Chemical and Physical Denudation Rates in the Poços de Caldas Alkaline Massif, Minas Gerais State, Brazil
Abstract
1. Introduction
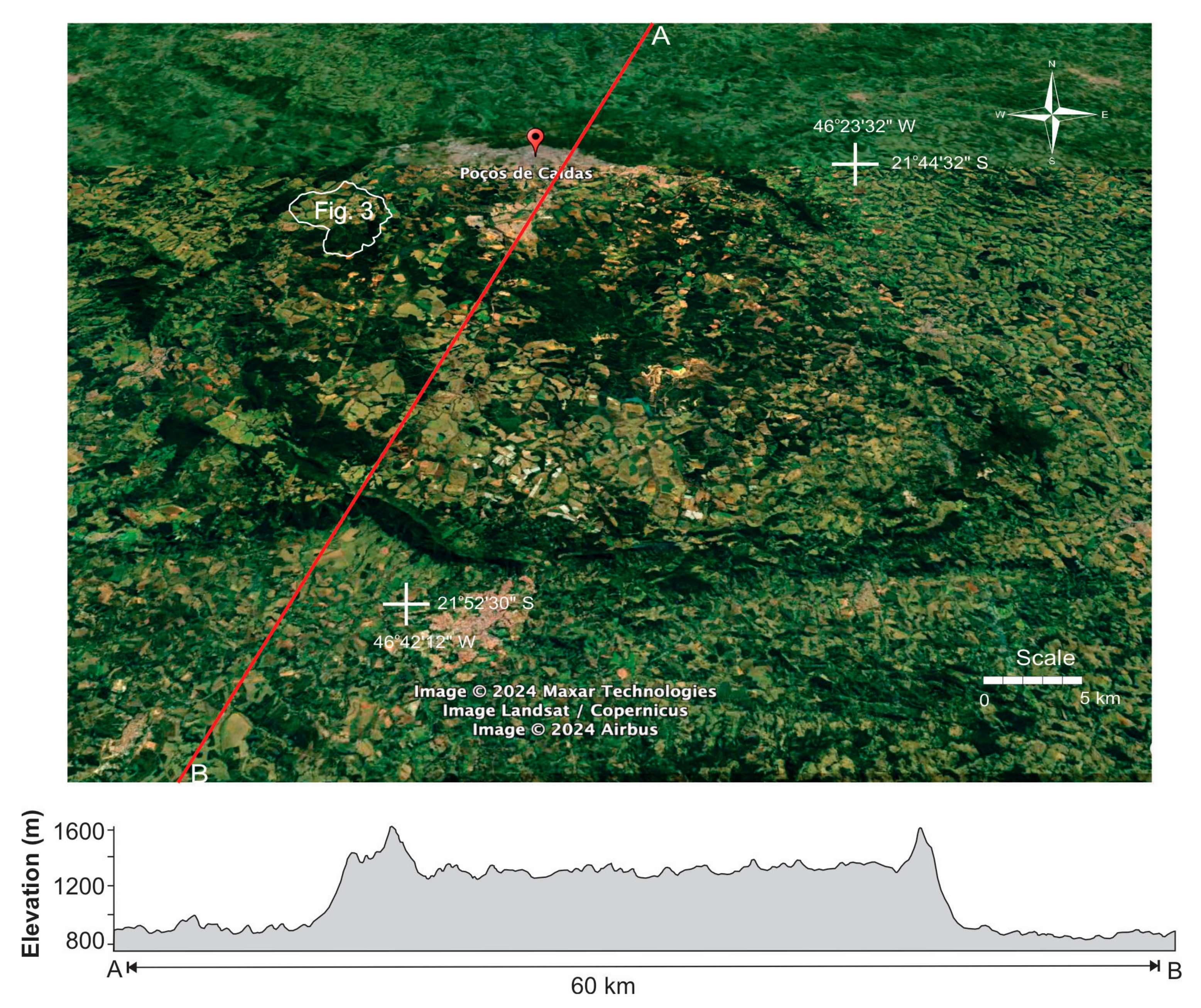
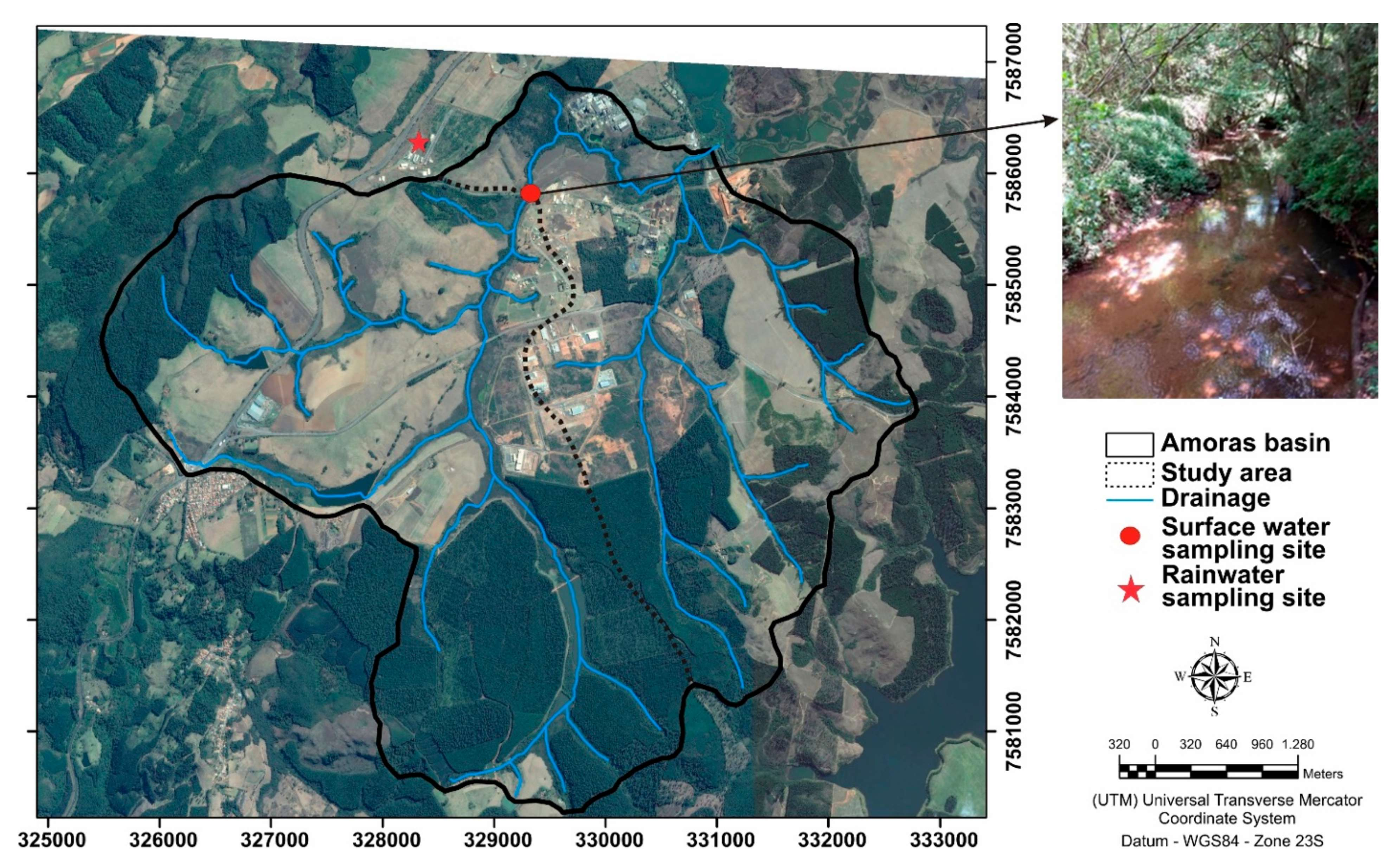
2. Study Area
3. Sampling and Analytical Techniques
4. Results
4.1. Surface Waters
4.2. Rainwaters
5. Discussion
5.1. Natural and Anthropogenic Inputs into Annual Fluxes of Dissolved Elements
5.2. Chemical Denudation Rate in the Poços de Caldas Alkaline Massif
5.3. Physical Denudation Rate in the Poços de Caldas Alkaline Massif
6. Conclusions
- The concentration of dissolved cations, anions, and silica in surface waters increased during the dry period in relation to the wet period. The same behavior is observed for pH, EC, temperature, TDS, and TSS. The relationship between the [TDS] and the Q was inverse, representing