Characterization of Kazakhstan’s Clays by Mössbauer Spectroscopy and X-ray Diffraction
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Fluorescence Analysis
3.2. X-ray Diffraction
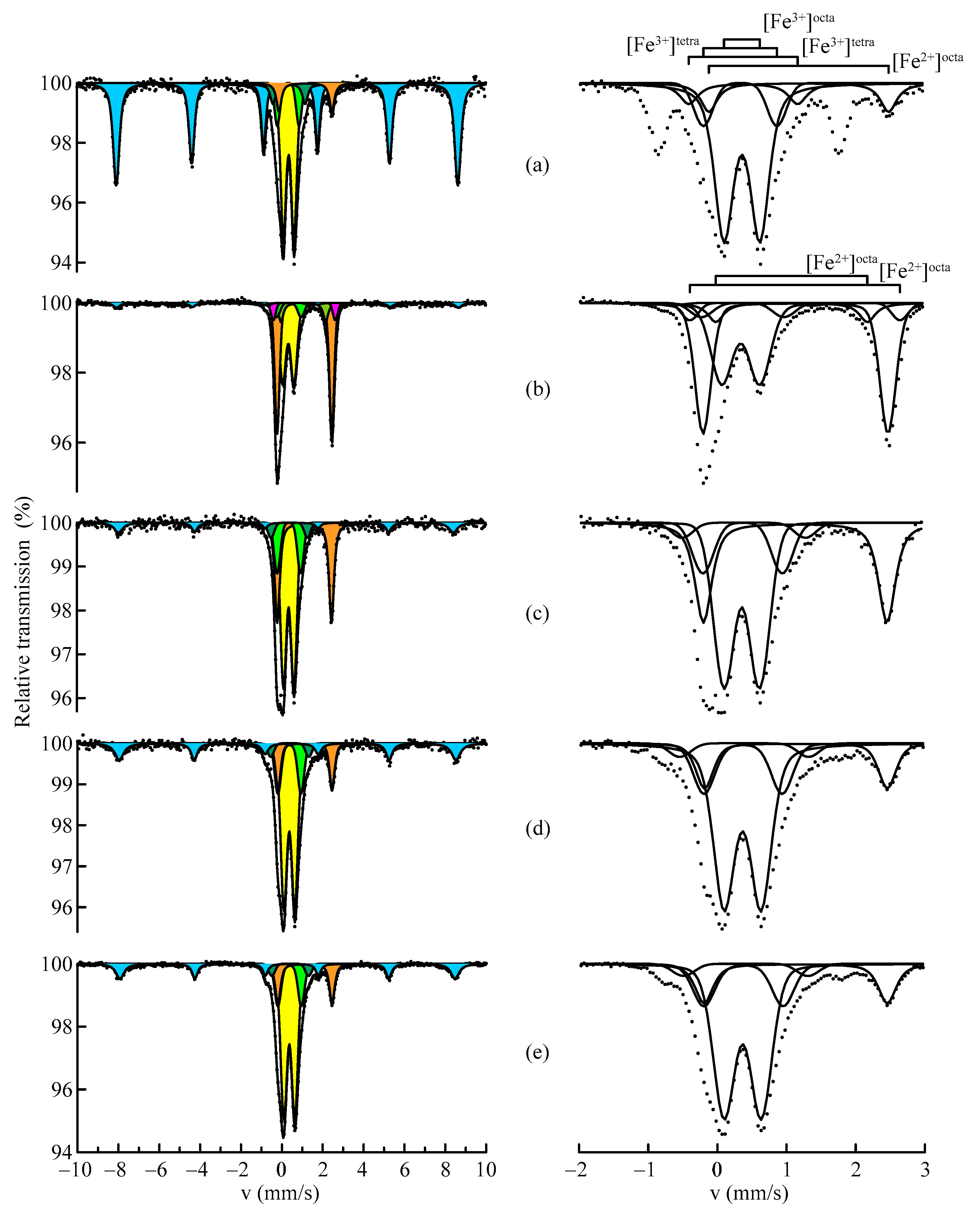
3.3. Mössbauer Spectroscopy
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tangari, A.C.; Le Pera, E.; Andò, S.; Garzanti, E.; Piluso, E.; Marinangeli, L.; Scarciglia, F. Soil-formation in the central Mediterranean: Insight from heavy minerals. Catena 2021, 197, 104998. [Google Scholar] [CrossRef]
- Biondino, D.; Borrelli, L.; Critelli, S.; Muto, F.; Apollaro, C.; Coniglio, S.; Tripodi, V.; Perri, F. A multidisciplinary approach to investigate weathering processes affecting gneissic rocks (Calabria, southern Italy). Catena 2020, 187, 104732. [Google Scholar] [CrossRef]
- Scarciglia, F.; Le Pera, E.; Critelli, S. The onset of the sedimentary cycle in a mid-latitude upland environment: Weathering, pedogenesis, and geomorphic processes on plutonic rocks (Sila Massif, Calabria). In Sedimentary Provenance and Petrogenesis: Perspectives from Petrography and Geochemistry; Arribas, J., Critelli, S., Johnsson, M.J., Geol, S., Am, S., Eds.; GeoScienceWorld: McLean, VA, USA, 2007; Volume 420, pp. 149–166. [Google Scholar]
- Bergaya, F.; Lagaly, G. Chapter 1—General introduction: Clays, clay minerals and clay science. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Oxford, UK, 2013; Volume 5, pp. 1–19. [Google Scholar]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. In Clay and Clay Miner; Do Nascimento, G., Ed.; Intech Open Journals: London, UK, 2021; pp. 1–29. [Google Scholar]
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA nomenclature and CMS nomenclature committees. Clay Miner. 1995, 30, 257–259. [Google Scholar] [CrossRef]
- Arsenovic, M.V.; Pezo, L.; Radojevic, Z.M.; Stankovic, S.M. Serbian heavy clays behavior: Application in rough ceramics. Hem. Ind. 2013, 67, 811–822. [Google Scholar] [CrossRef]
- Barton, C.D.; Karathanasis, A.D. Clay Minerals. In Encyclopedia of Soil Science, 2nd ed.; Lal, R., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 187–192. [Google Scholar]
- Varadachari, C. Clay mineral equilibra: Fuzzy phase diagrams. Clay Res. 2017, 36, 1–16. [Google Scholar]
- Schoonheydt, R.A. Reflections on the material science of clay minerals. Appl. Clay Sci. 2016, 131, 107–112. [Google Scholar] [CrossRef]
- Dai, T.; Liu, T.; Zheng, T.; Fang, C.; Zheng, S.; Lei, G. Effect of waste clay brick powder on microstructure and properties in blended oil well cement pastes at HTHP conditions. Geoenergy Sci. Eng. 2024, 237, 212823. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Xi, Y. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Muñoz, P.; Letelier, V.; Muñoz, L.; Gencel, O.; Sutcu, M.; Vasić, M. Assessing technological properties and environmental impact of fired bricks made by partially adding bottom ash from an industrial approach. Constr. Build. Mater. 2023, 396, 132338. [Google Scholar] [CrossRef]
