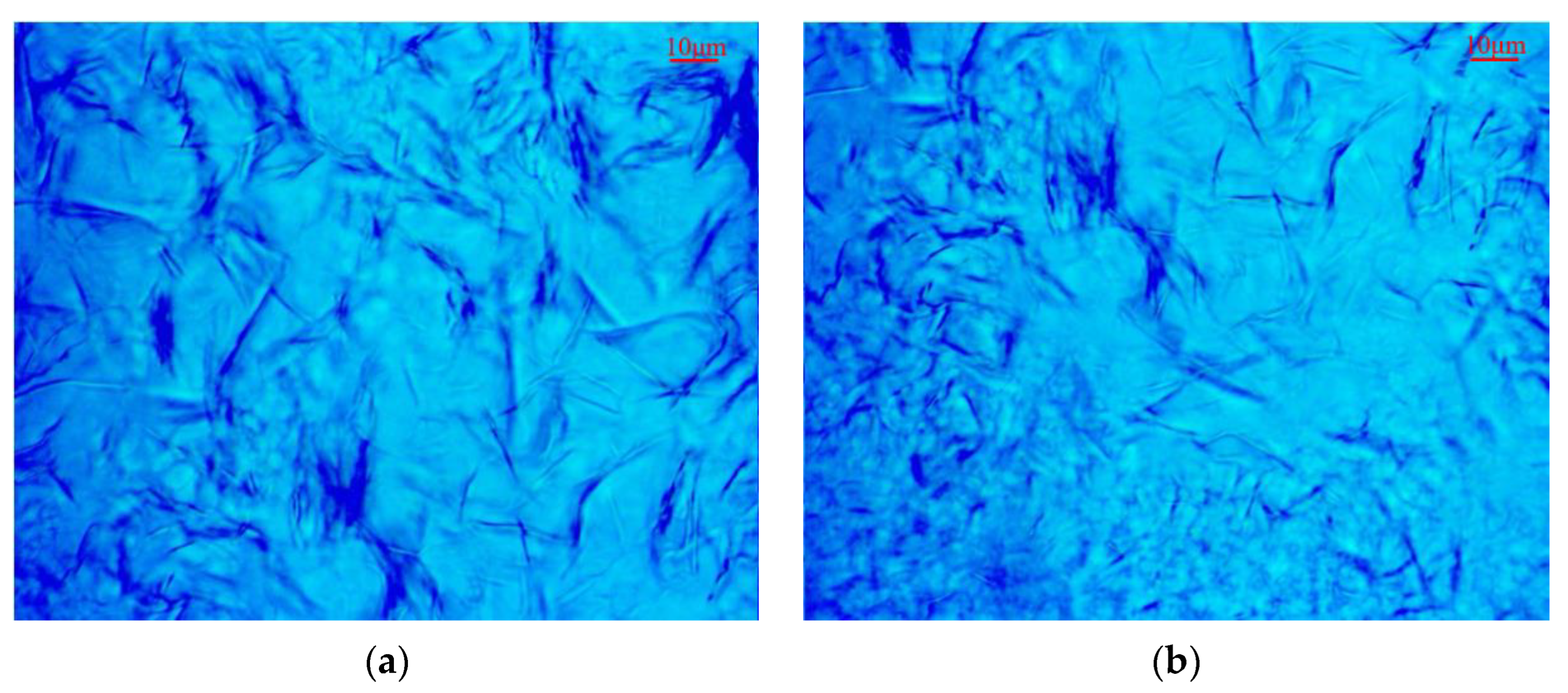
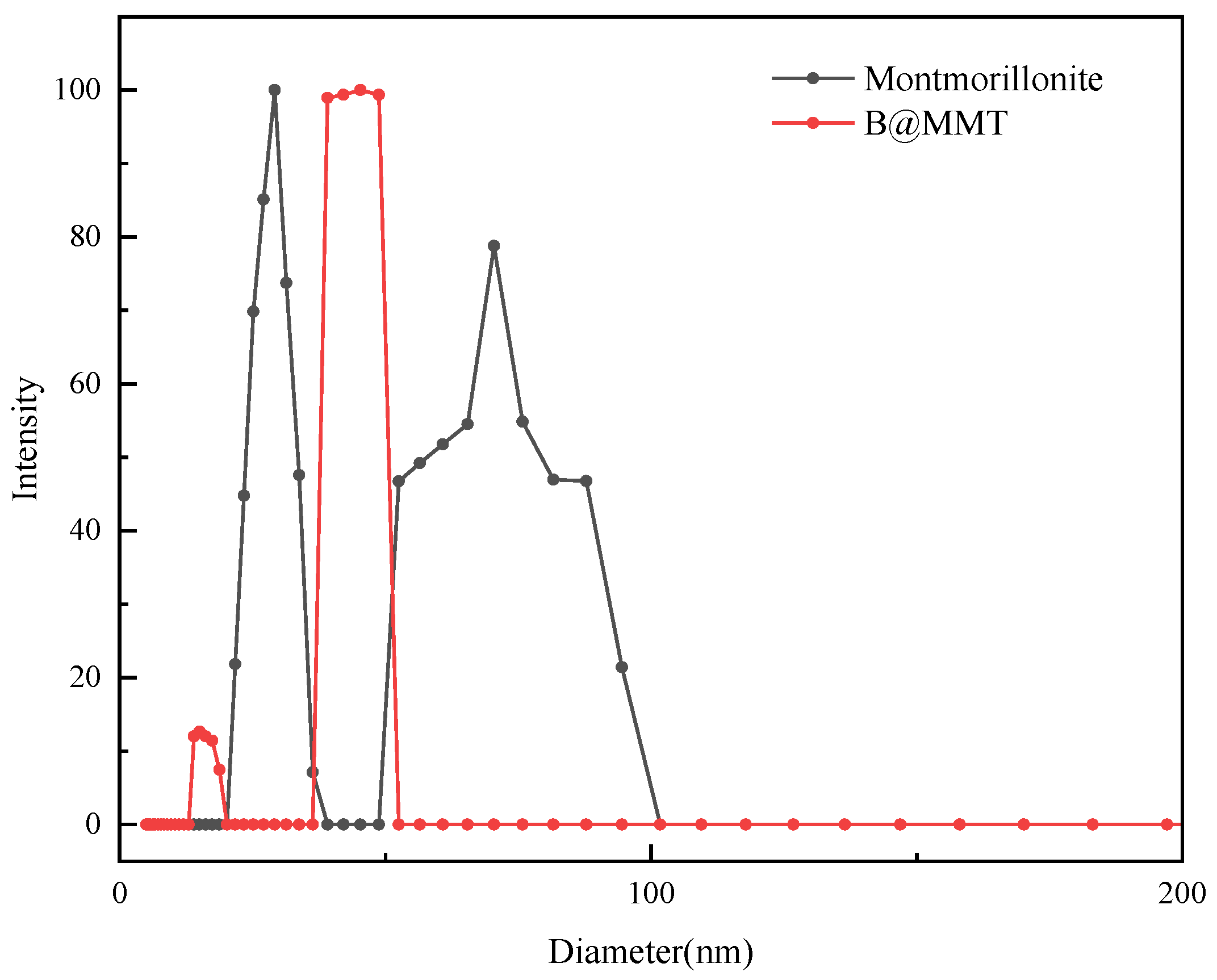


| Crude Oil | Pour Point /°C | Saturated HC /% | Aromatic HC /% | Asphaltene /% | Resin /% |
|---|---|---|---|---|---|
| J76 | 20.5 ± 0.25 | 49.52 ± 1.00 | 31.43 ± 1.00 | 7.23 ± 0.25 | 11.82 ± 0.25 |
| GN | 49.0 ± 0.25 | 62.45 ± 1.50 | 20.12 ± 0.50 | 10.41 ± 0.25 | 7.02 ± 0.25 |
| YL | 31.0 ± 0.25 | 45.63 ± 1.00 | 33.24 ± 1.00 | 8.31 ± 0.25 | 12.82 ± 0.25 |
| GMX | 28.0 ± 0.25 | 31.16 ± 1.00 | 28.73 ± 0.50 | 23.44 ± 0.50 | 16.67 ± 0.25 |
| HQ | 15.5 ± 0.25 | 30.05 ± 1.00 | 21.15 ± 0.50 | 30.17 ± 1.00 | 18.63 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Slaný, M.; Golian-Struhárová, A.; Wang, H.; Zhang, L.; Fu, J.; Chen, G.; Du, Y. Surface-Functionalized Nano-Montmorillonite and Its Application as Crude Oil Flow Improver. Minerals 2024, 14, 696. https://doi.org/10.3390/min14070696
Liu K, Slaný M, Golian-Struhárová A, Wang H, Zhang L, Fu J, Chen G, Du Y. Surface-Functionalized Nano-Montmorillonite and Its Application as Crude Oil Flow Improver. Minerals. 2024; 14(7):696. https://doi.org/10.3390/min14070696
Chicago/Turabian StyleLiu, Kechen, Michal Slaný, Alena Golian-Struhárová, Hailong Wang, Liyuan Zhang, Jiyou Fu, Gang Chen, and Yingna Du. 2024. "Surface-Functionalized Nano-Montmorillonite and Its Application as Crude Oil Flow Improver" Minerals 14, no. 7: 696. https://doi.org/10.3390/min14070696