the chemostatic behavior. The [TSS] was directly related to the discharge, indicating an enrichment behavior. The higher [TSS] occurring in the wet period is due to soil erosion after rainfall events.
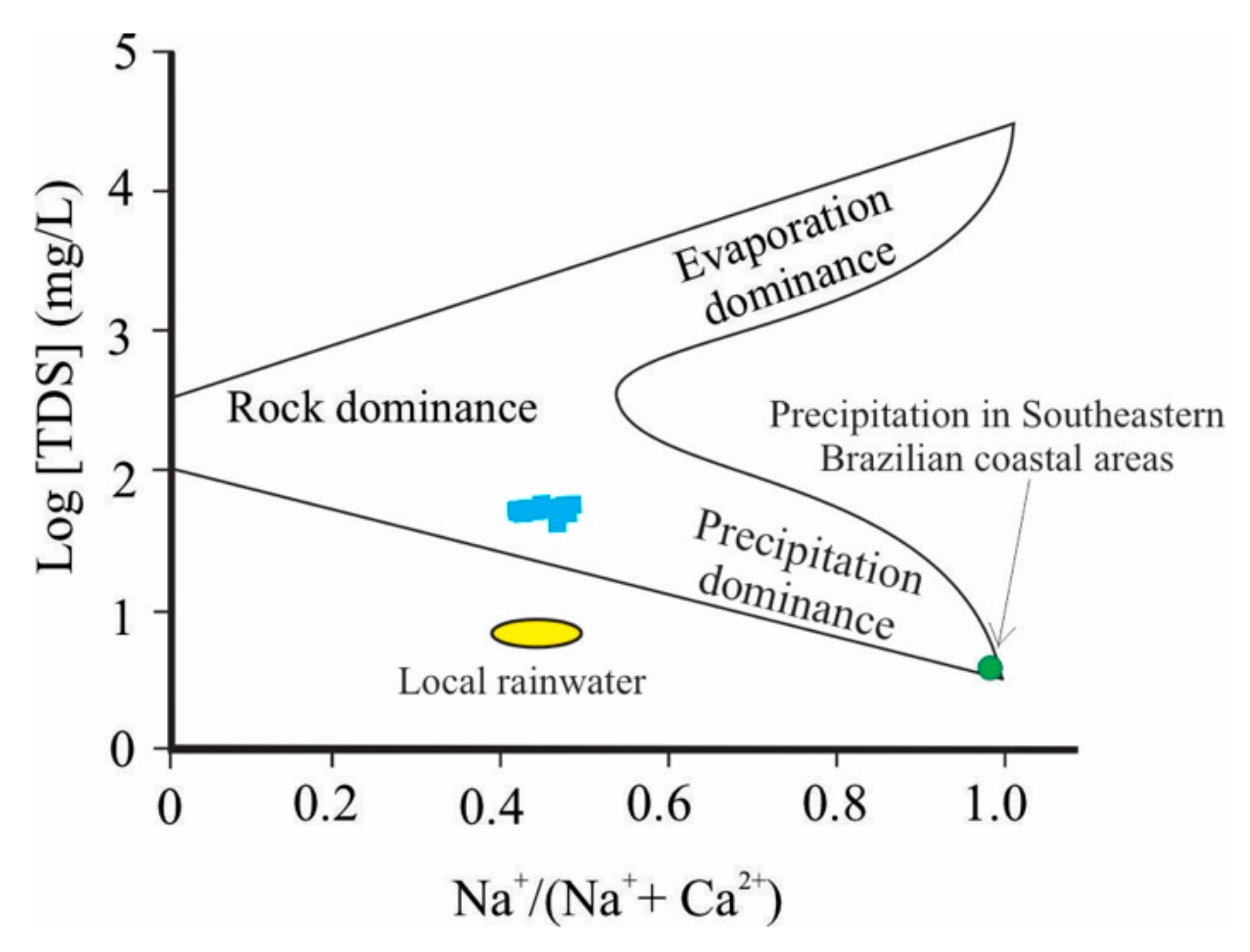
- The pH values in rainwaters vary between 5.1 and 6.4, with a weighted average of 5.7, indicating that the pH values are close to the “clean” rainwater (5.6) due to the partial solubilization of carbon dioxide to form carbonic acid (H2O + CO2 → H2CO3). Na+ and HCO3− are responsible for 40% and 60% of the total cation and anion sum, respectively, suggesting that they are the most abundant ions in the rainwater. The chemical composition of rainwater samples should be less influenced by sea salt than rainwater sampled from coastal areas, due to the distance of PC from the Atlantic Ocean.
