Study of a Copper Oxide Leaching in Alkaline Monosodium Glutamate Solution
Abstract
:1. Introduction
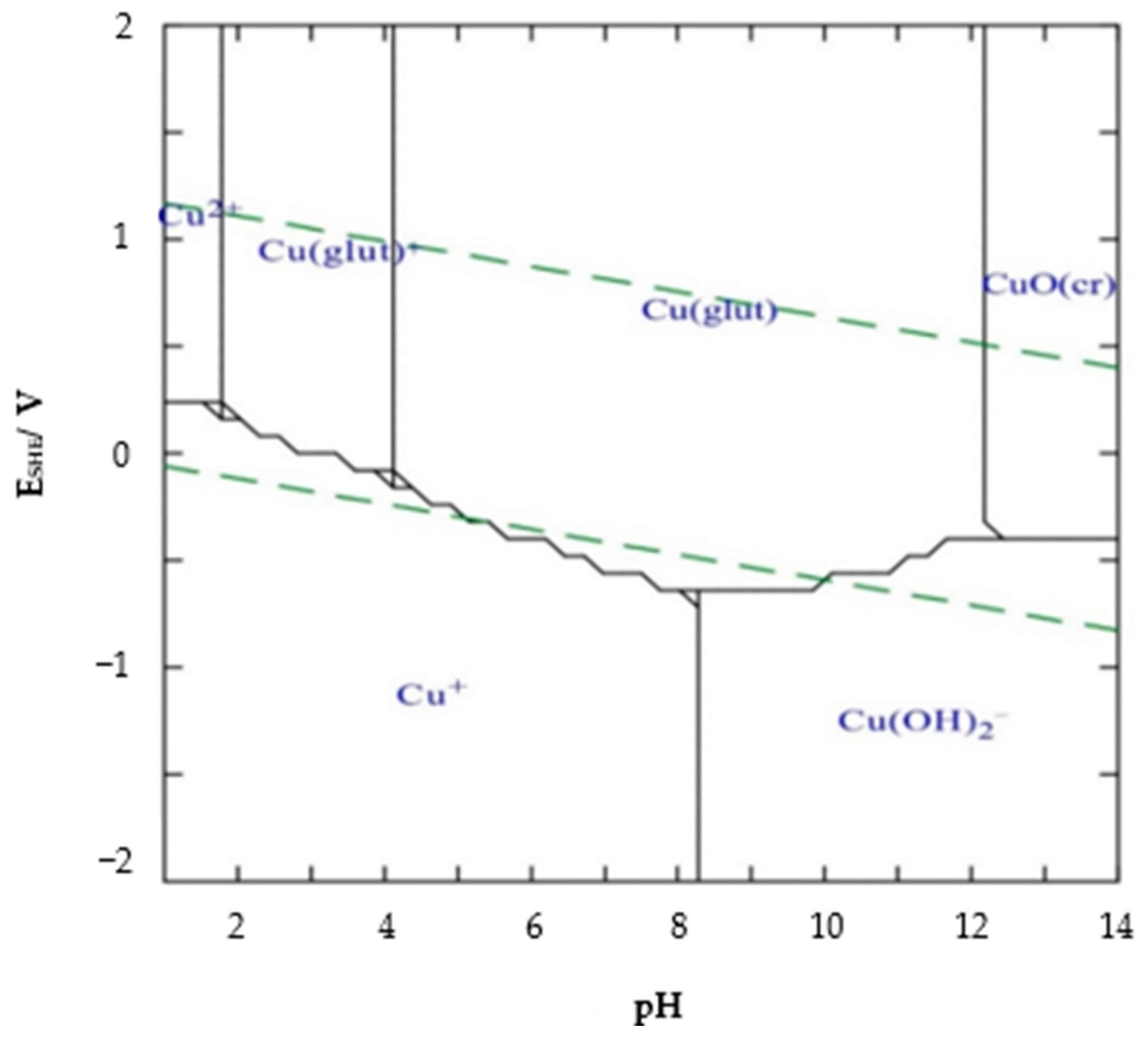
Chemical Composition and Leaching Mechanisms for Tenorite
2. Materials and Methods
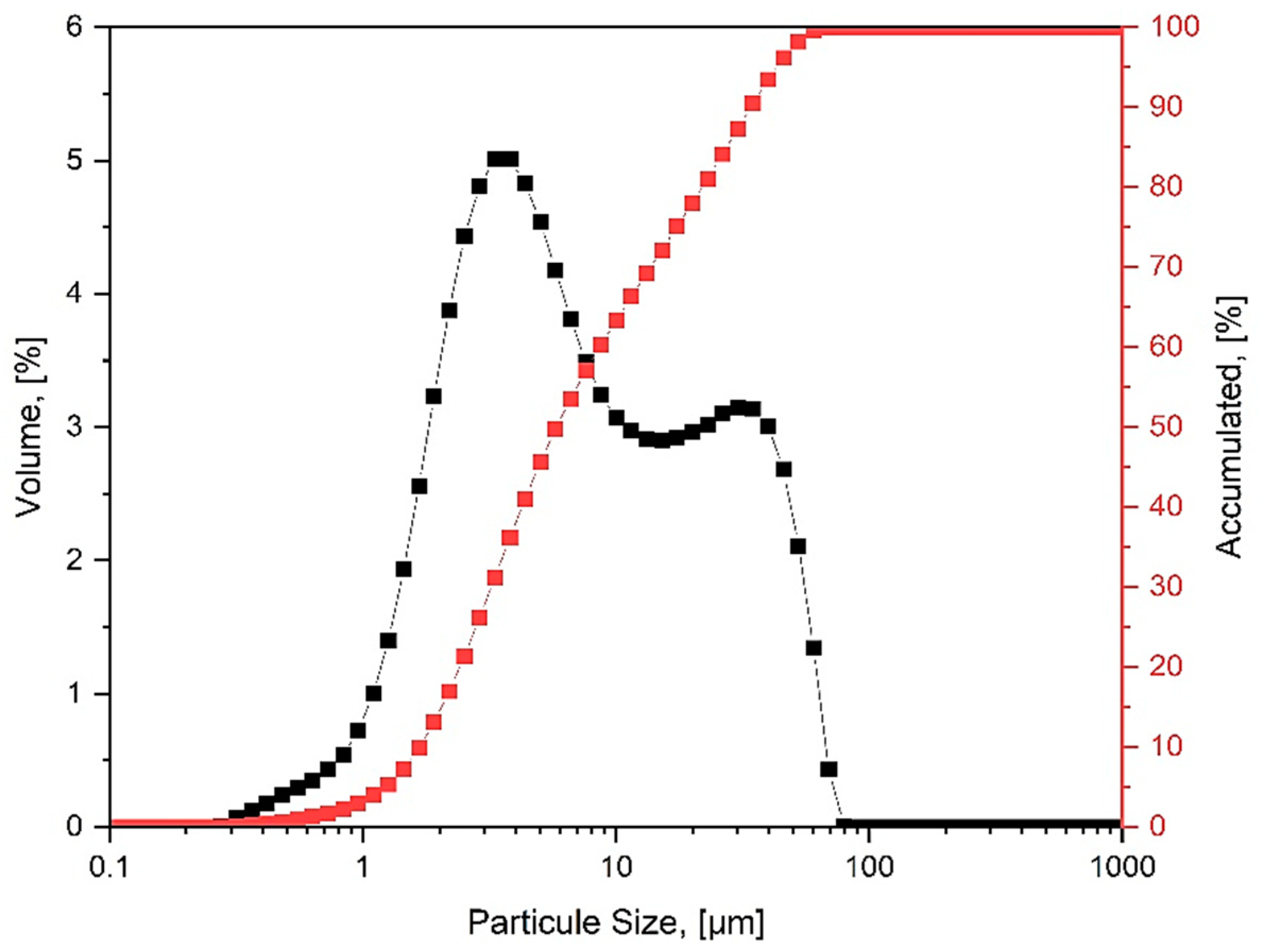
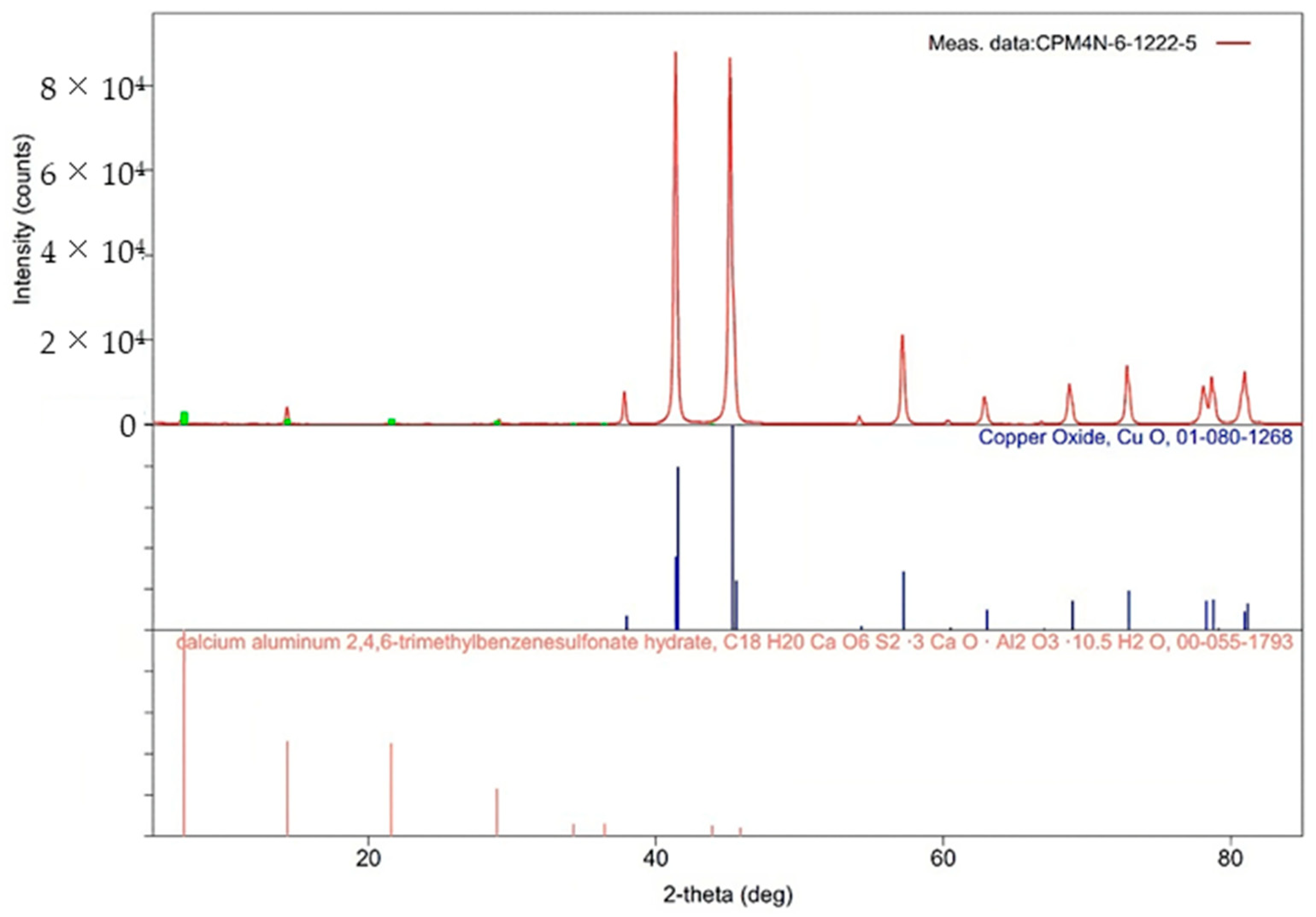
2.1. Materials