- Meseguer, S.; Pardo, F.; Jordan, M.M.; Sanfeliu, T.; González, I. Ceramic behaviour of some kaolins from Cauquenes Province (VII Region of Maule, Chile). Appl. Clay Sci. 2011, 52, 414–418. [Google Scholar] [CrossRef]
- Ferrage, E.; Hubert, F.; Dabat, T.; Asaad, A.; Dazas, B.; Grégoire, B.; Savoye, S.; Tertre, E. Anisotropy in particle orientation controls water diffusion in clay materials. Appl. Clay Sci. 2023, 244, 107117. [Google Scholar] [CrossRef]
- Charlet, L.; Alt-Epping, P.; Wersin, P.; Gilbert, B. Diffusive transport and reaction in clay rocks: A storage (nuclear waste, CO2, H2), energy (shale gas) and water quality issue. Adv. Water Resour. 2017, 106, 39–59. [Google Scholar] [CrossRef]
- Das, P.; Bharat, T.V. Kaolin based protective barrier in municipal landfills against adverse chemo-mechanical loadings. Sci. Rep. 2021, 11, 10354. [Google Scholar] [CrossRef]
- Smith, M.; Montgomery, C. Hydraulic Fracturing (Emerging Trends and Technologies in Petroleum Engineering), 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; 812p. [Google Scholar]
- Speight, J.G. Handbook of Hydraulic Fracturing, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; 299p. [Google Scholar]
- Miskimins, J. Hydraulic Fracturing: Fundamentals and Advancements; Society of Petroleum Engineers: Richardson, TX, USA, 2019; 784p. [Google Scholar]
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.; Li, L. A comprehensive review on proppant technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Montgomery, C.T.; Smith, M.B. Hydraulic fracturing: History of an enduring technology. J. Petrol. Technol. 2010, 62, 26–41. [Google Scholar] [CrossRef]
- Yarmonov, A.N. Study of the use of high alumina cement in the production of proppants for hydraulic fracturing. Bull. PNRPU Mech. Eng. Mater. Sci. 2018, 20, 95–107. [Google Scholar] [CrossRef]
- Biryukova, A.A.; Dzhienalyev, T.D.; Tikhonova, T.A. Ceramic proppants based on Kazakhstan natural alumosilicate resources. Refract. Ind. Ceram. 2017, 58, 269–275. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Lin, S.; Wu, W. Composition and Method for Producing an Ultra-Lightweight Ceramic Proppant. U.S. Patent US8727003B2, 20 May 2014. [Google Scholar]
- Rusinov, P.; Balashov, A. Ceramic Proppant and Its Production Method. Russia Patent RU2615563C9, 5 April 2017. [Google Scholar]
- Marinangeli, L.; Pompilio, L.; Baliva, A.; Billotta, S.; Bonanno, G.; Domeneghetti, M.C.; Fioretti, A.M.; Menozzi, O.; Nestola, F.; Piluso, E.; et al. Development of an ultra-miniaturised XRD/XRF instrument for the in situ mineralogical and chemical analysis of planetary soils and rocks: Implication for archaeometry. Rend. Fis. Acc. Lincei 2015, 26, 529–537. [Google Scholar] [CrossRef]
- Środoń, J. Chapter 12.2—Identification and quantitative analysis of clay minerals. In Handbook of Clay Science; Bergaya, F., Benny, K.G., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Oxford, UK, 2006; Volume 1, pp. 765–787. [Google Scholar]
- Bohor, B.F.; Randall, H.E. Scanning electron microscopy of clays and clay minerals. Clays Clay Miner. 1971, 19, 49–54. [Google Scholar] [CrossRef]
