The Effects of Wind Velocity on the Binding Properties of Ash, Bottom Ash, and Additives: A Wind Tunnel Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Characterization of the Initial FA and BA Samples
2.2.2. Sample Preparation Protocol for Geo-Mechanical Testing
- base (initial) test specimens containing only ash and water without additives,
- test specimens with additive (ash + additive + water), and
- test specimens with additive and bottom ash (ash + bottom ash + additive + water).
- Water content (% w/w): 5%, 8%, 10%, 15%, 20%, 25%;
- Additive content (% w/w): 1, 2, and 3.
- Measurement of the required quantities of water, fly ash, bottom ash, and additives according to the specifications outlined in Table 1.
- Components were mixed and homogenized in a laboratory planetary mixer (manufactured by “Toni Technik”) for a duration of 5 min.
- Addition of the specified amount of water to the mixture, followed by further stirring for an additional 5 min.
- After 30 min of aging, the prepared mixture was poured into a steel mold (with dimensions of D × H = 50 × 81 mm) and subjected to a compacting force of 120 kN/m2 using a laboratory hydraulic press (manufactured by W. Feddeler, Essen, Germany).
- Following extrusion from the mold, test specimens were measured, and their dimensions and weight were recorded. They were then appropriately labeled; subsequently, the test specimens were placed for aging under laboratory conditions, with ambient temperature maintained between 20 and 21 °C and relative humidity between 40 and 50%, while ensuring no exposure to direct sunlight.
- The aging period was 1, 7, and 28 days, following which the test specimens were subjected to geo-mechanical tests. Prior to subsequent investigation, the test specimens were measured after aging, and their weight and dimensions were recorded.
- The formed test specimens exhibited a cylindrical shape, with a diameter of 50 mm and a height of 51 ± 1 mm, as illustrated in Figure 1.
2.2.3. Preparation of New Mixture Samples for Wind Tunnel Testing
2.2.4. Measurement of Total Suspended Particles (TSPs)
2.2.5. Measurement of Concentration of PM10 Particles
2.2.6. Calculating the Level of Suspended Particles Emission
2.2.7. Wind Tunnel
- axial fan with frequency regulator;
- section for air flow conditioning;
- section for directing air flow;
- section with samples;
- section for measurement and sampling.

3. Results
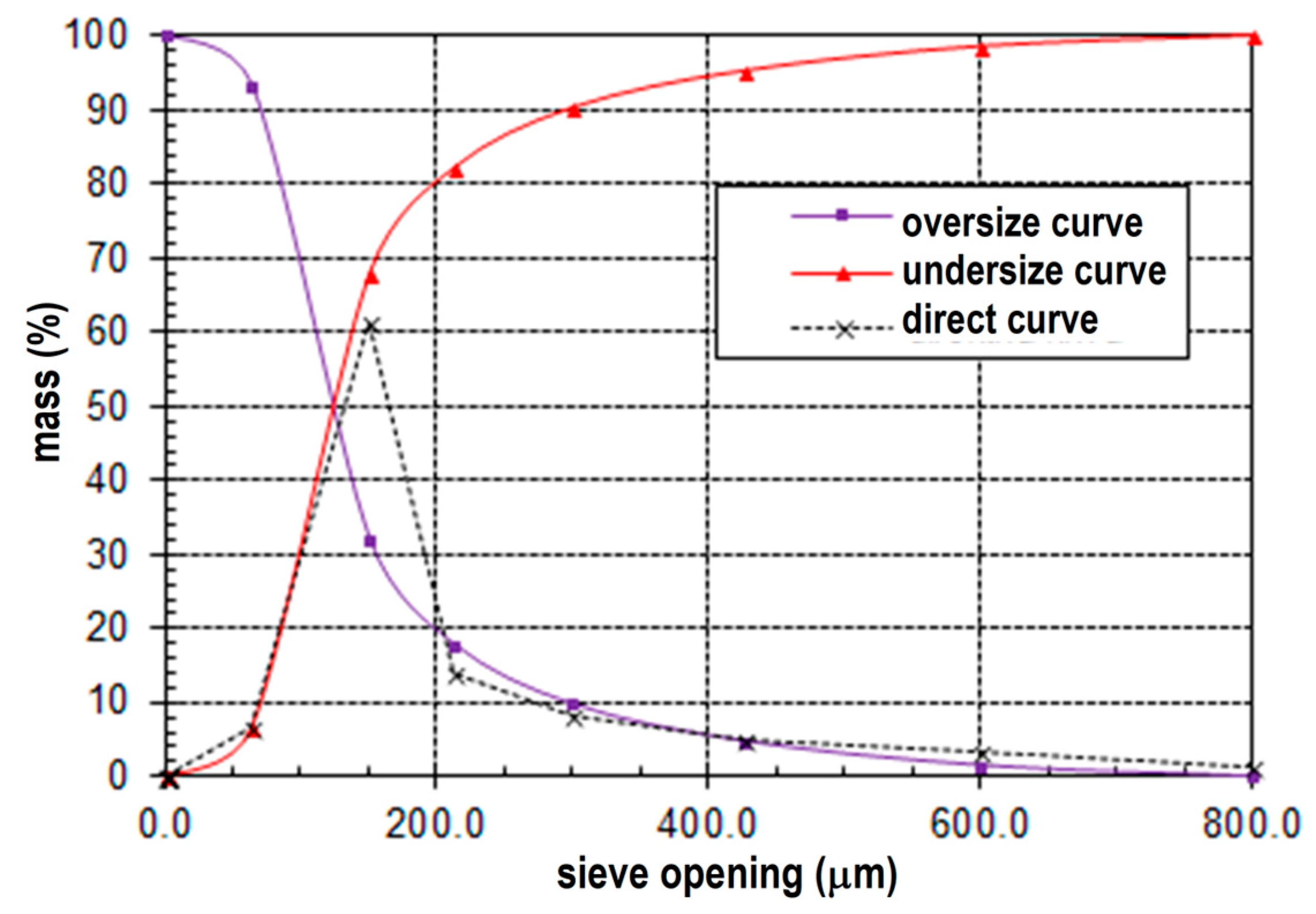
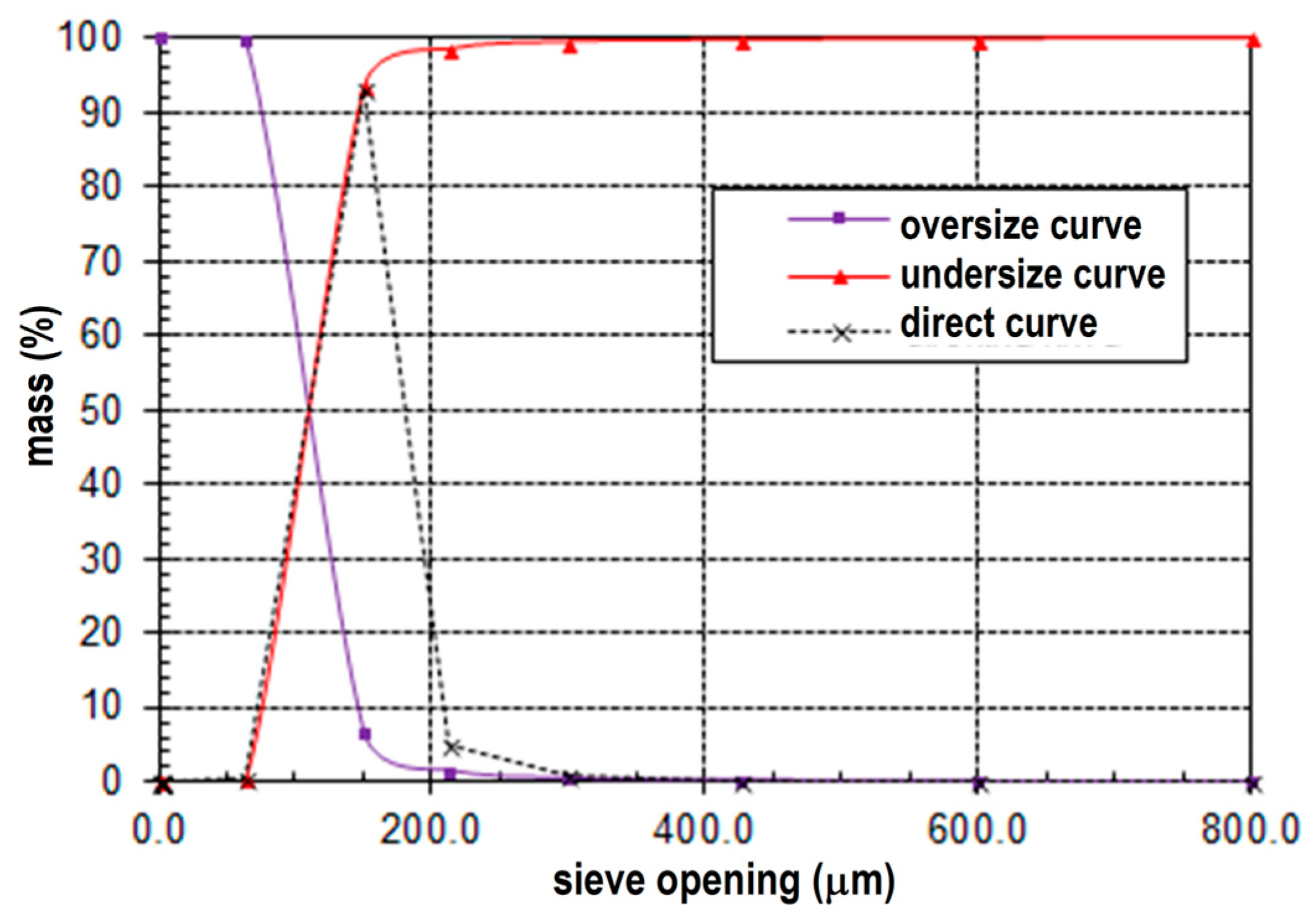
3.1. Particle Size Analysis
- The mean particle diameter (d50) is determined to be 125 µm.
