Modes of Occurrence, Migration, and Evolution Pathways of Lithium and Gallium during Combustion of an Al-Rich Coal, Inner Mongolia, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Methods
2.2.1. Coal Combustion Experiments
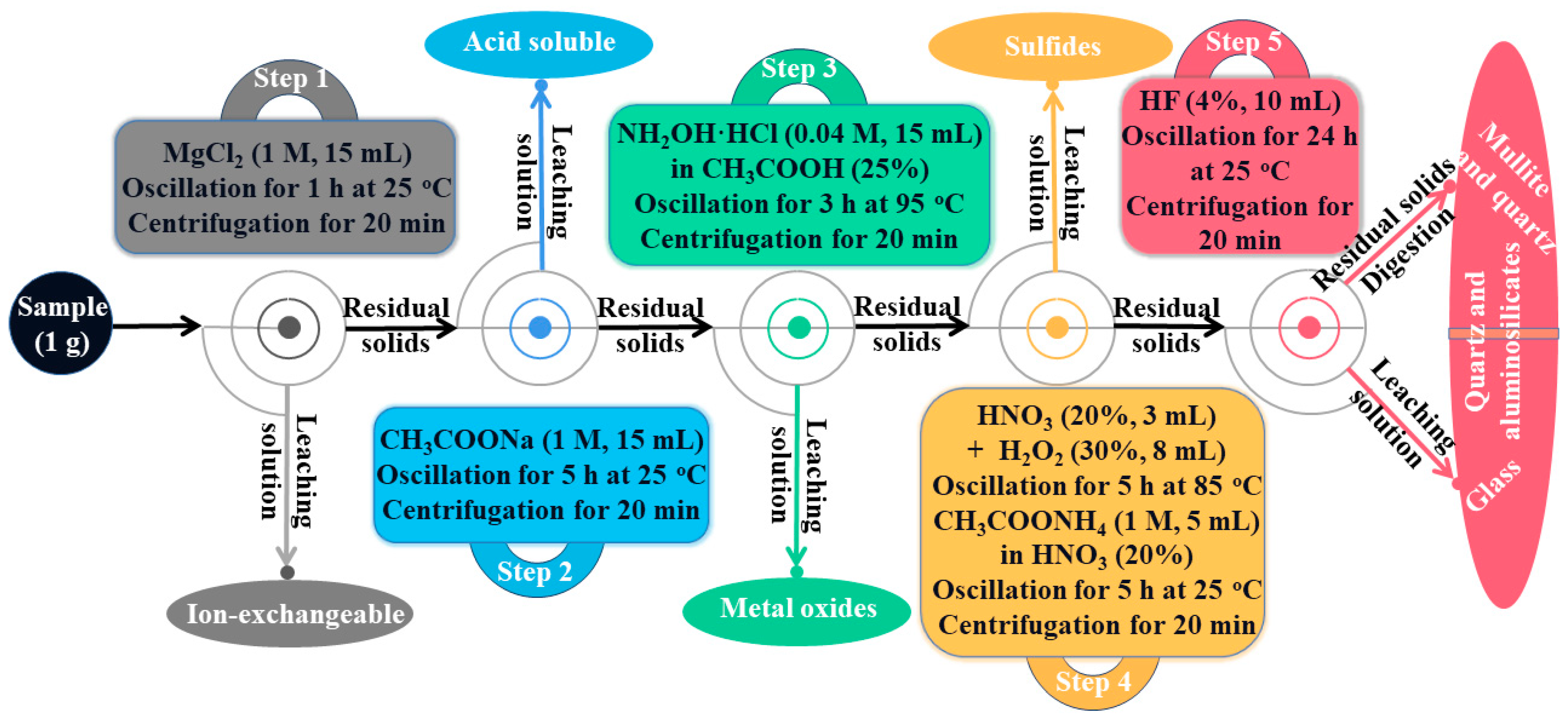
2.2.2. Sequential Chemical Extraction
2.2.3. Chemical and Mineralogical Analysis
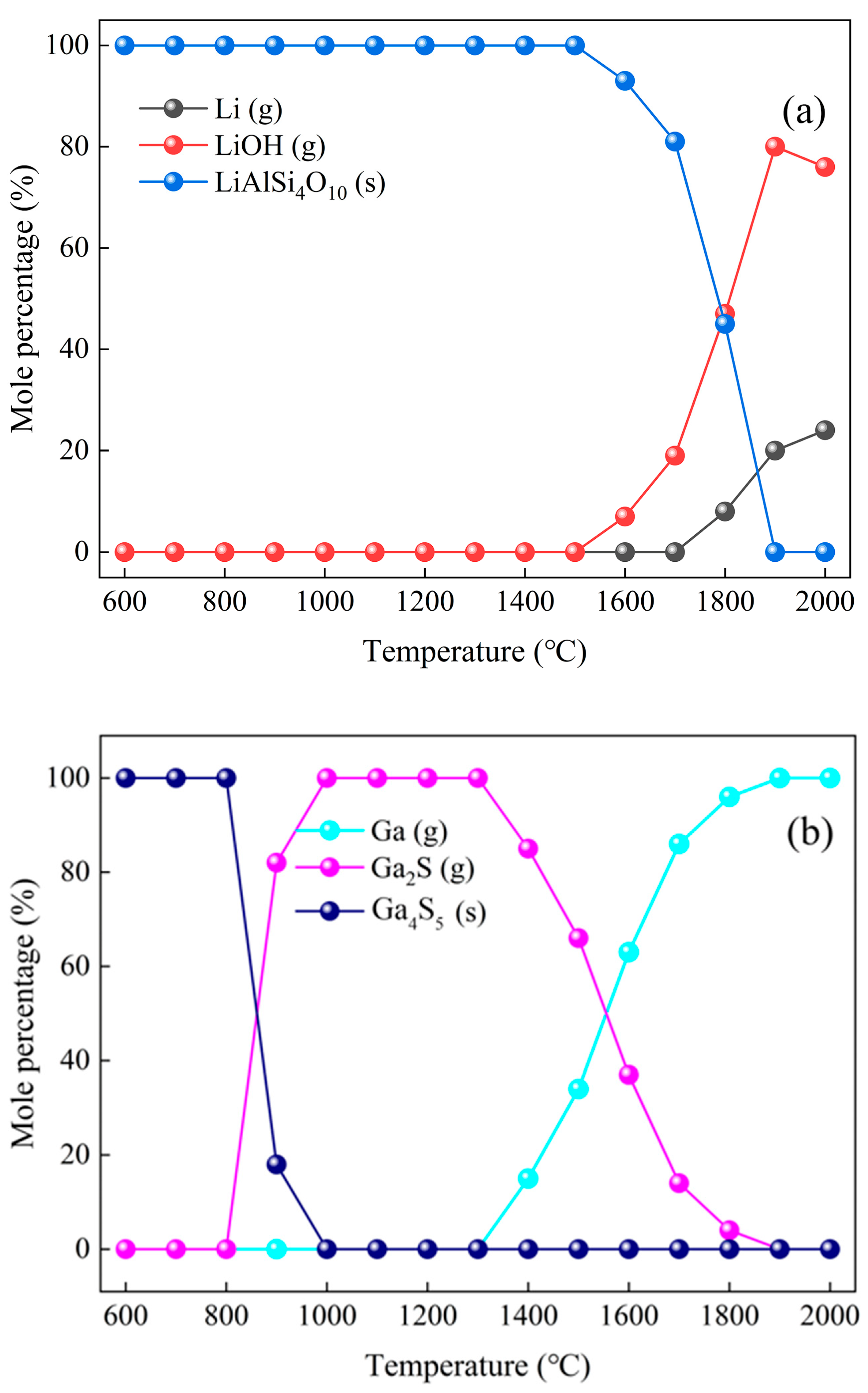
2.2.4. Thermodynamic Calculations
3. Results and Discussion
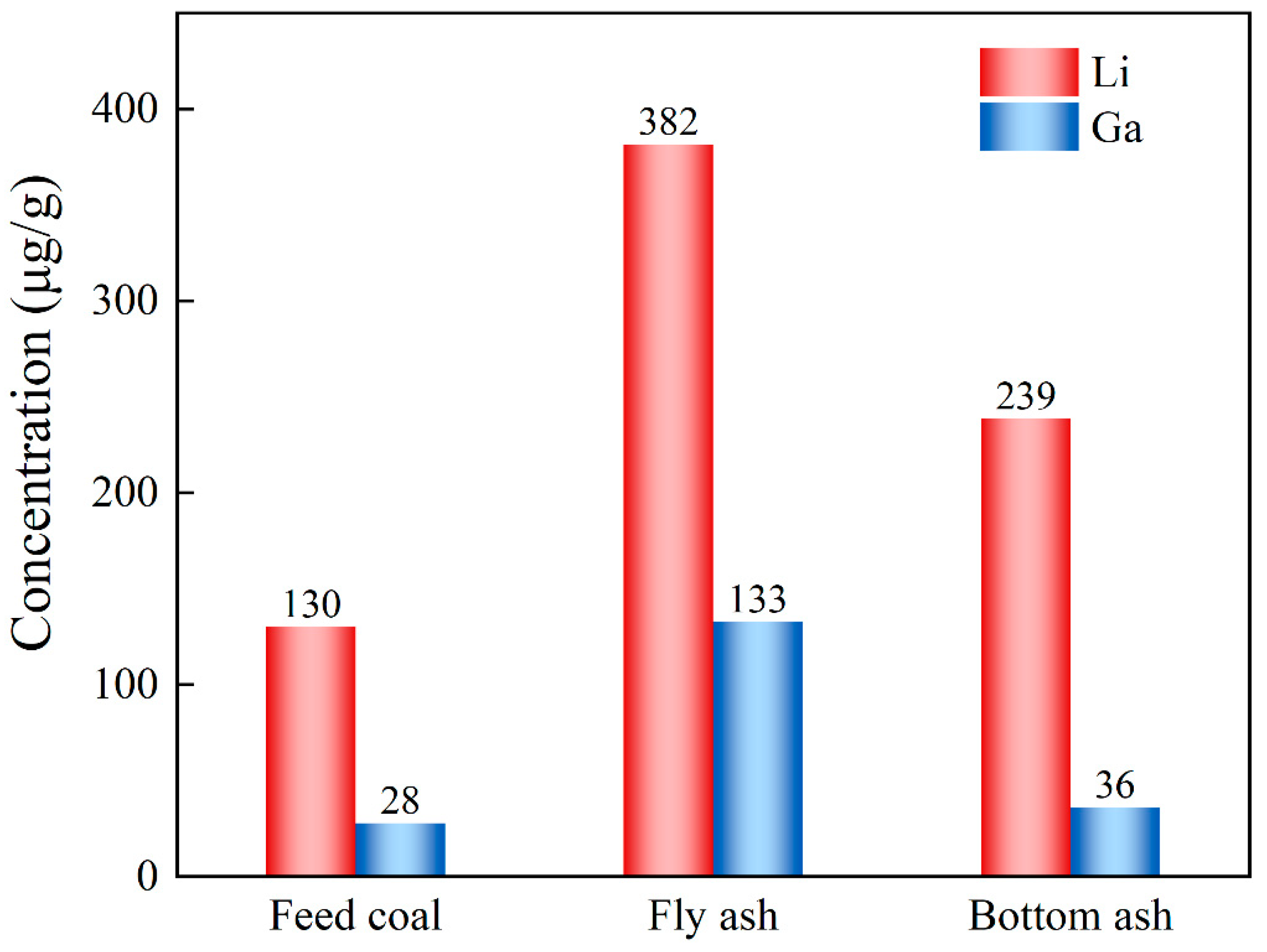
3.1. Properties of Feed Coal and Industrial Ash
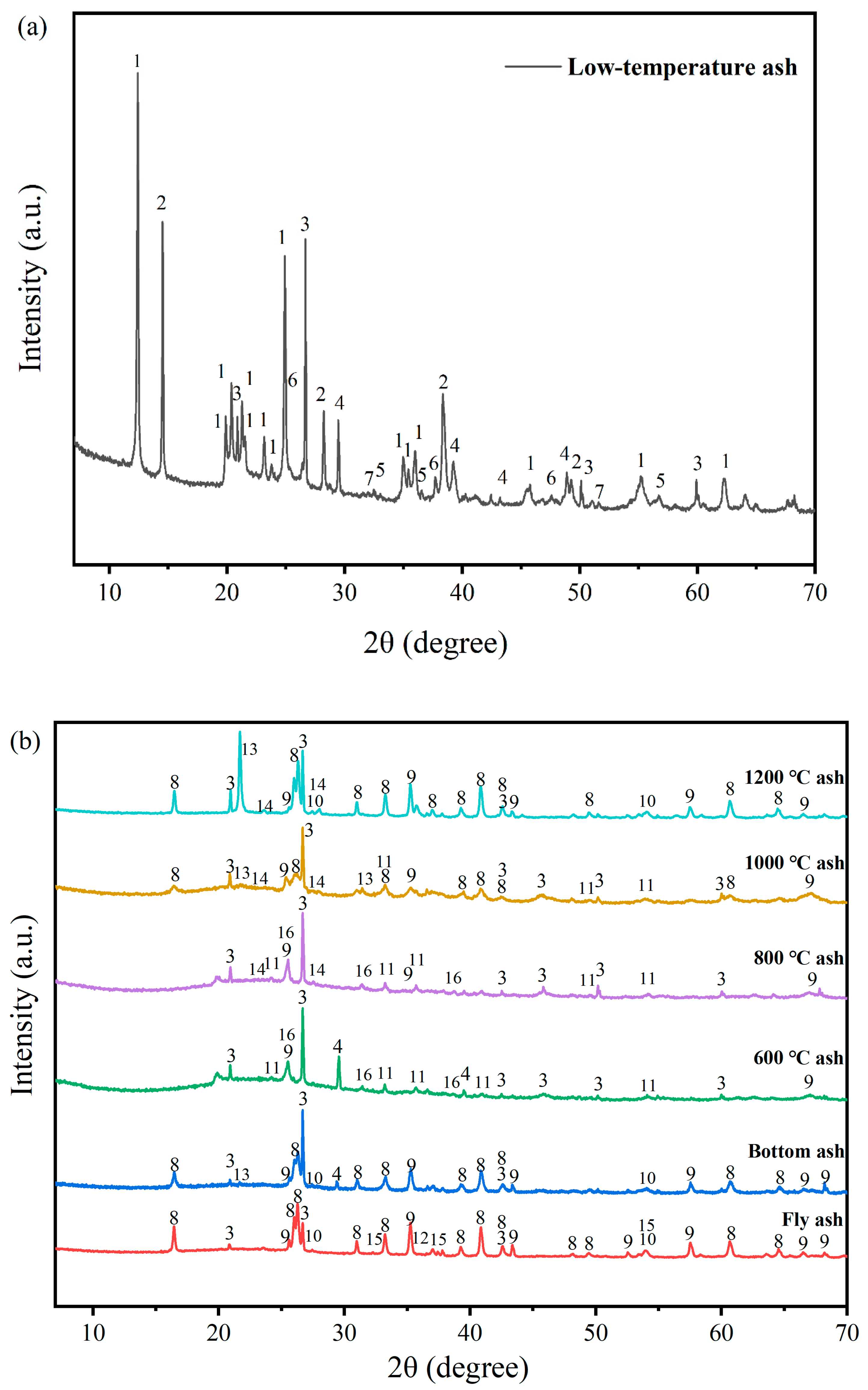
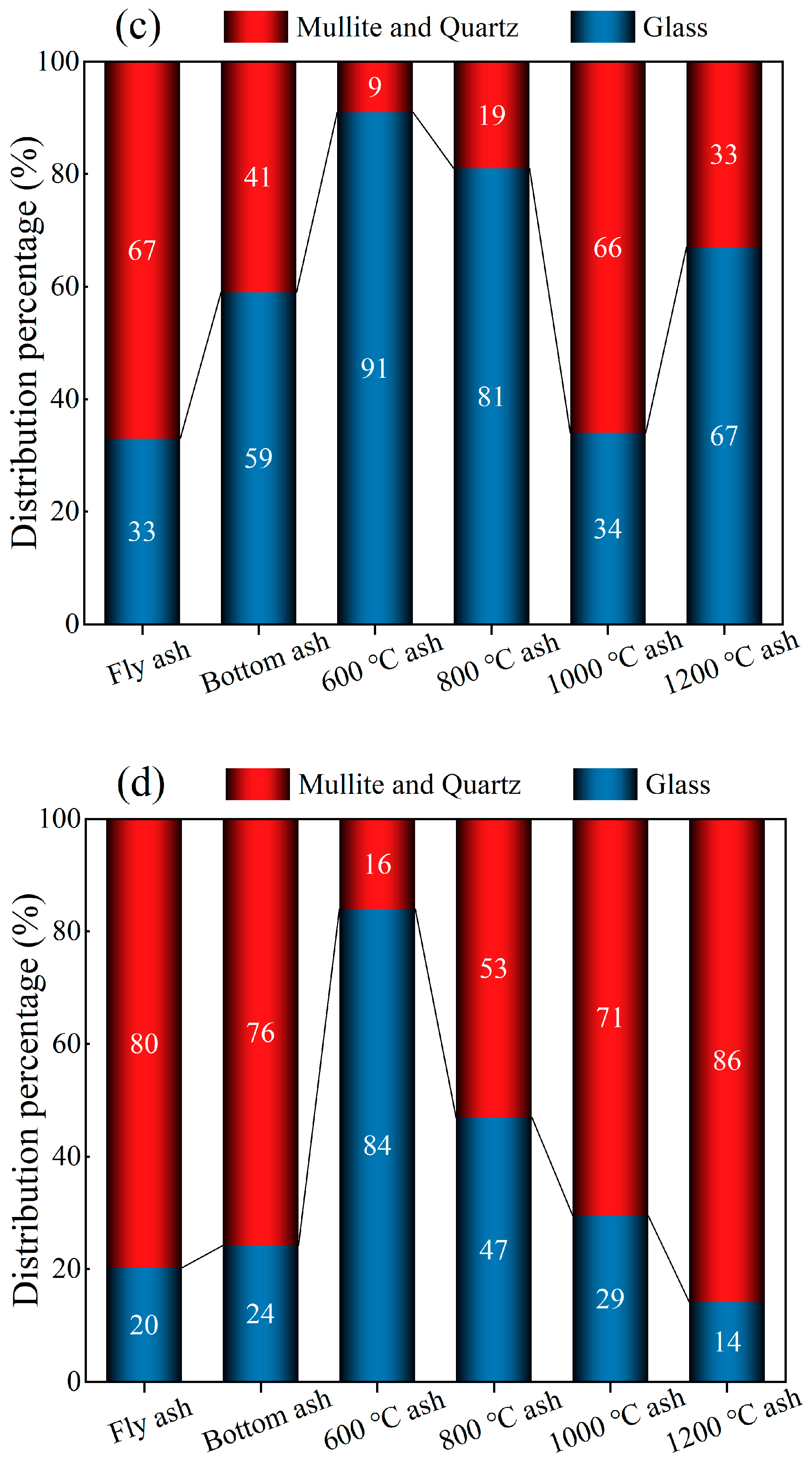
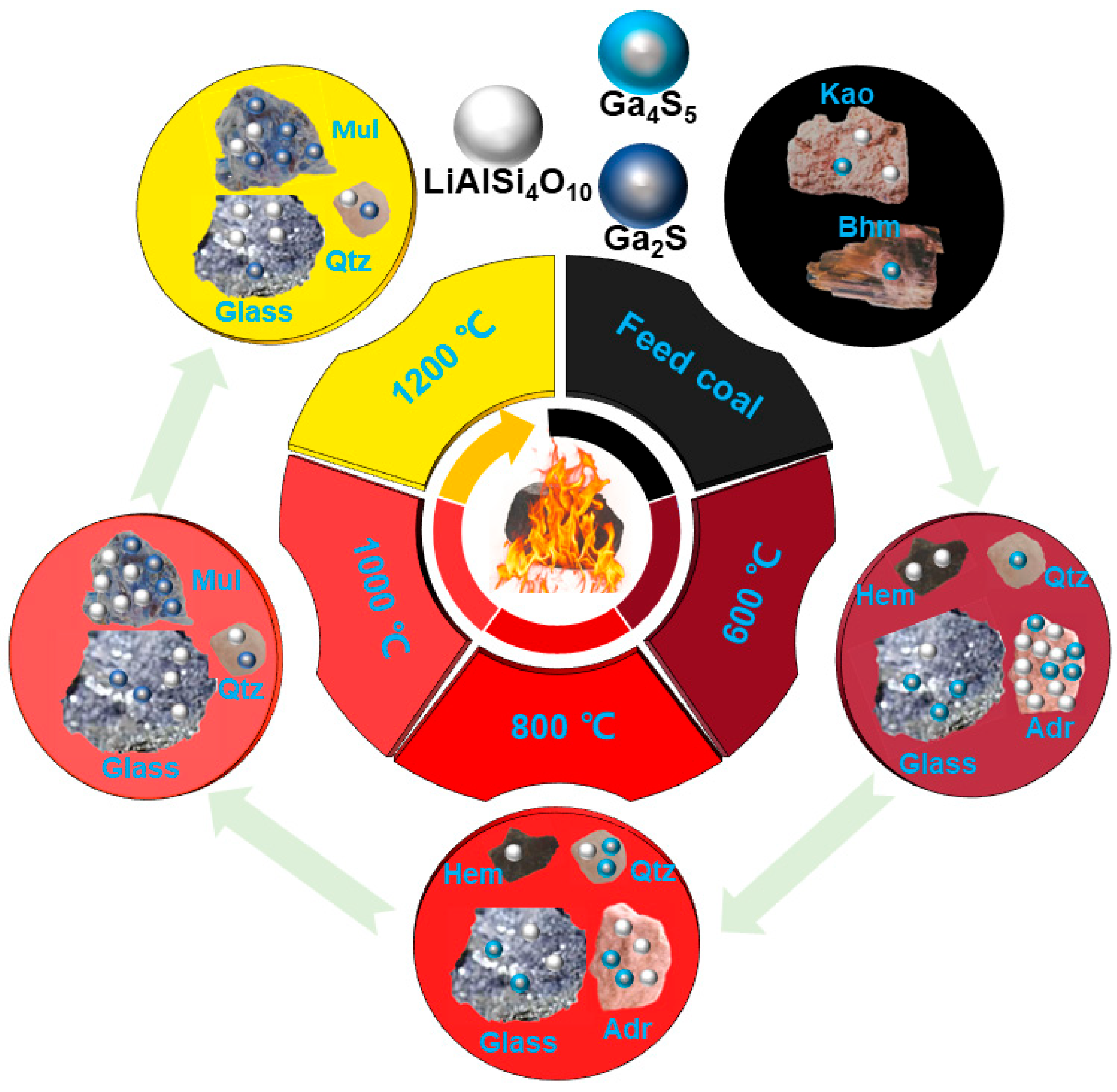
3.2. Transformation of Minerals during Coal Combustion
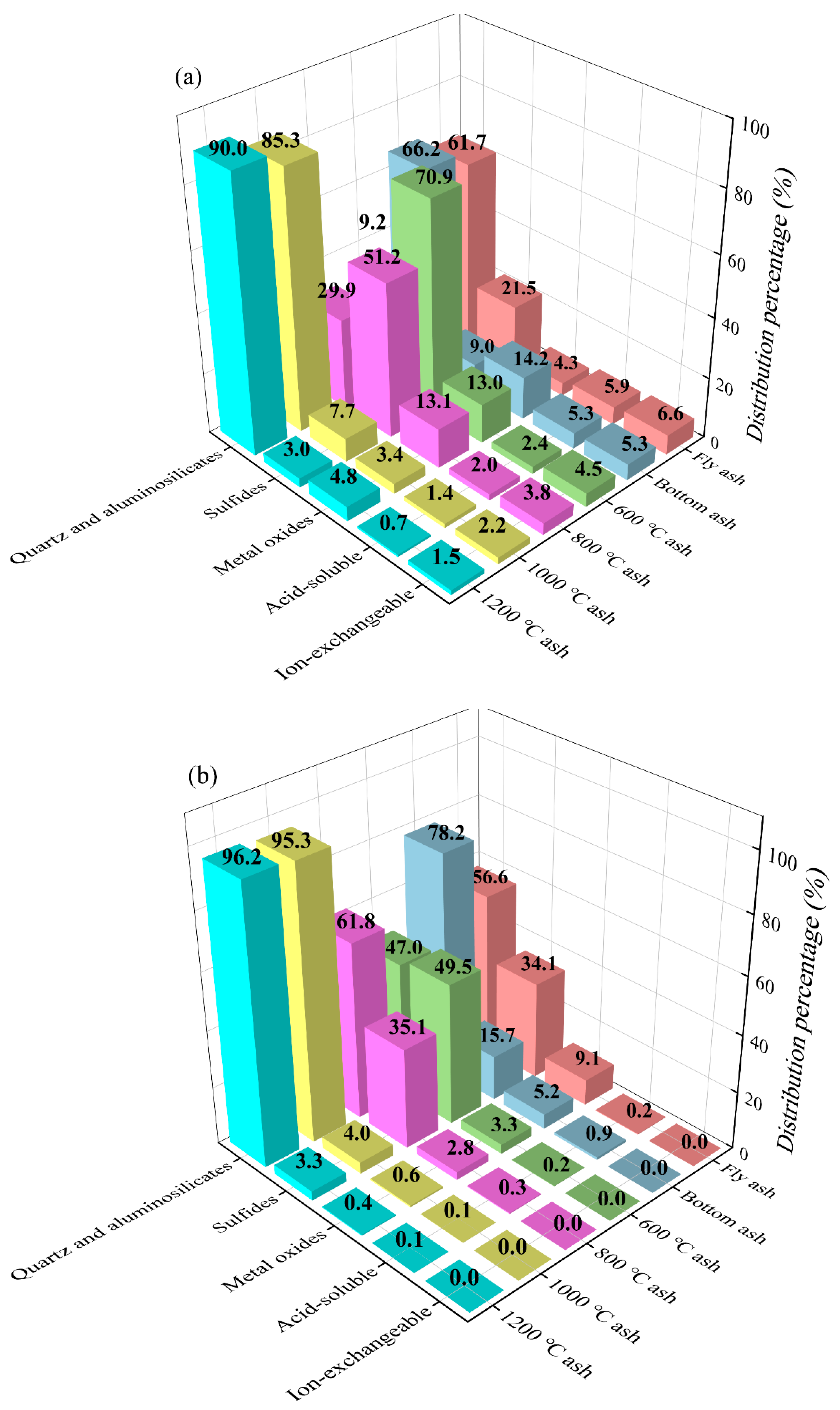
3.3. Modes of Occurrence of Li and Ga Indicated by SCEP Results
3.4. Evolution of Li and Ga Based on Thermodynamic Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research-global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Kim, S.H.; Hui, K.N.; Kim, Y.J.; Lim, T.S.; Yang, D.Y.; Kim, K.B.; Kim, Y.J.; Jang, G.J.; Yang, S.S. Fabrication of pre-alloyed Al-Li powders with high Li content via thermal dehydrogenation of LiH and rapid solidification process. Mater. Des. 2016, 94, 159–165. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.C.; Pan, J.H.; Wen, Z.P.; Shi, S.L.; Long, X.; Zhou, C.C. The effect of physical separation and calcination on enrichment and recovery of critical elements from coal gangue. Minerals 2022, 12, 1371. [Google Scholar] [CrossRef]
