Trace Element Compositions of Galena and Cerussite from the Bou Dahar MVT District, Morocco: Insights from LA-ICP-MS Analyses
Abstract
:1. Introduction
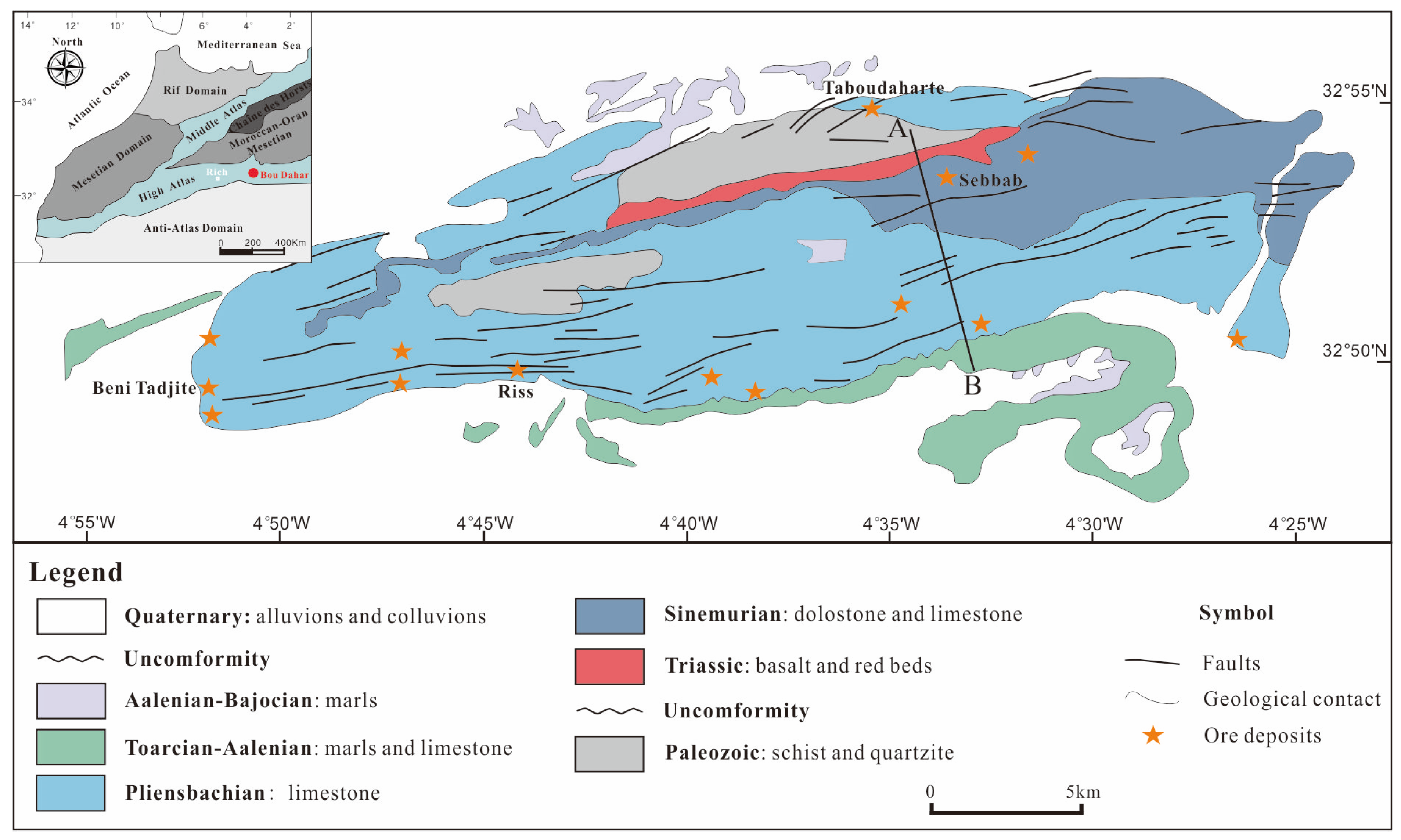
2. Regional Geology
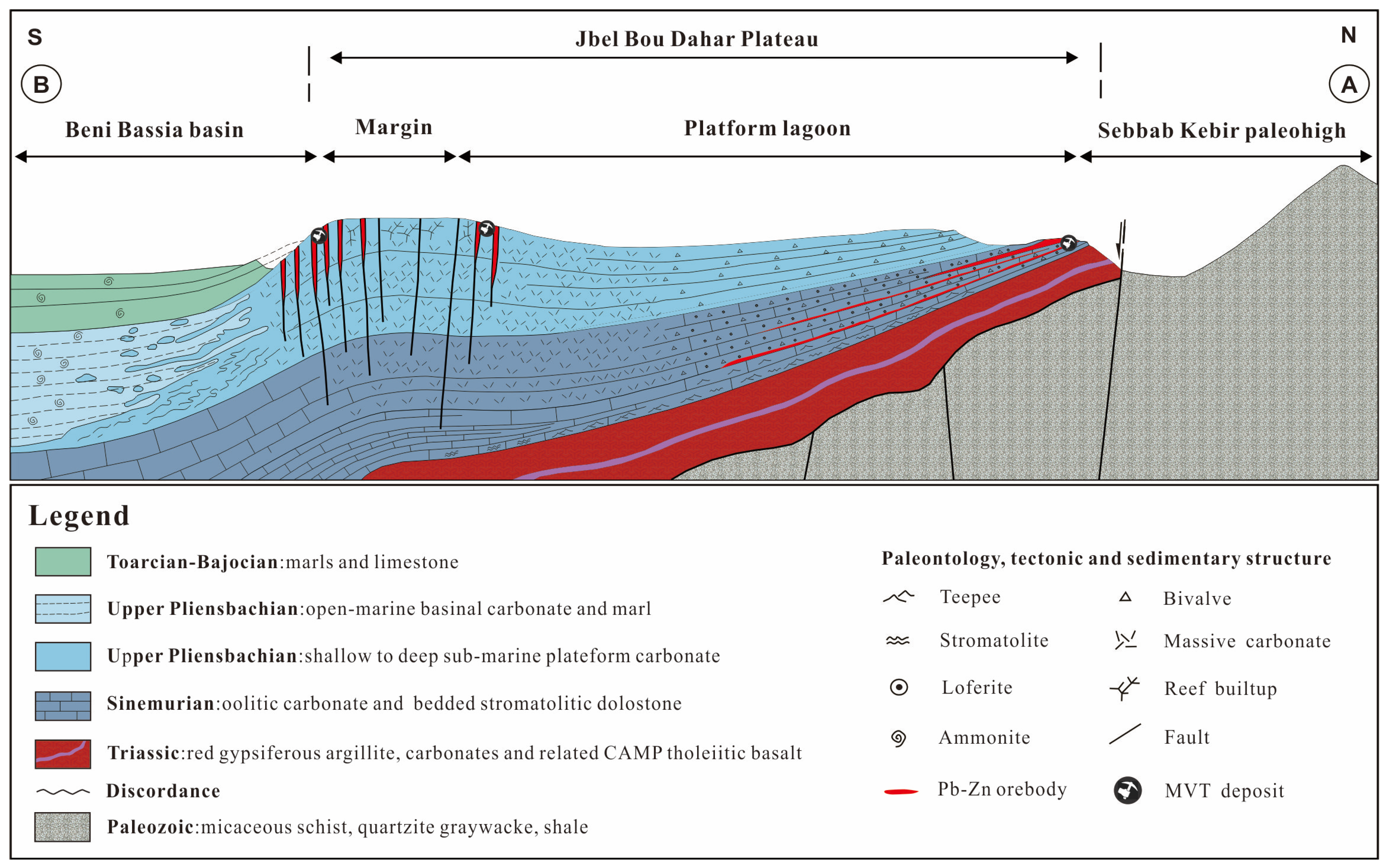
3. Deposit Geology
4. Sampling and Analytical Methods
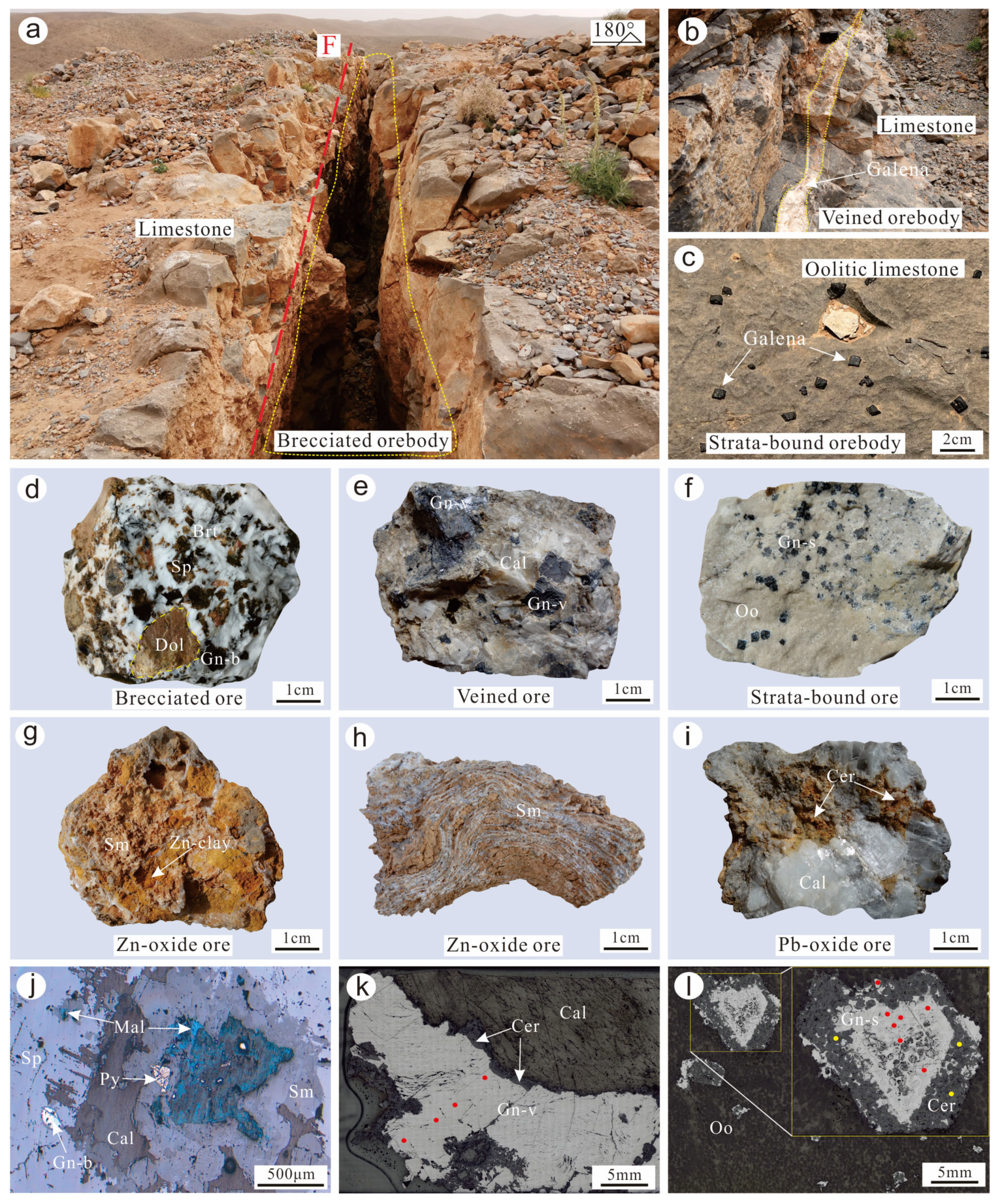
4.1. Sample Description
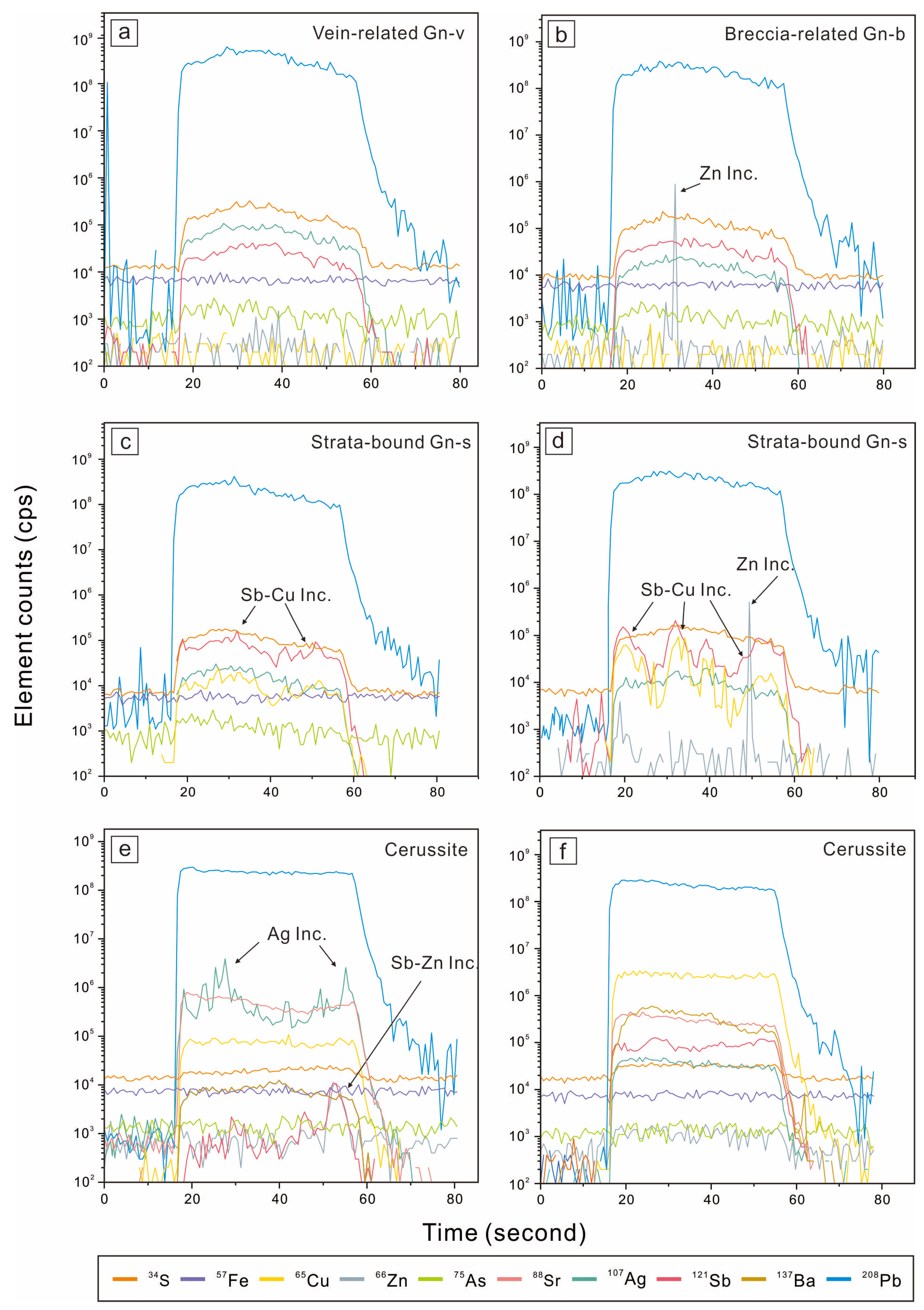
4.2. In Situ LA-ICP-MS Analysis
4.3. Principal Component Analysis (PCA)
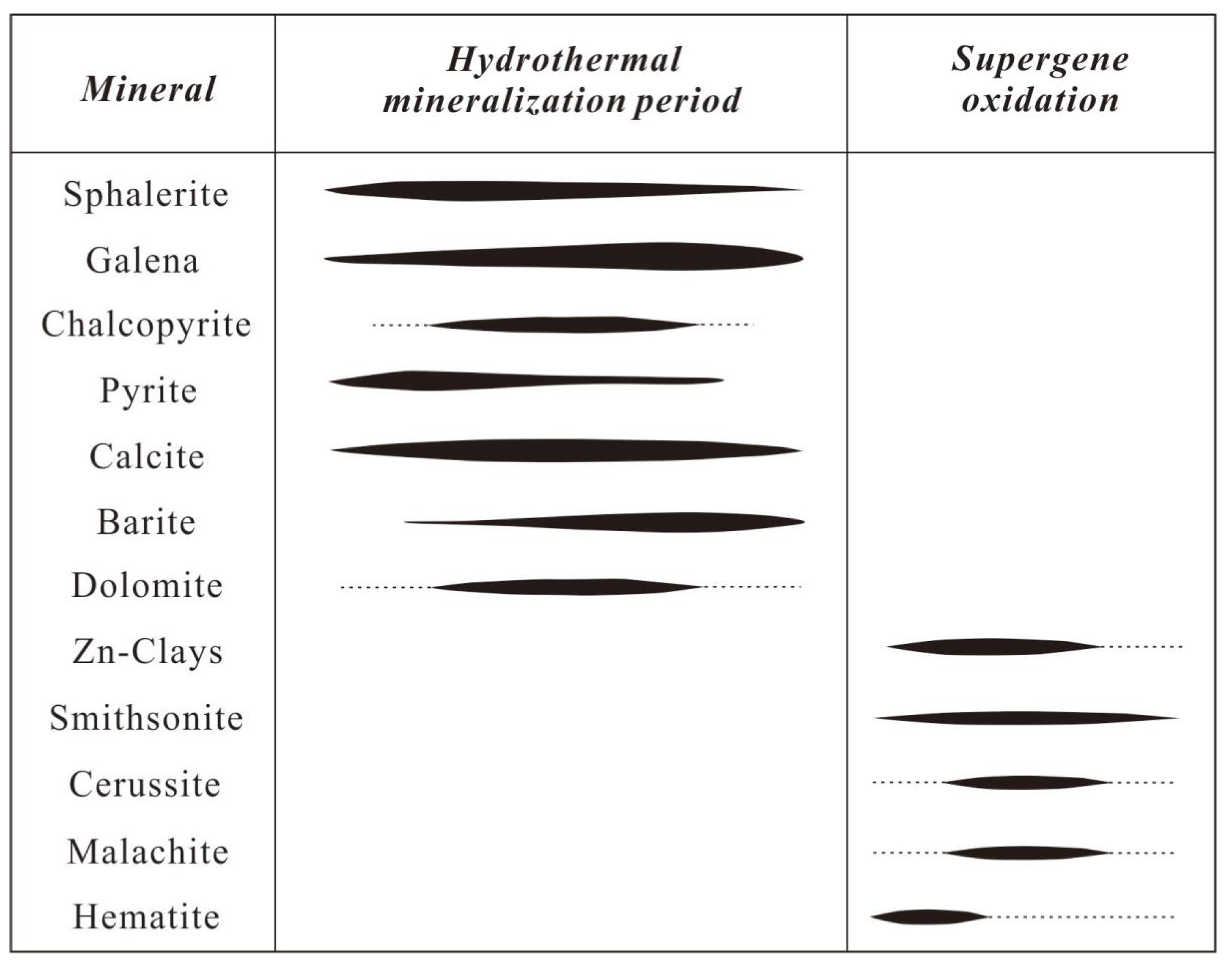
5. Results
5.1. Trace Element Contents in Galena
5.2. Trace Elements Contents in Cerussite
6. Discussion
6.1. The Substitution Mechanism of Trace Elements in Galena
6.2. Distribution and Substitutions of Trace Elements in Cerussite
6.3. Implications for Ore Genesis and Exploration
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Zhuang, L.; Song, Y.; Liu, Y.; Fard, M.; Hou, Z. Major and trace elements and sulfur isotopes in two stages of sphalerite from the world-class Angouran Zn–Pb deposit, Iran: Implications for mineralization conditions and type. Ore Geol. Rev. 2019, 109, 184–200. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Hu, Y.; Huang, Z.; Danyushevsky, L.; Wang, H. LA-ICP-MS analyses of trace elements in base metal sulfides from carbonate-hosted Zn-Pb deposits, South China: A case study of the Maoping deposit. Ore Geol. Rev. 2021, 130, 103945. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.J.; Ulrich, T.; Zhang, J.; Wang, J. Trace element compositions of sulfides from Pb-Zn deposits in the Northeast Yunnan and northwest Guizhou Provinces, SW China: Insights from LA-ICP-MS analyses of sphalerite and pyrite. Ore Geol. Rev. 2022, 141, 104639. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Wei, J.; Ulrich, T. Trace element compositions of galena in an MVT deposit from the Sichuan-Yunnan-Guizhou metallogenic province, SW China: Constraints from LA-ICP-MS spot analysis and elemental mapping. Ore Geol. Rev. 2022, 150, 105123. [Google Scholar] [CrossRef]
- George, L.; Cook, N.J.; Cristiana, L.C.; Wade, B.P. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in co-crystallized sphalerite-galena-chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Minor and trace elements in natural tetrahedrite-tennantite: Effects on element partitioning among base metal sulphides. Minerals 2017, 7, 17. [Google Scholar] [CrossRef]