- The upper size limit (d95) is observed to be 405 µm.
- The mean particle diameter (d50) is measured to be 110 µm.
- The upper size limit (d95) is determined to be 180 µm.
- The mean particle diameter (d50) is found to be 110 µm.
- The upper size limit (d95) is identified as 160 µm.
- The mean particle diameter (d50) is 700 µm.
- The upper size limit (d95) is observed to be 3800 µm.
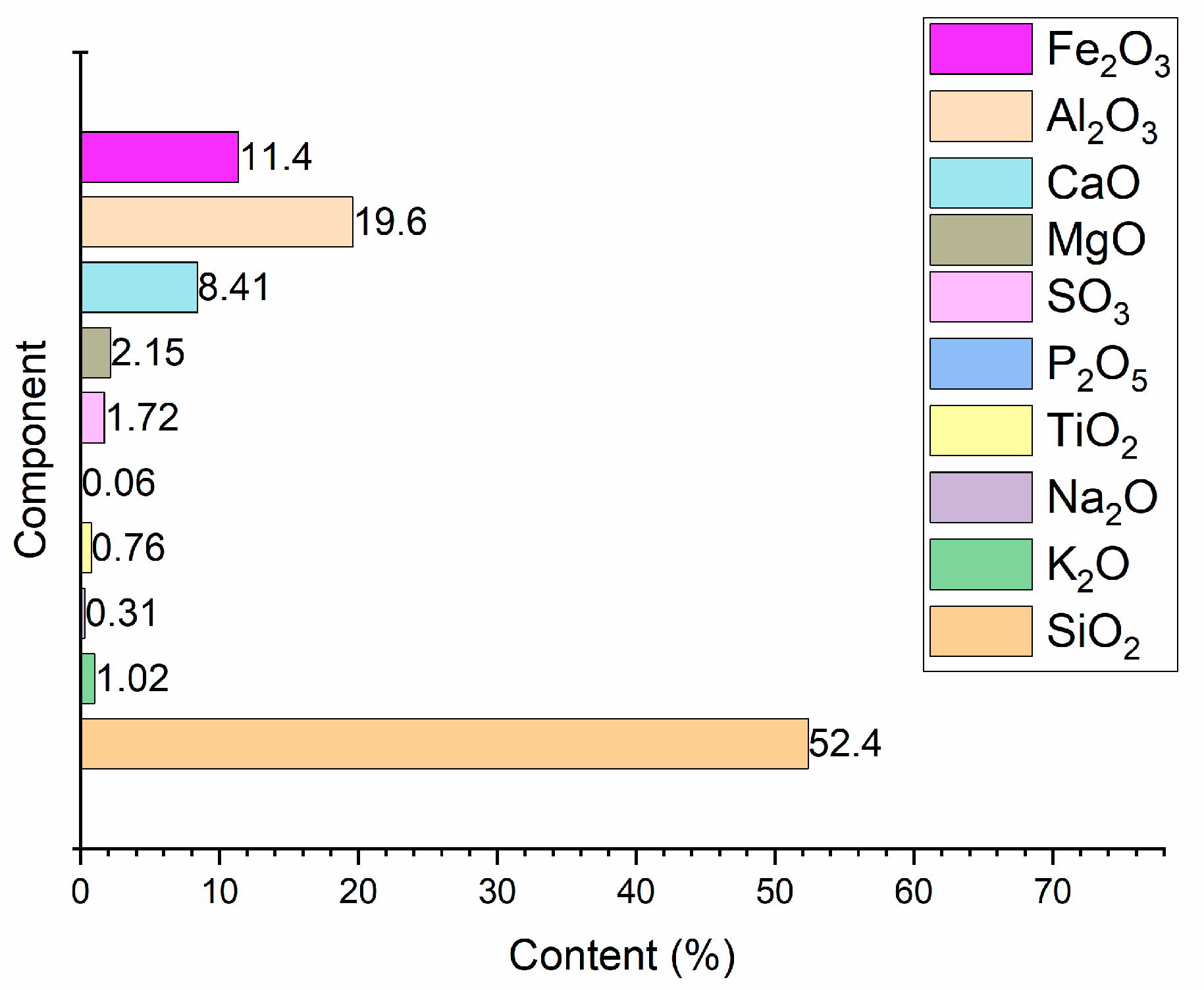
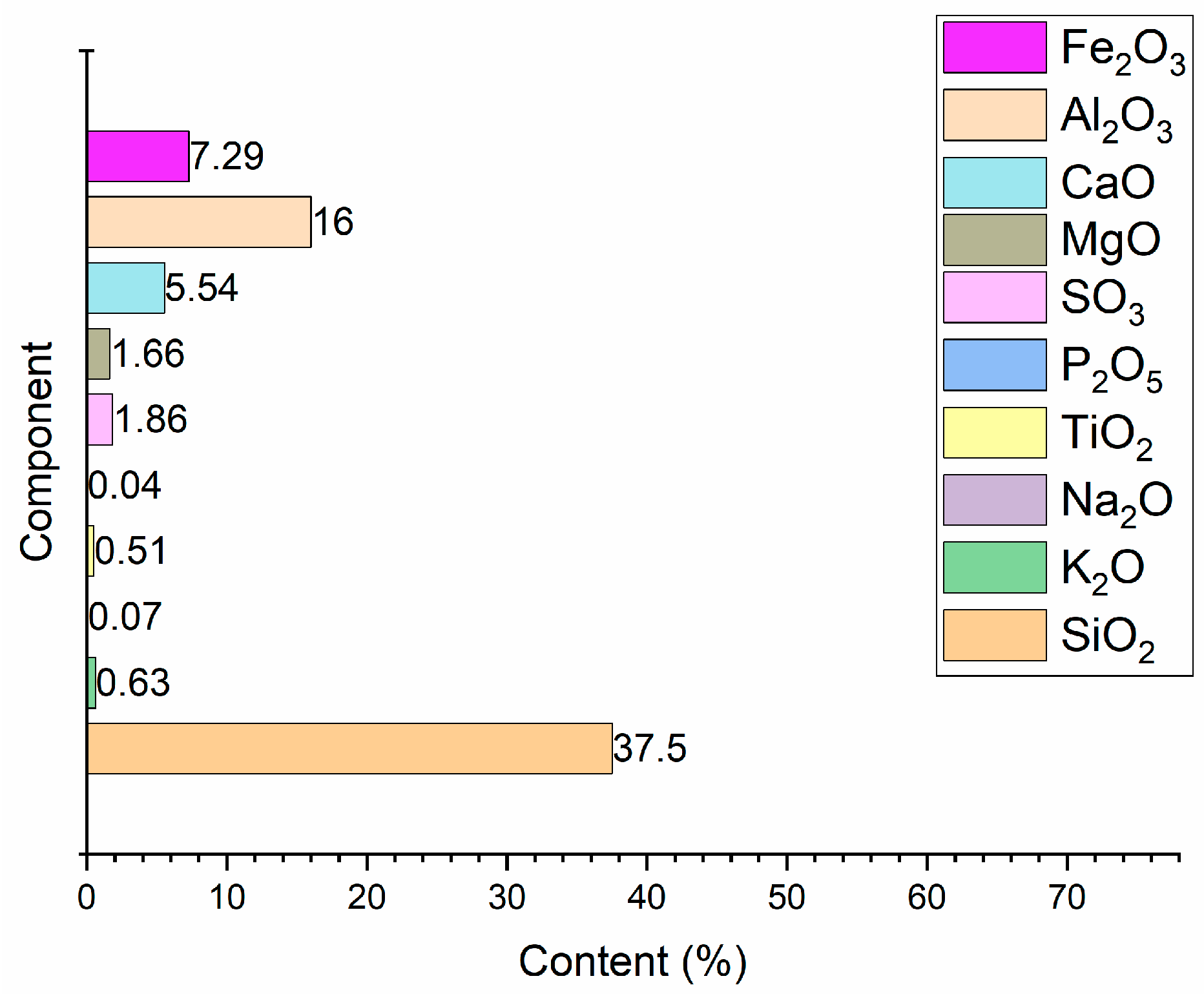
3.2. Results of Chemical Analysis
3.3. Results of Bulk Density
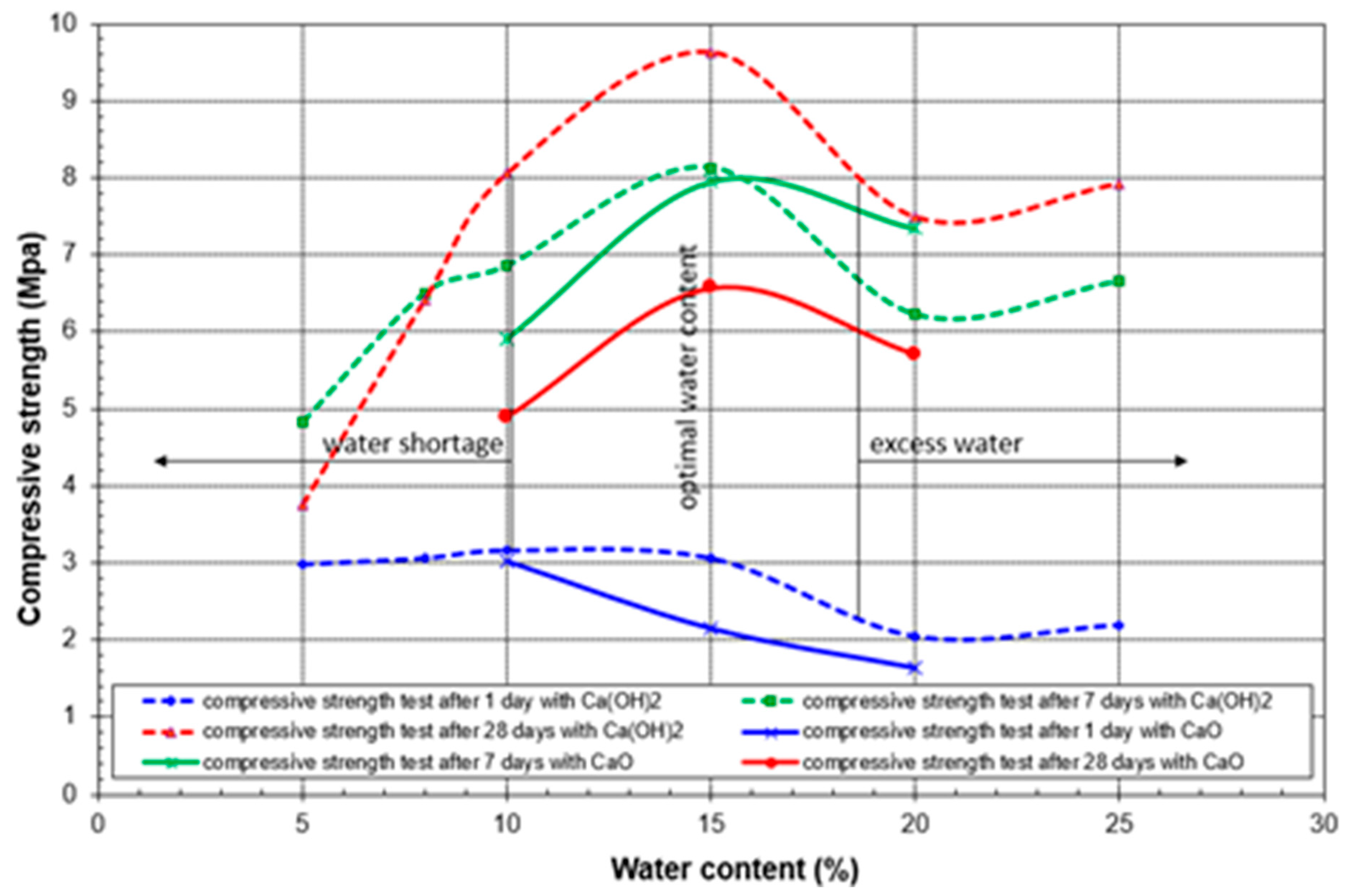
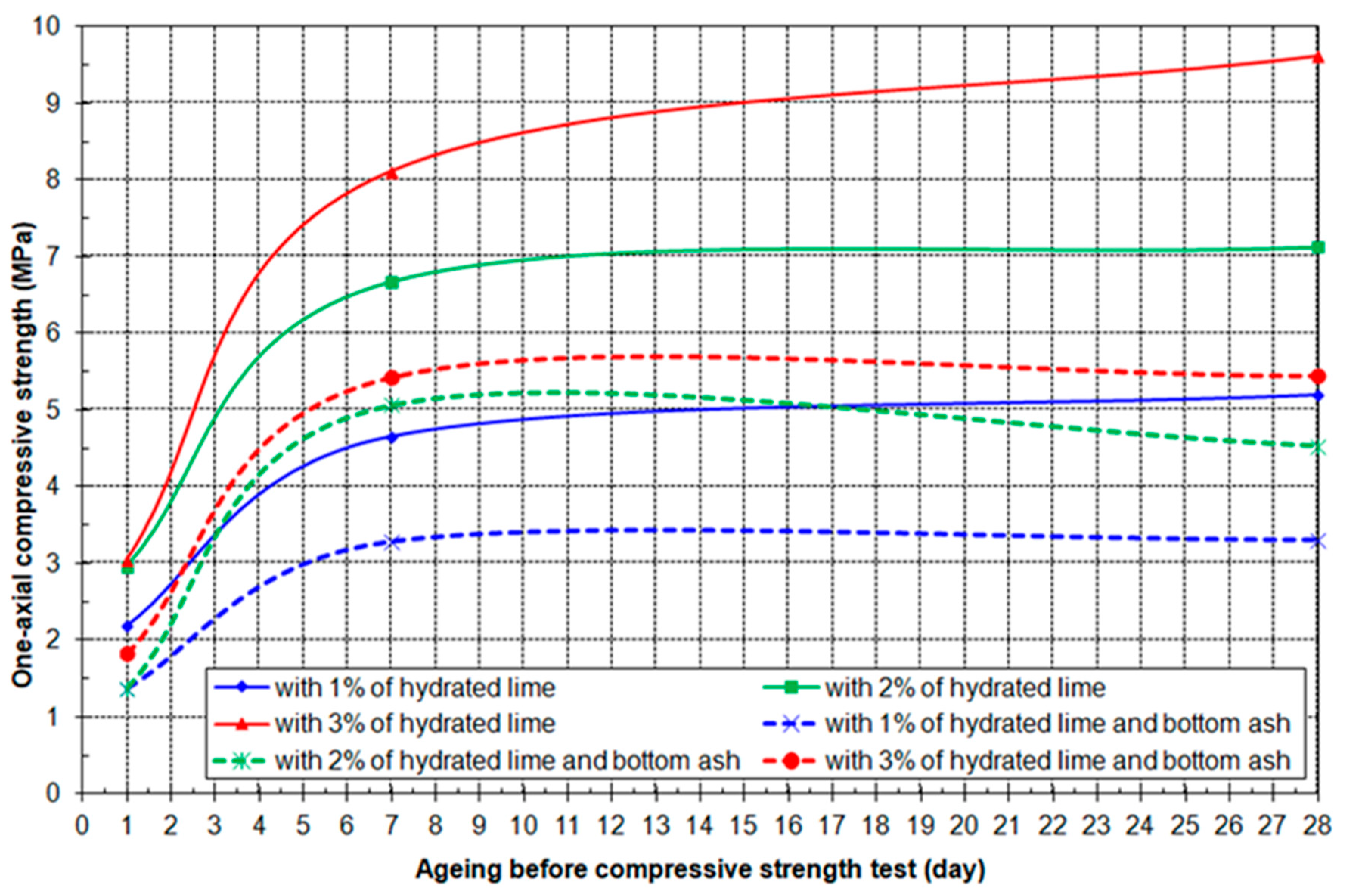
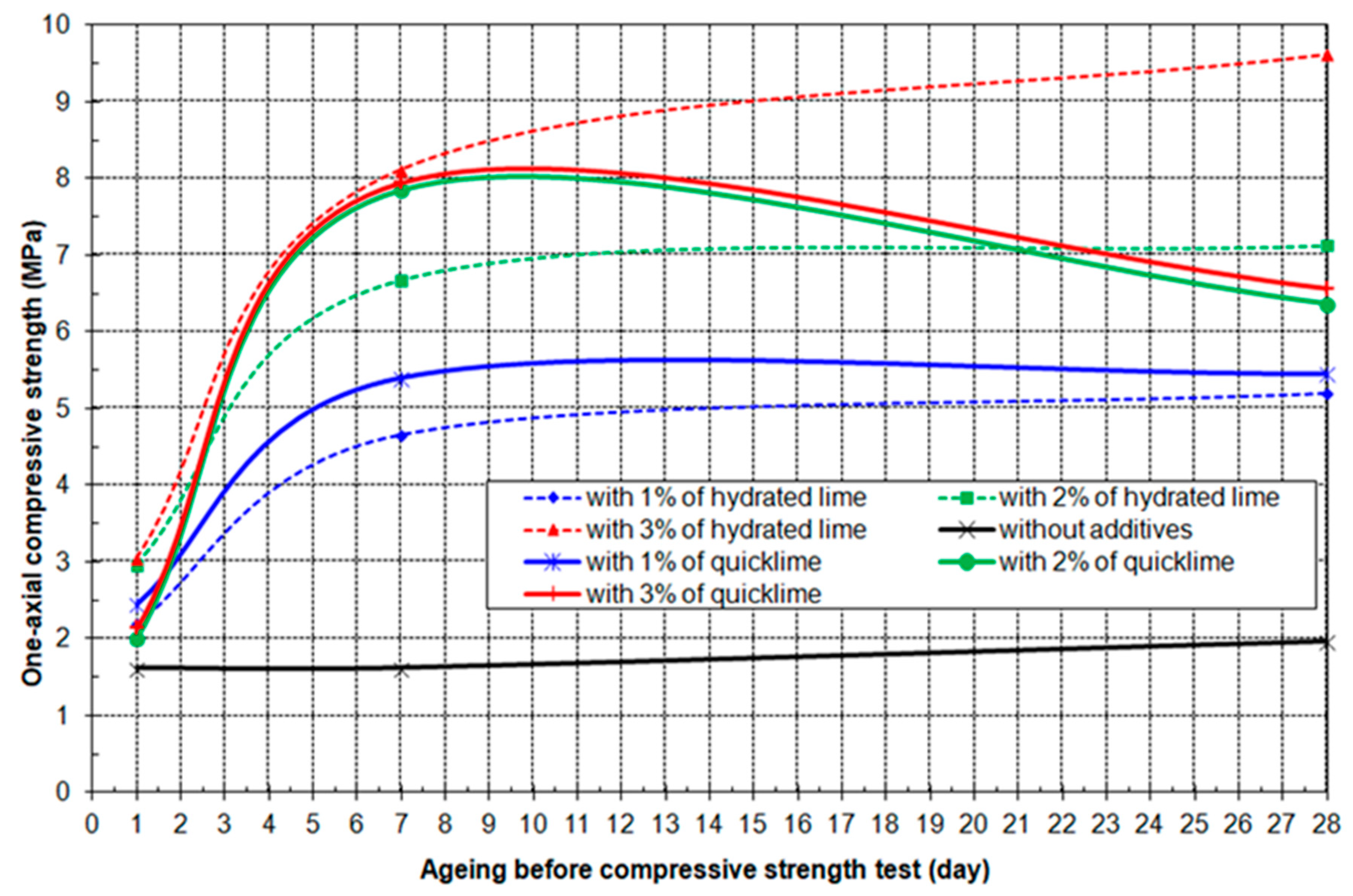
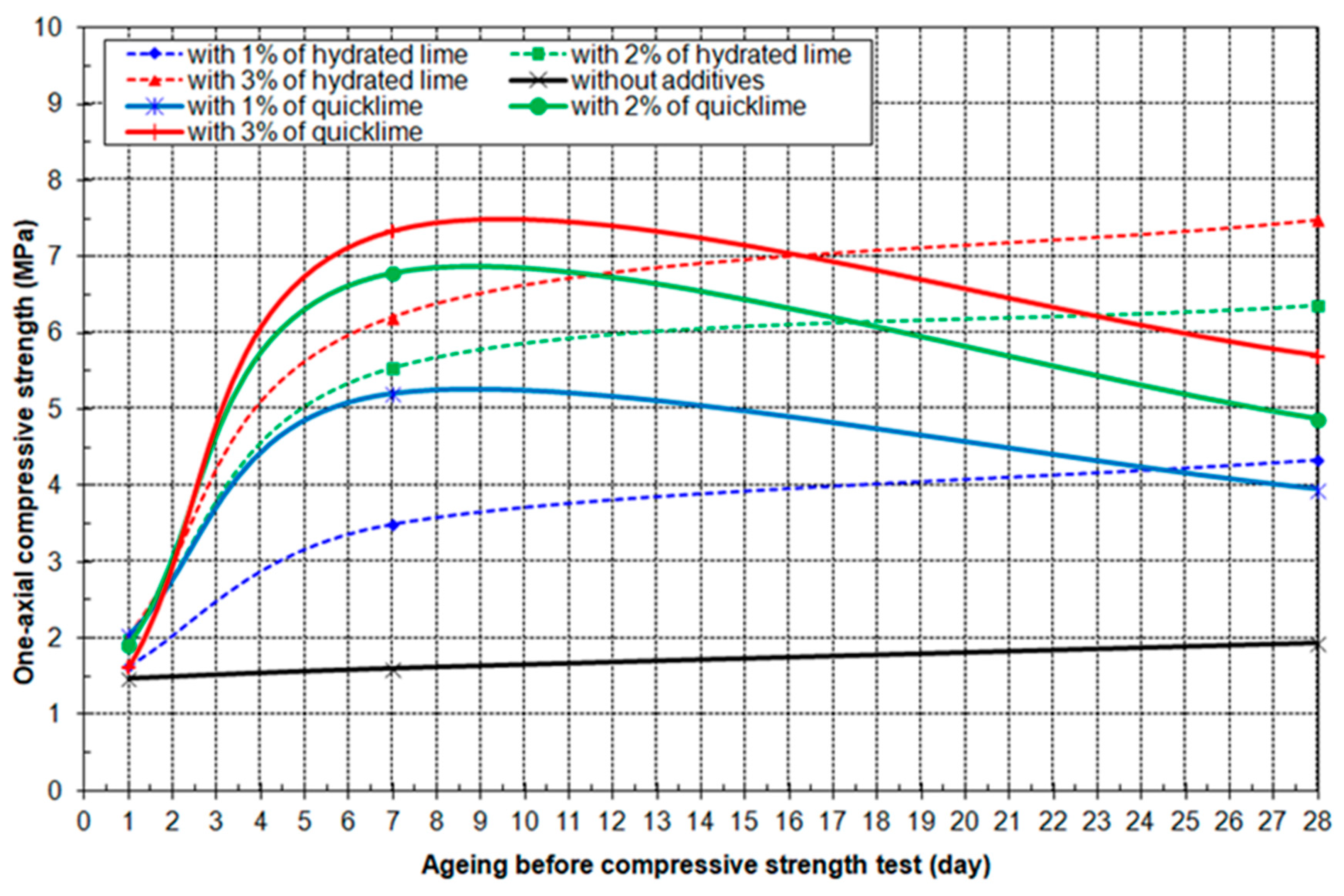
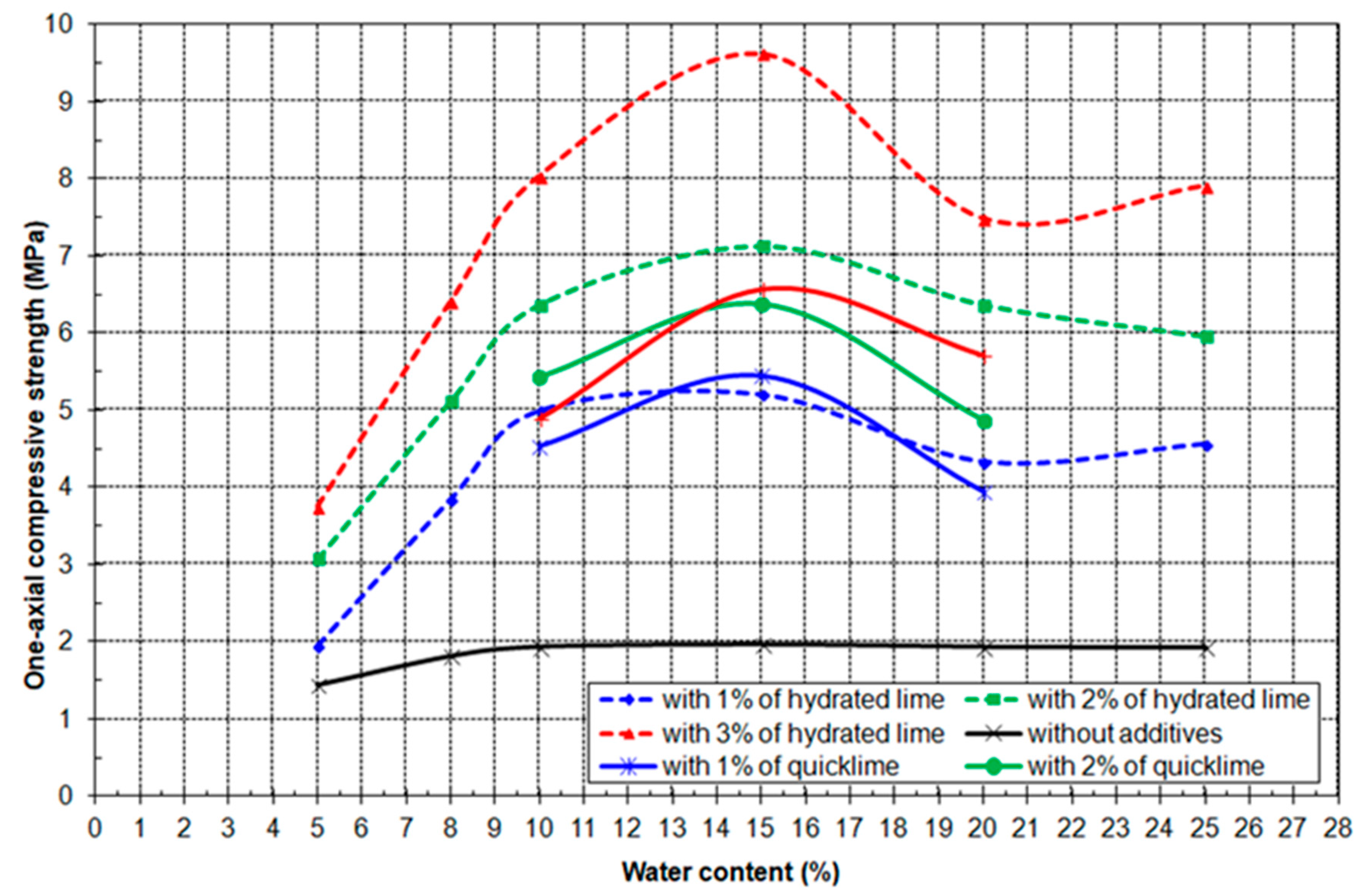
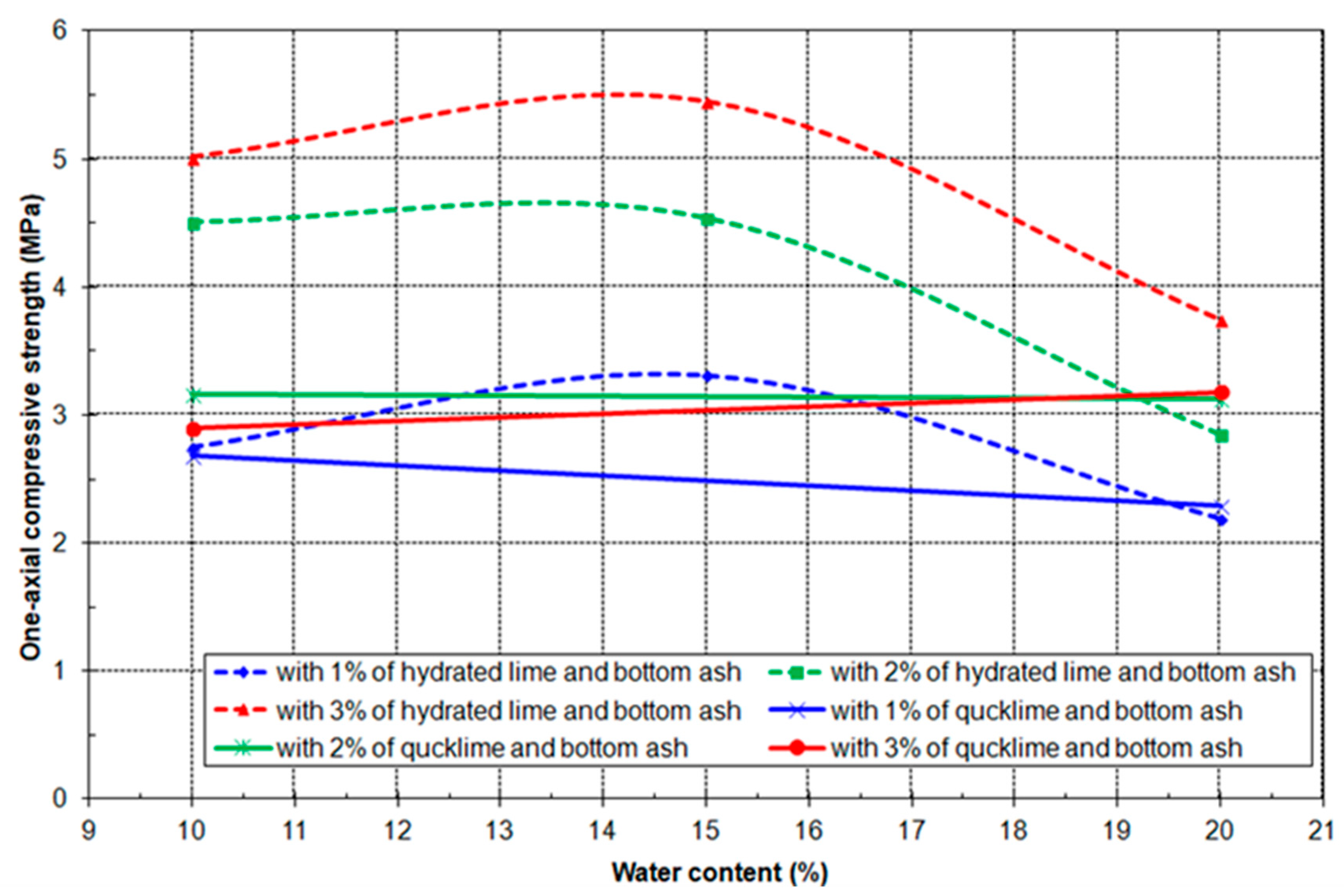
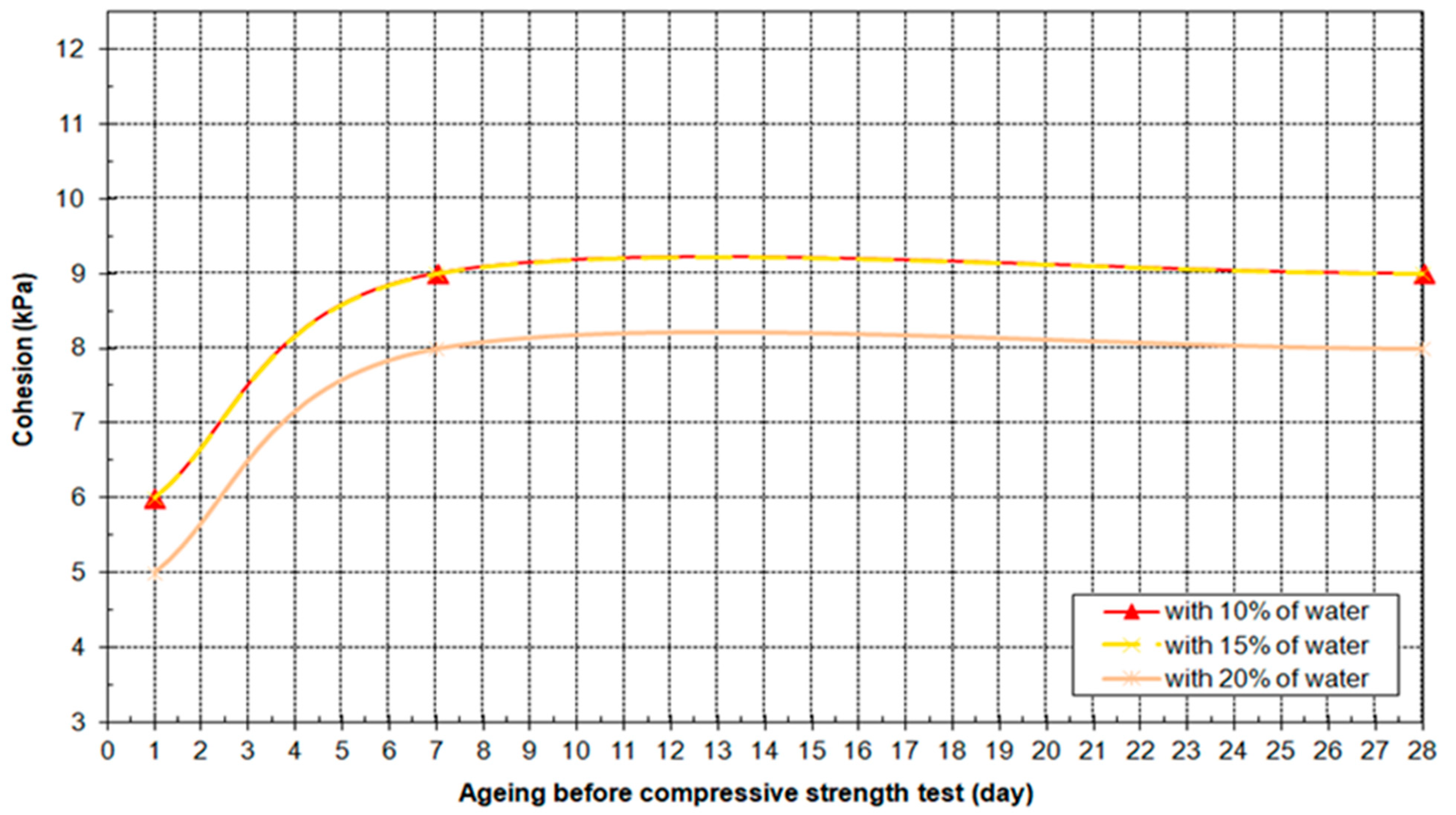
3.4. Results of Geo-Mechanical Testing
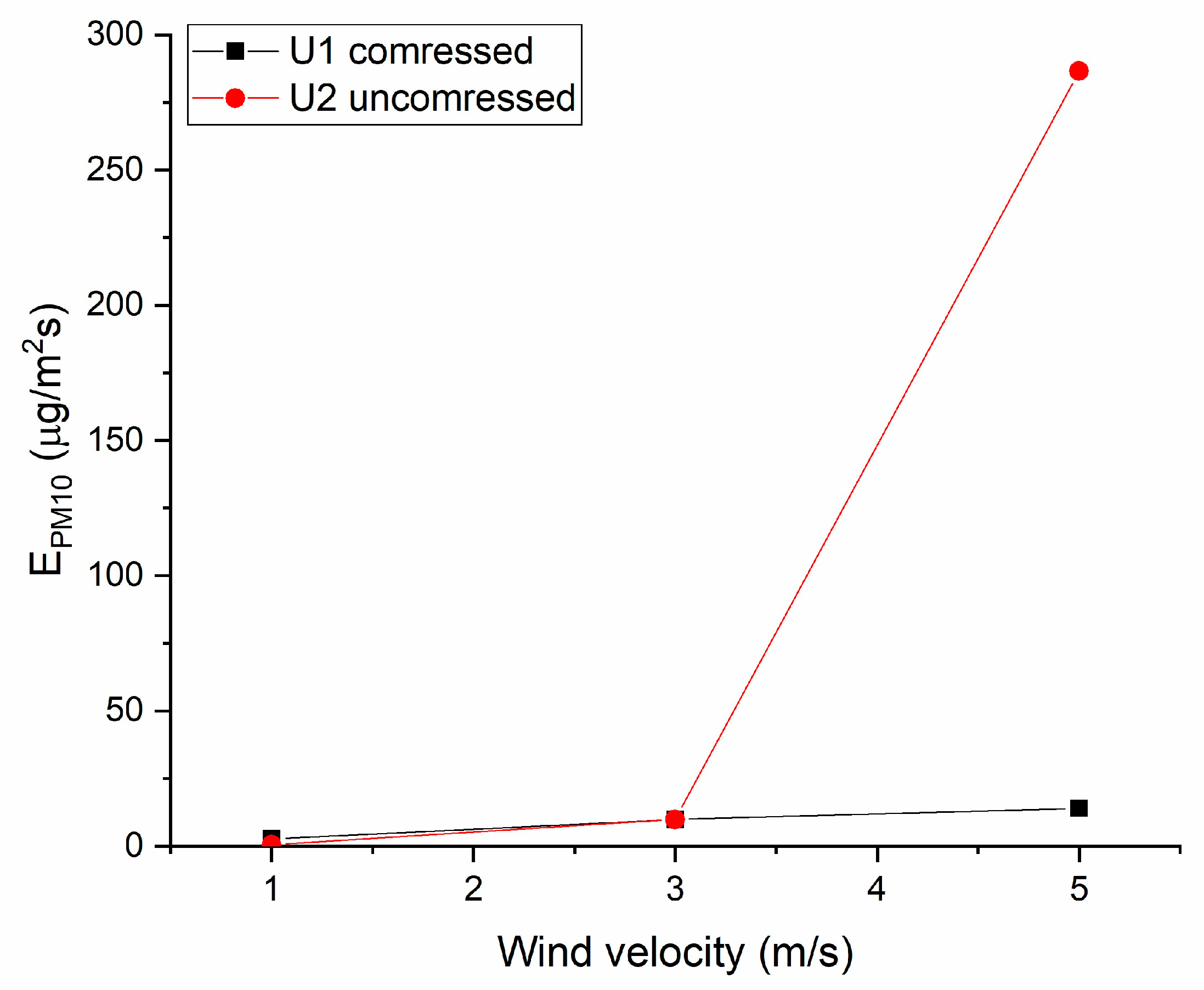
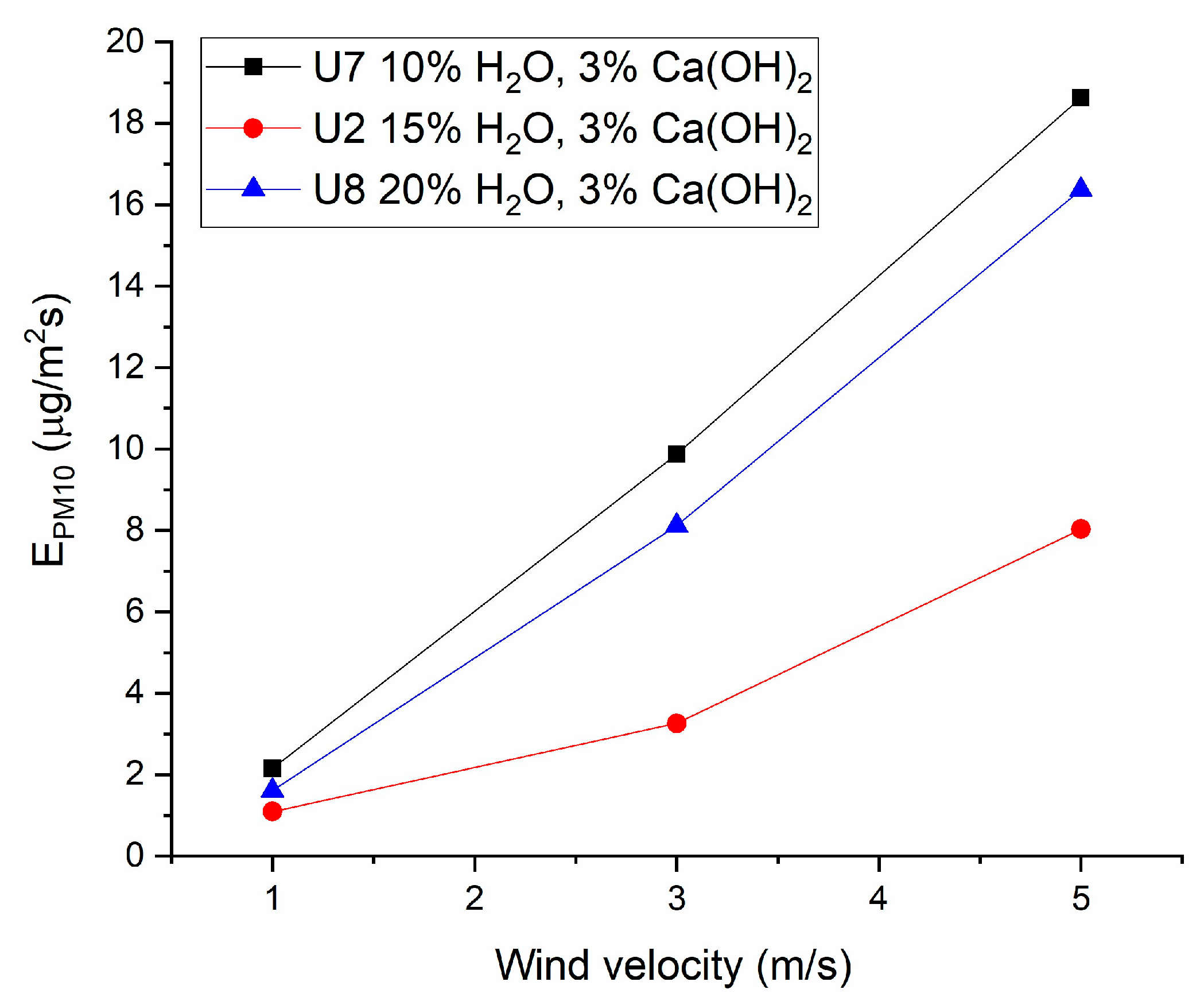