2.2. Methods
2.3. Kinetic Model
2.3.1. Rate Controlling Factor Based on the Shrinking Core Model (SCM)
- Diffusion from the main body of the solution to the solid surface through the liquid film;
- Reaction between the solid and reactant at the surface;
- Diffusion from the solid surface into the solution through the film.
- The particles are assumed to have an average size of less than 39 μm;
- Spherical particles are assumed. That is, the effects that the shape of the particle may generate are neglected;
- The effect of product concentration is neglected;
- The concentration of MSG is assumed constant because, with the excess of MSG and the low content of solids used, the change in concentration would be too small, which can be considered negligible.
2.3.2. Rate Controlling Factor Based on the Activation Energies
2.3.3. The Effect of PSD on the SCM
3. Results and Discussion
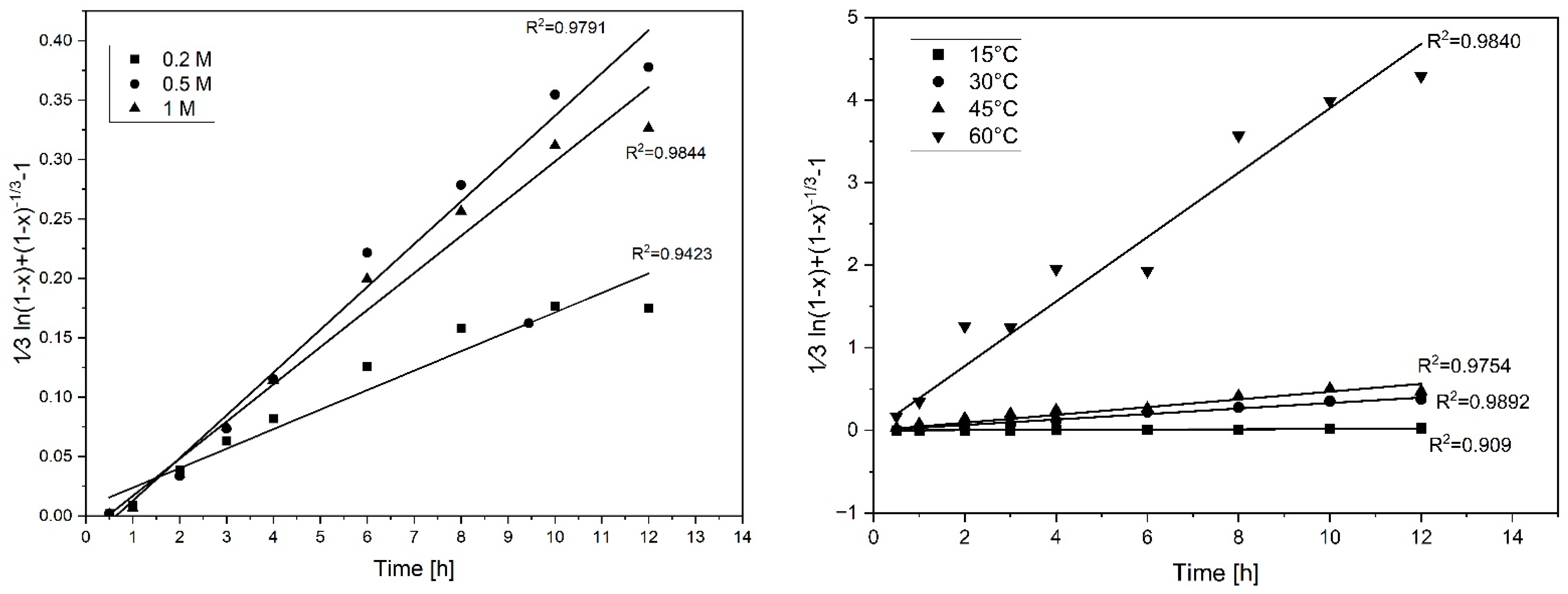
3.1. Assessment of Monosodium Glutamate Concentration