- An, F.Q.; Zhao, H.L.; Cheng, Z.; Qiu, J.Y.C.; Zhou, W.N.; Li, P. Development status and research progress of power battery for pure electric vehicles. Chin. J. Eng. 2021, 41, 22–41. [Google Scholar]
- Frenzel, M.; Ketris, M.P.; Seifert, T.; Gutzmer, J. On the current and future availability of gallium. Res. Pol. 2016, 47, 38–50. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 2, temporally and spatially resolved life-cycle assessment modeling. J. Ind. Ecol. 2020, 24, 90. [Google Scholar] [CrossRef]
- Long, J.; Zhang, S.X.; Luo, K.L. Discovery of anomalous gallium enriched in stone coal: Significance, provenance and recommendations. Geosci. Front. 2023, 14, 101538. [Google Scholar] [CrossRef]
- Diallo, M.S.; Kotte, M.R.; Cho, M. Mining critical metals and elements from seawater: Opportunities and Challenges. Environ. Sci. Technol. 2015, 49, 9390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, J.M.; Guo, Y.X.; Cheng, F.Q. Energy-efficient leaching process for preparation of aluminum sulfate and synergistic extraction of Li and Ga from circulating fluidized bed fly ash. Energy Sources Part A-Recov. Util. Environ. Eff. 2022, 44, 4398–4410. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.Z.; Wang, C.Y.; Chen, Y.Q. Extraction of valuable components from coal gangue through thermal activation and HNO3 leaching. J. Ind. Eng. Chem. 2022, 113, 564–574. [Google Scholar] [CrossRef]
- Huang, Z.X.; Fan, M.; Tiand, H.J. Coal and coal byproducts: A large and developable unconventional resource for critical materials-rare earth elements. J. Rare Earths 2018, 36, 337–338. [Google Scholar] [CrossRef]
- Dai, S.F.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M. Perspectives for the recovery of critical elements from future energy-efficient refrigeration materials. J. Clean. Prod. 2018, 197, 232–241. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, S.C.; Coulon, F.; Jiang, Y.; Wagland, S. Rare earth elements and critical metal content of extracted landfilled material and potential recovery opportunities. Waste Manag. 2015, 42, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, B.J.; Huang, C.J.; Xu, Y. Comprehensive utilization of resources of titanium dioxide at home and abroad. Adv. Mater. Res. 2013, 734–737, 2248–2251. [Google Scholar] [CrossRef]
- Hower, J.C.; Fu, B.; Dai, S.F. Geochemical partitioning from pulverized coal to fly ash and bottom ash. Fuel 2020, 279, 118542. [Google Scholar] [CrossRef]