3.5. Results from Wind Tunnel Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Xu, G.; Huang, J.; Eksteen, J.; Liu, X.; Zhao, Z. Characterization of coal particles wettability in surfactant solution by using four laboratory static tests. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 304–312. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yang, H.; Li, H.; Yu, M.; He, T.; Luo, Z.; Liu, F. A novel biomass thermoresponsive konjac glucomannan composite gel developed to control the coal spontaneous combustion: Fire prevention and extinguishing properties. Fuel 2021, 306, 121757. [Google Scholar] [CrossRef]
- International Energy Agency. Global Coal Consumption Rises by 1.4% in 2023 to Record 8.5 Billion Metric Tons. 2024. Available online: https://www.iea.org/reports/coal-2023/executive-summary (accessed on 1 June 2024).
- Chen, J.; Cheng, S.; Song, M.; Wang, J. Interregional differences of coal carbon dioxide emissions in China. Energy Policy 2016, 96, 1–13. [Google Scholar] [CrossRef]
- International Energy Agency. Global Coal Demand Is Set to Return to Its All-Time High in 2022. 2022. Available online: https://www.iea.org/news/global-coal-demand-is-set-to-return-to-its-all-time-high-in-2022 (accessed on 30 May 2024).
- Han, W.; Zhou, G.; Wang, J.; Jiang, W.; Zhang, Q.; Kong, Y.; Miao, Y. Experimental investigation on combined modification for micro physicochemical characteristics of coal by compound reagents and liquid nitrogen freeze-thaw cycle. Fuel 2021, 292, 120287. [Google Scholar] [CrossRef]
- Wang, P.; Han, H.; Liu, R.; Gao, R.; Wu, G. Effect of outlet diameter on atomization characteristics and dust reduction performance of X-swirl pressure nozzle. Process Saf. Environ. Prot. 2020, 137, 340–351. [Google Scholar] [CrossRef]
- Hall, N.B.; Blackley, D.J.; Halldin, C.N.; Laney, A.S. Current review of pneumoconiosis among US coal miners. Curr. Environ. Health Rep. 2019, 6, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Fidanchevski, E.; Angjusheva, B.; Jovanov, V.; Murtanovski, P.; Vladiceska, L.; Stamatovska Aluloska, N.; Krneta