Crystallization Sequence of the Spodumene-Rich Alijó Pegmatite (Northern Portugal) and Related Metasomatism on Its Host Rock
Abstract
1. Introduction
2. Geological Setting
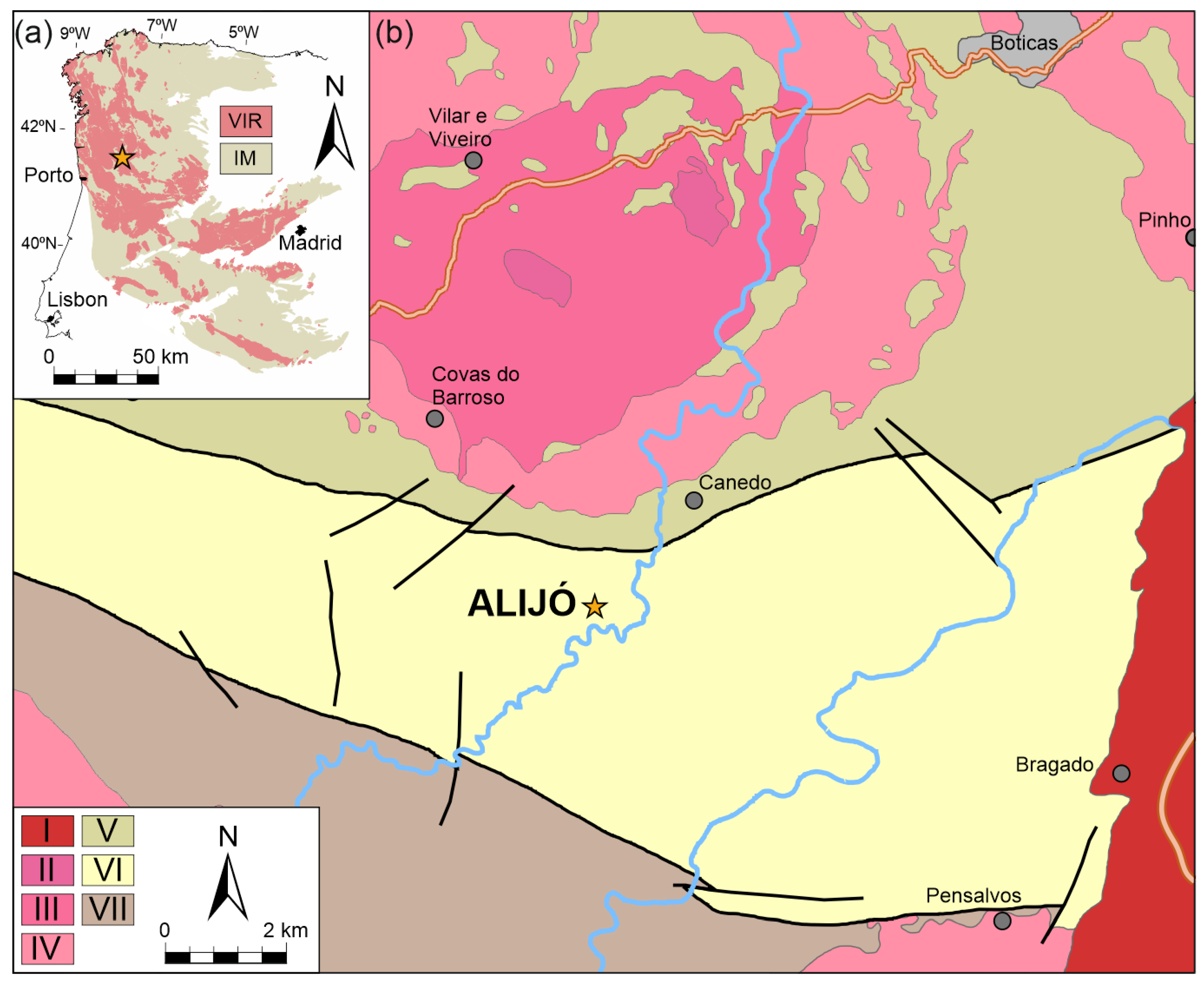
Pegmatites from Barroso–Alvão
- (1)
- Intragranitic pegmatites with quartz, feldspar, muscovite, biotite, minor tourmaline, beryl, garnet, fluorapatite, chlorite, and zircon.
- (2)
- Barren pegmatites with quartz, feldspar, muscovite, minor biotite, fluorapatite, beryl, tourmaline, chlorite, zircon, pyrite, and monazite-(Ce).
- (3)
- Spodumene pegmatites with quartz, feldspar, spodumene, muscovite, minor columbite group minerals (CGM), fluorapatite, montebrasite, triphylite, phosphoferrite, dufrénite, fairfieldite, chlorite, tourmaline, zircon, uraninite and sphalerite. The selected aplite-pegmatite body from Alijó belongs to this third group.
- (4)