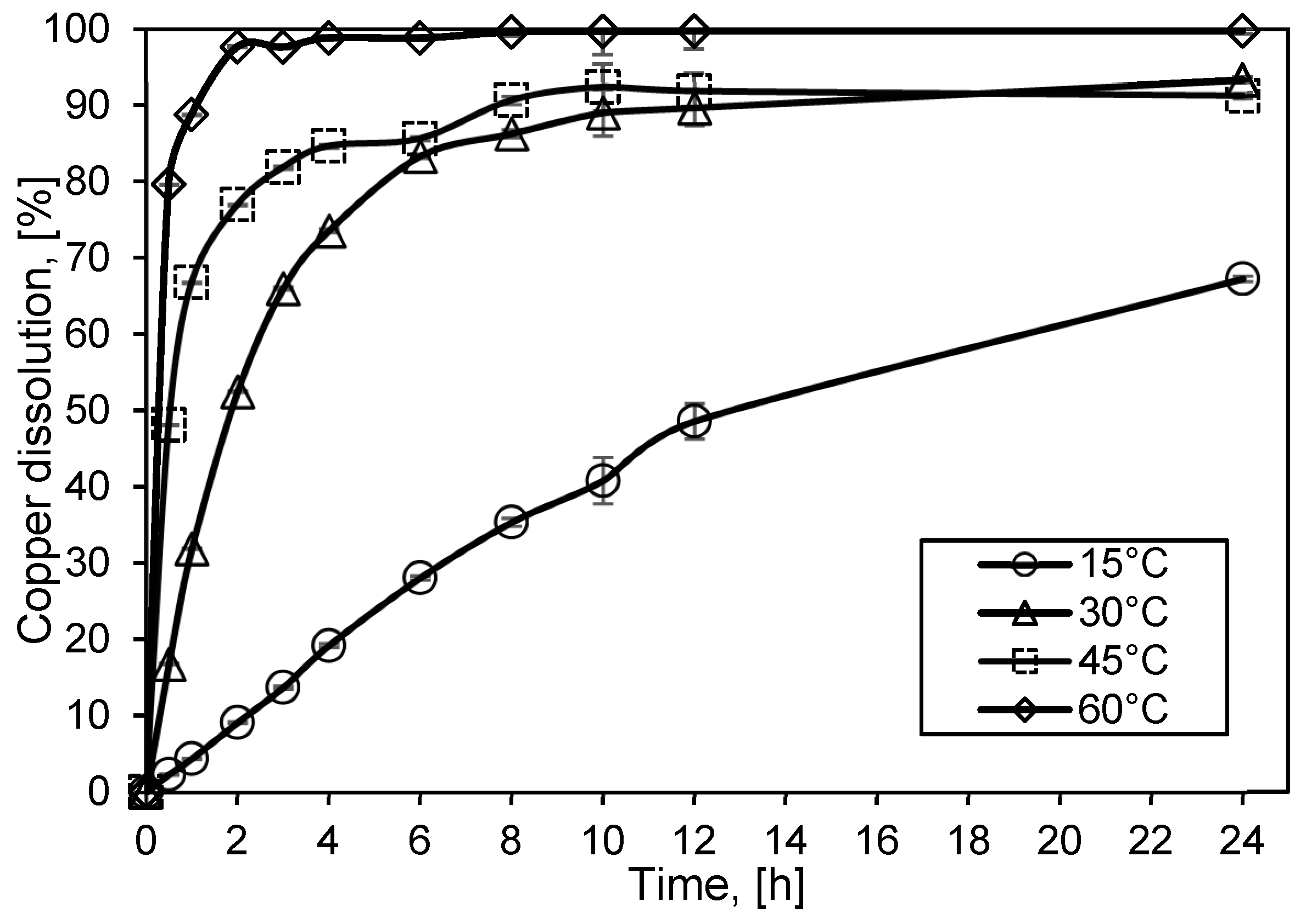
3.2. Assessment of Temperature
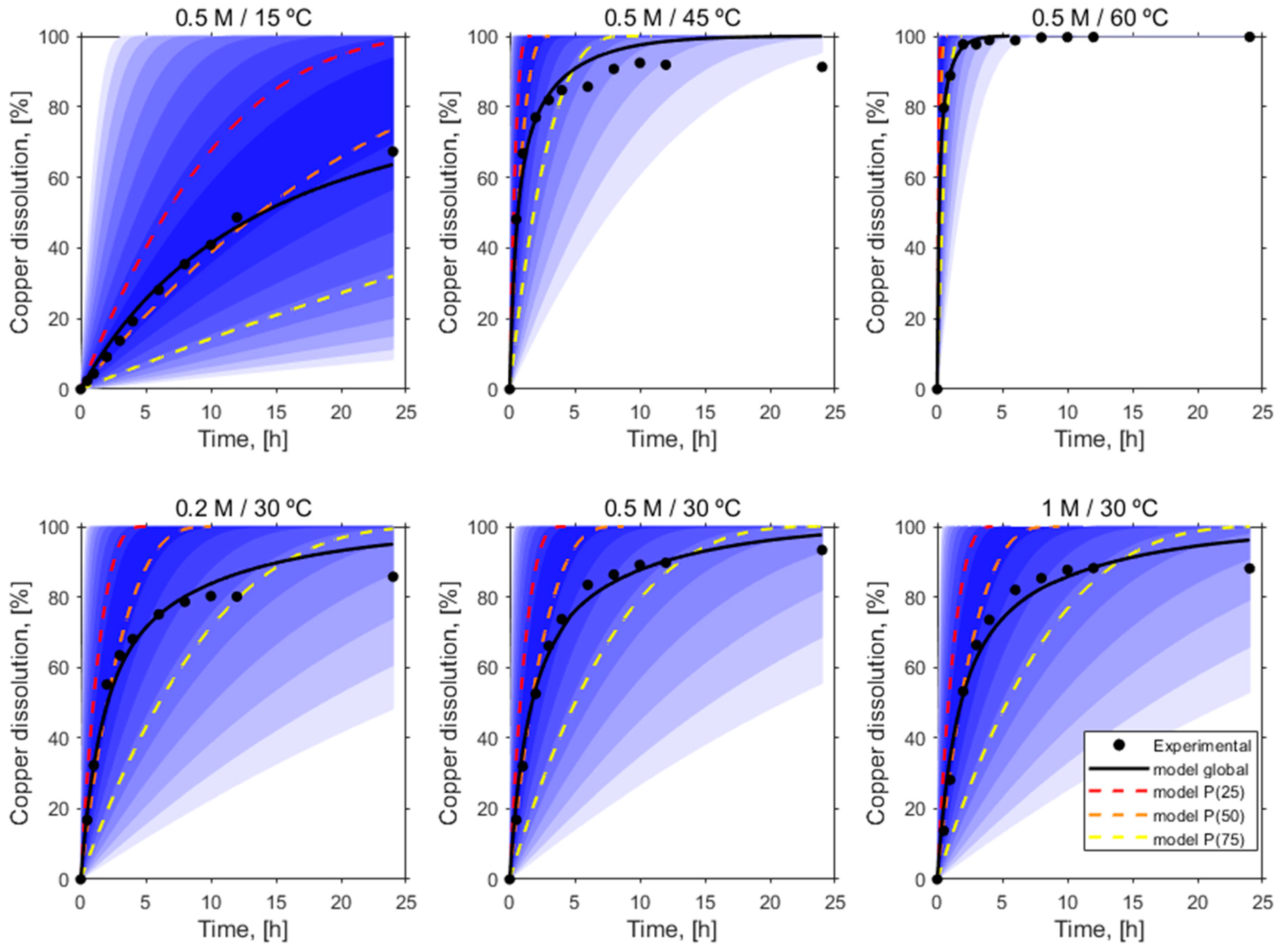
3.3. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey Copper Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/copper-statistics-and-information (accessed on 20 January 2023).
