Abstract
Given that particle and bubble size, as well as their surface properties, are pivotal in froth flotation, surfactants have been extensively employed due to their impact on bubble size and froth stabilization. This study aimed to investigate the influence of surfactants on the performance of apatite flotation in column. Three different categories of surfactants were examined: anionic, amphoteric, and nonionic, specifically Lupromin, Genagen, and Triton X-100, respectively. The critical coalescence concentration (CCC) and surface tension of each surfactant were determined. The impact of these surfactants on reducing bubble size was quantified, and their subsequent effects on apatite flotation in a column were assessed. The most favorable flotation response for Genagen was achieved at CCC and pH 11, resulting in the highest apatite recovery and the smallest bubble size. For Triton X-100, the best condition was attained at ¼ CCC and pH 11. However, overall, Lupromin was the surfactant that yielded the best flotation results (at ¼ CCC and pH 11). The superior performance of this anionic surfactant was corroborated by chemical adsorption results, as demonstrated by FTIR analyses.
1. Introduction
Phosphate rocks serve as the primary global reservoirs of phosphorus used in the production of phosphatic fertilizers [1]. Phosphorus fertilizers play a crucial role in enhancing agricultural productivity and ensuring food security for a growing global population. Its application extends to the food, pharmacy, and chemical industries, and produces phosphoric acid, detergents, food additives, etc., [1]. Phosphorus is also critical in a host of industrial applications and is a nonrenewable resource that is sourced primarily from the phosphatic mineral apatite, hosted in either sedimentary deposits or igneous ores [2]. Phosphorus within phosphate rocks is consistently bound to other elements in the form of phosphate minerals, with the most prevalent and widely distributed minerals belonging to the apatite group [2].
Igneous deposits, primarily extracted in Russia, South Africa, Brazil, Finland, and Zimbabwe, consist of fluorapatite ores predominantly associated with carbonatites and various types of alkaline intrusions [2]. However, these igneous phosphate ores typically have a low grade. With global reserves of high-grade phosphorus ore rapidly diminishing, there is an increasing reliance on low-grade phosphate ores, which depends on the development of efficient processes to yield high-quality apatite concentrates. This has made the study of phosphorus ore flotation an essential method for upgrading ore quality. A deep understanding of flotation techniques can significantly enhance the efficiency of phosphorus recovery, ensuring a more sustainable supply to meet global demands.
Froth flotation is a common technique in the mining industry used to separate and concentrate ores. In this process, target particles are separated from a liquid phase based on the varying ability of air bubbles to selectively adhere to their surfaces due to differences in hydrophobicity [3]. Consequently, the hydrophobic particles, referred to as “floatable”, preferentially attach to the surface of air bubbles. These bubbles ascend from the pulp zone to the foam zone, where they are collected in the concentrate stream. In contrast, the gangue material, consisting of more hydrophilic particles, remains in the pulp phase and is gathered in the tailings [3].
Due to the progressive depletion of igneous deposits, processing plants have resorted to extensive grinding to increase the release of phosphorus. This practice, however, has led to the production of a significant amount of fines [4]. Particle size is a crucial parameter in the flotation process, as it significantly impacts flotation performance. Inefficient flotation can result in substantial revenue loss and the unnecessary depletion of reserves. In this context, understanding the interaction between bubbles and particles and finding ways to enhance it become exceptionally important for improving the efficiency of the apatite flotation process [5,6].
The collection of particles by bubbles in the pulp involves bubble–particle collision, attachment, and detachment [7]. The relationship between particle size and bubble diameter plays a pivotal role in determining the likelihood of particles colliding with bubbles, adhering to bubbles after collision, and remaining attached within the pulp phase [7]. However, the mineral particles of interest often lack inherent hydrophobic properties, and the use of reagents can enhance flotation performance. Therefore, the judicious selection of reagents such as collectors, depressants, frothers, and pH modifiers is a fundamental step to enhance the selectivity of this process [8].
Various factors can influence the distribution of bubble sizes, including gas flow rate, solid concentration, temperature, and the addition of surfactants [9]. Surfactants are compounds with both a polar group and a hydrocarbon chain and are generally introduced into the process to regulate bubble size distribution and facilitate froth stabilization [10,11]. Additionally, frothers exhibit characteristics similar to collectors, with the primary distinction being the functionality of the polar group. Collectors tend to have greater adsorption on mineral surfaces, while frothers possess a lyophilic radical that is more attracted to water [12].
Given the significant impact of bubble size in froth flotation, various researchers have investigated this variable [13,14,15,16,17,18,19,20]. For instance, Reis et al. [18] explored the effect of bubble size on the flotation of fine particles in a low-grade Brazilian apatite ore and found that the optimal bubble size range for the particle sizes under investigation was 800–1000 µm. Li et al. [19] examined bubble size distribution in the presence of a surfactant and observed that the Sauter mean diameter (d32) significantly decreased when Triton X-100 was introduced into the air–water system (without ore). The frother’s role was to prevent coalescence by adsorbing onto the surface of the bubbles [20]. However, most of these studies were conducted in the context of an air–water system, as obtaining precise data on bubble size distribution becomes challenging when ore is introduced into the system. Therefore, assessing the influence of various categories of surfactants on bubble size within a system containing ore and its subsequent impact on flotation performance can offer a means to enhance the process for different particle sizes [20].
The objective of this study was to examine the impact of three distinct categories of surfactants (anionic, amphoteric, and nonionic) on the reduction in bubble diameter and, consequently, their influence on the performance of apatite flotation in a column. The critical coalescence concentration and surface tension were determined for each surfactant. The findings presented here may offer insights into identifying the most suitable type of surfactant for achieving optimal apatite froth flotation results.
2. Materials and Methods
2.1. Experimental Apparatus
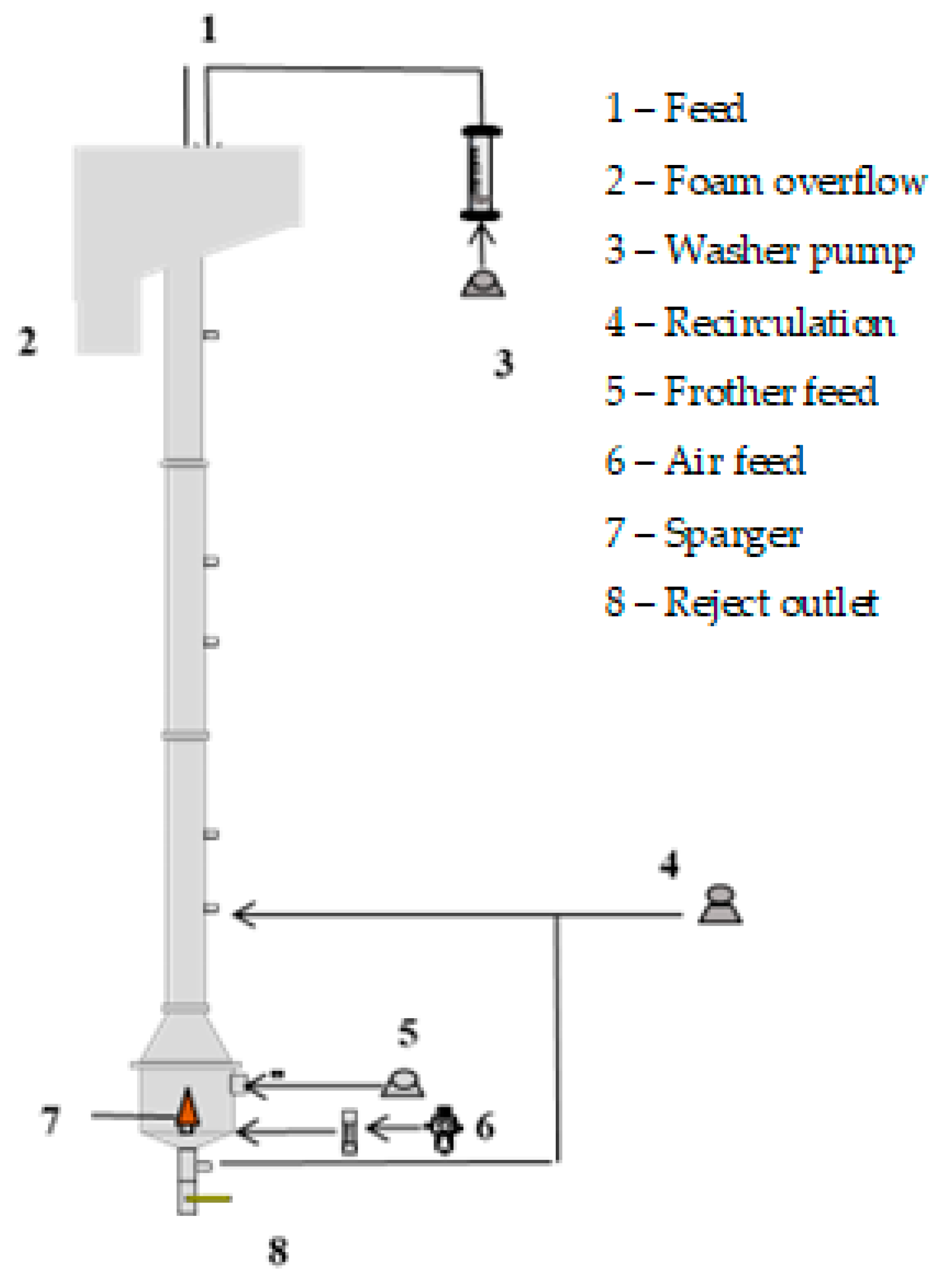
The batch flotation tests were conducted in an acrylic column divided into three sections: a cylindrical section with a diameter of 4 cm and a length of 150 cm, followed by a frustoconical section with a height of 9.5 cm, and, finally, another cylindrical section with a length of 12 cm and a diameter of 10 cm (Figure 1). At the base of the column, an air sparger was installed. This sparger consisted of a porous cone made of sintered bronze, designed by MetalSinter (São Paulo, Brazil) to generate the desired distribution of bubble sizes. The column was fed from the top, where a froth washer water system was also located. Additionally, there was a recirculation load at the bottom of the column to ensure that the feed particles passed through the collection zone.