- Mielenz, R.C.; Schieltz, N.C.; King, M.E. Thermogravimetric analysis of clay and clay-like minerals. Clays Clay Miner. 1953, 2, 285–314. [Google Scholar] [CrossRef]
- Goldanskii, V.; Herber, R. Chemical Applications of Mössbauer Spectroscopy; Academic Press: New York, NY, USA, 1968; 701p. [Google Scholar]
- Gütlich, P.; Bill, E.; Trautwein, A.X. Mössbauer Spectroscopy and Transition Metal Chemistry: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2011; 569p. [Google Scholar]
- Shokanov, A.; Vereshchak, M.; Manakova, I. Mössbauer and X-ray studies of phase composition of fly ashes formed after combustion of Ekibastuz coal (Kazakhstan). Metals 2020, 10, 929. [Google Scholar] [CrossRef]
- Vereshchak, M.; Shokanov, A.; Manakova, I. Mössbauer studies of narrow fractions of fly ash formed after combustion of Ekibastuz coal. Materials 2021, 14, 7437. [Google Scholar] [CrossRef]
- Vereshchak, M.; Shokanov, A.; Manakova, I.; Migunova, A. Mössbauer and X-ray diffraction spectroscopy of high-iron bauxites from Kazakhstan. Materials 2023, 16, 6706. [Google Scholar] [CrossRef]
- Ikeoka, R.A.; Appoloni, C.R.; Scorzelli, R.B.; Santos, E.; Rizzutto, M.A.; Bandeira, A.M. Study of ancient pottery from the Brazilian Amazon coast by EDXRF, PIXE, XRD, Mössbauer spectroscopy and computed radiography. Minerals 2022, 12, 1302. [Google Scholar] [CrossRef]
- Wagner, F.E.; Wagner, U. Mössbauer spectra of slays and seramics. Hyperfine Interact. 2004, 154, 35–82. [Google Scholar] [CrossRef]
- Häusler, W. Firing of slays studied by X-ray diffraction and Mössbauer spectroscopy. Hyperfine Interact. 2004, 154, 121–141. [Google Scholar] [CrossRef]
- Kuzmann, E.; Nagy, S.; Vértes, A. Critical review of analytical applications of Mössbauer spectroscopy illustrated by mineralogical and geological examples (IUPAC Technical Report). Pure Appll. Chem. 2003, 75, 801–858. [Google Scholar] [CrossRef]
- Murad, E.; Cashion, J. Mössbauer Spectroscopy of Environmental Materials and Their Industrial Utilization, 1st ed.; Springer: New York, NY, USA, 2004; 436p. [Google Scholar]
- Murad, E.; Wagner, U. Mössbauer spectra of kaolinite, halloysite and the firing products of kalonite: New results and a reappraisal of published work. Neues Jahrb. Miner. 1991, 162, 281–309. [Google Scholar]
- Murad, E.; Fabris, J.D. Kaolin mining and beneficiation: The role of iron. J. Phys. Conf. Ser. 2010, 217, 012066. [Google Scholar] [CrossRef]
- St. Pierre, T.G.; Singh, B.; Webb, J.; Gilkes, B. Mössbauer spectra of soil kaolins from South-Western Australia. Clays Clay Miner. 1992, 40, 341–346. [Google Scholar] [CrossRef]
- Komusinski, J.; Stoch, L.; Dubiel, S.M. Application of electron paramagnetic resonance and Mossbauer spectroscopy in the investigation of kaolinite-group minerals. Clays Clay Miner. 1981, 29, 23–30. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Shvanskaya, L.V.; Volkova, O.S.; Koshelev, A.V.; Zvereva, E.A.; Raganyan, G.V.; Presniakov, I.; Sobolev, A.V.; Abakumov, A.M.; Lvov, Y.M. Magnetism of natural composite of halloysite clay nanotubes Al2Si2O5(OH)4 and amorphous hematite Fe2O3. Mater. Charact. 2017, 129, 179–185. [Google Scholar] [CrossRef]