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and Critical Metals Production from Porphyry Ores and E-Wastes: A Review of Resource Availability, Processing/Recycling Challenges, Socio-Environmental Aspects, and Sustainability Issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating Global Copper Demand until 2100 with Regression and Stock Dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Mwanat, M.H.M.; Kasongo, K.B.; Muliangala, M.F.; Kayembe, M.M.; Kapiamba, K.F.; Ngenda, B.R. Simulation of Simultaneous Leaching of Copper and Cobalt Minerals in Acid-Reductive Media: Sensitivity Analysis and Optimization. J. Sustain. Metall. 2022, 8, 837–850. [Google Scholar] [CrossRef]
- Aracena, A.; Vivar, Y.; Jerez, O.; Vásquez, D. Kinetics of Dissolution of Tenorite in Ammonium Media. Miner. Process. Extr. Metall. Rev. 2015, 36, 317–323. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An Investigation into the Leaching Behaviour of Copper Oxide Minerals in Aqueous Alkaline Glycine Solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Shabani, M.A.; Irannajad, M.; Azadmehr, A.R. Investigation on Leaching of Malachite by Citric Acid. Int. J. Miner. Metall. Mater. 2012, 19, 782–786. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of Copper Oxide with Different Acid Solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Biswas, S.; Mulaba-bafubiandi, A.F. Extraction of Copper and Cobalt from Oxidized Ore Using Organic Acids. In Proceedings of the Hydrometallurgy Conference 2016: Sustainable Hydrometallurgical Extraction of Metals, Cape Town, South Africa, 1–3 August 2016; Available online: https://www.researchgate.net/publication/305851241_Extraction_of_Copper_and_Cobalt_from_Oxidized_Ore_using_Organic_Acids (accessed on 22 June 2024).
- Deng, J.; Wen, S.; Deng, J.; Wu, D.; Yang, J. Extracting Copper by Lactic Acid from Copper Oxide Ore and Dissolution Kinetics. J. Chem. Eng. Jpn. 2015, 48, 538–544. [Google Scholar] [CrossRef]
- Rojas, N.; Bustamante, O. Disolución de Cobre En Cianuración Convencional Proveniente de Ferrita Cúprica. Dyna 2007, 74, 151–157. [Google Scholar]
- Oudenne, P.D.; Olson, F.A. Leaching Kinetics of Malachite in Ammonium Carbonate Solutions. Metall. Trans. B 1983, 14, 33–40. [Google Scholar] [CrossRef]
- Jamett, I.; Carrasco, P.; Olmos, M. Glycine/Glutamate: “Green” Alternatives to Recover Metals from Minerals/Residues—Review of Current Research. Minerals 2023, 13, 22. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Oraby, E.; Eksteen, J. Amino Acids as Lixiviants for Metals Extraction from Natural and Secondary Resources with Emphasis on Glycine: A Literature Review. Hydrometallurgy 2023, 216, 106008. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal Recovery from Waste Printed Circuit Boards: A Review for Current Status and Perspectives. Resour. Conserv. Recycl. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of Copper and the Co-Leaching Behaviour of Other Metals from Waste Printed Circuit Boards Using Alkaline Glycine Solutions. Resour. Conserv. Recycl. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A.; Tanda, B.C. A Conceptual Process for Copper Extraction from Chalcopyrite in Alkaline Glycinate Solutions. Miner. Eng. 2017, 108, 53–66. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A.; O’Connor, G.M. The Kinetics of Chalcopyrite Leaching in Alkaline Glycine/Glycinate Solutions. Miner. Eng. 2019, 135, 118–128. [Google Scholar] [CrossRef]
- Conejeros, V.; Pérez, K.; Jeldres, R.I.; Castillo, J.; Hernández, P.; Toro, N. Novel Treatment for Mixed Copper Ores: Leaching Ammonia–Precipitation–Flotation (L.A.P.F.). Miner. Eng. 2020, 149, 106242. [Google Scholar] [CrossRef]
- Tanda, B.C.; Oraby, E.A.; Eksteen, J.J. Kinetics of Malachite Leaching in Alkaline Glycine Solutions. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2018, 130, 16–24. [Google Scholar] [CrossRef]
- Perea, C.G.; Restrepo, O.J. Uso De Aminoácidos Para La Disolución De Cobre a Partir De Residuos De Aparatos Eléctricos Y Electrónicos_V2. In Proceedings of the VII Latinometalurgia Conference, Cusco, Peru, 25–27 October 2017; Available online: https://www.scribd.com/document/363047178/VII-LATINOMETALURGIA-CONGRESO-INTERNACIONAL-EN-CUSCO-PERU-Libro-de-Ponencias-y-Resumenes (accessed on 10 July 2024).