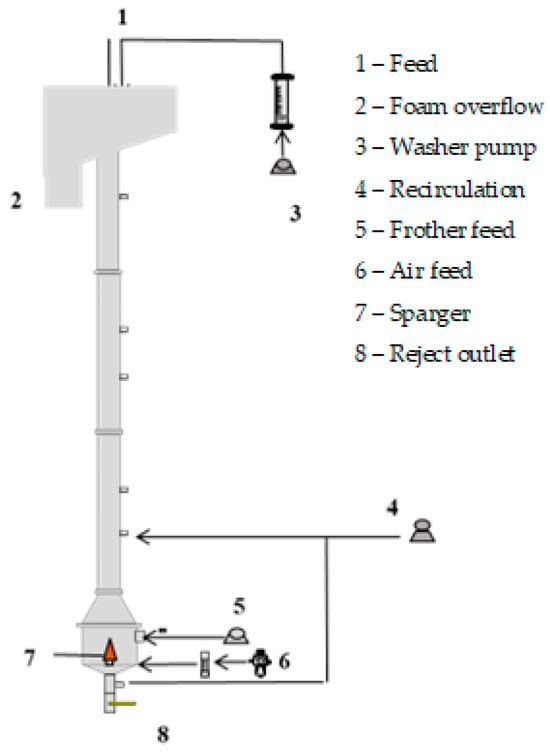
Figure 1.
Experimental apparatus.
2.2. Bubble Size Measurements
Bubble sizes were assessed using a Fastec IL5 high-speed digital camera (Fastec Imaging, San Diego, CA, USA). The bubbles were recorded in a dynamic state (1000 frames per second), and the acquired images were analyzed with the assistance of ImageJ software 1.49 (National Institutes of Health, New York, NY, USA). This software allowed us to measure the diameter sphere with the same projected area. Detailed descriptions of the measurement procedures can be found in our previous publication [21]. The Sauter mean diameter (d32) was determined from the bubble size distribution obtained in each test using Equation (1).
where n is the number of measured bubbles, di is the bubble diameter of bubble i, and ni is the number of bubbles with diameter di. In each test, around 500 bubbles were measured.
2.3. Surfactants and Air–Water System
In this study, the surfactants utilized included Lupromin (sodium sulfosuccinate from BASF, Ludwigshafen, Germany), Genagen (from Clariant, Muttenz, Switzerland), and Triton X-100 (polyethylene glycol tert-octylphenyl ether from Sigma Aldrich, St. Louis, MO, USA) at concentrations of 5, 10, 20, 30, 40, and 50 ppm. These specific surfactants were selected because apatite exhibits negative surface activity. Lupromin is categorized as an anionic surfactant, Genagen as an amphoteric surfactant, and Triton X-100 as a nonionic surfactant. The surface tension of all surfactant solutions was determined using a Krüss K6 tensiometer (Krüss, Hamburg, Germany).
The impact of these surfactants on bubble size in the air–water system was studied under the operating conditions specified in Table 1.

Table 1.
Fixed operating conditions for the air–water system.
The critical coalescence concentration (CCC) for each surfactant was determined by plotting the d32 of the bubbles against the concentration of the surfactant solution. The CCC was considered reached when the bubble sizes remained relatively unchanged across different concentrations [22,23].
2.4. Characterization of the Phosphate Ore Sample
The igneous apatite samples for this study were sourced from the Barreiro carbonatite complex in Araxá, Minas Gerais state, Brazil, which is owned and operated by Mosaic Fertilizantes do Brasil Ltd., São Paulo, Brazil. These samples were collected from the feed circuit of the apatite flotation column.
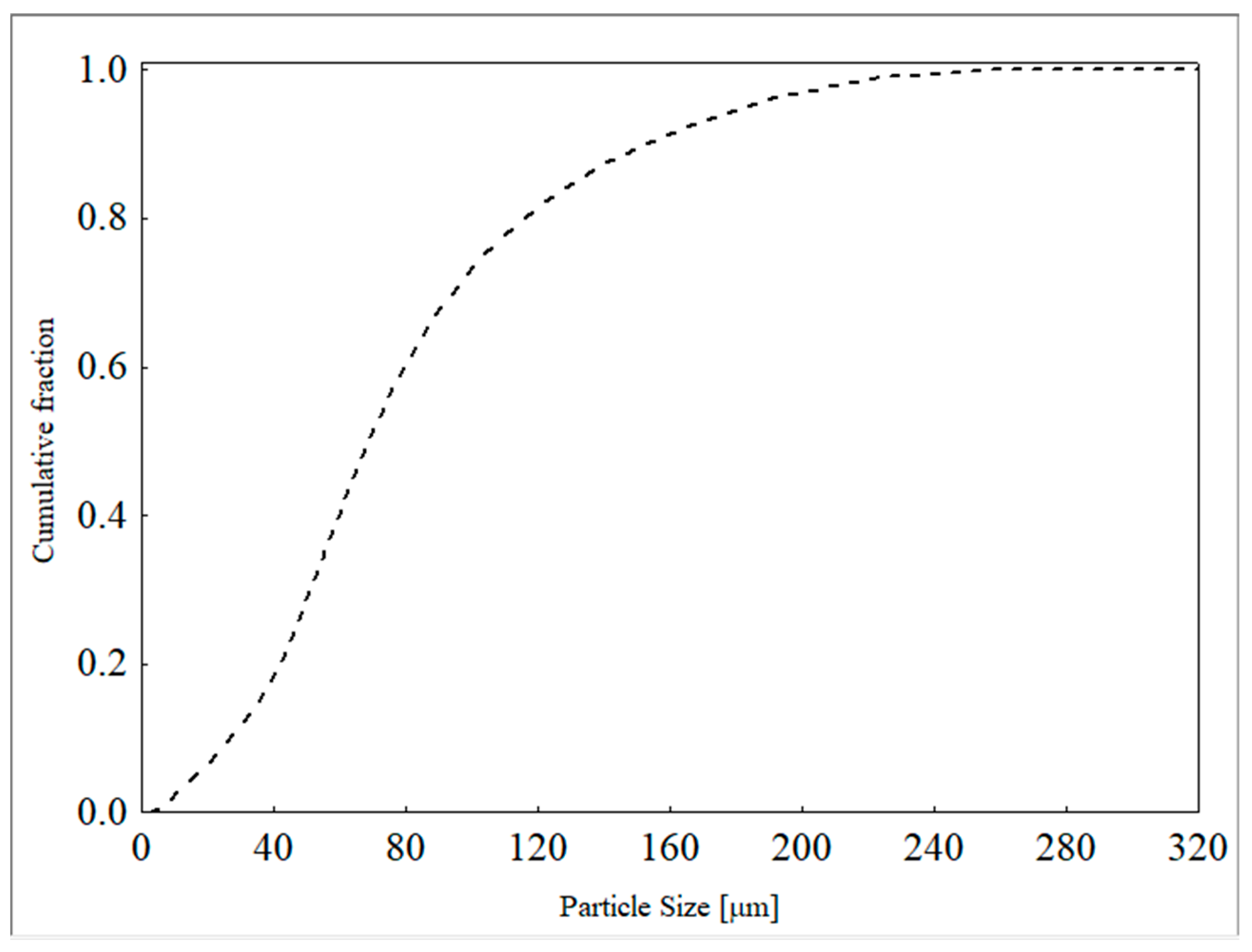
The particle size distribution of the ore was determined using a laser diffraction technique with a Mastersize Microplus MAF 5001® particle size analyzer from Malvern Panalytical Ltd., Malvern, UK. The Rosin–Rammler–Bennet (RRB) model (Equation (2)) provided the best fit to the experimental data, with estimated parameter values of d63.2 = 87.63 µm and n = 1.83 (r2 = 0.9984). The particle size distribution curve is presented in Figure 2.
where dp is the particle diameter and X is the cumulative fraction.
