LiDonit®—A Potential Secondary Raw Material for Ceramic Applications in Concentrated Solar Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. LiDonit
2.2. Sample Preparation
2.3. Characterization Methods
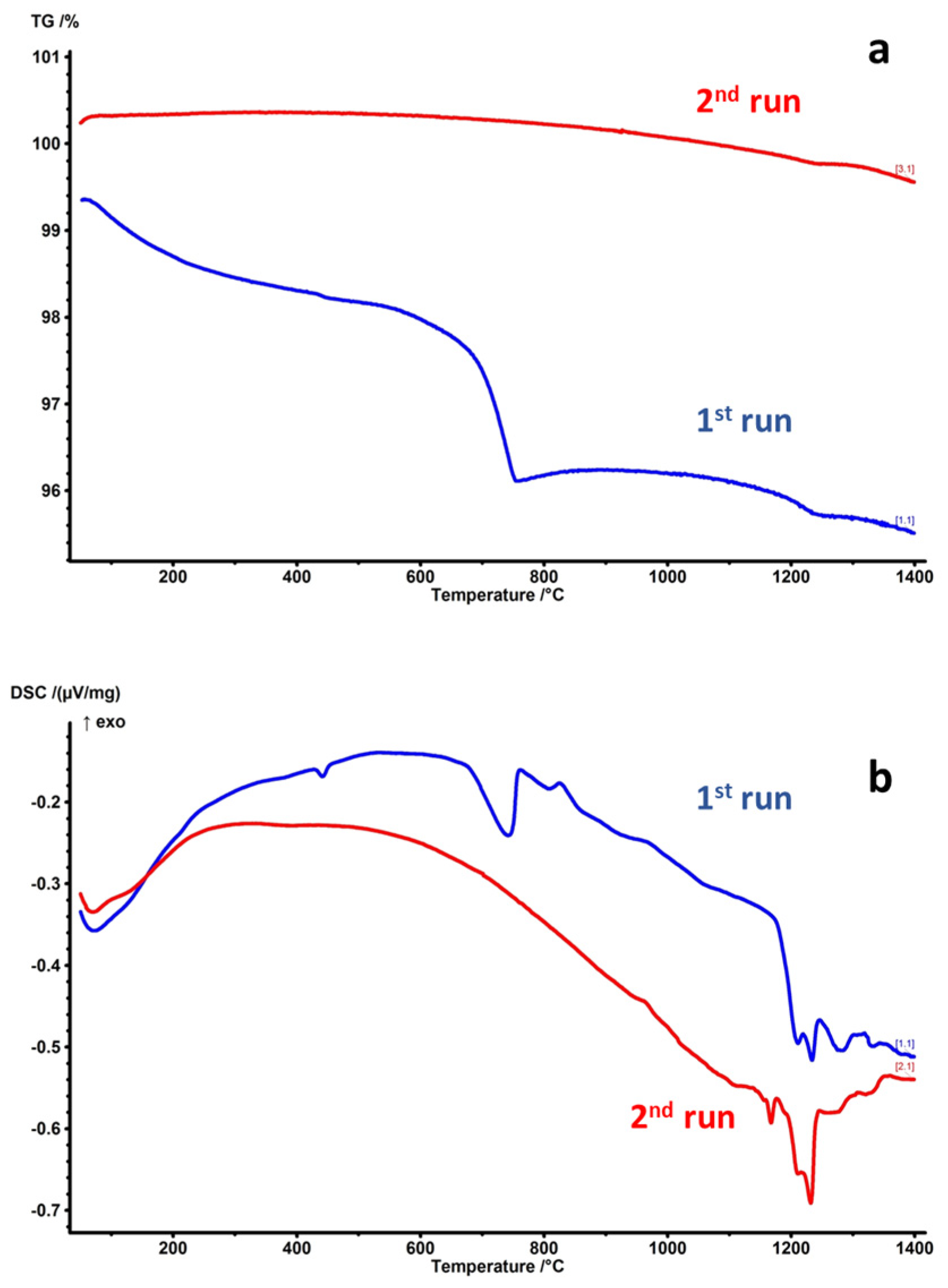
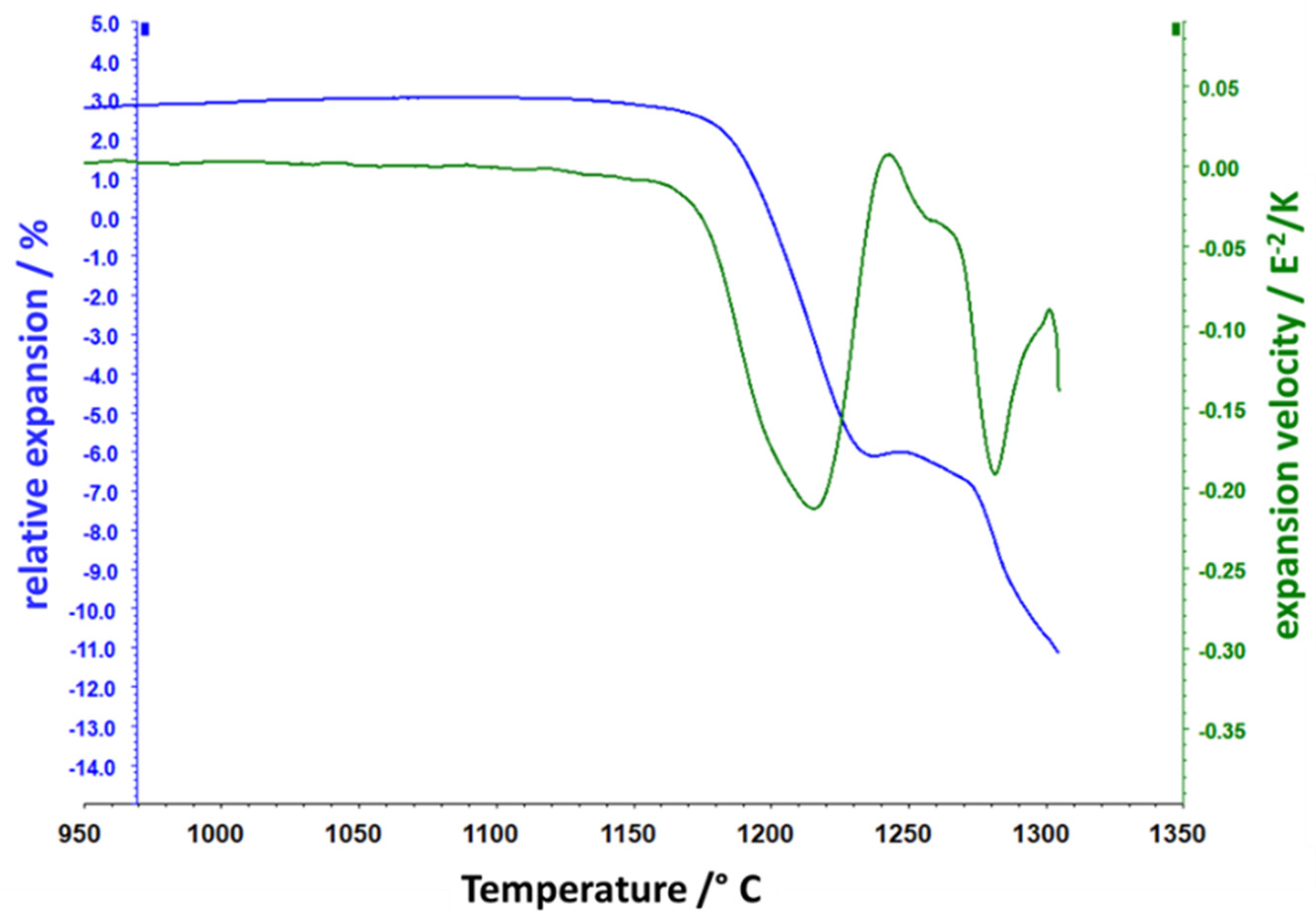
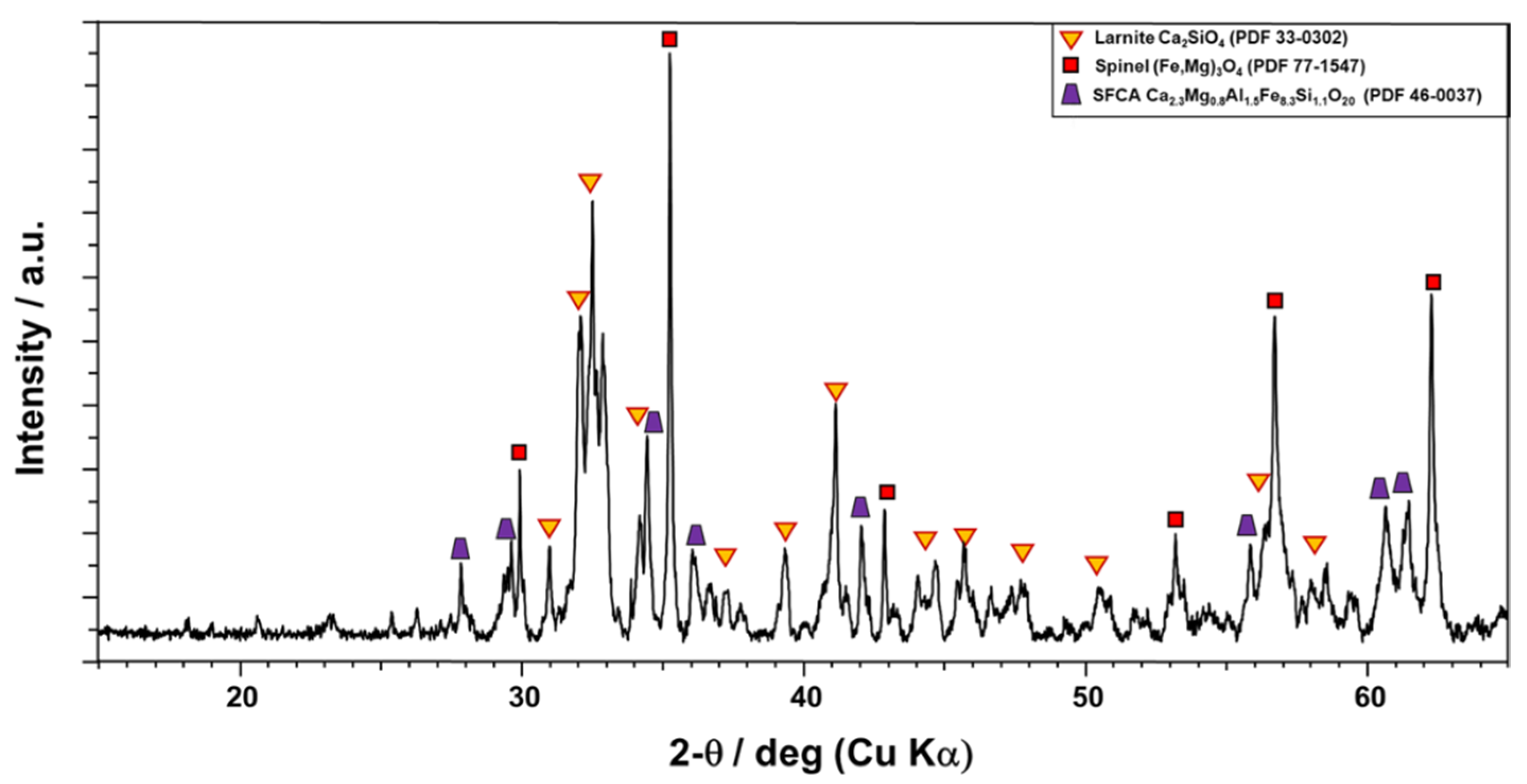
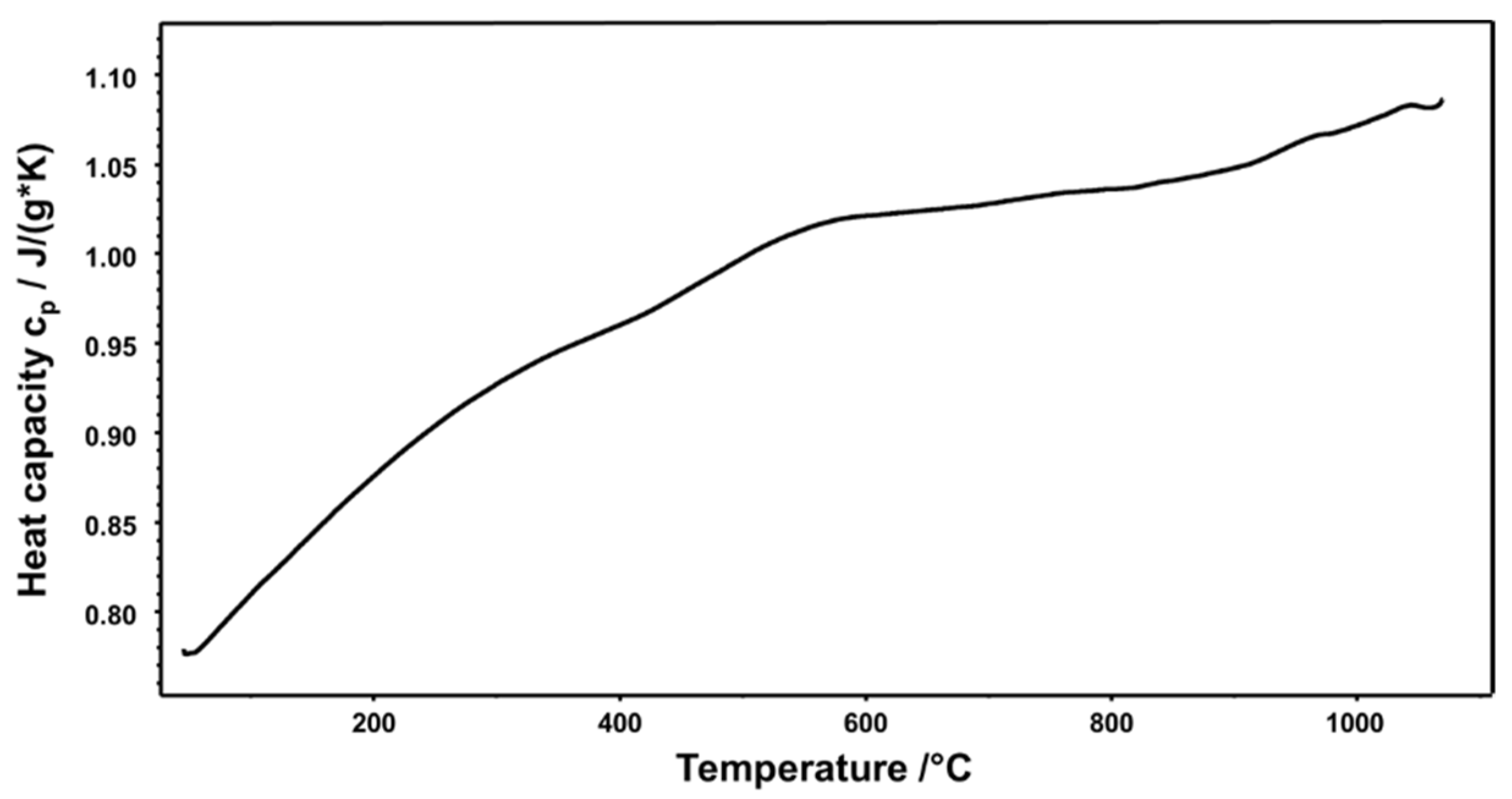
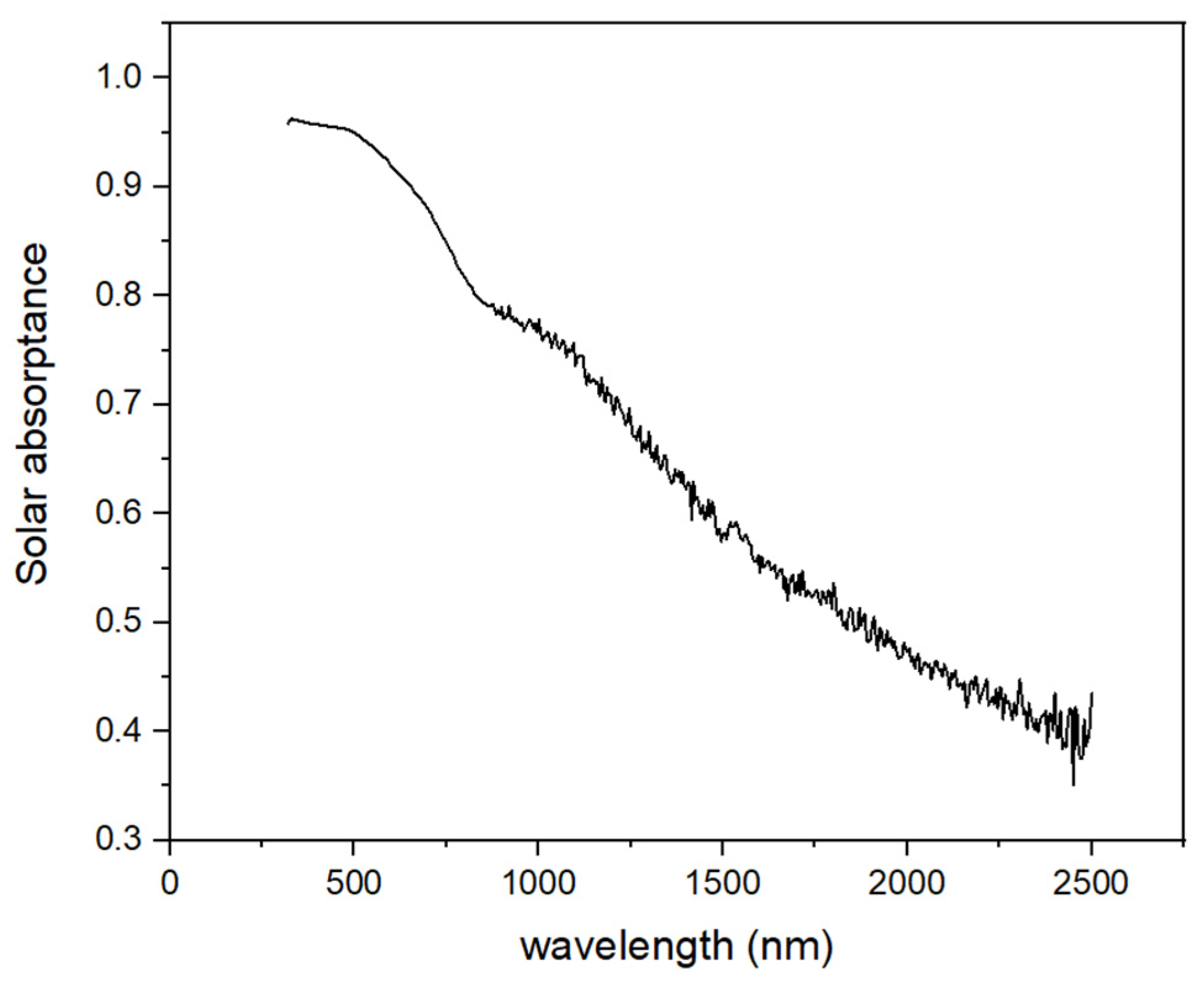
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Barrasso, M.; Langella, G.; Amoresano, A.; Iodice, P. Latest Advances in Thermal Energy Storage for Solar Plants. Processes 2023, 11, 1832. [Google Scholar] [CrossRef]
- Nie, F.; Bai, F.; Wang, Z.; Li, X.; Yang, R. Solid particle solar receivers in the next-generation concentrated solar power plant. EcoMat 2022, 4, e12207. [Google Scholar] [CrossRef]
- Buck, R.; Giuliano, S. Solar Tower System Temperature Range Optimization for reduced LCOE. AIP Conf. Proc. 2019, 2126, 030010. [Google Scholar]
- Palacios, A.; Barreneche, C.; Navarro, M.E.; Ding, Y. Thermal Energy Storage Technologies for Concentrated Solar Power—A Review from a Materials Perspective. Renew. Energy 2020, 156, 1244–1265. [Google Scholar] [CrossRef]