- Heller-Kallai, L.; Rosenson, I. The use of Mössbauer spectroscopy of iron in clay mineralogy. Phys. Chem. Miner. 1981, 7, 223–238. [Google Scholar] [CrossRef]
- Mashlan, M.; Martinec, P.; Kašlík, J.; Kovářová, E.; Scucka, J. Mössbauer study of transformation of Fe cations during thermal treatment of glauconite in air. IP Conf. Proc. 2012, 1489, 169–173. [Google Scholar]
- Dyar, M.D.; Agresti, D.G.; Schaefer, M.W.; Grant, C.A.; Sklute, E.C. Mössbauer spectroscopy of earth and planetary materials. Annu. Rev. Earth Planet. Sci. 2006, 34, 83–125. [Google Scholar] [CrossRef]
- Ali, A.M.; Hsia, Y.; Liu, R.C.; Zhang, J.; Duan, W.; Chen, L. A Mössbauer study of evolution of glauconite from Chinese seas. Spectrosc. Lett. 2001, 34, 701–708. [Google Scholar] [CrossRef]
- Murad, E.; Wagner, U. Mössbauer study of pure illite and its firing products. Hyperfine Interact. 1994, 91, 685–688. [Google Scholar] [CrossRef]
- Murad, E.; Wagner, U. The Mössbauer spectrum of illite. Clay Miner. 1994, 29, 1–10. [Google Scholar] [CrossRef]
- Pyataev, A.V.; Valiulina, S.I.; Ivanova, A.G.; Manapov, R.A.; Voronina, E.V. Traditional pottery raw Materials of the Bilyarsk monocentric agglomeration. Bull. Russ. Acad. Sci. Phys. 2015, 79, 1058–1061. [Google Scholar] [CrossRef]
- Valiulina, S.I.; Pyataev, A.V.; Ivanova, A.G.; Manapov, R.A.; Voronina, E.V. Mössbauer studies of moulded Kama–Cis-Urals ceramics. Archaeometry 2018, 60, 1237–1250. [Google Scholar] [CrossRef]
- Shabani, A.A.T.; Rancourt, D.G.; Lalonde, A.E. Determination of cis and trans Fe2+ populations in 2M1 muscovite by Mössbauer spectroscopy. Hyperfine Interact. 1998, 117, 117–129. [Google Scholar] [CrossRef]
- Schmidt, W.; Pietzsch, C. Iron distribution and geochemistry of pegmatitic dioctaedral 2M1 micas. Chem. Erde 1990, 50, 27–38. [Google Scholar]
- Hunziker, J.C.; Frey, M.; Clauer, N.; Dallmeyer, R.D.; Friedriehsen, H.; Flehmig, W.; Hoehstrasser, K.; Roggwiler, P.; Schwander, H. The evolution of illite to muscovite: Mineralogical and isotopic data from the Glarus Alps, Switzerland. Contrib. Mineral. Petrol. 1986, 92, 157–180. [Google Scholar] [CrossRef]
- Bogdanov, A.A. Basic features of the Paleozoic structure of Central Kazakhstan. Int. Geol. Rev. 1960, 2, 781–810. [Google Scholar] [CrossRef]
- Burtman, V.S.; Dvorova, A.V. Kokchetau-Issykkul terrain of the Kazakhstan Paleozoic continent and the Ordovician latitude of the continent. Lithosph. (Russ. Fed.) 2019, 19, 519–532. [Google Scholar] [CrossRef]
- Thomas, J.C.; Cobbold, P.R.; Shein, V.S.; Le Douaran, S. Sedimentary record of late Paleozoic to recent tectonism in central Asia—Analysis of subsurface data from the Turan and south Kazak domains. Tectonophysics 1999, 313, 243–263. [Google Scholar] [CrossRef]
- Natal’in, B.A.; Şengör, A.M.C. Late Palaeozoic to Triassic evolution of the Turan and Scythian platforms: The pre-history of the Palaeo-Tethyan closure. Tectonophysics 2005, 404, 175–202. [Google Scholar] [CrossRef]