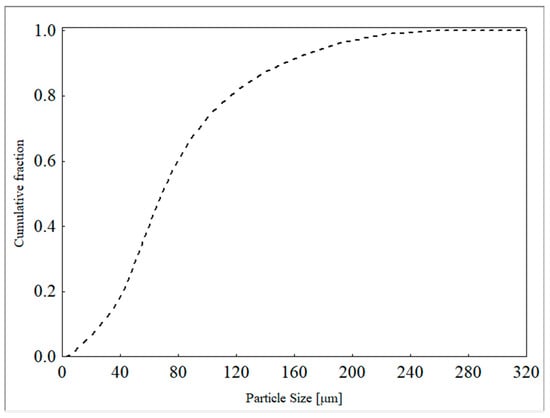
Figure 2.
Particle size distribution of the feed samples.
The chemical composition of the fed sample was determined by X-ray fluorescence spectrometry using an S8 Tiger spectrometer (Bruker, Germany). It was mainly composed of 20.66% of P2O5, 27.57% of CaO, 24.45% of Fe2O3, 12.44% of SiO2, 8.41% of TiO2, 2.74% of BaO, and 1.27% of Al2O3. Then, the highest proportion of gangue minerals combined with apatite were iron and silicate minerals.
2.5. Reagents and Flotation Test Procedures
In the ore-conditioning step, flotation reagents were introduced, including a collector, which was a fatty acid soap derived from rough rice oil, which was saponified with NaOH at 60 °C and applied at a concentration of 360 g/t. Additionally, a gangue depressant was employed, consisting of gelatinized cornstarch, which was used at a concentration of 100 g/t. Both the collector and depressant were diluted in water to reach concentrations of 2.5% and 3%, respectively. The pH was controlled by using 10% NaOH and 1 M HCl solutions. These reagents were chosen based on prior investigations related to apatite froth flotation [24,25,26,27]. Additionally, a solution of Lupromin, Genagen, or Triton X-100 was included in the flotation tests as a surfactant to control the bubble size distribution. Tests were conducted for each type of surfactant, with their concentrations varying between ¼ CCC and CCC, and at pH levels of 9 and 11. The remaining flotation parameters remained consistent across all experiments.
The batch tests were conducted in the column depicted in Figure 1. After setting the air flow rate at 80 L/h and the wash water flow at 0.15 L/min using flow meters, the preconditioned pulp, consisting of 13.68% solids, was introduced at the top of the column. The equipment operated with a circulating load of 0.50 L/min, ensuring the suspension of ore particles and allowing them to pass through the collection zone. Both the concentrate and the tailings were discharged from the flotation column. Subsequently, the products of each flotation experiment, namely, the concentrate and the tailings, underwent oven-drying at 110 ± 0.5 °C for 24 h. They were then weighed, and their chemical composition was analyzed through X-ray fluorescence spectrometry. Table 2 outlines the fixed operating conditions, which were determined based on prior studies [18,21,24,25,26] and preliminary tests. For each test, the size of bubbles was measured, and the corresponding d32 value was calculated.

Table 2.
Fixed operating conditions in the flotation.
The quality of the concentrated product was assessed based on the P2O5 grade, while the productivity of the flotation process was determined by the apatite recovery. For phosphate flotation of igneous ores, the industrial benchmark for satisfactory results is typically defined as having a minimum of 30% apatite grade and a 60% recovery [26].
To examine the adsorption of the surfactant within the system, the product from each flotation process was analyzed using Fourier transform infrared spectroscopy (FTIR) with a Miracle ATR/Spectrum Two FT-IR spectrometer from Pike Technologies, USA. FTIR spectra of apatite, both with and without surfactant, were recorded within the range of 400 to 1900 cm−1.
2.6. Index of Flotation Response
The performance of apatite flotation was assessed using the index of flotation response, which factors in both the P2O5 grade and the apatite recovery. This index considers the industrial benchmarks for grade and recovery, as represented by Equation (3):
In cases where the P2O5 grade and apatite recovery were both below 30% and 60%, respectively, i.e., falling short of the industrial reference values, the index of flotation response was assigned a value of zero
3. Results
3.1. Effect of Surfactant on the Air–Water System
It is an undeniable fact that mineral flotation, as we understand it today, relies heavily on the essential role of reagents [27]. These flotation reagents include collectors, depressants, frothers, and pH regulators, which serve to control the physical and chemical conditions of the three phases involved in the process.
Surface-active agents, commonly referred to as frothers, are amphipathic compounds capable of adsorbing at the air–water interface. These surfactants accumulate at the interface between water and air bubbles, forming a protective layer around the bubbles to prevent their collapse [28,29]. The arrangement of surfactant molecules at the air–water interface involves positioning the hydrophilic or polar groups towards the water phase, while the hydrophobic or nonpolar chains face the air phase [30]. Surfactants exhibit various charge characteristics depending on their properties, which can be anionic, cationic, amphoteric, or nonionic, with the type of surfactant determined by their hydrophilic chain [30].
In this study, three types of surfactants were chosen due to the active surface of apatite being characterized by an excess of negative charges. These surfactants included anionic (Lupromin), amphoteric (Genagen), and nonionic (Triton X-100), while the cationic surfactant was not tested because it carries an excess of positive charges, which would hinder its adsorption onto apatite [31].
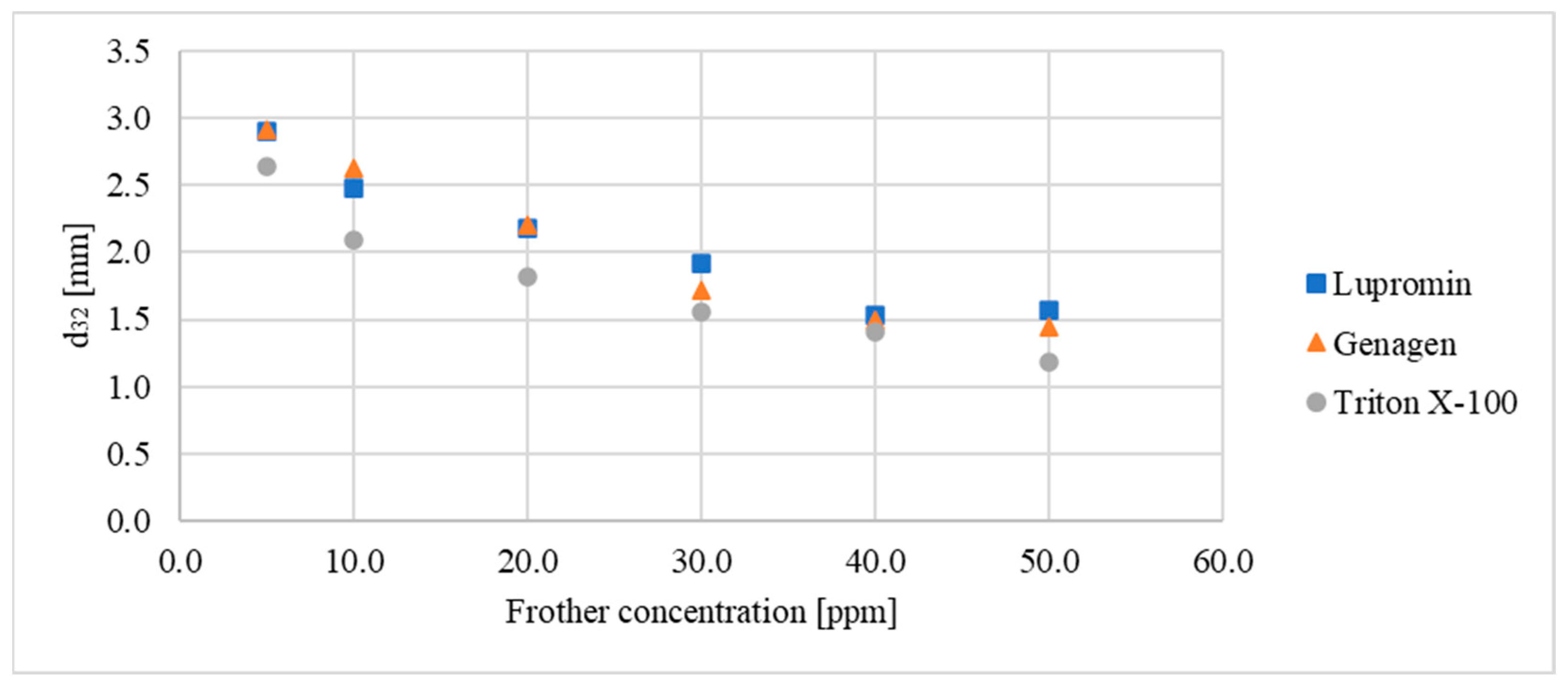
Initially, the impact of varying the concentration of these three surfactants on bubble sizes in a system without ore (air–water system) was examined. Figure 3 illustrates the relationship between the d32 of the bubbles and the concentration of each surfactant. The data indicate that the d32 decreased linearly with increasing concentrations of Lupromin and Genagen until the critical coalescence concentration (CCC) was reached at 40 ppm. Beyond this concentration, the bubble size remained constant, with slight fluctuation in the Sauter mean diameter falling within the margin of measurement error. Preliminary tests indicated that the CCC for Triton X-100 was approximately 50 ppm.