- Petalite pegmatites with quartz, feldspar, petalite, muscovite, minor cassiterite, CGM, fluorapatite, montebrasite, ferrisicklerite, eosphorite, pyrite, sphalerite, uraninite, monazite-(Ce), autunite and xenotime.
- (5)
- Lepidolite pegmatites with albite, lepidolite, and muscovite, minor cassiterite, CGM, fluorapatite, zircon, and goyazite.
3. Materials and Methods
3.1. Electron Microprobe (EMP) Analysis
3.2. Whole-Rock Geochemical Analysis
4. Results
4.1. Field Characterization of the Spodumene-Bearing Dyke from Alijó
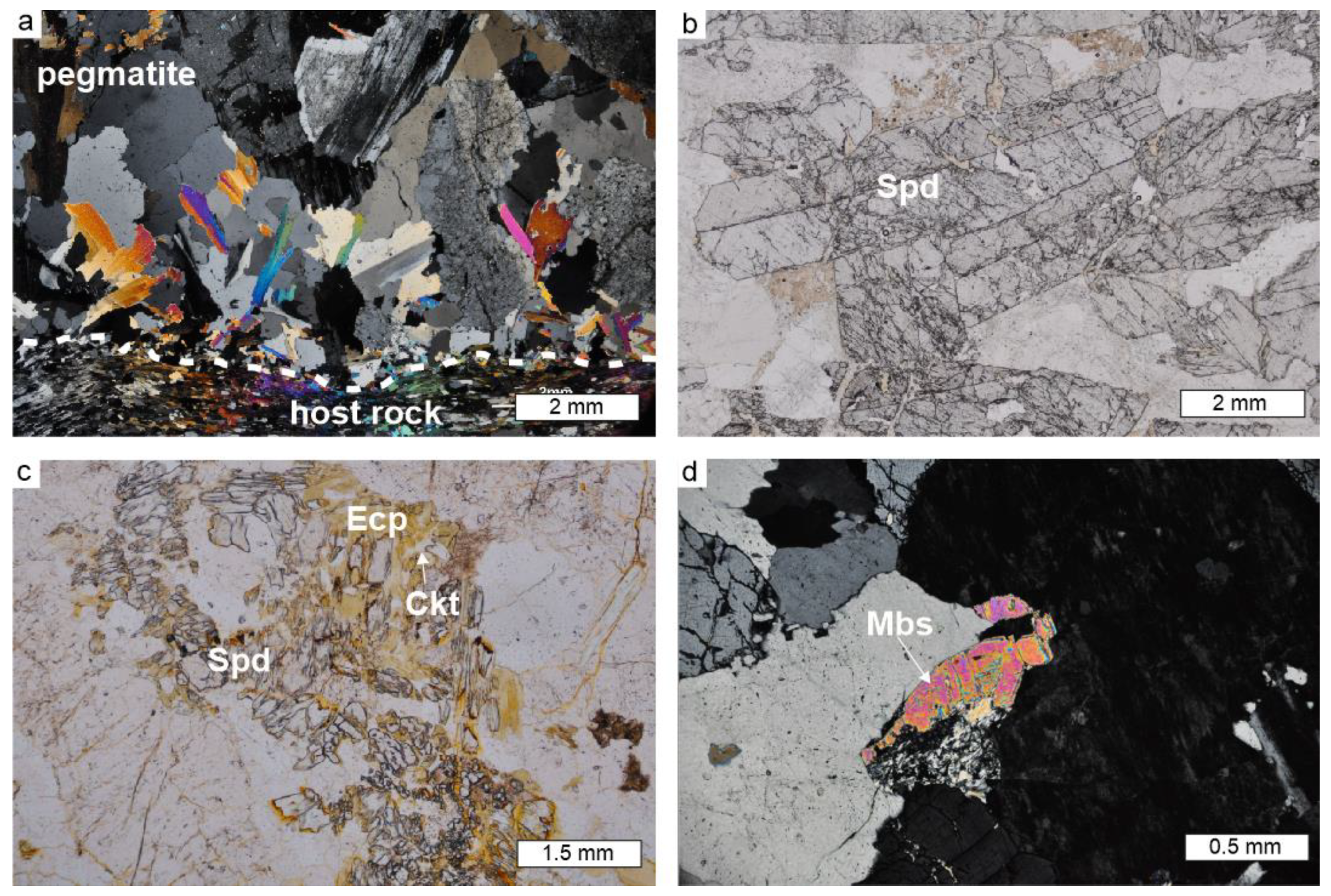
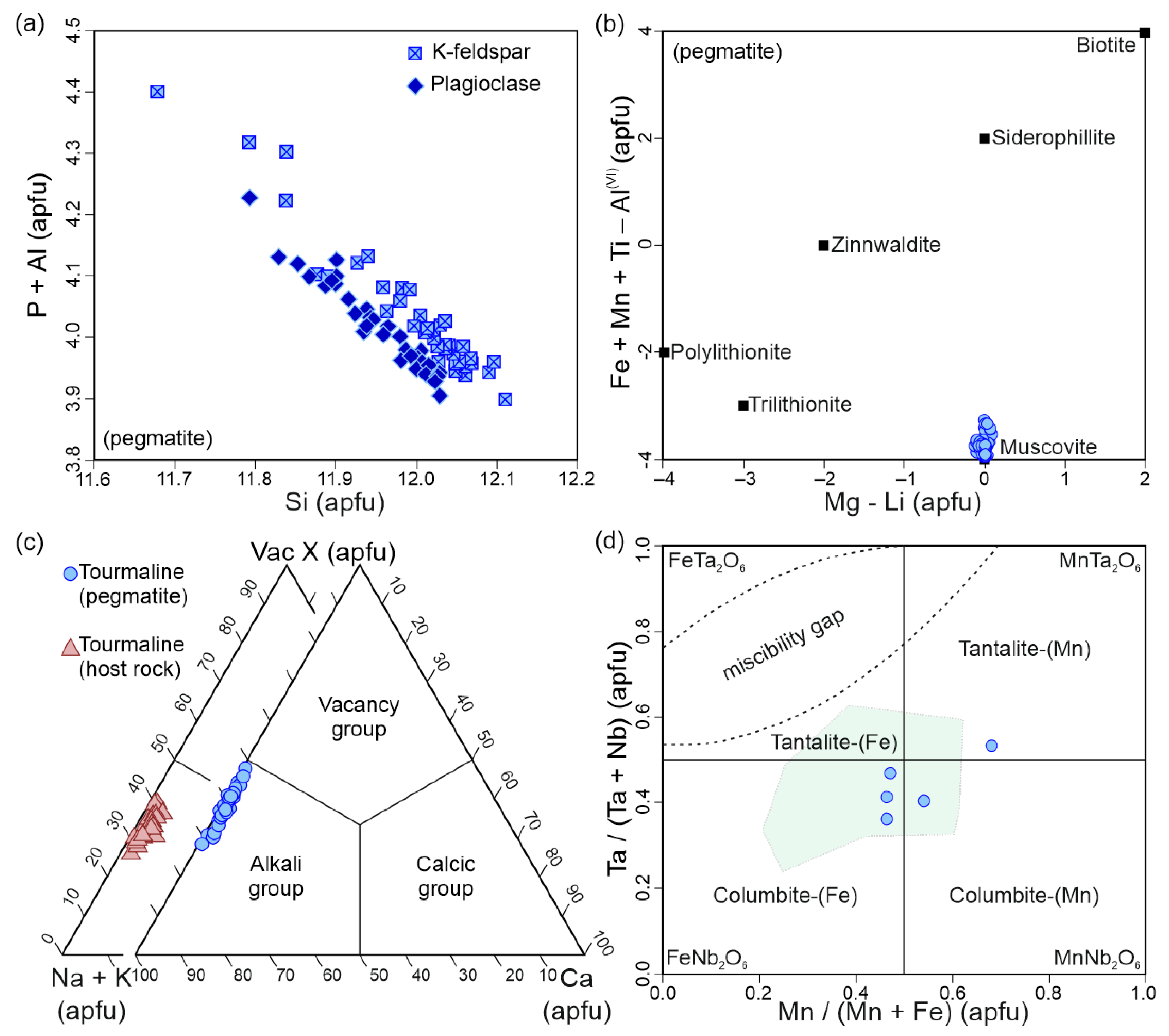
4.2. Petrography and Mineral Chemistry of the Spodumene Pegmatite
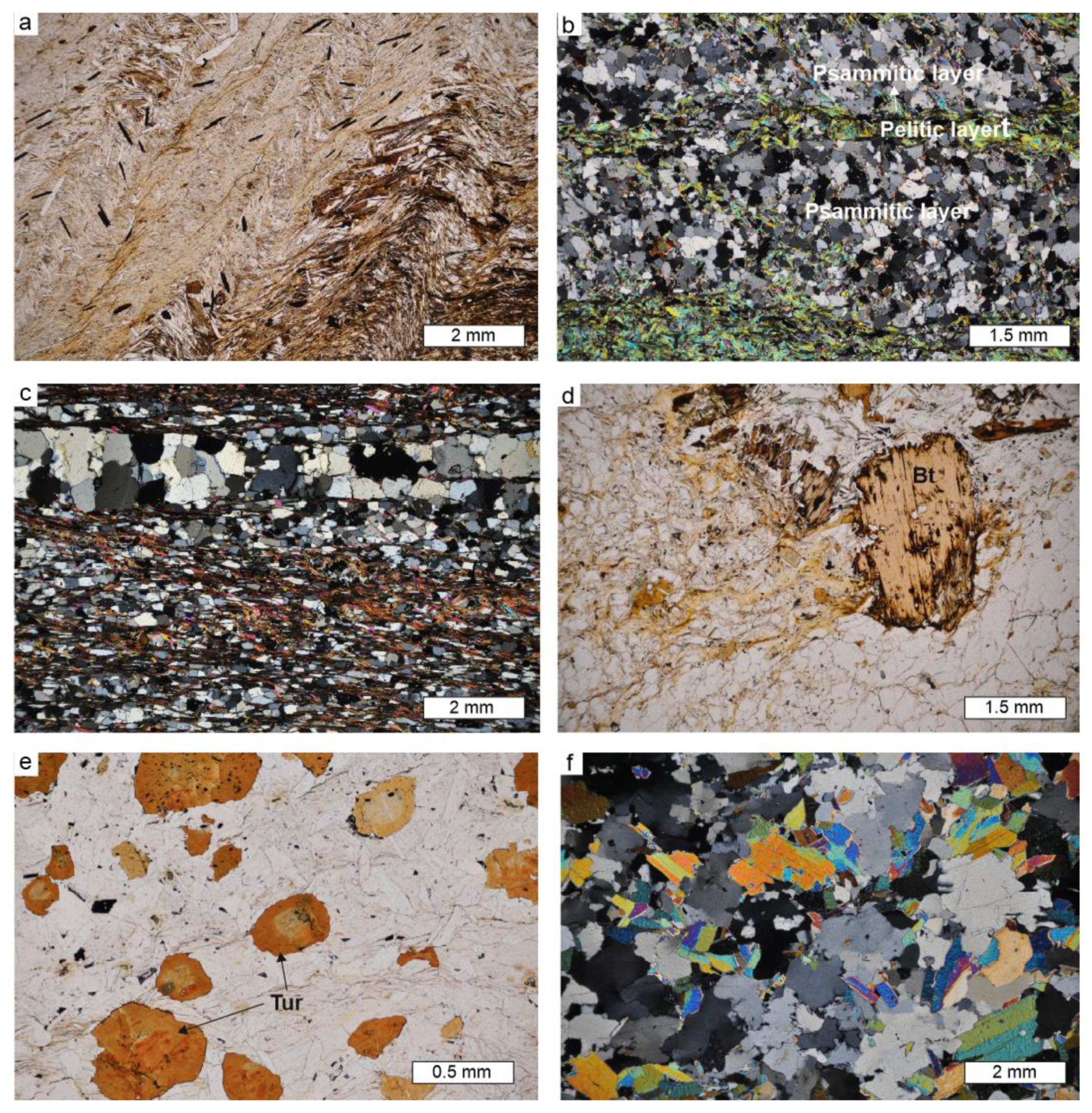
4.3. Petrography and Mineral Chemistry of the Host Rock
4.4. Whole Rock Geochemistry
5. Discussion
5.1. Internal Evolution of the Alijó Pegmatite
5.2. External Metasomatism Caused by the Alijó Pegmatite
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaskula, B.W. Minerals Yearbook: Lithium; U.S. Geological Survey: Reston, VA, USA, 2017.