- Calderón, A.; Barreneche, C.; Palacios, A.; Segarra, M.; Prietto, C.; Rodriguez-Sanchez, A.; Fernandez, A.I. Review of solid particle materials for heat transfer fluid and thermal energy storage in solar thermal power plants. Energy Storage 2019, 1, e63. [Google Scholar] [CrossRef]
- Siegel, N.; Gross, M.; Ho, C.; Phan, T.; Yuan, J. Physical Properties of Solid Particle Thermal Energy Storage Media for Concentrating Solar Power Applications. Energy Procedia 2014, 49, 1015–1023. [Google Scholar] [CrossRef]
- Alkan, G.; Mechnich, P.; Barbri, H.; Flucht, F.; Sergeev, D.; Müller, M. Evaluation of Ceramic Proppants as Heat Transfer and Storage medium. In Proceedings of the 27th SolarPACES Conference, Online, 27 September–1 October 2021. [Google Scholar]
- Roop, J.; Jeter, S.; Abdel-Khalik, S.; Ho, C. Optical properties of select particulates after high-temperature treatment exposure. In Proceedings of the 8th International Conference on Energy Sustainability, Boston, MA, USA, 30 June–2 July 2014. [Google Scholar]
- Gobereit, B.; Amsbeck, L.; Happich, C.; Schmücker, M. Assessment and improvement of optical properties of particles for solid particle receiver. Sol. Energy 2020, 199, 844–851. [Google Scholar] [CrossRef]