- Wu, J.; Zhai, D.; Zhao, Q.; Zhang, H.; Hong, J.; Zhao, G.; Liu, J. In situ trace element compositions of sulfides constraining the genesis of the worldclass Shuangjianzishan Ag-Pb-Zn. Ore Geol. Rev. 2023, 162, 105675. [Google Scholar] [CrossRef]
- Mouguina, E.M.; Daoudi, L. Minéralisation Pb-Zn du type MVT de la région d’Ali ou Daoud (Haut Atlas Central, Maroc): Caractérisations du gîte et relations avec les cortèges de minéraux argileux. Estud. Geol. 2008, 64, 135–150. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Niedermann, S.; Velasco, F. The Touissit-Bou Beker Mississippi Valley-type district of northeastern Morocco: Relationships to the Messinian salinity crisis, late Neogene-Quaternary alkaline magmatism, and buoyancy-driven fluid convection. Econ. Geol. 2015, 110, 1455–1484. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Sangster, D.F. Geology, geochemistry, and current genetic models for major Mississippi Valley-type Pb–Zn deposits of Morocco. In Mineral Deposits of North Africa; Springer: Berlin/Heidelberg, Germany, 2016; pp. 463–495. [Google Scholar]
- Rddad, L.; Bouhlel, S. The Bou Dahar Jurassic carbonate-hosted Pb–Zn–Ba deposits (Oriental High Atlas, Morocco): Fluid-inclusion and C-O-S-Pb isotope studies. Ore Geol. Rev. 2016, 72, 1072–1087. [Google Scholar] [CrossRef]
- Rddad, L.; Mouguina, E.M.; Muchez, P.; Darling, R.S. The genesis of the Ali Ou Daoud Jurassic carbonate Zn-Pb Mississippi Valley-type deposit, Moroccan central High Atlas: Constraints from bulk stable C-O-S, in situ radiogenic Pb isotopes, and fluid inclusion studies. Ore Geol. Rev. 2018, 99, 365–379. [Google Scholar] [CrossRef]
- Agard, J.; Du, D.R. Les gîtes plombo-zincifères du jbel Bou Dahar près de Beni-Tajjite (Haut Atlas oriental). Notes Mém. Serv. Géol. Maroc. 1965, 181, 135–166. [Google Scholar]
- Leblanc, M. Etude géologique et métallogénique du jbel Bou-Arhous et de son prolongement oriental (Haut Atlas marocian oriental). Notes Mem. Serv. Geol. Maroc. 1968, 206, 117–206. [Google Scholar]
- Bazin, D. Etude géologique et métallogénique des chaînons atlasiques du Tizi n’Firest au Nord de Ksar-es-Souk (Errachidia). Notes Mém. Serv. Géol. Maroc. 1968, 206, 37–116. [Google Scholar]
- Rddad, L.; Bouhlel, S. Fluid inclusions studies of Pb-Zn-Ba (±Sr) mineralization of Bou Dahar district (Oriental High Atlas, Morocco). In Proceedings of the 1st Lybian Mining Conference, Tripoli, Libya, 17–20 March 1997. [Google Scholar]
- Rddad, L. Géodynamique au Jurassique et pétrologie des matériaux magmatiques, carbonatés et sulfurés: Exemple des districts de Tigrinine et de Bou Dahar (Haut-Atlas central, Maroc) (Thèse de Doctorat), Université Tunis II, Fac. Science 1998, 303. (In French) [Google Scholar]