- Gourcerol, B.; Gloaguen, E.; Melleton, J.; Tuduri, J.; Galieguea, X. Re-assessing the European lithium resource potential—A review of hard-rock resources and metallogeny. Ore Geol. Rev. 2019, 109, 494–519. [Google Scholar] [CrossRef]
- Bibienne, T.; Magnan, J.-F.; Rupp, A.; Laroche, N. From Mine to Mind and Mobiles: Society’s Increasing Dependence on Lithium. Elements 2020, 16, 265–270. [Google Scholar] [CrossRef]
- Moss, R.L.; Tzimas, E.; Kara, H.; Willis, P.; Kooroshy, J. Critical Metals in Strategic Energy Technologies; Institute for Energy and Transport European Commission: Luxembourg, 2011. [Google Scholar]
- Linnen, R.L.; Van Litchervelde, M.; Černý, P. Granitic pegmatites as sources of strategic metals. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- Simons, B.; Andersen, J.C.Ø.; Shail, R.K.; Jenner, F.E. Fractionation of Li, Be, Ga, Nb, Ta, In, Sn, Sb, W and Bi in the peraluminous Early Permian Variscan granites of the Cornubian Batholith: Precursor processes to magmatic-hydrothermal mineralization. Lithos 2017, 278–281, 491–512. [Google Scholar] [CrossRef]
- European Comission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Müller, A.; Reimer, W.; Wall, F.; Williamson, B.; Menuge, J.; Brönner, M.; Haase, C.; Brauch, K.; Pohl, C.; Lima, A.; et al. GREENPEG—Exploration for pegmatite minerals to feed the energy transition: First steps towards the Green Stone Age. Geol. Soc. Lond. Spec. Publ. 2022, 526, 27. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I.; Martins, T.; Torres-Ruiz, J. Geology and mineralogy of Li mineralization in the Central Iberian Zone (Spain and Portugal). Mineral. Mag. 2016, 80, 103–126. [Google Scholar] [CrossRef]
- Simmons, W.B.; Webber, K.L. Pegmatite genesis: State of the art. Eur. J. Mineral. 2008, 20, 421–438. [Google Scholar] [CrossRef]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Partitioning of trace elements between crystals and melts. Earth Planet. Sci. Lett. 2003, 210, 383–397. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Villaseca, C.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I. Petrogenetic relationships between variscan granitoids and Li–(F–P)-rich aplite-pegmatites in the Central Iberian Zone: Geological and geochemical constraints and implications for other regions from the European variscides. Ore Geol. Rev. 2018, 95, 408–430. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Korbelová, Z.; Lima, A.; Vašinová Galiová, M.; Hložková, M.; Dosbaba, M. Rock textures and mineralzoning—A clue to understanding rare-metal granite evolution: Argemela stock, Central-Eastern Portugal. Lithos 2022, 410–411, 106562. [Google Scholar] [CrossRef]
- Linnen, R.; McNeil, A.; Flemming, R. Some thoughts on metasomatism in pegmatites. Can. Mineral. 2019, 57, 765–766. [Google Scholar] [CrossRef]
- Roza-Llera, A.; Fuertes-Fuente, M.; Cepedal, A.; Martin-Izard, A. Barren and Li–Sn–Ta Mineralized Pegmatites from NW Spain (Central Galicia): A Comparative Study of Their Mineralogy, Geochemistry, and Wallrock Metasomatism. Minerals 2019, 9, 739. [Google Scholar] [CrossRef]
- Barros, R.; Kaeter, D.; Menuge, J.F.; Škoda, R. Controls on chemical evolution and rare element enrichment in crystallising albite-spodumene pegmatite and wallrocks: Constraints from mineral chemistry. Lithos 2020, 352–353, 105289. [Google Scholar] [CrossRef]
- Errandonea-Martin, J.; Garate-Olave, I.; Roda-Robles, E.; Cardoso-Fernandes, J.; Lima, A.; Ribeiro, M.A.; Teodoro, A.C. Metasomatic effect of Li-bearing aplite pegmatites on psammitic and pelitic metasediments: Geochemical constraints on critical raw material exploration at the Fregeneda–Almendra Pegmatite Field (Spain and Portugal). Ore Geol. Rev. 2022, 150, 105155. [Google Scholar] [CrossRef]
- Lima, A. Estrutura, Mineralogia e Génese dos Filões Aplitopegmatíticos com Espodumena da Região do Barroso-Alvão (Norte de Portugal). Ph.D. Thesis, University Porto, Porto, Portugal, INPL, Nancy, France, 2000. [Google Scholar]
- Charoy, B.; Noronha, F.; Lima, A.M.C. Spodumene—Petalite—Eucryptite: Mutual relationships and alteration style in Li-rich aplite–Pegmatite dykes from northern Portugal. Can. Mineral. 2001, 39, 729–746. [Google Scholar] [CrossRef]
- Martins, T. Multidisciplinary Study of Pegmatites and Associated Li and Sn–Nb–Ta Mineralisation from the Barroso-Alvão Region. Ph.D. Thesis, University Porto, Porto, Portugal, 2009. [Google Scholar]
- Farias, P.; Gallastegui, G.; González Lodeiro, F.; Marquínez, J.; Martín-Parra, L.M.; Martínez Catalán, J.R.; Pablo Maciá, J.G.; Rodríguez-Fernández, L.R. Aportaciones al conocimiento de la litoestratigrafía y estructura de Galicia Central. In Memorias del Museo y Laboratorio Mineralógico y Geológico, de la Facultad de Ciencias; Universidad de Oporto: Porto, Portugal, 1987; Volume 1, pp. 411–431. [Google Scholar]
- Julivert, M.; Marcos, A.; Truyols, J. L’évolution paléogéographique du NW de l’Espagne pendant l’Ordovicien–Silurien. Bull. Soc. Géol. Mineral. Bretagne 1972, 4, 1–7. [Google Scholar]
- Ribeiro, A.; Pereira, E.; Dias, R.; Gil Ibarguchi, J.I.; Arenas, R. Allochthonous Sequences. In Pre-Mesozoic Geology of Iberia. IGCP-Project 233; Dallmeyer, R.D., Garcia, E.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar] [CrossRef]
- Valverde-Vaquero, P.; Marcos, A.; Farias, P. U–Pb dating of Ordovician felsic volcanics in the Schistose domain of the Galicia-Trás-os-Montes Zone near Cabo Ortegal (NW Spain). Geol. Acta 2005, 3, 27–37. [Google Scholar] [CrossRef]