- Alkan, G.; Mechnich, P.; Pernpeinter, J. Improved Performance of Ceramic Solar Absorber Particles Coated with Black Oxide Pigment Deposited by Resonant Acoustic Mixing and Reaction Sintering. Coatings 2022, 12, 757. [Google Scholar] [CrossRef]
- Alkan, G.; Mechnich, P.; Pernpeinter, J. Using an Al-Incorporated Deep Black Pigment Coating to Enhance the Solar Absorptance of Iron Oxide-Rich Particles. Coatings 2023, 13, 1925. [Google Scholar] [CrossRef]
- Farchado, M.; Vicente, G.S.; Barandica, N.; Sutter, F.; Alkan, G.; Sánchez-Señorán, D.; Morales, Á. Performance improvement of CSP particle receivers by depositing spinel absorber coatings. Sol. Energy Mater. Sol. Cells 2024, 266, 112681. [Google Scholar] [CrossRef]
- Erregueragui, Z.; Tizliouine, A.; Ouhsaine, L.; Chafi, M.; El Hachami Omari, L. Selection and performance evaluation of waste and by-product materials for thermal storage applications. Int. J. Low Carbon Technol. 2022, 17, 888–899. [Google Scholar] [CrossRef]
- Ferber, N.L.; Pham, M.D.; Falcoz, Q.; Meffre, A.; Tessier-Doyen, N.; Nzihou, A.; Goetz, V. Ceramics from municipal waste incinerator bottom ash and wasted clay for sensible heat storage at high temperature. Waste Biomass Valorization 2020, 11, 3107–3120. [Google Scholar] [CrossRef]
- Gutierrez, A.; Miró, L.; Gil, A.; Rodríguez-Aseguinolaza, J.; Barreneche, C.; Calvet, N.; Py, X.; Inés Fernández, A.; Grágeda, M.; Ushak, S.; et al. Advances in the valorization of waste and by-product materials as thermal energy storage (TES) materials. Renew. Sustain. Energy Rev. 2016, 59, 763–783. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, H.; Zhou, J.; Cen, K. Thermal properties and friction behaviors of slag as energy storage material in concentrate solar power plants. Sol. Energy Mater. Sol. Cells 2018, 182, 21–29. [Google Scholar] [CrossRef]
- Krüger, M.; Haunstetter, J.; Knödler, P.; Zunft, S. Slag as an Inventory Material for Heat Storage in a Concentrated Solar Tower Power Plant: Design Studies and Systematic Comparative Assessment. Appl. Sci. 2019, 9, 1833. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Calvet, N.; Gil, A.; Rodríguez-Aseguinolaza, J.; Faik, A.; D’Aguanno, B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, D.; Pinuela-Noval, J.; Gomez-Rodríguez, C.; Fernandez