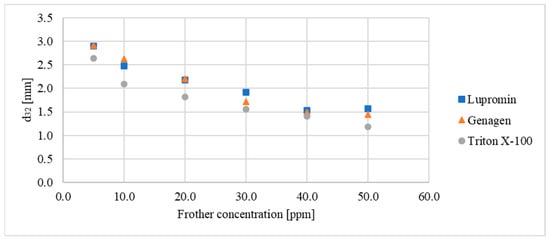
Figure 3.
Relationship between Sauter mean diameter and surfactant concentration for each surfactant for the air–water system.
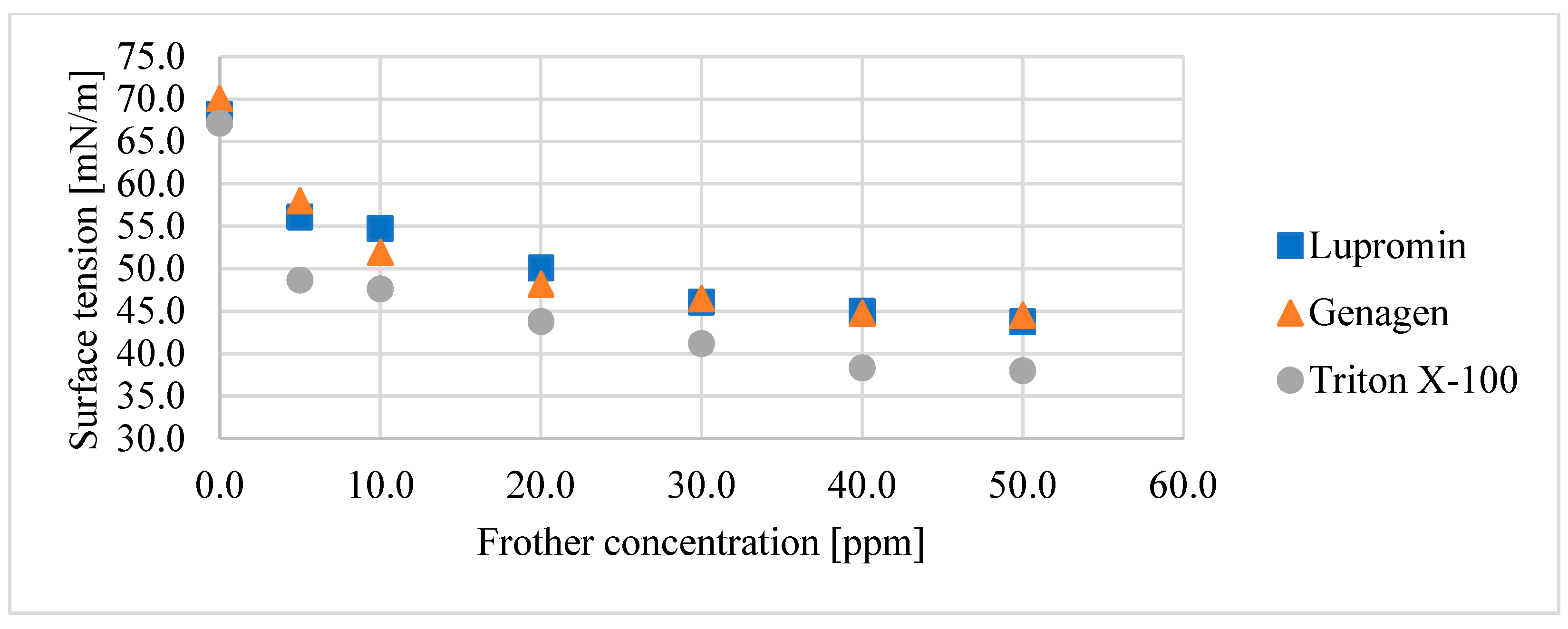
Figure 4 displays the reduction in surface tension with increasing surfactant concentration for all three surfactants under investigation. It is evident that the effect of these surfactants on bubble size reduction corresponds to the same behavior on respective surface tension. Triton X-100 resulted in the lowest surface tension and the smallest d32, followed by Lupromin and Genagen, which exhibited very similar responses.
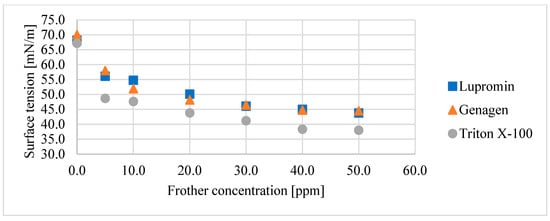
Figure 4.
Relationship between surface tension and surfactant concentration for each surfactant for the air–water system.
3.2. Influence of Surfactant in Apatite Flotation
Each of the surfactants used in this study, namely, Lupromin (an anionic surfactant), Genagen (an amphoteric surfactant), and Triton X-100 (a nonionic surfactant), was assessed for its impact on flotation performance. Table 3 provides details of the conditions and results for each apatite flotation test. It is evident that all the conditions investigated achieved a P2O5 grade and apatite recovery exceeding 30% and 60%, respectively, surpassing the industrial reference values. The most favorable flotation outcome was attained at a pH of 11 using a surfactant concentration equivalent to ¼ CCC (test F4). Under these conditions, the highest flotation response index was recorded, with a value of 3.58. Moreover, this condition led to the highest apatite recovery at 74.73%, with a P2O5 grade of 32.57%. The d32 value of the bubbles decreased by a factor of 1.27 compared to the system without surfactant, transitioning from coarse to medium-sized bubbles.

Table 3.
Conditions and results of each apatite flotation test.
This study involved a statistical quantification of the effects of independent variables on the index of flotation response for each surfactant. This analysis revealed that when Lupromin and Genagen were used, the effect of pH was six times more significant than the effect of surfactant concentration. However, in the tests using Triton X-100 as the surfactant, the effect of pH was twice as prominent as the effect of surfactant concentration. Hence, it becomes evident that a more comprehensive examination of the interaction between the collector and surfactant is required to gain a deeper understanding of this phenomenon. This investigation could provide valuable insights for identifying the optimal conditions for the apatite flotation process.
As evident from the results presented in Table 3, the type of surfactant had varying impacts on the selectivity and efficiency of apatite flotation. The index of flotation response ranged from highest to lowest in the following order: anionic (Lupromin), amphoteric (Genagen), and nonionic (Triton X-100). This suggests that the choice of surfactant can significantly influence the performance of the apatite flotation process, with anionic surfactants demonstrating the most favorable outcomes in this case. A deeper analysis for each surfactant is presented below.
3.2.1. Lupromin
The results from the first six tests (F1 to F6) reveal that the addition of Lupromin to the process led to a reduction in the d32 of the bubbles and an increase in apatite recovery. However, the P2O5 grade did not exhibit a significant change compared to the test without surfactant (F1 and F2). Since Lupromin is classified as an anionic surfactant, it is possible that it competed with the collector and influenced the hydrophobicity of apatite. This suggests that there might be an interaction between the collector and the surfactant. The most favorable flotation outcome was achieved at pH 11, using a surfactant concentration equivalent to ¼ CCC (test F4). Under these conditions, the highest index of flotation response was recorded, along with the smallest d32 of the bubbles.