Nikolic, J.; Ipavec, A.; Šter, K.; Mrak, M.; et al. Technical and radiological characterisation of fly ash and bottom ash from thermal power plant. J. Radioanal. Nucl. Chem. 2021, 330, 685–694. [Google Scholar] [CrossRef]
- Singh, N.; Shehnazdeep, A.B. Reviewing the role of coal bottom ash as an alternative of cement. Constr. Build. Mater. 2020, 233, 117276. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X.; Li, X.; Zhang, J. Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad. Dev. 2020, 31, 1317–1324. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process Eng. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Lunts, A.; Kreyling, W.; Cox, C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health 2002, 65, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Mahamaya, M.; Das, S.K.; Reddy, K.R.; Jain, S. Interaction of biopolymer with dispersive geomaterial and its characterization: An eco-friendly approach for erosion control. J. Clean. Prod. 2021, 312, 127778. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. 2006, 137, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Poon, C.S.; Sun, H.; Lo, I.M.C.; Kirk, D.W. Heavy Metal Specifications and Leaching Behaviors in Cement Based Solidified/Stabilized Waste Materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.; Andronescu, C.; Zahanagiu, A. Immobilization of harmful substances in binder matrices. Part I. Immobilization capacity. Influence on binding properties. Rev. Rom. Mater. 2006, 3, 189–197. [Google Scholar]
- Vijan, A.C.; Badanoiu, A.; Voicu, G.; Nicoara, A.I. Phosphate cements based on calcined dolomite: Influence of calcination temperature and silica addition. Materials 2021, 14, 3838. [Google Scholar] [CrossRef] [PubMed]
- Nešković, J.; Stjepanović, P.; Milojković, N.; Lazić, D.; Konc Janković, K. Solidification/stabilization technology of by products (ash) from power plants. In Proceedings of the 8th Balkan Mining Congress, Belgrade, Serbia, 28–30 September 2022; pp. 345–354, ISBN 978-86-82673-21-7. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, X.; Pang, Y. Wind Dynamic Characteristics and Wind Tunnel Simulation of Subgrade Sand Hazard in the Shannan Wide Valley of the Sichuan–Tibet Railway. Int. J. Environ. Res. Public Health 2022, 19, 8341. [Google Scholar] [CrossRef] [PubMed]
- Demarco, G.; Martins, L.G.N.; Bodmann, B.E.J.; Puhales, F.S.; Acevedo, O.C.; Wittwer, A.R.; Costa, F.D.; Roberti, D.R.; Loredo-Souza, A.M.; Degrazia, F.C.; et al. Analysis of thermal and roughness effects on the turbulent characteristics of experimentally simulated boundary layers in a wind tunnel. Int. J. Environ. Res. Public Health 2022, 19, 5134. [Google Scholar] [CrossRef]
- Zhao, Z.; Chang, P.; Ghosh, A.; Xu, G.; Liu, Y.; Morla, R. The influence of surfactants’ ionicity on the performance of coal dust suppression. Adv. Powder Technol. 2024, 35, 104295. [Google Scholar] [CrossRef]
- EN 1097-3:1999; Tests for Mechanical and Physical Properties of Aggregates. Determination of Loose Bulk Density And Voids. iTeh, Inc.: Newark, DE, USA, 1998.
