Gypsum on Mars: A Detailed View at Gale Crater
Abstract
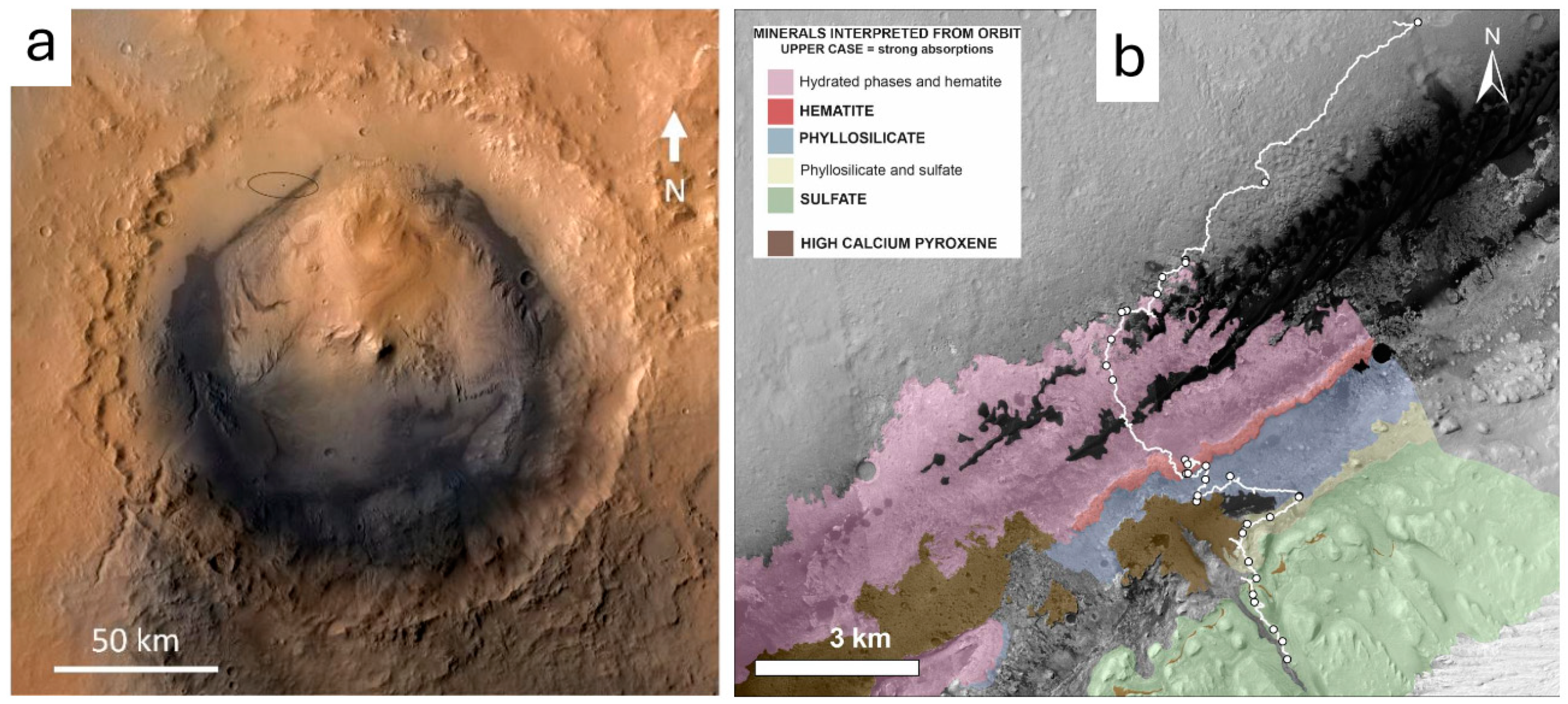
:1. Introduction
2. Methods
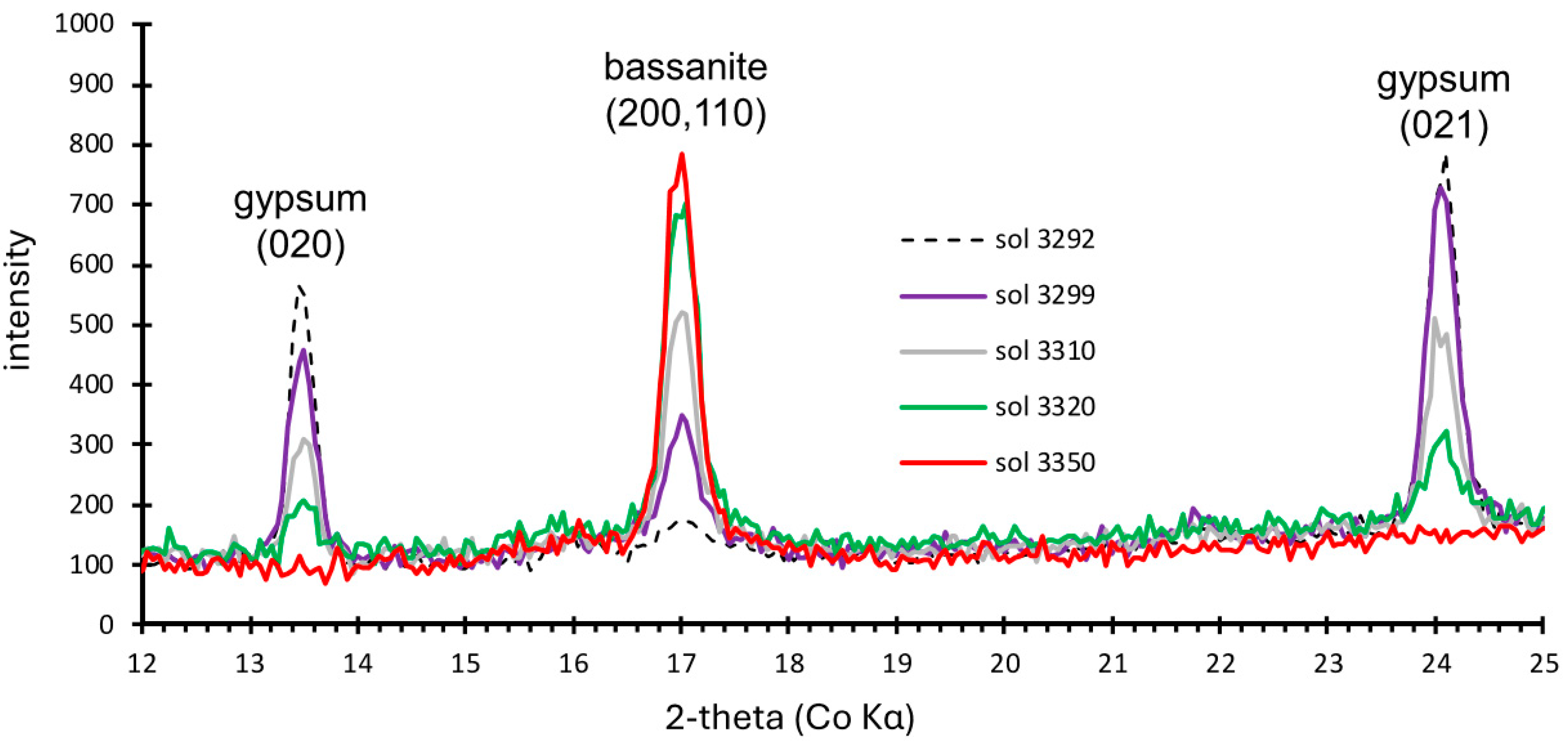
2.1. X-Ray Diffraction, with Evidence of Gypsum Dehydration to Bassanite
2.2. APXS
2.3. ChemCam
3. Discussion: Mineralogy in Stratigraphic Context at Gale Crater
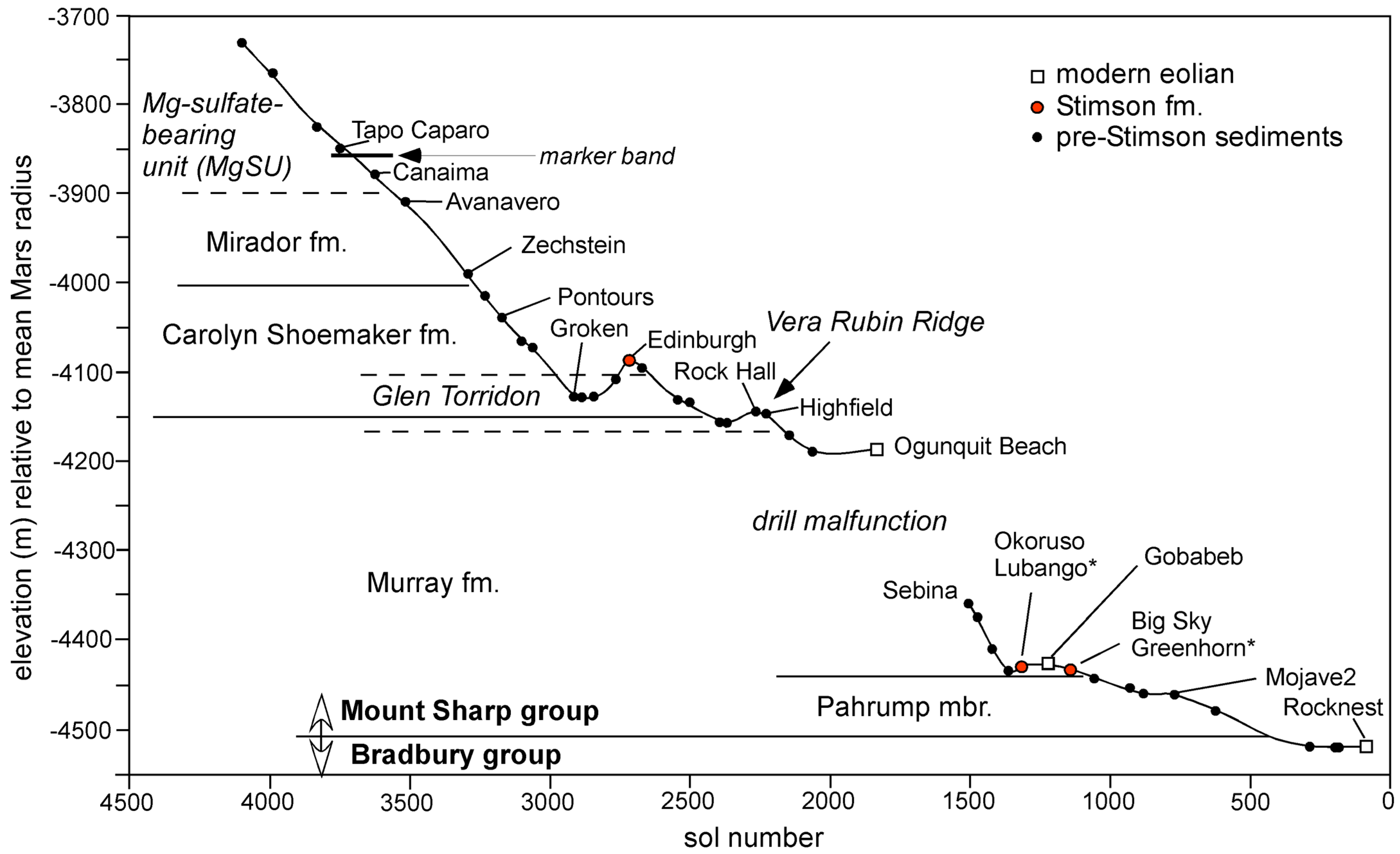
3.1. Stratigraphic Overview
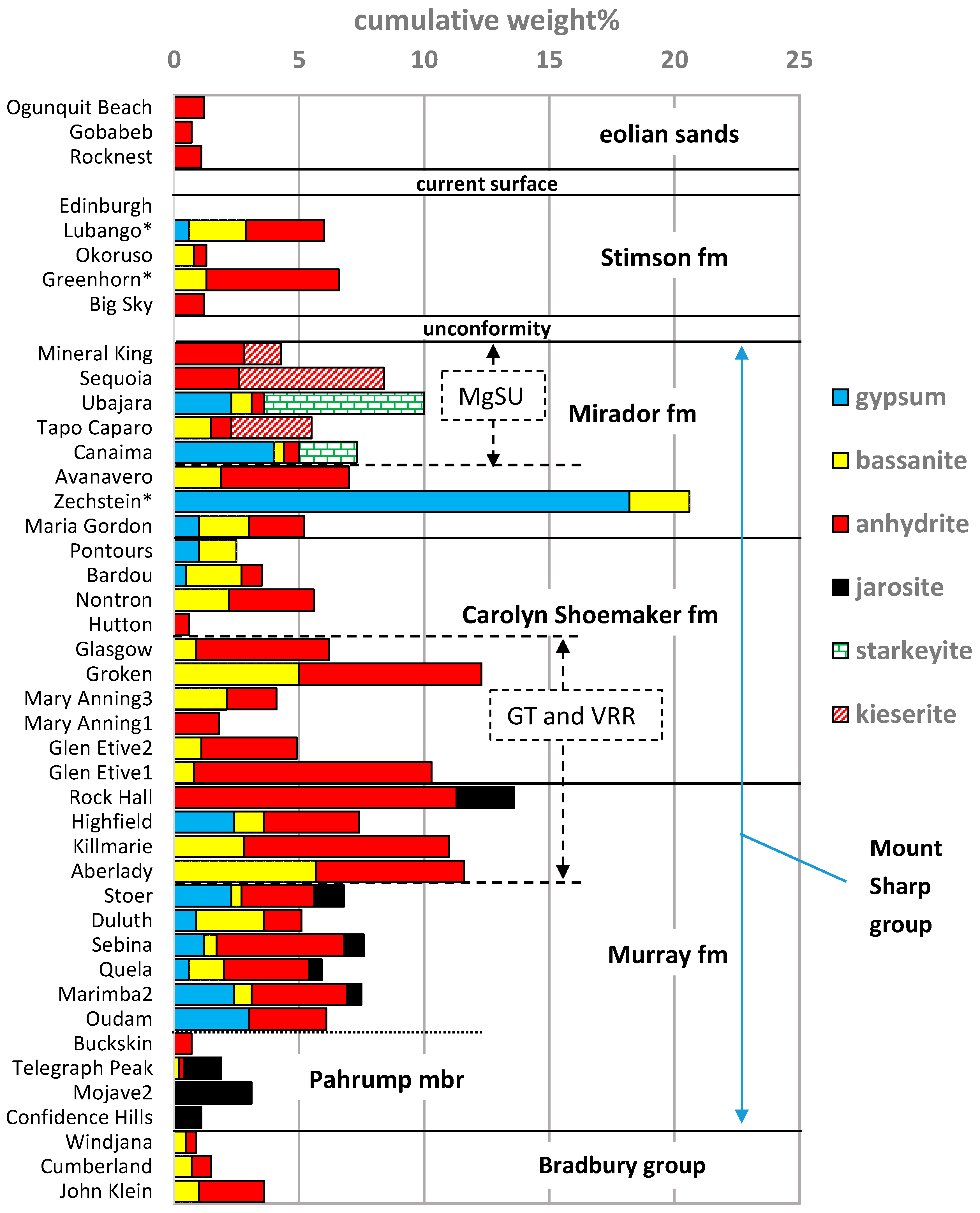
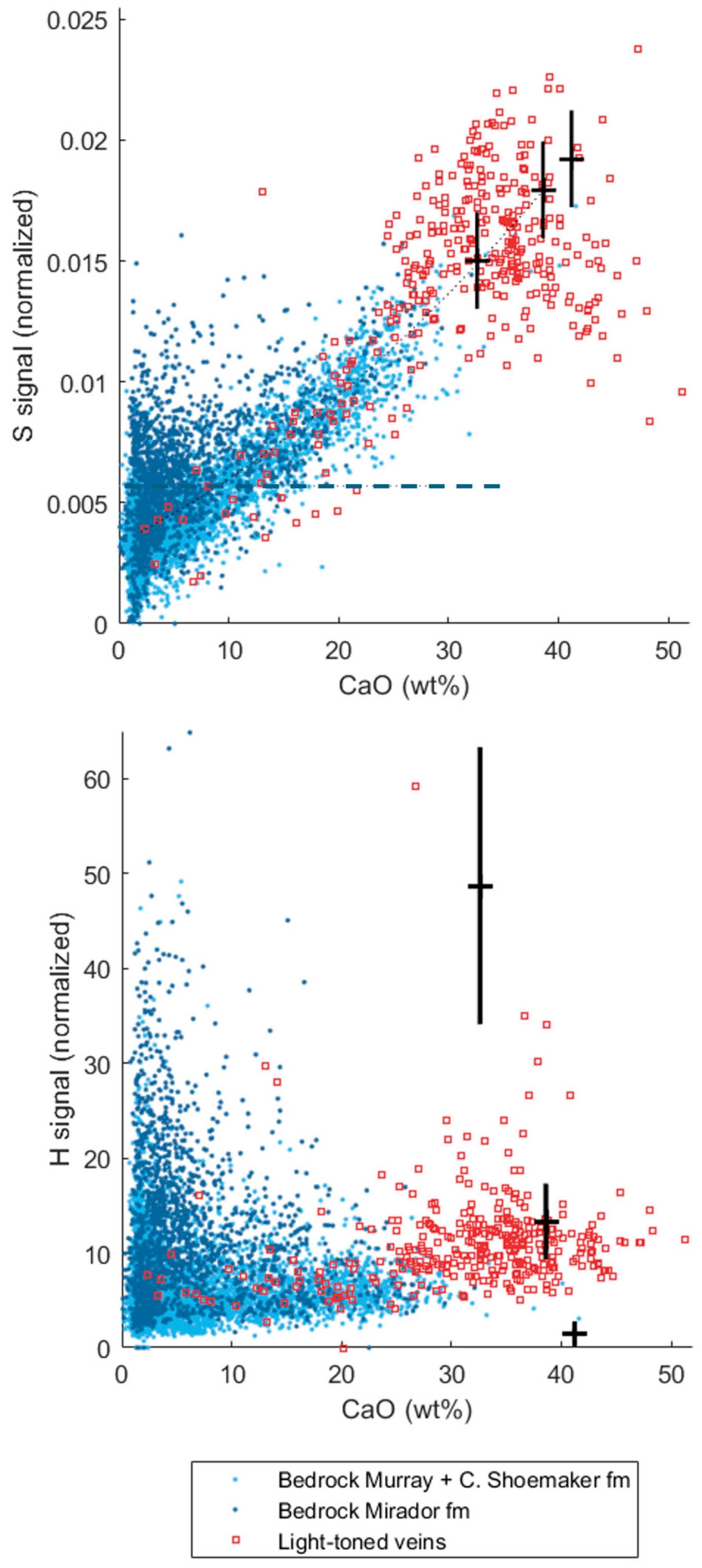
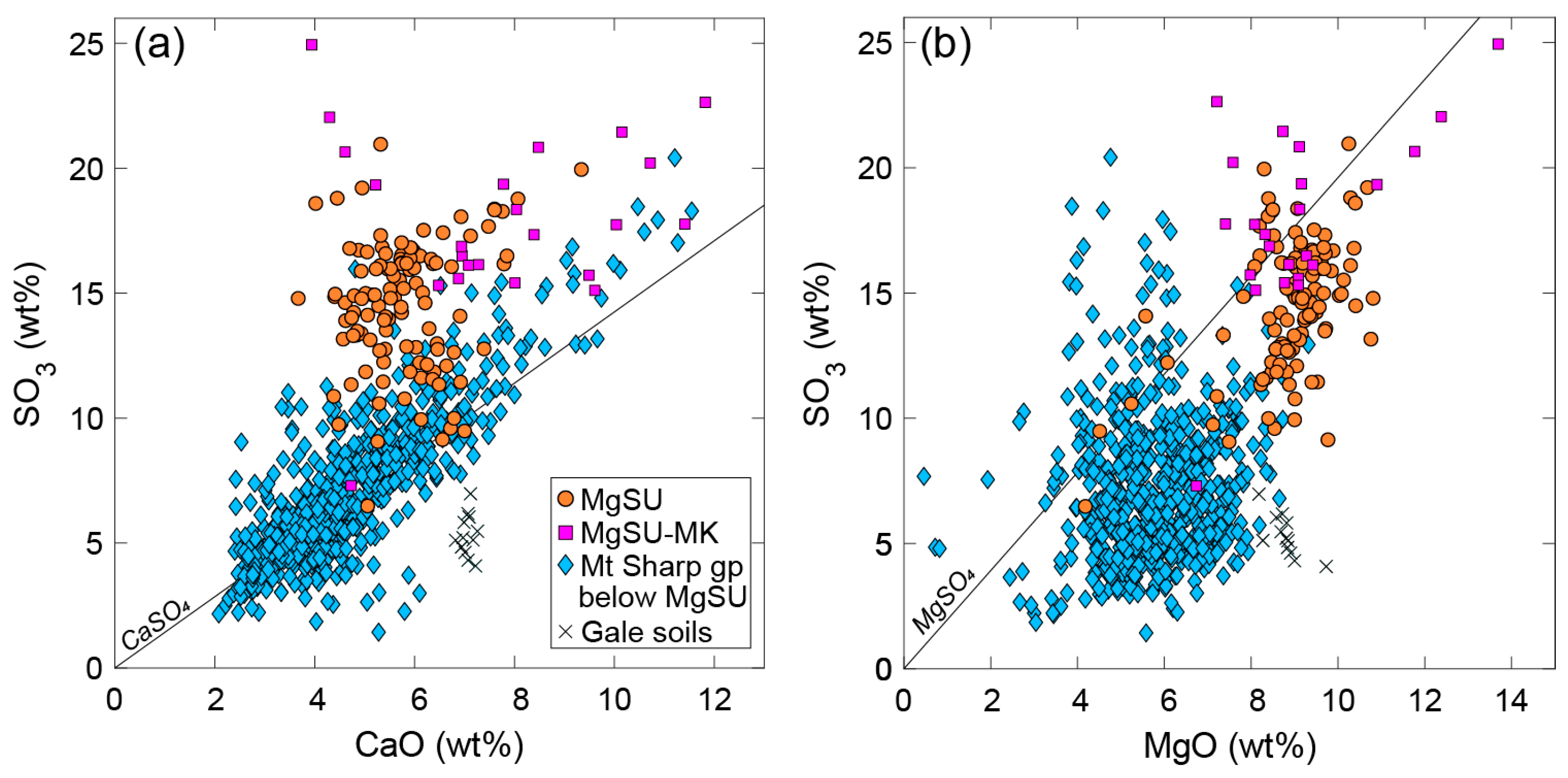
3.2. A Sulfate Mineral Tour through Gale Crater Strata
4. Broader Considerations
4.1. Gypsum and Other Ca-Sulfates at Gale Crater Are Persistent through Strata and Time
4.2. Gypsum Is at the Edge of Stability near the Martian Equator
4.3. Gypsum Is More Stable and More Abundant at Higher Latitudes
4.4. Gypsum and the Water Inventory of Mars
4.5. Gypsum and Associated Sulfate Origins at Gale Crater
4.6. Gypsum and the Future Exploration of Mars
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibring, J.-P.; Langevin, Y.; Gendrin, A.; Gondet, B.; Poulet, F.; Berthé, M.; Soufflot, A.; Arvidson, R.; Mangold, N.; Mustard, J.; et al. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 2005, 307, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Murchie, S.; Arvidson, R.; Bedini, P.; Beisser, K.; Bibring, J.-P.; Bishop, J.; Boldt, J.; Cavender, P.; Choo, T.; Clancy, R.T.; et al. Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) on Mars Reconnaissance Orbiter (MRO). J. Geophys. Res. Planets 2007, 112, E05S03. [Google Scholar] [CrossRef]
- Jamieson, C.S.; Noe Dobrea, E.Z.; Dalton III, J.B.; Pitman, K.M.; Abbey, W.J. The spectral variability of kieserite (MgSO4·H2O) with temperature and grain size and its application to the Martian surface. J. Geophys. Res. Planets 2014, 119, 1218–1237. [Google Scholar] [CrossRef]
- Wray, J.J.; Squyres, S.W.; Roach, L.H.; Bishop, J.; Mustard, J.F.; Noe Dobrea, E.Z. Identification of the Ca-sulfate bassanite in Mawrth Vallis, Mars. Icarus 2010, 209, 416–421. [Google Scholar] [CrossRef]
- Karunatillake, S.; Wray, J.J.; Gasnault, O.; McLennan, S.M.; Rogers, A.D.; Squyres, S.W.; Boynton, W.V.; Skok, J.R.; Ojha, L.; Olsen, N. Sulfates hydrating bulk soil in the Martian low and middle latitudes. Geophys. Res. Lett. 2014, 41, 7987–7996. [Google Scholar] [CrossRef]
- Milliken, R.E.; Grotzinger, J.P.; Thompson, B.J. Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophys. Res. Lett. 2010, 37, 4. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Milliken, R.E. The sedimentary rock record of Mars: Distribution, origins, and global stratigraphy. In Sedimentary Geology of Mars; Society for Sedimentary Geology (SEPM): Claremore, OK, USA, 2012; Special Pub. No. 102; pp. 1–48, Print ISBN 978-1-56576-312-8, CD/DVD ISBN 978-1-56576-313-5. [Google Scholar]
- Fraeman, A.A.; Ehlmann, B.L.; Arvidson, R.E.; Edwards, C.S.; Grotzinger, J.P.; Milliken, R.E.; Quinn, D.P.; Rice, M.S. The stratigraphy and evolution of lower Mount Sharp from spectral, morphological, and thermophysical orbital data sets. J. Geophys. Res. Planets 2016, 121, 1713–1736. [Google Scholar] [CrossRef] [PubMed]