- Adil, S.; Bouabdellah, M.; Grandia, F.; Cardellach, E.; Canals, À. Caractérisation géochimique des fluides associés aux minéralisations PbZn de Bou-Dahar (Maroc). Comptes Rendus Geosci. 2004, 336, 1265–1272. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Boukirou, W.; Potra, A.; Melchiorre, E.; Bouzahzah, H.; Yans, J.; Zaid, K.; Idbaroud, M.; Poot, J.; Dekoninck, A.; et al. Origin of the Moroccan Touissit-Bou Beker and Jbel Bou Dahar supergene non-sulfide biomineralization and its relevance to microbiological activity, late Miocene uplift and climate changes. Minerals 2021, 11, 401. [Google Scholar] [CrossRef]
- Auajjar, J.; Boulègue, J. Les minéralisations Pb-Zn (Cu) Ba du socle paléozoïque et de la plate-forme liasique du district de Tazekka (Taza, Maroc oriental): Une synthèse. Chron. Rech. Min. 1999, 536–537, 121–135. [Google Scholar]
- Caïa, J. Roches eruptives básiques et minéralisations en plomb, zinc et strontium de la region de Tirrhist (Haute Atlas de Midelt). Notes Mem. Serv. Geol. Maroc. 1968, 206, 7–30. [Google Scholar]
- Chèvremont, P. Les Roches Eruptives Basiques des Boutonnières de Tassent et Tasraft et Leurs Indices Métallifères dans Leur Cadre Géologique (Haut-Atlas Central, Maroc). Ph.D. Thesis, L’université Claude Bernard, Lyon, France, 1975. [Google Scholar]
- Rddad, L.; Bouhlel, S.; Laridhi Ouazaa, N. Gîtologie et inclusions fluides des minérallisations plombo-zincifères du district minier de Bou Dahar (Haut Atlas oriental, Maroc). 3 éme Congr. Nat. Sci. Terre Tunisp. 1995, 134. [Google Scholar]
- Bouabdellah, M.; Sangster, D.F.; Leach, D.L.; Brown, A.C.; Johnson, C.A.; Emsbo, P. Genesis of the Touissit-Bou Beker Mississippi valley-type district (Morocco-Algeria) and its relationship to the Africa-Europe collision. Econ. Geol. 2012, 107, 117–146. [Google Scholar] [CrossRef]
- Rddad, L.; Mouguina, E.M. Sulfur and lead isotopic compositions of ore sulfides and mining economic potential of the High Atlas Mississippi Valley-type ore province, Morocco. J. Geochem. Explor. 2021, 226, 106765. [Google Scholar] [CrossRef]
- Ovtracht, A. Province plombo-zincifère du Haut Atlas central. Mines Geol. Energ. Rabat 1978, 44, 103–109. [Google Scholar]
- Choulet, F.; Charles, N.; Barbanson, L.; Branquet, Y.; Sizaret, S.; Ennaciri, A.; Chen, Y. Non-sulfide zinc deposits of the Moroccan High Atlas: Multi-scale characterization and origin. Ore Geol. Rev. 2014, 56, 115–140. [Google Scholar] [CrossRef]