- Martínez Catalán, J.R.; Rubio Pascual, F.J.; Díez Montes, A.; Díez Fernández, R.; Gómez Barreiro, J.; Dias da Silva, I.; González Clavijo, E.; Ayarza, P.; Alcock, J.E. The late Variscan HT/LP metamorphic event in NW and Central Iberia: Relationships to crustal thickening, extension, orocline development and crustal evolution. In The Variscan Orogeny: Extent, Timescale and the Formation of the European Crust; Schulmann, K., Martínez Catalán, J.R., Lardeaux, J.M., Janousek, V., Oggiano, G., Eds.; Geological Society: London, UK, 2014; pp. 225–247. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Martins, H.C.; Almeida, A.; Noronha, F. Notícia Explicativa da Carta Geológica de Portugal na Escala 1:50,000—Folha 06C—Cabeceiras de Basto; Serviços Geológicos de Portugal: Amadora, Portugal, 2000. [Google Scholar]
- Noronha, F.; Ramos, J.M.F.; Rebelo, J.; Ribeiro, A.; Ribeiro, M.L. Essai de corrélation des phases de d’eformation hercyniennes dans le NW de la péninsule Ibérique. Leidse Geol. Meded. 1981, 52, 87–91. [Google Scholar]
- Dias, R.; Ribeiro, A. The Ibero-Armorican Arc: A collisional effect against an irregular continent? Tectonophysics 1995, 246, 113–128. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Ramos, R.; Noronha, F. Pegmatite-aplite veins of Barroso-Alvão Field. Lithostratigraphy and metamorphism of host rocks. In Granitic Pegmatites: The State of the Art, Field Trip Guidebook; Lima, A., Roda-Robles, E., Eds.; Universidade do Porto: Porto, Portugal, 2007. [Google Scholar]
- Noronha, F.; Ribeiro, M.L. Notícia Explicativa da Carta Geológica de Portugal na Escala 1:50,000—Folha 06A—Montalegre; Serviços Geológicos de Portugal: Amadora, Portugal, 1983. [Google Scholar]
- Teixeira, C. Notícia Explicativa da Carta Geológica de Portugal na Escala 1:50,000—Folha 06B—Chaves; Serviços Geológicos de Portugal: Amadora, Portugal, 1974. [Google Scholar]
- Sant’Ovaia, H.; Ribeiro, M.A.; Martins, H.C.B.; Noronha, F. Notícia Explicativa da Carta Geológica de Portugal na Escala 1:50,000—Folha 06D—Vila Pouca de Aguiar; Serviços Geológicos de Portugal: Amadora, Portugal, 2011. [Google Scholar]
- Ferreira, N.; Iglesias, M.; Noronha, F.; Pereira, E.; Ribeiro, A.; Ribeiro, M.L. Granitoides da Zona Centro Ibérica e o seu enquadramento geodinâmico. In Geología de los Granitóides y Rocas Asociadas del Macizo Hesperico. Homenage a L.C. García de Figuerola; Bea, F., Carnicero, A., Gonzalo, J.C., López-Plaza, M., Rodríguez Alonso, M.D., Eds.; Libro Editorial Rueda: Madrid, Spain, 1987; pp. 37–51. [Google Scholar]
- Dias, G.; Leterrier, J.; Mendes, A.; Simões, P.P.; Bertrand, J.M. U–Pb zircon and monazite geochronology of post-collisional Hercynian granitoids from the Central Iberian Zone (Northern Portugal). Lithos 1998, 45, 349–369. [Google Scholar] [CrossRef]
- Sant’Ovaia, H.; Bouchez, J.L.; Noronha, F.; Leblanc, D.; Vigneresse, J.L. Composite-laccolith emplacement of the post-tectonic Vila Pouca de Aguiar granite pluton (northern Portugal): A combined AMS and gravity study. In The Fourth Hutton Symposium on the Origin of Granites and Related Rocks; Barbarin, B., Stephens, W.E., Bonin, B., Bouchez, J.-L., Clarke, D.B., Cuney, M., Martin, H., Eds.; Geological Society of America: Boulder, CO, USA, 2000; Volume 350. [Google Scholar] [CrossRef]
- Almeida, A.; Martins, H.C.; Noronha, F. Hercynian acid magmatism and related mineralizations in northern Portugal. Gondwana Res. 2002, 5, 423–434. [Google Scholar] [CrossRef]
- Cruz, C.; Sant’Ovaia, H.; Barolomeu Raposo, M.I.; Lourenço, J.M.; Almeida, F.; Noronha, F. Unraveling the emplacement history of a Portuguese post-tectonic Variscan pluton using magnetic fabrics and gravimetry. J. Struct. Geotech. 2021, 153, 104470. [Google Scholar] [CrossRef]
- Almeida, A.; Leterrier, J.; Noronha, F.; Bertrand, J.M. U-Pb zircon and monazite geochronology of the Hercynian two mica granite composite pluton of Cabeceiras de Basto (Northern Portugal). C. R. Acad. Sci. 1998, 326, 779–785. [Google Scholar] [CrossRef]
- Mendes, A.; Dias, G. Mantle-like Sr-Nd isotope composition of Fe-K subalkaline granites: The Peneda-Gerês Variscan massif (NW Iberian Peninsula). Terra Nova 2004, 16, 109–115. [Google Scholar] [CrossRef]
- Teixeira, R.J.S.; Neiva, A.M.R.; Gomes, M.E.P.; Corfu, F.; Cuesta, A.; Croudace, I.W. The role of fractional crystallization in the genesis of early syn-D3, tin-mineralized Variscan two-mica granites from the Carrazeda de Ansiães area, northern Portugal. Lithos 2012, 153, 177–191. [Google Scholar] [CrossRef]
- Dias, G.; Simões, P.P.; Ferreira, N.; Leterrier, J. Mantle and Crustal Sources in the Genesis of Late-Hercynian Granitoids (NW Portugal): Geochemical and Sr-Nd Isotopic Constraints. Gondwana Res. 2002, 5, 287–305. [Google Scholar] [CrossRef]
- Villaseca, C.; Bellido, F.; Pérez-Soba, C.; Billström, K. Multiple crustal sources for post-tectonic I-type granites in the Hercynian Iberian Belt. Miner. Petrol. 2009, 96, 197–211. [Google Scholar] [CrossRef]
- Martins, T.; Lima, A.; Simmons, B.; Falster, A.U.; Noronha, F. Geochemical fractionation of Nb-Ta oxides in Li-bearing pegmatites from the Barroso-Alvão Pegmatite Field, northern Portugal. Can. Mineral. 2011, 49, 777–791. [Google Scholar] [CrossRef]
- Martins, T.; Roda-Robles, E.; Lima, A.; Parseval, P. Geochemistry and evolution of micas in the Barroso-Alvão pegmatite field, northern Portugal. Can. Mineral. 2012, 50, 1117–1129. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. “PAP” φ(ρZ) procedure for improved quantitative microanalysis. In Microbean Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Cerný, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Tischendorf, G.; Gottesmann, B.; Förster, H.-J.; Trumbull, R.B. On Li-bearing micas; estimating Li from electron microprobe analyses and an improved diagram for graphical representation. Mineral. Mag. 1997, 61, 809–834. [Google Scholar] [CrossRef]