- Positive mass balance values are found for Na+, K+, Mg2+, Ca2+, SiO2, HCO3−, TDS, and TSS in the PC. The RE of 2.47 reveals that the chemical weathering is the main water/rock interactions occurring in the PC under tropical climate, with partial removal of SiO2 from the profile and complete leaching of Na+, K+, Mg2+, and Ca2+. Hydrolysis of orthoclase and sanidine forms kaolinite. Silicate incongruent dissolution (nefeline, aegerine, augirine–augite, and phogopite) forms kaolinite and goethite. The Fw values that are negative or close to 0 indicate that annual fluxes for Al3+, Cl−, NO3−, and SO42− are associated with atmospheric pollution (aluminum production chain and burnt fossil fuel from vehicles).
- The Fw value due to [TDS] was 26 t/km2/a, a similar value to those obtained for the Paraná CBF province and Tapira and Catalão I alkaline-carbonatite rocks, areas located under tropical climate in Brazil. The FCO2 associated with chemical weathering was 1.6 × 105 mol/km2/a in the PC, also similar to the FCO2 world continental average. The chemical weathering rate was 4 ± 0.8 m/Ma, this rate being practically similar to the chemical denudation rate obtained for the Paraná CFB province and Tapira and Catalão I alkaline-carbonatite rocks.
- The Fw value associated with soil removal was 6 t/km2/a or 16 kg/km2/day, which can be classified as low daily TSS flux due to flat relief occurring within the PC. The physical denudation rate was 3.0 ± 0.6 m/Ma in the PC. The difference between the chemical and physical denudation rates indicated that under the current climatic condition, the weathering profile is in dynamic equilibrium. The LULCC are responsible for the increase in the soil removal in the PC, showing clearly that the human–landscape system affects physical denudation in the PC.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. The global control of silicate weathering rates and the coupling with physical erosion: New insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 2002, 196, 83–98. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, P.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Dupré, B.; Dessert, C.; Oliva, P.; Goddéris, Y.; Viers, J.; François, L.; Millot, R.; Gaillardet, J. Rivers, chemical weathering and Earth’s climate. C. R. Geosci. 2003, 335, 1141–1160. [Google Scholar] [CrossRef]
- Lerman, A.; Wu, L.; Mackenzie, F.T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in global carbon balance. Mar. Chem. 2007, 106, 326–350. [Google Scholar] [CrossRef]
- Johnson, N.M.; Likens, G.E.; Bormann, F.H.; Pierce, P.S. Rate of chemical weathering of silicate minerals in New Hampshire. Geochim. Cosmochim. Acta 1968, 32, 531–545. [Google Scholar] [CrossRef]
- Tardy, Y. Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs. Chem. Geol. 1971, 7, 253–271. [Google Scholar] [CrossRef]
- Probst, J.L. Dissolved and suspended matter transported by the Girou River (France): Mechanical and chemical erosion rates in a calcareous molasse basin. Hydrolog. Sci. J. 1986, 31, 61–79. [Google Scholar] [CrossRef][Green Version]
- Kattan, Z.; Gac, J.I.; Probst, J.L. Suspended sediment load and mechanical erosion in the Senegal Basin—Estimation of the surface runoff concentration and relative contributions of channel and slope erosion. J. Hydrol. 1987, 92, 59–76. [Google Scholar] [CrossRef]
- Probst, J.L.; Nkounkou, R.R.; Krempp, G.; Bricquet, J.P.; Thiébaux, J.P.; Olivry, J.C. Dissolved major elements exported by the Congo and the Ubangui rivers during the period 1987–1989. J. Hydrol. 1992, 135, 237–257. [Google Scholar] [CrossRef]
- Amiotte-Suchet, P.; Probst, J.L. Modelling of atmospheric CO2 consumption by chemical weathering of rocks: Application to the Garonne, Congo and Amazon basins. Chem. Geol. 1993, 107, 205–210. [Google Scholar] [CrossRef]
- Probst, J.L.; Mortatti, J.; Tardy, Y. Carbon river fluxes and weathering CO2 consumption in the Congo and Amazon rivers basins. Appl. Geochem. 1994, 9, 1–13. [Google Scholar] [CrossRef]
- White, A.F.; Blum, A.E. Effects of climate on chemical weathering in watersheds. Geochim. Cosmochim. Acta 1995, 59, 1729–1747. [Google Scholar] [CrossRef]
