Study of the Geological Context of the 7th–6th Century BC Phoenician Era Shipwreck “Mazarrón 2” (Murcia, Spain)
Abstract
:1. Introduction
2. Historical and Geographical Setting
3. Materials and Methods
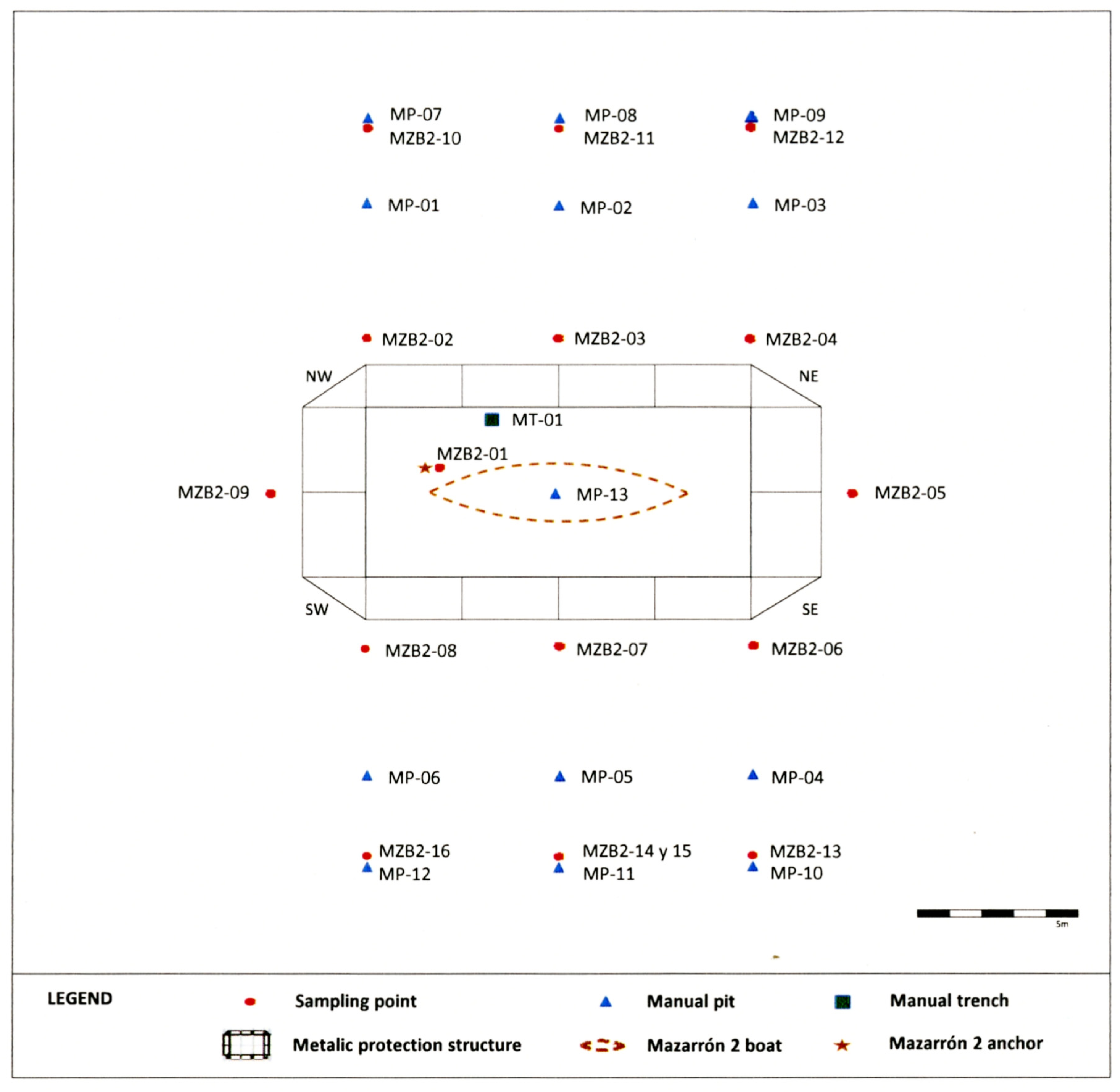
3.1. Sampling
3.2. Instrumentation
- (1)
- Optical microscopy: The sediments and wood samples were directly examined under an optical microscope. The sediment samples consisted of dried sand–silt–clay dispersed with a thin needle on a glass slide.
- (2)
- Scanning electron microscopy-X-ray microanalysis: The same subsamples examined under an optical microscope were mounted on aluminum disks and carbon-coated before acquiring images and X-ray spectra. A second series of sediments were finely powdered in an agate mortar and mounted on aluminum discs to perform the quantitative elemental analysis. The ZAF method of correction of the interelemental effects on intensity values was applied in each X-ray spectrum for every element. Three X-ray spectra from an area of 2.4 × 2.4 mm were acquired on three replicates of each sediment. The oxide wt% values of the different elements were calculated using the AztecOne software.A third series of subsamples were prepared as cross-sections. A small amount of sediment was embedded in polyester resin (Glasspol 328, Glasspol Composites SL, Valencia, Spain) and polished with abrasive dishes of SiC (Struers, Champigny sur Marne, France) until a uniform cross-section was obtained. The X-ray spectra acquired from these specimens enabled the characterization of the mineral composition of individual grains to avoid the interfering effect of the NaCl microcrystals deposited on the surface during drying in the laboratory (Figure S1). The stoichiometry of the mineral is deduced from the molar ratio among the identified elements, which is provided by the quantitative processing of the detected X-ray counts.
- (3)
- FTIR spectroscopy: sediments and wood subsamples were finely powdered in an agate mortar.
- (4)
- X-ray diffraction: sediment subsamples were finely powdered in an agate mortar.
- (5)
- X-ray microscopy: dried wood fragments of a few mm were examined by an X-ray microscope.
3.3. Physical Tests
3.3.1. Density
3.3.2. Sediment Color Determination
3.3.3. Granulometric Analysis
3.3.4. Geotechnical Characterization of the Seabed
3.4. Degree of Pyritization
- Normally oxygenated (oxic) environment: DOP < 0.45;
- Oxygen-restricted (dysoxic) environment: DOP ranges 0.4 < DOP < 0.7;
- Oxygen-depleted (anoxic to euxinic): DOP > 0.7.
