Geochronology, Geochemistry, and In Situ Sr-Nd-Hf Isotopic Compositions of a Tourmaline-Bearing Leucogranite in Eastern Tethyan Himalaya: Implications for Tectonic Setting and Rare Metal Mineralization
Abstract
:1. Introduction
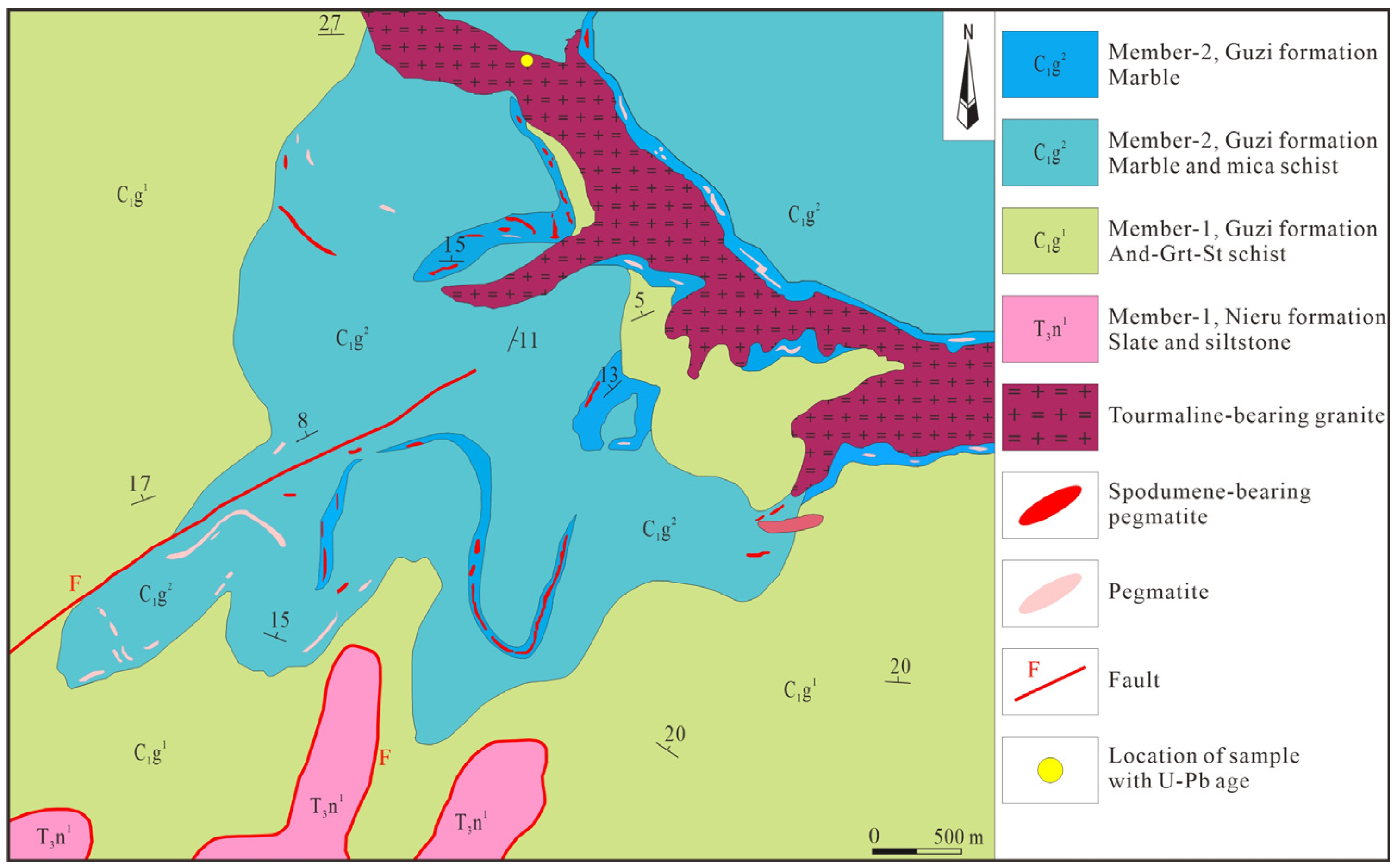
2. Geological Background
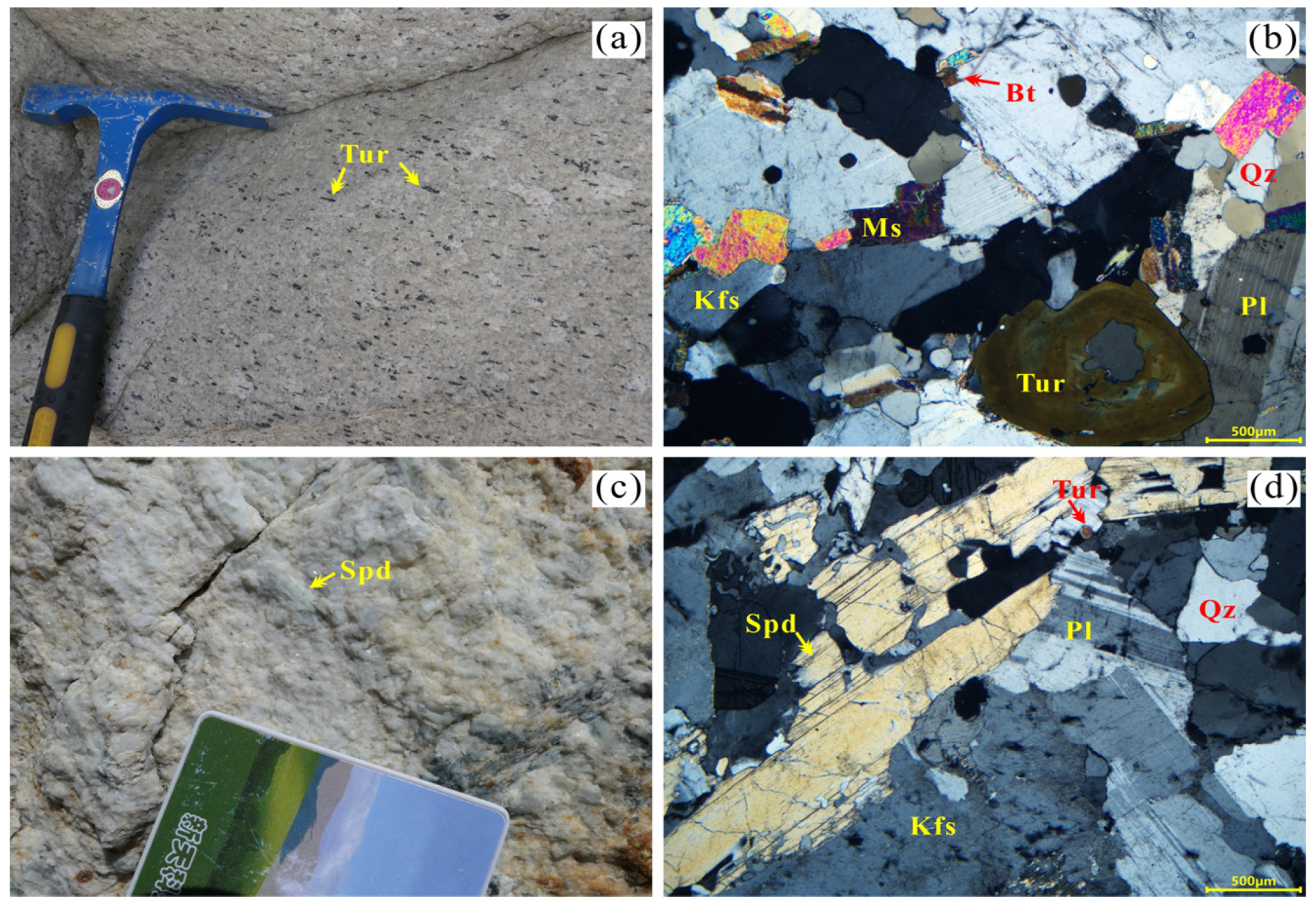
3. Petrography
4. Materials and Methods
4.1. Materials
4.2. Zircon and Monazite U-Pb Dating
4.3. Whole-Rock Major Oxides and Trace Elements Analysis
4.4. In Situ Sr-Nd-Hf Isotopic Analysis
5. Results
5.1. Zircon and Monazite U-Pb Ages
| Spot | Th | U | Ti | Th/U | TTi-in-zircon | 207Pb/206Pb | 207Pb/235U | 206Pb/238U | 207Pb/206Pb | 207Pb/235U | 206Pb/238U | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | 1 s | Ratio | 1 s | Ratio | 1 s | Age | 1 s | Age | 1 s | Age | 1 s | ||||||
| TGL01-4Z-01 | 424 | 9884 | 8.06 | 0.04 | 722 | 0.0463 | 0.0018 | 0.0184 | 0.0007 | 0.0029 | 0.0000 | 12 | 78 | 18.5 | 0.7 | 18.5 | 0.2 |
| TGL01-4Z-02 | 341 | 10,156 | 4.25 | 0.03 | 671 | 0.0494 | 0.0017 | 0.0198 | 0.0007 | 0.0029 | 0.0000 | 168 | 81 | 19.9 | 0.7 | 18.5 | 0.2 |
| TGL01-4Z-03 | 308 | 4642 | 8.86 | 0.07 | 730 | 0.0521 | 0.0022 | 0.0214 | 0.0009 | 0.0030 | 0.0000 | 289 | 94 | 21.5 | 0.9 | 19.0 | 0.2 |
| TGL01-4Z-04 | 254 | 10,156 | - | 0.02 | 0.0475 | 0.0015 | 0.0189 | 0.0005 | 0.0029 | 0.0000 | 76 | 63 | 19 | 0.5 | 18.5 | 0.2 | |
| TGL01-4Z-05 | 879 | 23,739 | 5.97 | 0.04 | 698 | 0.0538 | 0.0013 | 0.0226 | 0.0008 | 0.0030 | 0.0001 | 85 | 95 | 19.8 | 0.7 | 19.2 | 0.4 |
| TGL01-4Z-06 | 3206 | 7793 | - | 0.41 | 0.0554 | 0.0019 | 0.0223 | 0.0007 | 0.0029 | 0.0000 | 428 | 74 | 22.4 | 0.7 | 18.6 | 0.2 | |
| TGL01-4Z-07 | 524 | 9968 | 13.1 | 0.05 | 765 | 0.0463 | 0.0015 | 0.0187 | 0.0006 | 0.0029 | 0.0000 | 15 | 66 | 18.8 | 0.6 | 18.7 | 0.2 |
| TGL01-4Z-08 | 617 | 23,710 | 2.76 | 0.03 | 639 | 0.0483 | 0.0011 | 0.0193 | 0.0004 | 0.0029 | 0.0000 | 115 | 52 | 19.4 | 0.4 | 18.6 | 0.2 |
| TGL01-4Z-09 | 146 | 4418 | 2.84 | 0.03 | 641 | 0.0488 | 0.0020 | 0.0195 | 0.0008 | 0.0029 | 0.0000 | 136 | 93 | 19.6 | 0.8 | 18.6 | 0.2 |
| TGL01-4Z-10 | 718 | 21,044 | 8.86 | 0.03 | 730 | 0.1225 | 0.0034 | 5.7557 | 0.1545 | 0.3395 | 0.0031 | 1993 | 46 | 1940 | 23 | 1884 | 15 |
| TGL01-4Z-11 | 71.8 | 4772 | 6.59 | 0.02 | 706 | 0.0509 | 0.0020 | 0.0198 | 0.0008 | 0.0028 | 0.0000 | 100 | 87 | 18.7 | 0.7 | 18.0 | 0.2 |
| TGL01-4Z-12 | 315 | 6443 | 3.45 | 0.05 | 655 | 0.0478 | 0.0015 | 0.0196 | 0.0006 | 0.0030 | 0.0000 | 88 | 70 | 19.7 | 0.6 | 19.0 | 0.2 |
| TGL01-4Z-13 | 231 | 8303 | 2.33 | 0.03 | 627 | 0.0497 | 0.0015 | 0.0201 | 0.0006 | 0.0029 | 0.0000 | 181 | 69 | 20.2 | 0.6 | 18.7 | 0.2 |
| TGL01-4Z-14 | 268 | 13,532 | 5.96 | 0.02 | 697 | 0.0543 | 0.0014 | 0.0220 | 0.0006 | 0.0029 | 0.0000 | 66 | 67 | 19 | 0.5 | 18.6 | 0.2 |
| TGL01-4Z-15 | 548 | 13,776 | - | 0.04 | 0.0471 | 0.0012 | 0.0192 | 0.0005 | 0.0029 | 0.0000 | 55 | 54 | 19.3 | 0.5 | 18.9 | 0.2 | |