- Louvat, P.; Allègre, C.J. Present denudation rates on the island of Réunion determined by river geochemistry: Basalt weathering and mass budget between chemical and mechanical erosions. Geochim. Cosmochim. Acta 1997, 61, 3645–3669. [Google Scholar] [CrossRef]
- Louvat, P.; Allègre, C.J. Riverine erosion rates on Sao Miguel volcanic island, Azores archipelago. Chem. Geol. 1998, 148, 177–200. [Google Scholar] [CrossRef]
- Boeglin, J.L.; Probst, J.L. Physical and chemical weathering rates and CO2 consumption in a tropical lateritic environment: The upper Niger basin. Chem. Geol. 1998, 148, 137–156. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Braun, J.J.; Deberdt, S.; Angeletti, B.; Ngoupayou, J.N.; Michard, A. Major and trace element abundances, and strontium isotopes in the Nyong basin rivers (Cameroon): Constraints on chemical weathering processes and elements transport mechanisms in humid tropical environments. Chem. Geol. 2000, 169, 211–241. [Google Scholar] [CrossRef]
- Dessert, C.; Dupré, B.; François, L.M.; Schott, J.; Gaillardet, J.; Chakrapani, G.; Bajpai, S. Erosion of Deccan Traps determined by river geochemistry: Impact on the global climate and the 87Sr/86Sr ratio of seawater. Earth Planet. Sci. Lett. 2001, 188, 459–474. [Google Scholar] [CrossRef]
- West, A.J.; Bickle, M.J.; Collins, R.; Brasington, J. A small catchment perspective on Himalayan weathering fluxes. Geology 2002, 30, 355–358. [Google Scholar] [CrossRef]
- Dessert, C.; Dupré, B.; Gaillardet, J.; François, L.M.; Allègre, C.J. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 2003, 202, 257–273. [Google Scholar] [CrossRef]
- Oliva, P.; Viers, J.; Dupré, B. Chemical weathering in granitic environments. Chem. Geol. 2003, 202, 225–256. [Google Scholar] [CrossRef]
- Gaillardet, J.; Millot, R.; Dupré, B. Chemical denudation rates of the western Canadian orogenic belt: The Stikine terrane. Chem. Geol. 2003, 201, 257–279. [Google Scholar] [CrossRef]
- West, A.J.; Galy, A.; Bickle, M. Tectonic and climatic controls on silicate weathering. Earth Planet. Sci. Lett. 2005, 235, 211–228. [Google Scholar] [CrossRef]
- Louvat, P.; Gislason, S.R.; Allègre, C.J. Chemical and mechanical erosion rates in Iceland as deduced from river dissolved and solid material. Am. J. Sci. 2008, 308, 679–726. [Google Scholar] [CrossRef]
- Gibbs, R.J. The geochemistry of the Amazon River System. Part 1. The factors that control the salinity and the composition and concentration of suspended solids. Geol. Soc. Am. Bull. 1967, 78, 1203–1232. [Google Scholar] [CrossRef]
- Moreira-Nordemann, L.M. Use of 234U/238U disequilibrium in measuring chemical weathering rate of rocks. Geochim. Cosmochim. Acta 1980, 44, 103–108. [Google Scholar] [CrossRef][Green Version]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon Basin. 2. The influence of geology and weathering environment on the dissolved load. J. Geophys. Res. 1983, 88, 9671–9688. [Google Scholar] [CrossRef]
- Moreira-Nordemann, L.M. Salinity and weathering rate of rocks in a semi-arid region. J. Hydrol. 1984, 71, 131–147. [Google Scholar] [CrossRef]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon Basin. 3. Weathering chemistry and limits to dissolved inputs. J. Geophys. Res. 1987, 92, 8293–8302. [Google Scholar] [CrossRef]
- Freyssinet, P.; Farah, A.S. Geochemical mass balance and weathering rates of ultramafic schists in Amazonia. Chem. Geol. 2000, 170, 133–151. [Google Scholar] [CrossRef]
- Mortatti, J.; Probst, J.L. Silicate rock weathering and atmospheric/soil CO2 uptake in the Amazon basin estimated from river water geochemistry: Seasonal and spatial variations. Chem. Geol. 2003, 197, 177–196. [Google Scholar] [CrossRef]
- Conceição, F.T.; Bonotto, D.M. Use of U-isotopes disequilibrium to evaluate the weathering rates and fertilizer derived uranium at São Paulo State, Brazil. Environ. Geol. 2003, 44, 408–418. [Google Scholar] [CrossRef]
- Conceição, F.T.; Bonotto, D.M. Weathering rates and anthropogenic influences in a sedimentary basin, São Paulo State, Brazil. Appl. Geochem. 2004, 19, 575–591. [Google Scholar] [CrossRef]
- Sardinha, D.S.; Bonotto, D.M.; Conceição, F.T. Weathering rates at Alto Sorocaba basin, Brazil, using U-isotopes and major cations. Environ. Earth Sci. 2010, 61, 1025–1036. [Google Scholar] [CrossRef]