4. Results
4.1. Topography of the Seabed
4.2. Characterization of Sediments
4.2.1. Physical Properties
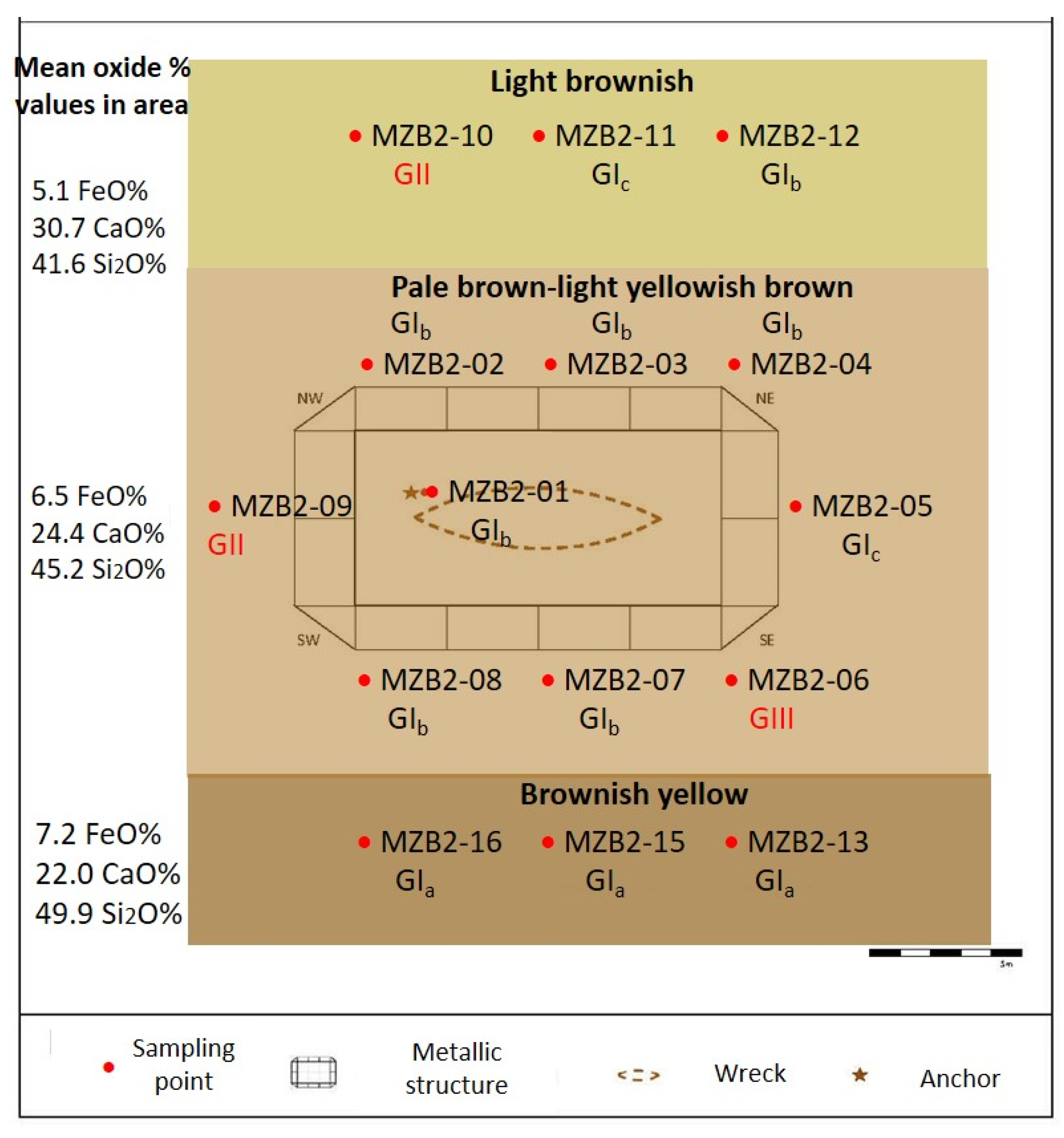
4.2.2. Chemical and Mineralogical Composition
4.2.3. Redox Status
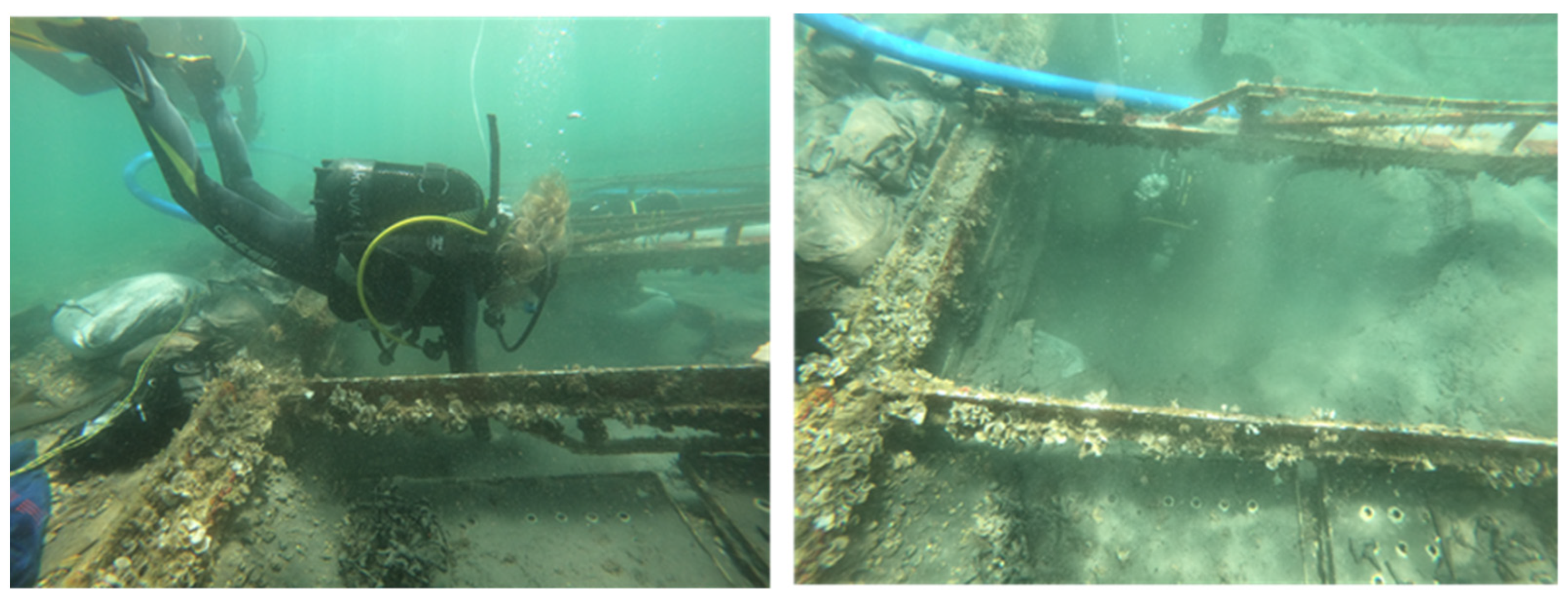
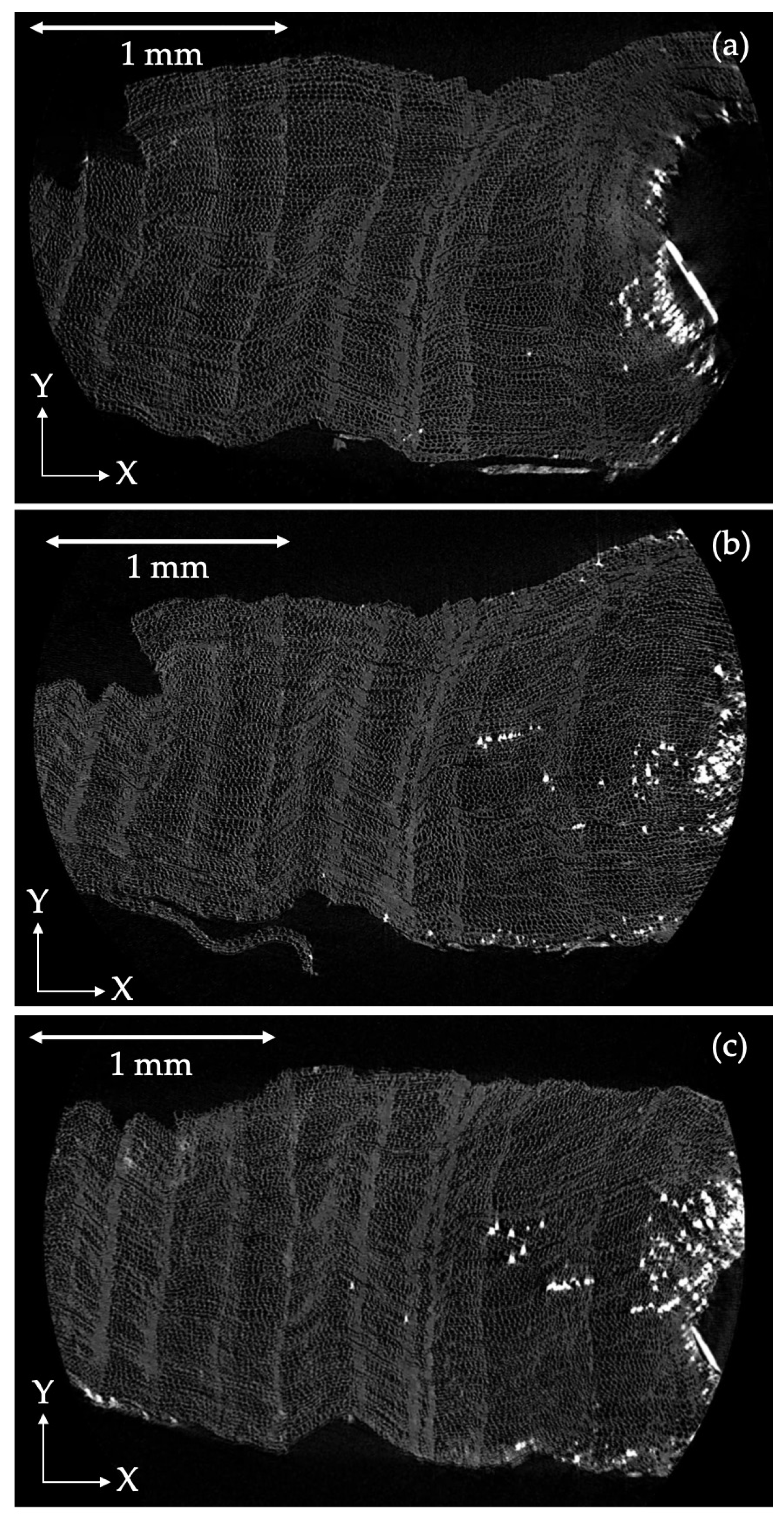
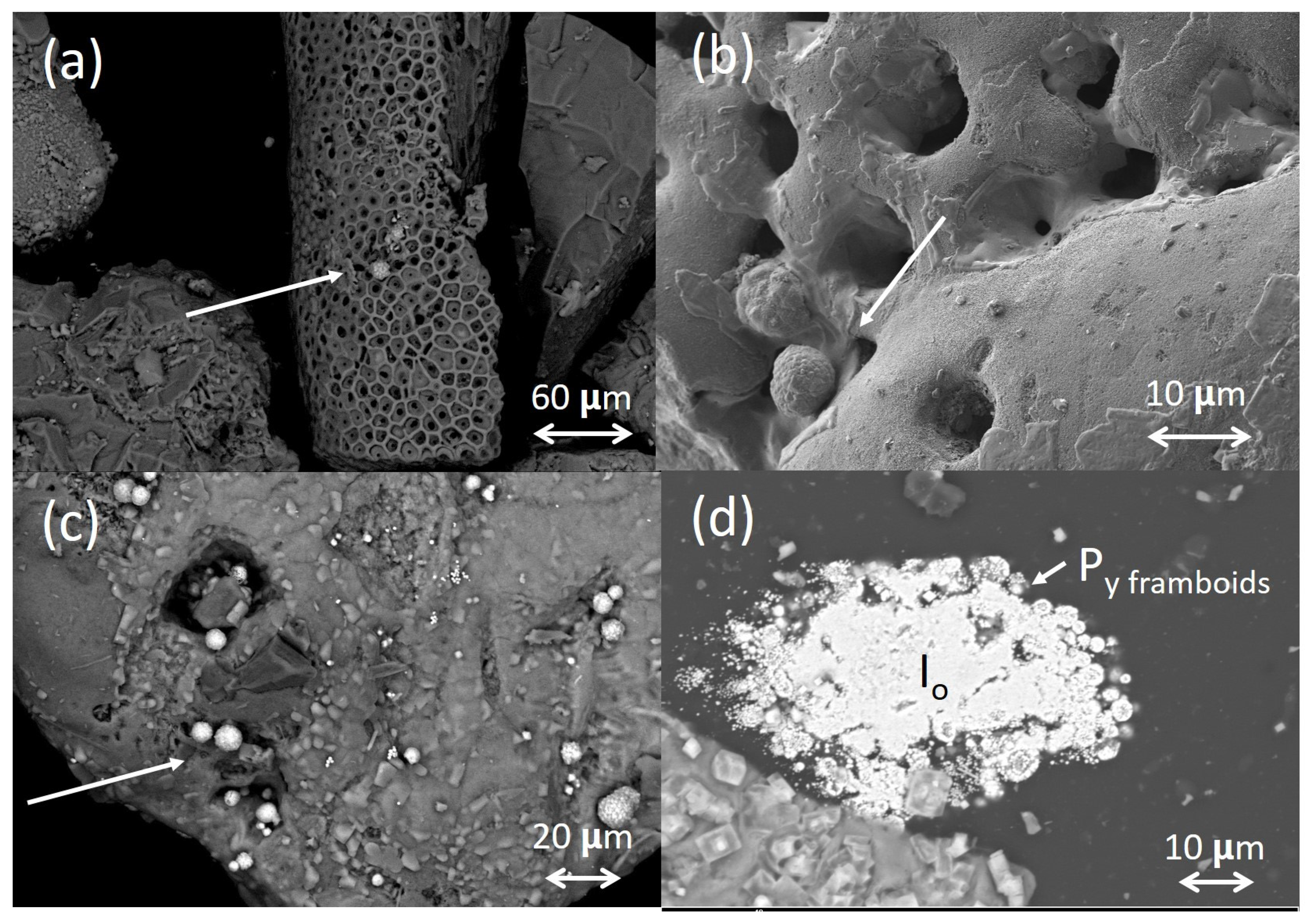
4.3. Wood Samples
5. Discussion
5.1. Sediment Biogeochemistry
5.2. Chemical Processes in Sediments
5.3. Chemical Processes in Wood
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, D.; Jensen, P.; Strætkvern, K. Conservation and in situ preservation of wooden shipwrecks from marine environments. J. Cult. Herit. 2012, 13, S139–S148. [Google Scholar] [CrossRef]
- Burdige, D.J. Geochemistry of Marine Sediments; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Lehtoranta, J.; Petri Ekholm, P.; Pitkänen, H. Coastal Eutrophication Thresholds: A Matter of Sediment Microbial Processes. AMBIO J. Hum. Environ. 2009, 38, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, O.V.; Sevastyanov, V.S.; Timerbaev, A.R. What are the current analytical approaches for sediment analysis related to the study of diagenesis? Highlights from 2010 to 2018. Talanta 2019, 191, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Nigam, R. Geological/paleontological applications in marine archeology: Few examples from Indian waters. In The Role of Tropics in Climate Change; Khare, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 419–439. [Google Scholar]
- Romano, E.; Magno, M.C.; Bergamin, L. Grain Size Data Analysis of Marine Sediments, From Sampling to Measuring and Classifying. A Critical Review. In Proceedings of the IMEKO International Conference on Metrology for The Sea, Naples, Italy, 11–13 October 2017. [Google Scholar]
- Jensen, P.; Gregory, D.J. Selected physical parameters to characterize the state of preservation of waterlogged archaeological wood: A practical guide for their determination. J. Archaeol. Sci. 2006, 33, 551–559. [Google Scholar] [CrossRef]
- Łucejko, J.J.; Modugno, F.; Ribechini, E.; del Río, J.C. Characterisation of archaeological waterlogged wood by pyrolytic and mass spectrometric techniques. Anal. Chim. Acta 2009, 654, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, B.; Giachi, G.; Fiorentino, L. Reasoned use of chemical parameters for the diagnostic evaluation of the state of preservation of waterlogged archaeological wood. J. Archaeol. Sci. 2013, 40, 1673–1680. [Google Scholar] [CrossRef]
- Macchioni, N.; Pizzo, B.; Capretti, C.H.; Giachi, G. How an integrated diagnostic approach can help in a correct evaluation of the state of preservation of waterlogged archaeological wooden artefacts. J. Archaeol. Sci. 2012, 39, 3255–3263. [Google Scholar] [CrossRef]
- Łucejko, J.J.; Modugno, F.; Ribechini, E.; Tamburinia, D.; Colombini, M.P. Analytical Instrumental Techniques to Study Archaeological Wood Degradation. Appl. Spectrosc. Rev. 2015, 50, 584–625. [Google Scholar] [CrossRef]