- Laville, E. A multiple releasing and restraining stepover model for the Jurassic strike-slip basin of the Central High Atlas (Morocco). In Developments in Geotectonics; Elsevier: Amsterdam, The Netherlands, 1988; Volume 22, pp. 499–523. [Google Scholar]
- Giese, P.; Jacobshagen, V. Inversion tectonics of intracontinental ranges: High and Middle Atlas, Morocco. Geol. Rundsch. 1992, 81, 249–259. [Google Scholar] [CrossRef]
- Laville, E. Évolution Sédimentaire, Tectonique et Magmatique du Bassin Jurassique du Haut Atlas (Maroc): Modèle en Relais Multiples de Décrochements. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 1985. Volume 166. (In French). [Google Scholar]
- Frizon de Lamotte, D.; Zizi, M.; Missenard, Y.; Hafid, M.; Azzouzi, M.E.; Maury, R.C.; Michard, A. The atlas system. In Continental Evolution: The Geology of Morocco: Structure, Stratigraphy, and Tectonics of the Africa-Atlantic-Mediterranean Triple Junction; Springer: Berlin/Heidelberg, Germany, 2008; pp. 133–202. [Google Scholar]
- Laville, E.; Pique, A.; Amrhar, M.; Charroud, M. A restatement of the Mesozoic Atlasic rifting (Morocco). J. Afr. Earth Sci. 2004, 38, 145–153. [Google Scholar] [CrossRef]
- Warme, J.E. Jurassic carbonate facies of the central and eastern High Atlas rift, Morocco. In The Atlas System of Morocco: Studies on its Geodynamic Evolution; Springer: Berlin/Heidelberg, Germany, 1988; pp. 169–199. [Google Scholar]
- Saddiqi, O.; El Haimer, F.Z.; Michard, A.; Barbarand, J.; Ruiz, G.M.H.; Mansour, E.M.; de Lamotte, D.F. Apatite fission-track analyses on basement granites from south-western Meseta, Morocco: Paleogeographic implications and interpretation of AFT age discrepancies. Tectonophysics 2009, 475, 29–37. [Google Scholar] [CrossRef]
- Charrière, A.; Haddoumi, H.; Mojon, P.O. Découverte de Jurassique supérieur et d’un niveau marin du Barrémien dans les «couches rouges» continentales du Haut Atlas central marocain: Implications paléogéographiques et structurales. Comptes Rendus Palevol. 2005, 4, 385–394. [Google Scholar] [CrossRef]
- de Lamotte, D.F.; Leturmy, P.; Missenard, Y.; Khomsi, S.; Ruiz, G.; Saddiqi, O.; Michard, A. Mesozoic and Cenozoic vertical movements in the Atlas system (Algeria, Morocco, Tunisia): An overview. Tectonophysics 2009, 475, 9–28. [Google Scholar] [CrossRef]
- Blomeier, D.P.; Reijmer, J.J. Drowning of a Lower Jurassic carbonate platform: Jbel Bou Dahar, High Atlas, Morocco. Facies 1999, 41, 81–110. [Google Scholar] [CrossRef]