Valdes, A.; Verdeja Gonzalez, L.F. Implications of renewable energy sources in metallurgy: Utilization of concentrated solar energy in recycling metallurgical wastes. Appl. Therm. Eng. 2024, 250, 123511. [Google Scholar] [CrossRef]
- Faik, A.; Guillot, S.; Lambert, J.; Veron, E.; Ory, S.; Bessada, C.; Echegut, P.; Py, X. Thermal storage material from inertized wastes: Evolution of structural and radiative properties with temperature. Sol. Energy 2012, 86, 139–146. [Google Scholar] [CrossRef]
- Glosser, D.; Striby, P.; Bellusci, M.; Bottem, R.; Amroun, S.; Suraneni, P. Characterization of optical and physiochemical properties of reclaimed fly ash for use as sensible thermal energy storage material in concentrating solar power systems. Sol. Energy 2023, 263, 111944. [Google Scholar] [CrossRef]
- Glosser, D.; Bellusci, M.; Oos, K.; Johnson, D.; Suraneni, P. Solar absorptance of fly ashes cycled in oxidizing and non-oxidizing high temperature conditions. J. Energy Storage 2024, 94, 112487. [Google Scholar] [CrossRef]
- Calderon-Vasquez, I.; Segovia, V.; Cardemil, J.M.; Barraza, R. Assessing the use of copper slags as thermal energy storage material for packed-bed systems. Energy 2021, 227, 120370. [Google Scholar] [CrossRef]
