Alteration and Non-Formula Elements Uptake of Zircon from Um Ara Granite, South Eastern Desert, Egypt
Abstract
:1. Introduction
2. Geological and Mineralogical Background
3. Sample Preparation and Analytical Procedures
4. Results
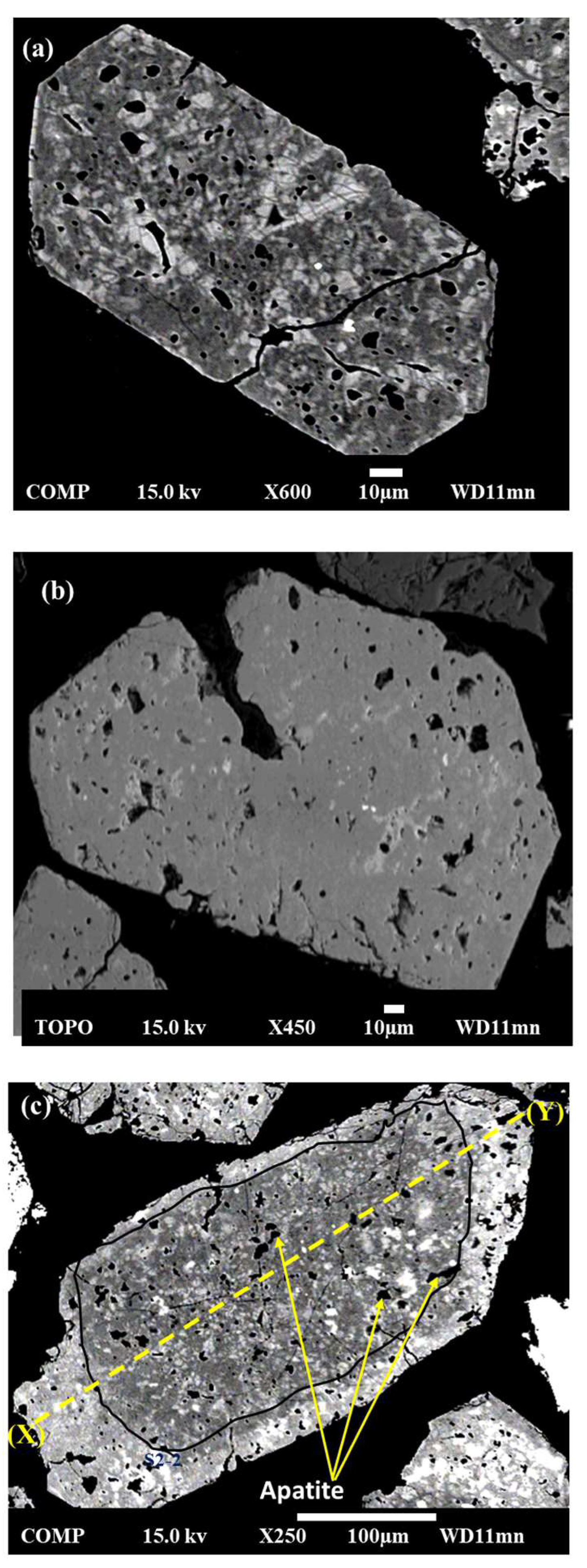
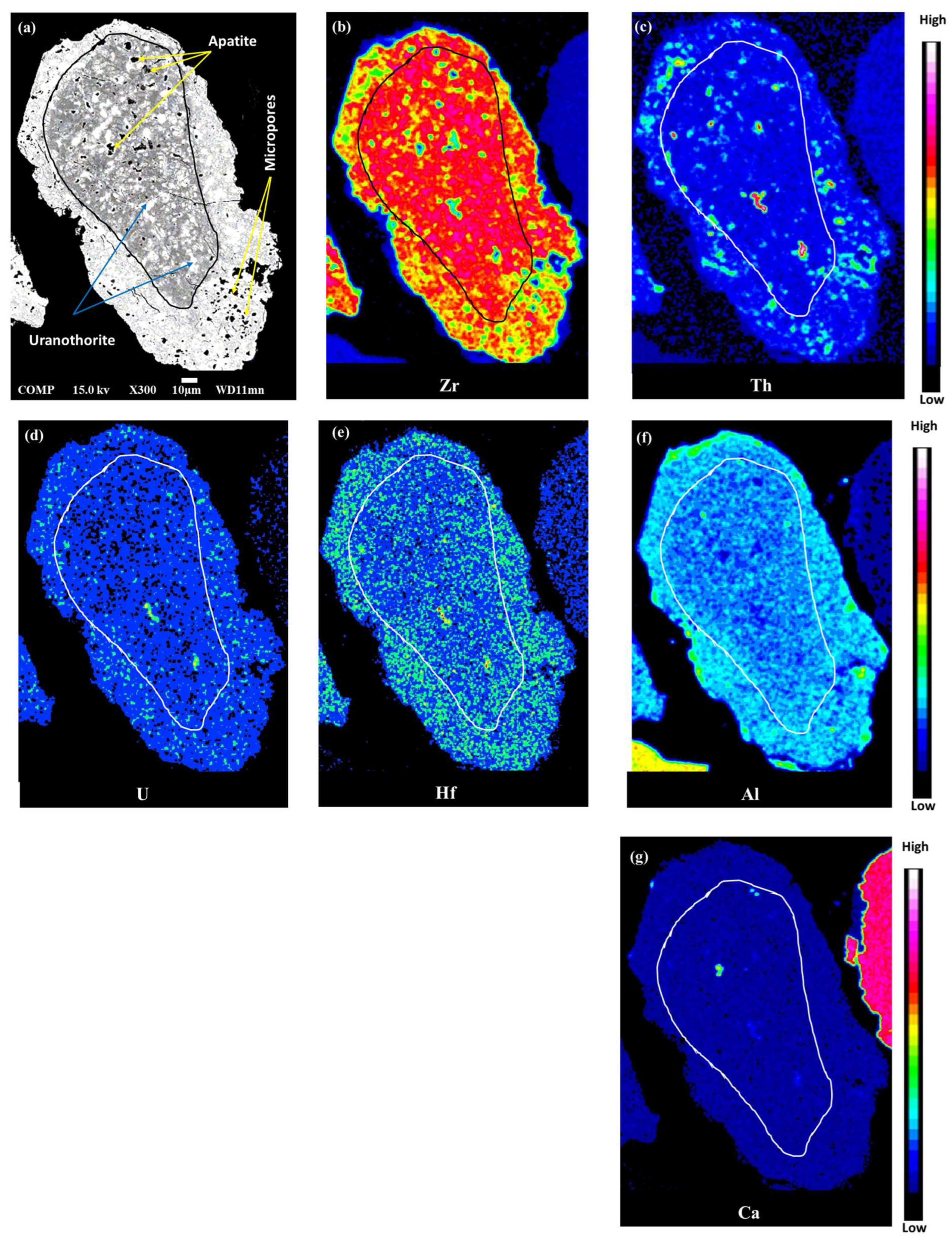
4.1. Textural Observations
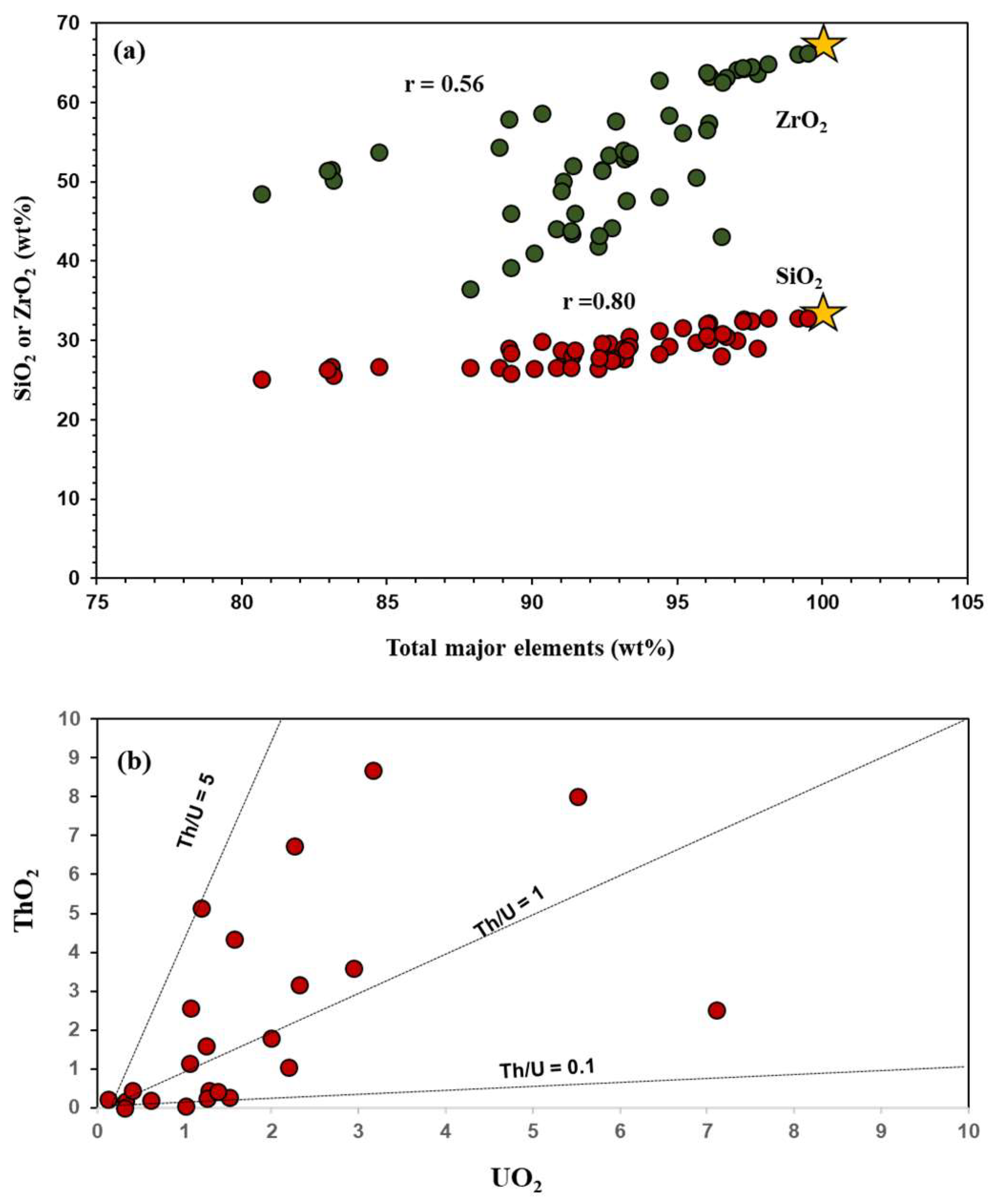
4.2. Major and Trace Elements Composition
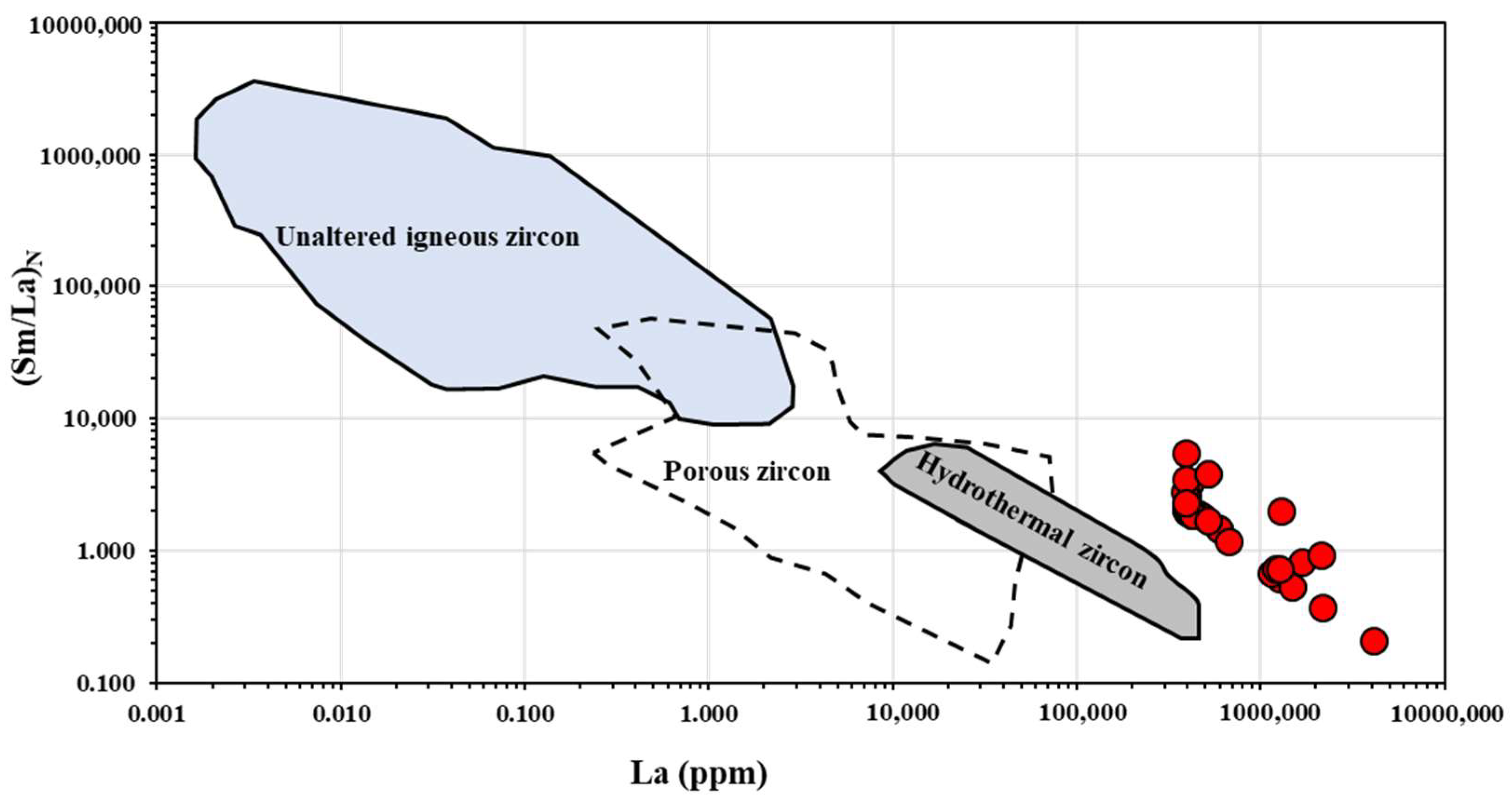
4.3. REE Pattern of Zircon
5. Discussion
5.1. Zircon Alteration
5.2. Timing of ‘Non-Formula’ Element Uptake in Um Ara Zircon
- (i)
- An early stage of hydrothermal activity took place between 603 and 530 Ma ago. The granitic rocks in the Um Ara area formed during this period, following intense mountain-building. Zircon crystals extracted from these granites have been dated to 603 ± 14 Ma, confirming this timeframe [16]. The intrusion of these granites seems to be influenced by deep shear zones and faulting in the Earth’s crust. These shear zones acted as pathways for rare