- Campbell, A.E.; Stafleu, J. Seismic modeling of an Early Jurassic, drowned carbonate platform: Djebel Bou Dahar, High Atlas, Morocco. AAPG BuIl. 1992, 76, 1760–1777. [Google Scholar]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Enzweiler, J. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanalytical Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Wu, S.; Wörner, G.; Jochum, K.P.; Stoll, B.; Simon, K.; Kronz, A. The preparation and preliminary characterisation of three synthetic andesite reference glass materials (ARM-1, ARM-2, ARM-3) for in situ microanalysis. Geostand. Geoanalytical Res. 2019, 43, 567–584. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Iolite, J.H. Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Heidel, C.; Tichomirowa, M. Galena oxidation investigations on oxygen and sulphur isotopes. Isot. Environ. Health Stud. 2011, 47, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.M.; Barton, P.B. Distribution of some minor elements between coexisting sulfide minerals. Econ. Geol. 1971, 66, 140–163. [Google Scholar] [CrossRef]
- Tauson, V.L.; Parkhomenko, I.Y.; Babkin, D.N.; Men shikov, V.I.; Lustenberg, E.E. Cadmium and mercury uptake by galena crystals under hydrothermal growth: A spectroscopic and element thermo-release atomic absorption study. Eur. J. Mineral. 2005, 17, 599–610. [Google Scholar] [CrossRef]
- Foord, E.E.; Shawe, D.R.; Conklin, N.M. Coexisting galena, PbS and sulfosalts: Evidence for multiple episodes of mineralization in the Round Mountain and Manhattan gold districts, Nevada. Can. Mineral. 1988, 26, 355–376. [Google Scholar]
- Foard, E.E.; Shawe, D.R. THE Pb-Bi-Ag-Cu-(Hg) chenistry of galena and some associated sulfosalta: A review and some new data from Colorado, California and Pennsylvania. Can. Mineral. 1989, 27, 363–382. [Google Scholar]
- Lueth, V.W.; Megaw, P.K.; Pingitore, N.E.; Goodell, P.C. Systematic variation in galena solid-solution compositions at Santa Eulalia, Chihuahua, Mexico. Econ. Geol. 2000, 95, 1673–1687. [Google Scholar] [CrossRef]
- Chutas, N.I.; Kress, V.C.; Ghiorso, M.S.; Sack, R.O. A solution model for high-temperature PbS-AgSbS2-AgBiS2 galena. Am. Mineral. 2008, 93, 1630–1640. [Google Scholar] [CrossRef]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Yuping, L.; Qian, Z.; Tiegeng, L.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- Zhai, D.; Bindi, L.; Voudouris, P.C.; Liu, J.; Tombros, S.F.; Li, K. Discovery of Se-rich canfieldite, Ag8Sn (S,Se)6, from the Shuangjianzishan Ag–Pb–Zn deposit, NE China: A multimethodic chemical and structural study. Mineral. Mag. 2019, 83, 419–426. [Google Scholar] [CrossRef]