- Chipera, S.J.; Vaniman, D.T.; Rampe, E.B.; Bristow, T.F.; Martínez, G.; Tu, V.M.; Peretyazhko, T.S.; Yen, A.S.; Gellert, R.; Berger, J.A.; et al. Mineralogical investigation of Mg-sulfate at the Canaima drill site, Gale crater, Mars. J. Geophys. Res. Planets 2023, 128, e2023JE008041. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Martínez, G.M.; Rampe, E.B.; Bristow, T.F.; Blake, D.F.; Yen, A.S.; Ming, D.W.; Rapin, W.; Meslin, P.-Y.; Morookian, J.M.; et al. Gypsum, bassanite, and anhydrite at Gale crater, Mars. Am. Min. 2018, 103, 1011–1020. [Google Scholar] [CrossRef]
- Blake, D.; Tu, V.; Bristow, T.; Rampe, E.; Vaniman, D.; Chipera, S.; Sarrazin, P.; Morris, R.; Morrison, S.; Yen, A.; et al. The Chemistry and Mineralogy (CheMin) X-ray diffractometer on the MSL Curiosity rover: A decade of mineralogy from Gale crater, Mars. Minerals 2024, 14, 568. [Google Scholar] [CrossRef]
- Morrison, S.M.; Downs, R.T.; Blake, D.F.; Vaniman, D.T.; Ming, D.W.; Hazen, R.M.; Treiman, A.H.; Achilles, C.N.; Yen, A.S.; Morris, R.V.; et al. Crystal chemistry of Martian minerals from Bradbury Landing through Naukluft Plateau, Gale crater, Mars. Am. Min. 2018, 103, 857–871. [Google Scholar] [CrossRef]
- Morrison, S.M.; Blake, D.F.; Bristow, T.F.; Castle, N.; Chipera, S.J.; Craig, P.I.; Downs, R.T.; Eleish, E.; Hazen, R.M.; Meusburger, J.M.; et al. Expanded insights into Martian mineralogy: Updated analysis of Gale crater’s mineral composition via CheMin crystal chemical investigations. Minerals 2024, 14, 773. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. FULLPAT: A full-pattern quantitative analysis program for X-ray powder diffraction using measured and calculated patterns. J. Appl. Cryst. 2002, 35, 744–749. [Google Scholar] [CrossRef]
- Anderson, R.C.; Jandura, L.; Okon, A.B.; Sunshine, D.; Roumeliotis, C.; Beegle, L.W.; Hurowitz, J.; Kennedy, B.; Limonadi, D.; McCloskey, S.; et al. Collecting samples in Gale crater, Mars; an overview of the Mars Science Laboratory Sample Acquisition, Sample Processing and Handling System. Space Sci. Rev. 2012, 170, 57–75. [Google Scholar] [CrossRef]
- Rapin, W.; Meslin, P.-Y.; Maurice, S.; Vaniman, D.; Nachon, M.; Mangold, N.; Schröder, S.; Gasnault, O.; Forni, O.; Wiens, R.C.; et al. Hydration state of calcium sulfates in Gale crater, Mars: Identification of bassanite veins. Earth Planet. Sci. Lett. 2016, 452, 197–205. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Chipera, S.J.; Martínez, G.; Rapin, W.; Rampe, E.B.; Bristow, T.; Blake, D.F.; Meusberger, J.; Ming, D.W.; Downs, R.T.; et al. Near-surface dehydration of salt hydrates at Gale crater, Mars. In Proceedings of the 55th Lunar and Planetary Science Conference (LPSC 2024), Houston, TX, USA, 11–15 March 2024; Volume 55. abst. no. 1327. [Google Scholar]
- Gellert, R.; Rieder, R.; Brückner, J.; Clark, B.C.; Dreibus, G.; Klingelhöfer, G.; Lugmair, G.; Ming, D.W.; Wänke, H.; Yen, A.; et al. Alpha Particle X-Ray Spectrometer (APXS): Results from Gusev crater and calibration report. J. Geophys. Res. 2006, 111, E02S05. [Google Scholar] [CrossRef]