- Perea, C.G.; Restrepo Baena, O.J.; Ihle, C.F.; Estay, H. Copper Leaching from Wastes Electrical and Electronic Equipment (WEEE) Using Alkaline Monosodium Glutamate: Thermodynamics and Dissolution Tests. Clean. Eng. Technol. 2021, 5, 100312. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C.; Nurjaman, F.; Al Muttaqii, M.; Handoko, A.S.; Bahfie, F.; Mufakhir, F.R. Monosodium Glutamate as Selective Lixiviant for Alkaline Leaching of Zinc and Copper from Electric Arc Furnace Dust. Metals 2020, 10, 644. [Google Scholar] [CrossRef]
- Khetwunchai, N.; Akeprathumchai, S.; Thiravetyan, P. Recovery of Copper and Gold from Waste Printed Circuit Boards Using Monosodium Glutamate Supplemented with Hydrogen Peroxide. Minerals 2023, 13, 321. [Google Scholar] [CrossRef]
- Perea, C.G.; Restrepo, O.J. Use of Amino Acids for Gold Dissolution. Hydrometallurgy 2018, 177, 79–85. [Google Scholar] [CrossRef]
- Bahfie, F.; Supriadi, M.R.; Oediyani, S.; Nurjaman, F.; Astuti, W.; Prasetyo, E.; Susanti, D. Use of Monosodium Glutamate (MSG) for Green Leaching Process: An Overview. Period. Mineral. 2022, 91, 155–162. [Google Scholar] [CrossRef]
- Perea, C.G.; Ihle, C.; Estay, H.; Dyer, L. An Investigation into the Leaching Behavior of Tenorite in Aqueous Alkaline Monosodium Glutamate Solutions. In Proceedings of the Copper 2022 International Conference; The Chilean Institute of Mining Engineers, (IIMCh), Ed.; The Chilean Institute of Mining Engineers: Santiago, Chile, 2022; Volume 4, pp. 254–262. [Google Scholar]
- KTH Royal Institute of Technology; Puigdomenech, I. Hydra-Medusa 2015. Available online: https://www.kth.se/che/medusa/downloads-1.386254 (accessed on 10 July 2024).
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. Kinetics of Chalcocite Leaching in Oxygenated Alkaline Glycine Solutions. Hydrometallurgy 2018, 178, 264–273. [Google Scholar] [CrossRef]
- Wadsworth, M.E.; Miller, J.D. Hydrometallurgical Processes. In Rate Processes of Extractive Metallurgy; Sohn, H.Y., Wadsworth, M.E., Eds.; Springer: Berlin, Germany, 1979; pp. 133–244. [Google Scholar]
- Bartlett, R.W. Solution Mining: Leaching and Fluid Recovery of Materials; Routledge: London, UK; Psychology Press: Hove, UK, 1998; ISBN 9056996339. [Google Scholar]
- Levenspiel, O. Ingeniería De Las Reacciones Químicas, 3rd ed.; Conesa, J., Arriola Guevara, E., Eds.; Limusa Wiley: México City, México, 2004; ISBN 968-18-5860-3. [Google Scholar]
- Dickinson, C.F.; Heal, G.R. Solid-Liquid Diffusion Controlled Rate Equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
- Marsden, J.O.; House, C.I. Principles of Gold Hydrometallurgy. In The Chemistry of Gold Extraction; Society for Mining Metallurgy: Colorado, CO, USA, 2006; pp. 111–145. [Google Scholar]
- Gbor, P.K.; Jia, C.Q. Critical Evaluation of Coupling Particle Size Distribution with the Shrinking Core Model. Chem. Eng. Sci. 2004, 59, 1979–1987. [Google Scholar] [CrossRef]
- Veloso, T.C.; Peixoto, J.J.M.; Pereira, M.S.; Leao, V.A. Kinetics of Chalcopyrite Leaching in Either Ferric Sulphate or Cupric Sulphate Media in the Presence of NaCl. Int. J. Miner. Process. 2016, 148, 147–154. [Google Scholar] [CrossRef]
- Ranjbar, M.; Hamghavandi, M.R.; Fazaelipoor, M.H.; Schaffie, M.; Manafi, Z. Development of a Kinetic Model of the Bacterial Dissolution of Copper Concentrate. Min. Eng. 2019, 71, 55–56. [Google Scholar] [CrossRef]
- Elmisaoui, S.; Latifi, A.M.; Khamar, L. Analysis of the Dissolution of Phosphate Ore Particles in Phosphoric Acid: Influence of Particle Size Distribution. Hydrometallurgy 2024, 223, 106197. [Google Scholar] [CrossRef]



| Material | Reaction Conditions | Metal Recovery (wt%) | References |