metal-bearing hydrothermal fluids. The granites contain various accessory minerals, like columbite, ilmenite, zircon, xenotime, thorite, monazite, and apatite. These minerals behave differently when they are altered by fluids. Some zircon crystals have compositions close to ideal stoichiometry, while others show signs of alteration. Normally, elements like uranium, thorium, and REEs would not be easily incorporated into zircon crystals, especially at low temperatures. So, it is likely that something changed the zircon crystals (metamictization) to allow them to accommodate these elements. This event likely involved the release of hot fluids during the final stages of magma cooling and crystallization, leading to Na- and K-metasomatism within the granite [14]. However, this phase was insufficient to alter zircon and other refractory minerals (xenotime, thorite, monazite, and apatite) due to their inherent resistance to alteration.
- (ii)
- Late-stage hydrothermal event (ca. 20 Ma), linked to the main rifting phase of the Red Sea. The prolonged metamictization of the refractory minerals could have eventually weakened them, rendering them susceptible to modification by this later hydrothermal event. This event may have led to the uptake of non-formula elements in the zircon, as supported by previous studies on metamict zircon crystals from the Gabel Hamradom in the Egyptian Eastern Desert. The time of this hydrothermal event is determined to be around 17.9 + 6.9 − 7.4 to 22.2 + 5.4 − 4.8 Ma [6].
- (iii)
- Surficial weathering event that led to the alteration of the Um Ara granites. Low-temperature alteration by oxic groundwater may have contributed to further uptake of non-formula elements in the zircon. The 230Th/234U ages of 50,000 to 159,000 years for uranophane from the Um Ara granites match up with periods of heavy rainfall (pluvial periods) known as the Kubbaniyan and Nabtian that occurred in the Egyptian Eastern Desert [56]. A similar low-temperature weathering event has been suggested as a mechanism for element uptake in metamict zircons [57,58].