- Babinski, L.; Izdebska-Mucha, D.; Waliszewska, B. Evaluation of the state of preservation of waterlogged archaeological wood based on its physical properties: Basic density vs. wood substance density. J. Archeol. Sci. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Čufar, K.; Gričar, J.; Zupančič, M.; Koch, G.; Schmitt, U. Anatomy, cell wall structure and topochemistry of water-logged archaeological wood aged 5200 and 4500 years. IAWA J. 2008, 29, 55–68. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T.; Daniel, G. Microbial decay of waterlogged archaeological wood found in Sweden. Applicable to archaeology and conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Christensen, M.; Frosch, M.; Jensen, P.; Schnell, U.; Shashoua, Y.; Nielsen, O.F. Waterlogged archaeological wood—Chemical changes by conservation and degradation. J. Raman Spectrosc. 2006, 37, 1171–1178. [Google Scholar] [CrossRef]
- Li, R.; Guo, J.; Macchioni, N.; Pizzo, B.; Xi, G.; Tian, X.; Chen, J.; Sun, J.; Jiang, X.; Cao, J.; et al. Characterisation of waterlogged archaeological wood from Nanhai No. 1 shipwreck by multidisciplinary diagnostic methods. J. Cult. Herit. 2022, 56, 25–35. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Martínez Cortizas, A. Chemometric tools for identification of wood from different oak species and their potential for provenancing of Iberian shipwrecks (16th–18th centuries AD). J. Archeol. Sci. 2018, 100, 372–383. [Google Scholar] [CrossRef]
- Giachi, G.; Bettazzi, F.; Chimichi, S.; Staccioli, G. Chemical characterisation of degraded wood in ships discovered in a recent excavation of the Etruscan and Roman harbour of Pisa. J. Cult. Herit. 2004, 4, 75–83. [Google Scholar] [CrossRef]
- Shen, D.; Li, N.; Fu, Y.; Macchioni, N.; Sozzi, L.; Tian, X.; Liu, J. Study on wood preservation state of Chinese ancient shipwreck Huaguangjiao I. J. Cult. Herit. 2018, 32, 53–59. [Google Scholar] [CrossRef]
- Zoia, L.; Salanti, A.; Orlandi, M. Chemical characterization of archaeological wood: Softwood Vasa and hardwood Riksapplet case studies. J. Cult. Herit. 2015, 16, 428–437. [Google Scholar] [CrossRef]
- MacLeod, I.D. Conservation of waterlogged timbers from the Batavia 1629. Bull. Aust. Marit. Archeol. 1990, 14, 1–8. [Google Scholar]
- Zhang, H.; Shen, D.; Zhang, Z.; Kang, H.; Ma, Q. Comparison of iron deposits removing material from the marine archaeological wood of Nanhai I shipwreck. J. Cult. Herit. 2024, 66, 59–67, and references therein. [Google Scholar] [CrossRef]
- Bjurhager, I.; Halonen, H.; Lindfors, E.L.; Iversen, T.; Almkvist, G.; Gamstedt, E.K.; Berglund, L.A. State of degradation in archeological oak from the 17th century Vasa ship: Substantial strength loss correlates with reduction in (holo)cellulose molecular weight. Biomacromolecules 2012, 13, 2521–2527. [Google Scholar] [CrossRef]
- Fors, Y.; Jalilehvand, F.; Risberg, E.D.; Björdal, C.; Phillips, E.; Sandström, M. Sulfur and iron analyses of marine archaeological wood in shipwrecks from the Baltic Sea and Scandinavian waters. J. Archeol. Sci. 2012, 39, 2521–2532. [Google Scholar] [CrossRef]