- Majlis, A.B.K.; Habib, M.A.; Khan, R.; Phoungthong, K.; Techato, K.; Islam, M.A.; Nakashima, S.; Islam, A.R.M.T.; Hood, M.M.; Hower, J.C. Intrinsic characteristics of coal combustion residues and their environmental impacts: A case study for Bangladesh. Fuel 2022, 324, 124711. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.F.; Finkelman, R.B.; French, D.; Graham, I.T.; Yang, Y.C.; Li, J.X.; Yang, P. Leaching behavior of trace elements from fly ashes of five chinese coal power plants. Int. J. Coal Geol. 2020, 219, 103381. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhao, L.; Peng, S.P.; Chou, C.L.; Wang, X.B.; Zhang, Y.; Li, D.; Sun, Y.Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Gong, Y.B.; Sun, J.M.; Zhang, Y.M.; Zhang, Y.F.; Zhang, T.A. Dependence on the distribution of valuable elements and chemical characterizations based on different particle sizes of high alumina fly ash. Fuel 2021, 291, 120225. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Hsu-Kim, H.; Taggart, R.K. Distribution of rare earth elements in fly ash derived from the combustion of Illinois Basin coals. Fuel 2021, 289, 119990. [Google Scholar] [CrossRef]
- Zhou, C.C.; Du, J.; Zhang, Y.L.; Sun, J.K.; Wu, W.T.; Liu, G.J. Redistribution and transformation mechanisms of gallium and germanium during coal combustion. Fuel 2021, 305, 121532. [Google Scholar] [CrossRef]