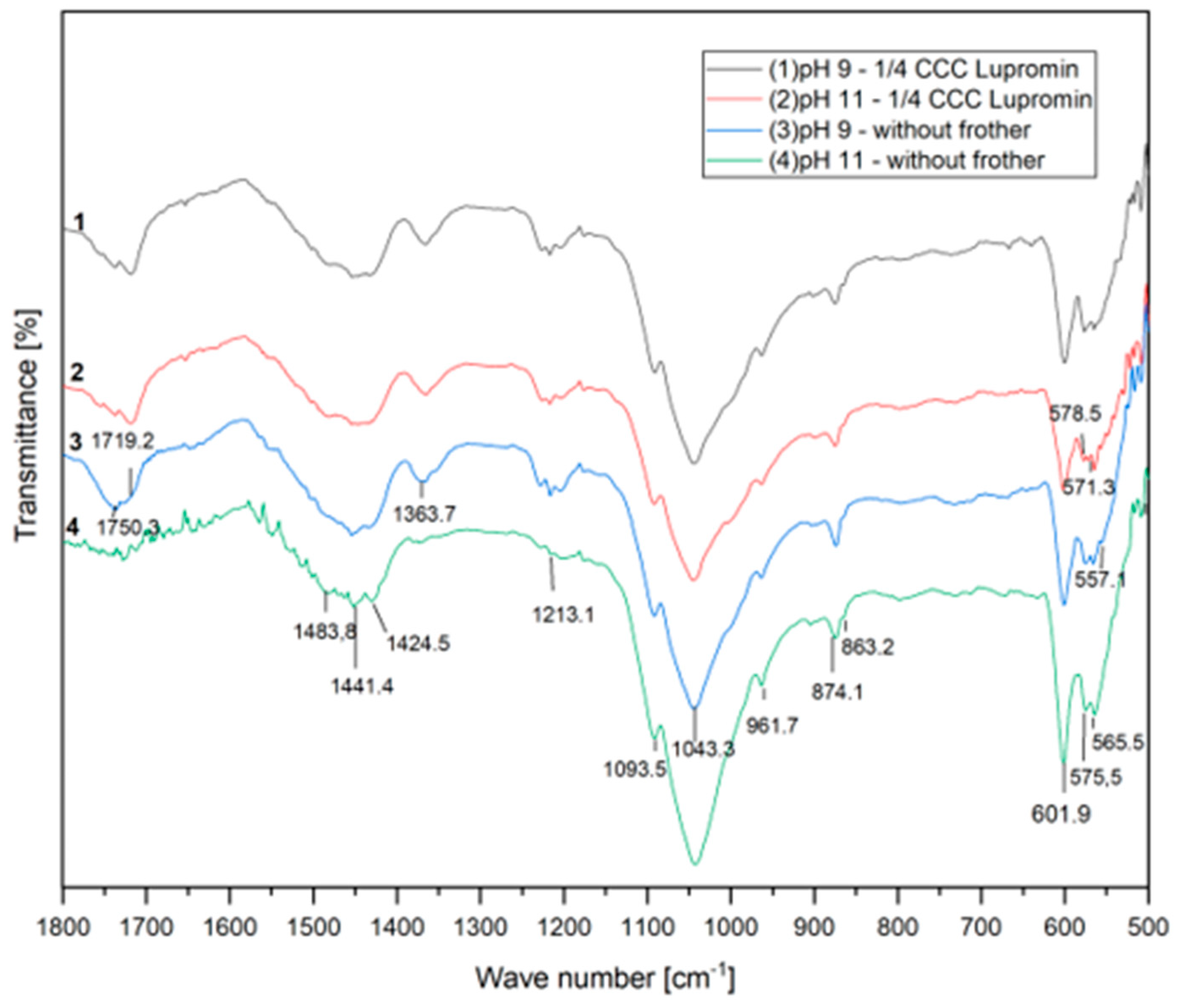
Figure 5 presents the infrared absorption spectra of the floated samples for the experiments conducted with the anionic surfactant (¼ CCC of Lupromin) at both pH 9 and 11, as well as without surfactant under the same conditions. Theoretical analysis indicates the presence of four vibrational modes for phosphate ions, namely, υ1, υ2, υ3, and υ4.
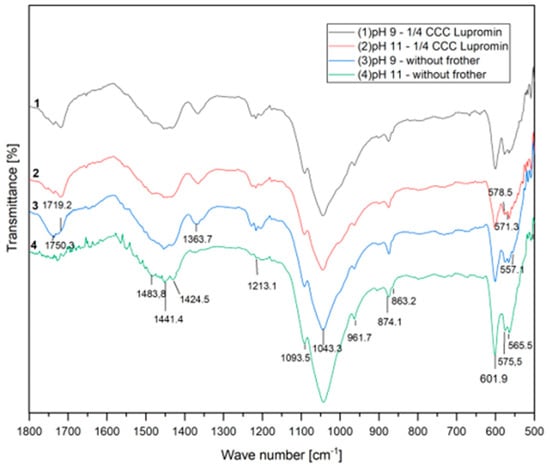
Figure 5.
FTIR spectra with Lupromin.
In the spectra related to flotation without surfactant, characteristic phosphate bands within the apatite domain are observable. These bands correspond to the vibration of PO43− ions, including asymmetric bending of P–O in υ4 at 601.9 and 575.5 cm−1, symmetric valence oscillation of υ1 at 961.7 cm−1, and asymmetric valence oscillations of υ3 attributed to the asymmetric stretching of P–O at 1093.5 and 1043.3 cm−1 [32,33].
At pH 9, no chemical or physical adsorption of Lupromin was observed. However, at pH 11, a different behavior was noted, characterized by the appearance of new peaks at 571.3 and 578.5 cm−1 within the vibrational mode υ4 of phosphate. This suggests the occurrence of chemical adsorption because the P2O5 grade remained nearly constant, while apatite recovery increased, attributed to true flotation and entrainment caused by the addition of Lupromin at pH 11. It is also important to mention that no adsorption was observed in the domain ranging from 1750 to 1400 cm−1, which pertains to the carboxylate group. This implies that Lupromin may have interacted with the active surface of apatite after the conditioning process, subsequently enhancing the flotation process.
The observed behaviors provide an explanation for the superior result obtained in test F4. This enhanced performance can be attributed to true flotation, brought about by the chemical adsorption of the surfactant. Furthermore, this process was facilitated by the smaller d32 of the bubbles and the entrainment mechanism. The highest apatite recovery achieved in this condition may also be linked to the formation of a more stable froth, as visually observed during the test, which inhibited the detachment mechanism. All these results collectively demonstrate that the anionic surfactant (Lupromin) was chemically adsorbed onto the apatite surface and that its presence also influenced the stability of the froth.
3.2.2. Genagen
The results obtained with the addition of Genagen to the flotation tests (tests F7 to F10 in Table 3) indicate that, in all the studied conditions, the P2O5 grade and apatite recovery exceeded the industrial reference values of 30% and 60%, respectively. The most favorable index of flotation response was achieved in test F10, conducted at pH 11 with a surfactant concentration equivalent to the CCC value.
Table 3 provides clear evidence of the amphoteric surfactant’s effect on reducing bubble size. Test F10, which exhibited the best flotation performance for this surfactant, also resulted in the smallest d32 value for the bubbles (0.66 mm). At alkaline pH levels, this amphoteric surfactant behaves similarly to anionic surfactants [34] and possesses more frothing properties, evident from its stability and the smaller d32 of the bubbles.
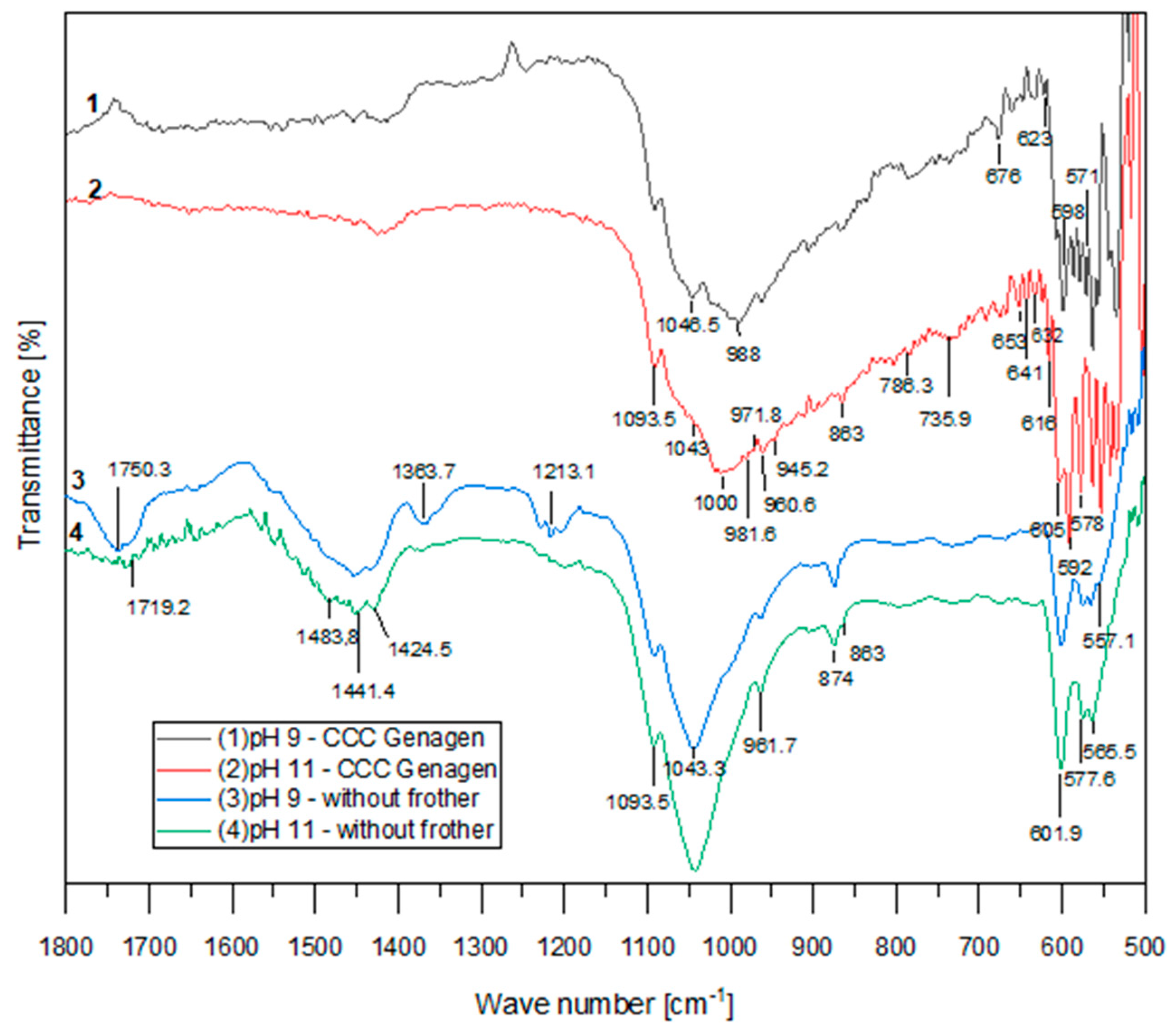
The infrared absorption spectra of the floated samples from the experiments conducted with and without the amphoteric surfactant (at CCC of Genagen) at pH 9 and 11 are presented in Figure 6. These spectra clearly demonstrate that the addition of Genagen had a significant impact on the behavior of apatite flotation.