- Wang, K.; Zhai, D.; Liu, J.; Wu, H. LA-ICP-MS trace element analysis of pyrite from the Dafang gold deposit, South China: Implications for ore genesis. Ore Geol. Rev. 2021, 139, 104507. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Sun, H.; Koua, K.A.D.; Lyu, C. LA-ICP-MS trace element analysis of sphalerite and pyrite from the Beishan Pb-Zn ore district, south China: Implications for ore genesis. Ore Geol. Rev. 2022, 150, 105128. [Google Scholar] [CrossRef]
- Krismer, M.; Vavtar, F.; Tropper, P.; Sartory, B.; Kaindl, R. Mineralogy, mineral chemistry and petrology of the ag-bearing cu-fe-pb-zn sulfide mineralizations of the pfunderer berg (south Tyrol, Italy). Austrian J. Earth Sci. 2011, 104, 36–48. [Google Scholar]
- Keim, M.F.; Vaudrin, R.; Markl, G. Redistribution of silver during supergene oxidation of argentiferous galena: A case study from the Schwarzwald, SW Germany. Neues JB Min. Abh 2016, 193, 295–309. [Google Scholar] [CrossRef]
- Cave, B.; Lilly, R.; Barovich, K. Textural and geochemical analysis of chalcopyrite, galena and sphalerite across the Mount Isa Cu to Pb-Zn transition: Implications for a zoned Cu-Pb-Zn system. Ore Geol. Rev. 2020, 124, 103647. [Google Scholar] [CrossRef]
- Cama, J.; Acero, P. Dissolution of minor sulphides present in a pyritic sludge at pH 3 and 25 °C. Geol. Acta 2005, 3, 15–26. [Google Scholar]
- Domènech, C.; De Pablo, J.; Ayora, C. Oxidative dissolution of pyritic sludge from the Aznalcóllar mine (SW Spain). Chem. Geol. 2002, 190, 339–353. [Google Scholar] [CrossRef]
- Chen, X.; Duan, D.; Zhang, Y.; Zhou, F.; Yuan, X.; Wu, Y. Genesis of the Giant Huoshaoyun Non-Sulfide Zinc-Lead Deposit in Karakoram, Xinjiang: Constraints from Mineralogy and Trace Element Geochemistry. Minerals 2023, 13, 842. [Google Scholar] [CrossRef]
- Szczerba, M.; Sawłowicz, Z. Remarks on the origin of cerussite in the Upper Silesian Zn-Pb deposits, Poland. Mineralogia 2009, 40, 54–64. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. The orthorhombic structure of CaCO3, SrCO3, PbCO3 and BaCO3: Linear structural trends. Can. Mineral. 2009, 47, 1245–1255. [Google Scholar] [CrossRef]
- Zemann, J.; Wildner, M.; Jarosch, D.; Heger, G.; Giester, G.; Chevrier, G. Neutron single-crystal refinement of cerussite, PbCO3, and comparison with other aragonite-type carbonates. Z. Krist.-Cryst. Mater. 1992, 199, 67–74. [Google Scholar] [CrossRef]