- Hu, P.P.; Hou, X.J.; Zhang, J.B.; Li, S.P.; Wu, H.; Damø, A.J.; Li, H.Q.; Wu, Q.S.; Xi, X.G. Distribution and occurrence of Lithium in high-alumina-coal fly ash. Int. J. Coal Geol. 2018, 189, 27–34. [Google Scholar] [CrossRef]
- Xu, F.; Qin, S.J.; Li, S.Y.; Wang, J.X.; Qi, D.E.; Lu, Q.F.; Xing, J.K. Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the chongqing power plant. J. Clean. Prod. 2022, 342, 130910. [Google Scholar] [CrossRef]
- Wu, G.Q.; Shi, N.; Wang, T.; Cheng, C.M.; Wang, J.W.; Tian, C.X.; Pan, W.P. Enrichment and occurrence form of rare earth elements during coal and coal gangue combustion. Environ. Sci. Pollut. Res. 2022, 29, 44709–44722. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Zhou, C.C.; Tang, M.C.; Cao, S.S.; Liu, C.; Zhang, N.N.; Wen, M.Z.; Luo, Y.L.; Hu, T.T.; Ji, W.S. Study on the modes of occurrence of rare earth elements in coal fly ash by statistics and a sequential chemical extraction procedure. Fuel 2019, 237, 555–565. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, S.F.; Zou, J.H.; French, D.; Graham, I.T. Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Hower, J.C. Petrographic examination of coal-combustion fly ash. Int. J. Coal Geol. 2012, 92, 90–97. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Qin, S.J.; Sun, Y.Z.; Li, Y.H.; Wang, J.X.; Zhao, C.L.; Gao, K. Coal deposits as promising alternative sources for gallium. Earth-Sci. Rev. 2015, 150, 95–101. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Li, S.S.; Jiang, Y.F. Mineralogy and geochemistry of the No. 6 coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Dai, S.F.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D.Y.; Ma, Y.W.; Sun, Y.Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhao, L.; Hower, J.C.; Johnston, M.N.; Song, W.J.; Wang, P.P.; Zhang, S.F. Petrology, Mineralogy, and Chemistry of Size-Fractioned Fly Ash from the Jungar Power Plant, Inner Mongolia, China, with Emphasis on the Distribution of Rare Earth Elements. Energy Fuels 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- Karayığıt, A.İ.; Oskay, R.G.; Gayer, R.A. Mineralogy and geochemistry of feed coals and combustion residues of the Kangal power plant (Sivas, Turkey). Turk. J. Earth Sci. 2019, 28, 438–456. [Google Scholar] [CrossRef]
- Ward, C.R.; French, D. Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffractometry. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Querol, X.; Fernandez Turiel, J.L.; Lopez Soler, A. The behaviour of mineral matter during combustion of Spanish subbituminous and brown coals. Mineral. Mag. 1994, 58, 119–133. [Google Scholar] [CrossRef]
- Reifenstein, A.P.; Kahraman, H.; Coin, C.D.A.; Calos, N.J.; Miller, G.; Uwins, P. Behaviour of selected minerals in an improved ash fusion test: Quartz, potassium feldspar, sodium feldspar, kaolinite, illite, calcite, dolomite, siderite, pyrite and apatite. Fuel 1999, 78, 1449–1461. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.J.; Han, Y.X.; Gao, P.; Gong, G.C. Investigation on calcination behaviors of coal gangue by fluidized calcination in comparison with static calcination. Minerals 2017, 7, 19. [Google Scholar] [CrossRef]
- Zhao, Y.; Frost, R.L.; Martens, W.N.; Zhu, H.Y. XRD, TEM and thermal analysis of Fe doped boehmite nanofibres and nanosheets. J. Therm. Anal. Calorim. 2007, 3, 755–760. [Google Scholar] [CrossRef]
| Proximate Analysis (wt%) | Ultimate Analysis (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Vd | Ad | FCd | C | H | O | N | S |
| 3.03 | 25.30 | 37.40 | 37.30 | 47.80 | 3.02 | 30.6 | 0.99 | 0.62 |
| Sample | Al2O3 | SiO2 | TiO2 | Fe2O3 | CaO | K2O | Na2O | MgO |
|---|---|---|---|---|---|---|---|---|
| Feed coal | 36.27 | 40.04 | 5.33 | 4.13 | 3.85 | 1.15 | 0.18 | 0.21 |
| Fly ash | 47.72 | 41.50 | 2.87 | 2.33 | 3.80 | 0.50 | 0.17 | 0.26 |
| Bottom ash | 46.70 | 42.64 | 2.26 | 2.23 | 4.45 | 0.49 | 0.21 | 0.21 |
| Sample | Feed Coal | Fly Ash | Bottom Ash | 600 °C Ash | 800 °C Ash | 1000 °C Ash | 1200 °C Ash |
|---|---|---|---|---|---|---|---|
| Qtz | 8.1 | 2.1 | 5.2 | 2.7 | 3.6 | 2.7 | 4.8 |