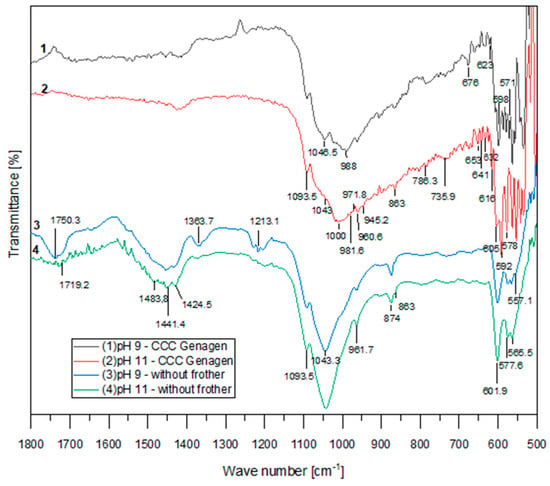
Figure 6.
FTIR spectra with Genagen.
In all the curves, bands corresponding to the apatite domain of PO43− ions can be observed. However, when Genagen was introduced into the system, there were noticeable changes in the shape of the curves, and new peaks appeared at 605, 598, 592, 578, and 571 cm−1. These new peaks are associated with the vibration of PO43− ions, particularly between asymmetric formations of υ4 related to P–O bonds. Additionally, new peaks were identified in the region corresponding to the symmetric valence oscillation of υ1 and the asymmetric valence oscillations of υ3 at 1046.5, 1043, 1000, 988, and 981.6 cm−1. The peak at 874 cm−1, attributed to HPO42−, disappeared when Genagen was added to the system, and the peaks in the range of 1750 to 1400 cm−1, corresponding to CO32− ion oscillations, also vanished. These observations suggest that Genagen did not adsorb onto the calcium mineral surface and point to the existence of chemical adsorption of Genagen on the active surface of apatite, as indicated by the region where the new peaks appeared.
3.2.3. Triton X-100
The conditions and results of each flotation test using Triton X-100 are provided in Table 3, encompassing tests F11 to F14. Notably, the addition of the nonionic surfactant substantially reduced the d32 of the bubbles. However, it is evident that neither the surfactant concentration nor the pH had a significant impact on the bubble size distribution
In terms of the P2O5 grade, there was a slight increase in this response when the experiments were conducted at pH 11 for Triton X-100. This increase might be attributed to the physical adsorption of the surfactant and the potential interaction between the collector and the surfactant under these conditions. The most favorable flotation outcome was achieved at pH 11 using a surfactant concentration equivalent to ¼ CCC (test F12).
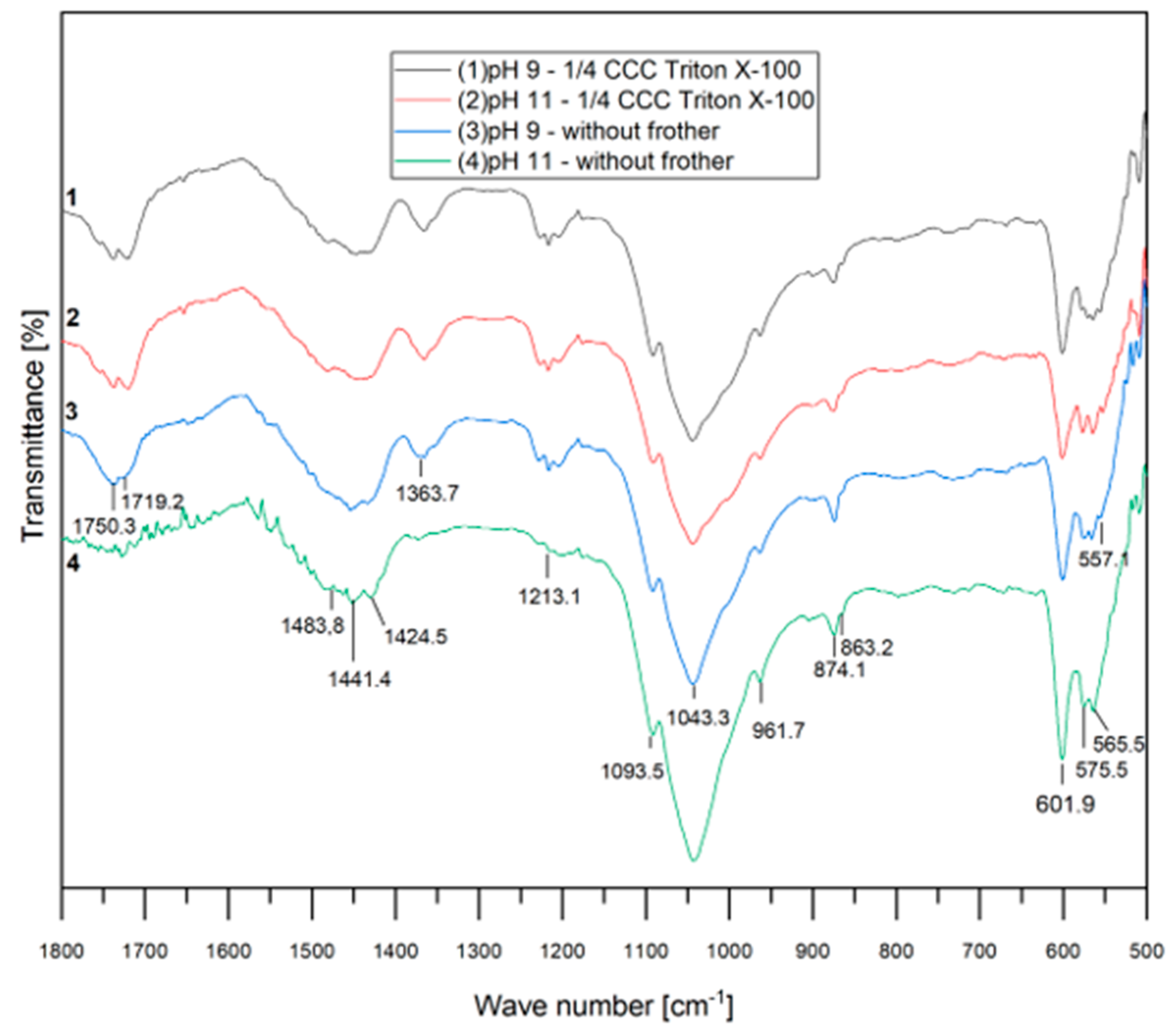
Figure 7 presents the FTIR spectra for each test conducted without and with the nonionic surfactant (at ¼ CCC of Triton X-100) at pH 9 and 11. The curves appear very similar, indicating the absence of chemical adsorption of Triton X-100 in the system. This figure also highlights the regions associated with the carboxylate and apatite groups, as previously described [32,33].
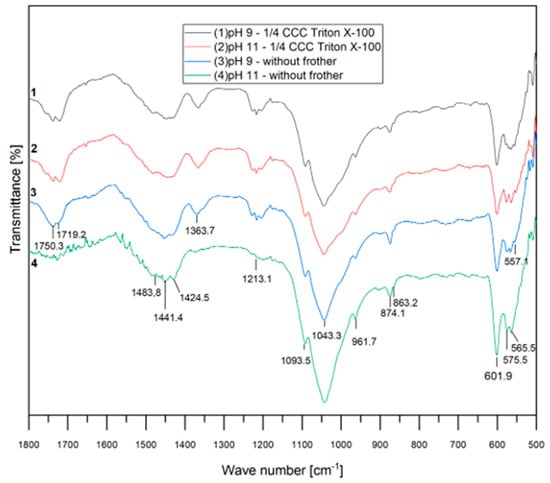
Figure 7.