- Knipe, S.W. Role of sulphide surfaces in sorption of precious metals from hydrothermal fluids. Inst. Min. Metall. Trans. Sect. B Appl. Earth Sci. 1992, 101, 83–88. [Google Scholar]
- Larocque, A.C.; Jackman, J.A.; Cabri, L.J.; Hodgson, C.J. Calibration of the ion microprobe for the determination of silver in pyrite and chalcopyrite from the Mobrun VMS deposit, Rouyn-Noranda, Quebec. Can. Mineralogist. 1995, 33, 361. [Google Scholar]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Foster, J. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Pfaff, K.; Koenig, A.; Wenzel, T.; Ridley, I.; Hildebrandt, L.H.; Leach, D.L.; Markl, G. Trace and minor element variations and sulfur isotopes in crystalline and colloform ZnS: Incorporation mechanisms and implications for their genesis. Chem. Geol. 2011, 286, 118–134. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhai, D.; Williams-Jones, A.E.; Liu, J. Trace element and isotopic (S, Pb) constraints on the formation of the giant Chalukou porphyry Mo deposit, NE China. Am. Mineral. 2023, 108, 160–177. [Google Scholar] [CrossRef]
- Wind, S.C.; Schneider, D.A.; Hannington, M.D.; McFarlane, C.R. Regional similarities in lead isotopes and trace elements in galena of the Cyclades Mineral District, Greece with implications for the underlying basement. Lithos 2020, 366, 105559. [Google Scholar] [CrossRef]
- Li, Z.; Ye, L.; Hu, Y.; Wei, C.; Huang, Z.; Yang, Y.; Danyushevsky, L. Trace elements in sulfides from the Maozu Pb-Zn deposit, Yunnan Province, China: Implications for trace-element incorporation mechanisms and ore genesis. Am. Mineral. 2020, 105, 1734–1751. [Google Scholar] [CrossRef]
- Grant, H.L.; Layton-Matthews, D.; Peter, J.M. Distribution and controls on silver mineralization in the Hackett River Main Zone, Nunavut, Canada: An Ag-and Pb-enriched archean volcanogenic massive sulfide deposit. Econ. Geol. 2015, 110, 943–982. [Google Scholar] [CrossRef]
- Tooth, B.; Etschmann, B.; Pokrovski, G.S.; Testemale, D.; Hazemann, J.L.; Grundler, P.V.; Brugger, J. Bismuth speciation in hydrothermal fluids: An X-ray absorption spectroscopy and solubility study. Geochim. Cosmochim. Acta 2013, 101, 156–172. [Google Scholar] [CrossRef]

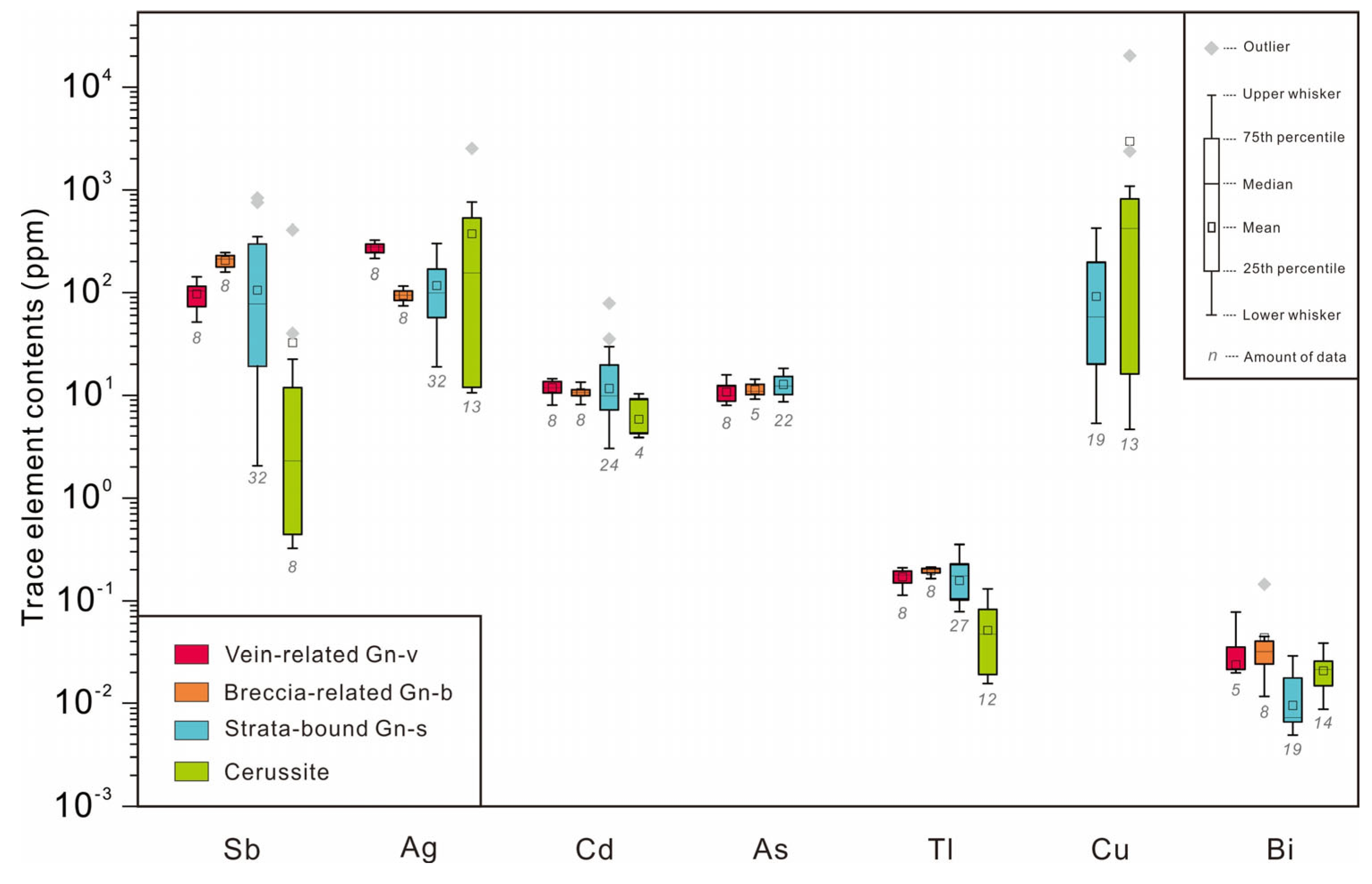


| Mineral | Sb | Ag | Cu | Cd | As | Tl | Zn | Bi | Sn | Au | Sr | Ba | (Ag + Cu) (Mol%)/Sb (Mol%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |||