- Zonenshain, L.P.; Korinevsky, V.G.; Kazmin, V.G.; Pechersky, D.M.; Khain, V.V.; Matveenkov, V.V. Plate tectonic model of the South Urals development. Tectonophysics 1984, 109, 95–135. [Google Scholar] [CrossRef]
- Grachev, A.F.; Nikolaev, V.A.; Nikolaev, V.G. East European platform development in the Late Precambrian and Paleozoic: Structure and sedimentation. Russ. J. Earth Sci. 2006, 8, 1–22. [Google Scholar] [CrossRef][Green Version]
- Kheraskova, T.N.; Volozh, Y.A.; Antipov, M.P.; Bykadorov, V.A.; Postnikova, I.S. Features of the structure and development of the southeastern part of the East European platform and the Caspian basin in the late Precambrian-early Paleozoic. Geotectonics 2020, 54, 628–651. [Google Scholar] [CrossRef]
- Brookfield, M.E. Geological development and Phanerozoic crustal accretion in the western segment of the southern Tien Shan (Kyrgyzstan, Uzbekistan and Tajikistan). Tectonophysics 2000, 328, 1–14. [Google Scholar] [CrossRef]
- Biske, Y.S.; Konopelko, D.L.; Seltmann, R. Paleozoic collisional belt of the South Tien Shan: A review. Earth-Sci. Rev. 2024, 248, 104637. [Google Scholar] [CrossRef]
- Silachyov, I.; Akymbek, Y. Element content of the samples of medieval ceramics from Southern Kazakhstan: Searching the way of preliminary differentiation. J. Radioanal. Nucl. Chem. 2023, 332, 3799–3811. [Google Scholar] [CrossRef]
- Solodukhin, V.; Silachyov, I.; Poznyak, V.; Gorlachyov, I. Development of the complex of nuclear-physical methods of analysis for geology and technology tasks in Kazakhstan. J. Radioanal. Nucl. Chem. 2016, 309, 125–134. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar]
- Xiao, J.; Song, Y.; Li, Y. Comparison of quantitative X-ray diffraction mineral analysis Methods. Minerals 2023, 13, 566. [Google Scholar] [CrossRef]
- Cuevas, J.; Cabrera, M.A.; Fernandez, C.; Mota-Heredia, C.; Fernandez, R.; Torres, E.; Turrero, M.J.; Ruiz, A.I. Bentonite powder XRD quantitative analysis using Rietveld refinement: Revisiting and updating bulk semiquantitative mineralogical compositions. Minerals 2022, 12, 772. [Google Scholar] [CrossRef]
- Mashlan, M.; Kholmetskii, A.; Yevdokimov, V.; Pechousek, J.; Verich, O.; Zboril, R.; Tsonchev, R. Mössbauer spectrometer with resonant detector. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 243, 241–246. [Google Scholar] [CrossRef]
- Belyaev, A.A.; Volodin, V.S.; Irkaev, S.M.; Panchuk, V.V.; Semenov, V.G. Application of resonant detectors in Mössbauer spectroscopy. Bull. Russ. Acad. Sci. Phys. 2010, 74, 412–415. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489, 178–185. [Google Scholar]
- Stevens, J.G.; Khasanov, A.M.; Miller, J.W.; Pollak, H.; Li, Z. Mössbauer Mineral Handbook; Mössbauer Effect Data Center: Asheville, NC, USA, 1998; 527p. [Google Scholar]
- Betekhtin, A.G. A Mineralogy Course; House University: Moscow, Russia, 2008; 736p. [Google Scholar]
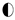 —hematite,
—hematite,  —kaolinite,
—kaolinite,  —muscovite,
—muscovite,  —illite,
—illite,  —quartz,
—quartz,  —albite,
—albite,  —calcite,
—calcite, 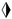 —dolomite,
—dolomite,  —microcline, and
—microcline, and  —dellaite.