6. Conclusions
- Initial zircon crystallization occurred around 603 Ma in the Um Ara granitic source, accompanied by the formation of other U- and Th-bearing minerals such as xenotime, thorite, monazite, and apatite.
- Long-term metamictization of the primary zircon crystals, as well as the associated U- and Th-bearing minerals, resulted in the formation of fractures and cracks within these minerals. These fractures and cracks facilitated the subsequent alteration processes by allowing fluid circulation and chemical changes.
- A major hydrothermal event, contemporaneous with the rifting of the Red Sea around 20 Ma, led to fluid-rock interaction and the release of Zr, Si, U, Th, Hf, Fe, Al, Ca, Ti, P, As, and REEs from the metamicted zircon and associated U- and Th-bearing minerals. As the hydrothermal alteration progressed, voids or altered remnants were left behind within the zircon grains. The enrichment of the hydrothermal fluids with non-formula elements, such as REEs, P, Al, Ca, Fe, and Ti, facilitated their incorporation into the modified zircon crystal structure, filling the voids and altered zones.
- Subsequent pluvial periods in the Kubbaniyan and Nabtian periods (around 50,000–159,000 years ago) may have allowed further uptake of non-formula elements during low-temperature alteration. The shear zones within the Um Ara granites facilitated the mobilization and transport of non-formula element-bearing fluids, likely as carbonate and fluoride complexes.
- Subsequent pluvial periods in the Kubbaniyan and Nabtian periods (around 50,000–159,000 years ago) may have had a limited role in incorporating additional elements into zircon. The shear zones within the Um Ara granites facilitated the mobilization and transport of non-formula element-bearing fluids, likely as carbonate and fluoride complexes.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of zircon in aqueous fluids and melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Lenting, C.; Geisler, T.; Gerdes, A.; Kooijman, E.; Scherer, E.E.; Zeh, A. The behavior of the Hf isotope system in radiation damaged zircon during experimental hydrothermal alteration. Am. Miner. 2010, 95, 1343–1348. [Google Scholar] [CrossRef]
- Lewerentz, A.; Harlov, D.E.; Scherstén, A.; Whitehouse, M.J. Baddeleyite formation in zircon by Ca-bearing fluids in silica-saturated systems in nature and experiment: Resetting of the U–Pb geochronometer. Contrib. Miner. Pet. 2019, 174, 64. [Google Scholar] [CrossRef]
- Harlov, D.E.; Anczkiewicz, R.; Dunkley, D.J. Metasomatic alteration of zircon at lower crustal P-T conditions utilizing alkali- and F-bearing fluids: Trace element incorporation, depletion, and resetting the zircon geochronometer. Geochim. Cosmochim. Acta 2023, 352, 222–235. [Google Scholar] [CrossRef]
- Pidgeon, R.T.; O’Neil, J.R.; Silver, L.T. Uranium and Lead Isotopic Stability in a Metamict Zircon under Experimental Hydrothermal Conditions. Science 1966, 154, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Geisler, T.; Rashwan, A.A.; Rahn, M.K.W.; Poller, U.; Zwingmann, H.; Pidgeon, R.T.; Schleicher, H.; Tomaschek, F. Low-temperature hydrothermal alteration of natural metamict zircons from the Eastern Desert, Egypt. Miner. Mag. 2003, 67, 485–508. [Google Scholar] [CrossRef]
- Tomaschek, F.; Kennedy, A.K.; Villa, I.M.; Lagos, M.; Ballhaus, C. Zircons from Syros, Cyclades, Greece—Recrystallization and mobilization of zircon during high-pressure metamorphism. J. Pet. 2003, 44, 1977–2002. [Google Scholar] [CrossRef]
- Kapsiotis, A.; Rassios, A.E.; Antonelou, A.; Tzamos, E. Genesis and Multi-Episodic Alteration of Zircon-Bearing Chromitites from the Ayios Stefanos Mine, Othris Massif, Greece: Assessment of an unconventional hypothesis on the origin of zircon in ophiolitic chromitites. Minerals 2016, 6, 124. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, L.; Jiao, Y.; Rong, H.; Zhang, F. Alteration and elements migration of detrital zircons from the Daying uranium deposit in the Ordos Basin, China. Ore Geol. Rev. 2021, 139, 104418. [Google Scholar] [CrossRef]