| Gn-v | Max | 142 | 324 | <2.25 | 14.4 | 15.8 | 0.21 | <3.46 | 0.08 | 0.43 | <0.21 | 10.46 | <0.50 | 2.58 |
| Min | 51.5 | 214 | <1.63 | 8.03 | 7.99 | 0.11 | <6.02 | <0.01 | <0.29 | <0.02 | <0.109 | <0.06 | 4.70 | |
| Mean (8) | 97.3 | 272 | - | 11.8 | 10.7 | 0.17 | - | 0.04 | - | - | - | - | 3.16 | |
| Median (8) | 104 | 278 | - | 11.9 | 9.97 | 0.18 | - | 0.04 | - | - | - | - | 3.02 | |
| Gn-b | Max | 245 | 116 | <3.17 | 13.3 | 14.25 | 0.21 | 43.73 | 0.14 | <0.72 | <0.21 | <0.17 | <0.94 | 0.54 |
| Min | 158 | 74.3 | <2.11 | 8.13 | 2.31 | 0.16 | <3.92 | 0.01 | <0.48 | <0.03 | <0.07 | <0.08 | 0.53 | |
| Mean (8) | 204 | 94.4 | - | 10.7 | 9.84 | 0.19 | - | 0.04 | - | - | - | - | 0.52 | |
| Median (8) | 211 | 94.5 | - | 10.7 | 11.88 | 0.20 | - | 0.03 | - | - | - | - | 0.51 | |
| Gn-s | Max | 835 | 309 | 425 | 78.8 | 18.2 | 0.35 | 27.4 | 0.03 | 0.43 | 0.07 | 15.75 | 0.19 | 35.00 |
| Min | 2.05 | 19.0 | <2.50 | 4.62 | 8.15 | 0.05 | <3.15 | <0.01 | <0.20 | <0.03 | <0.02 | <0.10 | 0.78 | |
| Mean (32) | 178 | 116 | 89.9 | 13.2 | 13.0 | 0.19 | - | - | - | - | - | - | 4.04 | |
| Median (32) | 128 | 101 | 13.3 | 8.4 | 13.0 | 0.19 | - | - | - | - | - | - | 2.27 | |
| Cer | Max | 403 | 2527 | 37,435 | 14.1 | <11.50 | 0.13 | 42.0 | 0.04 | <0.80 | 6.27 | 1273 | 5296 | |
| Min | <0.37 | 2.34 | <1.57 | 2.87 | <7.21 | 0.02 | <5.72 | 0.01 | <0.46 | <0.13 | 454 | 7.59 | ||
| Mean (14) | 34.7 | 401 | 3181 | 7.93 | - | 0.07 | 11.86 | 0.02 | - | - | 692 | 898 | ||
| Median (14) | 0.65 | 159 | 433 | 7.36 | - | 0.06 | 8.63 | 0.02 | - | - | 673 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Wu, F.; Cheng, X.; Cheng, S.; Wu, J.; He, Y.; Wang, C.; Lkebir, N.; Cui, S.; Hu, P.; et al. Trace Element Compositions of Galena and Cerussite from the Bou Dahar MVT District, Morocco: Insights from LA-ICP-MS Analyses. Minerals 2024, 14, 748. https://doi.org/10.3390/min14080748
Zhao K, Wu F, Cheng X, Cheng S, Wu J, He Y, Wang C, Lkebir N, Cui S, Hu P, et al. Trace Element Compositions of Galena and Cerussite from the Bou Dahar MVT District, Morocco: Insights from LA-ICP-MS Analyses. Minerals. 2024; 14(8):748. https://doi.org/10.3390/min14080748
Chicago/Turabian StyleZhao, Kai, Fafu Wu, Xiang Cheng, Shunbo Cheng, Jinchao Wu, Yaoyan He, Chenggang Wang, Noura Lkebir, Sen Cui, Peng Hu, and et al. 2024. "Trace Element Compositions of Galena and Cerussite from the Bou Dahar MVT District, Morocco: Insights from LA-ICP-MS Analyses" Minerals 14, no. 8: 748. https://doi.org/10.3390/min14080748