- Henry, D.J.; Novak, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the tourmaline-supergroup minerals. Am. Mineral. 2011, 9, 895–913. [Google Scholar] [CrossRef]
- Bosi, F. Tourmaline crystal chemistry. Am. Mineral. 2018, 2018103, 298–306. [Google Scholar] [CrossRef]
- Debon, F.; Le Fort, P. A chemical–mineralogical classification of common plutonic rocks and associations. Trans. R. Soc. Edinburgh Earth Sci. 1983, 73, 135–149. [Google Scholar] [CrossRef]
- Villaseca, C.; Barbero, L.; Herreros, V. A re-examination of the typology of peraluminous granite types in intracontinental orogenic belts. Trans. R. Soc. Edinburgh Earth Sci. 1998, 89, 113–119. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier Science: Oxford, UK, 2014; Volume 4, pp. 1–51. [Google Scholar] [CrossRef]
- Herron, M.M. Geochemical classification of terrigenous sands and shales from core or log data. J. Sediment. Petrol. 1988, 58, 820–829. [Google Scholar] [CrossRef]
- Ugidos, J.M.; Sánchez-Santos, J.M.; Barba, P.; Valladares, M.I. Upper Neoproterozoic series in the Central Iberian, Cantabrian and West Asturian Leonese Zones (Spain): Geochemical data and statistical results as evidence for a shared homogenised source area. Precambrian Res. 2010, 178, 51–58. [Google Scholar] [CrossRef]
- Villaseca, C.; Merino, E.; Oyarzun, R.; Orejana, D.; Pérez-Soba, C.; Chicharro, E. Contrasting chemical and isotopic signatures from Neoproterozoic metasedimentary rocks in the Central Iberian Zone (Spain) of pre-Variscan Europe: Implications for terrane analysis and early Ordovician magmatic belts. Precambrian Res. 2014, 245, 131–145. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Neiva, A.M.R. Distribution of trace elements in feldspars granitic aplites and pegmatites from Alijó-Sanfins, northern Portugal. Mineral. Mag. 1995, 59, 35–45. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. Mica and feldspar as indicators of the evolution of a highly evolved granite-pegmatite system in the Tres Arroyos area (Central Iberian Zone, Spain). J. Iberian Geol. 2018, 44, 375–403. [Google Scholar] [CrossRef]
- London, D.; Hunt, L.E.; Schwing, C.R.; Gutter, B.M. Feldspar thermometry in pegmatites: Truth and consequences. Contrib. Mineral. Petr. 2020, 175, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, K.; Zhao, J.; Zhou, O.; Shi, R. Feldspar traces mineralization processes in the Qongjiagang giant lithium ore district, Himalaya, Tibet. Ore Geol. Rev. 2023, 157, 105451. [Google Scholar] [CrossRef]
- Tuttle, O.F.; Bowen, N.L. Origin of Granite in the Light of Experimental Studies in the System NaAlSi3O8–KAlSi3O8–SiO2–H2O. Geol. Soc. Am. Mem. 1958, 74, 1–146. [Google Scholar] [CrossRef]
- Parson, I. Feldspars defined and described: A pair of posters published by the Mineralogical Society. Sources and supporting information. Mineral. Mag. 2010, 74, 529–551. [Google Scholar] [CrossRef][Green Version]
- Carmichael, I.S.E.; Turner, F.J.; Verhoogen, J. Igneous Petrology; McGraw—Hill: New York, NY, USA, 1974; p. 739. [Google Scholar]
- Tuttle, O.F. Origin of the contrasting mineralogy of extrusive and plutonic salic rocks. J. Geol. 1952, 60, 107–124. [Google Scholar] [CrossRef]
- Smith, J.V. Chemical and textural properties. In Feldspar Minerals; Smith, J.V., Ed.; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Ribbe, P.H. Feldspar Mineralogy, 2nd ed.; De Gruyter: Berlin, Germany, 1983; p. 362. [Google Scholar]
- London, D. Experimental phase equilibria in the system LiAlSiO4-SiO2-H2O: A petrogenetic grid for lithium-rich pegmatites. Am. Mineral. 1984, 69, 995–1004. [Google Scholar]
- Černý, P.; Ferguson, R.B. The Tanco pegmatite at Bernic Lake, Manitoba. IV. Petalite and spodumene relations. Can. Mineral. 1972, 11, 660–678. [Google Scholar]
- Rossovskyi, L.N.; Matrosov, I.I. Pseudomorphs of quartz and spodumene after petalite and their importance to the pegmatite-forming process. Dokl. Akad. Nauk SSSR 1974, 216, 1135–1137. [Google Scholar]
- Dias, F.; Ribeiro, R.; Gonçalves, F.; Lima, A.; Roda-Robles, E.; Martins, T. Calibrating a Handheld LIBS for Li Exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite. Minerals 2023, 13, 470. [Google Scholar] [CrossRef]
- Dias, F.; Lima, A.; Roda-Robles, E. Mutual relationships between spodumene and petalite from the Iberian Massif pegmatites: More than PT changes? Can. Mineral. 2019, 57, 731–732. [Google Scholar] [CrossRef]
- Roda, E.; Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J.; Fontan, F. Origin and internal evolution of the Li-F-Be-B-P-bearing Pinilla de Fermoselle pegmatite (Central Iberian Zone, Zamora, Spain). Am. Mineral. 2005, 90, 1887–1899. [Google Scholar] [CrossRef]
- Marchal, K.L.; Simmons, W.B.; Falster, A.U.; Webber, K.L.; Roda-Robles, E. Geochemistry, mineralogy, and evolution of Li-Al micas and feldspars from the Mount Mica pegmatite, Maine, USA. Can. Mineral. 2014, 52, 221–233. [Google Scholar] [CrossRef]
- Cao, C.; Shen, P.; Bai, Y.; Luo, Y.; Feng, H.; Li, C.; Pan, H. Chemical evolution of micas and Nb-Ta oxides from the Koktokay pegmatites, Altay, NW China: Insights into rare-metal mineralization and genetic relationships. Ore Geol. Rev. 2022, 146, 104933. [Google Scholar] [CrossRef]