- Smith, A.D.; Jones, M.; Berko, A.; Chadwick, A.V.; Newport, R.J.; Skinner, T.; Salomé, M.; Fredrick, J.; Mosselmans, W. An investigation of the Sulfur–Iron chemistry in timbers of the sixteenth century warship, the Mary Rose, by Synchrotron micro-X-ray spectroscopy. In Proceedings of the 37th International Symposium on Archaeometry, Siena, Italy, 13–16 May 2008; pp. 389–394. [Google Scholar]
- North, N.A.; MacLeod, I.D. Corrosion of metals. In Conservation of Marine Archaeological Objects; Pearson, C., Ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Almkvist, G.; Persson, I. Distribution of iron and sulfur and their speciation in relation to degradation processes in wood from the Swedish warship Vasa. New. J. Chem. 2011, 35, 1491–1502. [Google Scholar] [CrossRef]
- Sandström, M.; Jalilehvand, F.; Damian, E.; Fors, Y.; Gelius, U.; Jones, M.; Murielle, S. Sulfur accumulation in the timbers of King Henry VIII’s warship Mary Rose: A pathway in the sulfur cycle of conservation concern. Proceedings of the National Academy of Sciences 2005, 102, 14165–14170. [Google Scholar] [CrossRef] [PubMed]
- Bettazzi. F.; Giachi, G.; Staccioli, G.; Chimichi, S. Chemical characterisation of wood of Roman Ships brought to light in the recently discovered ancient Harbour of Pisa (Tuscany, Italy). Holzforschung 2003, 57, 373–376. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Tran, K.; Guilminot, E.; Conforto, E.; Refait, P. Study of iron (II) sulphides by environmental scanning electron microscopy (ESEM) and micro-Raman spectroscopy in waterlogged archaeological woods. In Proceedings of the International Conference on Non-Destructive Investigations & Microanalysis for the Diagnostics & Conservation of Cultural & Environmental Heritage, Florence, Italy, 13–15 April 2011; pp. 297–307. [Google Scholar]
- Rémazeilles, C.; Meunier, L.; Lévêque, F.; Plasson, N.; Conforto, E.; Crouzet, M.; Refalt, P.; Caillat, L. Posttreatment study of Iron/Sulfur-containing compounds in the wreck of Lyon Saint-Georges 4 (Second Century ACE). Stud. Conserv. 2019, 65, 28–36. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Li, N.S.; Tian, X.L.; Liu, J.; Shen, D.W. Research on the removal of the iron sulfides in the Qing Dynasty marine shipwreck, Ningbo Xiaobaijiao No. 1. Sci. Conserv. Archaeol. 2014, 26, 30–38. [Google Scholar]
- Vieira, H.C.; Bordalo, M.D.; Osten, J.R.V.; Soares, A.M.; Abreu, S.N.; Morgado, F. Can a 16th Century Shipwreck Be Considered a Mercury Source in the 21st Century?—A Case Study in the Azores Archipelago (Portugal). J. Mar. Sci. Eng. 2023, 11, 276. [Google Scholar] [CrossRef]
- Negueruela, I.; Pinedo, J.; Gómez, M.; Miñano, A.; Arellano, I.; Barba, J.S. Seventh-century BC Phoenician vessel discovered at Playa de la Isla, Mazarron, Spain. Int. J. Naut. Archaeol. 1995, 24, 189–197. [Google Scholar] [CrossRef]
- Negueruela, I. Protection of shipwrecks: The experience of the Spanish National Maritime Archaeological Museum. In Underwater Archaeology and Coastal Management. Focus on Alexandria; Hassan, M., Grimal, N., Nakashima, D., Eds.; UNESCO: París, France, 2000; pp. 111–116. [Google Scholar]