- Campbell, J.L.; Perrett, G.M.; Gellert, R.; Andrushenko, S.M.; Boyd, N.I.; Maxwell, J.A.; King, P.L.; Schofield, C.D. Calibration of the Mars Science Laboratory Alpha Particle X-ray Spectrometer. Space Sci. Rev. 2012, 170, 319–340. [Google Scholar] [CrossRef]
- Maurice, S.; Clegg, S.M.; Wiens, R.C.; Gasnault, O.; Rapin, W.; Forni, O. ChemCam activities and discoveries during the nominal mission of the Mars Science Laboratory in Gale crater, Mars. J. Anal. At. Spectrom. 2016, 31, 863–889. [Google Scholar] [CrossRef]
- Clegg, S.M.; Wiens, R.C.; Anderson, R.; Forni, O.; Frydenvang, J.; Lasue, J.; Maurice, S. Recalibration of the Mars Science Laboratory ChemCam instrument with an expanded geochemical database. Spectrochim. Acta Part B At. Spectrosc. 2017, 129, 64–85. [Google Scholar] [CrossRef]
- Rapin, W.; Meslin, P.-Y.; Maurice, S.; Wiens, R.C.; Laporte, D.; Chauviré, B.; Thomas, N.H. Quantification of water content by laser induced breakdown spectroscopy on Mars. Spectrochim. Acta Part B At. Spectrosc. 2017, 130, 82–100. [Google Scholar] [CrossRef]
- Rapin, W.; Ehlmann, B.L.; Dromart, G.; Schieber, J.; Thomas, N.H.; Fischer, W.W.; Vasavada, A.R. An interval of high salinity in ancient Gale crater lake on Mars. Nat. Geosci. 2019, 12, 889–895. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Sumner, D.Y.; Kah, L.C.; Stack, K.; Gupta, S.; Edgar, L.; Rubin, D.; Lewis, K.; Schieber, J.; Mangold, N.; et al. A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale crater, Mars. Science 2014, 343, 1242777. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Bish, D.L.; Ming, D.W.; Bristow, T.F.; Morris, R.V.; Blake, D.F.; Chipera, S.J.; Morrison, S.M.; Treiman, A.H.; Rampe, E.B.; et al. Mineralogy of a mudstone at Gale crater, Yellowknife Bay, Mars. Science 2014, 343, 1243480. [Google Scholar] [CrossRef] [PubMed]
- Bish, D.L.; Blake, D.F.; Vaniman, D.T.; Chipera, S.J.; Morris, R.V.; Ming, D.W.; Treiman, A.H.; Sarrazin, P.; Morrison, S.M.; Downs, R.T.; et al. X-ray diffraction results from Mars Science Laboratory: Mineralogy of Rocknest at Gale crater. Science 2013, 341, 1238932. [Google Scholar] [CrossRef] [PubMed]
- Achilles, C.N.; Downs, R.T.; Ming, D.W.; Rampe, E.B.; Morris, R.V.; Treiman, A.H.; Morrison, S.M.; Blake, D.F.; Vaniman, D.T.; Ewing, R.C.; et al. Mineralogy of an active eolian sediment from the Namib dune, Gale crater, Mars. J. Geophys. Res. Planets 2017, 122, 2344–2361. [Google Scholar] [CrossRef]
- Rampe, E.B.; Lapotre, M.G.A.; Bristow, T.F.; Arvidson, R.E.; Morris, R.V.; Achilles, C.N.; Weitz, C.; Blake, D.F.; Ming, D.W.; Morrison, S.M.; et al. Sand mineralogy within the Bagnold Dunes, Gale crater, as observed in situ and from orbit. Geophys. Res. Lett. 2018, 45, 9488–9497. [Google Scholar] [CrossRef]
- Treiman, A.H.; Bish, D.L.; Vaniman, D.T.; Chipera, S.J.; Blake, D.F.; Ming, D.W.; Morris, R.V.; Bristow, T.F.; Morrison, S.M.; Baker, M.B.; et al. Mineralogy, provenance, and diagenesis of a potassic basaltic sandstone on Mars: CheMin X-ray diffraction of the Windjana sample (Kimberley area, Gale crater). J. Geophys. Res. Planets 2016, 121, 75–106. [Google Scholar] [CrossRef] [PubMed]