- Vieira, R.; Roda-Robles, E.; Pesquera, A.; Lima, A. Chemical variation and significance of micas from the Fregeneda-Almendra pegmatitic field (Central-Iberian Zone, Spain and Portugal). Am. Mineral. 2011, 96, 637–645. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Vieira, R.; Lima, A.; Errandonea-Martin, J.; Pesquera, A.; Cardoso-Fernandes, J.; Garate-Olave, I. Li-rich pegmatites and related peraluminous granites of the Fregeneda-Almendra field (Spain-Portugal): A case study of magmatic signature for Li enrichment. Lithos 2023, 452–453, 107195. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A.; Errandonea-Martin, J. The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology. Minerals 2020, 10, 1008. [Google Scholar] [CrossRef]
- Keller, P.; Von Knorring, O. Pegmatites at the Okatjimukuju farm, Karibib, Namibia Part I: Phosphate mineral associations of the Clementine II pegmatite. Eur. J. Mineral. 1989, 1, 567–593. [Google Scholar] [CrossRef]
- Baijot, M.; Hatert, F.; Philippo, S. Mineralogy and geochemistry of phosphates and silicates in the Sapucaia pegmatite, Minas Gerais, Brazil: Genetic implications. Can. Mineral. 2012, 50, 1531–1554. [Google Scholar] [CrossRef]
- Vignola, P.; Zucali, M.; Rotiroti, N.; Marotta, G.; Risplendente, A.; Pavese, A.; Boscardin, M.; Mattioli, V.; Bertoldi, G. The chrysoberyl- and phosphate-bearing albite pegmatite of Malga Garbella Val Di Rabbi, Trento province, Italy. Can. Mineral. 2018, 56, 411–424. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A.; Lima, A.; Garate-Olave, I.; Merino-Martínez, E.; Cardoso-Fernandes, J.; Errandonea-Martin, J. Compositional Variations in Apatite and Petrogenetic Significance: Examples from Peraluminous Granites and Related Pegmatites and Hydrothermal Veins from the Central Iberian Zone (Spain and Portugal). Minerals 2022, 12, 1401. [Google Scholar] [CrossRef]
- Piccoli, P.; Candela, P. Apatite in Igneous Systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Linnen, R.L.; Salvi, S.; Beziat, D. The role of metagabbro rafts on tantalum mineralization in the Tanco pegmatite, Manitoba. Can. Mineral. 2006, 44, 625–644. [Google Scholar] [CrossRef]
- London, D.; Morgan Vi, G.B.; Wolf, M.B. Amblygonite-montebrasite solid solutions as monitors of fluorine in evolved granitic and pegmatitic melts. Am. Mineral. 2001, 86, 225–233. [Google Scholar] [CrossRef]
- Kaeter, D.; Barros, R.; Menuge, J.F.; Chew, D.M. The magmatic hydrothermal transition in rare-element pegmatites from southeast Ireland: LA-ICP-MS chemical mapping of muscovite and columbite-tantalite. Geochim. Cosmochim. Acta 2018, 240, 96–130. [Google Scholar] [CrossRef]
- Ballouard, C.; Elburg, M.A.; Tappe, S.; Reinke, C.; Ueckermann, H.; Doggart, S. Magmatic-hydrothermal evolution of rare metal pegmatites from the Mesoproterozoic Orange River pegmatite belt (Namaqualand, South Africa). Ore Geol. Rev. 2020, 116, 103252. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Muchez, P. Tracing fluid saturation during pegmatite differentiation by studying the fluid inclusion evolution and multiphase cassiterite mineralisation of the Gatumba pegmatite dyke system (NW Rwanda). Lithos 2020, 354–355, 105285. [Google Scholar] [CrossRef]
- Shaw, R.A.; Goodenough, K.M.; Deady, E.; Nex, P.; Ruzvidzo, B.; Rushton, J.C.; Mounteney, I. The Magmatic–Hydrothermal Transition in Lithium Pegmatites: Petrographic and Geochemical Characteristics of Pegmatites from the Kamativi Area, Zimbabwe. Can. Mineral. 2022, 60, 957–987. [Google Scholar] [CrossRef]
- Candela, P.A. A Review of Shallow, Ore-related Granites: Textures, Volatiles, and Ore Metals. J. Petrol. 1997, 38, 1619–1633. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Water in granite and pegmatite-forming melts. Ore Geol. Rev. 2012, 46, 32–46. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Revisiting complete miscibility between silicate melts and hydrous fluids, and the extreme enrichment of some elements in the supercritical state—Consequences for the formation of pegmatites and ore deposits. Ore Geol. Rev. 2016, 72, 1088–1101. [Google Scholar] [CrossRef]
- Selway, J.B.; Breaks, F.W.; Tindle, A.G. A Review of Rare-Element (Li-Cs-Ta) Pegmatite Exploration Techniques for the Superior Province, Canada, and Large Worldwide Tantalum Deposits. Explor. Min. Geol. 2005, 14, 1–30. [Google Scholar] [CrossRef]
- Martins, T.; Linnen, R.L.; Fedikow, M.A.F.; Singh, J. Whole-rock and mineral geochemistry as exploration tools for rare-element pegmatite in Manitoba: Examples from the Cat Lake–Winnipeg River and Wekusko Lake pegmatite fields (parts of NTS 52L6, 63J13). In Report of Activities 2017; Manitoba Growth, Enterprise and Trade, Manitoba Geological Survey: Winnipeg, MB, Canada, 2017; pp. 42–51. [Google Scholar]
- Webber, K.L.; Falster, A.U.; Simmons, W.B.; Foord, E.E. The role of diffusion-controlled oscillatory nucleation in the formation of line rock in pegmatite-aplite dikes. J. Petrol. 1997, 38, 1777–1791. [Google Scholar] [CrossRef]
- Webber, K.L.; Simmons, W.B.; Falster, A.U.; Foord, E.E. Cooling rates and crystallization dynamics of shallow level pegmatite-aplite dikes, San Diego County, California. Am. Mineral. 1999, 84, 708–717. [Google Scholar] [CrossRef]
- Sirbescu, M.-L.C.; Hartwick, E.E.; Student, J.J. Rapid crystallization of the Animikie Red Ace Pegmatite, Florence county, northeastern Wisconsin: Inclusion microthermometry and conductive-cooling modeling. Contrib. Mineral. Petrol. 2008, 156, 289–305. [Google Scholar] [CrossRef]
- Nabelek, P.I.; Whittington, A.G.; Sirbescu, M.-L.C. The role of H2O in rapid emplacement and crystallization of granite pegmatites: Resolving the paradox of large crystals in highly undercooled melts. Contrib. Mineral. Petrol. 2010, 160, 313–325. [Google Scholar] [CrossRef]