- Negueruela, I. The Phoenician Ships of Mazarrón. In Assyria to Iberia at the Dawn of the Classical Age; Aruz, J., Graff, S.B., Rakic, Y., Eds.; The Metropolitan Museum of Art: New York, NY, USA, 2014; pp. 243–245. [Google Scholar]
- Castillo, R.; Pérez, S.; Buendía, M. Una historiografía del pecio Mazarrón 2. In Actas de la Reunión Internacional de Expertos Sobre la Extracción y Conservación del Pecio Mazarrón 2; Ministerio de Cultura: Madrid, Spain, 2023; pp. 29–99. [Google Scholar]
- de Juan, C. El pecio de Mazarrón 2 (Murcia) y la arquitectura naval venida del Levante. In Actas de la Reunión Internacional de Expertos Sobre la Extracción y Conservación del Pecio Mazarrón 2; Ministerio de Cultura: Madrid, Spain, 2023; pp. 15–28. [Google Scholar]
- Doménech, T. Analytical techniques and sampling strategies for establishing the state of conservation of underwater archaeological objects. In Actas de la Reunión Internacional de Expertos Sobre la Extracción y Conservación del Pecio Mazarrón 2; Ministerio de Cultura: Madrid, Spain, 2023; pp. 271–278. [Google Scholar]
- Negueruela, I. Hacia la comprensión de la construcción naval fenicia según el barco “Mazarrón-2” del siglo VII a. C. In La Navegación Fenicia. Tecnología Naval y Derroteros. Encuentro Entre Marinos, Arqueólogos e Historiadores; Centro de Estudios Fenicios y Púnicos, Universidad Complutense de Madrid: Madrid, Spain, 2004; pp. 227–278. [Google Scholar]
- Gianfrotta, P.A. Le “ancore d’argento” dei mercanti fenici (Diod. V 35, 4): Espediente di carico e precauzione daziaria. Quad. Aristonothos. Riv. Studi Sul. Mediterr. Antico. 2021, 17, 253–275. [Google Scholar]
- Pomey, P. Le dossier de l’épave du Golo (Mariana, Haute-corse). Archaeonautica 2012, 17, 11–30. [Google Scholar] [CrossRef]
- de Juan, C. Los pecios de Mazarrón y la familia arquitectónica ibérica. Los ejemplos más antiguos de la arquitectura naval indígena en la Península Ibérica. In Mazarrón II. Contexto Arqueológico, Viabilidad Científica y Perspectiva Patri–monial del Barco B-2 De La Bahía de Mazarrón (Murcia). En Homenaje a Julio Mas García; Universidad Autónoma de Madrid: Madrid, Spain, 2017; pp. 229–254. [Google Scholar]
- Ramón, J. Eivissa Fenícia I Les Comunitats Indígenas Del Sud-Est. Contactes: Indígenes i fenicis a la Mediterrània occidental entre els sigles VIII y VI ane. In Simposi d’Arqueologia d’Alcanar, 24–26 November 2006; Signes, Disseny i Comunicación: Barcelona, Spain, 2008; pp. 39–53. [Google Scholar]
- Ramón, J. La cerámica fenicia del Mediterráneo extremo-occidental y del Atlántico (s. VIII-1r. 1/3 del VI a. C.). Problemas y perspectivas actuales. In Motya and the Phoenician Ceramic Repertoire between the Levant and the West 9–6th Century BC: Proceedings of the International Conference Held in Rome, 26 February 2010; Nigro, L., Ed.; pp. 211–253.
- Miñano, A.I. El Barco 2 de Mazarrón. 2014. Available online: https://www.cultura.gob.es/fragatamercedes/dam/jcr:8af3ffff-0e26-426f-8d34-d4e5b2d273cc/barco-mazarron-2.pdf (accessed on 13 May 2024).