FTIR spectra with Triton X-100.
Nonionic surfactants are commonly incorporated into the flotation process due to their ability to reduce bubble size and stabilize mineralized froths. Furthermore, they are known for generating robust and stable foams without engaging in ionic interactions with surfaces, a characteristic that aligns with the findings of our FTIR analyses.
3.3. Effect of Surfactant on Bubble Size for Both Systems
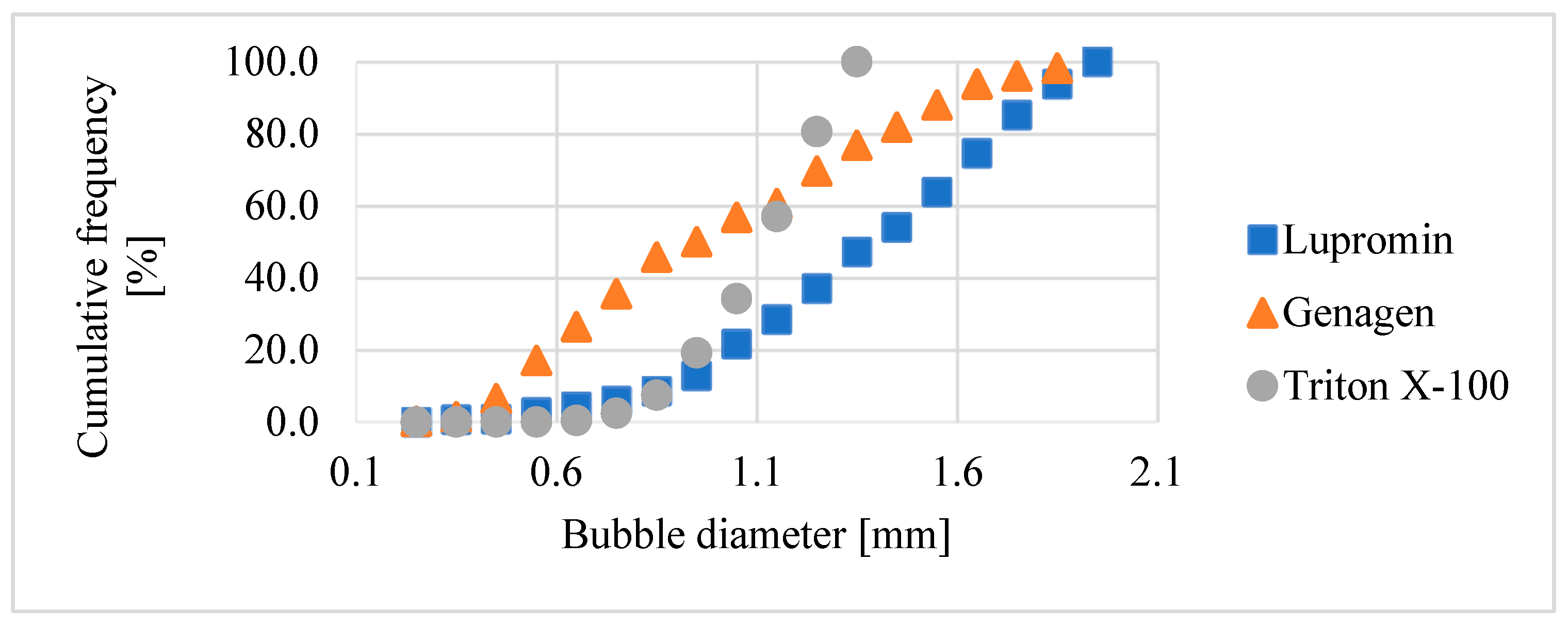
This section provides a comparison of the impact of the three investigated surfactants on bubble size for systems both without ore (air–water) and with ore (air–water–ore). Figure 8 illustrates the bubble size distribution in the air–water system for each type of surfactant at a concentration of 50 ppm, which corresponded to the CCC of Triton X-100. As depicted, each type of surfactant (anionic, amphoteric, and nonionic) had varying effects on reducing bubble size, resulting in distinct bubble size distributions.
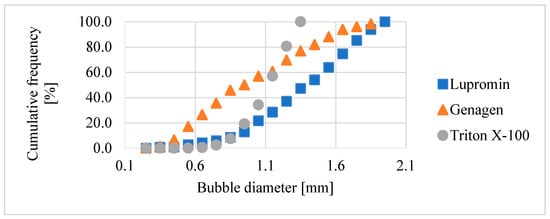
Figure 8.
Bubble size distribution for the air–water system at 50 ppm.
The effect of the surfactant on bubble size in the system with ore was notably different compared to the system without ore (the air–water system). In the presence of ore particles, the mineralized bubbles had their size reduced compared to the system without ore. This reduction in bubble size occurred because the presence of ore particles attached to the surface of the bubbles prevented coalescence, which was a result of the lower surface tension under these conditions [34]. It is known that the concentration of surfactants has a considerable impact on bubbles size, likely due to the adsorption of the surfactant at the gas–liquid interface. Within a pulp, these molecules adsorb at this interface, with their polar groups facing the liquid phase, thereby forming a structured molecular layer. This layer covers the surface of the aqueous solution with nonpolar groups. As the concentration of surfactant increases, there is a corresponding rise in the accumulation of nonpolar groups on the surface, resulting in a reduction in surface tension until a minimum value is achieved. This behavior is consistently observed [35,36].
Figure 9 shows the bubble sizes in the air–water system using Lupromin at ¼ CCC, while Figure 10 illustrates the system with ore using Lupromin at the same concentration and at pH 11. This particular condition was selected because it yielded the highest index of flotation response (i.e., 3.58) among all the conditions analyzed in this study. It is evident that the air–water system had bubbles with irregular shapes due to the coalescence phenomenon, while the system with ore featured more uniform and round-shaped bubbles. Additionally, the d32 of the bubbles in the system without ore was 2.79 times larger than in the system with ore.

Figure 9.
Images of the best condition reached by the addition of Lupromin: ¼ CCC for the air–water system.
Figure 10.
Images of the best condition reached by the addition of Lupromin: ¼ CCC and pH 11 for the system with ore.
The results in Table 3 demonstrate that all three surfactants were effective in reducing bubble sizes, a crucial variable that significantly impacts the probability of bubble–particle interactions, attachment, and retention in the pulp phase. This, in turn, has a substantial influence on the flotation performance. However, it is worth noting that the type of surfactant had varying effects on the selectivity and efficiency of apatite flotation. The anionic surfactant (Lupromin) and the amphoteric surfactant (Genagen) proved to be more effective in enhancing the flotation performance, likely due to their chemical adsorption on the active surface of apatite.
3.4. Global Analysis of the Effect of Surfactant on Apatite Flotation
The best results in apatite flotation, i.e., the highest index of flotation response (3.58), were achieved in test F4. In this test, an anionic surfactant (Lupromin) was employed at a concentration equivalent to ¼ CCC and a pH of 11. This condition yielded a P2O5 grade of 32.57% and an apatite recovery of 74.73%, both significantly exceeding the industrial reference values. In terms of bubble size (d32), it was 32% smaller than that obtained in the best result of the flotation test conducted without a surfactant (test F1).
The superior performance at pH 11 can be attributed to its favoring of chemical adsorption, as indicated in the FTIR analyses (Figure 5 and Figure 6), and likely the collector–surfactant interaction [36].
The impact of surfactants on the flotation process depends on their chemical properties and the applied conditions. Surfactants that reduce superficial tension the most may not necessarily deliver the best performance for the flotation process. In our study, although Triton X-100 exhibited the lowest surface tension in the air–water system, followed by Lupromin and Genagen, its performance in the air–water–ore system varied significantly. This discrepancy can be attributed to the influence of the collector–surfactant interaction on the formation of bubble–particle aggregates, a factor that cannot be overlooked. The nonionic surfactant (Triton X-100) was found to be physically adsorbed on the active surface of apatite (Figure 7).
4. Conclusions
In this study, we examined the impact of various surfactants on the performance of apatite flotation in a column. In the air–water system, it was evident that all the surfactants tested were capable of significantly reducing surface tension, leading to a reduction in bubble size. Notably, the bubble size in the system without ore (air–water) was 2.79 times larger than that in the system with ore (air–water–ore).
During the flotation tests, it was observed that under all conditions examined, the P2O5 grade and apatite recovery exceeded the industrial reference values (30% and 60%, respectively). However, the anionic surfactant (Lupromin) and the amphoteric surfactant (Genagen) demonstrated higher efficiency in enhancing the flotation performance, primarily due to their chemical adsorption onto the active surface of apatite. Furthermore, we observed a collector–surfactant interaction that influenced the formation of bubble–particle aggregates, ultimately favoring true flotation, improved selectivity, and enhanced flotation efficiency.