- Vujić, S.; Radosavljević, M.; Makar, N.; Marijanac, S.; Nešković, J.; Gigov, M.; Žarković, M.; Hafner Ljubenović, T.; Belić, D.; Bužalo, A. Fifty-five years of the Mining Institute. Bull. Mines 2015, CXII, 1–27. [Google Scholar]
- BS EN 12341:2023; Ambient Air. Standard Gravimetric Measurement Method for the Determination of the PM10 or PM2.5 Mass Concentration of Suspended Particulate Matter. European Standards: Plzen, Czech Republic, 2023.
- Scott Van Pelt, R.; Zobeck, M.T. Chapter 3, Portable Wind Tunnels for Field Testing of Soils and Natural Surfaces, Wind Tunnel Designs and Their Diverse Engineering Applications; IntechOpen: London, UK, 2013; Volume 17. [Google Scholar]
- ISO 16911-1:2013; Stationary Source Emissions Manual and Automatic Determination of Velocity and Volume Flow Rate in Ducts Part 1: Manual Reference Method. International Standards: Geneva, Switzerland, 2013.
- Tennekes, H. The logarithmic wind profile. J. Atmos. Sci. 1973, 30, 234–238. [Google Scholar] [CrossRef]
- White, B.R. Laboratory simulation of aeolian sand transport and physical modeling of flow around dunes. Ann. Arid Zone 1996, 35, 187–213. [Google Scholar]
- Etyemezian, V.; Gillies, J.A.; Mastin, L.G.; Crawford, A.; Hasson, R.; Van Eaton, A.R.; Nikolich, G. Laboratory experiments of volcanic ash resuspension by wind. J. Geophys. Res. Atmos. 2019, 124, 9534–9560. [Google Scholar] [CrossRef]
- Goossens, D.; Buck, B. Dust dynamics in off-road vehicle trails: Measurements on 16 arid soil types, Nevada, USA. J. Environ. Manag. 2009, 90, 3458–3469. [Google Scholar] [CrossRef]
- Macpherson, T.; Nickling, W.G.; Gillies, J.A.; Etyemezian, V. Dust emissions from undisturbed and disturbed supply-limited desert surfaces. J. Geophys. Res. Earth Surf. 2008, 113, F02S04. [Google Scholar] [CrossRef]
- ASTM Standard C618-22; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2023.
- European Standard EN 459-1; Building Lime Definitions, Specifications and Conformity Criteria. iTeh, Inc.: Newark, DE, USA, 2015.
- Regulation on Conditions for Monitoring and Air Quality Requirements; no. 11/10, 75/10 and 63/13; Official Gazette of the Republic of Serbia: Belgrade, Serbia, 2013.









| Velocity in Wind Tunnel, m/s | Wind Velocity at 10 m, m/s |
|---|---|
| 1 | 2.82 |
| 3 | 8.45 |
| where: | |
| 10 m | |
| 0.0003 m—according to the European Wind Atlas for sand surfaces (smooth) | |
| 0.05 m—the height in the wind tunnel at which the velocity is measured | |
| Sample No. | Fly Ash Content % | Water Content % | CaO % | Ca(OH)2 % | Bottom Ash Content % |
|---|---|---|---|---|---|
| U1 | 79 | 15 | - | - | 6 |
| U2 | 76 | 15 | - | 3 | 6 |
| U3 | 76 | 15 | 3 | - | 6 |
| U4 | 74 | 15 | - | 5 | 6 |
| U5 | 84 | 10 | - | - | 6 |
| U6 | 82 | 10 | - | 2 | 6 |
| U7 | 81 | 10 | - | 3 | 6 |
| U8 | 71 | 20 | - | 3 | 6 |
| Bulk Density | Loose State | Compacted State |
|---|---|---|
| Sample | (g/dm3) | (g/dm3) |
| Ash | 812.53 | 1032.63 |
| Bottom ash | 467.70 | 533.47 |
| Ca(OH)2 | 497.80 | 671.30 |
| CaO | 642.70 | 849.60 |
| Sample | Ash | Bottom Ash | Ca(OH)2 | CaO |
|---|---|---|---|---|
| Density (g/cm3) | 2.1 | 2.1 | 2.4 | 3.6 |
| Sample No. | Wind Velocity (m/s) | EPM1 (µg/m2s) | EPM10/E0 (max) |
|---|---|---|---|
| U0 | 1 | 0.6 | - |
| 3 | 3.3 | ||
| 5 | 13.8 | ||
| U1 | 1 | 2.7 | 4.8 |
| 3 | 9.9 | ||
| 5 | 13.9 | ||
| U2 | 1 | 1.1 | 1.9 |
| 3 | 3.3 | ||
| 5 | 8.0 | ||
| U3 | 1 | 1.1 | 1.9 |
| 3 | 3.3 | ||
| 5 | 8.4 | ||
| U4 | 1 | 0.6 | 20.8 |
| 3 | 9.9 | ||
| 5 | 286.6 | ||
| U5 | 1 | 3.4 | 5.9 |
| 3 | 13.2 | ||
| 5 | 29.9 | ||
| U6 | 1 | 2.7 | 4.8 |
| 3 | 10.1 | ||
| 5 | 22.0 | ||
| U7 | 1 | 2.1 | 3.8 |
| 3 | 9.9 | ||
| 5 | 18.6 | ||
| U8 | 1 | 1.6 | 2.8 |
| 3 | 8.1 | ||
| 5 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petković Papalazarou, S.; Nešković, J.; Ćorluka, S.; Polavder, S.; Mitrašinović, A.; Stjepanović, P. The Effects of Wind Velocity on the Binding Properties of Ash, Bottom Ash, and Additives: A Wind Tunnel Study. Minerals 2024, 14, 809. https://doi.org/10.3390/min14080809
Petković Papalazarou S, Nešković J, Ćorluka S, Polavder S, Mitrašinović A, Stjepanović P. The Effects of Wind Velocity on the Binding Properties of Ash, Bottom Ash, and Additives: A Wind Tunnel Study. Minerals. 2024; 14(8):809. https://doi.org/10.3390/min14080809
Chicago/Turabian StylePetković Papalazarou, Sandra, Jasmina Nešković, Stevan Ćorluka, Svetlana Polavder, Aleksandar Mitrašinović, and Pavle Stjepanović. 2024. "The Effects of Wind Velocity on the Binding Properties of Ash, Bottom Ash, and Additives: A Wind Tunnel Study" Minerals 14, no. 8: 809. https://doi.org/10.3390/min14080809
APA StylePetković Papalazarou, S., Nešković, J., Ćorluka, S., Polavder, S., Mitrašinović, A., & Stjepanović, P. (2024). The Effects of Wind Velocity on the Binding Properties of Ash, Bottom Ash, and Additives: A Wind Tunnel Study. Minerals, 14(8), 809. https://doi.org/10.3390/min14080809