- Agüera, S.; Iniesta, A.; Martínez, M. El coto minero de San Cristóbal y Los Perules (Mazarrón). Patrimonio Histórico Arqueológico e Industrial. In Memorias de Arqueología de la Región de Murcia 8; Editora Regional de Murcia: Murcia, Spain, 1999; pp. 523–550. [Google Scholar]
- Ramallo, S.; Arana, R. La minería romana en Mazarrón (Murcia). Aspectos arqueológicos y geológicos. An. Prehist. Arqueol. 1985, 1, 49–68. [Google Scholar]
- Guillén, M.C. Las minas de Mazarrón: El paradigma de un paisaje cultural. Entre la desidia de las administraciones públicas y el absentismo de la población. Rev. Murc. Antropol. 2018, 25, 95–114. [Google Scholar]
- Ros, M.M. Nuevos datos en torno a la presencia fenicia en la Bahía de Mazarrón (Sureste Ibérico). In El Oriente de Occidente. Fenicios y Púnicos en el Área Ibérica; Prados, F., Sala, F., Eds.; Servicio de Publicaciones de la Universidad de Alicante: Alicante, Spain, 2017; pp. 79–104. [Google Scholar]
- Arellano, I.; Barba, J.S.; Gómez, M.; Miñano, A.I.; Negueruela, I.; Pinedo, J. Proyecto Nave Fenicia: 2ª campaña. In Memorias de Arqueología de la Región de Murcia 9; Editora Regional de Murcia: Murcia, Spain, 1999; pp. 219–222. [Google Scholar]
- Renzi, M.; Montero, I.; Bode, M. Non-ferrous metallurgy from the Phoenician site of La Fonteta (Alicante, Spain): A study of provenance. J. Archaeol. Sci. 2009, 36, 2584–2596. [Google Scholar] [CrossRef]
- Egea, P.M. Una perspectiva social de la minería contemporánea en Mazarrón. In Phicaria. III Encuentros Internacionales del Mediterráneo. Minería y Metalurgia en el Mediterráneo y su Periferia Oceánica; Universidad Popular de Mazarrón: Mazarrón, Spain, 2015; pp. 209–228. [Google Scholar]
- Guillén, M.C. Mazarrón 1900, 2nd ed.; Ayuntamiento de Mazarrón: Mazarrón, Spain, 2022. [Google Scholar]
- Dabrio, C.J. Playas. In Sedimentología: Del Proceso Físico a la Cuenca Sedimentaria; Arche, A., Ed.; CSIC: Madrid, Spain, 2010; pp. 441–501. [Google Scholar]
- Dabrio, C.J.; Polo, M.D. Dinámica litoral y evolución costera del puerto de Mazarrón (Murcia). Bol. R. Soc. Esp. Hist. Nat. 1981, 79, 225–234. [Google Scholar]
- Munsell. Munsell Soil Color Charts; Munsell: Boston, MA, USA, 2009. [Google Scholar]
- Hema, B.; Jagadeeswaran, R. A comparative study on soil colour determination through munsell colour chart and hyper spectral remote sensing technique. Int. J. Chem. Stud. 2018, 6, 2420–2429. [Google Scholar]
- UNE-EN ISO 17892-4:2019; Investigación y Ensayos Geotécnicos. Ensayos de Laboratorio De Suelos. Parte 4. Determinación de la Distribución Granulométrica. (ISO 17892-4:2016). Asociación Española de Normalización: Madrid, Spain, 2019.
- D6913-04; Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis. ASTM: West Conshohocken, PA, USA, 2009.
- Seibold, E.; Berger, W.H. Sources and Composition of Marine Sediments. In The Sea Floor; Springer: Berlin/Heidelberg, Germany, 1996; pp. 69–95. [Google Scholar]
- D 2487-83; Classification of Soils for Engineerring Purposes: Annual Book os ASTM Standards. American Society for Testing and Materials: West Conshohocken, PA, USA, 1985.