| TGL01-4Z-16 | 327 | 9733 | - | 0.03 | 0.0462 | 0.0015 | 0.0189 | 0.0006 | 0.0030 | 0.0000 | 7 | 64 | 19.1 | 0.6 | 19.1 | 0.2 | |
| TGL01-4Z-17 | 93.4 | 5536 | 4.38 | 0.02 | 673 | 0.0479 | 0.0021 | 0.0186 | 0.0008 | 0.0028 | 0.0000 | 95 | 94 | 18.7 | 0.8 | 18.1 | 0.2 |

| Spot | Th | U | Th/U | 207Pb/206Pb | 207Pb/235U | 206Pb/238U | 207Pb/235U | 206Pb/238U | 208Pb/232Th | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | 1 s | Ratio | 1 s | Ratio | 1 s | Age | 1 s | Age | 1 s | Age | 1 s | ||||
| TGL01-4M-01 | 62,701 | 9647 | 6.50 | 0.0663 | 0.0032 | 0.0276 | 0.0014 | 0.0030 | 0.0000 | 27.6 | 1.4 | 18.9 | 0.3 | 18.3 | 0.2 |
| TGL01-4M-02 | 73,408 | 4923 | 14.91 | 0.0798 | 0.0053 | 0.0319 | 0.0019 | 0.0030 | 0.0001 | 31.9 | 1.8 | 18.6 | 0.3 | 18.9 | 0.2 |
| TGL01-4M-03 | 83,213 | 8999 | 9.25 | 0.0691 | 0.0038 | 0.0278 | 0.0014 | 0.0030 | 0.0000 | 27.8 | 1.4 | 18.6 | 0.3 | 18.4 | 0.2 |
| TGL01-4M-04 | 71,771 | 4699 | 15.27 | 0.0729 | 0.0042 | 0.0303 | 0.0017 | 0.0031 | 0.0001 | 30.4 | 1.7 | 19.1 | 0.4 | 18.7 | 0.2 |
| TGL01-4M-05 | 79,742 | 7001 | 11.39 | 0.0767 | 0.0043 | 0.0303 | 0.0015 | 0.0029 | 0.0000 | 30.3 | 1.5 | 18.1 | 0.3 | 18.4 | 0.2 |
| TGL01-4M-06 | 75,073 | 7379 | 10.17 | 0.0691 | 0.0037 | 0.0277 | 0.0015 | 0.0029 | 0.0000 | 27.8 | 1.4 | 18.4 | 0.3 | 18.5 | 0.2 |
| TGL01-4M-07 | 64,482 | 4101 | 15.72 | 0.0764 | 0.0048 | 0.0315 | 0.0019 | 0.0031 | 0.0001 | 31.5 | 1.8 | 19.0 | 0.3 | 17.9 | 0.2 |
| TGL01-4M-08 | 77,132 | 6874 | 11.22 | 0.0709 | 0.0040 | 0.0283 | 0.0015 | 0.0029 | 0.0000 | 28.4 | 1.5 | 18.4 | 0.3 | 18.4 | 0.2 |
| TGL01-4M-09 | 74,297 | 5652 | 13.14 | 0.0777 | 0.0045 | 0.0314 | 0.0018 | 0.0030 | 0.0000 | 31.4 | 1.7 | 18.3 | 0.3 | 18.2 | 0.2 |
| TGL01-4M-10 | 86,244 | 4800 | 17.97 | 0.0841 | 0.0046 | 0.0358 | 0.0019 | 0.0031 | 0.0001 | 35.7 | 1.8 | 19.3 | 0.4 | 18.1 | 0.2 |
| TGL01-4M-11 | 88,744.0 | 5447 | 16.29 | 0.0818 | 0.0045 | 0.0327 | 0.0017 | 0.0029 | 0.0000 | 32.6 | 1.7 | 18.0 | 0.3 | 18.1 | 0.2 |
| TGL01-4M-12 | 52,578 | 4041 | 13.01 | 0.0672 | 0.0047 | 0.0275 | 0.0018 | 0.0030 | 0.0001 | 27.6 | 1.7 | 18.8 | 0.3 | 18.8 | 0.2 |
| TGL01-4M-13 | 53,956 | 3573 | 15.10 | 0.0741 | 0.0048 | 0.0304 | 0.0019 | 0.0030 | 0.0001 | 30.4 | 1.9 | 18.6 | 0.3 | 17.8 | 0.2 |
| TGL01-4M-14 | 74,487 | 3672 | 20.29 | 0.0894 | 0.0054 | 0.0372 | 0.0021 | 0.0031 | 0.0001 | 37.1 | 2.0 | 18.8 | 0.4 | 18.2 | 0.2 |
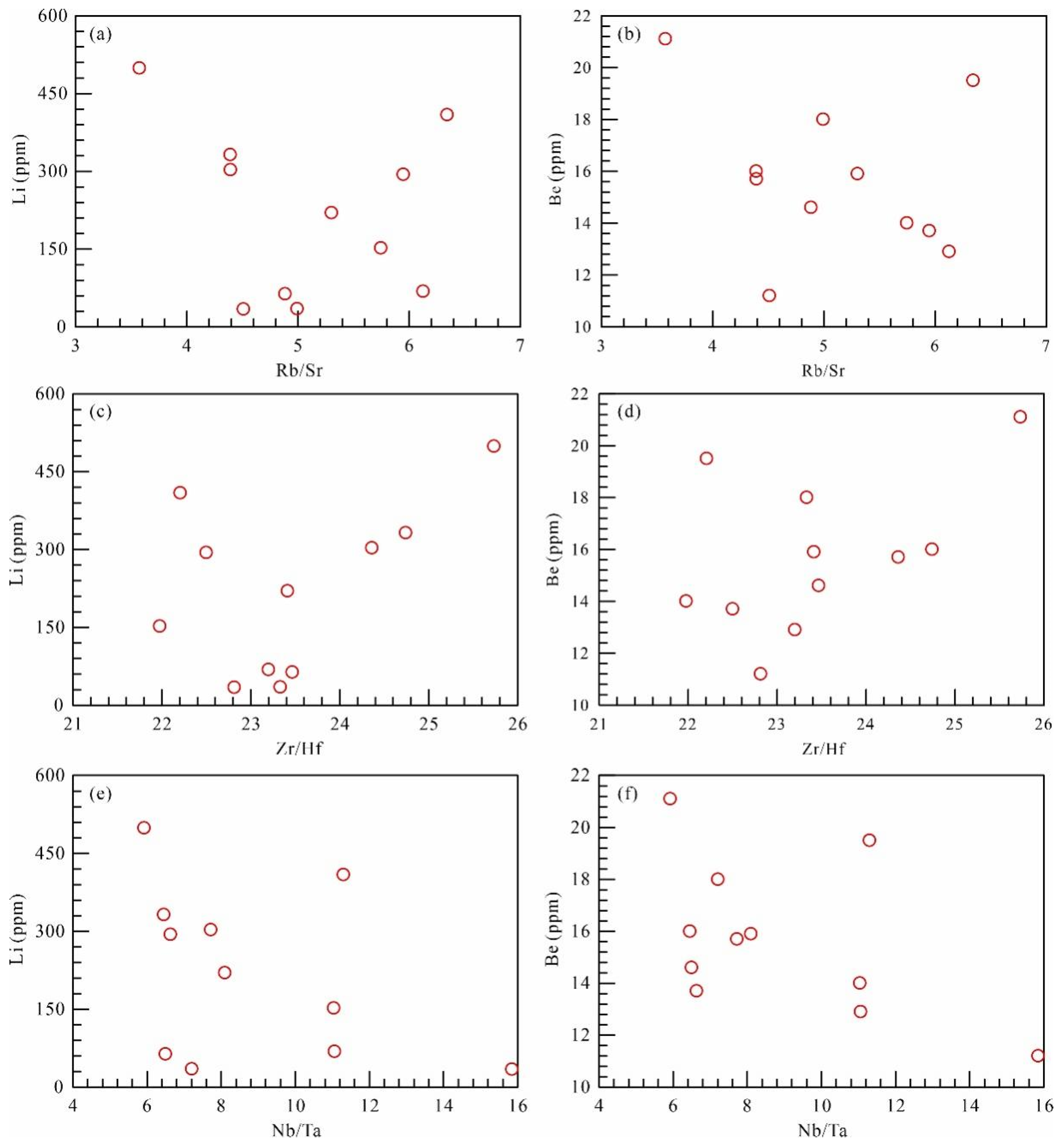
5.2. Whole-Rock Major Oxides and Trace Elements
| Sample | TGL01-2 | TGL01-3 | TGL01-4 | TGL04-1 | TGL05-2 | TGL06-1 | TGL07-1 | TGL07-3 | TGL09-1 | TGL10 | TGL11 | Average | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rock type | Tourmaline-bearing leucogranite | |||||||||||||
| Major element (wt.%) | ||||||||||||||
| SiO2 | 73.16 | 73.90 | 73.82 | 73.57 | 73.63 | 73.79 | 73.99 | 73.41 | 73.35 | 73.46 | 73.76 | 73.62 | 73.16 | 73.99 |
| TiO2 | 0.079 | 0.066 | 0.069 | 0.077 | 0.052 | 0.079 | 0.069 | 0.056 | 0.095 | 0.084 | 0.081 | 0.07 | 0.05 | 0.10 |