- Conceição, F.T.; Sardinha, D.S.; Souza, A.D.G.; Navarro, G.R.B. Anthropogenic influences on annual flux of cations and anions at Meio Stream basin, São Paulo State, Brazil. Water Air Soil Pollut. 2010, 205, 79–91. [Google Scholar] [CrossRef]
- Conceição, F.T.; Santos, C.M.; Sardinha, D.S.; Navarro, G.R.B.; Godoy, L.H. Chemical weathering rate, denudation rate, and atmospheric and soil CO2 consumption of Paraná flood basalts in São Paulo state, Brazil. Geomorphology 2015, 233, 41–51. [Google Scholar] [CrossRef]
- Horbe, A.M.C.; Lages, A.S.; Moquet, J.S.; Santos, R.V.; Seyler, P.; Cochonneau, G. Geochemistry of organic-rich river waters in Amazonia: Insights on weathering processes of intertropical cratonic terrain. Appl. Geochem. 2016, 65, 22–35. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Conceição, F.T.; Spatti, E.P., Jr.; Sardinha, D.S.; Mortatti, J. Chemical weathering rates and atmospheric/soil CO2 consumption of igneous and metamorphic rocks under tropical climate in southeastern Brazil. Chem. Geol. 2016, 443, 54–66. [Google Scholar] [CrossRef]
- Spatti Júnior, E.P.; Conceição, F.T.; Fernandes, A.M.; Sardinha, D.S.; Moruzzi, R.B. Chemical weathering rates of clastic sedimentary rocks from the Paraná Basin in the Paulista Peripheral Depression, Brazil. J. South Am. Earth Sci. 2019, 96, 102369. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Conceição, F.T.; Spatti Júnior, E.P.; Couto Júnior, A.A.; Hissler, C.; Mortatti, J. Human influences on the present denudation rates of the Paulista Peripheral Depression, Brazil. Geomorphology 2020, 351, 106955. [Google Scholar] [CrossRef]
- Conceição, F.T.; Vasconcelos, P.M.; Godoy, L.H.; Navarro, G.R.B.; Montibeller, C.C.; Sardinha, D.S. Water/rock interactions, chemical weathering and erosion, and supergene enrichment in the Tapira and Catalão I alkaline-carbonatite complexes, Brazil. J. Geochem. Explor. 2022, 237, 106999. [Google Scholar] [CrossRef]
- Comin-Chiaramonti, P.; Gomes, C.B. Mesozoic to Cenozoic Alkaline Magmatism in the Brazilian Platform; Editora da Universidade de São Paulo: São Paulo, Brazil, 2005. [Google Scholar]
- Ulbrich, H.H.G.J.; Gomes, C.B. Alkaline rocks from continental Brazil. Earth Sci. Rev. 1981, 17, 135–154. [Google Scholar] [CrossRef]
- Valeton, I.; Schumann, A.; Vinx, R.; Wieneke, M. Supergene alteration since the upper cretaceous on alkaline igneous and metasomatic rocks of the Poços de Caldas ring complex, Minas Gerais, Brazil. Appl. Geochem. 1997, 12, 133–154. [Google Scholar] [CrossRef]
- Holmwe, D.C.; Pitty, A.E.; Noy, D.J. Geomorphological and hydrogeological features of the Poços de Caldas caldera analogue study sites. J. Geochem. Explor. 1992, 45, 215–247. [Google Scholar] [CrossRef]
- Departamento Nacional de Produíão Mineral (DNPM). Sumário Mineral—2016; Departamento Nacional de Produção Mineral (DNPM): Brasília, Brazil, 2018. [Google Scholar]
- Köppen, W. Climatologia; Fondo de Cultura Econômica: Mexico City, Mexico, 1948. [Google Scholar]
- Antunes, M.L.P.; Couperthwaite, S.J.; Conceição, F.T.; Jesus, C.P.C.; Kiyohara, P.K.; Coelho, A.C.V.; Frost, R.L. Red mud from Brazil: Thermal behavior and physical properties. Ind. Eng. Chem. Res. 2012, 51, 775–779. [Google Scholar] [CrossRef]
- Sardinha, D.S.; Godoy, L.H.; Conceição, F.T.; Spatti Júnior, E.P.; Fernandes, A.M.; Victal, F.A.C.A.; Costa, D.A.T. Geoquímica fluvial e balanço de denudação em Tinguaítos de Poço de Caldas, Minas Gerais. Geologia USP 2018, 18, 259–272. [Google Scholar] [CrossRef][Green Version]
- Edmond, J.M. High precision determination of titration alkalinity and total carbon dioxide content of seawater by potentiometric titration. Deep-Sea Res. I Oceanogr. Res. Pap. 1970, 17, 737–750. [Google Scholar] [CrossRef]
- Mosello, R.; Bianchi, M.; Geiss, H.; Marchetto, A.; Serrini, G.; Serini Lanza, G.; Tartari, G.A.; Muntau, H. AQUACON-MedBas Subproject No. 6: Acid Rain Analysis; Environment Institute: Luxembourg, 1996. [Google Scholar]
- Godsey, S.E.; Kirchner, J.W.; Clow, D.W. Concentration-discharge relationship reflect chemostatic characteristics of US catchments. Hydrol. Process. 2009, 23, 1844–1864. [Google Scholar] [CrossRef]