- Folk, R.L.; Ward, W.C. Brazos river bar: A study in the significance of grain size parameters. J. Sed. Petr. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Royse, C. Introduction To Sedimentary Analysis; Arizona State University Publications: Tempe, AZ, USA, 1970. [Google Scholar]
- D2487-17e1; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- Berner, R. Sedimentary pyrite formation. Am. J. Sci. 1970, 268, 1–23. [Google Scholar] [CrossRef]
- Álvarez-Iglesias, P.; Rubio, B. Early diagenesis of organic-matter-rich sediments in a ría environment: Organic matter sources, pyrites morphology and limitation of pyritization at depth. Estuar. Coast. Shelf Sci. 2012, 100, 113–123. [Google Scholar] [CrossRef]
- Leventhal, J.; Taylor, C. Comparison of methods to determine degree of pyritization. Geochim. Cosmochim. Acta 1990, 54, 2621–2625. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Morse, J.W. Quantitative Method for Determination of Trace Metal Concentrations in Sedimentary Pyrite. Mar. Chem. 1990, 29, 119–144. [Google Scholar] [CrossRef]
- Erdey, L.; Bodor, E. Ascorbic Acid in Analytical Chemistry. Determination of Ferric Irons. Anal. Chem. 1952, 24, 418–420. [Google Scholar] [CrossRef]
- Metodiev, L.; Georgieva, M.; Stoylkova, T. Degree of Pyritization (DOP) and Indicator of Anoxicity (IA) in Jurassic sedimentary rocks from Bulgaria. Rev. Bulg. Geol. Soc. 2022, 83, 149–152. [Google Scholar] [CrossRef]
- Raiswell, R.; Newton, R.; Wignall, P.B. An indicator of water-column anoxia: Resolution of biofacies variations in the Kimmeridge clay (upper Jurassic, U.K.). J. Sediment. Res. 2001, 71, 286–294. [Google Scholar]
- Silva, P.G.; Roquero, E.; Rodríguez-Pascua, M.A.; Bardají, T.; Huerta, P.; Giner, J.L.; Pérez-López, R. Development of a numerical system and field-survey charts for earthquake environmental effects based on the Munsell Soil Color Charts. INQUA Focus Group on Paleoseismology and Active Tectonics. In Proceedings of the 4th International INQUA Meeting on Paleoseismology, Active Tectonics and Archeoseismology (PATA), Aachen, Germany, 9–14 October 2013. [Google Scholar]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Nota, D.J.G. Sediments of the western Guyana shelf. In Report of Orinoco Shelf Expedition, 2. Mendedel; Landbomvhogedrool: Wegeningen, The Netherlands, 1958; Volume 58, pp. 1–98. [Google Scholar]
- Izzo, F.C.; Kratter, M.; Nevin, A.; Zendri, E. A Critical Review on the Analysis of Metal Soaps in Oil Paintings. ChemistryOpen 2021, 10, 904–921. [Google Scholar] [CrossRef]
- López Ruíz, J.; Rodríguez Badiola, E. La región volcánica neógena del sudeste de España. Cons. Super. Investig. Cient. 1980, 36, 5–53. [Google Scholar]
- López Ruíz, J.; Rodríguez Badiola, E.; Arroyo, A.; Coy-YII, R. Los óxidos de Fe-Ti de las Rocas Calco-alcalinas del sureste de España. Estud. Geol. 1984, 40, 269–279. [Google Scholar] [CrossRef]
- Li, Y.-H.; Schoonmaker, J.E. Chemical Composition and Mineralogy of Marine Sediments. Treatise Geochem. 2003, 7, 1–35. [Google Scholar]
- Liu, C.; Wang, W.; Wang, H.; Zhu, C.; Ren, B. A Review on Removal of Iron Impurities from Quartz Mineral. Minerals 2023, 13, 1128. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D.; Kądziołka-Gaweł, M.; Krzykawski, T. Chemical and mineral composition of furnace slags produced in the combustion process of hard coal. Int. J. Environ. Sci. Technol. 2019, 16, 5387–5396. [Google Scholar] [CrossRef]
- Velazco, F. Shallow marine sediments in the bay of Callao, Perú. Inst. Mar. Perú 2011, 26, 75–82. [Google Scholar]
- Chang, J.; Li, Y.; Lu, H. The Morphological Characteristics of Authigenic Pyrite Formed in Marine Sediments. J. Mar. Sci. Eng. 2022, 10, 1533. [Google Scholar] [CrossRef]
- Burdige, D.J.; Komada, T. Iron redox cycling, sediment resuspension and role of sediments in low oxygen environments as sources of iron to wáter column. Mar. Chem. 2020, 223, 103793. [Google Scholar] [CrossRef]
- Canfield, D.E.; Raiswell, R.; Bottrell, S. The Reactivity of Sedimentary iron minerals towards sufide. Am. J. Sci. 1992, 292, 659–683. [Google Scholar] [CrossRef]
- Rickard, D. Kinetics and mechanism of pyrite formation at low temperatures. Am. J. Sci. 1975, 275, 636–652. [Google Scholar] [CrossRef]
- Yücel, M.; Konovalov, S.K.; Moore, T.S.; Janzen, C.P.; Luther, G.W., III. Sulfur speciation in the upper Black Sea sediments. Chem. Geol. 2010, 269, 364–375. [Google Scholar] [CrossRef]
- Ranganath, N.; Padhi, A.K.; Padhi, A.K.; Rai, A.K. Framboidal—Colloform—Recrystallised pyrite in the granitoids of Wahkyn area, West Khasi hills, Meghalaya. J. Geol. Soc. India 2009, 74, 591–596. [Google Scholar] [CrossRef]