| Al2O3 | 15.16 | 15.24 | 15.08 | 15.12 | 15.12 | 15.05 | 15.15 | 15.10 | 15.21 | 15.14 | 15.13 | 15.14 | 15.05 | 15.24 |
| TFe2O3 | 0.83 | 0.78 | 0.71 | 0.78 | 0.64 | 0.80 | 0.80 | 0.75 | 0.78 | 0.82 | 0.83 | 0.78 | 0.64 | 0.83 |
| MnO | 0.011 | 0.010 | 0.012 | 0.012 | 0.010 | 0.021 | 0.020 | 0.015 | 0.025 | 0.014 | 0.015 | 0.01 | 0.01 | 0.02 |
| MgO | 0.12 | 0.12 | 0.12 | 0.15 | 0.12 | 0.15 | 0.12 | 0.11 | 0.20 | 0.16 | 0.14 | 0.14 | 0.11 | 0.20 |
| CaO | 0.97 | 0.85 | 0.92 | 0.92 | 0.80 | 0.96 | 0.93 | 0.95 | 1.22 | 1.03 | 1.02 | 0.96 | 0.80 | 1.22 |
| Na2O | 3.97 | 3.81 | 3.81 | 3.64 | 4.15 | 3.73 | 3.86 | 3.82 | 4.09 | 3.61 | 3.57 | 3.82 | 3.57 | 4.15 |
| K2O | 4.41 | 4.85 | 4.87 | 4.64 | 4.28 | 4.72 | 4.46 | 4.60 | 3.98 | 4.64 | 4.65 | 4.55 | 3.98 | 4.87 |
| P2O5 | 0.10 | 0.09 | 0.10 | 0.08 | 0.08 | 0.09 | 0.08 | 0.09 | 0.08 | 0.10 | 0.10 | 0.09 | 0.08 | 0.10 |
| LOI | 0.43 | 0.46 | 0.37 | 0.54 | 0.53 | 0.74 | 0.69 | 0.59 | 0.68 | 0.62 | 0.61 | 0.57 | 0.37 | 0.74 |
| SUM | 99.24 | 100.18 | 99.88 | 99.52 | 99.41 | 100.11 | 100.17 | 99.48 | 99.72 | 99.68 | 99.91 | 99.75 | 99.24 | 100.18 |
| Trace element (ppm) | ||||||||||||||
| Li | 34.1 | 68.4 | 152 | 220 | 63.5 | 34.8 | 409 | 294 | 499 | 303 | 332 | 219 | 34.1 | 499 |
| Be | 11.2 | 12.9 | 14.0 | 15.9 | 14.6 | 18.0 | 19.5 | 13.7 | 21.1 | 15.7 | 16.0 | 15.7 | 11.2 | 21.1 |
| Sc | 1.77 | 1.47 | 1.51 | 1.40 | 0.98 | 1.29 | 1.37 | 1.00 | 1.34 | 1.26 | 1.54 | 1.36 | 0.98 | 1.77 |
| V | 2.35 | 2.22 | 2.05 | 2.05 | 2.42 | 2.04 | 1.67 | 1.14 | 6.95 | 1.74 | 1.59 | 2.39 | 1.14 | 6.95 |
| Cr | 0.88 | 0.80 | 0.58 | 0.76 | 0.69 | 0.87 | 0.46 | 0.22 | 3.28 | 0.28 | 0.54 | 0.85 | 0.22 | 3.28 |
| Co | 0.48 | 0.42 | 0.39 | 0.53 | 0.34 | 0.47 | 0.33 | 0.33 | 0.74 | 0.43 | 0.47 | 0.45 | 0.33 | 0.74 |
| Ni | 0.65 | 0.43 | 0.35 | 0.36 | 0.30 | 0.40 | 0.14 | 0.18 | 1.20 | 0.21 | 0.34 | 0.41 | 0.14 | 1.20 |
| Cu | 0.81 | 0.72 | 0.51 | 0.58 | 1.10 | 0.49 | 0.37 | 0.34 | 0.42 | 0.40 | 0.37 | 0.56 | 0.34 | 1.10 |
| Zn | 50.4 | 60.7 | 48.2 | 53.1 | 43.7 | 44.1 | 55.1 | 51.1 | 55.2 | 54.8 | 50.0 | 51.5 | 43.7 | 60.7 |
| Ga | 33.2 | 29.6 | 29.9 | 31.3 | 25.6 | 30.2 | 33.9 | 31.9 | 27.5 | 33.7 | 31.3 | 30.8 | 25.6 | 33.9 |
| Rb | 382 | 435 | 427 | 395 | 338 | 393 | 444 | 417 | 454 | 380 | 372 | 403 | 338 | 454 |
| Sr | 84.7 | 71.0 | 74.3 | 74.5 | 69.2 | 78.7 | 70.0 | 70.1 | 127 | 86.5 | 84.7 | 81.0 | 69.2 | 127 |
| Y | 6.44 | 5.45 | 6.10 | 6.13 | 7.85 | 6.51 | 5.93 | 6.68 | 6.27 | 5.74 | 7.14 | 6.39 | 5.45 | 7.85 |
| Zr | 39.7 | 34.8 | 40.0 | 38.4 | 40.6 | 39.2 | 34.2 | 35.1 | 50.7 | 40.2 | 38.6 | 39.2 | 34.2 | 50.7 |
| Nb | 9.51 | 8.52 | 7.84 | 8.02 | 2.99 | 10.6 | 13.0 | 9.82 | 9.30 | 8.42 | 9.49 | 8.87 | 2.99 | 13.0 |
| Sn | 10.0 | 8.74 | 7.60 | 11.6 | 10.3 | 15.6 | 20.0 | 15.2 | 11.7 | 15.7 | 14.1 | 12.8 | 7.60 | 20.0 |
| Cs | 15.2 | 14.2 | 12.8 | 41.2 | 85.8 | 60.5 | 77.0 | 57.7 | 97.2 | 56.8 | 56.6 | 52.3 | 12.8 | 97.2 |