- Pollard, P.J.; Pichavant, M.; Charoy, B. Contrasting evolution of fluorine- and boron-rich tin systems. Mineral. Depos. 1987, 22, 315–321. [Google Scholar] [CrossRef]
- Thomas, R.; Webster, J.D.; Heinrich, W. Melt inclusions in pegmatite quartz: Complete miscibility between silicate melts and hydrous fluids at low pressure. Contrib. Mineral. Petrol. 2000, 139, 394–401. [Google Scholar] [CrossRef]
- Thomas, R.; Förster, H.-J.; Heinrich, W. The behaviour of boron in a peraluminous granite–pegmatite system and associated hydrothermal solutions: A melt and fluid inclusion study. Contrib. Mineral. Petrol. 2003, 144, 457–472. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Mineral. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]

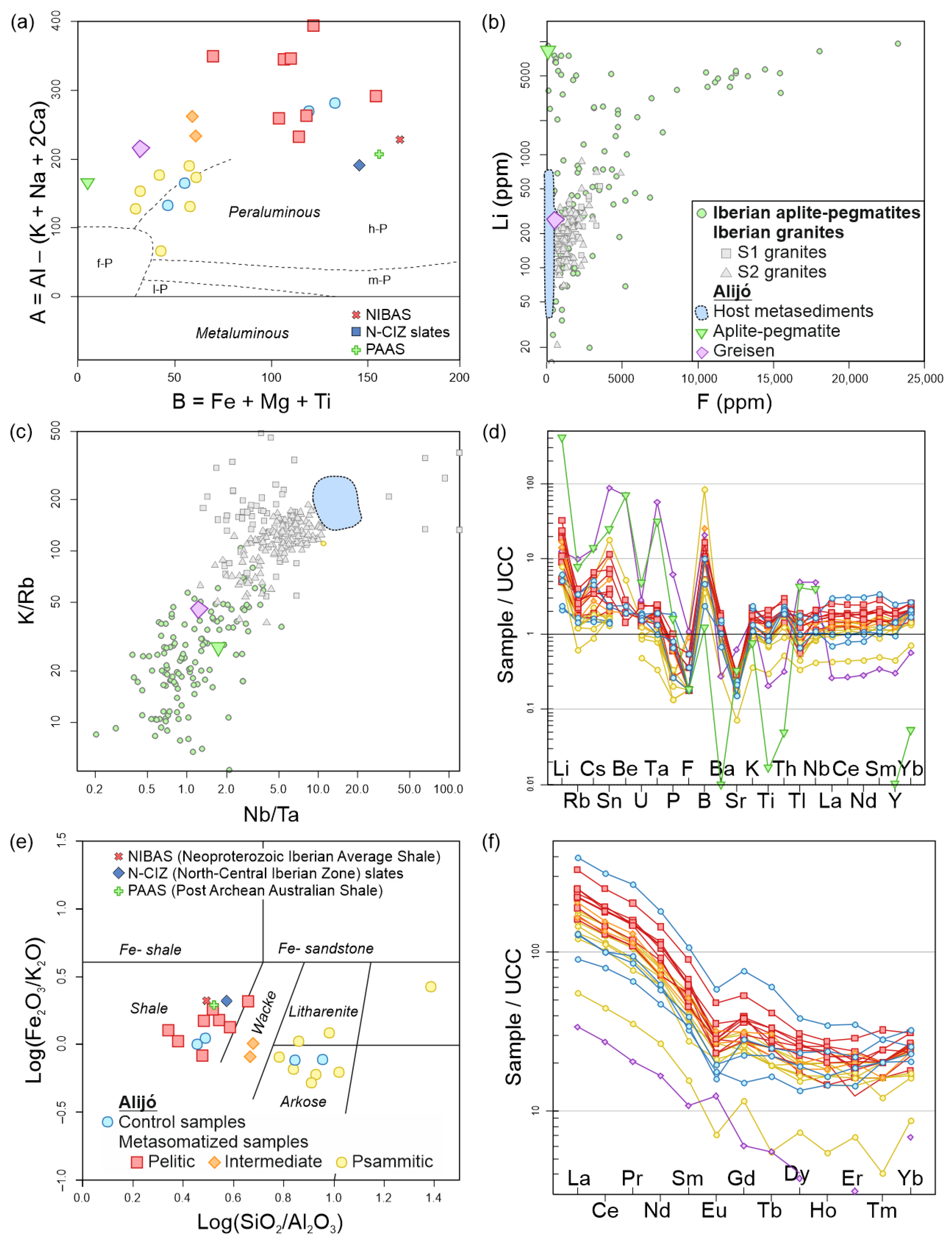

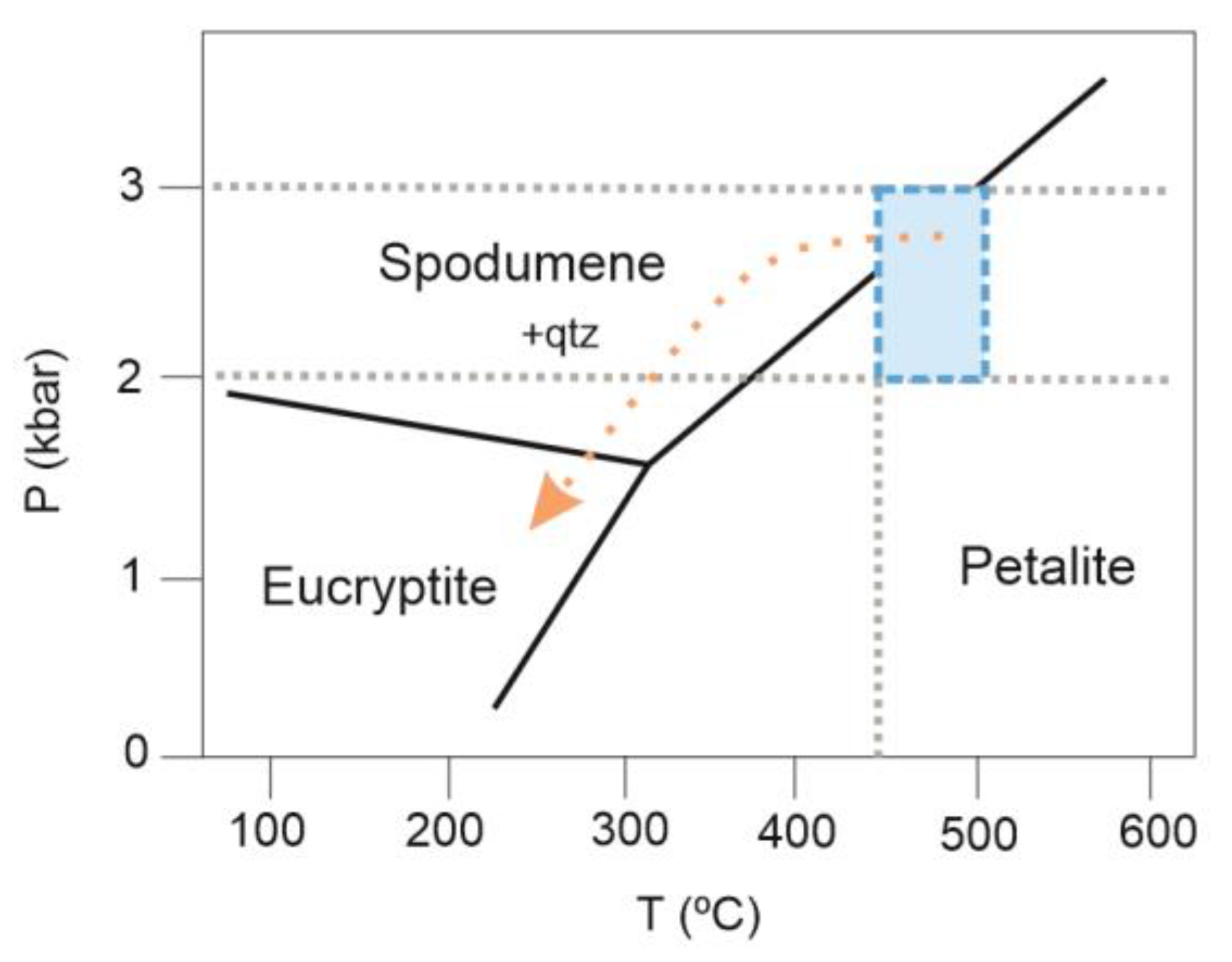
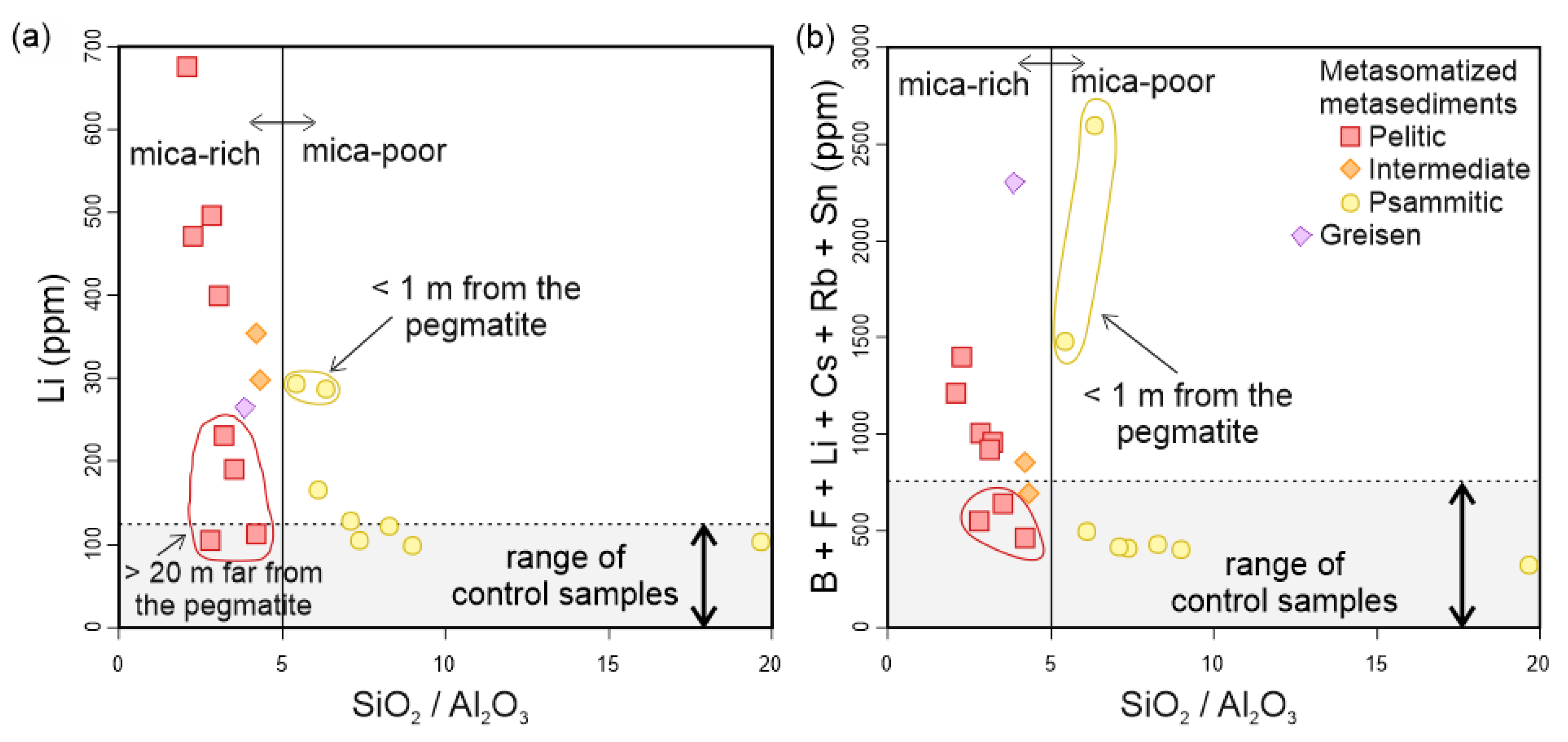
| (a) | ||||||||||||
| Type | met. | met. | met. | met. | met. | met. | met. | met. | met. | met. | met. | met. |
| Class. | lith. | wacke | sub-lith. | shale | lith. | lith. | lith. | shale | shale | shale | greisen | Shale |
| Sample | B-09 | B-10 | B-11 | B-12 | B-13 | B-14 | B-15 | B-16 | B-17 | B-18 | B-19 | B-20 |
| Dist. (m) | 0 W | 0.5 W | 1 W | 2 W | 3 W | 6 W | 12 W | 20 W | 35 W | 50 W | 0 E | 0.5 E |
| SiO2 | 78.36 | 75.49 | 90.49 | 61.3 | 84.53 | 82.18 | 81.62 | 64.49 | 65.6 | 67.91 | 70.1 | 56.41 |
| TiO2 | 0.54 | 0.56 | 0.19 | 0.9 | 0.45 | 0.47 | 0.46 | 1.18 | 0.82 | 0.56 | 0.13 | 0.96 |
| Al2O3 | 12.37 | 13.99 | 4.61 | 20.1 | 9.43 | 11.21 | 9.89 | 23.05 | 18.51 | 16.21 | 18.01 | 24.83 |
| Fe2O3t | 3.08 | 2.95 | 2.69 | 8.02 | 1.56 | 1.71 | 3.31 | 3.65 | 5.92 | 7.57 | 2.06 | 6.54 |
| MnO | 0.024 | 0.026 | 0.026 | 0.085 | 0.026 | 0.015 | 0.037 | 0.055 | 0.044 | 0.052 | 0.04 | 0.026 |
| MgO | 0.62 | 0.54 | 0.26 | 1.69 | 0.18 | 0.18 | 0.42 | 0.37 | 0.77 | 0.49 | 0.2 | 0.58 |
| CaO | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.79 | <0.01 |
| Na2O | 0.22 | 0.2 | 0.06 | 0.23 | 0.14 | 0.16 | 0.17 | 0.28 | 0.28 | 0.23 | 0.37 | 0.28 |
| K2O | 2.9 | 3.68 | 1.01 | 4.47 | 2.49 | 2.85 | 2.71 | 4.42 | 4.45 | 3.7 | 4.62 | 6.22 |
| P2O5 | 0.04 | 0.04 | 0.02 | 0.04 | 0.04 | 0.03 | 0.05 | 0.09 | 0.1 | 0.1 | 0.93 | 0.11 |
| LOI | 1.82 | 2.26 | 0.71 | 3.79 | 1.49 | 1.79 | 1.69 | 2.69 | 4.09 | 3.65 | 2.73 | 4.7 |
| Total | 99.97 | 99.74 | 100.09 | 100.63 | 100.34 | 100.6 | 100.36 | 100.28 | 100.58 | 100.47 | 99.98 | 100.66 |
| F | 600 | 500 | <100 | 200 | <100 | <100 | <100 | <100 | <100 | <100 | 600 | 300 |
| As | 22 | 66 | 12 | 30 | 15 | 12 | 8 | 104 | 46 | 19 | 14 | 32 |
| B | 1430 | 430 | 60 | 80 | 90 | 90 | 90 | 180 | 150 | 90 | 350 | 240 |
| Ba | 481 | 642 | 179 | 823 | 477 | 532 | 539 | 814 | 940 | 689 | 170 | 1170 |
| Be | 11 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | 147 | 6 |
| Cr | 90 | 110 | 110 | 150 | 80 | 100 | 90 | 130 | 130 | 120 | 70 | 150 |
| Cs | 26.8 | 22.8 | 4.3 | 16.8 | 8.8 | 8.2 | 11 | 9.1 | 17.1 | 7.9 | 66.5 | 30.2 |
| Ga | 15.9 | 19.8 | 6 | 31.4 | 15.9 | 13.3 | 15.9 | 29.9 | 25.4 | 24.5 | 30.3 | 32.6 |
| Ge | 2.8 | 3.1 | 1.3 | 3.7 | 2.3 | 2.1 | 2.7 | 2.9 | 2.7 | 2.6 | 6.4 | 3.3 |
| Li | 288 | 294 | 104 | 400 | 99 | 105 | 123 | 105 | 190 | 113 | 264 | 472 |
| Mo | 5 | 6 | 9 | 3 | 3 | 6 | 3 | 1 | <1 | 3 | 2 | 2 |
| Nb | 12.5 | 11.8 | 5 | 24.6 | 10.8 | 9.8 | 10 | 20.5 | 17.7 | 13.7 | 58.3 | 19.3 |
| Ni | 40 | 40 | 40 | 50 | 20 | 30 | 20 | 30 | 50 | 40 | 140 | 20 |
| Pb | 27.9 | 19.6 | 17.2 | 25.2 | 18.3 | 21.4 | 19.9 | 32.2 | 26.5 | 26 | 19.4 | 34.8 |
| Rb | 215 | 216 | 52.1 | 229 | 104 | 104 | 102 | 147 | 178 | 146 | 834 | 334 |
| Sn | 38 | 22.8 | 3.5 | 7.6 | 3.7 | 2.7 | 3 | 8.3 | 5 | 4.3 | 185 | 24.4 |
| Sr | 58 | 53 | 23 | 69 | 52 | 54 | 56 | 82 | 62 | 60 | 197 | 85 |
| Ta | 1.3 | 0.9 | 0.3 | 1.8 | 0.8 | 1.1 | 0.9 | 2.1 | 1.5 | 1.2 | 51.4 | 1.8 |
| Th | 17.5 | 14.4 | 5.5 | 20.4 | 18.3 | 14.9 | 16.2 | 30.9 | 19.5 | 14.7 | 3.3 | 22 |
| Tl | 1.5 | 1.6 | 0.3 | 1.5 | 0.5 | 0.6 | 0.5 | 0.6 | 0.8 | 0.5 | 4.4 | 1.7 |
| U | 4 | 3.6 | 1.3 | 3.5 | 2.6 | 2.5 | 3.3 | 6.6 | 4.5 | 4.1 | 7.6 | 4.2 |
| V | 52 | 63 | 17 | 128 | 32 | 35 | 38 | 71 | 78 | 71 | 16 | 121 |
| W | 5.9 | 5.8 | 1.9 | 6.9 | 3.6 | 5.1 | 4.3 | 5.7 | 7.6 | 5.4 | 5.4 | 16.9 |
| Y | 32 | 24.7 | 9.3 | 35 | 29.4 | 24 | 28.9 | 41.5 | 30.4 | 25.7 | 6.3 | 38.2 |
| Zn | 60 | 30 | 30 | 80 | <30 | 30 | 30 | 40 | 40 | 60 | 60 | 100 |
| La | 41.8 | 40.3 | 13.2 | 59.3 | 36.5 | 34.6 | 43.3 | 55.5 | 45 | 38.1 | 8 | 59.2 |
| Ce | 90.6 | 80.8 | 27.3 | 116 | 77.7 | 70.2 | 91 | 119 | 89.3 | 79.2 | 16.7 | 112 |
| Pr | 11.1 | 10.1 | 3.3 | 14.8 | 9.8 | 8.5 | 10.5 | 13.8 | 11.1 | 10.2 | 1.9 | 13.9 |
| Nd | 37 | 32.9 | 12.2 | 48.1 | 32.1 | 32 | 35.9 | 52.9 | 40.5 | 32.6 | 7.6 | 50.5 |
| Sm | 8.3 | 8 | 2.3 | 9.1 | 6 | 5.2 | 7 | 9.1 | 7.1 | 8.2 | 1.6 | 10 |
| Eu | 1.6 | 1.6 | 0.4 | 1.6 | 1.3 | 1.5 | 1.6 | 1.7 | 1.3 | 1.3 | 0.7 | 1.5 |
| Gd | 6.3 | 5.4 | 2.3 | 7.9 | 5.4 | 4.8 | 6 | 7.7 | 6 | 5.5 | 1.2 | 7.2 |
| Tb | 0.9 | 0.8 | 0.2 | 0.9 | 0.9 | 0.7 | 0.7 | 1.2 | 1 | 0.9 | 0.2 | 1.1 |
| Dy | 5.1 | 4.8 | 1.8 | 5.9 | 4.8 | 4.2 | 4.9 | 6.9 | 5.5 | 4.3 | 1 | 6.3 |
| Ho | 1 | 1.1 | 0.3 | 1.3 | 1.1 | 0.8 | 1.1 | 1.4 | 1.1 | 0.8 | <0.2 | 1.4 |
| Er | 3.2 | 2 | 1.1 | 2.9 | 2.6 | 2.3 | 3.2 | 3.3 | 3.3 | 2.6 | 0.5 | 3.8 |
| Tm | 0.5 | 0.4 | 0.1 | 0.5 | 0.5 | 0.4 | 0.5 | 0.6 | 0.5 | 0.4 | <0.1 | 0.5 |
| Yb | 4 | 3.6 | 1.4 | 4.2 | 3.8 | 2.7 | 4.3 | 5 | 4.3 | 2.9 | 1.1 | 3.9 |
| (b) | ||||||||||||
| Type | met. | met. | met. | met. | met. | met. | met. | Ctrl. | Ctrl. | Ctrl. | Ctrl. | Peg. |
| Class. | wacke | shale | lith. | lith. | wacke | shale | shale | lith. | shale | lith. | shale | Spd. Peg. |