—dellaite.
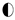 —hematite,
—hematite,  —kaolinite,
—kaolinite,  —muscovite,
—muscovite,  —illite,
—illite,  —quartz,
—quartz,  —albite,
—albite,  —calcite,
—calcite, 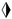 —dolomite,
—dolomite,  —microcline, and
—microcline, and  —dellaite.
—dellaite.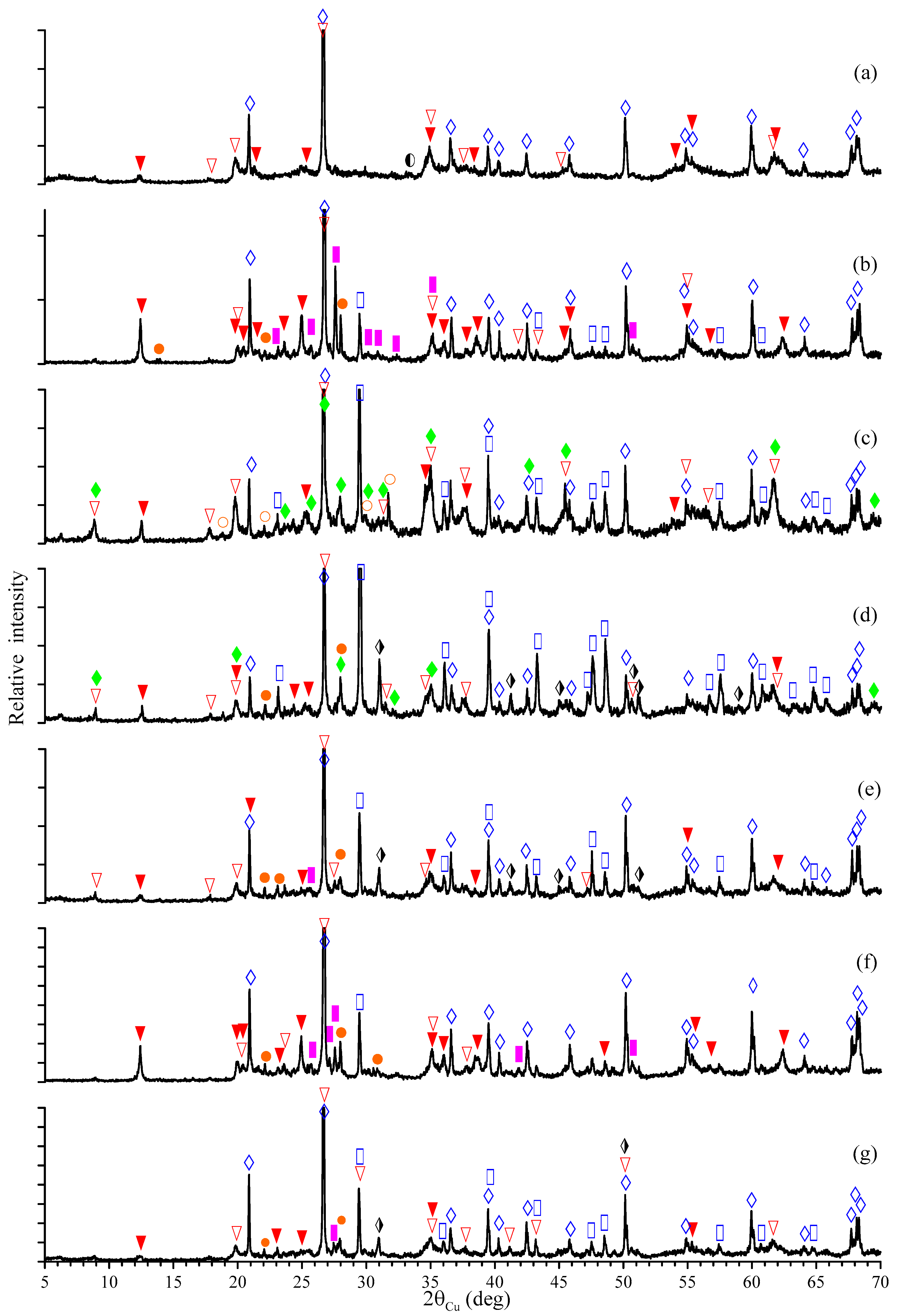
| Sample | Deposit | Geographic Coordinates |
|---|---|---|
| 1 | Kalzhat, Kalzhat village, Almaty region | 43°30′32″ N, 80°40′22″ E |
| 2 | Koskudyk, Kunaev city, Almaty region | 44°04′37″ N, 77°24′49″ E |
| 3 | Aktash, Tashkent region | 41°36′51″ N, 69°46′08″ E |
| 4 | Turkestan, Turkestan region | 43°15′53″ N, 68°23′14″ E |
| 5 | Soyuznoye, Aktobe region | 50°50′05″ N, 60°09′33″ E |
| 6 | Alekseevskoye, Akmola region | 53°30′35″ N, 68°31′12″ E |
| 7 | Aktobe, Aktobe region | 50°21′28″ N, 57°07′09″ E |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | MgO | Na2O | MnO | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57.16 | 16.95 | 7.46 | 0.37 | 3.02 | 1.23 | - | - | - | - | 13.44 |
| 2 | 61.87 | 20.94 | 0.67 | 3.27 | 2.72 | 0.57 | 0.57 | - | - | - | 9.25 |
| 3 | 53.63 | 16.12 | 5.43 | 5.94 | 5.80 | 0.73 | 0.33 | - | - | - | 11.76 |
| 4 | 43.70 | 9.29 | 5.28 | 19.46 | 3.62 | 0.57 | 0.76 | 0.99 | 0.99 | - | 16.82 |
| 5 | 52.83 | 13.72 | 6.08 | 7.36 | 3.51 | 0.87 | 0.63 | 0.68 | 0.68 | - | 13.53 |
| 6 | 57.65 | 18.36 | 0.78 | 8.16 | 2.58 | 1.00 | 0.69 | - | - | - | 10.54 |
| 7 | 52.61 | 13.20 | 6.27 | 7.51 | 3.59 | 0.92 | 0.51 | 0.61 | 0.61 | - | 14.38 |
| Sample | Hematite | Kaolinite | Muscovite | Illite | Glauconite | Quartz | Albite | Calcite | Dolomite | Microcline | Dellaite |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.22 | 55.73 | - | 24.14 | 1.30 | 18.61 | - | - | - | - | - |