| Ba | 173 | 163 | 139 | 161 | 124 | 173 | 135 | 103 | 222 | 138 | 160 | 154 | 103 | 222 |
| La | 13.8 | 9.87 | 12.1 | 12.6 | 12.2 | 9.15 | 11.6 | 9.45 | 14.6 | 13.4 | 13.5 | 12.0 | 9.15 | 14.6 |
| Ce | 30.2 | 21.5 | 26.6 | 27.2 | 26.3 | 19.9 | 25.5 | 20.8 | 31.2 | 29.3 | 29.7 | 26.2 | 19.9 | 31.2 |
| Pr | 3.49 | 2.47 | 2.98 | 3.08 | 3.03 | 2.32 | 2.90 | 2.39 | 3.60 | 3.37 | 3.42 | 3.00 | 2.32 | 3.60 |
| Nd | 12.4 | 8.72 | 10.6 | 11.1 | 10.3 | 8.26 | 10.2 | 8.47 | 12.7 | 12.3 | 12.2 | 10.7 | 8.26 | 12.7 |
| Sm | 3.94 | 2.86 | 3.65 | 3.69 | 3.35 | 2.64 | 3.33 | 3.12 | 3.32 | 3.97 | 4.03 | 3.44 | 2.64 | 4.03 |
| Eu | 0.68 | 0.54 | 0.56 | 0.59 | 0.51 | 0.63 | 0.58 | 0.50 | 0.64 | 0.65 | 0.66 | 0.59 | 0.50 | 0.68 |
| Gd | 3.42 | 2.38 | 2.91 | 2.84 | 2.99 | 2.45 | 2.98 | 2.75 | 2.69 | 3.06 | 3.31 | 2.89 | 2.38 | 3.42 |
| Tb | 0.46 | 0.35 | 0.43 | 0.40 | 0.42 | 0.35 | 0.38 | 0.36 | 0.35 | 0.37 | 0.43 | 0.39 | 0.35 | 0.46 |
| Dy | 1.83 | 1.43 | 1.59 | 1.51 | 1.87 | 1.60 | 1.51 | 1.60 | 1.53 | 1.48 | 1.82 | 1.62 | 1.43 | 1.87 |
| Ho | 0.23 | 0.19 | 0.23 | 0.22 | 0.28 | 0.22 | 0.20 | 0.21 | 0.21 | 0.19 | 0.24 | 0.22 | 0.19 | 0.28 |
| Er | 0.45 | 0.39 | 0.45 | 0.46 | 0.59 | 0.45 | 0.41 | 0.49 | 0.42 | 0.43 | 0.54 | 0.46 | 0.39 | 0.59 |
| Tm | 0.051 | 0.040 | 0.048 | 0.052 | 0.080 | 0.057 | 0.046 | 0.059 | 0.054 | 0.049 | 0.065 | 0.05 | 0.04 | 0.08 |
| Yb | 0.30 | 0.26 | 0.28 | 0.31 | 0.50 | 0.31 | 0.28 | 0.36 | 0.33 | 0.30 | 0.38 | 0.33 | 0.26 | 0.50 |
| Lu | 0.039 | 0.035 | 0.040 | 0.044 | 0.067 | 0.042 | 0.036 | 0.045 | 0.040 | 0.037 | 0.050 | 0.04 | 0.04 | 0.07 |
| Hf | 1.74 | 1.50 | 1.82 | 1.64 | 1.73 | 1.68 | 1.54 | 1.56 | 1.97 | 1.65 | 1.56 | 1.67 | 1.50 | 1.97 |
| Ta | 0.60 | 0.77 | 0.71 | 0.99 | 0.46 | 1.47 | 1.15 | 1.48 | 1.57 | 1.09 | 1.47 | 1.07 | 0.46 | 1.57 |
| Tl | 2.25 | 2.67 | 2.46 | 2.21 | 1.97 | 2.18 | 2.58 | 2.47 | 2.93 | 2.23 | 2.19 | 2.38 | 1.97 | 2.93 |
| Pb | 89.8 | 94.3 | 98.7 | 91.0 | 88.8 | 96.1 | 85.5 | 92.2 | 83.0 | 86.7 | 95.7 | 91.1 | 83.0 | 98.7 |
| Th | 7.66 | 5.84 | 7.26 | 7.81 | 6.86 | 7.93 | 7.07 | 5.46 | 9.09 | 7.37 | 8.04 | 7.31 | 5.46 | 9.09 |
| U | 3.16 | 10.1 | 9.56 | 11.5 | 8.38 | 4.29 | 12.0 | 3.68 | 17.9 | 2.72 | 6.40 | 8.15 | 2.72 | 17.9 |
| CIPW Norms | ||||||||||||||
| Q | 31.11 | 31.07 | 30.88 | 32.51 | 31.42 | 31.72 | 32.19 | 31.56 | 31.47 | 32.36 | 32.83 | 31.7 | 30.9 | 32.8 |
| C | 2.36 | 2.40 | 2.10 | 2.62 | 2.41 | 2.27 | 2.48 | 2.33 | 2.16 | 2.56 | 2.61 | 2.39 | 2.10 | 2.62 |
| Ab | 34.00 | 32.31 | 32.41 | 31.14 | 35.51 | 31.78 | 32.86 | 32.67 | 34.96 | 30.86 | 30.41 | 32.6 | 30.4 | 35.5 |
| An | 4.19 | 3.64 | 3.96 | 4.11 | 3.53 | 4.25 | 4.11 | 4.24 | 5.58 | 4.52 | 4.46 | 4.23 | 3.53 | 5.58 |
| Or | 26.45 | 28.82 | 28.99 | 27.81 | 25.62 | 28.13 | 26.57 | 27.52 | 23.84 | 27.73 | 27.76 | 27.2 | 23.8 | 29.0 |
| Hy | 1.34 | 1.28 | 1.17 | 1.33 | 1.11 | 1.36 | 1.32 | 1.24 | 1.47 | 1.41 | 1.38 | 1.31 | 1.11 | 1.47 |
| Il | 0.15 | 0.13 | 0.13 | 0.15 | 0.10 | 0.15 | 0.13 | 0.11 | 0.18 | 0.16 | 0.16 | 0.14 | 0.10 | 0.18 |