|---|---|---|---|
| Electrical and electronic waste | 0.5 M MSG, 0.03 M H2O2, pH 9.4, for 2 h at room temperature. | 92% Cu | [21,22] |
| Electrical and electronic waste | 0.5 M MSG, 0.03 M KMnO4, pH 9.4, after 20 h at room temperature. | 79% Cu | [21,22] |
| Electric arc furnace dust | 1 M MSG, pH 9, solid–liquid ratio: 50 g/L, at room temperature, 2 and 4 h for Zn and Cu, respectively. | 86% Cu and 99% Zn | [23] |
| Printed circuit boards waste | 0.75 M MSG, 0.5% (w/v) H2O2 initial pH 7, 150 rpm, for 3 h at 30 °C. | 93% Cu | [24] |
| Printed circuit boards waste | 1 M MSG, 0.25% (w/v) H2O2 initial pH 7, 150 rpm, for 3 h at 30 °C. | 86% Au | [24] |
| Electrical and electronic waste | 0.5 M MSG, 0.03 M KMnO4, pH 9.4, after 24 h at room temperature. | 11.8% Au | [25] |
| Sample | Al | Si | P | Cl | Ca | Cu | Th | LE |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 0.72 | 0.28 | 1.96 | 0.18 | 75.53 | 0.79 | 19.77 |
| 2 | 0.63 | 0.69 | 0.27 | 191 | 0.17 | 73.62 | 0.75 | 21.8 |
| 3 | 0.55 | 0.7 | 0.27 | 1.91 | 0.17 | 72.48 | 0.74 | 23.01 |
| Average | 0.59 | 0.7 | 0.27 | 1.93 | 0.17 | 73.88 | 0.76 | 21.52 |
| # Test | Variables | T (°C) | Glutamate Concentration (M) |
|---|---|---|---|
| 1 | Temperature | 15 | 0.5 |
| 2 | 30 | 0.5 | |
| 3 | 45 | 0.5 | |
| 4 | 60 | 0.5 | |
| 5 | Glutamate concentration | 30 | 0.2 |
| 6 | 30 | 1 |
| Coefficient of Variation for the Evaluated Models: R2 | ||||
|---|---|---|---|---|
| Variables | Chemical Reaction Controls | Diffusion through Product Layer Controls | Mixed Control | No Product Layer Formation-Film Diffusion Controls |
| Glutamate concentration | ||||
| 0.2 | 0.8927 | 0.9471 | 0.9423 | 0.8147 |
| 0.5 | 0.9309 | 0.9716 | 0.9844 | 0.8505 |
| 1 | 0.9245 | 0.9661 | 0.9791 | 0.8466 |
| Temperature | ||||
| 15 | 0.9993 | 0.9410 | 0.9090 | 0.9984 |
| 30 | 0.9309 | 0.9716 | 0.9892 | 0.9005 |
| 45 | 0.8341 | 0.8944 | 0.9754 | 0.7928 |
| 60 | 0.7833 | 0.5607 | 0.9840 | 0.7185 |
| Coefficient of Variation for the Evaluated Models: R2 | |||
|---|---|---|---|
| Variables | Chemical Reaction Controls | Diffusion through Product Layer Controls | Mixed Control |
| Glutamate concentration | |||
| 0.2 | 0.99 | 0.89 | 0.99 |
| 0.5 | 0.96 | 0.83 | 0.96 |
| 1 | 0.96 | 0.82 | 0.96 |
| Temperature | |||
| 15 | 0.97 | 0.75 | 0.97 |
| 30 | 0.96 | 0.83 | 0.96 |
| 45 | 1.00 | 0.91 | 1.00 |
| 60 | 0.97 | 0.68 | 0.97 |
| Variables | Chemical Reaction Controls | Mixed Control | |||
|---|---|---|---|---|---|
| Krn | MSE | Krn1 | Krn2 | MSE | |
| Glutamate concentration | |||||
| 0.2 | 0.0537 | 0.007 | 1.00 × 10−16 | 0.0554 | 0.007 |
| 0.5 | 0.1213 | 0.043 | 1.00 × 10−16 | 0.1190 | 0.041 |
| 1 | 0.2606 | 0.044 | 1.00 × 10−16 | 0.2606 | 0.044 |
| Temperature | |||||
| 15 | 1.1074 | 0.013 | 1.00 × 10−16 | 1.0975 | 0.012 |
| 30 | 0.1213 | 0.043 | 1.00 × 10−16 | 0.1190 | 0.041 |
| 45 | 0.0383 | 0.003 | 1.00 × 10−16 | 0.0397 | 0.004 |
| 60 | 0.0026 | 0.029 | 1.00 × 10−16 | 0.0050 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perea, C.G.; Ihle, C.; Dyer, L.; Díaz Quezada, S.; Estay, H. Study of a Copper Oxide Leaching in Alkaline Monosodium Glutamate Solution. Minerals 2024, 14, 714. https://doi.org/10.3390/min14070714
Perea CG, Ihle C, Dyer L, Díaz Quezada S, Estay H. Study of a Copper Oxide Leaching in Alkaline Monosodium Glutamate Solution. Minerals. 2024; 14(7):714. https://doi.org/10.3390/min14070714
Chicago/Turabian StylePerea, Carlos G., Christian Ihle, Laurence Dyer, Simón Díaz Quezada, and Humberto Estay. 2024. "Study of a Copper Oxide Leaching in Alkaline Monosodium Glutamate Solution" Minerals 14, no. 7: 714. https://doi.org/10.3390/min14070714