- L’Haridon, J.; Mangold, N.; Meslin, P.-Y.; Johnson, J.R.; Rapin, W.; Forni, O.; Wiens, R.C. Chemical variability in mineralized veins observed by ChemCam on the lower slopes of Mount Sharp in Gale crater, Mars. Icarus 2018, 311, 69–86. [Google Scholar] [CrossRef]
- Berger, J.A.; Gellert, R.; McCraig, M.A.; O’Connell-Cooper, C.D.; Spray, J.G.; Thompson, L.M.; VanBommel, S.J.; Yen, A.S.; Rampe, E.B.; Clark, J.V. Isochemical Characteristics of the Clay-Sulfate Transition in Gale Crater, Mars: APXS Results from Mont Mercou to the Marker Band Valley. In Proceedings of the 54th Lunar and Planetary Science Conference (LPSC 2023), Houston, TX, USA, 13–17 March 2023; Volume 54. abst. no. 1662. [Google Scholar]
- Rampe, E.B.; Ming, D.W.; Blake, D.F.; Bristow, T.F.; Chipera, S.J.; Grotzinger, J.P.; Morris, R.V.; Morrison, S.M.; Vaniman, D.T.; Yen, A.S.; et al. Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 172–185. [Google Scholar] [CrossRef]
- Kah, L.C.; Stack, K.M.; Eigenbrode, J.L.; Yingst, R.A.; Edgett, K.S. Syndepositional precipitation of calcium sulfate in Gale Crater, Mars. Terra Nova 2018, 30, 431–439. [Google Scholar] [CrossRef]
- Martin, P.E.; Farley, K.A.; Baker, M.B.; Malespin, C.A.; Schwenzer, S.P.; Cohen, B.A.; Mahaffy, P.R.; McAdam, A.C.; Ming, D.W.; Vasconcelos, P.M.; et al. A two-Step K-Ar experiment on Mars: Dating the diagenetic formation of Jarosite from Amazonian groundwaters. J. Geophys. Res. Planets 2017, 122, 2803–2818. [Google Scholar] [CrossRef]
- Le Deit, L.; Hauber, E.; Fueten, F.; Pondrelli, M.; Rossi, A.P.; Jaumann, R. Sequence of infilling events in Gale Crater, Mars: Results from morphology, stratigraphy, and mineralogy. J. Geophys. Res. Planets 2013, 118, 2439–2773. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Gupta, S.; Malin, M.C.; Rubin, D.M.; Schieber, J.; Siebach, K.; Sumner, D.Y.; Stack, K.M.; Vasavada, A.R.; Arvidson, R.E.; et al. Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science 2015, 350, aac7575. [Google Scholar] [CrossRef] [PubMed]
- Gellert, R.; Berger, J.A.; Boyd, N.; Campbell, J.L.; Desouza, E.D.; Elliott, B.; Fisk, M.; Pavri, B.; Perrett, G.M.; Schmidt, M.; et al. Chemical evidence for an aqueous history at Pahrump, Gale crater, Mars, as seen by the APXS. In Proceedings of the 46th Lunar and Planetary Science Conference (LPSC 2015), Houston, TX, USA, 16–20 March 2015; Volume 46. abst. no. 1855. [Google Scholar]
- VanBommel, S.J.; Gellert, R.; Berger, J.A.; Campbell, J.L.; Thompson, L.M.; Edgett, K.S.; McBride, M.J.; Minitti, M.E.; Pradlera, I.; Boyd, N.I. Deconvolution of distinct lithology chemistry through oversampling with the Mars Science Laboratory Alpha Particle X-Ray Spectrometer. X-ray Spectrom. 2016, 45, 155–161. [Google Scholar] [CrossRef]
- Schieber, J.; Bohacs, K.M.; Coleman, M.; Bish, D.; Reed, M.H.; Thompson, L.; Rapin, W.; Zalami, Y. Mars is a mirror—Understanding the Pahrump Hills mudstones from a perspective of Earth analogues. Sedimentology 2022, 69, 2371–2435. [Google Scholar] [CrossRef]