| Mt | 0.15 | 0.14 | 0.12 | 0.14 | 0.11 | 0.14 | 0.14 | 0.13 | 0.14 | 0.14 | 0.15 | 0.14 | 0.11 | 0.15 |
| Ap | 0.24 | 0.21 | 0.23 | 0.19 | 0.18 | 0.20 | 0.20 | 0.20 | 0.20 | 0.24 | 0.24 | 0.21 | 0.18 | 0.24 |
| Zr/Ti | 0.08 | 0.09 | 0.10 | 0.08 | 0.13 | 0.08 | 0.08 | 0.10 | 0.09 | 0.08 | 0.08 | 0.09 | 0.11 | 0.09 |
| Na2O + K2O | 8.38 | 8.66 | 8.68 | 8.29 | 8.42 | 8.45 | 8.32 | 8.41 | 8.07 | 8.25 | 8.22 | 8.38 | 8.07 | 8.68 |
| K2O/Na2O | 1.11 | 1.27 | 1.28 | 1.28 | 1.03 | 1.26 | 1.16 | 1.20 | 0.97 | 1.28 | 1.30 | 1.20 | 0.97 | 1.30 |
| A/CNK | 1.16 | 1.17 | 1.14 | 1.19 | 1.17 | 1.16 | 1.18 | 1.16 | 1.15 | 1.18 | 1.19 | 1.17 | 1.14 | 1.19 |
| A/NK | 1.34 | 1.32 | 1.31 | 1.37 | 1.32 | 1.34 | 1.36 | 1.34 | 1.38 | 1.38 | 1.39 | 1.35 | 1.31 | 1.39 |
| Al2O3/TiO2 | 192 | 232 | 220 | 197 | 291 | 191 | 221 | 269 | 159 | 180 | 186 | 213 | 159 | 291 |
| CaO/Na2O | 0.24 | 0.22 | 0.24 | 0.25 | 0.19 | 0.26 | 0.24 | 0.25 | 0.30 | 0.29 | 0.29 | 0.25 | 0.19 | 0.30 |
| Nb/Ta | 15.9 | 11.1 | 11.0 | 8.06 | 6.52 | 7.22 | 11.3 | 6.65 | 5.92 | 7.72 | 6.44 | 8.90 | 5.92 | 15.9 |
| Zr/Hf | 22.8 | 23.2 | 22.0 | 23.4 | 23.5 | 23.3 | 22.2 | 22.5 | 25.8 | 24.3 | 24.7 | 23.4 | 22.0 | 25.8 |
| (La/Yb)N | 32.5 | 27.7 | 31.4 | 29.1 | 17.7 | 20.9 | 30.2 | 19.0 | 31.3 | 32.4 | 25.6 | 27.1 | 17.7 | 32.5 |
| δEu | 0.57 | 0.63 | 0.52 | 0.56 | 0.49 | 0.76 | 0.56 | 0.52 | 0.65 | 0.57 | 0.55 | 0.58 | 0.49 | 0.76 |
| Rb/Sr | 4.51 | 6.13 | 5.75 | 5.30 | 4.89 | 4.99 | 6.35 | 5.95 | 3.58 | 4.40 | 4.39 | 5.11 | 3.58 | 6.35 |
| Rb/Ba | 2.21 | 2.68 | 3.08 | 2.45 | 2.72 | 2.27 | 3.28 | 4.05 | 2.05 | 2.75 | 2.32 | 2.72 | 2.05 | 4.05 |
| TZr (°C) | 686 | 677 | 686 | 686 | 689 | 686 | 677 | 678 | 703 | 689 | 686 | 686 | 677 | 703 |
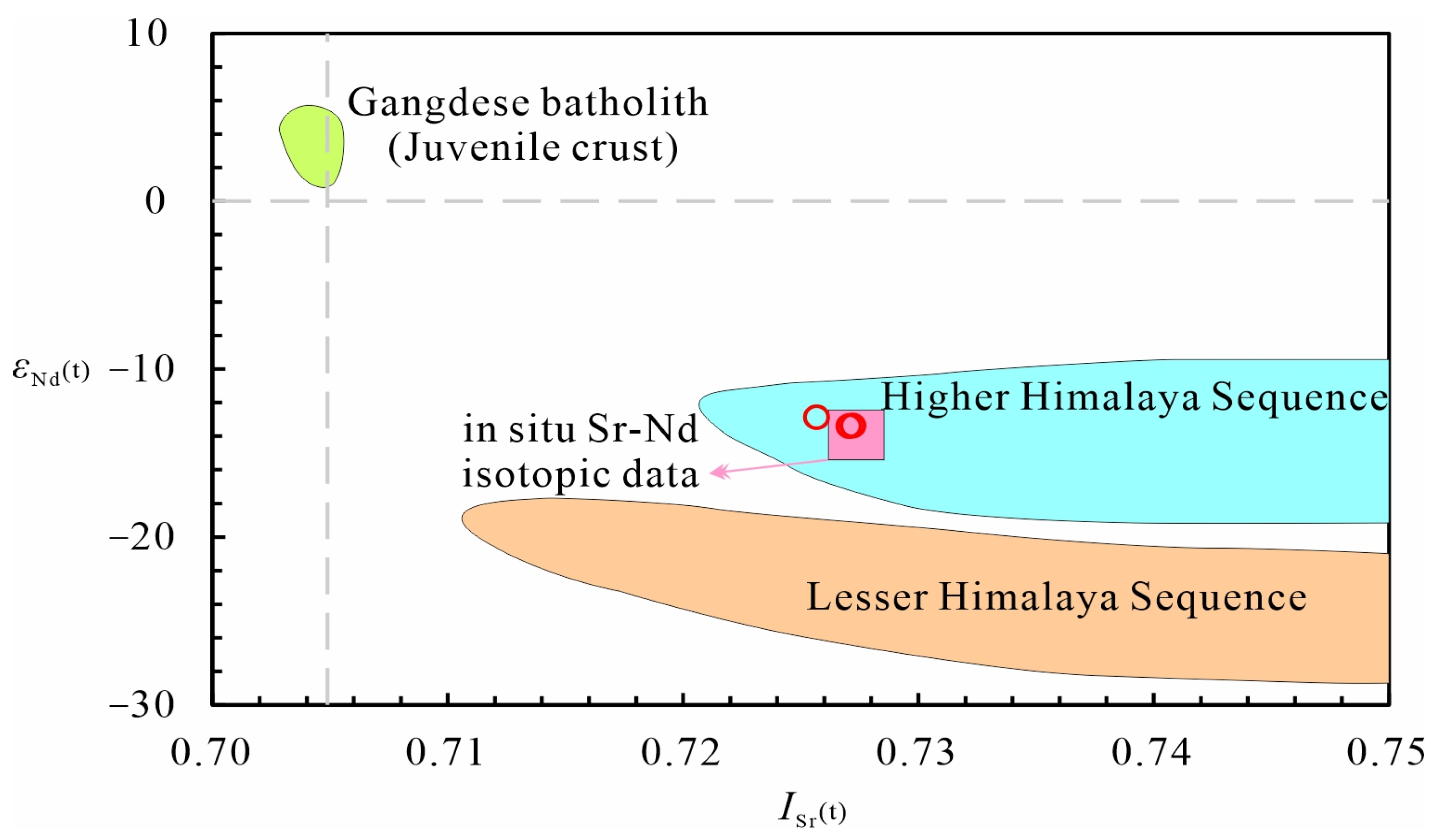
5.3. In Situ Sr-Nd-Hf Isotopic Compositions
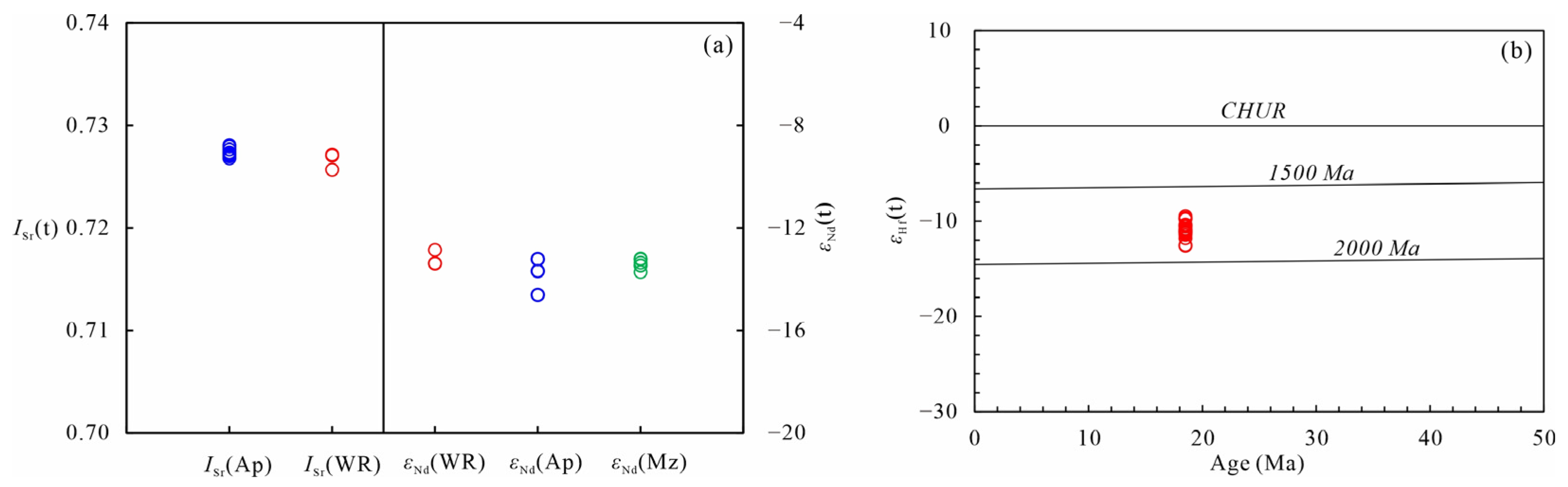
5.3.1. Apatite Sr Isotopic Compositions
5.3.2. Apatite and Monazite Nd Isotopic Compositions
5.3.3. Zircon Hf Isotopic Compositions
6. Discussion
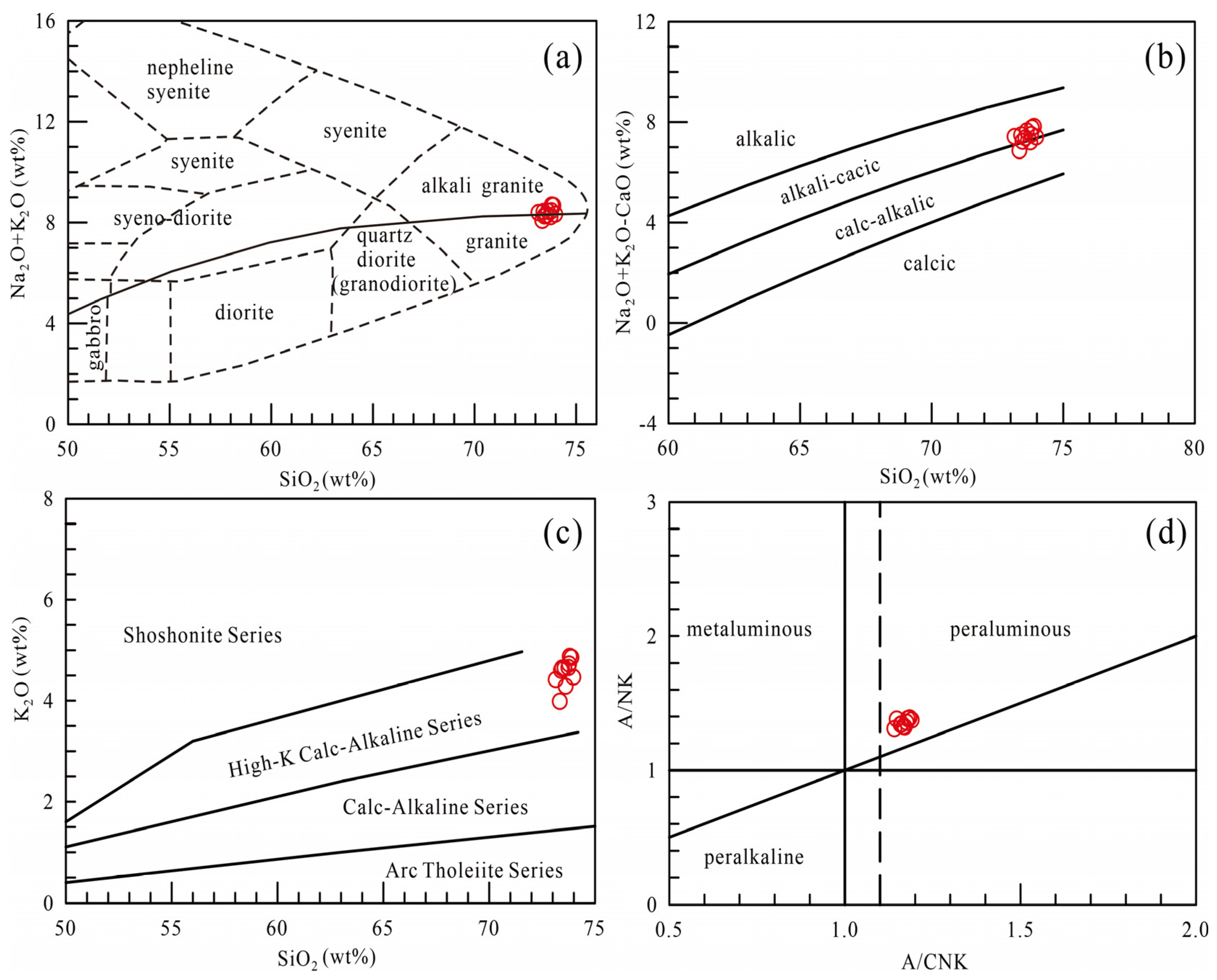
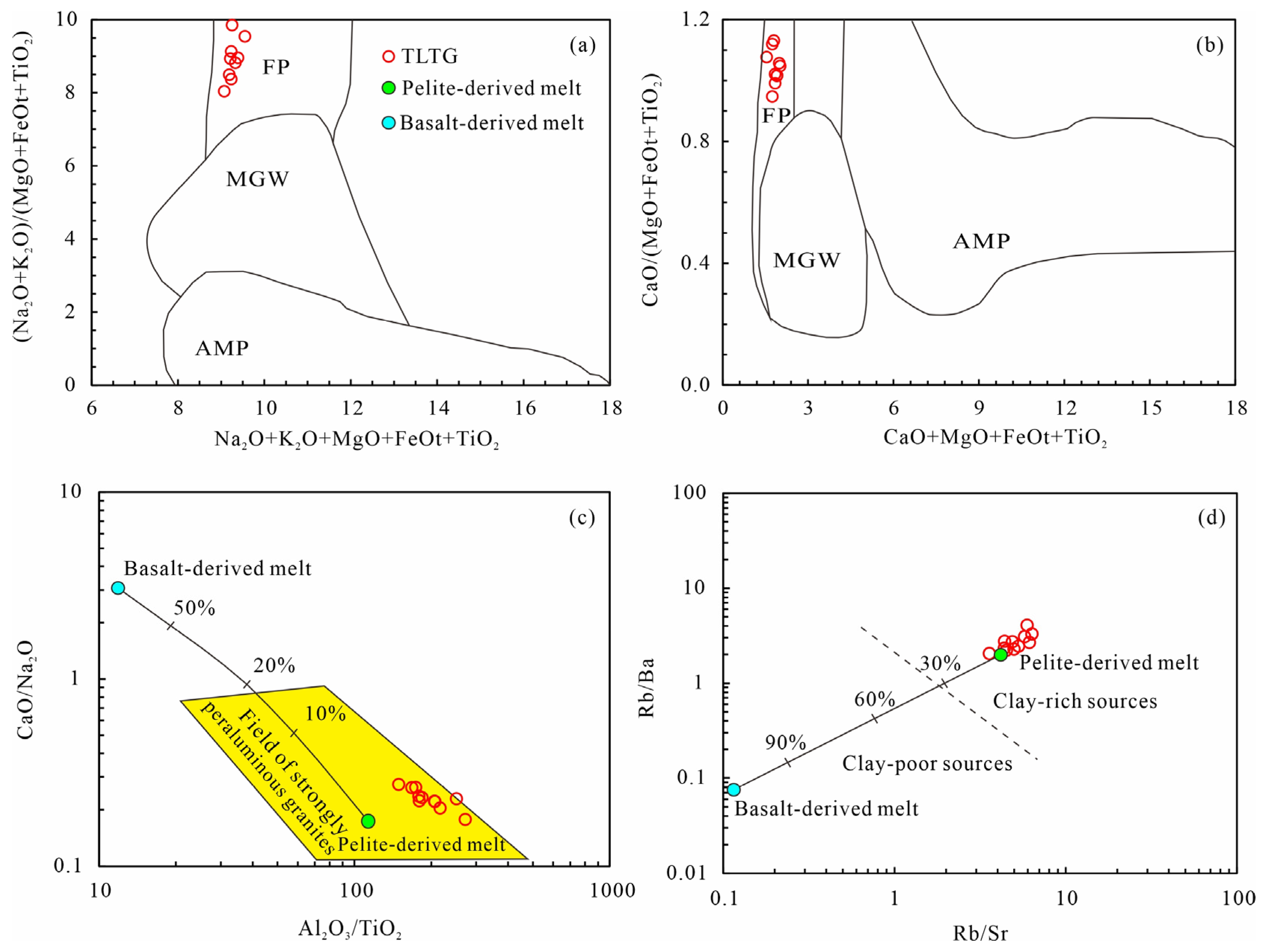
6.1. Genetic Classification of the LTLGs
6.2. Petrogenesis of the LTLGs
6.3. Tectonic Implications
7. Summary of Findings
- Zircon and monazite dating of tourmaline-bearing leucogranites from the Luozha area in South Tibet yielded identical results, with weighted mean ages of 18.66 ± 0.16 Ma and 18.59 ± 0.22 Ma, respectively.
- Whole-rock geochemical and in situ Sr-Nd-Hf isotopic data indicate that the tourmaline-bearing leucogranites are characterized by high SiO2, Al2O3, Na2O, and K2O contents and A/CNK, Al2O3/TiO2, and Rb/Sr ratios, and low TiO2, Fe2O3t, MgO, CaO, and MnO contents and CaO/TiO2 and Eu/Eu* ratios, typical of S-type granite. The samples analyzed share similar features in their LREE and LILE enrichment and HREE and HFSE depletion, with homogeneous and high I Sr (t) but low εNd(t) and εHf(t).