| 2 | - | 38.43 | - | 12.17 | - | 30.98 | 6.16 | 3.54 | - | 8.72 | - |
| 3 | - | 11.72 | 10.06 | 37.77 | - | 10.96 | 2.83 | 7.16 | - | - | 19.50 |
| 4 | - | 10.84 | 10.10 | 27.15 | - | 13.79 | 6.19 | 25.06 | 6.87 | - | - |
| 5 | - | 24.61 | - | 26.64 | 2.49 | 25.31 | 6.17 | 7.26 | 3.54 | 3.98 | - |
| 6 | - | 39.07 | - | 8.05 | - | 33.38 | 6.09 | 0.83 | - | 12.58 | - |
| 7 | - | 16.43 | - | 30.16 | 1.33 | 29.57 | 7.26 | 7.94 | 4.23 | 3.08 | - |
| Sample | Kaolinite | ||
|---|---|---|---|
| a | b | c | |
| 1 | 5.179 | 8.980 | 7.738 |
| 2 | 5.159 | 8.933 | 7.438 |
| 3 | 5.190 | 8.980 | 7.440 |
| 4 | 5.190 | 8.980 | 7.440 |
| 5 | 5.181 | 8.978 | 7.381 |
| 6 | 5.161 | 8.940 | 7.403 |
| 7 | 5.175 | 8.978 | 7.387 |
| Sample | Component | A, aт.% | δ, mm/s | Δ/2ε, mm/s | H, kOe | Fe2+/Fe3+ |
|---|---|---|---|---|---|---|
| 1 | S | 16 ± 1 | 0.36 ± 0.04 | −0.21 ± 0.04 | 511 ± 1 | 0.12 |
| D1 | 41 ± 4 | 0.36 ± 0.04 | 0.45 ± 0.04 | |||
| D2 | 18 ± 3 | 0.71 ± 0.04 | ||||
| D3 | 10 ± 2 | 1.12 ± 0.04 | ||||
| D4 | 6 ± 1 | 1.50 ± 0.04 | ||||
| D5 | 9 ± 1 | 1.12 ± 0.04 | 2.66 ± 0.04 | |||
| 3 | S | 6 ± 1 | 0.38 ± 0.05 | −0.21 ± 0.05 | 514 ± 3 | 1.29 |
| D1 | 12 ± 1 | 0.33 ± 0.04 | 0.49 ± 0.04 | |||
| D2 | 23 ± 1 | 0.62 ± 0.04 | ||||
| D3 | 5 ± 1 | 1.05 ± 0.04 | ||||
| D4 | 1 ± 1 | 1.61 ± 0.05 | ||||
| D5 | 45 ± 2 | 1.14 ± 0.04 | 2.67 ± 0.04 | |||
| D6 | 5 ± 1 | 1.94 ± 0.04 | ||||
| D7 | 3 ± 2 | 2.97 ± 0.06 | ||||
| 4 | S | 8 ± 1 | 0.35 ± 0.04 | −0.25 ± 0.04 | 509 ± 2 | 0.46 |
| D1 | 16 ± 1 | 0.36 ± 0.04 | 0.40 ± 0.07 | |||
| D2 | 29 ± 1 | 0.58 ± 0.04 | ||||
| D3 | 13 ± 1 | 1.16 ± 0.04 | ||||
| D4 | 5 ± 1 | 1.79 ± 0.04 | ||||
| D5 | 26 ± 1 | 1.14 ± 0.04 | 2.66 ± 0.04 | |||
| D6 | 3 ± 1 | 2.03 ± 0.04 | ||||