- Wei, H.; Algeo, T.J.; Yu, H.; Wang, J.; Guo, C.; Shi, G. Episodic euxinia in the Changhsingian (late Permian) of South China: Evidence from framboidal pyrite and geochemical data. Sediment. Geol. 2015, 319, 78–97. [Google Scholar] [CrossRef]
- Liu, K.; Huang, F.; Gao, S.; Zhang, Z.; Ren, Y.; An, B. Morphology of framboidal pyrite and its textural evolution: Evidence from the Logatchev area, Mid-Atlantic Ridge. Ore Geol. Rev. 2022, 141, 104630. [Google Scholar] [CrossRef]
- Raiswell, R.; Buckley, F.; Berner, R.A.; Anderson, T.F. Degree of pyritization of iron as a paleoenvironmental indicator of bottom-water oxygenation. J. Sediment. Petrol. 1988, 58, 812–819. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochem. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]








| Oxide | MZB2-01 | MZB2-02 | MZB2-03 | MZB2-04 | MZB2-05 | MZB2-06 | MZB2-07 | MZB2-08 | MZB2-09 |
| Na2O | 3.1 (0.9) | 3.2 (0.2) | 3.41 (0.03) | 3.2 (0.3) | 3.4 (0.3) | 2.1 (0.1) | 3.4 (0.1) | 2.2 (0.3) | 2.1 (0.2) |
| MgO | 4.9 (0.5) | 4.6 (0.2) | 5.0 (0.2) | 4.37 (0.06) | 5.2 (0.3) | 6.0 (0.5) | 5.1 (0.2) | 5.8 (0.2) | 6.0 (0.1) |
| Al2O3 | 8.3 (0.7) | 10.4 (0.1) | 9.68 (0.07) | 10.4 (0.7) | 9.5 (0.3) | 5.8 (0.3) | 8.2 (0.3) | 6.8 (0.1) | 8.9 (0.4) |
| SiO2 | 53 (1) | 46.3 (0.9) | 42 (2) | 43 (2) | 42.3 (0.5) | 50 (2) | 47 (1) | 47 (1) | 44.4 (0.5) |
| SO3 | 1.5 (0.3) | 0.3 (0.5) | 1.4 (0.2) | n.d. | 1.6 (0.3) | 0.7 (0.6) | 1.1 (0.2) | 0.7 (0.2) | 1.2 (0.2) |
| K2O | 1.9 (0.1) | 2.63 (0.04) | 2.6 (0.1) | 2.6 (0.1) | 2.6 (0.1) | 1.6 (0.1) | 2.1 (0.2) | 1.8 (0.1) | 2.2 (0.1) |
| CaO | 21 (1) | 21.2 (0.1) | 24 (2) | 23 (2) | 24.3 (0.3) | 27 (1) | 24.7 (2) | 27.6 (0.8) | 26.8 (0.6) |
| TiO2 | 0.6 (0.1) | 0.8 (0.1) | 0.7 (0.3) | 1.0 (0.5) | 0.6 (0.1) | 0.3 (0.3) | 0.4 (0.3) | 0.6 (0.2) | n.d. |
| MnO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| FeO | 3.4 (0.2) | 8.5 (0.2) | 8.25 (0.03) | 9.9 (0.2) | 7.8 (0.2) | 5.5 (0.2) | 4.8 (0.4) | 5.6 (0.2) | 6.7 (0.2) |
| CuO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.2 (0.2) | n.d. | n.d. |
| ZnO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| PbO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BaO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oxide | MZB2-10 | MZB2-11 | MZB2-12 | MZB2-13 | MZB2-15 | MZB2-16 | MZCG-01 | MZPI-01 | |
| Na2O | 2.4 (0.4) | 3.5 (0.2) | 1.8 (0.1) | 2.7 (0.1) | 2.3 (0.1) | 2.5 (0.1) | 4.3 (0.1) | 2.7 (0.3) | |
| MgO | 5.6 (0.4) | 7.0 (0.4) | 7.7 (0.2) | 4.4 (0.5) | 4.6 (0.3) | 3.9 (0.5) | 3.5 (0.1) | 8.4 (0.8) | |
| Al2O3 | 8.8 (0.3) | 7.2 (0.3) | 7.2 (0.4) | 10.2 (0.2) | 8.4 (0.2) | 9.0 (0.6) | 9.6 (0.2) | 4.1 (0.2) | |
| SiO2 | 42.6 (0.6) | 42 (2) | 40.1 (0.8) | 48.1 (0.6) | 50.3 (0.8) | 51.4 (0.5) | 52 (1) | 17.4 (0.4) | |
| SO3 | 1.1 (0.2) | 1.3 (0.3) | 1.1 (0.1) | 0.4 (0.3) | 0.7 (0.3) | 0.8 (0.2) | 1.05 (0.08) | 2.5 (0.2) | |
| K2O | 2.5 (0.1) | 1.8 (0.2) | 1.8 (0.1) | 2.5 (0.2) | 2.07 (0.02) | 2.4 (0.2) | 2.0 (0.1) | 0.8 (0.1) | |
| CaO | 28.5 (0.3) | 29.2 (0.8) | 34.4 (0.7) | 21.4 (0.4) | 24.6 (0.4) | 20 (1) | 14.2 (0.3) | 35 (1) | |
| TiO2 | n.d. | n.d. | 0.2 (0.2) | 0.7 (0.2) | 0.6 (0.4) | 0.4 (0.1) | 0.56 (0.09) | 0.3 (0.1) | |
| MnO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 (0.1) | 0.3 (0.3) | |
| FeO | 6.1 (0.1) | 4.6 (0.3) | 4.7 (0.1) | 8.2 (0.5) | 5.5 (0.2) | 8 (1) | 9.7 (0.7) | 24.4 (0.3) | |
| CuO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.3 (0.7) | |
| ZnO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.2 (0.2) | 0.8 (0.9) | |
| PbO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.3 (0.5) | |
| BaO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.5 (0.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doménech-Carbó, M.-T.; Guasch-Ferré, N.; Álvarez-Romero, C.; Castillo-Belinchón, R.; Pérez-Mateo, S.; Buendía-Ortuño, M. Study of the Geological Context of the 7th–6th Century BC Phoenician Era Shipwreck “Mazarrón 2” (Murcia, Spain). Minerals 2024, 14, 778. https://doi.org/10.3390/min14080778
Doménech-Carbó M-T, Guasch-Ferré N, Álvarez-Romero C, Castillo-Belinchón R, Pérez-Mateo S, Buendía-Ortuño M. Study of the Geological Context of the 7th–6th Century BC Phoenician Era Shipwreck “Mazarrón 2” (Murcia, Spain). Minerals. 2024; 14(8):778. https://doi.org/10.3390/min14080778
Chicago/Turabian StyleDoménech-Carbó, María-Teresa, Nuria Guasch-Ferré, Carla Álvarez-Romero, Rocío Castillo-Belinchón, Soledad Pérez-Mateo, and Milagros Buendía-Ortuño. 2024. "Study of the Geological Context of the 7th–6th Century BC Phoenician Era Shipwreck “Mazarrón 2” (Murcia, Spain)" Minerals 14, no. 8: 778. https://doi.org/10.3390/min14080778