- Walsh, J.M.J.; Spandler, C. The role of zircon in hydrothermal heavy REE mineralisation: The case for unconformity-related ore deposits of north-west Australia. Chem. Geol. 2023, 629, 121493. [Google Scholar] [CrossRef]
- Levashova, E.V.; Skublov, S.G.; Zamyatin, D.A.; Li, Q.; Levashov, D.S.; Li, X. Tetrad Effect of Rare Earth Element Fractionation in Zircon from the Pegmatite of the Adui Massif, Middle Urals. Geosciences 2024, 14, 7. [Google Scholar] [CrossRef]
- Levashova, E.V.; Mamykina, M.E.; Skublov, S.G.; Galankina, O.L.; Li, Q.-L.; Li, X.-H. Geochemistry (TE, REE, Oxygen) of Zircon from Leucogranites of the Belokurikhinsky Massif, Gorny Altai, as Indicator of Formation Conditions. Geochem. Int. 2023, 61, 1323–1339. [Google Scholar] [CrossRef]
- Machevariani, M.M.; Alekseenko, A.V.; Bech, J. Complex Characteristic of Zircon from Granitoids of the Verkhneurmiysky Massif (Amur Region). Minerals 2021, 11, 86. [Google Scholar] [CrossRef]
- Abdalla, H.M.; Ishihara, S.; Matsueda, H.; Abdel-Monem, A.A. On the albite-enriched granitoids at Um Ara area, Southeastern Desert, Egypt. 1. Geochemical, ore potentiality and fluid inclusion studies. J. Geochem. Explor. 1996, 57, 127–138. [Google Scholar] [CrossRef]
- Abd El-Naby, H. High and low temperature alteration of uranium and thorium minerals, Um Ara granites, south Eastern Desert, Egypt. Ore Geol. Rev. 2009, 35, 436–446. [Google Scholar] [CrossRef]
- Moussa, E.M.; Stern, R.J.; Manton, W.I.; Ali, K.A. SHRIMP zircon dating and Sm/Nd isotopic investigations of Neoproterozoic granitoids, Eastern Desert, Egypt. Precambrian Res. 2007, 160, 341–356. [Google Scholar] [CrossRef]
- Törnroos, R. Metamict zircon from Mozambique. Bull. Geol. Soc. Finl. 1985, 57, 181–195. [Google Scholar] [CrossRef]
- Smith, D.G.W.; de St. Jorre, L.; Reed, S.J.B.; Long, J.V.P. Zonally metamictized and other zircons from Thor Lake, Northwest Territories. Can. Miner. 1991, 29, 301–309. [Google Scholar]
- Geisler, T.; Pidgeon, R.T.; Kurtz, R.; van Bronswijk, W.; Schleicher, H. Experimental hydrothermal alteration of partially metamict zircon. Am. Miner. 2003, 86, 1496–1518. [Google Scholar] [CrossRef]
- Pointer, C.M.; Ashworth, J.R.; Ixer, R.A. The zircon-thorite mineral group in metasomatized granite, Ririwai, Nigeria 1. Zoning, alteration and exsolution in zircon. Miner. Pet. 1988, 39, 21–37. [Google Scholar] [CrossRef]
- Kempe, D.; Gruner, T.; Nasdala, L.; Wolf, D. Relevance of cathodoluminescence for the interpretation of U-Pb zircon ages, with an example of an application to a study of zircons from the Saxonian Granulite Complex, Germany. In Cathodoluminescence in Geosciences; Pagel, M., Barbin, V., Blanc, P., Ohnenstetter, D., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000; pp. 415–455. [Google Scholar]
- Nasdala, L.; Kronz, A.; Wirth, R.; Váczi, T.; Pérez-Soba, C.; Willner, A.; Kennedy, A.K. The phenomenon of deficient electron microprobe totals in radiation-damaged and altered zircon. Geochim. Cosmochim. Acta 2009, 73, 1637–1650. [Google Scholar] [CrossRef]
- Pérez-Soba, C.; Villaseca, C.; Del Tánago, J.G.; Nasdala, L. The composition of zircon in the peraluminous Hercynian granites of the Spanish Central System batholith. Can. Miner. 2007, 45, 509–527. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The composition of zircon and igneous and metamorphic petrogenesis. Rev. Miner. Geochem. 2003, 53, 27–62. [Google Scholar] [CrossRef]
- Kirkland, C.; Smithies, R.; Taylor, R.; Evans, N.; McDonald, B. Zircon Th/U ratios in magmatic environs. Lithos 2015, 212, 397–414. [Google Scholar] [CrossRef]
- Rubatto, D. Zircon: The metamorphic mineral. Rev. Miner. Geochem. 2017, 83, 261–295. [Google Scholar] [CrossRef]
- Yakymchuk, C.; Kirkland, C.L.; Clark, C. Th/U ratios in metamorphic zircon. J. Metamorph. Geol. 2018, 36, 715–737. [Google Scholar] [CrossRef]
- Zhai, W.; Zhang, E.; Zheng, S.; Santosh, M.; Sun, X.; Niu, H.; Fu, B.; Fu, Y.; Li, D.; Jiang, Y.; et al. Hydrothermal zircon: Characteristics, genesis and metallogenic implications. Ore Geol. Rev. 2022, 149, 105111. [Google Scholar] [CrossRef]
- Mohanty, S.; Papadopoulos, A.; Petrelli, M.; Papadopoulou, L.; Sengupta, D. Geochemical Studies of Detrital Zircon Grains from the River Banks and Beach Placers of Coastal Odisha, India. Minerals 2023, 13, 192. [Google Scholar] [CrossRef]