| 5 | S | 14 ± 1 | 0.38 ± 0.04 | −0.20 ± 0.04 | 512 ± 1 | 0.18 |
| D1 | 33 ± 3 | 0.36 ± 0.04 | 0.50 ± 0.04 | |||
| D2 | 23 ± 1 | 0.60 ± 0.04 | ||||
| D3 | 11 ± 3 | 1.16 ± 0.04 | ||||
| D4 | 6 ± 1 | 1.92 ± 0.04 | ||||
| D5 | 13 ± 1 | 1.14 ± 0.04 | 2.63 ± 0.04 | |||
| 7 | S | 13 ± 1 | 0.38 ± 0.04 | −0.20 ± 0.04 | 510 ± 1 | 0.19 |
| D1 | 28 ± 2 | 0.36 ± 0.04 | 0.49 ± 0.04 | |||
| D2 | 30 ± 1 | 0.59 ± 0.04 | ||||
| D3 | 11 ± 2 | 1.23 ± 0.04 | ||||
| D4 | 4 ± 1 | 1.98 ± 0.04 | ||||
| D5 | 12 ± 1 | 1.14 ± 0.04 | 2.65 ± 0.04 | |||
| D6 | 2 ± 1 | 1.81 ± 0.04 |
| Sample | Hematite | Kaolinite | Muscovite | Illite | Glauconite |
|---|---|---|---|---|---|
| 1 | 16 | 46 | - | 32 | 6 |
| 3 | 6 | 16 | 13 | 55 | 10 |
| 4 | 8 | 23 | - | 60 | 9 |
| 5 | 14 | 38 | - | 40 | 8 |
| 7 | 13 | 32 | - | 48 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokanov, A.; Manakova, I.; Vereshchak, M.; Migunova, A. Characterization of Kazakhstan’s Clays by Mössbauer Spectroscopy and X-ray Diffraction. Minerals 2024, 14, 713. https://doi.org/10.3390/min14070713
Shokanov A, Manakova I, Vereshchak M, Migunova A. Characterization of Kazakhstan’s Clays by Mössbauer Spectroscopy and X-ray Diffraction. Minerals. 2024; 14(7):713. https://doi.org/10.3390/min14070713
Chicago/Turabian StyleShokanov, Adilkhan, Irina Manakova, Mikhail Vereshchak, and Anastassiya Migunova. 2024. "Characterization of Kazakhstan’s Clays by Mössbauer Spectroscopy and X-ray Diffraction" Minerals 14, no. 7: 713. https://doi.org/10.3390/min14070713
APA StyleShokanov, A., Manakova, I., Vereshchak, M., & Migunova, A. (2024). Characterization of Kazakhstan’s Clays by Mössbauer Spectroscopy and X-ray Diffraction. Minerals, 14(7), 713. https://doi.org/10.3390/min14070713