- Zhong, J.; Li, S.; Ibarra, D.E.; Ding, H.; Liu, C. Solute production and transport processes in Chinese monsoonal rivers: Implications for global climate change. Global Biogeochem. Cycles 2020, 34, e2020GB006541. [Google Scholar] [CrossRef]
- Conceição, F.T.; Fernandes, A.M.; Moruzzi, R.B.; Mortatti, J. Dynamics of dissolved inorganic carbon (DIC) and stable C isotope ratios (δ13CDIC) in a tropical watershed with diversified land use in São Paulo State, Brazil. Sci. Total Environ. 2024, 933, 173071. [Google Scholar] [CrossRef] [PubMed]
- Walling, D.E.; Fang, D. Recent trends in the suspended sediment loads of the world’s rivers. Glob. Planet. Change 2003, 39, 111–126. [Google Scholar] [CrossRef]
- Berner, E.K.; Berner, R.A. The Global Water Cycle: Geochemistry and Environment; Prentice-Hall: Englewood Cliffs, NJ, USA, 1987. [Google Scholar]
- Moon, S.; Chamberlain, C.P.; Hilley, G.E. New estimates of silicate weathering rates and their uncertainties in global rivers. Geochim. Cosmochim. Acta 2014, 134, 257–274. [Google Scholar] [CrossRef]
- Sardinha, D.S.; Conceição, F.T.; Souza, A.D.G.; Silveira, A.; de Julio, M.; Gonçalves, J.C.S.I. Evaluation of the water quality and auto-purification from the Meio Stream, Leme (SP). Eng. Sanit. Ambient. 2008, 13, 329–338. [Google Scholar] [CrossRef]
- Conceição, F.T.; Sardinha, D.S.; Souza, A.D.G.; Bonotto, D.M. Hydrochemical relationship at Meio Stream watershed (Leme city), São Paulo State, Brazil. Rev. Bras. Geociênc. 2007, 37, 389–400. [Google Scholar] [CrossRef][Green Version]
- Galloway, J.N.; Likens, G.E.; Keene, W.C.; Miller, J.M. The composition of precipitation in remote areas of the world. J. Geophys. Res. 1982, 87, 8771–8786. [Google Scholar] [CrossRef]
- Souza, P.A.; Mello, W.Z.; Maldonado, J.; Evangelista, H. Composição química da chuva e aporte atmosférico na Ilha Grande, RJ. Quím. Nova 2006, 29, 471–476. [Google Scholar] [CrossRef]
- Mello, W.Z. Precipitation chemistry in the coast of the Metropolitan Region of Rio de Janeiro, Brazil. Environ. Pollut. 2001, 114, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Danelon, O.M.; Moreira-Nordemann, L.M. Ocorrência natural e antropogênica de Cl−, Na+, NO3−, NH4+ E SO42+ na Bacia do Rio Quilombo—(Cubatão—SP). Rev. Bras. Geociênc. 1991, 21, 96–101. [Google Scholar] [CrossRef]
- Conceição, F.T.; Sardinha, D.S.; Navarro, G.R.B.; Antunes, M.L.P.; Angelucci, V.A. Rainwater chemical composition and annual atmospheric deposition at Alto Sorocaba basin. Quím. Nova 2011, 34, 610–616. [Google Scholar] [CrossRef]
- Conceição, F.T.; Litholdo, T.; Sardinha, D.S.; Moruzzi, R.B.; Navarro, G.R.B.; Godoy, L.H. The influence of phosphate mining on the chemical composition of annual atmospheric deposition in Catalão (GO) and Tapira (MG). Water Air Soil Pollut. 2016, 227, 69. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world river water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.G.S.M. Análise Comparativa dos Impactos Ambientais e dos Aspectos Tecnológicos da Produção de Alumínio primário em Minas Gerais. Master’s Thesis, Instituto de Ciências Exatas e Biológicas, Universidade Federal de Ouro Preto, Ouro Preto, Brazil, 2011. [Google Scholar]
- Cronan, C.S. Major Cations (Ca, Mg, Na, K, Al). In Encyclopedia of Inland Waters; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 45–51. [Google Scholar] [CrossRef]
- CETESB. Relatório de Atendimento a Mortandade de Peixes—Represa Bortolan—Poços de Caldas, MG—DMAE; CETESB: São Paulo, Brazil, 1985. [Google Scholar]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Meybeck, M.; Laroche, L.; Dürr, H.H.; Syvitski, J.P.M. Global variability of daily total suspended solids and their fluxes in rivers. Glob. Planet. Change 2003, 39, 65–93. [Google Scholar] [CrossRef]
- Doranti-Tiritan, C.; Hackspacher, P.C.; Ribeiro, M.C.S.; Glasmacher, U.A.; Souza, D.H. Evolução do relevo da região de planalto de Poços de Caldas (SP/MG) baseado em dados de termocronologia de baixa temperatura e modelagem termocinemática 3D. Rev. Bras. Geomorfol. 2014, 15, 291–310. [Google Scholar] [CrossRef]
- Couto Júnior, A.A.; Conceição, F.T.; Fernades, A.M.; Spatti Júnior, E.P.; Lupinacci, C.M.; Moruzzi, R.B. Land use changes associated with the expansion of sugar cane crops and their influences on soil removal in a tropical watershed in São Paulo State (Brazil). Catena 2019, 172, 313–323. [Google Scholar] [CrossRef]