- Frydenvang, J.; Gasda, P.J.; Hurowitz, J.A.; Grotzinger, J.P.; Wiens, R.C.; Newsom, H.E.; Edgett, K.S.; Watkins, J.; Bridges, J.C.; Maurice, S.; et al. Diagenetic silica enrichment and late-stage groundwater activity in Gale crater, Mars. Geophys. Res. Lett. 2017, 44, 4716–4724. [Google Scholar] [CrossRef]
- Morris, R.V.; Vaniman, D.T.; Blake, D.F.; Gellert, R.; Chipera, S.J.; Rampe, E.B.; Ming, D.W.; Morrison, S.M.; Downs, R.T.; Treiman, A.H.; et al. Silicic volcanism on Mars evidenced by tridymite in high-SiO2 sedimentary rock at Gale crater. Proc. Nat. Acad. Sci. USA 2016, 113, 7071–7076. [Google Scholar] [CrossRef] [PubMed]
- Payré, V.; Siebach, K.L.; Thorpe, M.T.; Antoshechkina, P.; Rampe, E.B. Tridymite in a lacustrine mudstone in Gale crater, Mars: Evidence for an explosive silicic eruption during the Hesperian. Earth Planetary Sci. Lett. 2022, 594, 117694. [Google Scholar] [CrossRef]
- Yen, A.S.; Morris, R.V.; Ming, D.W.; Schwenzer, S.P.; Sutter, B.; Vaniman, D.T.; Treiman, A.H.; Gellert, R.; Achilles, C.N.; Berger, J.A.; et al. Formation of tridymite and evidence for a hydrothermal history at Gale crater, Mars. J. Geophys. Res. Planets 2021, 126, e2020JE006569. [Google Scholar] [CrossRef]
- Fraeman, A.A.; Edgar, L.A.; Rampe, E.B.; Thompson, L.M.; Frydenvang, J.; Fedo, C.M.; Catalano, J.G.; Dietrich, W.E.; Gabriel, T.S.J.; Vasavada, A.R.; et al. Evidence for a diagenetic origin of Vera Rubin Ridge, Gale crater, Mars: Summary and synthesis of Curiosity’s exploration campaign. J. Geophys. Res. Planets 2020, 125, e2020JE006527. [Google Scholar] [CrossRef] [PubMed]
- Rampe, E.B.; Bristow, T.F.; Morris, R.V.; Morrison, S.M.; Achilles, C.N.; Ming, D.W.; Vaniman, D.T.; Blake, D.F.; Tu, V.M.; Chipera, S.J.; et al. Mineralogy of Vera Rubin Ridge from the Mars Science Laboratory CheMin instrument. J. Geophys. Res. Planets 2020, 125, e2019JE006306. [Google Scholar] [CrossRef]
- Thorpe, M.T.; Bristow, T.F.; Rampe, E.B.; Tosca, N.J.; Grotzinger, J.P.; Bennett, K.A.; Achilles, C.N.; Blake, D.F.; Chipera, S.J.; Downs, G.; et al. Mars Science Laboratory CheMin data from the Glen Torridon region and the significance of lake-groundwater interactions in interpreting mineralogy and sedimentary history. J. Geophys. Res. Planets 2022, 127, e2021JE007099. [Google Scholar] [CrossRef]
- L’Haridon, J.; Mangold, N.; Fraeman, A.A.; Johnson, J.R.; Cousin, A.; Rapin, W.; Wiens, R.C. Iron mobility during diagenesis at Vera Rubin Ridge, Gale crater, Mars. J. Geophys. Res. Planets 2020, 125, e2019JE006299. [Google Scholar] [CrossRef]
- Gasda, P.J.; Comellas, J.; Essunfeld, A.; Das, D.; Bryk, A.B.; Dehouck, E.; Schwenzer, S.P.; Crossey, L.; Herkenhoff, K.; Johnson, J.R.; et al. Overview of the morphology and chemistry of diagenetic features in the clay-rich Glen Torridon unit of Gale crater, Mars. J. Geophys. Res. Planets 2022, 127, e2021JE007097. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Chipera, S.J. Transformations of Mg- and Ca-sulfate hydrates in Mars regolith. Am. Min. 2006, 91, 1628–1642. [Google Scholar] [CrossRef]
- Turner, S.M.R.; Schwenzer, S.P.; Bridges, J.C.; Rampe, E.B.; Bedford, C.C.; Achilles, C.N. Early diagenesis at and below Vera Rubin Ridge, Gale crater, Mars. Meteoritics Planet. Sci. 2021, 56, 1905–1932. [Google Scholar] [CrossRef]