- Navarro, M.E.; Martinez, M.; Gil, A.; Fernandez, A.I.; Cabeza, L.F.; Olives, R.; Py, X. Selection and characterization of recycled materials for sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 131–135. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, M.; Yang, S.; Mweemba, S.; Huang, A.; Liu, X.; Zhang, X. Application of copper slags in encapsulating high-temperature phase change thermal storage particles. Sol. Energy Mater. Sol. Cells 2023, 254, 112257. [Google Scholar] [CrossRef]
- Alkan, G.; Mechnich, P.; Lucas, H.; Knoblauch, N.; Sommerfeld, M.; Flucht, F.; Pernpeintner, J.; Sergeev, D.; Müller, M.; Friedrich, B. Assessment of Metallurgical Slags as Solar Heat Absorber Particles. Minerals 2022, 12, 121. [Google Scholar] [CrossRef]
- Lai, Z.; Cen, K.; Zhoum, H. Applicability of coal slag for application as packed bed thermal energy storage materials. Sol. Energy 2022, 236, 733–742. [Google Scholar] [CrossRef]
- Jonczy, I. Microstructures of Metallurgical Slags. Arch. Metall. Mater. 2016, 61, 61–66. [Google Scholar] [CrossRef][Green Version]
- Schlackenmanagement, LiDonit®. thyssenkrupp MillServices & Systems GmbH: Oberhausen, Germany.
- Wu, L.; Li, H.; Mei, H.; Rao, L.; Wang, H.; Lv, N. Generation, utilization, and environmental impact of ladle furnace slag: A minor review. Sci. Total Environ. 2023, 895, 165070. [Google Scholar] [CrossRef]
- Aysal, N.; Kurt, Y.; Öztürk, H.; Ildiz, G.O.; Yesiltas, M.; Laçin, D.; Öngen, S.; Nikitin, T.; Fausto, R. Crystallization Kinetics: Relationship between Crystal Morphology and the Cooling Rate—Applications for Different Geological Materials. Crystals 2023, 13, 1130. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Studer, A.J.; Manuel, J.R.; Kimpton, A.K. Fundamentals of Silico-Ferrite of Calcium and Aluminum (SFCA) and SFCA-I Iron Ore Sinter Bonding Phase Formation: Effects of CaO:SiO2 Ratio. Metall. Mater. Trans. B 2014, 45, 2097–2105. [Google Scholar] [CrossRef]
- Kahlenberg, V.; Krüger, H.; Tribus, M.; Anwander, B. SFCA-II type Ca2.46Fe3+8.57 Fe2+0.52Al5.45O24—An improved structural model for an iron-ore sinter phase. Mineral. Petrol. 2021, 115, 137–147. [Google Scholar] [CrossRef]




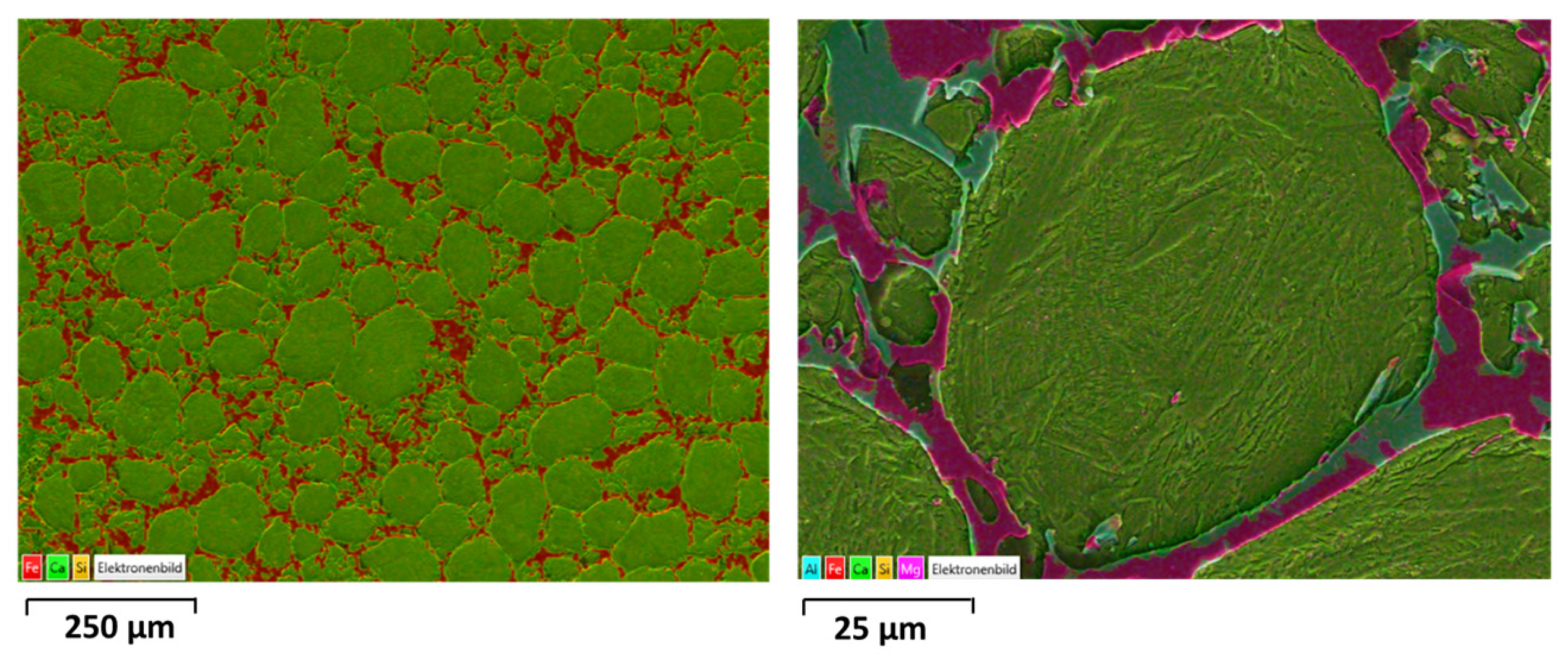