- The tourmaline-bearing leucogranites were derived from the muscovite dehydration melting of an ancient metapelitic source within the Higher Himalayan Sequence, and wall-rock contamination played only a negligible role in their formation.
- The leucogranites were formed in regional extension due to the activity of the STDS, which contributed to the formation of Neo-Himalayan leucogranites and associated rare-metal mineralization.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yin, A.; Harrison, T.M. Geologic evolution of the Himalayan–Tibetan orogen. Annu. Rev. Earth Planet. Sci. 2000, 28, 211–280. [Google Scholar] [CrossRef]
- Yin, A. Cenozoic tectonic evolution of Asia: A preliminary synthesis. Tectonophysics 2010, 488, 293–325. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Qu, X.M.; Yang, Z.S.; Meng, X.J.; Li, Z.Q.; Yang, Z.M.; Zheng, M.P.; Zheng, Y.Y.; Nie, F.J.; Gao, Y.F.; et al. Metallogenesis in Tibetan collisional orogenic belt: Ⅲ. Mineralization in post-collisional extension setting. Miner. Depos. 2006, 25, 629–651, (In Chinese with English Abstract). [Google Scholar]
- Wu, F.Y.; Liu, Z.C.; Liu, X.C.; Ji, W.Q. Himalayan leucogranite: Petrogenesis and implications to orogenesis and plateau uplift. Acta Petrol. Sin. 2015, 31, 1–36, (In Chinese with English Abstract). [Google Scholar]
- Wu, F.Y.; Liu, X.C.; Liu, Z.C.; Wang, R.C.; Xie, L.; Wang, J.M.; Ji, W.Q.; Yang, L.; Liu, C.; Khanal, G.P.; et al. Highly fractionated Himalayan leucogranites and associated rare-metal mineralization. Lithos 2020, 352, 105319. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Yang, J.S.; Hou, Z.Q.; Zhang, Z.M.; Zeng, L.S.; Li, H.B.; Zhang, J.X.; Li, Z.H.; Ma, X.X. The progress in the study of continental dynamics of the Tibetan Plateau. Geol. China 2016, 43, 1–42, (In Chinese with English Abstract). [Google Scholar]
- Zeng, L.S.; Gao, L.E. Cenozoic crustal anatexis and the leucogranites in the Himalayan collisional orogenic belt. Acta Petrol. Sin. 2017, 33, 1420–1444, (In Chinese with English Abstract). [Google Scholar]
- Deng, J.F.; Zhao, H.L.; Lai, S.C.; Liu, H.X.; Luo, Z.H. Generation of muscovite/two-mica granite and intracontinental subduction. Earth Sci. J. China Univ. Geosci. 1994, 19, 139–147, (In Chinese with English Abstract). [Google Scholar]
- Visonà, D.; Lombardo, B. Two-mica and tourmaline leucogranites from the Everest-Makalu region (Nepal-Tibet). Himalayan leucogranite genesis by isobaric heating? Lithos 2002, 62, 125–150. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liao, Q.A.; Li, D.W. Geochemical features of the high Himalayan leucogranites of Dingjie Area, Tibet: Implication for magma sources. Geol. Sci. Technol. Inf. 2004, 22, 9–14, (In Chinese with English Abstract). [Google Scholar]
- Guo, S.S.; Li, S.G. Petrological and geochemical constraints on the origin of leucogranites. Earth Sci. Front. 2007, 14, 290–298. [Google Scholar]
- Liu, Z.C.; Wu, F.Y.; Liu, X.C.; Wang, J.G.; Yin, R.; Qiu, Z.L.; Ji, W.Q.; Yang, L. Mineralogical evidence for fractionation processes in the Himalayan leucogranites of the Ramba Dome, southern Tibet. Lithos 2019, 340, 71–86. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Liu, S.A.; Liu, Z.C.; Zheng, Y.C.; Wu, F.Y. Extreme Mg and Zn isotope fractionation recorded in the Himalayan leucogranites. Geochim. Cosmochim. Acta 2019, 278, 305–321. [Google Scholar] [CrossRef]
- Yan, H.; Yu, D.; Wang, S.; Ma, C. Magmatic Garnet and Magma Evolution in Cuonadong Leucogranites: Constraints from Petrology and Mineral Geochemistry. Minerals 2022, 12, 1275. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.C.; Linnen, R.L.; Wu, F.Y.; Xie, L.; Liu, X.C. Continuous Be mineralization from two-mica granite to pegmatite: Critical element enrichment processes in a Himalayan leucogranite pluton. Am. Mineral. 2023, 108, 31–41. [Google Scholar] [CrossRef]
- Yang, X.S.; Jin, Z.M.; Huenges, E.; Frank, R.S.; Wunder, B. Experimental study on dehydration melting of natural biotite-plagioclase gneiss from high Himalayas and implications for Himalayan crust anatexis. Chin. Sci. Bull. 2001, 46, 867–872. [Google Scholar] [CrossRef]
- Guo, Z.F.; Wilson, M. The Himalayan leucogranites: Constraints on the nature of their crustal source region and geodynamic setting. Gondwana Res. 2012, 22, 360–376. [Google Scholar] [CrossRef]
- Gou, Z.B.; Zhang, Z.M.; Dong, X.; Xiang, H.; Ding, H.X.; Tian, Z.L.; Lei, H.C. Petrogenesis and tectonic implications of the Yadong leucogranites, southern Himalaya. Lithos 2016, 256, 300–310. [Google Scholar] [CrossRef]
- Gao, P.; Zheng, Y.F.; Zhao, Z.F.; Sun, G.C. Source diversity in controlling the compositional diversity of the Cenozoic granites in the Tethyan Himalaya. Lithos 2021, 388, 106072. [Google Scholar] [CrossRef]
- Aoya, M.; Wallis, S.R.; Terada, K.; Lee, J.; Kawakami, T.; Wang, Y.; Heizler, M. North-south extension in the Tibetan crust triggered by granite emplacement. Geology 2005, 33, 853–856. [Google Scholar] [CrossRef]
- King, J.; Harris, N.; Argles, T.; Parrish, R.; Zhang, H.F. Contribution of crustal anatexis to the tectonic evolution of indian crust beneath southern Tibet. Geol. Soc. Am. Bull. 2011, 123, 218–239. [Google Scholar] [CrossRef]
- Li, G.M.; Zhang, L.K.; Jiao, Y.J.; Xia, X.B.; Dong, S.L.; Fu, J.G.; Liang, W.; Zhang, Z.; Wu, J.Y.; Dong, L.; et al. First discovery and implications of Cuonadong superlarge Be-W-Sn polymetallic deposit in Himalayan metallogenic belt, southern Tibet. Miner. Depos. 2017, 36, 1003–1008, (In Chinese with English Abstract). [Google Scholar]
- Qin, K.Z.; Zhao, J.X.; He, C.T.; Shi, R.Z. Discovery of the Qongjiagang giant lithium pegmatite deposit in Himalaya, Tibet, China. Acta Petrol. Sin. 2021, 37, 3277–3286, (In Chinese with English Abstract). [Google Scholar]
- Wu, F.Y.; Wang, R.C.; Liu, X.C.; Xie, L. New breakthroughs in the studies of Himalayan rare-metal mineralization. Acta Petrol. Sin. 2021, 37, 3261–3276, (In Chinese with English Abstract). [Google Scholar]
- Li, G.M.; Fu, J.G.; Guo, W.K.; Zhang, H.; Zhang, L.K.; Dong, S.L.; Li, Y.X.; Wu, J.Y.; Jiao, Y.J.; Jin, C.H.; et al. Discovery of the Gabo granitic pegmatite-type lithium deposit in the Kulagangri Dome, eastern Himalayan metallogenic belt, and its prospecting implication. Acta Petrol. Mineral. 2022, 41, 1109–1119, (In Chinese with English Abstract). [Google Scholar]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Liu, Y.S.; Gao, S.; Hu, Z.C.; Gao, C.G.; Zong, K.Q.; Wang, D.B. Continental and oceanic crust recycling-induced melt-peridotite interactions in the Trans-North China Orogen: U-Pb dating, Hf isotopes and trace elements in zircons of mantle xenoliths. J. Petrol. 2010, 51, 537–571. [Google Scholar] [CrossRef]
- Ludwig, K.R. A Geochronological Toolkit for Microsoft Excel; Isoplot: Berkeley, CA, USA, 2003; pp. 1–77. [Google Scholar]
- GB/T 14506.30-2010; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and Standardization Administration of the People’s Republic of China. Methods for Chemical Analysis of Silicate Rocks—Part 30: Determination of 44 Elements. Standards Press of China: Beijing, China, 2010; 14p.