- Mees, F.; De Dapper, M. Vertical variations in bassanite distribution patterns in near-surface sediments, southern Egypt. Sed. Geol. 2005, 181, 225–229. [Google Scholar] [CrossRef]
- Rapin, W.; Dromart, G.; Clark, B.C.; Schieber, J.; Kite, E.S.; Kah, L.C.; Thompson, L.M.; Gasnault, O.; Lasue, J.; Meslin, P.-Y.; et al. Sustained wet–dry cycling on early Mars. Nature 2023, 620, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Weitz, C.M.; Lewis, K.W.; Kite, W.S.; Dietrich, W.E.; Thompson, L.M.; O’Connell-Cooper, C.D.; Schieber, J.; Rubin, D.; Gasda, P.; Mondro, C.; et al. The Marker Band in Gale crater: A synthesis of orbital and ground observations. In Proceedings of the 54th Lunar and Planetary Science Conference (LPSC 2023), Houston, TX, USA, 13–17 March 2023; Volume 54. abst. no. 1560. [Google Scholar]
- Weitz, C.M.; Lewis, K.W.; Bishop, J.L.; Thomson, B.J.; Arvidson, R.E.; Grant, J.A.; Seelos, K.D.; Ettenborough, I. Orbital observations of a marker horizon at Gale crater. J. Geophys. Res. Planets 2022, 127, e2022JE007211. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Hausrath, E.M.; Kite, E.S.; Rampe, E.B.; Bristow, T.F.; Downs, R.T.; Treiman, A.; Peretyazhko, T.S.; Thorpe, M.T.; Grotzinger, J.P.; et al. In situ evidence of an active carbon cycle on ancient Mars. AGU Fall Meet. Abstr. 2023, 2023, P43A-06. [Google Scholar]
- Moiola, R.J.; Glover, E.D. Recent anhydrite from Clayton playa, Nevada. Am. Min. 1965, 50, 2063–2069. [Google Scholar]
- Yen, A.S.; Ming, D.W.; Vaniman, D.T.; Gellert, R.; Blake, D.F.; Morris, R.V.; Morrison, S.M.; Bristow, T.F.; Chipera, S.J.; Edgett, K.S.; et al. Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Siljeström, S.; Czaja, A.D.; Corpolongo, A.; Berger, E.L.; Li, A.Y.; Cardarelli, E.; Abbey, W.; Asher, S.A.; Beegle, L.W.; Benison, K.C.; et al. Evidence of sulfate-rich fluid alteration in Jezero crater floor, Mars. J. Geophys. Res. Planets 2024, 129, e2023JE007989. [Google Scholar] [CrossRef]
- Lopez-Reyes, G.; Nachon, M.; Veneranda, M.; Beyssac, O.; Madariaga, J.M.; Manrique, J.A.; Clavé, E.; Ollila, A.; Castro, K.; Sharma, S.K.; et al. Anhydrite detections by Raman spectroscopy with SuperCam at the Jezero delta, Mars. In Proceedings of the 55th Lunar and Planetary Science Conference (LPSC 2024), Houston, TX, USA, 11–15 March 2024; Volume 55. abst. no. 1721. [Google Scholar]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.F.; Arvidson, R.; et al. Sulfates in Martian layered terrains: The OMEGA/Mars Express view. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Squyres, S.W.; Arvidson, R.E.; Bell, J.F., III; Calef, F., III; Clark, B.C.; Cohen, B.A.; Crumpler, L.A.; de Souza, P.A., Jr.; Farrand, W.H.; Gellert, R.; et al. Ancient impact and aqueous processes at Endeavour crater, Mars. Science 2012, 336, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kounaves, S.P.; Hecht, M.H.; Kapit, J.; Quinn, R.C.; Catling, D.C.; Clark, B.C.; Ming, D.W.; Gospodinova, K.; Hredzak, P.; McElhoney, K.; et al. Soluble sulfate in the Martian soil at the Phoenix landing site. Geophys. Res. Lett. 2010, 37, L09201. [Google Scholar] [CrossRef]