The current work identified that the best results in apatite flotation, indicated by the highest index of flotation response (3.58), were achieved when utilizing the anionic surfactant (Lupromin) at a concentration equivalent to ¼ CCC and a pH of 11. However, to build on these findings and further enhance the understanding of surfactants behavior in flotation process, it is recommended that future research explores the application of a broader range of surfactants. This could involve measuring the surface tension of the pulp associated with each surfactant under controlled conditions, leading to more efficient and cost-effective flotation processes.
Author Contributions
Conceptualization, M.A.S.B. and T.F.M.; methodology, T.F.M. and A.S.R.; software, T.F.M.; validation, A.S.R., M.A.S.B. and T.F.M.; formal analysis, M.A.S.B. and T.F.M.; investigation, T.F.M. and A.S.R.; resources, M.A.S.B.; data curation, T.F.M.; writing—original draft preparation, T.F.M. and A.S.R.; writing—review and editing, A.C.S. and M.A.S.B.; visualization, T.F.M. and A.S.R.; supervision, A.C.S. and M.A.S.B.; project administration, M.A.S.B.; funding acquisition, M.A.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian research funding agencies CNPq (Brazilian National Council for Scientific and Technological Development), CAPES (Brazilian Federal Agency for the Support and Improvement of Higher Education), and FAPEMIG (State of Minas Gerais Research Support Foundation) with funding following number TEC—APQ-00803-17.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Acknowledgments
We would like to thank the Faculty of Chemical Engineering (FEQUI) and the Federal University of Uberlândia for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ruan, Y.; He, D.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef]
- Decrée, S.; Savolainen, M.; Mercadier, J.; Debaille, V.; Höhn, S.; Frimmel, H.; Baele, J.M. Geochemical and spectroscopic investigation of apatite in the Siilinjärvi Carbonatite Complex: Keys to understanding apatite forming processes and assessing potential for rare earth elements. Appl. Geochem. 2020, 123, 104778. [Google Scholar] [CrossRef]
- Brabcová, Z.; Karapantsios, T.; Kostoglou, M.; Basarová, P.; Matis, K. Bubble-particle collision interaction in flotation systems. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 95–103. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M. Nanobubbles effect on the mechanical flotation of phosphate ore fine particles. Physicochem. Probl. Miner. Process. 2017, 54, 278–279. [Google Scholar] [CrossRef]
- Cheng, G.; Shi, C.; Yan, X.; Zhang, Z.; Xu, H.; Lu, Y. A study of bubble-particle interactions in a column flotation process. Physicochem. Probl. Miner. Process. 2017, 53, 17–33. [Google Scholar] [CrossRef]
- Eskanlou, A.; Khalesi, M.R.; Abdollahy, M.; Chegeni, M.H. Interactional effects of bubble size, particle size, and collector dosage on bubble loading in column flotation. J. Min. Environ. 2018, 9, 107–116. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Xu, M.; Li, C.; Wang, Y.; Zhang, H. Investigation on mechanism of intensifying coal fly ash froth flotation by pretreatment of non-ionic surfactant. Fuel 2019, 254, 115601. [Google Scholar] [CrossRef]
- Momen, M.S.; Etefagh, M.; Roshan, A.H.; Koohestani, H. The effect of different auxiliary collector in flotation of phosphate ore. J. Phys. Theor. Chem. 2021, 17, 145–150. [Google Scholar] [CrossRef]
- Finch, J.A.; Nesset, J.E.; Acuña, C. Role of frother on bubble production and behaviour in flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Norori-McCormac, A.; Brito-Parada, P.R.; Hadler, K.; Cole, K.; Cilliers, J.J. The effect of particle size distribution on froth stability in flotation. Sep. Purif. Technol. 2017, 184, 240–247. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Yan, X.; Wang, L.; Wang, Y.; Zhang, H. Regulation of bubble size in flotation: A review. J. Environ. Chem. Eng. 2020, 8, 104070. [Google Scholar] [CrossRef]
- Santana, R.C.; Ribeiro, J.A.; Santos, M.A.; Reis, A.S.; Ataíde, C.H.; Barrozo, M.A.S. Flotation of fine apatitic ore using microbubbles. Sep. Purif. Technol. 2012, 98, 402–409. [Google Scholar] [CrossRef]
- Bournival, G.; Ata, S.; Jameson, G.J. Bubble and froth stabilizing agents in froth flotation. Miner. Process. Extr. Metall. Rev. 2017, 38, 366–387. [Google Scholar] [CrossRef]
- Aldrich, C.; Feng, D. The effect of mothers on bubble size distributions in flotation pulp phases and surface froths. Miner. Eng. 2000, 13, 1049–1057. [Google Scholar] [CrossRef]
- Tao, D. Role of bubble size in flotation of coarse and fine particles—A review. Sep. Sci. Technol. 2005, 39, 741–760. [Google Scholar] [CrossRef]
- Bournival, G.; Ata, S.; Wanless, E.J. The roles of particles in multiphase processes: Particles on bubble surfaces. Adv. Colloid Interface Sci. 2015, 225, 114–133. [Google Scholar] [CrossRef]
- Reis, A.S.; Reis Filho, A.M.; Demuner, L.R.; Barrozo, M.A.S. Effect of bubble size on the performance flotation of fine particles of a low-grade Brazilian apatite ore. Powder Technol. 2019, 356, 884–891. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhu, T.; Rhuan, X. Imaging study on bubble size distribution in presence of frother in froth flotation. In Proceedings of the International Symposium on Water Resource and Environmental Protection, Xi’an, China, 20–22 May 2011; pp. 1191–1194. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, J.K. Effect of microbubbles and particle size on the particle collection in the column flotation. Korean J. Chem. Eng. 2002, 19, 703–710. [Google Scholar] [CrossRef]
- Reis, A.S. Study of the Influence of the Bubble Size on Apatite Flotation Performance for Different Granulometries. Ph.D. Thesis, University Federal of Uberlandia, Uberlandia, Brazil, 2019. [Google Scholar]
- Cho, Y.S.; Laskowski, L.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araújo, A.C.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Santana, R.C.; Duarte, C.R.; Ataide, C.H.; Barrozo, M.A.S. Flotation selectivity of phosphate ore: Effects of particle size and reagent concentration. Sep. Sci. Technol. 2011, 46, 1511–1518. [Google Scholar] [CrossRef]
- Santana, R.C.; Farnese, A.C.C.; Fortes, M.C.B.; Ataide, C.H.; Barrozo, M.A.S. Influence of particle size and reagent dosage on the performance of apatite flotation. Sep. Purif. Technol. 2008, 64, 8–15. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Queiroz, G.M.; Guimarães, R.C.; Ataide, C.H.; Barrozo, M.A.S. Selectivity in phosphate column flotation. Miner. Eng. 2007, 20, 197–199. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Z.; Xie, Y.; Ai, M.; Zhang, G.; Gui, W. Data-driven adaptive modelling method for industrial processes and its application in flotation reagent control. ISA Trans. 2021, 108, 305–316. [Google Scholar] [CrossRef]
- Li, Y.; Weng, W. Surface modification of hydroxyapatite by stearic acid: Characterization and in vitro behaviors. J. Mater. Sci. Mater. Med. 2008, 19, 19–25. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Bournival, G.; Ata, S. Analysis of bubble coalescence dynamics and postrupture oscillation of capillary-held bubbles in water. Ind. Eng. Chem. Res. 2017, 56, 14781–14792. [Google Scholar] [CrossRef]
- Butt, H.J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef]
- Dal Sasso, G.; Asscher, Y.; Angelini, I.; Nodari, L.; Artioli, G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci. Rep. 2018, 8, 12025. [Google Scholar] [CrossRef]
- Back, S.; Shin, D.; Kim, G.; Lee, A.; Noh, J.; Choi, B.; Huh, S.; Jong, H.; Sung, Y. Influence of amphoteric and anionic surfactants on stability, surface tension, and thermal conductivity of Al2O3/water nanofluids. Case Stud. Therm. Eng. 2021, 25, 100995. [Google Scholar] [CrossRef]
- Rajapakse, N.; Zargar, M.; Sen, T.; Khiadani, M. Effects of liquid phase physicochemical characteristics on microbubble dynamics in dissolved air flotation: A review. Sep. Purif. Technol. 2022, 289, 120772. [Google Scholar] [CrossRef]
- Jia, J.; Yang, S.; Li, J.; Liang, Y.; Li, R.; Tsuji, T.; Niu, B.; Peng, B. Review of the Interfacial Structure and Properties of Surfactants in Petroleum Production and Geological Storage Systems from a Molecular Scale Perspective. Molecules 2024, 29, 3230. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).