- Conceição, F.T.; Vasconcelos, P.M.; Navarro, G.R.B.; Farley, K.A. 40Ar/39Ar and (U-Th)/He constraints on emplacement, exhumation, and weathering of alkaline-carbonatite complexes in the Alto Paranaíba Igneous Province (APIP), Brazil. Gondwana Res. 2024, 130, 116–130. [Google Scholar] [CrossRef]
- Vasconcelos, P.M.; Carmo, I.O. Calibrating denudation chronology through 40Ar/39Ar weathering geochronology. Earth Sci. Rev. 2018, 179, 411–435. [Google Scholar] [CrossRef]




| Sampling | Q 1 | pH | EC 2 | T 3 | Na+ | K+ | Mg2+ | Ca2+ | Al3+ | SiO2 | HCO3− | NO3− | PO43− | Cl− | SO42− | TDS 4 | TSS 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m3/s) | (µS/cm) | (°C) | (mg/L) | ||||||||||||||
| 01/26 | 1.92 | 6.2 | 22 | 27.0 | 3.0 | 1.6 | 1.0 | 2.0 | 0.18 | 1.0 | 14.4 | 0.5 | 0.06 | 1.4 | 0.6 | 25 | 10 |
| 02/16 | 1.81 | 6.0 | 23 | 25.0 | 3.1 | 0.7 | 0.7 | 2.1 | 0.20 | 4.0 | 15.7 | 0.2 | 0.07 | 0.8 | 0.4 | 28 | 12 |
| 03/08 | 1.27 | 6.3 | 22 | 24.0 | 2.9 | 1.2 | 1.2 | 2.1 | 0.60 | 3.5 | 16.6 | 0.4 | 0.04 | 1.3 | 0.6 | 30 | 15 |
| 04/05 | 0.48 | 6.4 | 21 | 24.0 | 2.9 | 1.0 | 1.5 | 2.4 | 0.43 | 4.4 | 19.1 | 0.3 | 0.05 | 1.1 | 0.3 | 33 | 3 |
| 05/09 | 0.30 | 6.8 | 19 | 25.0 | 2.7 | 1.4 | 1.4 | 2.6 | 0.50 | 2.5 | 19.4 | 0.6 | 0.03 | 1.0 | 0.4 | 33 | 4 |
| 06/17 | 0.47 | 6.3 | 14 | 24.0 | 3.1 | 1.7 | 1.7 | 2.3 | 0.30 | 1.4 | 21.3 | 0.6 | 0.06 | 1.7 | 0.7 | 35 | 5 |
| 07/17 | 0.20 | 6.1 | 14 | 26.0 | 3.2 | 1.7 | 1.7 | 2.9 | 0.19 | 1.8 | 22.9 | 0.6 | 0.02 | 1.5 | 0.6 | 37 | 3 |
| 08/12 | 0.19 | 6.7 | 12 | 20.0 | 3.7 | 1.9 | 1.9 | 3.0 | 0.38 | 3.1 | 24.6 | 0.7 | 0.03 | 1.6 | 0.8 | 42 | 3 |
| 09/15 | 0.08 | 5.9 | 18 | 33.0 | 3.3 | 1.8 | 1.8 | 3.2 | 0.40 | 1.8 | 23.7 | 1.0 | 0,04 | 1.2 | 0.5 | 39 | 4 |
| 10/13 | 0.30 | 6.3 | 20 | 35.0 | 3.3 | 2.0 | 2.0 | 3.5 | 0.46 | 1.9 | 25.1 | 1.2 | 0.08 | 1.2 | 0.7 | 41 | 4 |
| 11/04 | 0.18 | 6.7 | 21 | 33.0 | 3.0 | 1.9 | 1.5 | 3.2 | 0.45 | 2.1 | 23.5 | 0.9 | 0.05 | 1.3 | 0.8 | 39 | 6 |
| 12/20 | 0.58 | 6.4 | 22 | 23.0 | 2.9 | 1.0 | 1.0 | 2.8 | 0.41 | 1.7 | 21.0 | 0.4 | 0.01 | 0.9 | 0.6 | 33 | 5 |
| CE | 0.65 | 6.2 | 19 | 27.1 | 3.1 | 1.5 | 1.4 | 2.7 | 0.37 | 2.4 | 20.6 | 0.6 | 0.05 | 1.2 | 0.6 | 33 | 7 |
| Sampling | P | pH | EC | Na+ | K+ | Mg2+ | Ca2+ | Al3+ | SiO2 | HCO3− | NO3− | PO43− | Cl− | SO42− | TDS | TSS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mm) | (µS/cm) | (mg/L) | ||||||||||||||
| 01/25 | 16.6 | 5.6 | 19 | 1.6 | 1.3 | 0.3 | 1.2 | 0.20 | <0.02 | 3.7 | 0.5 | 0.03 | 2.5 | 0.2 | 11 | 2 |
| 02/15 | 26.2 | 5.7 | 8 | 1.4 | 0.1 | 0.1 | 1.0 | 0.21 | <0.02 | 3.2 | 0.3 | 0.04 | 0.4 | 0.2 | 7 | 2 |
| 02/17 | 8.2 | 5.3 | 9 | 1.2 | 0.9 | 0.1 | 0.7 | 0.18 | <0.02 | 3.3 | 0.8 | 0.02 | 0.5 | 0.4 | 8 | 1 |
| 02/21 | 22.6 | 5.7 | 6 | 0.7 | 0.4 | 0.1 | 1.0 | 0.17 | <0.02 | 2.8 | 0.5 | 0.04 | 0.3 | 0.2 | 6 | <1 |
| 02/23 | 18.6 | 5.8 | 5 | 1.4 | 0.2 | 0.1 | 0.9 | 0.15 | <0.02 | 3.8 | 0.4 | 0.04 | 0.3 | 0.1 | 8 | <1 |
| 02/27 | 9.4 | 5.6 | 20 | 1.4 | 1.7 | 0.2 | 1.4 | 0.18 | <0.02 | 3.6 | 0.6 | 0.02 | 2.3 | 0.2 | 12 | <1 |
| 03/03 | 52.6 | 5.6 | 5 | 0.9 | 0.9 | 0.1 | 0.8 | 0.20 | <0.02 | 1.2 | 0.5 | 0.03 | 1.0 | 0.4 | 6 | <1 |
| 03/10 | 8.6 | 6.2 | 8 | 1.2 | 0.8 | 0.2 | 1.0 | 0.18 | <0.02 | 3.3 | 0.2 | 0.01 | 1.0 | 0.7 | 8 | <1 |
| 03/16 | 27.4 | 5.9 | 5 | 0.9 | 0.4 | 0.1 | 0.6 | 0.21 | <0.02 | 2.4 | 0.3 | 0.03 | 0.5 | 0.1 | 6 | <1 |
| 05/31 | 24.4 | 5.8 | 7 | 0.7 | 0.2 | 0.2 | 0.8 | 0.12 | <0.02 | 3.1 | 0.9 | 0.01 | 0.3 | 0.5 | 7 | <1 |
| 06/02 | 40.0 | 5.1 | 15 | 1.4 | 0.8 | 0.1 | 1.0 | 0.24 | <0.02 | 1.4 | 0.7 | 0.06 | 1.2 | 0.3 | 8 | <1 |
| 06/06 | 20.2 | 5.6 | 10 | 1.0 | 0.6 | 0.4 | 1.0 | 0.12 | <0.02 | 2.9 | 1.1 | 0.03 | 1.1 | 0.1 | 9 | <1 |
| 06/07 | 29.0 | 6.3 | 17 | 1.3 | 1.2 | 0.2 | 0.9 | 0.23 | <0.02 | 3.1 | 1.3 | 0.03 | 1.9 | 0.3 | 10 | <1 |
| 08/21 | 34.2 | 5.1 | 5 | 1.2 | 0.2 | 0.3 | 1.1 | 0.20 | <0.02 | 2.3 | 1.5 | 0.01 | 0.4 | 0.5 | 8 | <1 |
| 10/24 | 22.4 | 5.7 | 11 | 1.5 | 1.3 | 0.2 | 1.1 | 0.21 | <0.02 | 2.4 | 1.0 | 0.08 | 1.2 | 0.7 | 10 | 2 |
| 10/25 | 38.8 | 6.4 | 7 | 1.4 | 0.2 | 0.2 | 1.0 | 0.20 | <0.02 | 1.8 | 0.9 | 0.01 | 0.2 | 0.5 | 6 | 1 |