- Zhi, J.; Lei, R.; Chen, B.; Muhtar, M.N.; Feng, Z.; Zhang, K.; Cai, Y.; Wu, C. Zircon Genesis and Geochronology for the Zhangbaoshan Super-Large Rubidium Deposit in the Eastern Tianshan, NW China: Implication to Magmatic-Hydrothermal Evolution and Mineralization Processes. Front. Earth Sci. 2021, 9, 682720. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Y.-H.; Zhao, P.; Liao, S.-Y.; Jin, G.-D.; Liu, Z.; Jia, R.-Y. SHRIMP U-Pb dating on hydrothermal zircons: Evidence for an Early Cretaceous epithermal event in the Middle Jurassic Dexing porphyry copper deposit, Southeast China. Econ. Geol. 2012, 107, 1507–1514. [Google Scholar] [CrossRef]
- Hoskin, P.W.O. Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochim. Cosmochim. Acta 2005, 69, 637–648. [Google Scholar] [CrossRef]
- Pettke, T.; Audetat, A.; Schaltegger, U.; Heinrich, C.A. Magmatic-to-hydrothermal crystallization in the W-Sn mineralized Mole Granite (NSW, Australia)—Part II: Evolving zircon and thorite trace element chemistry. Chem. Geol. 2005, 220, 191–213. [Google Scholar] [CrossRef]
- Skublov, S.G.; Berezin, A.V.; Li, X.-H.; Li, Q.-L.; Salimgaraeva, L.I.; Travin, V.V.; Rezvukhin, D.I. Zircons from a Pegmatite Cutting Eclogite (Gridino, Belomorian Mobile Belt): U-Pb-O and Trace Element Constraints on Eclogite Metamorphism and Fluid Activity. Geosciences 2020, 10, 197. [Google Scholar] [CrossRef]
- Claiborne, L.L.; Miller, C.F.; Wooden, J.L. Trace element composition of igneous zircon: A thermal and compositional record of the accumulation and evolution of a large silicic batholith, Spirit Mountain, Nevada. Contrib. Miner. Pet. 2010, 160, 511–531. [Google Scholar] [CrossRef]
- Trail, D.; Watson, E.B.; Tailby, N.D. Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochim. Cosmochim. Acta 2012, 97, 70–87. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Grimes, C.B.; John, B.E.; Cheadle, M.J.; Mazdab, F.K.; Wooden, J.L.; Swapp, S.; Schwartz, J.J. On the occurrence, trace element geochemistry, and crystallization history of zircon from in situ ocean lithosphere. Contrib. Miner. Pet. 2009, 158, 757–783. [Google Scholar] [CrossRef]
- Bouvier, A.-S.; Ushikubo, T.; Kita, N.T.; Cavoise, A.J.; Kozdon, R.; Valley, J.W. Isotopes and trace elements as a petrogenetic tracer in zircon: Insights from Archean TTGs and sanukitoids. Contrib. Miner. Pet. 2012, 163, 745–768. [Google Scholar] [CrossRef]
- Murakami, T.; Chakoumakos, B.C.; Ewing, R.C.; Lumpkin, G.R.; Weber, W.J. Alpha-decay event damage in zircon. Am. Miner. 1991, 76, 1510–1532. [Google Scholar]
- Ewing, R.C.; Meldrum, A.; Wang, L.; Weber, W.J.; Corrales, L.R. Radiation effects in zircon. Rev. Miner. Geochem. 2003, 53, 387–425. [Google Scholar] [CrossRef]
- Marsellos, A.E.; Garver, J.I. Radiation damage and uranium concentration in zircon as assessed by Raman spectroscopy and neutron irradiation. Am. Miner. 2010, 95, 1192–1201. [Google Scholar] [CrossRef]
- Lee, J.K.W.; Tromp, J. Self-induced fracture generation in zircon. J. Geophys. Res. 1995, 100, 17753–17770. [Google Scholar] [CrossRef]
- Reddy, S.M.; Timms, N.; Kinny, P.D.; Buchan, C.; Trimby, P.; Blake, K. Crystal-plastic deformation of zircon: A defect in the assumption of chemical robustness. Geology 2006, 34, 257–260. [Google Scholar] [CrossRef]
- Soman, A.; Geisler, T.; Tomaschek, F.; Grange, M.; Berndt, J. Alteration of crystalline zircon solid solutions: A case study on zircon from an alkaline pegmatite from Zomba-Malosa, Malawi. Contrib. Miner. Pet. 2010, 160, 909–930. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Bingen, B.; Paquette, J.L.; Bosse, V. Nanoscale evidence for uranium mobility in zircon and the discordance of U-Pb chronometers. Earth Planet. Sci. Lett. 2015, 409, 43–48. [Google Scholar] [CrossRef]
- Hassan, M. Genesis of Miocene polymetal mineralization, western Red Sea coastal zone. Egypt. Sedimentol. Egypt 2002, 10, 1–16. [Google Scholar]
- El Agami, N.L. Manganese Mineralization Related to the Red Sea Rift System: Examples from the Red Sea Coast and Sinai, Egypt. In Geological Setting, Palaeoenvironment and Archaeology of the Red Sea; Rasul, N., Stewart, I., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Frondel, C. Hydroxyl substitution in thorite and zircon. Am. Miner. 1953, 38, 1007–1018. [Google Scholar]