| Sample | B-21 | B-22 | B-23 | B-24 | B-25 | B-26 | B-27 | ALI-01 | ALI-03 | ALI-04 | ALI-05 | ALI-02 |
| Dist. (m) | 1 E | 2 E | 3 E | 6 E | 12 E | 20 E | 35 E | > 200 | > 200 | > 100 | > 100 | > 100 |
| SiO2 | 71.31 | 54.76 | 78.18 | 81.2 | 73.52 | 63.31 | 63.12 | 77.92 | 60.16 | 81.57 | 59.14 | 75.62 |
| TiO2 | 0.76 | 1.34 | 0.43 | 0.45 | 0.69 | 0.87 | 0.84 | 0.6 | 0.86 | 0.52 | 0.88 | 0.01 |
| Al2O3 | 16.96 | 26.2 | 12.89 | 11.47 | 17.02 | 22.32 | 19.57 | 12.73 | 21.1 | 10.53 | 22.19 | 16.05 |
| Fe2O3t | 3.5 | 6.67 | 2.19 | 1.63 | 3.14 | 5.89 | 7.41 | 2.9 | 6.82 | 2.36 | 6.74 | 0.34 |
| MnO | 0.024 | 0.056 | 0.016 | 0.015 | 0.026 | 0.051 | 0.048 | 0.054 | 0.065 | 0.039 | 0.074 | 0.029 |
| MgO | 0.3 | 0.85 | 0.36 | 0.24 | 0.44 | 0.87 | 0.62 | 0.44 | 0.91 | 0.41 | 1.48 | 0.03 |
| CaO | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.02 | <0.01 | <0.01 | 0.13 | 0.11 | 0.06 | 0.1 |
| Na2O | 0.21 | 0.24 | 0.16 | 0.15 | 0.18 | 0.24 | 0.38 | 0.14 | 0.26 | 0.15 | 0.28 | 3.18 |
| K2O | 4.34 | 5.28 | 3.33 | 3.11 | 3.1 | 3.96 | 4.93 | 3.73 | 6.13 | 3.03 | 6.69 | 2.05 |
| P2O5 | 0.09 | 0.15 | 0.04 | 0.02 | 0.04 | 0.1 | 0.1 | 0.04 | 0.27 | 0.12 | 0.1 | 0.23 |
| LOI | 2.93 | 4.32 | 2.12 | 1.8 | 2.24 | 2.93 | 3.69 | 1.83 | 3.36 | 1.52 | 3.07 | 0.84 |
| Total | 100.42 | 99.87 | 99.72 | 100.09 | 100.41 | 100.56 | 100.71 | 100.38 | 100.07 | 100.36 | 100.7 | 98.48 |
| F | <100 | <100 | <100 | <100 | <100 | <100 | 200 | <100 | 300 | <100 | 200 | <100 |
| As | 16 | 102 | 15 | 19 | 22 | 78 | 17 | 18 | 175 | 24 | 40 | <5 |
| B | 170 | 180 | 90 | 80 | 150 | 170 | 280 | 40 | 80 | 170 | 170 | 20 |
| Ba | 788 | 1020 | 614 | 560 | 568 | 755 | 1090 | 647 | 900 | 422 | 968 | 6 |
| Be | <3 | 3 | <3 | <3 | 4 | 4 | <3 | <3 | 4 | <3 | 5 | 141 |
| Cr | 150 | 160 | 80 | 90 | 100 | 160 | 130 | 80 | 160 | 100 | 140 | 70 |
| Cs | 17.1 | 17 | 8.8 | 5.8 | 13.5 | 32.6 | 19.6 | 7.8 | 25.2 | 7.2 | 22.5 | 65.5 |
| Ga | 22.7 | 33.4 | 16.6 | 17.5 | 21.7 | 28.8 | 27.9 | 15.4 | 34.9 | 15 | 30.8 | 17.5 |
| Ge | 3 | 3.8 | 2.4 | 2.3 | 3 | 3.3 | 2.7 | 1.6 | 3.2 | 2.1 | 2.5 | 6.8 |
| Li | 355 | 676 | 167 | 129 | 298 | 497 | 231 | 44 | 108 | 50 | 130 | 8480 |
| Mo | 4 | <1 | 1 | 2 | <1 | 3 | <1 | <1 | <1 | 2 | 2 | 2 |
| Nb | 16.5 | 24.9 | 10.8 | 11.4 | 12.4 | 18.6 | 18.6 | 12.5 | 20.2 | 13.1 | 19.6 | 46.5 |
| Ni | 50 | 30 | 20 | 20 | 40 | 70 | 30 | 20 | 30 | 30 | 40 | 20 |
| Pb | 26.8 | 43.7 | 23.5 | 22 | 22.1 | 28.2 | 33 | 17.2 | 31.1 | 18.8 | 28.5 | 12 |
| Rb | 200 | 232 | 132 | 101 | 127 | 190 | 214 | 138 | 289 | 121 | 287 | 628 |
| Sn | 11.1 | 7 | 3.5 | 3.4 | 4.2 | 15.1 | 13.3 | 3.1 | 4.8 | 2.9 | 5 | 50.1 |
| Sr | 85 | 89 | 69 | 57 | 66 | 99 | 91 | 49 | 76 | 48 | 68 | 103 |
| Ta | 1.7 | 2.2 | 0.7 | 0.8 | 1.4 | 1.7 | 1.4 | 1.2 | 1.7 | 0.9 | 1.7 | 28.8 |
| Th | 20.5 | 27.7 | 9.4 | 12.9 | 22.5 | 19.8 | 21 | 24 | 22.5 | 21.3 | 19.7 | 0.5 |
| Tl | 1.2 | 1.1 | 0.7 | 0.4 | 0.8 | 1.1 | 1.1 | 0.9 | 1.3 | 0.6 | 1.6 | 3.7 |
| U | 4.1 | 6.3 | 2.3 | 2.6 | 4.2 | 4.4 | 4.6 | 5.1 | 4.9 | 4.5 | 4.1 | 12.7 |
| V | 63 | 113 | 38 | 35 | 46 | 82 | 94 | 37 | 104 | 36 | 101 | <5 |
| W | 10.9 | 11 | 7 | 5.8 | 4.4 | 8.7 | 7.2 | 4.4 | 7.6 | 3 | 8.9 | 1.3 |
| Y | 30.8 | 42.7 | 26.2 | 22.7 | 26 | 39.2 | 33 | 19.7 | 52.1 | 30.8 | 30 | 0.2 |
| Zn | 40 | 90 | 30 | <30 | 60 | 80 | 110 | <30 | 70 | 40 | 60 | 40 |
| La | 48.6 | 78.2 | 28.7 | 31.1 | 39.1 | 52.1 | 52.6 | 30.7 | 93.5 | 21.5 | 31 | <0.4 |
| Ce | 95.5 | 154 | 63 | 69.2 | 83 | 110 | 111 | 61.6 | 193 | 48.9 | 61.6 | <0.8 |
| Pr | 12.1 | 18.9 | 7.2 | 8.2 | 10.6 | 14.2 | 13.7 | 8.8 | 24.8 | 6.1 | 7.9 | <0.1 |
| Nd | 37.2 | 66 | 28.3 | 28.2 | 32.9 | 50.8 | 42 | 28.6 | 83.4 | 21.5 | 26.6 | <0.4 |
| Sm | 7.6 | 13.3 | 4.1 | 5.7 | 6.5 | 9.2 | 6.7 | 4.8 | 15.9 | 5.1 | 5.8 | <0.1 |
| Eu | 1.8 | 2.7 | 1.2 | 1.1 | 1.5 | 2 | 1.6 | 1 | 3.3 | 0.9 | 1.1 | <0.1 |
| Gd | 6.2 | 10.6 | 4.7 | 5.2 | 5.5 | 7.5 | 7.6 | 3 | 15.2 | 5.6 | 4.5 | 0.2 |
| Tb | 1.1 | 1.5 | 0.7 | 0.7 | 0.8 | 1.2 | 1 | 0.6 | 2.2 | 0.9 | 0.8 | <0.1 |
| Dy | 4.9 | 7.6 | 3.8 | 4.1 | 4.5 | 6.6 | 6.3 | 3.3 | 9.5 | 5.7 | 4.7 | <0.3 |
| Ho | 1.2 | 1.5 | 0.9 | 0.9 | 1 | 1.2 | 1.3 | 0.8 | 1.9 | 1.3 | 0.9 | <0.2 |
| Er | 3.7 | 4.6 | 2.8 | 2.6 | 3.2 | 3.5 | 3 | 2.3 | 5.6 | 3.5 | 3 | <0.1 |
| Tm | 0.5 | 0.8 | 0.3 | 0.4 | 0.4 | 0.5 | 0.6 | 0.5 | 0.7 | 0.7 | 0.5 | <0.1 |
| Yb | 4 | 5 | 2.6 | 2.8 | 3.8 | 4.1 | 3.8 | 3.7 | 5.2 | 4.1 | 3.3 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garate-Olave, I.; Roda-Robles, E.; Santos-Loyola, N.; Martins, T.; Lima, A.; Errandonea-Martin, J. Crystallization Sequence of the Spodumene-Rich Alijó Pegmatite (Northern Portugal) and Related Metasomatism on Its Host Rock. Minerals 2024, 14, 701. https://doi.org/10.3390/min14070701
Garate-Olave I, Roda-Robles E, Santos-Loyola N, Martins T, Lima A, Errandonea-Martin J. Crystallization Sequence of the Spodumene-Rich Alijó Pegmatite (Northern Portugal) and Related Metasomatism on Its Host Rock. Minerals. 2024; 14(7):701. https://doi.org/10.3390/min14070701
Chicago/Turabian StyleGarate-Olave, Idoia, Encarnación Roda-Robles, Nora Santos-Loyola, Tania Martins, Alexandre Lima, and Jon Errandonea-Martin. 2024. "Crystallization Sequence of the Spodumene-Rich Alijó Pegmatite (Northern Portugal) and Related Metasomatism on Its Host Rock" Minerals 14, no. 7: 701. https://doi.org/10.3390/min14070701
APA StyleGarate-Olave, I., Roda-Robles, E., Santos-Loyola, N., Martins, T., Lima, A., & Errandonea-Martin, J. (2024). Crystallization Sequence of the Spodumene-Rich Alijó Pegmatite (Northern Portugal) and Related Metasomatism on Its Host Rock. Minerals, 14(7), 701. https://doi.org/10.3390/min14070701