- Zhang, W.; Hu, Z.; Spectroscopy, A. Estimation of Isotopic Reference Values for Pure Materials and Geological Reference Materials. At. Spectrosc. 2020, 41, 93–102. [Google Scholar] [CrossRef]
- Tong, X.R.; Liu, Y.S.; Hu, Z.C.; Chen, H.H.; Zhou, L.; Hu, Q.H.; Xu, R.; Deng, L.X.; Chen, C.F.; Yang, L.; et al. Accurate determination of Sr isotopic compositions in clinopyroxene and silicate glasses by LA-MC-ICP-MS. Geostand. Geoanalytical Res. 2016, 40, 85–99. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Z.; Liu, Y.; Wu, T.; Deng, X.; Guo, J.; Han, Z. Improved in situ Sr isotopic analysis by a 257 nm femtosecond laser in combination with the addition of nitrogen for geological minerals. Chem. Geol. 2018, 479, 10–21. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wu, F.Y.; Yang, J.H.; Chew, D.M.; Xie, L.W.; Chu, Z.Y.; Zhang, Y.B.; Huang, C. Sr and Nd isotopic compositions of apatite reference materials used in U-Th-Pb geochronology. Chem. Geol. 2014, 385, 35–55. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Z.C.; Zhang, W.; Yang, L.; Liu, Y.S.; Gao, S. In situ Nd isotope analyses in geological materials with signal enhancement and non-linear mass dependent fractionation reduction using laser ablation MC-ICP-MS. J. Anal. At. Spectrom. 2015, 30, 232–244. [Google Scholar] [CrossRef]
- Wu, Y.B.; Zheng, Y.F. Genesis of zircon and its constraints on interpretation of U-Pb age. Chin. Sci. Bull. 2004, 49, 1554–1569. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Mineral. Petrol. 2006, 151, 413–433. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Wilson, M. Igneous Petrogenesis: A Global Tectonic Approach; Unwin Hyman: London, UK, 1989; 466p. [Google Scholar]
- Frost, B.R.; Barnes, C.G.; Collins, W.J.; Arculus, R.J.; Ellis, D.J.; Frost, C.D. A geochemical classification for granitic rocks. J. Petrol. 2001, 42, 2033–2048. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Maniar, P.D.; Piccoli, P.M. Tectonic discrimination of granitoids. Geol. Soc. Am. Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- MDonough, W.F.; Sun, S.S. Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Huang, C.M.; Zhao, Z.D.; Zhu, D.C.; Liu, D.; Huang, Y.; Dong, M.C.; Hu, Z.C.; Zheng, J.P. Geochemistry, zircon U-Pb chronology and Hf isotope of Luozha leucogranite, southern Tibet: Implication for petrogenesis. Acta Petrol. Sin. 2013, 29, 3689–3702. [Google Scholar]
- Chappell, B.W.; White, A. Two contrasting granite types. Pac. Geol. 1974, 8, 173–174. [Google Scholar]
- Loiselle, M.C.; Wones, D.R. Characteristics of Anorogenic Granites. Geol. Soc. Am. Abstr. Programs 1979, 11, 468. [Google Scholar]
- Wu, F.Y.; Li, X.H.; Yang, J.H.; Zheng, Y.F. Discussion on the petrogenesis of granites. Acta Petrol. Sin. 2007, 23, 1217–1238, (In Chinese with English Abstract). [Google Scholar]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Petrol. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Chappell, B.W. Aluminium saturation in I-and S-type granites and the characterization of fractionated haplogranites. Lithos 1999, 46, 535–551. [Google Scholar] [CrossRef]
- Li, X.H.; Li, W.X.; Li, Z.X. On genetic types and tectonic significance of early Yanshanian granites in Nanling. Chin. Sci. Bull. 2007, 9, 981–991. [Google Scholar]
- Acosta-Vigil, A.; London, D.; Morgan, V.I. Experiments on the kinetics of partial melting of a leucogranite at 200 MPa H2O and 690–800 °C: Compositional variability of melts during the onset of H2O-saturated crustal anatexis. Contrib. Mineral. Petrol. 2006, 151, 539–557. [Google Scholar] [CrossRef]
- Searle, M.P.; Cottle, J.M.; Streule, M.J.; Waters, D.J. Crustal melt granites and migmatites along the Himalaya: Melt source, segregation, transport and granite emplacement mechanisms. Earth Environ. Sci. Trans. R. Soc. Edinb. 2009, 100, 219–233. [Google Scholar] [CrossRef]
- Teixeira, R.J.S.; Neiva, A.M.R.; Gomes, M.E.P.; Corfu, F.; Cuesta, A.; Croudace, I.W. The role of fractional crystallization in the genesis of early syn-D3, tin-mineralized Variscan two-mica granites from the Carrazeda de Ansiães area, northern Portugal. Lithos 2012, 153, 177–191. [Google Scholar] [CrossRef]
- Gao, L.E.; Zeng, L.S. Fluxed melting of metapelite and the formation of Miocene high-CaO two-mica granites in the Malashan gneiss dome, southern Tibet. Geochim. Cosmochim. Acta 2014, 130, 136–155. [Google Scholar] [CrossRef]
- Huang, F.; Bai, R.; Deng, G.; Liu, X.; Li, X. Barium isotope evidence for the role of magmatic fluids in the origin of Himalayan leucogranites. Sci. Bull. 2021, 66, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.E.; Zeng, L.; Zhao, L.; Yan, L. Sequential melting of deep crustal source rocks in a rift system: An example from southern Tibet. Chem. Geol. 2023, 618, 121295. [Google Scholar] [CrossRef]
- Wu, F.Y.; Sun, D.Y.; Li, H.; Jahn, B.M.; Wilde, S. A-type granites in northeastern China: Age and geochemical constraints on their petrogenesis. Chem. Geol. 2002, 187, 143–173. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wu, F.Y.; Ji, W.Q.; Wang, J.G.; Liu, C.Z. Petrogenesis of the Ramba leucogranite in the Tethyan Himalaya and constraints on the channel flow model. Lithos 2014, 208, 118–136. [Google Scholar] [CrossRef]
- Liu, Z.C.; Liu, X.C.; Yu, L.J.; Wang, J. Highly fractionated origin and magmatic-hydrothermal evolution of the Kampa leucogranites in the Tethyan Himalaya. J. Nanjing Univ. Nat. Sci. 2020, 56, 800–814, (In Chinese with English Abstract). [Google Scholar]
- Kaygusuz, A.; Siebel, W.; Şen, C.; Satir, M. Petrochemistry and petrology of I-type granitoids in an arc setting: The composite Torul pluton, Eastern Pontides, NE Turkey. Int. J. Earth Sci. 2008, 97, 739–764. [Google Scholar] [CrossRef]
- Sylvester, P.J. Post-collisional strongly peraluminous granites. Lithos 1998, 45, 29–44. [Google Scholar] [CrossRef]
- Gao, L.E.; Zeng, L.S.; Asimow, P.D. Contrasting geochemical signatures of fluidabsent versus fluid-fluxed melting of muscovite in metasedimentary sources: The Himalayan leucogranites. Geology 2017, 45, 39–42. [Google Scholar] [CrossRef]
- Richards, A.; Parrish, R.; Harris, N.; Argles, T.; Zhang, L. Correlation of lithotectonic units across the eastern Himalaya, Bhutan. Geology 2006, 34, 341–344. [Google Scholar] [CrossRef]
- Zeng, L.S.; Gao, L.E.; Tang, S.H.; Hou, K.J.; Guo, C.L.; Hu, G.Y. Eocene magmatism in the Tethyan Himalaya, Southern Tibet. Geol. Soc. Spec. Publ. 2014, 412, 287–316. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Cao, H.W.; Pei, Q.M.; Santosh, M.; Li, G.M.; Zhang, L.K.; Zhang, X.F.; Zhang, Y.H.; Zou, H.; Dai, Z.W.; Lin, B.; et al. Himalayan leucogranites: A review of geochemical and isotopic characteristics, timing of formation, genesis, and rare metal mineralization. Earth-Sci. Rev. 2022, 234, 104229. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Cao, H.; Zhang, X.; Li, Y.; Gao, K.; Dong, L.; Zhang, K.; Liu, X. Origin of Himalayan Eocene Adakitic Rocks and Leucogranites: Constraints from Geochemistry, U-Pb Geochronology and Sr-Nd-Pb-Hf Isotopes. Minerals 2023, 13, 1204. [Google Scholar] [CrossRef]
- Hu, X.M.; Garzanti, E.; Wang, J.G.; Huang, W.T.; Wei, A.; Webb, A. The timing of India-Asia collision onset—Facts, theories, controversies. Earth Sci. Rev. 2016, 160, 264–299. [Google Scholar] [CrossRef]
- Zeng, L.S.; Gao, L.E.; Xie, K.J.; Jing, L.Z. Mid-Eocene high Sr/Y granites in the Northern Himalayan Gneiss Domes: Melting thickened lower continental crust. Earth Planet. Sci. Lett. 2011, 303, 251–266. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Zheng, Y.C.; Zeng, L.S.; Gao, L.E.; Huang, K.X.; Li, W.; Li, Q.Y.; Fu, Q.; Liang, W.; Sun, Q.Z. Eocene-Oligocene granitoids in southern Tibet: Constraints on crustal anatexis and tectonic evolution of the Himalayan orogen. Earth Planet. Sci. Lett. 2012, 349, 38–52. [Google Scholar] [CrossRef]
- Hodges, K.V. Tectonics of the Himalaya and southern Tibet from two perspectives. Geol. Soc. Am. Bull. 2000, 112, 324–350. [Google Scholar] [CrossRef]
- Zhang, H.F.; Harris, N.; Parrish, R.; Zhang, L.; Zhao, Z.D. U–Pb ages of Kude and Sajia leucogranites in Sajia dome from north Himalaya and their geological implications. Chin. Sci. Bull. 2004, 49, 2087–2092. [Google Scholar] [CrossRef]
- Zhang, L.K.; Zhang, Z.; Li, G.M.; Dong, S.L.; Xia, X.B.; Liang, W.; Fu, J.G.; Cao, H.W. Rock assemblage, structural characteristics and genesis mechanism of the Cuonadong dome, Tethys Himalaya. Earth Sci. 2018, 43, 2664–2683, (In Chinese with English Abstract). [Google Scholar]