- Fishbaugh, K.E.; Poulet, F.; Chevrier, V.; Langevin, Y.; Bibring, J.-P. On the origin of gypsum in the Mars north polar region. J. Geophys. Res. 2007, 112, E07002. [Google Scholar] [CrossRef]
- Massé, M.; Bourgeois, O.; Le Mouélic, S.; Verpoorter, C.; Spiga, A.; Le Deit, L. Wide distribution and glacial origin of polar gypsum on Mars. Earth Planet. Sci. Lett. 2012, 317–318, 44–55. [Google Scholar] [CrossRef]
- Parente, M.; Bishop, J.L.; Saranathan, A.M.; Szynkiewicz, A.; Fenton, L. Detection of bassanite in the north polar dunes of Mars and implications for aqueous activity. In Proceedings of the 53rd Lunar and Planetary Science Conference (LPSC 2022), Houston, TX, USA, 7–11 March 2022; Volume 53. abst. no. 2342. [Google Scholar]
- Szynkiewicz, A.; Bishop, J.L.; Fenton, L.; Parente, M. Evidence for groundwater activity and sulfate origin in the basal unit and Olympia Undae dunes on Mars. In Proceedings of the 53rd Lunar and Planetary Science Conference (LPSC 2022), Houston, TX, USA, 7–11 March 2022; Volume 53. abst. no. 1615. [Google Scholar]
- Cardenas, B.T.; Lamb, M.P. Paleogeographic reconstructions of an ocean margin on Mars based on deltaic sedimentology at Aeolis Dorsa. J. Geophys. Res. Planets 2022, 127, e2022JE007390. [Google Scholar] [CrossRef]
- Feldman, W.C.; Pathare, A.; Maurice, S.; Prettyman, T.H.; Lawrence, D.J.; Milliken, R.E.; Travis, B.J. Mars Odyssey neutron data: 2. Search for buried excess water ice deposits at nonpolar latitudes on Mars. J. Geophys. Res. Planets 2011, 116, E11009. [Google Scholar] [CrossRef]
- Feldman, W.C.; Bourke, M.C.; Elphic, R.C.; Maurice, S.; Prettyman, T.H.; Lawrence, D.J.; Hagerty, J.J. Constraints on the structure and composition of sand dunes within Olympia Undae using Mars Odyssey neutron spectrometer data. In Proceedings of the 38th Lunar and Planetary Science Conference (LPSC 2007), Houston, TX, USA, 12–16 March 2007; Volume 38. abst. no. 2311. [Google Scholar]
- Martín-Torres, F.J.; Zorzano, M.-P.; Valentín-Serrano, P.; Harri, A.-M.; Genzer, M.; Kemppinen, O.; Rivera-Valentin, E.-G.; Jun, I.; Wray, J.; Madsen, M.B.; et al. Transient liquid water and water activity at Gale crater on Mars. Nat. Geosci. 2015, 8, 357–361. [Google Scholar] [CrossRef]
- Bish, D.L.; Carey, J.W.; Vaniman, D.T.; Chipera, S.J. Stability of hydrous minerals on the Martian surface. Icarus 2003, 164, 96–103. [Google Scholar] [CrossRef]
- Siebach, K.L.; Grotzinger, J.P. Volumetric estimates of ancient water on Mount Sharp based on boxwork deposits, Gale crater, Mars. J. Geophys. Res. Planets 2014, 119, 189–198. [Google Scholar] [CrossRef]
- Sutter, B.; McAdam, A.C.; Mahaffy, P.R.; Ming, D.W.; Edgett, K.S.; Rampe, E.B.; Eigenbrode, J.L.; Franz, H.B.; Freissinet, C.; Grotzinger, J.P.; et al. Evolved gas analyses of sedimentary rocks and eolian sediment in Gale crater, Mars: Results of the Curiosity rover’s sample analysis at Mars instrument from Yellowknife Bay to the Namib Dune. J. Geophys. Res. Planets 2017, 122, 2574–2609. [Google Scholar] [CrossRef]