| at. % | O | Ca | Fe | Si | Mg | Al | Mn | Phase |
|---|---|---|---|---|---|---|---|---|
| 1 | 66.3 | 20 | 0.5 | 11.3 | 1.5 | 0.1 | 0.2 | Larnite |
| 2 | 61.6 | 0.8 | 24.1 | 0.1 | 8.5 | 1 | 3.5 | Magnetite |
| 3 | 63.8 | 6.2 | 22.1 | 2.8 | 1.6 | 2.2 | 1 | SFCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkan, G.; Mechnich, P.; Pernpeintner, J. LiDonit®—A Potential Secondary Raw Material for Ceramic Applications in Concentrated Solar Energy. Minerals 2024, 14, 752. https://doi.org/10.3390/min14080752
Alkan G, Mechnich P, Pernpeintner J. LiDonit®—A Potential Secondary Raw Material for Ceramic Applications in Concentrated Solar Energy. Minerals. 2024; 14(8):752. https://doi.org/10.3390/min14080752
Chicago/Turabian StyleAlkan, Gözde, Peter Mechnich, and Johannes Pernpeintner. 2024. "LiDonit®—A Potential Secondary Raw Material for Ceramic Applications in Concentrated Solar Energy" Minerals 14, no. 8: 752. https://doi.org/10.3390/min14080752
APA StyleAlkan, G., Mechnich, P., & Pernpeintner, J. (2024). LiDonit®—A Potential Secondary Raw Material for Ceramic Applications in Concentrated Solar Energy. Minerals, 14(8), 752. https://doi.org/10.3390/min14080752