- Larson, K.P.; Godin, L.; Davis, W.J.; Davis, D.W. Out-of-sequence deformation and expansion of the Himalayan orogenic wedge: Insight from the Changgo culmination, south Central Tibet. Tectonics 2010, 29, 1–30. [Google Scholar] [CrossRef]
- Wang, J.M.; Wu, F.Y.; Rubatto, D.; Liu, K.; Zhang, J.J.; Liu, X.C. Early Miocene rapid exhumation in southern Tibet: Insights from P–T–t–D–magmatism path of Yardoi dome. Lithos 2018, 30, 38–56. [Google Scholar] [CrossRef]
- Godin, L.; Grujic, D.; Law, R.D.; Searle, M.P. Channel Flow, Ductile Extrusion and Exhumation in Continental Collision Zones: An Introduction; Special Publications: London, UK, 2008; pp. 1–23. [Google Scholar]
- Wang, X.X.; Zhang, J.J.; Liu, J.; Yan, S.Y.; Wang, J.M. Middle-Miocene transformation of tectonic regime in the Himalayan orogen. Chin. Sci. Bull. 2013, 58, 108–117. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–51. [Google Scholar]
- Gao, L.E.; Zeng, L.S.; Hu, G.Y.; Gao, J.H.; Zhao, L.H.; Wang, Y.Y. Rare Metal Enrichment in Leucogranite within Nariyongcuo Gneiss Dome, South Tibet. Earth Sci. 2019, 44, 1860–1875. [Google Scholar]
- He, C.T.; Qin, K.Z.; Li, A.; Zhou, Q.F.; Zhao, J.X.; Li, G.M. Preliminary study on occurrence status of berylium andgenetic mechanism in Cuonadong tungsten-tin-beryllium deposit, eastern Himalaya. Acta Petrol. Sin. 2020, 36, 3593–3606. [Google Scholar]
- Zhao, J.X.; He, C.T.; Shi, R.Z.; Qin, K.Z.; Yu, K.L.; Qiu, J.T.; Li, Z.; Zhou, Q.F. Mineralogical characteristics of the leucogranite-pegmatite in the Yardoi Gneiss Dome, Himalaya, Tibet: Implication to the rare-metal mineralization. Acta Petrol. Sin. 2022, 38, 1981–2002. [Google Scholar]
- Zhou, W.; Xie, L.; Wang, R.C.; Wu, F.Y.; Tian, E.N.; Liu, C.; Liu, X.C. The study on the micas in the Gyirong leucogranite-pegmatite from Himalaya: Implications for the lithium enrichment. Acta Petrol. Sin. 2022, 38, 2153–2173. [Google Scholar]
- Fu, J.G.; Li, G.M.; Wang, G.; Guo, W.K.; Dong, S.L.; Li, Y.; Zhang, H.; Liang, W.; Jiao, Y. Geochemical Evidence for Genesis of Nb–Ta–Be Rare Metal Mineralization in Highly Fractionated Leucogranites at the Lalong Dome, Tethyan Himalaya, China. Minerals 2023, 13, 1456. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Qin, K.Z.; Liu, Y.C.; He, C.T.; Zhao, J.X.; Li, J.Y.; Zhu, L.O.; Zhao, Y.N.; Zhang, X. Cassiterite of the Kuqu spodumene-bearing pegmatites in the eastern Himalaya, Tibet, and its implication. Acta Petrol. Sin. 2024, 40, 433–449, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Cao, H.W.; Li, G.M.; Zhang, R.Q.; Zhang, Y.H.; Zhang, L.K.; Dai, Z.W.; Xia, X.B. Genesis of the Cuonadong tin polymetallic deposit in the Tethyan Himalaya: Evidence from geology, geochronology, fluid inclusions and multiple isotopes. Gondwana Res. 2021, 92, 72–101. [Google Scholar] [CrossRef]
- Fu, J.G.; Li, G.M.; Guo, W.K.; Zhang, H.; Zhang, L.K.; Dong, S.L.; Zhou, L.M.; Li, Y.X.; Jiao, Y.J.; Shi, H.Z. Mineralogical characteristics of columbite group minerals and its implications for magmatic-hydrothermal transition in the Gabo lithium deposit, Himalayan metallogenic belt. Earth Sci. Front. 2023, 30, 134–150, (In Chinese with English Abstract). [Google Scholar]




| Sample | 87Rb/86Sr | 87Sr/86Sr | 2s | ISr (t) | 147Sm/144Nd | 143Nd/144Nd | 2s | 143Nd/144Nd(t) | εNd (t) | T2DM (Ga) |
|---|---|---|---|---|---|---|---|---|---|---|
| Apatite | ||||||||||
| Ap01 | 0.0001 | 0.727667 | 0.000337 | 0.7277 | 0.3806 | 0.511911 | 0.000029 | 0.511865 | −14.6 | 1.720 |
| Ap02 | 0.0047 | 0.726974 | 0.000284 | 0.7270 | 0.3633 | 0.511957 | 0.000024 | 0.511913 | −13.7 | 1.656 |
| Ap03 | 0.0501 | 0.728079 | 0.000288 | 0.7281 | 0.3768 | 0.511982 | 0.000033 | 0.511937 | −13.2 | 1.624 |
| Ap04 | 0.0140 | 0.726803 | 0.000427 | 0.7268 | ||||||
| Ap05 | 0.0083 | 0.727367 | 0.000290 | 0.7274 | ||||||
| Ap06 | 0.0025 | 0.727219 | 0.000301 | 0.7272 | ||||||
| Ap07 | 0.0031 | 0.727108 | 0.000350 | 0.7271 | ||||||
| Ap08 | 0.0060 | 0.727930 | 0.000278 | 0.7279 | ||||||
| Monazite | ||||||||||
| Mz01 | 0.1216 | 0.511939 | 0.000022 | 0.511924 | −13.5 | 1.641 | ||||
| Mz02 | 0.1261 | 0.511953 | 0.000017 | 0.511938 | −13.2 | 1.622 | ||||
| Mz03 | 0.1400 | 0.511928 | 0.000020 | 0.511911 | −13.7 | 1.658 | ||||
| Mz04 | 0.1324 | 0.511952 | 0.000023 | 0.511936 | −13.2 | 1.625 | ||||
| Mz05 | 0.1309 | 0.511940 | 0.000018 | 0.511924 | −13.5 | 1.641 | ||||
| Mz06 | 0.1193 | 0.511944 | 0.000017 | 0.511930 | −13.4 | 1.633 | ||||
| Whole rock * | ||||||||||
| LZH1101 | 17.8085 | 0.730355 | 0.000018 | 0.7257 | 0.1798 | 0.511977 | 0.000010 | 0.511955 | −12.9 | 1.599 |
| LZH1103 | 15.3662 | 0.731090 | 0.000012 | 0.7271 | 0.1901 | 0.511951 | 0.000008 | 0.511928 | −13.4 | 1.635 |
| LZH1107 | 16.2554 | 0.731431 | 0.000010 | 0.7272 | 0.1763 | 0.511950 | 0.000004 | 0.511929 | −13.4 | 1.635 |
| Spot. No | 176Hf/177Hf | 1σ | 176Lu/177Hf | 1σ | 176Yb/177Hf | 1σ | 176Hf/177Hf(t) | εHf (0) | εHf (t) | 1σ | TDM (Ma) | TDM2 (Ma) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGL01-4-01 | 0.282437 | 0.000013 | 0.000973 | 0.000035 | 0.043089 | 0.001383 | 0.282437 | −11.9 | −11.5 | 0.4 | 1151 | 1819 |
| TGL01-4-02 | 0.282462 | 0.000017 | 0.001315 | 0.000054 | 0.056022 | 0.001598 | 0.282462 | −11.0 | −10.6 | 0.6 | 1126 | 1763 |
| TGL01-4-03 | 0.282455 | 0.000010 | 0.000727 | 0.000008 | 0.029321 | 0.000071 | 0.282455 | −11.2 | −10.8 | 0.3 | 1119 | 1779 |
| TGL01-4-04 | 0.282484 | 0.000010 | 0.001319 | 0.000019 | 0.057886 | 0.001049 | 0.282484 | −10.2 | −9.8 | 0.4 | 1095 | 1715 |
| TGL01-4-05 | 0.282449 | 0.000010 | 0.000769 | 0.000009 | 0.028968 | 0.000103 | 0.282449 | −11.4 | −11.0 | 0.3 | 1128 | 1792 |
| TGL01-4-06 | 0.282442 | 0.000020 | 0.001289 | 0.000012 | 0.055288 | 0.000348 | 0.282442 | −11.7 | −11.3 | 0.7 | 1153 | 1807 |
| TGL01-4-07 | 0.282493 | 0.000014 | 0.001552 | 0.000027 | 0.069375 | 0.000758 | 0.282493 | −9.9 | −9.47 | 0.5 | 1089 | 1694 |
| TGL01-4-08 | 0.282488 | 0.000012 | 0.002599 | 0.000023 | 0.125070 | 0.001412 | 0.282487 | −10.0 | −9.67 | 0.4 | 1128 | 1707 |
| TGL01-4-09 | 0.282447 | 0.000012 | 0.001001 | 0.000011 | 0.039564 | 0.000212 | 0.282447 | −11.5 | −11.1 | 0.4 | 1137 | 1796 |
| TGL01-4-10 | 0.282428 | 0.000022 | 0.001043 | 0.000008 | 0.045795 | 0.000544 | 0.282427 | −12.2 | −11.8 | 0.8 | 1166 | 1840 |
| TGL01-4-11 | 0.282468 | 0.000012 | 0.001532 | 0.000018 | 0.066801 | 0.000760 | 0.282468 | −10.7 | −10.4 | 0.4 | 1124 | 1750 |
| TGL01-4-12 | 0.282445 | 0.000013 | 0.001080 | 0.000010 | 0.045265 | 0.000351 | 0.282445 | −11.6 | −11.2 | 0.5 | 1143 | 1801 |
| TGL01-4-13 | 0.282485 | 0.000014 | 0.001005 | 0.000031 | 0.044787 | 0.001045 | 0.282485 | −10.1 | −9.7 | 0.5 | 1084 | 1712 |
| TGL01-4-14 | 0.282465 | 0.000011 | 0.000843 | 0.000003 | 0.037326 | 0.000143 | 0.282464 | −10.9 | −10.5 | 0.4 | 1109 | 1758 |
| TGL01-4-15 | 0.282407 | 0.000016 | 0.000920 | 0.000006 | 0.034614 | 0.000160 | 0.282406 | −12.9 | −12.5 | 0.6 | 1192 | 1886 |
| TGL01-4-16 | 0.282405 | 0.000010 | 0.000895 | 0.000014 | 0.033825 | 0.000193 | 0.282404 | −13.0 | −12.6 | 0.3 | 1194 | 1890 |
| TGL01-4-17 | 0.282453 | 0.000016 | 0.002122 | 0.000016 | 0.084481 | 0.000409 | 0.282452 | −11.3 | −10.9 | 0.6 | 1165 | 1785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drolma, Y.; Li, K.; Li, Y.; Zhang, J.; Yang, C.; Zhang, G.; Li, R.; Liu, D. Geochronology, Geochemistry, and In Situ Sr-Nd-Hf Isotopic Compositions of a Tourmaline-Bearing Leucogranite in Eastern Tethyan Himalaya: Implications for Tectonic Setting and Rare Metal Mineralization. Minerals 2024, 14, 755. https://doi.org/10.3390/min14080755
Drolma Y, Li K, Li Y, Zhang J, Yang C, Zhang G, Li R, Liu D. Geochronology, Geochemistry, and In Situ Sr-Nd-Hf Isotopic Compositions of a Tourmaline-Bearing Leucogranite in Eastern Tethyan Himalaya: Implications for Tectonic Setting and Rare Metal Mineralization. Minerals. 2024; 14(8):755. https://doi.org/10.3390/min14080755
Chicago/Turabian StyleDrolma, Yangchen, Kaijun Li, Yubin Li, Jinshu Zhang, Chengye Yang, Gen Zhang, Ruoming Li, and Duo Liu. 2024. "Geochronology, Geochemistry, and In Situ Sr-Nd-Hf Isotopic Compositions of a Tourmaline-Bearing Leucogranite in Eastern Tethyan Himalaya: Implications for Tectonic Setting and Rare Metal Mineralization" Minerals 14, no. 8: 755. https://doi.org/10.3390/min14080755