| 11/05 | 75.0 | 6.1 | 6 | 0.9 | 0.3 | 0.1 | 0.9 | 0.18 | <0.02 | 2.1 | 0.4 | 0.09 | 0.1 | 0.1 | 5 | 2 |
| 12/10 | 22.8 | 5.3 | 2 | 1.3 | 0.1 | 0.1 | 0.9 | 0.17 | <0.02 | 2.1 | 0.8 | 0.01 | 0.2 | 0.3 | 6 | 2 |
| PE | 27.6 | 5.7 | 9 | 1.1 | 0.6 | 0.1 | 0.9 | 0.19 | <0.02 | 2.7 | 0.7 | 0.03 | 0.8 | 0.3 | 8 | 1 |
| H+ | Na+ | K+ | Mg2+ | Ca2+ | Al3+ | HCO3− | Cl− | NO3− | PO43− | SO42− | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H+ | 1.00 | ||||||||||
| Na+ | −0.10 | 1.00 | |||||||||
| K+ | −0.05 | 0.39 | 1.00 | ||||||||
| Mg2+ | −0.11 | −0.01 | 0.47 | 1.00 | |||||||
| Ca2+ | −0.12 | 0.53 | 0.26 | 0.47 | 1.00 | ||||||
| Al3+ | 0.10 | 0.46 | 0.31 | 0.34 | 0.08 | 1.00 | |||||
| HCO3− | 0.14 | 0.21 | 0.23 | −0.34 | 0.26 | −0.37 | 1.00 | ||||
| Cl− | −0.04 | 0.47 | 0.89 | 0.44 | 0.58 | 0.29 | 0.32 | 1.00 | |||
| NO3− | −0.18 | 0.10 | 0.06 | 0.48 | 0.15 | −0.01 | −0.12 | 0.08 | 1.00 | ||
| PO43− | −0.26 | 0.26 | 0.13 | 0.06 | 0.11 | 0.27 | −0.14 | 0.05 | 0.23 | 1.00 | |
| SO42− | 0.08 | 0.14 | 0.14 | 0.04 | 0.07 | 0.14 | −0.19 | −0.03 | 0.29 | 0.45 | 1.00 |
| Flux | Na+ | K+ | Mg2+ | Ca2+ | Al3+ | SiO2 | HCO3− | Cl− | NO3− | PO43− | SO42− | TDS | TSS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR 1 | 3.5 | 1.7 | 1.6 | 3.0 | 0.4 | 2.7 | 23.4 | 1.4 | 0.7 | 0.1 | 0.7 | 39.3 | 7.0 |
| FA 2 | 2.1 | 1.1 | 0.3 | 1.7 | 0.3 | 0.0 | 4.7 | 1.5 | 1.3 | 0.1 | 0.6 | 13.3 | 1.0 |
| Fw 3 | 1.4 | 0.6 | 1.3 | 1.3 | 0.1 | 2.7 | 18.7 | −0.1 | −0.6 | 0.0 | 0.1 | 26.0 | 6.0 |
| Hydrolysis |
| 2 KAlSi3O8 (orthoclase) + 2 CO2 (aq) + 11 H2O (liq) → Al2Si2O5(OH)4 (kaolinite) + 2 K+ (aq) + 2 HCO3− (aq) + 4 H4SiO4 (aq) |
| 2 (K0.75,Na0.25)AlSi3O8 (sanidine) + 2 CO2 (aq) + 11 H2O (liq) → Al2Si2O5(OH)4 (kaolinite) + 1.5 K+ (aq) + 0.5 Na+ (aq)+ 2 HCO3− (aq) + 4 H4SiO4 (aq) |
| Incongruent Dissolution |
| (Na0.75,K0.25)AlSiO4 (nefeline) + 4 CO2 (aq) + 4 H2O (liq) → 0.75 Na+ (aq) + 0.25K+ (aq) + Al3+ (aq) + 4 HCO3− (aq) + H4SiO4 (aq) NaFe3+(Si2O6) (aegirine) + 4 CO2 (aq) + 6 H2O (liq) → Na+ (aq) + Fe3+ (aq) + 4 HCO3− (aq) + 2 H4SiO4 (aq) (Ca0.75,Na0.25)(Mg0.5,Fe2+0.25,Fe3+0.25)(Si2O6) (aegirine-augite) + 4 CO2 (aq) + 6 H2O (liq) → 0.75 Ca2+(aq) + 0.25 Na+ (aq) + 0.5 Mg+3 (aq) + 0.25 Fe2+ (aq) + 0.25 Fe3+(aq) + 4 HCO3− (aq) + 2 H4SiO4 (aq) KMg3AlSi3O10(OH)2 (phlogopite) + 10 CO2 (aq) + 10 H2O (liq) → K+ (aq) + 3 Mg2+ (aq) + Al3+ (aq) + 10 HCO3− (aq) + 3 H4SiO4 (aq) |
| Forming Kaolinite and Goethite |
| 3 Al3+ (aq) + H2O (liq) → 3 H+ (aq) + Al(OH)3 (aq) |
| 2 Al(OH)3 (aq) + 2 H4SiO4 (aq) → Al2Si2O5(OH)4 (kaolinite) + 5 H2O (liq) 4 Fe2+ (aq) + O2 (aq) + 6 H2O (aq) → 4 FeOOH (goethite) + 8 H+ (aq) Fe3+ (aq) + 2 H2O (liq) → FeOOH (goethite) + 3 H+ (aq) |
| Area | Fw (t/km2/a) | Temperature (°C) | Runoff (mm/a) | Reference |
|---|---|---|---|---|
| Poços de Caldas Alkaline Massif | 26 | 17.0 | 1139 | This study |
| Paraná CBF province | 30 | 22.5 | 565 | [35] |
| Tapira | 29 | 20.7 | 512 | [40] |
| Catalão I | 32 | 22.6 | 541 | [40] |
| São Miguel | 35 | 16.0 | 730 | [14] |
| Deccan traps | 37 | 25.0 | 460 | [17] |
| Iceland | 36 | 2.0 | 1883 | [23] |
| Réunion island | 102 | 18.7 | 2430 | [13] |
| Java island | 326 | 26.6 | 4050 | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, F.T.d.; Mello, R.C.A.d.; Fernandes, A.M.; Sardinha, D.d.S. Chemical and Physical Denudation Rates in the Poços de Caldas Alkaline Massif, Minas Gerais State, Brazil. Minerals 2024, 14, 700. https://doi.org/10.3390/min14070700
Conceição FTd, Mello RCAd, Fernandes AM, Sardinha DdS. Chemical and Physical Denudation Rates in the Poços de Caldas Alkaline Massif, Minas Gerais State, Brazil. Minerals. 2024; 14(7):700. https://doi.org/10.3390/min14070700
Chicago/Turabian StyleConceição, Fabiano Tomazini da, Rafael Carvalho Alves de Mello, Alexandre Martins Fernandes, and Diego de Souza Sardinha. 2024. "Chemical and Physical Denudation Rates in the Poços de Caldas Alkaline Massif, Minas Gerais State, Brazil" Minerals 14, no. 7: 700. https://doi.org/10.3390/min14070700
APA StyleConceição, F. T. d., Mello, R. C. A. d., Fernandes, A. M., & Sardinha, D. d. S. (2024). Chemical and Physical Denudation Rates in the Poços de Caldas Alkaline Massif, Minas Gerais State, Brazil. Minerals, 14(7), 700. https://doi.org/10.3390/min14070700