- Czarnecki, S.; Hardgrove, C.; Gasda, P.J.; Gabriel, T.S.J.; Starr, M.; Rice, M.S.; Frydenvang, J.; Wiens, R.C.; Rapin, W.; Nikiforov, S. Identification and description of a silicic volcaniclastic layer in Gale crater, Mars, using active neutron interrogation. J. Geophys. Res. Planets 2020, 125, e2019JE006180. [Google Scholar] [CrossRef]
- King, P.L.; McLennan, S.M. Sulfur on Mars. Elements 2010, 6, 107–112. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the Martian surface. Ann. Rev. Earth Planet. Sci. 2014, 42, 291–315. [Google Scholar] [CrossRef]
- Franz, H.B.; McAdam, A.C.; Ming, D.W.; Freissinet, C.; Mahaffy, P.R.; Eldridge, D.L.; Fischer, W.W.; Grotzinger, J.P.; House, C.H.; Hurowitz, J.A.; et al. Large sulfur isotope fractionations in Martian sediments at Gale crater. Nat. Geosci. 2017, 10, 658–663. [Google Scholar] [CrossRef]
- Bristow, T.F.; Grotzinger, J.P.; Rampe, E.B.; Cuadros, J.; Chipera, S.J.; Downs, G.W.; Fedo, C.M.; Frydenvang, J.; McAdam, A.C.; Morris, R.V.; et al. Brine-driven destruction of clay minerals in Gale crater, Mars. Science 2021, 373, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.H.; Grotzinger, J.P. Diagenesis of the clay-sulfate stratigraphic transition, Mount Sharp group, Gale crater, Mars. J. Geophys. Res. Planets. in press.
- Martin, P.E.; Farley, K.A.; Malespin, C.A.; Mahaffy, P.R.; Edgett, K.S.; Gupta, S.; Dietrich, W.E.; Malin, M.C.; Stack, K.M.; Vasconcelos, P.M. Billion-year exposure ages in Gale crater (Mars) indicate Mount Sharp formed before the Amazonian period. Earth Planet. Sci. Lett. 2021, 554, 116667. [Google Scholar] [CrossRef]
- Schwenzer, S.P.; Abramov, O.; Allen, C.C.; Bridges, J.C.; Clifford, S.M.; Filiberto, J.; Kring, D.A.; Lasue, J.; McGovern, P.J.; Newsom, H.E.; et al. Gale crater: Formation and post-impact hydrous environments. Planet. Space Sci. 2012, 70, 84–95. [Google Scholar] [CrossRef]
- Knoll, A.H.; Grotzinger, J. Water on Mars and the prospect of Martian life. Elements 2015, 2, 169–173. [Google Scholar] [CrossRef]
- Benison, K.C.; Karmanocky, F.J., III. Could microorganisms be preserved in Mars gypsum? Insights from terrestrial examples. Geology 2014, 42, 615–618. [Google Scholar] [CrossRef]
- Parnell, J.; Lee, P.; Cockell, C.S.; Osinski, G.R. Microbial colonization in impact-generated hydrothermal sulphate deposits, Haughton impact structure, and implications for sulphates on Mars. Int. J. Astrobiol. 2004, 3, 247–256. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaniman, D.; Chipera, S.; Rampe, E.; Bristow, T.; Blake, D.; Meusburger, J.; Peretyazhko, T.; Rapin, W.; Berger, J.; Ming, D.; et al. Gypsum on Mars: A Detailed View at Gale Crater. Minerals 2024, 14, 815. https://doi.org/10.3390/min14080815
Vaniman D, Chipera S, Rampe E, Bristow T, Blake D, Meusburger J, Peretyazhko T, Rapin W, Berger J, Ming D, et al. Gypsum on Mars: A Detailed View at Gale Crater. Minerals. 2024; 14(8):815. https://doi.org/10.3390/min14080815
Chicago/Turabian StyleVaniman, David, Steve Chipera, Elizabeth Rampe, Thomas Bristow, David Blake, Johannes Meusburger, Tanya Peretyazhko, William Rapin, Jeff Berger, Douglas Ming, and et al. 2024. "Gypsum on Mars: A Detailed View at Gale Crater" Minerals 14, no. 8: 815. https://doi.org/10.3390/min14080815