| Kao | 68.3 | ||||||
| Bhm | 17.6 | ||||||
| Py | 0.7 | ||||||
| Cal | 4.4 | 0.7 | 1.8 | ||||
| Sd | 0.4 | ||||||
| Ana | 0.5 | ||||||
| Rut | 0.2 | 0.2 | 0.5 | ||||
| Mul | 31.1 | 33.8 | 20.6 | 39.3 | |||
| Crn | 4.5 | 3.4 | 0.9 | 0.9 | 1.9 | 2.7 | |
| Hem | 1.3 | 1.9 | 1.1 | ||||
| Mag | 0.1 | ||||||
| Crs | 0.2 | 1 | 8.2 | ||||
| An | 1.5 | 1.3 | 0.3 | ||||
| Lm | 0.3 | ||||||
| Adr | 1.8 | 2.4 | |||||
| Glass | 61.6 | 56.5 | 91.5 | 89.6 | 71.4 | 44.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Bian, K.; Zhang, K.; Geng, J.; Zheng, Y.; Li, X. Modes of Occurrence, Migration, and Evolution Pathways of Lithium and Gallium during Combustion of an Al-Rich Coal, Inner Mongolia, China. Minerals 2024, 14, 771. https://doi.org/10.3390/min14080771
Feng L, Bian K, Zhang K, Geng J, Zheng Y, Li X. Modes of Occurrence, Migration, and Evolution Pathways of Lithium and Gallium during Combustion of an Al-Rich Coal, Inner Mongolia, China. Minerals. 2024; 14(8):771. https://doi.org/10.3390/min14080771
Chicago/Turabian StyleFeng, Lili, Kaixuan Bian, Kailong Zhang, Jiawei Geng, Yanmin Zheng, and Xiao Li. 2024. "Modes of Occurrence, Migration, and Evolution Pathways of Lithium and Gallium during Combustion of an Al-Rich Coal, Inner Mongolia, China" Minerals 14, no. 8: 771. https://doi.org/10.3390/min14080771