- Finch, R.J.; Hanchar, J.M. Structure and chemistry of zircon and zircon-group minerals. Rev. Miner. Geochem. 2003, 53, 1–25. [Google Scholar] [CrossRef]
- Speer, J.A. Zircon. Mineralogical Society of America. Rev. Miner. 1982, 5, 67–l12. [Google Scholar]
- Hoskin, P.W.O.; Kinny, P.D.; Wyborn, D.; Chappell, B.W. Identifying accessory mineral saturation during differentiation in granitoid magmas: An integrated approach. J. Petrol. 2000, 41, 1365–1396. [Google Scholar] [CrossRef]
- Caruba, R.; Iacconi, P. Les zircons de Narssârssuk (Groënland)—L’eau et les groupements OH dans les zircons métamictes. Chem. Geol. 1983, 38, 75–92. [Google Scholar] [CrossRef]
- Geisler, T. Isothermal annealing of partially metamict zircon: Evidence or a three-stage recovery process. Phys. Chem. Miner. 2002, 29, 420–429. [Google Scholar] [CrossRef]
- Geisler, T.; Burakov, B.E.; Zirlin, V.; Nikolaeva, L.; Pöml, P. A Raman spectroscopic study of high-uranium zircon from the Chernobyl “lava”. Eur. J. Miner. 2005, 17, 883–894. [Google Scholar] [CrossRef]
- Dawood, Y.H. Uranium-series disequilibrium dating of secondary uranium ore from south Eastern Desert of Egypt. Appl. Radiat. Isot. 2001, 881–887. [Google Scholar] [CrossRef]
- Delattre, S.; Utsunomiya, S.; Ewing, R.C.; Boeglin, J.-L.; Braun, J.; Balan, E.; Calas, G. Dissolution of radiation-damaged zircon in lateritic soils. Am. Miner. 2007, 92, 1978–1989. [Google Scholar] [CrossRef]
- Hay, D.C.; Dempster, T.J. Zircon behaviour during low-temperature metamorphism. J. Pet. 2009, 50, 571–589. [Google Scholar] [CrossRef]





| Element | Emission Line | Std. | Analyzer Crystal | Limit of Quantification (LOQ) (wt.%) |
|---|---|---|---|---|
| Si | Kα1 | Wollastonite | PETH | 0.066 |
| Zr | Lα1 | Zr oxide | PETJ | 0.042 |
| U | Mα1 | UO2 | PETH | 0.081 |
| Th | Mα1 | ThO2 | PETH | 0.102 |
| Hf | Lα1 | HfO2 | LIFH | 0.033 |
| Pb | Mα1 | PbVGe oxides | PETH | 0.015 |
| Fe | Kα1 | Fe2O3 | LIFH | 0.033 |
| Al | Kα1 | Y-garnet | TAP | 0.027 |
| Ca | Kα1 | Wollastonite | PETJ | 0.051 |
| Mg | Kα1 | MgO | TAP | 0.051 |
| Ti | Kα1 | Ilmenite | PETJ | 0.024 |
| P | Kα1 | LaPO4 | TAP | 0.081 |
| Y | Lα1 | Y-garnet | TAP | 0.039 |
| As | Lα1 | Cal-STD | LIF | 0.039 |
| La | Lα1 | LaPO4 | LIF | 0.045 |
| Ce | Lα1 | CePO4 | LIFH | 0.069 |
| Nd | Lβ1 | NdPO4 | LIFH | 0.036 |
| Sm | Lβ1 | SmPO4 | LIFH | 0.057 |
| Eu | Lβ1 | EuPO4 | LIFH | 0.036 |
| Gd | Lβ1 | GdPO4 | LIF | 0.075 |
| Tb | Lβ1 | TbPO4 | LIFH | 0.036 |
| Dy | Lβ1 | DyPO4 | LIFH | 0.075 |
| Ho | Lβ1 | HoPO4 | LIFH | 0.018 |
| Er | Lβ1 | ErPO4 | LIFH | 0.018 |
| Tm | Lα1 | TmPO4 | LIFH | 0.018 |
| Yb | Lα1 | YbPO4 | LIFH | 0.039 |
| Lu | Lα1 | LuPO4 | LIFH | 0.021 |
| Mineral Type | Less Altered Zircon | Altered Zircon | Uranophane | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | SP1 (N* = 1) | SP2-1 (N* = 2) | SP2-2 (N* = 8) | SP3-1 (N* = 7) | SP3-2 (N* = 8) | SP4-1 (N* = 8) | SP5 (N* = 8) | SP4-2B (N* = 8) | U1 (N = 3) | U3 (N = 3) |
| Oxides (wt.%) | ||||||||||
| SiO2 | 32.853 | 32.846 | 30.518 | 30.913 | 28.44 | 26.48 | 25.84 | 26.66 | 14.327 | 13.696 |
| ZrO2 | 66.273 | 64.848 | 63.184 | 62.605 | 50.04 | 41.08 | 39.2 | 36.52 | 0.037 | 0.006 |
| UO2 | 0.083 | 0.118 | 0.085 | 0.013 | 2.195 | 3.166 | 2.262 | 5.518 | 66.685 | 60.288 |
| ThO2 | 0.103 | 0.05 | - | 0.038 | 1.035 | 8.685 | 6.73 | 8.01 | - | - |
| HfO2 | - | 0.075 | 1.716 | 1.275 | 3.342 | 2.931 | 6.257 | 2.905 | 0.157 | - |
| PbO | - | - | - | 0.092 | - | - | 0.104 | - | - | |
| FeO | 0.152 | 0.145 | 0.035 | 0.625 | 0.992 | 1.086 | 2.035 | 1.662 | 0.219 | 0.284 |
| Al2O3 | - | - | 0.029 | - | 0.813 | 2.062 | 1.768 | 3.224 | 0.15 | 0.289 |
| CaO | 0.057 | 0.043 | - | 0.051 | 1.368 | 1.597 | 1.416 | 1.426 | 6.764 | 6.143 |
| MgO | - | - | - | - | 0.059 | 0.055 | - | 0.074 | 0.029 | 0.069 |
| TiO2 | - | - | 0.053 | 0.064 | 0.026 | - | - | - | 0.014 | - |
| P2O5 | - | - | - | - | 0.248 | 0.417 | 0.39 | 0.128 | - | - |
| Y2O3 | - | - | - | - | 0.518 | - | 0.564 | - | - | - |
| As2O3 | - | - | - | - | 0.06 | 0.061 | 0.22 | 0.128 | 0.034 | 0.055 |
| La2O3 | - | - | 0.046 | 0.047 | 0.07 | 0.15 | 0.25 | 0.054 | - | 0.056 |
| Ce2O3 | - | - | 0.079 | 0.07 | 0.103 | 1.024 | 0.941 | 0.856 | 0.183 | - |
| Nd2O3 | - | - | 0.106 | 0.126 | 0.04 | 0.039 | 0.037 | 0.036 | 0.118 | 0.06 |
| Sm2O3 | - | - | 0.059 | 0.075 | 0.063 | 0.184 | 0.144 | 0.066 | - | - |
| EU2O3 | - | - | 0.056 | 0.091 | 0.04 | 0.037 | 0.038 | 0.045 | - | - |
| Gd2O3 | - | - | 0.198 | 0.175 | 0.081 | 0.164 | 0.11 | 0.078 | - | - |
| Tb2O3 | - | - | 0.037 | 0.037 | 0.037 | 0.039 | 0.037 | 0.039 | - | 0.089 |
| Dy2O3 | - | - | 0.077 | 0.162 | 0.288 | 0.328 | 0.177 | 0.241 | - | - |
| Ho2O3 | - | - | 0.175 | 0.104 | 0.082 | 0.02 | 0.018 | 0.018 | - | - |
| Er2O3 | - | - | 0.02 | 0.02 | 0.356 | 0.053 | 0.413 | 0.076 | - | - |
| Tm2O3 | - | - | 0.062 | 0.023 | 0.193 | 0.018 | 0.037 | 0.072 | - | 0.035 |
| Yb2O3 | - | - | 0.26 | 0.078 | 0.489 | 0.393 | 0.395 | 0.044 | - | - |
| Lu2O3 | - | - | 0.112 | 0.033 | 0.182 | 0.089 | 0.03 | 0.021 | - | 0.075 |
| Total | 99.52 | 98.13 | 96.91 | 96.56 | 91.25 | 90.16 | 89.31 | 88.01 | 88.72 | 81.15 |
| Structural formula | Based on O = 4 | Based on O = 7 | ||||||||
| Si | 1.007 | 1.017 | 0.982 | 0.991 | 0.991 | 0.976 | 0.969 | 1.000 | 1.508 | 1.549 |
| Al | - | - | 0.001 | - | 0.033 | 0.090 | 0.078 | 0.142 | 0.019 | 0.039 |
| P | - | - | - | - | 0.007 | 0.013 | 0.012 | 0.004 | - | - |
| As | - | - | - | - | 0.001 | 0.001 | 0.005 | 0.003 | 0.002 | 0.004 |
| T-site | 1.007 | 1.017 | 0.982 | 0.991 | 1.033 | 1.080 | 1.064 | 1.150 | ||
| Zr | 0.990 | 0.979 | 0.991 | 0.979 | 0.850 | 0.738 | 0.717 | 0.668 | 0.002 | 0.000 |
| U | 0.001 | 0.001 | 0.001 | 0.000 | 0.017 | 0.026 | 0.019 | 0.046 | 1.562 | 1.517 |
| Th | 0.001 | 0.000 | - | 0.000 | 0.008 | 0.073 | 0.057 | 0.068 | - | - |
| Hf | - | 0.001 | 0.016 | 0.012 | 0.033 | 0.031 | 0.067 | 0.031 | 0.005 | - |
| Pb | - | - | - | - | 0.001 | 0.000 | - | 0.001 | - | - |
| Fe | 0.004 | 0.004 | 0.001 | 0.017 | 0.029 | 0.034 | 0.064 | 0.052 | 0.019 | 0.027 |
| Ca | 0.001 | 0.001 | - | 0.002 | 0.051 | 0.063 | 0.057 | 0.057 | 0.763 | 0.744 |
| Mg | - | - | - | - | 0.003 | 0.001 | - | 0.004 | 0.005 | 0.012 |
| Ti | - | - | 0.001 | 0.002 | 0.000 | - | - | - | 0.001 | - |
| Y | - | - | - | 0.000 | 0.010 | - | 0.011 | - | - | - |
| La | - | - | 0.000 | 0.000 | 0.001 | 0.002 | 0.004 | 0.000 | - | 0.002 |
| Ce | - | - | 0.001 | 0.001 | 0.001 | 0.014 | 0.013 | 0.012 | 0.007 | - |
| Nd | - | - | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.004 | 0.002 |
| Sm | - | - | 0.001 | 0.001 | 0.000 | 0.002 | 0.002 | 0.001 | - | - |
| Eu | - | - | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | - |
| Gd | - | - | 0.002 | 0.001 | 0.000 | 0.002 | 0.001 | 0.001 | - | - |
| Tb | - | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | 0.003 |
| Dy | - | - | 0.000 | 0.000 | 0.003 | 0.004 | 0.002 | 0.003 | - | - |
| Ho | - | - | 0.002 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | - | - |
| Er | - | - | 0.000 | 0.000 | 0.004 | 0.001 | 0.005 | 0.001 | - | - |
| Tm | - | - | 0.001 | 0.000 | 0.002 | 0.000 | 0.000 | 0.001 | - | 0.001 |
| Yb | - | - | 0.003 | 0.001 | 0.005 | 0.004 | 0.005 | 0.000 | - | - |
| Lu | - | - | 0.001 | 0.001 | 0.002 | 0.001 | 0.000 | 0.000 | - | 0.003 |
| A-Site | 0.996 | 0.986 | 1.022 | 1.019 | 1.023 | 0.996 | 1.025 | 0.948 | ||
| Total | 2.002 | 2.003 | 2.004 | 2.010 | 2.056 | 2.076 | 2.089 | 2.098 | 3.896 | 3.904 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Naby, H.H. Alteration and Non-Formula Elements Uptake of Zircon from Um Ara Granite, South Eastern Desert, Egypt. Minerals 2024, 14, 834. https://doi.org/10.3390/min14080834
Abd El-Naby HH. Alteration and Non-Formula Elements Uptake of Zircon from Um Ara Granite, South Eastern Desert, Egypt. Minerals. 2024; 14(8):834. https://doi.org/10.3390/min14080834
Chicago/Turabian StyleAbd El-Naby, Hamdy H. 2024. "Alteration and Non-Formula Elements Uptake of Zircon from Um Ara Granite, South Eastern Desert, Egypt" Minerals 14, no. 8: 834. https://doi.org/10.3390/min14080834
APA StyleAbd El-Naby, H. H. (2024). Alteration and Non-Formula Elements Uptake of Zircon from Um Ara Granite, South Eastern Desert, Egypt. Minerals, 14(8), 834. https://doi.org/10.3390/min14080834





